Key Points
Question
Are there any differences in the response to apheresis therapy for steroid refractory relapses among patients with histologically defined immunopathological patterns of multiple sclerosis?
Findings
This cohort study of 69 patients defined 3 patterns of multiple sclerosis and observed functional improvement after apheresis therapy in patients with histological pattern 1 (31%) and pattern 2 (55%), but not in patients with pattern 3. Secondary outcome parameters (magnetic resonance imaging and expanded disability status scale improvement) strongly supported the primary outcome.
Meaning
Response to apheresis therapy may be associated with immunopathological patterns and thus with pathological mechanisms of lesion development.
This cohort study evaluates the differences in response to apheresis treatment of patients with 3 histopathologically defined immunopathological patterns of multiple sclerosis.
Abstract
Importance
Plasma exchange and immunoadsorption are second-line apheresis therapies for patients experiencing multiple sclerosis relapses. Early active multiple sclerosis lesions can be classified into different histopathological patterns of demyelination. Pattern 1 and 2 lesions show T-cell– and macrophage–associated demyelination, and pattern 2 is selectively associated with immunoglobulin and complement deposits, suggesting a humoral immune response. Pattern 3 lesions show signs of oligodendrocyte degeneration. Thus it is possible that pathogenic heterogeneity might predict therapy response.
Objective
To evaluate the apheresis response in relation to histopathologically defined immunopathological patterns of multiple sclerosis.
Design, Setting and Participants
This single-center cohort study recruited 69 patients nationwide between 2005 and 2016. All included patients had a diagnosis of early active inflammatory demyelination consistent with multiple sclerosis; were classified into patterns 1, 2, or 3 based on brain biopsy analysis; and underwent apheresis treatments. Patients who had concomitant severe disease, neuromyelitis optica, or acute disseminated encephalomyelitis were excluded.
Main Outcomes and Measures
The primary therapy outcome was a functionally relevant improvement of the relapse-related neurological deficit. Radiological and Expanded Disability Status Scale changes were secondary outcome parameters.
Results
The mean (SD) age of patients was 36.6 (13.3) years; 46 of the 69 participants (67%) were female. Overall, 16 patients (23%) exhibited pattern 1 lesions, 40 (58%) had pattern 2 lesions, and 13 (19%) had pattern 3 lesions. A functional therapy response was observed in 5 of the 16 patients with pattern 1 disease (31%) and 22 of the 40 patients with pattern 2 disease (55%), but none of the 13 patients with pattern 3 disease exhibited improvement (pattern 2 vs 3 P < .001). Radiological improvements were found in 4 (25%), 22 (56%), and 1 (11%) of patients with patterns 1, 2, and 3, respectively. The respective rates of response measured by changes in Expanded Disability Status Scale scores were 25%, 40%, and 0%. Brainstem involvement was a negative predictive factor for the functional therapy response (logarithmic odds ratio [logOR], −1.43; 95% CI, −3.21 to 0.17; P = .03), while immunoadsorption (as compared with plasma exchange) might be a positive predictive factor (logOR, 3.26; 95% CI, 0.75 to 8.13; P = .01).
Conclusions and Relevance
This cohort study provides evidence that the response to apheresis treatment is associated with immunopathological patterns. Patients with both patterns 1 and 2 improved clinically after apheresis treatment, but pattern 2 patients who showed signs of a humoral immune response benefited most. Apheresis appears unlikely to benefit patients with pattern 3 lesions.
Introduction
Apheresis therapies, including plasma exchange (PLEX) and immunoadsorption (IA), are used to treat steroid-unresponsive multiple sclerosis (MS) relapses. Although the percentage of steroid-unresponsive cases is fairly low at 5%, PLEX and IA are important rescue therapies with response rates of 40% to 90%. The mechanism of action is assumed to be the removal of disease-causing agents such as antibodies and autoantibodies, immune complexes, and cytokines. As apheresis therapies are associated with potentially severe risks such as infections, predictors of treatment response are needed.
Histopathologically early active demyelinating MS lesions can be classified into 3 main, intraindividually stable immunopathological patterns of demyelination (patterns 1, 2, and 3), which suggests pathogenic heterogeneity between individuals and may also predict therapy response to PLEX and IA. Patterns 1 and 2 share similar features of demyelination. However, only pattern 2 lesions are selectively associated with immunoglobulins and complement deposited along myelin sheaths and present within macrophages, which suggests an antibody and complement-mediated mechanism of action. Pattern 3 lesions show a more degenerative character and possibly reflect primary oligodendrocytic damage.
Previously, an analysis of 19 patients showed that only patients with pattern 2 pathology responded to PLEX treatment, but none of the patients with patterns 1 or 3 did so. These results suggest that apheresis therapies may be beneficial for patients with pattern 2 lesions, which are characterized by a humoral immune response. This is in line with the known efficacy in antibody-mediated diseases such as neuromyelitis optica. We aimed to retrospectively analyze the PLEX and IA responses in a cohort of 69 patients with MS with histopathologically defined patterns of demyelination who were treated for steroid-unresponsive MS relapses.
Methods
This study was approved by the ethics committee of the University Medical Center Goettingen. Written informed consent was obtained from participants. All brain biopsies included in the analysis had been performed for differential diagnostic procedures in a clinical setting.
Study Cohort
The study cohort was recruited from our German brain biopsy databank, which includes 774 patients nationwide with histologically proven inflammatory demyelination consistent with MS. Not all biopsied patients can be classified into patterns 1, 2, or 3, because the presence of early active demyelination, which represents the earliest stages of lesions, is a prerequisite for classification. Artifacts or insufficient material may also hinder classification. A total of 386 cases in the databank were classifiable into immunopathological patterns, and of these, 69 patients met the inclusion criteria, which were defined as (1) histopathological diagnosis of inflammatory demyelination consistent with early active demyelinating MS, classified into immunopathological patterns 1, 2, or 3 per published criteria; (2) at least 2 treatments with PLEX and/or IA for steroid-unresponsive acute attacks of MS; (3) clinical documentation available for the analysis of the index attack and the therapeutic outcomes of PLEX and/or IA treatments; and (4) no concomitant severe diseases (for example, mental retardation). The index attack was defined as the relapse leading to apheresis therapy. In addition to persons with severe comorbidities, patients with a histopathological diagnosis of neuromyelitis optica or acute disseminated encephalomyelitis, defined according to published criteria, were excluded. This cohort does not overlap with the cohort on which Keegan et al have reported previously.
Histopathology and Classification of Lesions
Histological and immunohistochemical stainings were performed as indicated in the eTable in the Supplement. For the classification of lesions, the research team first determined the demyelinating activity based on published criteria describing early active demyelinating lesions containing myelin-laden macrophages immunoreactive for minor and major myelin proteins. Those lesions were subsequently classified into 1 of the immunopathological patterns 1, 2, and 3 by 2 board-certified neuropathologists (W.B. and I.M.), who were blinded to PLEX and IA treatment response.
Clinical and Radiological Follow-up
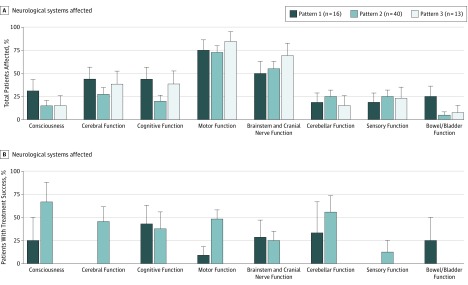
Data analysis was performed in a single center using a standardized protocol. Treatment response, magnetic resonance imaging (MRI) changes, and Expanded Disability Status Scale (EDSS) scores were assessed retrospectively by 1 study investigator (L.S.) and approved by a board-certified neurologist (I.M.), both of whom were blinded to immunopatterns. Clinical information was obtained from medical record review for all 69 participants. Radiological information before PLEX/IA treatment was available for 62 patients (90%) and after apheresis treatment for 46 patients (67%). Multiple sclerosis diagnosis and clinical course at the time of index PLEX/IA treatment were evaluated based on published criteria. Different neurological systems were evaluated to assess which deficits occurred with the index attack (Figure 1). Only new or worsening symptoms occurring with the index attack that influenced EDSS or significantly impacted function were considered.
Figure 1. Affected Neurological Systems and Functional Response to Apheresis Treatment.
A, Neurological systems affected during the index attack leading to PLEX/IA treatment, stratified by immunopathological patterns. Data are presented as the percentage of patients with the specified functional system affected in relation to all patients with the indicated disease pattern. Consciousness includes somnolence, sopor, and coma; cerebral function includes aphasia, apraxia, and other impairments; cognitive function includes memory dysfunction and disorientation. Bowel/bladder involvement differs significantly between patients in patterns 1 and 2 (4 of 16, or 25%, affected vs 2 of 40, or 5%; P = .04). B, Functional response of different systems, stratified per disease patterns. Bars show the percentage of patients with moderate or marked improvement after apheresis treatment in relation to all patients with the respective pattern and functional system affected. For some patients, clinical data were not sufficient to judge therapy response in single functional systems. Differences in functional responses of the motor system between patterns 1 and 2 are significant (improved: 1 of 11, or 9%, in pattern 1, vs 13 of 27, or 48%, in pattern 2; P = .002).
Primary Outcome
The functional outcome of the PLEX/IA treatments was evaluated using an established grading system focused on changes in the affected (targeted) neurological systems. This system defines the response as (1) none (ie, no gain in neurological function); (2) mild gain (ie, subjective or minimal change, without changes in function); (3) moderate gain (ie, gain in neurological status that effects function); and (4) marked gain (ie, substantial difference from baseline with major gain in function). Changes in each neurological system were evaluated separately within 30 days after apheresis treatment.
Secondary Outcome
Secondary outcomes included magnetic resonance imaging (MRI) and EDSS responses. Magnetic resonance imaging changes were investigated using T2-weighted images as well as gadolinium-enhanced T1-weighted images from primary centers that used different protocols. Scans by MRI at the time of index attack and before PLEX/IA treatment (a maximum of 3 weeks between the MRI and initiation of apheresis treatment) were analyzed for new or enlarging hyperintense T2-weighted lesions and gadolinium enhancement (1 or more index lesions). Within 30 days after PLEX/IA, MRI scans were evaluated for index lesion development. The EDSS scores were collected from charts or retrospectively estimated for 2 points: first, the highest EDSS score associated with the index attack before PLEX/IA treatment, and second, the EDSS score within 1 month after apheresis therapy. The EDSS treatment response was defined as a reduction in the EDSS score of 0.5 points or more for patients who had had an EDSS score of 6.0 or more at the time of the index attack, and a reduction of 1.0 or more for patients with an EDSS score of 5.5 or less before PLEX/IA treatment was started.
Statistical Analyses
Descriptive statistics are given for the cohort as a whole and for patients in each immunopattern strata. Comparisons for global group differences were made with the Fisher exact test and the Kruskal-Wallis test or 1-way analysis of variance. To adjust for relevant covariates, the association of the immunopatterns with treatment response in the primary and secondary outcomes were analyzed using logistic regression models. The Firth correction was used to avoid model fitting problems owing to very low response rates in some subgroups. Univariate and multivariate effect measures along with penalized likelihood profiles–based 95% confidence intervals as well as predicted probabilities for various subgroups are given. Selection of relevant covariates was initially done using least absolute shrinkage and selection operator (lasso) and subsequently by backward variable selection, eliminating less informative statistical variables to avoid overfitting. Only variables that significantly differed between the pattern strata were always kept in the model, unless major collinearities appeared. When addressing longitudinal measurements of serial PLEX/IA sessions within single patients, generalized estimation equations with a compound symmetry covariance structure were used to estimate whether a response is predictive for future responses while adjusting for relevant covariates. Statistical analyses were carried out with SAS, version 9.4 (SAS Institute) and R, version 3.1.2 (R Foundation for Statistical Computing). Two-tailed P values smaller than or equal to 0.05 were regarded as statistically significant.
Results
Patient Demographics and Baseline Clinical Features
Demographic data as well as clinical baseline characteristics of 69 eligible patients, stratified by immunopathological patterns, are summarized in Table 1. The groups showed no statistically significant differences in most demographical and clinical parameters listed. However, patients with pattern 3 lesions more frequently showed a clinically isolated syndrome (9 of 13 patients; 69%; in a comparison for all 3 groups, P = .02), whereas patients with pattern 2 lesions more often had a relapsing-remitting disease course (26 of 40 patients; 65%; P = .02). There was a tendency to longer time intervals between the start of the index attack and the apheresis therapy (PLEX/IA delay) in patients with pattern 3 lesions compared with patients exhibiting patterns 1 and 2 (P = .07). The disease duration (from new-symptom onset to apheresis therapy) tended to be longer for patients with pattern 2 lesions (P = .07). Subsequent analyses were corrected for these variables to exclude any possible influence on outcome measures.
Table 1. Demographic and Clinical Characteristics of Cohort at the Time of Plasma Exchange/Immunoadsorption Treatment.
| Characteristic | No. (%) | P Value | |||
|---|---|---|---|---|---|
| All (n = 69) | Pattern 1 (n = 16) | Pattern 2 (n = 40) | Pattern 3 (n = 13) | ||
| Age, y, mean (SD) | 36.6 (13.3) | 35.3 (13.1) | 38.4 (13.9) | 32.7 (11.3) | .38 |
| Female | 46 (67) | 10 (63) | 30 (75) | 6 (46) | .14 |
| Disease duration, y, median (range) | 0.2 (0.0-18.0) | 0.1 (0.0-17.0) | 0.6 (0.0-18.0) | 0.1 (0.0-16.0) | .07 |
| Disease course | |||||
| Clinically isolated syndrome | 28 (41) | 8 (50) | 11 (28) | 9 (69) | .02 |
| Remitting-relapsing | 36 (52) | 7 (44) | 26 (65) | 3 (23) | .02 |
| Secondary progressive | 5 (7) | 1 (6) | 3 (8) | 1 (8) | >.99 |
| Expanded Disability Status Scale score | |||||
| Score at baseline, median (range) | 1.0 (0.0-8.5) | 0.0 (0.0-6.5) | 2.0 (0.0-8.5) | 0.0 (0.0-8.5) | .20 |
| Score at index attack, median (range) | 6.0 (2.0-9.5) | 7.5 (3.5-9.5) | 5.0 (2.0-9.5) | 6.0 (3.0-9.0) | .12 |
| Immunoadsorption and plasma exchange treatment | |||||
| Initiation delay, d, mean (SD) | 25.4 (20.4) | 16.3 (13.6) | 26.5 (22.8) | 33.2 (16.1) | .07 |
| Therapy with high-dose corticosteroids before initiation | 63 (91) | 13 (81) | 37 (93) | 13 (100) | .19 |
| Therapy with disease-modifying drugs in the 3 mo before initiation, No./Total No. (%) | 17/67 (25) | 5/15 (33) | 11/39 (28) | 1 (8) | .25 |
At the time of apheresis therapy, more than two-thirds of the patients (51 of 69; 74%) had clinically definite MS according to the 2011 McDonald criteria. Index attack symptoms leading to PLEX/IA treatment are shown in Figure 1A. Most patients (58 of 69; 84%) presented with multifocal neurological deficits involving more than 1 functional system. A similar distribution of affected functional systems was observed when comparing the different pathological patterns.
Functional Improvement After Apheresis Therapy
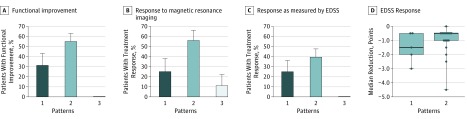
Functioning improved after apheresis therapy in a subgroup of patients exhibiting patterns 1 and 2, but not in patients with pattern 3 disease. Treatment success, defined as moderate or marked therapy response with a functional improvement of deficits, was found in 27 of 69 patients (39%) within 1 month of apheresis. This percentage is consistent with published data. Treatment success differed when comparing patients across the different immunopathological patterns (Figure 2A). The highest response rate to PLEX/IA was in patients with pattern 2 disease, 22 (55%) of whom experienced improvement compared with 0 of 13 patients with pattern 3 disease who experienced improvement (P < .001). One-third of the patients with pattern 1 disease also benefited from therapy (5 of 16 patients; pattern 1 vs 3 P = .03). Clinical improvement could be observed in most functional systems (Figure 1B).
Figure 2. Treatment Response to Apheresis Therapy Stratified by Immunopathological Patterns.
A, Functional response to apheresis therapy is presented as the percentage of patients successfully treated with apheresis treatment, stratified according to pathological patterns; differences between patterns 1 and 3 and between patterns 2 and 3 are significant (P = .03 and P < .001, respectively). B, The percentage of patients with lesion improvement, as established by magnetic resonance imaging is shown; the difference between patterns 2 and 3 is significant (P = .03). C, The percentage of patients with an Expanded Disability Status Scale (EDSS) score in response to apheresis therapy is shown; the difference between patterns 2 and 3 is significant (P = .003). D, The median reductions in EDSS score within 1 month after apheresis therapy in therapy responders with pattern 1 and 2 lesions are shown. Bars and boxes define the minimum, 25th quartile, 75th quartile, and maximum changes in scores. the dots represent individual patients. Data to evaluate MRI and/or EDSS response were omitted when patient data were incomplete.
Lesion Improvement on MRI
Most pronounced lesion improvements on MRI were in patients with pattern 2 lesions, followed by patients with pattern 1 lesions. Changes to MRI results were analyzed as a secondary outcome. Lesion improvement, defined as lesions becoming smaller and/or showing less gadolinium enhancement, was found in 18 of 46 patients (39%). Patients with pattern 2 disease showed lesion regression more often (14 of 25; 56%) compared with patients with pattern 3 disease (1 of 9 patients; 11%; P = .03; Figure 2B). In patients with pattern 1 lesions, lesion improvement was observed in 3 of 12 patients (25%).
EDSS Response
The overall rate of response as measured by EDSS was 28% (n = 19/67) and thus lower than the functional response observed in 39% of patients as described above. The EDSS focuses on the function of the lower extremities and has a poor sensitivity for disabilities that occur because of involvement of upper extremities or because of cognitive changes. Again, the highest EDSS response rate was found for patients exhibiting pattern 2 (15 of 38; 40%), followed by patients exhibiting pattern 1 (4 of 16; 25%). None of the patients with pattern 3 lesions showed an EDSS improvement (0 of 13; 0%; Figure 2C). Focusing on therapy responders, responders with pattern 2 lesions showed a median EDSS reduction of 0.5 points, while responders with pattern 1 lesions showed a median reduction of 1.5 points (Figure 2D). Thus, EDSS evaluation supports the conclusion that patients with patterns 1 and 2 lesions have a more favorable response to PLEX and IA than do patients exhibiting pattern 3, who showed no EDSS response.
Predictors of PLEX/IA
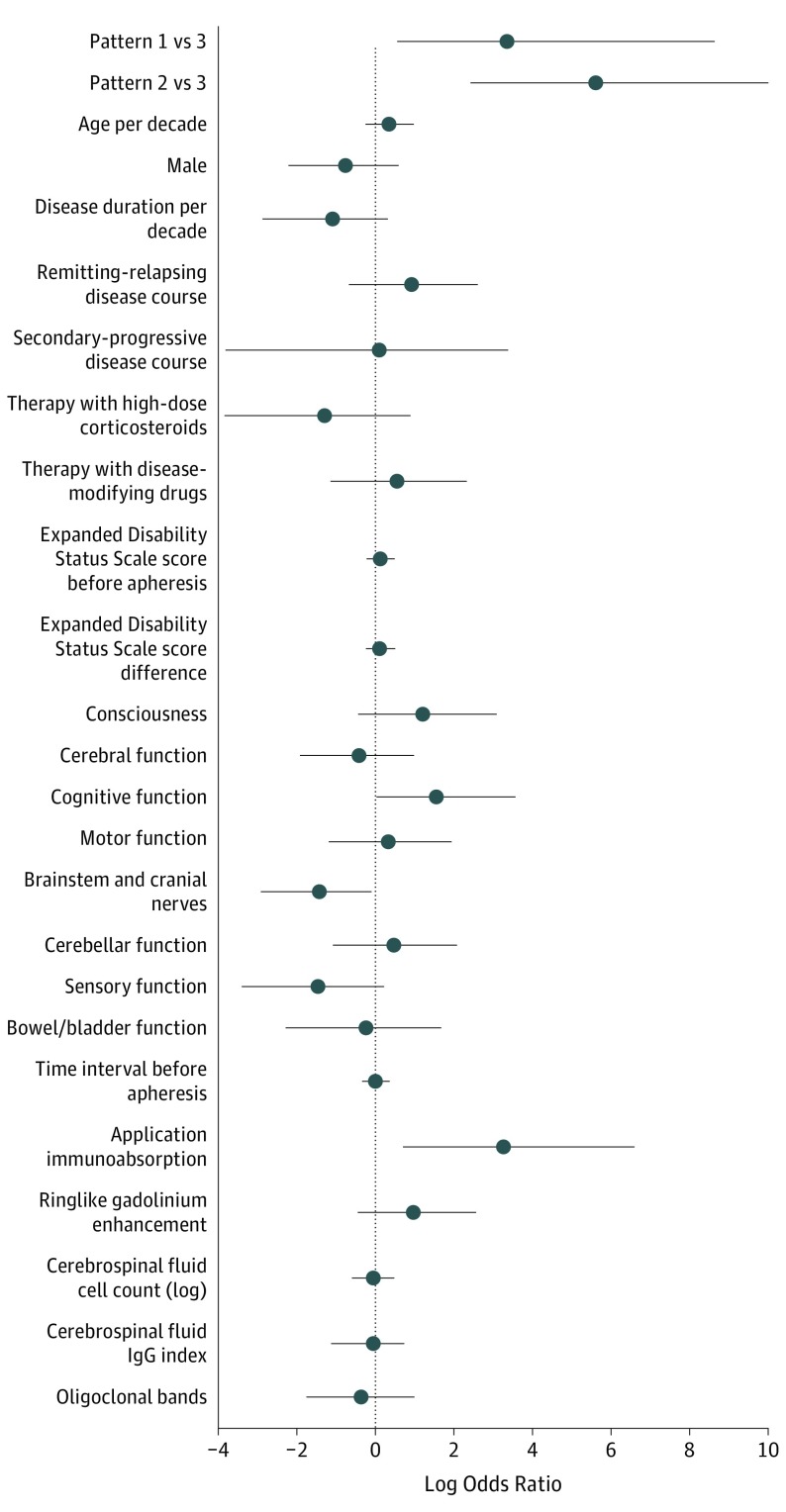
To analyze which factors predict PLEX/IA functional response, various parameters were tested in univariate and multivariate logistic regressions (Figure 3). Immunopattern 1 (logarithmic odds ratio [logOR], 3.35; 95% CI, 0.57-8.59; P = .01) and pattern 2 (logOR, 5.61; 95% CI, 2.49-11.32; P < .001) were found to be positive predictive factors for therapy response. In addition, therapy with IA, compared with PLEX, turned out to be a positive predictive factor for patients in patterns 1 and 2 (logOR, 3.26; 95% CI, 0.75-8.1; P = .01). Clinical involvement of the cognitive system also positively influences therapy response, although with a lower effect size (logOR, 1.56; 95% CI, 0.03-4.37; P = .046). In contrast, involvement of the brainstem emerged as a negative predictive factor (logOR, −1.43; 95% CI, −3.21 to −0.17; P = .03). Thus, we found a high predicted probability that patients would benefit from apheresis, for example, if they had pattern 2 disease without brainstem involvement and were treated with IA (99%; 90% CI, 0.83-1.0; Table 2). It should be noted that in peripheral subgroups, the predicted therapy response rate might be overfitted.
Figure 3. Logistic Regression Model of the Apheresis Treatment Response.
This figure presents estimates on functional response to apheresis therapy. Estimates were obtained by multivariate logistic regression using Firth correction. The estimated log odds ratio of relevant covariates, including penalized likelihood profiles-based 95% confidence intervals, are given per patient experience of moderate/marked functional improvement after apheresis. Covariates with a negative log odds ratio predict no response, while covariates with a positive log odds ratio predict therapy success. Estimates are significant when the 95% CI bars do not cross the log odd ratio 0. Multivariate adjustment included immunopattern; neurological system, brainstem, or cognitive involvement; therapy with immunoadsorption, disease duration, and apheresis treatment delay.
Table 2. Predicted Probability of Therapy Response to Apheresis Treatments, Stratified by Immunopathological Patterns, Brainstem Involvement, and Cognitive Change.
| Lesion Pattern | % (90% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Immunoadsorption Therapy | Plasma Exchange Therapy | |||||||
| Brainstem Affected | Brainstem Not Affected | Cognition Affected | Cognition Not Affected | Brainstem Affected | Brainstem Not Affected | Cognition Affected | Cognition Not Affected | |
| 1 | 65 (23-92) | 89 (49-98) | 91 (54-99) | 69 (26-94) | 7 (1-26) | 23 (8-53) | 29 (10-59) | 8 (2-30) |
| 2 | 95 (64-99) | 99 (83-100) | 99 (84-100) | 96 (68-100) | 41 (24-60) | 74 (54-88) | 80 (51-94) | 45 (31-61) |
Prior publications have found male sex, a shorter delay before PLEX initiation, a shorter disease duration, an EDSS of 5.0 or less at the time of PLEX initiation, and the presence of ringlike contrast enhancement on MRI to be associated with a better PLEX response, factors that were not predictive in our study. However, ringlike enhancement was significantly more often present in patients with pattern 2 lesions (13 of 39; 33%) compared with patients with pattern 3 lesions (0 of 13; 0%; P = .02). The absence of reflexes, which in prior studies was associated with a negative therapy response, was only observed in 1 patient with pattern 1 disease, and this patient showed no therapy response.
Fourteen patients received more than 1 PLEX/IA session. Longitudinal measurements of therapy responses to consecutive PLEX/IA sessions within 1 patient were not positively correlated (ρ = −0.27). Patients with pattern 3 disease did not respond to either the first or any following PLEX/IA sessions.
Discussion
Multiple sclerosis is a heterogeneous disease with respect to its clinical, genetic, radiographic and pathological features. Three main immunopathological patterns of early active MS lesions have been described, with lesions showing an intraindividual pathological homogeneity and interindividual heterogeneity that persists over time. However, this concept has been a matter of debate, and a time-dependent heterogeneity of lesions has been suggested. The clinical and therapeutic relevance of these histopathologically defined patterns is still poorly understood. We analyzed the efficiency of apheresis therapy for steroid-resistant relapses in patients with MS, stratified according to their pattern of early demyelination and therefore in relation to their assumed pathophysiological mechanisms of lesion development. In a large cohort of 69 patients, we confirmed the successful apheresis treatment in more than 50% of patients with pattern 2 lesions. Importantly, we showed that, in addition, every third patient with pattern 1 pathology also responded to the PLEX/IA therapy. This is in contrast to a prior report, which had found no therapy response in patients with pattern 1 lesions, most likely because only 3 patients with pattern 1 lesions were analyzed. Patients with pattern 3 lesions did not display any treatment response.
Several studies support the heterogeneity of early MS lesions by observing distinct chemokine receptor profiles in pattern 2 compared with pattern 3 lesions, and by noting mitochondrial defects only in patients with pattern 3 pathology. The histopathology of pattern 3 lesions resembles white matter stroke, and the mitochondrial changes described in these lesions suggest a hypoxia-like tissue injury rather than an inflammation-driven pathogenesis. This might explain the nonresponse to PLEX/IA treatment.
In contrast, the pathological features of pattern 1 and 2 lesions are similar. They are only distinguishable from each other by the presence of immunoglobulin and complement deposits in patients exhibiting pattern 2, suggesting antibody/complement-mediated demyelination. Antigen microarrays analyzing central nervous system proteins, lipid autoantigens, and heat shock proteins identified unique serum antibody signatures in patients exhibiting pattern 1 vs pattern 2. However, specific pathogenic autoantibodies in patients with MS have not been identified yet, although myelin oligodendrocyte glycoprotein–IgG antibodies may be pathogenic in a low percentage of adult patients with pattern 2 lesions. Removal of antibodies and circulating immune complexes is suggested as a mechanism of action of apheresis therapies, reducing serum antibodies by 85% from preapheresis levels.
In pattern 1 lesions, proinflammatory mediators such as cytokines and chemokines produced by activated microglia or macrophages and T cells were suggested to cause myelin damage. Elimination of these substances may be beneficial in patients with pattern 1 lesions, but data on the removal with PLEX are controversial. Cytokine levels were not lowered after PLEX in septic patients. In contrast, a reduction of interleukin 8 and tumor necrosis factor α cytokine levels was observed after PLEX therapy for thrombotic thrombocytopenic purpura. Fibrinogen and complement 3 were reduced in plasma after PLEX for MS relapses. Thus, elimination of factors other than antibodies may be relevant for the treatment outcomes observed in about one-third of patients with pattern 1 pathology.
In addition to the removal of pathological agents, changes in immune cell numbers, composition and activation after apheresis treatment can also be observed. Changes may occur either because of alterations in concentrations of soluble plasma factors, or because of the apheresis procedure itself.
Both the immunopathological patterns 1 and 2, as well as the application of IA (compared with PLEX), were strongly associated with a beneficial outcome after apheresis treatment. Prior studies have shown significant clinical improvement after IA in 73% to 85% of patients with MS compared with 40% to 70% after PLEX. A combination of both therapies may even be more effective.
Our study was limited by the low number of patients treated with IA (a total of 10, of whom 3 had pattern 1 lesions, 3 had pattern 2 lesions, and 4 had pattern 3 lesions), so that further studies are necessary to explore whether IA may even have superior treatment outcomes. Interestingly, IA not only removes antibodies but also other proteins that may be involved in MS pathogenesis.
Previous studies reported that lesions with ringlike enhancement on MRI were associated with a beneficial therapy response to PLEX, and they correlate with a macrophage rim at the lesion border in pattern 1 and 2 lesions. Although we found a ringlike contrast enhancement significantly more often in patients with pattern 2 than in patients with pattern 3 (none of the pattern 3 patients showed ring enhancement), it was not independently associated with a favorable outcome. This may be related to the limited number of patients (16 of 69; 23%) with available MRI scans and ringlike contrast enhancement in our study.
Limitations
Importantly, the immunopathological patterns can only be determined by histology, which is not routinely available. As there are inherent risks to biopsy, this should not be performed solely for apheresis treatment decisions. Further studies need to identify serological and MRI biomarkers to diagnose MS patterns without taking biopsies.
Another limitation of our study is that the longer delay to PLEX initation in patients with pattern 3 lesions could influence therapy outcome. However, the observed therapy responses in patients with pattern 1 and 2 up to 92 days after symptom onset argues against this possibility. Analyses were corrected for the PLEX delay.
A third limitation is that the biopsied cohort with MS is characterized by atypical clinical presentations, such as tumefactive lesions, which led to the neurosurgical diagnostic procedure. However, comparing patients with MS who undergo biopsy with a nonbiopsied cohort showed no significant differences in demographic parameters and subsequent clinical course. This suggests that, although biopsied patients are atypical in their first clinical presentation, studying them yields important pathological and clinical observations.
Finally, this study is based on brain biopsies and the question arises as to whether our results could be valid for other lesion locations, eg, in the spinal cord or optic nerve. As most of the patients in this study presented with multifocal symptoms involving these regions, it seems likely that these results are valid for other parts of the central nervous system.
Conclusions
In conclusion, this study shows that pathological differences in early MS lesions could not only explain different pathophysiological mechanisms and targets of tissue injury, but also variable responses to apheresis treatment. Therapy response is influenced by multiple factors, including histopathological patterns and possibly also factors associated with IA, brainstem involvement, and disturbed cognitive functions, all of which may be important for therapy planning.
eTable. Antibody list
References
- 1.Schwartz J, Winters JL, Padmanabhan A, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013;28(3):145-284. [DOI] [PubMed] [Google Scholar]
- 2.Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878-886. [DOI] [PubMed] [Google Scholar]
- 3.Magaña SM, Keegan BM, Weinshenker BG, et al. Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol. 2011;68(7):870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehler J, Koball S, Sauer M, et al. Response to therapeutic plasma exchange as a rescue treatment in clinically isolated syndromes and acute worsening of multiple sclerosis: a retrospective analysis of 90 patients. PLoS One. 2015;10(8):e0134583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heigl F, Hettich R, Arendt R, Durner J, Koehler J, Mauch E. Immunoadsorption in steroid-refractory multiple sclerosis: clinical experience in 60 patients. Atheroscler Suppl. 2013;14(1):167-173. [DOI] [PubMed] [Google Scholar]
- 6.Koziolek MJ, Kitze B, Mühlhausen J, Müller GA. Immunoadsorption in steroid-refractory multiple sclerosis. Atheroscler Suppl. 2013;14(1):175-178. [DOI] [PubMed] [Google Scholar]
- 7.Okafor C, Ward DM, Mokrzycki MH, Weinstein R, Clark P, Balogun RA. Introduction and overview of therapeutic apheresis. J Clin Apher. 2010;25(5):240-249. [DOI] [PubMed] [Google Scholar]
- 8.Williams ME, Balogun RA. Principles of separation: indications and therapeutic targets for plasma exchange. Clin J Am Soc Nephrol. 2014;9(1):181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLeod BC. Therapeutic apheresis: history, clinical application, and lingering uncertainties. Transfusion. 2010;50(7):1413-1426. [DOI] [PubMed] [Google Scholar]
- 10.Winters JL. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. Hematology Am Soc Hematol Educ Program. 2012;2012:7-12. [DOI] [PubMed] [Google Scholar]
- 11.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47(6):707-717. [DOI] [PubMed] [Google Scholar]
- 12.Keegan M, König F, McClelland R, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366(9485):579-582. [DOI] [PubMed] [Google Scholar]
- 13.Metz I, Weigand SD, Popescu BFG, et al. Pathologic heterogeneity persists in early active multiple sclerosis lesions. Ann Neurol. 2014;75(5):728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aboul-Enein F, Rauschka H, Kornek B, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62(1):25-33. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Kim W, Huh SY, Lee KY, Jung IJ, Kim HJ. Clinical efficacy of plasmapheresis in patients with neuromyelitis optica spectrum disorder and effects on circulating anti-aquaporin-4 antibody levels. J Clin Neurol. 2013;9(1):36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young NP, Weinshenker BG, Parisi JE, et al. Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain. 2010;133(pt 2):333-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brück W, Popescu B, Lucchinetti CF, et al. Neuromyelitis optica lesions may inform multiple sclerosis heterogeneity debate. Ann Neurol. 2012;72(3):385-394. [DOI] [PubMed] [Google Scholar]
- 18.Brück W, Porada P, Poser S, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38(5):788-796. [DOI] [PubMed] [Google Scholar]
- 19.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(suppl 1):1-5. [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Agency Committee for Medicinal Products for Human Use Guideline on clinical investigation of medical products for the treatment of multiple sclerosis, 2 edition. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500185161.pdf. Published March 2015. Accessed December 28, 2017.
- 22.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58(1):143-146. [DOI] [PubMed] [Google Scholar]
- 23.Llufriu S, Castillo J, Blanco Y, et al. Plasma exchange for acute attacks of CNS demyelination: Predictors of improvement at 6 months. Neurology. 2009;73(12):949-953. [DOI] [PubMed] [Google Scholar]
- 24.Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55(4):458-468. [DOI] [PubMed] [Google Scholar]
- 26.Mahad DJ, Trebst C, Kivisäkk P, et al. Expression of chemokine receptors CCR1 and CCR5 reflects differential activation of mononuclear phagocytes in pattern II and pattern III multiple sclerosis lesions. J Neuropathol Exp Neurol. 2004;63(3):262-273. [DOI] [PubMed] [Google Scholar]
- 27.Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008;131(pt 7):1722-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintana FJ, Farez MF, Viglietta V, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci U S A. 2008;105(48):18889-18894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarius S, Metz I, König FB, et al. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in ‘pattern II multiple sclerosis’ and brain biopsy findings in a MOG-IgG-positive case. Mult Scler. 2016;22(12):1541-1549. [DOI] [PubMed] [Google Scholar]
- 30.König FB, Wildemann B, Nessler S, et al. Persistence of immunopathological and radiological traits in multiple sclerosis. Arch Neurol. 2008;65(11):1527-1532. [DOI] [PubMed] [Google Scholar]
- 31.Spadaro M, Gerdes LA, Mayer MC, et al. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol. 2015;2(3):295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Pauli F, Höftberger R, Reindl M, et al. Fulminant demyelinating encephalomyelitis: insights from antibody studies and neuropathology. Neurol Neuroimmunol Neuroinflamm. 2015;2(6):e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleiter I, Gahlen A, Borisow N, et al. ; Neuromyelitis Optica Study Group . Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206-216. [DOI] [PubMed] [Google Scholar]
- 34.Popescu BFG, Pirko I, Lucchinetti CF. Pathology of multiple sclerosis: where do we stand? Continuum (Minneap Minn). 2013;19(4 Multiple Sclerosis):901-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br J Haematol. 2014;164(3):342-351. [DOI] [PubMed] [Google Scholar]
- 36.Hamishehkar H, Beigmohammadi MT, Abdollahi M, et al. Pro-inflammatory cytokine profile of critically ill septic patients following therapeutic plasma exchange. Transfus Apher Sci. 2013;48(1):75-78. [DOI] [PubMed] [Google Scholar]
- 37.Shariatmadar S, Nassiri M, Vincek V. Effect of plasma exchange on cytokines measured by multianalyte bead array in thrombotic thrombocytopenic purpura. Am J Hematol. 2005;79(2):83-88. [DOI] [PubMed] [Google Scholar]
- 38.Weiner HL, Dau PC, Khatri BO, et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosis. Neurology. 1989;39(9):1143-1149. [DOI] [PubMed] [Google Scholar]
- 39.Yoshii F, Shinohara Y. Lymphocyte subset proportions in Guillain-Barré syndrome patients treated with plasmapheresis. Eur Neurol. 2000;44(3):162-167. [DOI] [PubMed] [Google Scholar]
- 40.Ghio M, Contini P, Ansaldi F, et al. A possible role of soluble HLA-I molecule in the immunomodulatory effects of therapeutic apheresis. Blood Transfus. 2014;12(suppl 1):s167-s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mühlhausen J, Kitze B, Huppke P, Müller GA, Koziolek MJ. Apheresis in treatment of acute inflammatory demyelinating disorders. Atheroscler Suppl. 2015;18:251-256. [DOI] [PubMed] [Google Scholar]
- 42.Faissner S, Nikolayczik J, Chan A, et al. Plasmapheresis and immunoadsorption in patients with steroid refractory multiple sclerosis relapses. J Neurol. 2016;263(6):1092-1098. [DOI] [PubMed] [Google Scholar]
- 43.Brück W, Neubert K, Berger T, Weber JR. Clinical, radiological, immunological and pathological findings in inflammatory CNS demyelination—possible markers for an antibody-mediated process. Mult Scler. 2001;7(3):173-177. [DOI] [PubMed] [Google Scholar]
- 44.Pittock SJ, McClelland RL, Achenbach SJ, et al. Clinical course, pathological correlations, and outcome of biopsy proved inflammatory demyelinating disease. J Neurol Neurosurg Psychiatry. 2005;76(12):1693-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Antibody list