Key Points
Question
Is there an association between the magnetic resonance imaging characteristics of progressive multifocal leukoencephalopathy, especially lesion volume, and the results of polymerase chain reaction for JC virus in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis?
Findings
In this cross-sectional study that included 56 patients, patients with small progressive multifocal leukoencephalopathy lesion volumes had a significantly higher probability for undetectable JC virus DNA or low JC virus copy numbers in cerebrospinal fluid.
Meaning
Strict pharmacovigilance by magnetic resonance imaging will lead to identification of smaller progressive multifocal leukoencephalopathy lesions that associate with a higher likelihood of negative polymerase chain reaction results, which hampers a formal diagnosis of progressive multifocal leukoencephalopathy and may complicate patient treatment.
Abstract
Importance
The JC virus (JCV) was named after the first patient to be described with progressive multifocal leukoencephalopathy (PML), John Cunningham. Detection of JC virus DNA in cerebrospinal fluid (CSF) by polymerase chain reaction (PCR), and of specific lesions by brain magnetic resonance imaging (MRI), are both considered essential for the diagnosis of natalizumab-associated PML (NTZ-PML) in patients with multiple sclerosis. However, strict pharmacovigilance by MRI can result in detection of patients with small lesions and undetectable JCV DNA in CSF.
Objective
To investigate the association of PML lesion characteristics on MRI with both qualitative and quantitative JCV PCR results in CSF of patients with NTZ-PML.
Design, Setting and Participants
This was a retrospective, cross-sectional study conducted from January 2007 to December 2014 in patients considered to have NTZ-PML based on a set of predefined criteria. Follow-up was at least 6 months. Data of patients from the Dutch-Belgian NTZ-PML cohort and patients treated at multiple medical centers in Belgium and the Netherlands and selected for research purposes were included as a convenience sample.
Main Outcomes and Measures
Brain MRI scans were analyzed for PML lesion volume, location, dissemination, and signs of inflammation. Associations of the qualitative and quantitative CSF JCV PCR results with PML MRI characteristics were calculated.
Results
Of the 73 patients screened, 56 were included (37 were women). At inclusion, 9 patients (16.1%) had undetectable JCV DNA in CSF. Patients with a positive PCR had larger total PML lesion volumes than those with undetectable JCV DNA (median volume, 22.9 mL; interquartile range, 9.2-60.4 mL vs median volume, 6.7 mL; interquartile range, 4.9-14.7 mL; P = .008), and logistic regression showed that a lower PML lesion volume significantly increased the probability for undetectable JCV DNA. There was a positive correlation between PML lesion volume and JCV copy numbers (Spearman ρ, 0.32; P = .03). Progressive multifocal leukoencephalopathy lesion volume was higher in patients with PML symptoms and in patients with more widespread lesion dissemination. No association was found between PCR results and PML lesion dissemination, signs of inflammation, or PML symptoms.
Conclusions and Relevance
Smaller NTZ-PML lesions are associated with a higher likelihood of undetectable JCV DNA in CSF. This may preclude a formal diagnosis of PML and can complicate patient treatment in patients with small MRI lesions highly suggestive of PML detected early through pharmacovigilance.
This study investigates the association of progressive multifocal leukoencephalopathy lesion characteristics on magnetic resonance imaging with both qualitative and quantitative John Cunningham virus polymerase chain reaction results in cerebrospinal fluid of patients with natalizumab-associated progressive multifocal leukoencephalopathy.
Introduction
The JC virus (JCV) was named after the first patient to be described with progressive multifocal leukoencephalopathy (PML), John Cunningham. Treatment with natalizumab (NTZ) can lead to PML, a lytic infection of glial and neuronal cells by the John Cunningham virus (JCV).1,2,3 According to the consensus statement from the American Academy of Neurology Neuroinfectious Disease Section, the presence of clinical symptoms, magnetic resonance imaging (MRI) findings suggestive of PML, and JCV DNA in cerebrospinal fluid (CSF) detected by polymerase chain reaction (PCR) are all required to establish the diagnosis of definite PML.4
Early diagnosis of PML leads to improved survival and functional outcome.5 Because brain MRI may reveal PML lesions months before the onset of symptoms,6,7,8,9,10,11 MRI has been introduced as a screening tool in patients treated with NTZ classified as having high risk of developing PML.12,13,14 However, JCV DNA can be undetectable by PCR in CSF of patients with PML,15,16 and we hypothesize that rigorous pharmacovigilance may lead to detection of patients with smaller lesions and more frequently negative PCR results. To our knowledge, there have been no studies investigating an association between PML imaging findings and CSF JCV PCR results in patients with NTZ-PML. We investigated the association of MRI lesion characteristics and presence of PML symptoms with both qualitative and quantitative CSF JCV PCR results.
Methods
Study Design and Patient Selection
This was a retrospective cross-sectional study at the time of PML diagnosis in patients with multiple sclerosis (MS) treated with NTZ. Patients were recruited from a data set comprising patients from the Dutch-Belgian NTZ-PML cohort in multiple medical centers in Belgium and the Netherlands as well as data of patients treated at different medical centers selected for research purposes (Biogen Inc). A subset of the patients has been analyzed and reported previously.17,18,19 This study was approved by the medical ethical committee, VU University Medical Center, Amsterdam, The Netherlands, and written informed consent was obtained from all participants.
We defined inclusion criteria as a diagnosis of PML, meeting 1 of the following conditions:
According to the consensus statement from the American Academy of Neurology Neuroinfectious Disease Section (definite or probable PML [ie, a positive PCR and MRI findings suggestive of PML, with or without PML symptoms]),4 or
In the absence of a positive PCR, the presence of all 4 of the following features: high risk of PML development [ie, positive anti-JCV serostatus and NTZ treatment duration greater than 12 months]20; no MS disease activity prior to PML suspicion; MRI lesions highly suggestive of PML, with lesion characteristics as previously reported and absence of lesion characteristics suggestive of other diseases,21 as judged by an experienced neuroradiologist (M.P.W.); and a lesion evolution on follow-up MRI scans suggestive of PML including development of immune reconstitution inflammatory syndrome.18
Exclusion criteria were:
Absence of a qualitative CSF JCV PCR result (positive or negative) obtained for PML diagnostic workup.
Absence of a brain MRI scan of sufficient quality, including at least fluid-attenuated inversion recovery (FLAIR) or a T2 sequence, performed within 10 days of the CSF collection.
Absence of data on presence or absence of PML symptoms (as stated by the treating neurologist) at the time of CSF collection, and
Suspicion of concomitant granule cell neuronopathy or any other opportunistic infection.22
Cerebrospinal Fluid JCV PCR, MRI, and Clinical Data
The first available CSF JCV PCR result obtained for PML diagnostic workup was used in this study for association with MRI findings. In patients with a positive PCR, the JCV viral load expressed as number of copies per milliliter CSF was used as a quantitative PCR result. If CSF from 1 sample was analyzed in different laboratories, a patient was considered PCR positive when at least 1 PCR result was positive. In cases of more than 1 positive result, the analysis revealing the highest copy number was used for our study. The JCV PCR was performed in different laboratories: Institute of Neurological Disorders and Stroke/National Institutes of Health (NIH), Bethesda, Maryland, or Focus Laboratories (Focus Diagnostics Inc); Unilabs Laboratory, Copenhagen, Denmark; Institute for Virology, Heinrich Heine University, Düsseldorf, Germany; and the Department of Medical Microbiology and Infection Control, VU University Medical Center, Amsterdam, The Netherlands.
The MRI scan acquired closest to the date of CSF collection (per protocol, ≤10 days before or after) was analyzed for association with PCR results. Brain MRI scans were based on local protocols.
For all included patients it was determined whether symptoms suggestive of PML, as reported by the treating neurologist, were present at the time of CSF sampling (asymptomatic vs symptomatic patients with PML).
Image Analysis
All lesions considered as PML, referred to as PML lesions, were identified in consensus by 2 radiology raters with special expertise in the field of inflammatory diseases of the central nervous system (M.P.W. and M.T.W.).21,23 To verify PML lesion identification, the raters also reviewed preceding and subsequent MRI scans. The following characteristics were scored for each PML lesion: location (frontal, parietal, occipital, and temporal lobe; corpus callosum; basal ganglia; thalamus; brainstem; and cerebellum), tissue type involvement (cortical gray matter, juxtacortical white matter, deep white matter, deep gray matter, periventricular white matter, infratentorial white matter, and infratentorial gray matter), and signs suggestive of inflammation (presence of contrast enhancement or punctuate perivascular T2 lesions).24,25 For each patient, the lesion dissemination26 was scored as unilobar (lesions confined to 1 lobe), multilobar (lesions in 2 or more contiguous lobes), or widespread (lesions in 2 or more noncontiguous lobes or present in both hemispheres).
Next, 3 raters (M.T.W., I.K., and M.P.W.) manually delineated all identified PML lesions in consensus using Medical Imaging Processing, Analysis, and Visualization software (National Institues of Health Center for Information Technology), which then calculated lesion volumes. The volumes of all PML lesions in a patient were combined to calculate total PML lesion volume. Volumes of all lesions involving cortical gray matter, periventricular white matter, or infratentorial gray matter were combined to calculate the volume of PML lesions adjacent to CSF. Preferably, the measurements were performed on axial FLAIR images or, when axial FLAIR was unavailable, on sagittal or coronal FLAIR images because FLAIR has the highest sensitivity for the detection of PML lesions.26 When no FLAIR sequence was available, the measurements were performed on axial T2-weighted images.
Statistical Analyses
Using SPSS statistics software, version 22 (IBM Corp), we investigated the associations between qualitative and quantitative CSF JCV PCR results, PML MRI characteristics, and PML symptoms. Because the lesion volumes and quantitative PCR results were not normally distributed, nonparametric analyses (Mann-Whitney U and Kruskal-Wallis test) were used. To determine the association between JCV viral load and lesion volumes, Spearman ρ correlations were computed. For calculating the relation between JCV viral load and other variables, the patients with a negative PCR were excluded. For analysis of the relation between dichotomous and ordinal variables, we used a Pearson χ2 test or Fisher exact test.
We tested total PML lesion volume and both quantitative and qualitative PCR results for possible confounding by age, sex, treatment duration, and laboratory performing the PCR. Finally, we estimated the probability of the PCR being positive or negative depending on the total PML lesion volume via logistic regression. Results with a 2-sided P value of .05 or less were deemed statistically significant.
Results
Patients
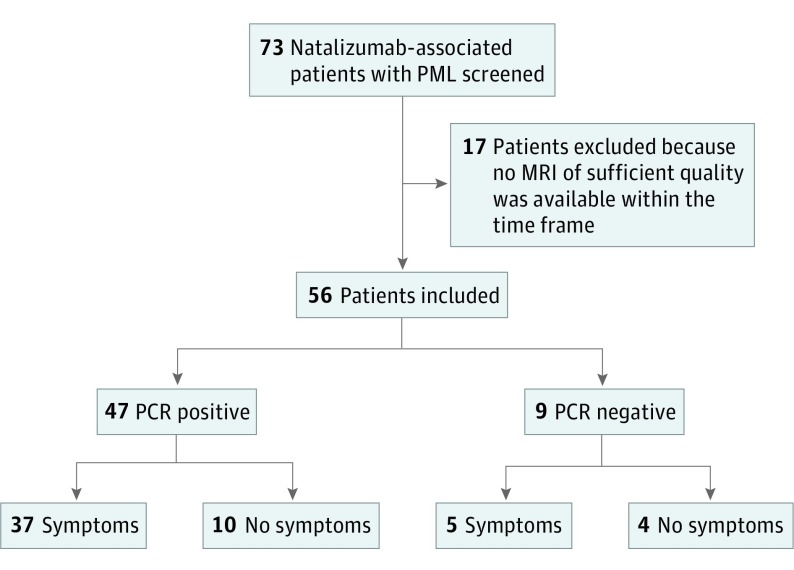
Of the 73 patients screened, 56 met the inclusion criteria (Figure 1). Most patients (n = 37; 65.5%) were female. At the time of PML diagnosis, the median age was 45 years (interquartile range [IQR], 40-53 years) and the median NTZ treatment duration was 43 months (IQR, 26-58 years). At inclusion, 9 of 56 patients (16.1%) were CSF JCV PCR negative and 14 (25%) were asymptomatic for PML (Figure 1). The median interval between CSF collection and MRI was 1 day (IQR, 1-5 days). A total of 133 PML lesions were detected. Table 1 shows information on the MRI sequences used for analysis. The PCR was performed at NIH or Focus Laboratories in 50 patients (89.3%; 43 positive and 7 negative), Unilabs in 4 patients (7.1%; 2 positive and 2 negative), Heinrich Heine University in Düsseldorf in 1 patient (1.8%; positive), and Department of Medical Microbiology and Infection Control, VU University Medical Center, Amsterdam, The Netherlands, in 1 patient (1.8%; positive). In 26 of 50 patients, we have data on whether samples were tested in both NIH and Focus Laboratories. In 5 patients, the samples were sent to both laboratories. In 4 patients, the results were concordant with regard to the qualitative PCR results. In 1 patient, JCV DNA was not detected at the Focus Laboratory but was detected at the NIH laboratory. As outlined in the Methods section, this patient is included in our study as CSF JCV PCR positive. In 4 of 9 PCR-negative patients, new CSF samples were collected during follow-up, turning positive in 2 of 4. The 7 patients without a positive PCR result were considered to have PML based on the presence of all 4 key features of inclusion criterion detailed in the Methods section.
Figure 1. Flowchart Presenting Patient Inclusion and Clinical Characteristics.
MRI indicates magnetic resonance imaging; PCR, polymerase chain reaction; and PML, progressive multifocal leukoencephalopathy.
Table 1. Details on MRI Sequences Available and Used This Study.
| MRI Sequence | No. |
|---|---|
| Available MRI sequences | |
| FLAIR | 55 |
| T2 | 56 |
| T1 with contrast | 52 |
| MRI sequence used for PML lesion volume measurement | |
| Axial FLAIR | 48 |
| Sagittal FLAIR | 4 |
| Coronal FLAIR | 3 |
| Axial T2 | 1 |
Abbreviations: FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy.
Association of PML Lesion Volume With CSF JCV PCR Results, PML Symptoms, and Lesion Dissemination
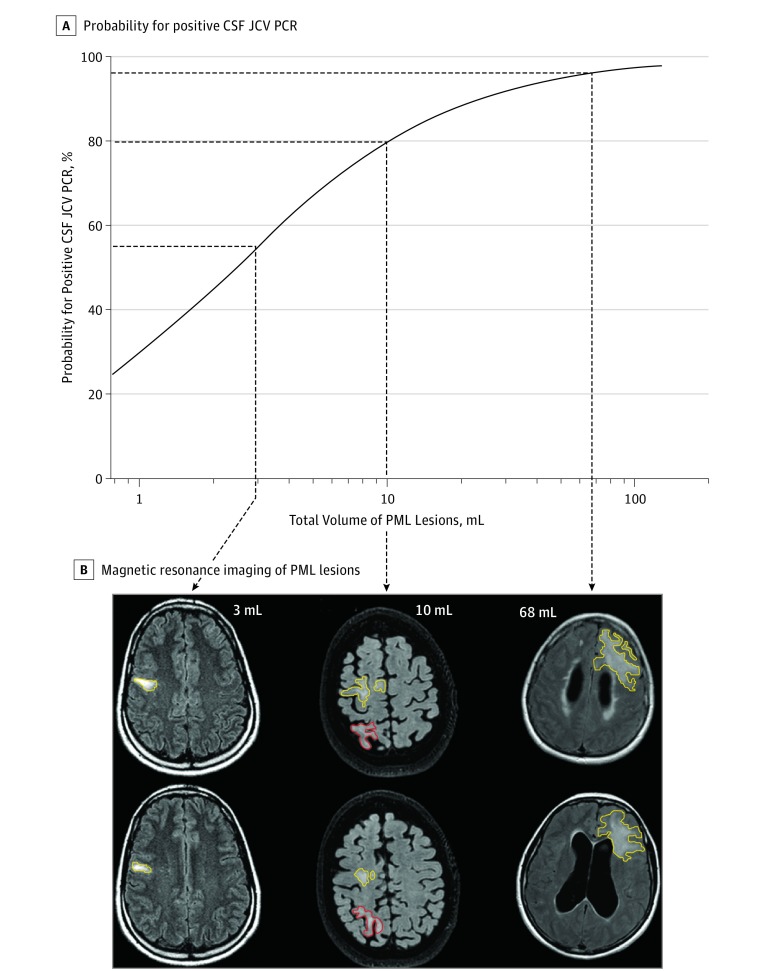
Table 2 shows the association of total PML lesion volume with both qualitative and quantitative CSF JCV PCR results (also illustrated by the eFigure in the Supplement), the presence of PML symptoms, and PML lesion dissemination. Patients with a positive PCR result had a larger total PML lesion volume than those with undetectable JCV DNA. Logistic regression showed that a lower PML lesion volume significantly increased the probability for a negative PCR result (Figure 2). Within the group of PCR-positive patients, there was a positive correlation between total PML lesion volume and the DNA copy number (Spearman ρ, 0.32; P = .03). None of the investigated potential confounders (treatment duration, age, sex, or laboratory performing the PCR) were found to interfere with these associations.
Table 2. Association of Total PML Lesion Volume With Qualitative and Quantitative JCV PCR Results in CSF and Clinical Findings.
| Association | Total PML Lesion Volume, Median (IQR), mL | P Value |
|---|---|---|
| Qualitative JCV PCR | ||
| JCV positive (n = 57) | 22.9 (9.2-60.4) | .008a |
| JCV negative (n = 9) | 6.7 (4.9-14.7) | |
| Presence of PML symptoms, volume, mL | ||
| Present (n = 42) | 28.9 (10.1-60.6) | .005a |
| Not present (n = 14) | 9.2 (3.8-18.3) | |
| Lesion dissemination | ||
| Unilobar (n = 16) | 8.9 (4.6-19.5) | .001b |
| Multilobar (n = 8) | 14.8 (5.3-22.5) | |
| Widespread (n = 32) | 45.9 (16.3-63.4) |
Abbreviations: CSF, cerebrospinal fluid; IQR, interquartile range; JCV, JC virus; PCR, polymerase chain reaction, PML, progressive multifocal leukoencephalopathy.
Calculated by Mann-Whitney U test.
Calculated by Kruskal-Wallis test.
Figure 2. Estimating the Probability for Positive Cerebrospinal Fluid (CSF) JC Virus (JCV) Polymerase Chain Reaction (PCR) Depending on Progressive Multifocal Leukoencephalopathy (PML) Lesion Volume via Logistic Regression.
Patients with PML symptoms had a larger total PML lesion volume than asymptomatic patients. In addition, PML lesion volume increased with lesion dissemination (widespread > multilobar > unilobar). Cerebrospinal fluid adjacency (lesion involvement of cortical gray matter, periventricular white matter, or infratentorial gray matter) was present in 105 of 133 lesions. The volume of PML lesions adjacent to CSF was also associated with qualitative PCR (median volume, 22.9 mL; IQR, 8.5-54.0 mL in PCR-positive patients vs median volume, 6.7 mL; IQR, 4.514.7 mL in PCR-negative patients; P = .01) and quantitative PCR (Spearman ρ, 0.31; P = .04) results.
Association of CSF JCV PCR With Imaging Signs Suggestive of Inflammation and PML Symptoms
No association between PCR results and imaging signs suggestive of inflammation (contrast enhancement and perivascular T2 lesions) or the presence of PML symptoms was detected (eTable in the Supplement). A trend was observed between lesion dissemination (widespread > multilobar > unilobar) and a positive CSF JCV PCR (widespread, 30 positive vs 2 negative; multilobar, 5 positive vs 3 negative; and unilobar, 12 positive vs 4 negative; P = .05).
Discussion
To our knowledge, this is the first study that shows an association between total PML lesion volume measured by brain MRI and CSF JCV PCR results in patients with NTZ-PML. This may have considerable implications for patient care. First, patients with a smaller PML lesion volume are more likely to have a negative test result for JCV, which may lead to a delayed diagnosis of PML. In addition, we show that patients with smaller PML lesion volumes are also more likely to be asymptomatic, which may further delay diagnosis. This can result in a therapeutic dilemma. Unjustly excluding PML may have serious consequences (eg, when switching from NTZ to even more potent immunosuppressive treatments, such as alemtuzumab). On the other hand, inconclusiveness on the presence of PML may also result in inadequate treatment of a patient with active MS. Finally, our data support the notion that the a priori chance for a positive PCR is low in patients without an apparent PML lesion; thus, CSF collection in such patients cannot be generally recommended.
In patients with a positive PCR result, we found a positive correlation between the JCV viral load and total PML lesion volume. In a previous study,27 no such correlation was observed, which may be owing to the smaller sample size. It has been demonstrated that the JCV viral load is negatively associated with survival.28,29 Although not formally tested because of the lack of data on survival and functional outcome, our study suggests that the PML lesion volume on MRI, in addition to the viral load in the CSF, might also serve as marker for disease severity and prognosis.30 However, longitudinal studies are needed to investigate this.
Remarkably, neither perivascular T2 lesions nor contrast enhancement were found to be associated with the PCR results. In addition, no significant association between PML symptoms and PCR results was detected, despite the fact that total PML lesion volume was significantly associated with both qualitative and quantitative PCR results as well as with the presence of PML symptoms. The observed trend that patients with more disseminated PML lesions have a higher risk for a positive PCR result and higher JCV viral load in CSF may be explained by higher PML lesion volumes in these patients and thus is not an independent finding. Cerebrospinal fluid adjacency of PML lesions does not appear to be associated with PCR results because the association between lesion volume of PML lesions with CSF contact and the PCR results equals the association of the total PML lesion volume with the PCR results.
Limitations
Our study has several limitations. First, we included 7 patients who never had a positive PCR result. However, we considered them as patients with PML based on the following considerations detailed in the inclusion critera of the Methods section. First, all 7 patients had lesion characteristics and lesion evolution compatible with PML and not suggestive of any other disease. Second, following immune reconstitution, all 7 patients developed symptoms and MRI findings fitting PML–immune reconstitution inflammatory syndrome. Nevertheless, in the absence of direct evidence of JCV as causative pathogen, the diagnosis of PML cannot be unequivocally established and we cannot completely rule out alternative diagnoses, such as return of MS disease activity, which may mimic MRI features of PML. Therefore, the use of these criteria for the diagnosis of NTZ-PML in patients with undetectable JCV DNA in CSF may be considered clinically premature because these criteria were applied retrospectively for research purposes. Second, this retrospective study included patients from different centers, and MRI acquisition, PCR procedures, and number of repeated CSF collections for JCV PCR in patients with a negative PCR result were therefore not standardized. This might have led to differences in the scoring of PML characteristics. However, we feel that the assessment of lesion characteristics by consensus has mitigated this concern to a large extent. Finally, although to our knowledge potential differences in PCR sensitivity between the laboratories have never been systematically tested, we feel this has not influenced our results. Indeed, the NIH, Focus Laboratories, and Unilabs Laboratories (comprising all negative PCR results in this study) all report very sensitive assays.
Conclusions
In conclusion, we demonstrate that in patients with NTZ-PML, both the probability for a positive CSF JCV PCR result and the JCV viral load are associated with the total PML lesion volume. As a consequence, patients with smaller PML lesion volumes are more likely to have undetectable JCV DNA, and PML can thus not reliably be excluded based on a negative PCR result. The intense pharmacovigilance by MRI of NTZ-treated patients with MS leads to detection of smaller and asymptomatic lesions suggestive of PML and thus more PCR-negative CSF results, which can lead to uncertainty about the diagnosis and clinical treatment. In these patients, meticulous clinical and MRI follow-up in combination with repeated CSF JCV PCR testing is warranted. Complementary PML diagnostic approaches, such as assessment of intrathecal antibody synthesis to JCV by determining the CSF JCV antibody index,31,32 may be of additional value and need to be studied for diagnostic value in PCR-negative patients with suspected PML based on MRI. Furthermore, undetectable JCV DNA does not completely preclude the presence of JCV DNA. Further development and improvement of ultrasensitive PCR assays may improve the diagnostic accuracy in the future.
eFigure. JCV PCR Copy Numbers vs Total Lesion Volume
eTable. Association of Qualitative and Quantitative CSF JCV PCR Results With PML Imaging Characteristics and Presence of PML Symptoms
References
- 1.Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9(4):438-446. [DOI] [PubMed] [Google Scholar]
- 2.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudick R, Polman C, Clifford D, Miller D, Steinman L. Natalizumab: bench to bedside and beyond. JAMA Neurol. 2013;70(2):172-182. [DOI] [PubMed] [Google Scholar]
- 4.Berger JR, Aksamit AJ, Clifford DB, et al. . PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong-Si T, Richman S, Wattjes MP, et al. . Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol. 2014;1(10):755-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havla J, Hohlfeld R, Kümpfel T, Kümpfel T. Unusual natalizumab-associated progressive multifocal leukoencephalopathy starting in the brainstem. J Neurol. 2014;261(1):232-234. [DOI] [PubMed] [Google Scholar]
- 7.Blair NF, Brew BJ, Halpern JP. Natalizumab-associated PML identified in the presymptomatic phase using MRI surveillance. Neurology. 2012;78(7):507-508. [DOI] [PubMed] [Google Scholar]
- 8.Phan-Ba R, Belachew S, Outteryck O, et al. . The earlier, the smaller, the better for natalizumab-associated PML: in MRI vigilance veritas? Neurology. 2012;79(10):1067-1069. [DOI] [PubMed] [Google Scholar]
- 9.Phan-Ba R, Lommers E, Tshibanda L, et al. . MRI preclinical detection and asymptomatic course of a progressive multifocal leucoencephalopathy (PML) under natalizumab therapy. J Neurol Neurosurg Psychiatry. 2012;83(2):224-226. [DOI] [PubMed] [Google Scholar]
- 10.Yousry TA, Pelletier D, Cadavid D, et al. . Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012;72(5):779-787. [DOI] [PubMed] [Google Scholar]
- 11.Wattjes MP, Richert ND, Killestein J, et al. . The chameleon of neuroinflammation: magnetic resonance imaging characteristics of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler. 2013;19(14):1826-1840. [DOI] [PubMed] [Google Scholar]
- 12.McGuigan C, Craner M, Guadagno J, et al. . Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. 2016;87(2):117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wattjes MP, Rovira À, Miller D, et al. ; MAGNIMS study group . Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis: establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606. [DOI] [PubMed] [Google Scholar]
- 14.Traboulsee A, Simon JH, Stone L, et al. . Revised recommendations of the Consortium of MS Centers Task Force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wattjes MP, Vennegoor A, Mostert J, van Oosten BW, Barkhof F, Killestein J. Diagnosis of asymptomatic natalizumab-associated PML: are we between a rock and a hard place? J Neurol. 2014;261(6):1139-1143. [DOI] [PubMed] [Google Scholar]
- 16.Kuhle J, Gosert R, Bühler R, et al. . Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology. 2011;77(23):2010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wattjes MP, Wijburg MT, Vennegoor A, et al. . Diagnostic performance of brain MRI in pharmacovigilance of natalizumab-treated MS patients. Mult Scler. 2016;22(9):1174-1183. [DOI] [PubMed] [Google Scholar]
- 18.Wattjes MP, Wijburg MT, Vennegoor A, et al. ; Dutch-Belgian Natalizumab-associated PML study group . MRI characteristics of early PML-IRIS after natalizumab treatment in patients with MS. J Neurol Neurosurg Psychiatry. 2016;87(8):879-884. [DOI] [PubMed] [Google Scholar]
- 19.Wattjes MP, Vennegoor A, Steenwijk MD, et al. . MRI pattern in asymptomatic natalizumab-associated PML. J Neurol Neurosurg Psychiatry. 2015;86(7):793-798. [DOI] [PubMed] [Google Scholar]
- 20.Borchardt J, Berger JR. Re-evaluating the incidence of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler Relat Disord. 2016;8:145-150. [DOI] [PubMed] [Google Scholar]
- 21.Wijburg MT, Witte BI, Vennegoor A, et al. . MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry. 2016;87(10):1138-1145. [DOI] [PubMed] [Google Scholar]
- 22.Wijburg MT, Siepman D, van Eijk JJ, Killestein J, Wattjes MP. Concomitant granule cell neuronopathy in patients with natalizumab-associated PML. J Neurol. 2016;263(4):649-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodel J, Outteryck O, Dubron C, et al. . Asymptomatic progressive multifocal leukoencephalopathy associated with natalizumab: diagnostic precision with MR imaging. Radiology. 2016;278(3):863-872. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschmidt-DeMasters BK, Miravalle A, Schowinsky J, Corboy J, Vollmer T. Update on PML and PML-IRIS occurring in multiple sclerosis patients treated with natalizumab. J Neuropathol Exp Neurol. 2012;71(7):604-617. [DOI] [PubMed] [Google Scholar]
- 25.Metz I, Radue EW, Oterino A, et al. . Pathology of immune reconstitution inflammatory syndrome in multiple sclerosis with natalizumab-associated progressive multifocal leukoencephalopathy. Acta Neuropathol. 2012;123(2):235-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richert N, Bloomgren G, Cadavid D, Dong-Si T, Richman S, Ticho B. Imaging findings for PML in natalizumab-treated MS patients. Mult Scler. 2012;18(Suppl. 4):27-28. [Google Scholar]
- 27.García De Viedma D, Díaz Infantes M, Miralles P, et al. . JC virus load in progressive multifocal leukoencephalopathy: analysis of the correlation between the viral burden in cerebrospinal fluid, patient survival, and the volume of neurological lesions. Clin Infect Dis. 2002;34(12):1568-1575. [DOI] [PubMed] [Google Scholar]
- 28.Dong-Si T, Gheuens S, Gangadharan A, et al. . Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol. 2015;21(6):637-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoepner R, Kolb EM, Dahlhaus S, et al. . Predictors of severity and functional outcome in natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler. 2017;23(6):830-835. [DOI] [PubMed] [Google Scholar]
- 30.Clifford DB, Nath A, Cinque P, et al. . A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol. 2013;19(4):351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warnke C, von Geldern G, Markwerth P, et al. . Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76(6):792-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warnke C, Wijburg MT, Hartung HP, Killestein J, Adams O, Wattjes MP. Application of the CSF JCV antibody index to early natalizumab-associated progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry. 2017;88(12):1092-1094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. JCV PCR Copy Numbers vs Total Lesion Volume
eTable. Association of Qualitative and Quantitative CSF JCV PCR Results With PML Imaging Characteristics and Presence of PML Symptoms