Key Points
Question
Are the associations between oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers altered by modifiable lifestyle characteristics?
Findings
In this population-based cohort study of more than 100 000 predominantly postmenopausal women, risk reductions for ovarian cancer associated with increasing duration of oral contraceptive use were generally consistent across health behaviors. For endometrial cancer, the reductions were strongest among current smokers, obese women, and those who exercised rarely; lack of associations with breast and colorectal cancer were consistent across health behaviors.
Meaning
The beneficial effects of oral contraceptive use on ovarian and endometrial cancer risk are apparent across most lifestyle factors.
This cohort study uses data from the prospective NIH-AARP Diet and Health Study to examine whether associations between duration of contraceptive use and ovarian, endometrial, breast, and colorectal cancer risk are altered by modifiable lifestyle characteristics.
Abstract
Importance
Although oral contraceptive (OC) use is common, the influence of OC use on carcinogenesis is not fully understood. A recent Agency for Healthcare Research and Quality report identified a need to understand the consistency of OC use and cancer associations across subpopulations, including smokers and obese women.
Objective
To determine whether associations between duration of OC use and risk of specific cancers were modified by lifestyle characteristics.
Design, Setting, and Participants
The prospective NIH-AARP Diet and Health Study (enrolled 1995-1996, followed until 2011), with population-based recruitment of AARP members in 6 states and 2 metropolitan areas. All analyses included at least 100 000 women who reported OC use at enrollment. We identified 1241 ovarian, 2337 endometrial, 11 114 breast, and 3507 colorectal cancer cases during follow-up. Data analysis was performed between September 2016 and April 2017.
Exposures
Duration of OC use (never or <1 year [reference], 1-4, 5-9, or ≥10 years).
Main Outcomes and Measures
Development of ovarian, endometrial, breast, and colorectal cancers. We examined effect modification by modifiable lifestyle characteristics: cigarette smoking, alcohol consumption, body mass index (BMI), and physical activity. We used Cox models adjusted for age, race, age at menarche, and the modifiers of interest.
Results
The analytic population was aged 50 to 71 years (median, 62 years) at enrollment and largely white (91%) and postmenopausal (96%). For ovarian cancer, OC use–associated risk reductions strengthened with duration of use (long-term OC use [≥10 years] HR, 0.60; 95% CI, 0.47-0.76; P < .001 for trend) and were similar across modifiable lifestyle factors. Risk reductions for endometrial cancer strengthened with duration of use (long-term OC use HR, 0.66; 95% CI, 0.56-0.78; P < .001 for trend); the most pronounced reductions were among long-term OC users who were smokers (HR, 0.47; 95% CI, 0.25-0.88), had obese BMIs (0.36; 95% CI, 0.25-0.52), and who exercised rarely (HR, 0.40; 95% CI, 0.29-0.56). Associations between OC use and breast and colorectal cancers were predominantly null.
Conclusions and Relevance
Long-term OC use is consistently associated with reduced ovarian cancer risk across lifestyle factors. We observed the greatest risk reductions for endometrial cancer among women at risk for chronic diseases (ie, smokers, obese BMI). Oral contraceptive use may be beneficial for chemoprevention for a range of women with differing baseline cancer risks.
Introduction
Oral contraceptive (OC) use is common in the United States, and there is much research on benefits and risks associated with OC use. The influence of OC use on the long pathogenesis of cancer needs further study. Increased risks of breast cancer among women currently using OCs are suggested, and risk reductions for ovarian, endometrial, and likely colorectal cancers are associated with increasing duration of OC use.
In 2013, the Agency for Healthcare Research and Quality (AHRQ) released a report on “Oral Contraceptive Use for the Primary Prevention of Ovarian Cancer,” which synthesized the findings from published literature on OC use and risks of several cancers. Data gaps were identified, including a need to understand OC-associated cancer risks in subpopulations, such as those stratified by smoking, obesity, or family history of cancer.
To address this data gap, we estimated associations between duration of OC use and ovarian, endometrial, breast, and colorectal cancer risk and determined whether these associations were modified by lifestyle factors that themselves are modifiable: smoking status, alcohol use, body mass index, and physical activity. We also considered effect modification by family history of breast, colon, and ovarian cancer.
Methods
Study Cohort
The NIH-AARP Diet and Health Study is a large prospective cohort study that enrolled participants in 1995 through 1996. The National Cancer Institute Special Studies Institutional Review Board approved the study. Approximately 3.5 million AARP members who were between the ages of 50 and 71 years and residing in 6 states (California, Florida, Louisiana, New Jersey, North Carolina, or Pennsylvania), Atlanta, Georgia, or Detroit, Michigan, were mailed a baseline questionnaire; 566 398 members completed questionnaires and provided informed consent. We excluded participants who completed questionnaires by proxy (n = 15 760), were men (n = 325 171), had a history of cancer other than nonmelanoma skin cancer (n = 23 998), were identified as having cancer through death reports only (n = 1430), showed disagreement with reported sex (n = 136), indicated their menses stopped due to chemotherapy or radiation (n = 157), and who did not provide information on OC use (n = 3210). The analytic population consisted of 196 536 women.
Information on the duration of OC use, demographic characteristics, and health and lifestyle history was obtained from the baseline questionnaire (before any cancer diagnosis)—including information about our effect modifiers of interest: current smoking status, alcohol use, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), and physical activity. Participants were queried about family history of breast and colon cancers. A questionnaire that provided information on family history of ovarian cancer was sent roughly 6 months after the baseline questionnaire and was used in models stratified by family history of ovarian cancer (information on family history of ovarian cancer was available for 624 cases and 77 254 noncases [51%] of 150 745 women included in the ovarian cancer analyses).
Cohort Follow-up and Case Ascertainment
Cancer cases were ascertained through linkage with state cancer registries for the original 8 states plus Arizona, Texas, and Nevada. Vital status was determined by linkage to the Social Security Administration Death Master File, the National Death Index Plus, and the registries. Participants were observed from enrollment until the first date of diagnosis for a given cancer of interest, the date of death, the end of study follow-up (December 31, 2011), or the date of loss to follow-up, whichever occurred first. Women were not censored until 1 of the prior conditions was met.
We independently examined risks for invasive ovarian, endometrial, breast, and colorectal cancers. For analyses of ovarian cancer, we excluded women with a diagnosis of nonepithelial ovarian cancers or those with unknown histologic type (n = 123) and those who had undergone a bilateral oophorectomy or were missing this information (n = 45 668). Ovarian cancer cases included women with cancers of the ovary (n = 1075), fallopian tube (n = 48), or peritoneum (n = 118; n = 1241 cases total). For endometrial cancer, we excluded those whose cancers were nonepithelial or of unknown histologic type (n = 81) and those who had undergone a hysterectomy or were missing this information (n = 81 932), resulting in 2337 cases. We excluded 23 women with nonepithelial or unknown histologic type breast cancers and 48 with nonepithelial or unknown histologic type colorectal cancers; there were 11 114 breast and 3507 colorectal cancer cases in our analyses.
Statistical Analyses
Participants reported duration of OC use as 1 to 4 years, 5 to 9 years, or 10 or more years, relative to never using OCs or using them for less than 1 year (nonusers). We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals for the associations between OC use and time to diagnosis of a cancer of interest (ovarian, endometrial, breast, or colorectal). Our survival time metric was age (age at baseline to age at diagnosis or end of follow-up); we also adjusted for baseline age to minimize the impact of left truncation and confounding by varying baseline ages. The exact method was used to handle ties, and we examined correlations between ranked failure times and Schoenfeld residuals to evaluate proportional hazards. Our intent was causal inference rather than predictive modeling; we examined our models only for convergence and proportional hazards violations. These were complete case analyses. Missingness was less than 5% for all covariates, except family history of ovarian cancer. We report P values for trends from χ2 tests of significance for the OC duration of use variable treated as continuous measure. All tests of significance were 2 sided and used an α of .05.
We first estimated the overall associations between duration of OC use and each cancer. We then stratified these models by our modifiers of interest: smoking status (nonsmoker, former smoker, or current smoker), alcohol use (nondrinkers, ≤2 servings per day, or >2 servings per day), BMI (<25, 25-29, or ≥30 or greater), and physical activity (periods of activity lasting ≥20 minutes and causing an increase in breathing or heart rate ≥3 times per week, 1-2 times per week, or ≤3 times per month). Although not modifiable, we also report associations stratified by a family history of breast, colon, or ovarian cancer among first-degree relatives for the breast, colorectal, and ovarian cancer outcomes, respectively. We assessed whether the relationship between duration of OC use and cancer risk differed across strata of our modifiers. For each cancer outcome, we used likelihood ratio tests to compare models with and without an interaction term for duration of OC use and each modifier (reported as P for interaction).
We used knowledge of the literature and directed acyclic graphs to select potential confounders a priori. Our models were adjusted for age, race, BMI, age at menarche, and the modifiers of interest. For the models stratified by the modifiers, we adjusted for these factors and the remaining modifiers. When breast or colorectal cancer was the outcome, we additionally adjusted for a family history of breast or colon cancer, respectively.
We performed the following sensitivity analyses to assess the robustness of our findings: for each cancer outcome, we examined the overall duration of OC use associations (1) by cancer histologic type (and receptor status for breast cancer); (2) among postmenopausal women; and (3) adjusted for menopausal hormone therapy use. Finally, given that clinically undetected malignant neoplasms may influence symptoms and reporting of information at baseline, we excluded the first 2 years of follow-up. We computed all statistical analyses with SAS 9.3 (SAS Institute Inc) and generated forest plots with R Studio.
Results
We present characteristics of our population in the eTable in the Supplement. Before exclusions for specific cancer outcomes were made, 60% of the participants were nonusers (n = 118 144); 34 866 women used OCs for 1 to 4 years, 24 564 women used them for 5 to 9 years, and 10% (n = 18 962) were long-term OC users (≥10 years). Compared with women who used OCs for 1 year or less, long-term users were more likely to be younger and premenopausal, have completed more years of education, drink alcohol, and have lower BMIs.
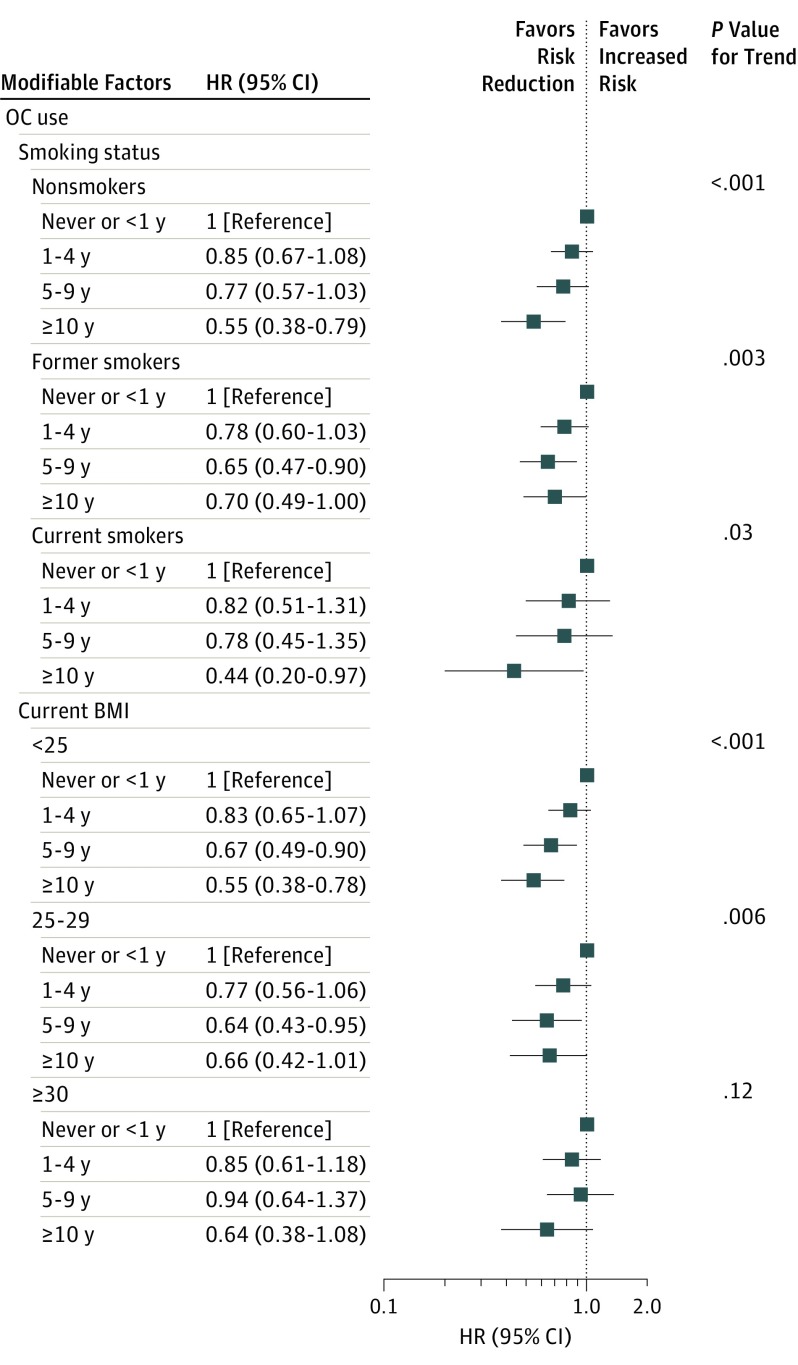
Long-term OC use reduced ovarian cancer risk by 40% (HR, 0.60; 95% CI, 0.47-0.76; P < .001 for trend) (eFigure 1 in the Supplement). We identified reductions in risk associated with increasing duration of OC use across most strata of our modifiers, with the greatest reductions among long-term users. Across strata of smoking status, long-term OC use reduced risk by 30% (former smokers) to 56% (current smokers; P = .08 for interaction) (Figure 1). Increasing duration of OC use was associated with stronger trends in risk reduction among women with BMIs of less than 25 (P < .001 for trend). We noted risk reductions among women not consuming alcohol and women drinking 2 servings of alcohol per day or less (eFigure 1 in the Supplement). The effect estimates among women who consumed more than 2 servings of alcohol per day were imprecise due to small numbers. We observed reduced ovarian cancer risks with long-term OC use across strata of physical activity and identified reductions for all durations of use among those who exercised infrequently (≤3 times per month).
Figure 1. Oral Contraceptive Use and Time to Ovarian Cancer Diagnosis Across Baseline Smoking Status and BMI.
Data are from the NIH-AARP Diet and Health Study. There were 1241 ovarian cancer cases and 149 504 women without ovarian cancer used for comparison in these analyses. Women with bilateral oophorectomy were excluded. Prior oral contraceptive use and lifestyle factors queried at baseline (age 50-71 years). BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; and OC, oral contraceptive.
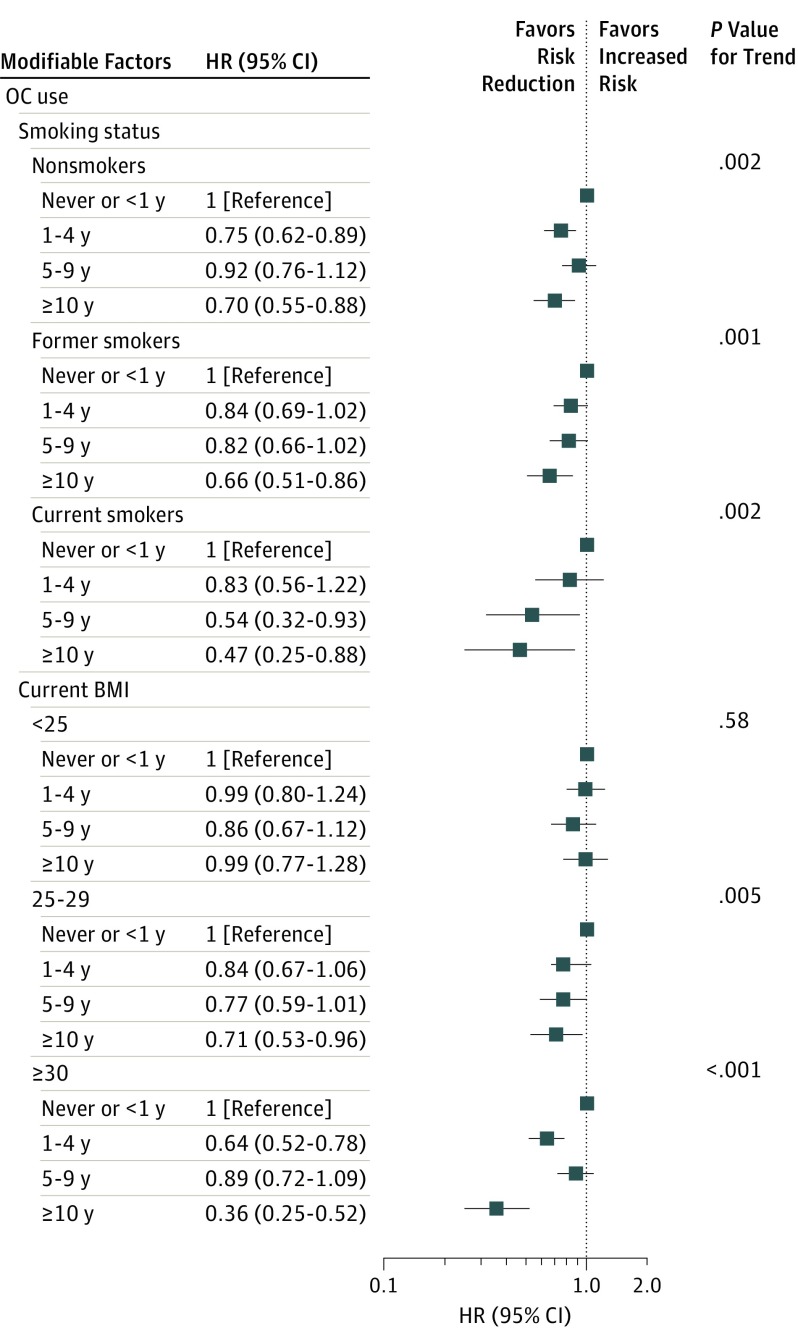
Overall, long-term OC use reduced endometrial cancer risk by 34% (95% CI, 0.56-0.78; P < .001 for trend) (eFigure 2 in the Supplement). We identified the strongest risk reductions among current smokers (long-term use HR, 0.47; 95% CI, 0.25-0.88; P = .002 for trend) but noted reductions among former and nonsmokers as well (Figure 2). We did not find reduced risks for endometrial cancer associated with OC use among women with BMIs of 25 or less. Increasing duration of use reduced risk among women with BMIs of 30 or greater (long-term OC use: HR, 0.36; 95% CI, 0.25-0.52; P < .001 for interaction). We observed OC-associated risk reductions among women consuming alcohol; reductions were strongest, but imprecise among those consuming more than 2 servings per day (eFigure 2 in the Supplement). Long-term OC use reduced endometrial cancer risk by 50% to 60% among those who exercised moderately (1-2 times per week) or infrequently (P < .001 for interaction).
Figure 2. Oral Contraceptive Use and Time to Endometrial Cancer Diagnosis Across Baseline Smoking Status and BMI.
Data are from the NIH-AARP Diet and Health Study. There were 2337 endometrial cancer cases and 112 186 women without endometrial cancer used for comparison in these analyses. Women with hysterectomy were excluded. Prior oral contraceptive use and lifestyle factors queried at baseline (age 50-71 years). BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; and OC, oral contraceptive.
Null associations between OC use and breast cancer were suggested across most strata of our modifiers, but the magnitudes of most associations were in the direction of a slight increase in risk (typically ranging from 0 to 8%) (eFigure 3 in the Supplement). However, long-term OC users who were current smokers were at increased breast cancer risk (HR, 1.21; 95% CI, 1.01-1.44). We identified a trend of increasing risks associated with longer OC use among moderate drinkers (long-term OC use: HR, 1.08; 95% CI, 1.00-1.17; P = .05 for trend).
Associations between OC use and colorectal cancer predominantly suggested no association with risk (eFigure 4 in the Supplement). Strong risk reductions were suggested among women who drank more than 2 servings of alcohol per day, but there was no trend with increasing duration and estimates were not statistically significant except among those who used OCs for 1 to 4 years.
No association between OC use and breast cancer was indicated, regardless of family history (eFigure 5A in the Supplement). Risk reductions for colorectal cancer that strengthened with duration of OC use were suggested among women with a family history of the disease (long-term use HR, 0.62; 95% CI, 0.38-1.01; P = .03 for trend) (eFigure 5B in the Supplement). Reductions in risk associated with longer OC use were also suggested for women with and without a family history of ovarian cancer (eFigure 5C in the Supplement).
Our results were comparable to our main findings when we performed the secondary analyses described in the Methods section (results not shown).
Discussion
We expanded on the AHRQ Evidence Report on OC use and cancer in several ways. Our overall risk reductions for ovarian and endometrial cancers are comparable to those in the report, but we also found risk reductions across strata of modifiable lifestyle factors. Comparing our findings on effect modification is difficult because it was not explored in many studies. Our identification of risk reductions for ovarian and endometrial cancer across most modifiable lifestyle factors suggests that OC use is beneficial for chemoprevention for a range of women with differing baseline risks for these cancers. Oral contraceptives may influence carcinogenesis as a result of the decreased production of and exposure to estradiol across the menstrual cycle—a consequence of the continuous progestin dose provided by OCs. These medications may actually influence long-term changes in hormone metabolism (B. Trabert, PhD, S.B. Coburn, MPH, J.E. Manson, MD, et al; unpublished data; 2017).
Risk reductions for ovarian cancer consistently strengthened with duration of OC use across most lifestyle characteristics evaluated. Although we stratified models for the examined cancers by all modifiers of interest, neither BMI, alcohol use, nor physical activity are considered strong risk factors for most ovarian cancer subtypes. The biologic rationale for any interactions between OC use and these factors on ovarian cancer risk should be further explored. A large collaborative study did not observe OC-effect heterogeneity (ie, interaction) across strata of BMI, tobacco, or alcohol use, but these researchers looked at the associations with duration of OC use only among those who used OCs, while we compared with non-OC users. Similar to our findings, the EPIC cohort noted that increasing duration of OC use conferred a stronger ovarian cancer risk reduction among those with BMIs less than 24. A case-control study similarly noted stronger OC-associated risk reductions among women with a family history of ovarian cancer.
Interestingly, we observed the strongest risk reductions for endometrial cancer among long-term OC users who were smokers, who were obese, or who exercised infrequently. We checked for participant overlap across all 4 of the lifestyle groups that would be deemed “least healthy”; only 29 women (noncases) were common across all groups, so the same women were likely not driving these estimates. A meta-analysis did not identify OC-effect heterogeneity across smoking status, BMI, or alcohol intake (evaluating this only among OC users), but a stronger reduction associated with any OC use among smokers was found in another large cohort. It is possible that our findings among smokers reflect a biologic antiestrogenic interaction between OCs and chemicals in tobacco smoke. The chemopreventive effects of OCs are, in part, attributed to the decrease in endogenous estradiol across the menstrual cycle. However, crediting a mechanism of synergistic interaction should be done cautiously, as the antiestrogenic effects attributed to smoking may differ by menopausal status and may also be mediated by smoking’s effect on obesity. Although we assessed BMI in late life, our BMI results indicate that OC use may help maintain endometrial health among obese women, despite increased risk for this cancer and concerns about hormonal contraceptive effectiveness.
We identified attenuated risks for breast cancer relative to those from the AHRQ report’s meta-analysis (any use odds ratio, 1.08; 95% CI, 1.00-1.17). This may be explained by the high proportion of postmenopausal women in our population, many of whom likely stopped using OCs years before enrollment. A large collaborative group found that increased risks for breast cancer are associated with current OC use and that these risks diminish after cessation. The strongest risk for breast cancer in our study was among long-term OC users who were current smokers. The interaction between OCs and smoking is likely complex; most OCs provide continual doses of both estrogen and progestin and may elevate breast cancer risk through the “estrogen plus progestin hypothesis” (ie, the idea that progestins stimulate proliferation in breast tissue).
We did not observe a risk reduction for colorectal cancer as strong as that identified in the AHRQ meta-analysis (any use odds ratio, 0.86; 95% CI, 0.79-0.95), but other large cohorts suggest no association. We identified potential OC-associated risk reductions among women with a family history of colon cancer. Our risk reduction among women who drank more than 2 servings of alcohol per day is interesting given that heavy alcohol consumption is associated with increased incidence of colorectal cancer—although it should be noted that there was no dose response with duration of OC use. Both OC use and alcohol consumption can decrease folate levels, but research suggests that folate reduces or increases risk for colorectal cancer depending on the presence of polyps, so the timing of these exposures may be integral in carcinogenesis.
Limitations
To our knowledge, the NIH-AARP Diet and Health study is the largest single US data source that has been used to evaluate OC use and cancer; previous analyses only report on effect modification by parity or use of menopausal hormone therapy. We were interested in evaluating the total effect of OC use on cancer risk. Therefore, we did not adjust for potential mediators such as parity (ie, on a causal path between OC use and cancer risk). Adjusting for a mediator may bias effect estimates or result in exposure enrichment (not evaluating the same association across strata of the mediator). For example, nulliparous women, compared with parous women, may have used different OCs, used OCs for different indications (eg, endometriosis), or used OCs differently (eg, longer uninterrupted periods of use). Therefore, the OC effect among nulliparous women is potentially measuring something different than among parous women. Unfortunately, information on parity before and after every episode of OC use, as well as mediation analysis methods (eg, inverse probability weighting or g-methods), is required to elucidate the complex and time-varying relationship between parity, OC use, and cancer risk. As with other studies on this subject, incomplete information prevents the use of mediation methods that can be used to control for confounding due to parity that is causally related to the choice to use these medications while also estimating the total effect of OC use on cancer risk that is in part due to these medications’ influence on parity. However, it is reassuring that our overall findings are consistent with most studies, many of which adjust for parity.
Conclusions
We did not have information on recency of OC use or OC formulation, but our participants were likely using first- and second-generation drugs (marketed before 1989); these drugs contained higher doses of estradiol and progestins with more androgenic activity compared with newer OCs. If the primary mechanism of these medications is altering hormone metabolism, the associations between newer medications and cancer risk may differ for future cohorts. Therefore, continued study of OC use as primary prevention for cancer is warranted. Ultimately, this study contributes to a body of literature that may enable clinicians to better counsel their patients about the risks and benefits of taking OCs.
eTable. Characteristics of the NIH-AARP Diet and Health Study population across categories of duration of oral contraceptive use
eFigure 1. Oral contraceptive use duration and time to ovarian cancer diagnosis across lifestyle characteristics
eFigure 2. Oral contraceptive use duration and time to endometrial cancer diagnosis across lifestyle characteristics
eFigure 3. Oral contraceptive use duration and time to breast cancer diagnosis across lifestyle characteristics
eFigure 4. Oral contraceptive use duration and time to colorectal cancer diagnosis across lifestyle characteristics
eFigure 5. Oral contraceptive use duration and time to breast (A), colorectal (B), or ovarian (C) cancer diagnosis across family history
References
- 1.De Leo V, Musacchio MC, Cappelli V, Piomboni P, Morgante G. Hormonal contraceptives: pharmacology tailored to women’s health. Hum Reprod Update. 2016;22(5):634-646. [DOI] [PubMed] [Google Scholar]
- 2.Petitti DB. Clinical practice. Combination estrogen-progestin oral contraceptives. N Engl J Med. 2003;349(15):1443-1450. [DOI] [PubMed] [Google Scholar]
- 3.Havrilesky LJ, Gierisch JM, Moorman PG, et al. Oral Contraceptive Use for the Primary Prevention of Ovarian Cancer. Evidence Report/Technology Assessment No. 212. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713-1727. [DOI] [PubMed] [Google Scholar]
- 5.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119-1125. [DOI] [PubMed] [Google Scholar]
- 6.Kleinbaum DG, Klein M. The Cox Proportional Hazards Model and Its Characteristics: Using Age as the Time Scale. Survival Analysis: A Self-Learning Text. 3rd ed New York, NY: Springer; 2012:97-160. [Google Scholar]
- 7.Kleinbaum DG, Klein M. Survival Analysis on the Computer (Part B: SAS): Assessing the PH Assumption With a Statistical Test. Survival Analysis: A Self-Learning Text. 3rd ed New York, NY: Springer; 2012:570-670. [Google Scholar]
- 8.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531-1543. [PubMed] [Google Scholar]
- 9.Chan MF, Dowsett M, Folkerd E, et al. Past oral contraceptive and hormone therapy use and endogenous hormone concentrations in postmenopausal women. Menopause. 2008;15(2):332-339. [DOI] [PubMed] [Google Scholar]
- 10.Beral V, Doll R, Hermon C, Peto R, Reeves G; Collaborative Group on Epidemiological Studies of Ovarian Cancer . Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303-314. [DOI] [PubMed] [Google Scholar]
- 11.Tsilidis KK, Allen NE, Key TJ, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2011;105(9):1436-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker GR, Schlesselman JJ, Ness RB. Family history of cancer, oral contraceptive use, and ovarian cancer risk. Am J Obstet Gynecol. 2002;186(1):8-14. [DOI] [PubMed] [Google Scholar]
- 13.Collaborative Group on Epidemiological Studies on Endometrial Cancer Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16(9):1061-1070. [DOI] [PubMed] [Google Scholar]
- 14.Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1-580.e9. [DOI] [PubMed] [Google Scholar]
- 15.Gu F, Caporaso NE, Schairer C, et al. Urinary concentrations of estrogens and estrogen metabolites and smoking in Caucasian women. Cancer Epidemiol Biomarkers Prev. 2013;22(1):58-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand JS, Chan MF, Dowsett M, et al. Cigarette smoking and endogenous sex hormones in postmenopausal women. J Clin Endocrinol Metab. 2011;96(10):3184-3192. [DOI] [PubMed] [Google Scholar]
- 17.Simmons KB, Edelman AB. Hormonal contraception and obesity. Fertil Steril. 2016;106(6):1282-1288. [DOI] [PubMed] [Google Scholar]
- 18.Pike MC, Spicer DV. Hormonal contraception and chemoprevention of female cancers. Endocr Relat Cancer. 2000;7(2):73-83. [DOI] [PubMed] [Google Scholar]
- 19.Tsilidis KK, Allen NE, Key TJ, et al. Oral contraceptives, reproductive history and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2010;103(11):1755-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho E, Lee JE, Rimm EB, Fuchs CS, Giovannucci EL. Alcohol consumption and the risk of colon cancer by family history of colorectal cancer. Am J Clin Nutr. 2012;95(2):413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari P, McKay JD, Jenab M, et al. Alcohol dehydrogenase and aldehyde dehydrogenase gene polymorphisms, alcohol intake and the risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr. 2012;66(12):1303-1308. [DOI] [PubMed] [Google Scholar]
- 22.Shere M, Bapat P, Nickel C, Kapur B, Koren G. Association between use of oral contraceptives and folate status: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2015;37(5):430-438. [DOI] [PubMed] [Google Scholar]
- 23.Lindenbaum J. Folate and vitamin B12 deficiencies in alcoholism. Semin Hematol. 1980;17(2):119-129. [PubMed] [Google Scholar]
- 24.Kim YI. Role of folate in colon cancer development and progression. J Nutr. 2003;133(11)(suppl 1):3731S-3739S. [DOI] [PubMed] [Google Scholar]
- 25.Chiang FF, Huang SC, Wang HM, Chen FP, Huang YC. High serum folate might have a potential dual effect on risk of colorectal cancer. Clin Nutr. 2015;34(5):986-990. [DOI] [PubMed] [Google Scholar]
- 26.Arem H, Park Y, Felix AS, et al. Reproductive and hormonal factors and mortality among women with colorectal cancer in the NIH-AARP Diet and Health Study. Br J Cancer. 2015;113(3):562-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodelon C, Wentzensen N, Schonfeld SJ, et al. Hormonal risk factors and invasive epithelial ovarian cancer risk by parity. Br J Cancer. 2013;109(3):769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17-32. [DOI] [PubMed] [Google Scholar]
- 29.Ahrens KA, Cole SR, Westreich D, Platt RW, Schisterman EF. A cautionary note about estimating effects of secondary exposures in cohort studies. Am J Epidemiol. 2015;181(3):198-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golobof A, Kiley J. The current status of oral contraceptives: progress and recent innovations. Semin Reprod Med. 2016;34(3):145-151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of the NIH-AARP Diet and Health Study population across categories of duration of oral contraceptive use
eFigure 1. Oral contraceptive use duration and time to ovarian cancer diagnosis across lifestyle characteristics
eFigure 2. Oral contraceptive use duration and time to endometrial cancer diagnosis across lifestyle characteristics
eFigure 3. Oral contraceptive use duration and time to breast cancer diagnosis across lifestyle characteristics
eFigure 4. Oral contraceptive use duration and time to colorectal cancer diagnosis across lifestyle characteristics
eFigure 5. Oral contraceptive use duration and time to breast (A), colorectal (B), or ovarian (C) cancer diagnosis across family history