Key Points
Question
Are the efficacy and safety of clopidogrel plus aspirin vs aspirin alone consistent in different infarction patterns after transient ischemic attack or minor stroke?
Findings
In the imaging substudy of the Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events randomized clinical trial that included 1089 transient ischemic attacks and minor strokes, a significant risk reduction of 50% in stroke recurrence was observed in patients with multiple acute infarctions administered clopidogrel plus aspirin compared with aspirin alone, and this finding was not observed in patients with a single acute infarction or no acute infarction. The bleeding risk was similar among treatment groups.
Meaning
Patients with multiple acute infarctions received the most pronounced clinical benefit from dual antiplatelet therapy.
Abstract
Importance
Infarction patterns may serve as important imaging markers to assess the probability of stroke recurrence in transient ischemic attack (TIA) and minor stroke. However, it is unclear whether patients with different infarction patterns benefit differently from dual antiplatelet therapy.
Objectives
To investigate whether infarction patterns can stratify the risk of recurrent stroke and whether the efficacy and safety of clopidogrel plus aspirin vs aspirin alone are consistent in different infarction patterns after TIA or minor stroke.
Design, Setting, and Participants
In this prespecified imaging substudy of the Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events (CHANCE) randomized clinical trial, a total of 1342 patients with noncardioembolic TIA or minor stroke at 45 sites of CHANCE from October 1, 2009, to July 30, 2012, were included in this substudy. The final analysis was conducted on July 30, 2016, and included 1089 patients with required magnetic resonance imaging sequences. Infarction patterns were grouped into multiple acute infarctions (MAIs), single acute infarction (SAI), and no acute infarction (NAI) according to diffusion-weighted imaging.
Main Outcomes and Measures
Primary and secondary efficacy outcomes were stroke recurrence and new clinical vascular event after 3 months, respectively. The safety outcome was moderate to severe bleeding risk after 3 months.
Results
Among 1089 patients, the mean (SD) age was 63.1 (10.7) years and 731 patients (65%) were men. Patients with MAIs (hazard ratio [HR], 5.8; 95% CI, 2.2-15.1; P < .001) and SAI (HR, 3.9; 95% CI, 1.5-10.5; P = .007) had higher risk of recurrent stroke than those with NAI after adjustment for potential confounders at 3-month follow-up. Stroke recurrence occurred in 15 (10.1%) and 25 (18.8%) of patients with MAIs administered clopidogrel plus aspirin and placebo plus aspirin, respectively (HR, 0.5; 95% CI, 0.3-0.96; P = .04), 24 (8.9%) and 24 (8.5%) of patients with SAI administered clopidogrel plus aspirin and placebo plus aspirin, respectively (HR, 1.1; 95% CI, 0.6-2.0; P = .71), and 3 (2.6%) and 2 (1.4%) of patients with NAI administered clopidogrel plus aspirin and placebo plus aspirin, respectively (HR, 1.7; 95% CI, 0.3-11.1; P = .56), with P = .04 for treatment × infarction pattern interaction effect. Clopidogrel plus aspirin did not increase moderate to severe bleeding risk.
Conclusions and Relevance
Infarction patterns can efficiently stratify the risk of recurrent stroke within 3 months of noncardioembolic TIA or minor ischemic stroke. Patients with MAIs received the most pronounced clinical benefit from dual antiplatelet therapy without increasing the risk of moderate to severe bleeding. However, even if after dual antiplatelet treatment, patients with MAIs still had a risk of stroke recurrence as high as those with SAI.
Trial Registration
clinicaltrials.gov Identifier: NCT00979589
This imaging substudy of a randomized clinical trial investigates whether infarction patterns can be used to stratify the risk of recurrent stroke within 3 months of a transient ischemic attack or minor ischemic stroke.
Introduction
Transient ischemic attack (TIA) and minor ischemic stroke are the most common manifestations of acute cerebrovascular disease.1 Previous studies demonstrated that patients with TIA or minor stroke have a high risk of stroke recurrence especially in the early period after stroke.2,3,4,5 A recent study from a worldwide registry of TIA and minor stroke indicated a more rapid reduction of stroke recurrence than previously reported owing to the implementation of standardized secondary stroke prevention strategies. This study also showed that patients with multiple acute infarctions (MAIs) have higher risk of stroke recurrence than those with single acute infarction (SAI) or no acute infarction (NAI) in TIA and minor ischemic stroke, implying that the acute infarction patterns identified using magnetic resonance imaging (MRI) may be used to stratify the risk of recurrent stroke.6
Stroke with different infarction patterns has very different pathogenetic origins. Multiple acute infarctions from embolism are common in patients with acute ischemic stroke.7,8,9 Artery-to-artery embolism, cardioembolism, and embolism from other undetermined sources are often the causes of MAIs.10 Single acute infarction characterized as lacunar infarction is usually caused by a single subcortical infarction from a small-vessel occlusion with underlying vascular changes, such as lipohyalinosis or fibrinoid necrosis of small perforating arteries,11,12,13,14 and SAI characterized as nonlacunar infarction is usually caused by cardiac embolism, artery-to-artery embolism, or embolism from other undetermined sources.11,12
The Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events (CHANCE) randomized clinical trial has demonstrated the superiority of clopidogrel and aspirin combination over aspirin alone in reducing the risk of recurrent stroke in the first 3 months after noncardioembolic TIA and minor stroke, without increasing hemorrhagic risk.15,16 In addition, there is evidence suggesting that dual antiplatelet therapy with clopidogrel plus aspirin is more effective than aspirin alone in reducing artery-to-artery microemboli in patients with TIA and ischemic stroke,17,18 and dual antiplatelet therapy may not be effective in patients with lacunar stroke characterized as an SAI.19,20,21 This evidence suggests that the efficacy of dual antiplatelet therapy may be different for stroke with distinct pathogenetic types.
In this prespecified imaging substudy of the CHANCE trial, we examine whether infarction patterns can be used to stratify the risk of recurrent stroke within 3 months of a TIA or minor ischemic stroke. We also evaluate the efficacy and safety of clopidogrel plus aspirin vs aspirin alone in TIA or minor stroke with different infarction patterns (NAI, SAI, and MAIs).
Methods
Overview of the CHANCE Trial
The detailed design and methods of the CHANCE trial have been previously described.15,16 Briefly, CHANCE was a randomized, double-blind, placebo-controlled clinical trial conducted at 114 clinical centers in China from October 1, 2009, to July 30, 2012, within 24 hours of noncardioembolic minor ischemic stroke or high-risk TIA onset. A total of 5170 patients were randomly assigned to either the clopidogrel plus aspirin (clopidogrel at an initial dose of 300 mg, followed by 75 mg/d for 90 days, plus aspirin at 75 mg/d for the first 21 days) the placebo plus aspirin (75 mg/d for 90 days). The trial was approved by the ethics committee of Beijing Tiantan Hospital. Written informed consent was obtained from all participants or their legal proxies.
Overview of the Imaging Substudy of the CHANCE Trial
This imaging study was a prespecified substudy of the CHANCE trial.22,23 Patients from 45 of 114 sites (39%) of the CHANCE trial were prospectively recruited into the imaging substudy from October 1, 2009, to July 30, 2012. All patients in this substudy were asked to complete the MRI examinations (3.0 or 1.5 T) during hospitalization. Patients with the following MRI sequences were included in the current analysis: T1-weighted imaging, T2-weighted imaging, diffusion-weighted imaging (DWI), and 3-dimensional time-of-flight magnetic resonance angiography. Those without a baseline MRI examination or any of the these sequences were excluded.
Image Analysis and Interpretation
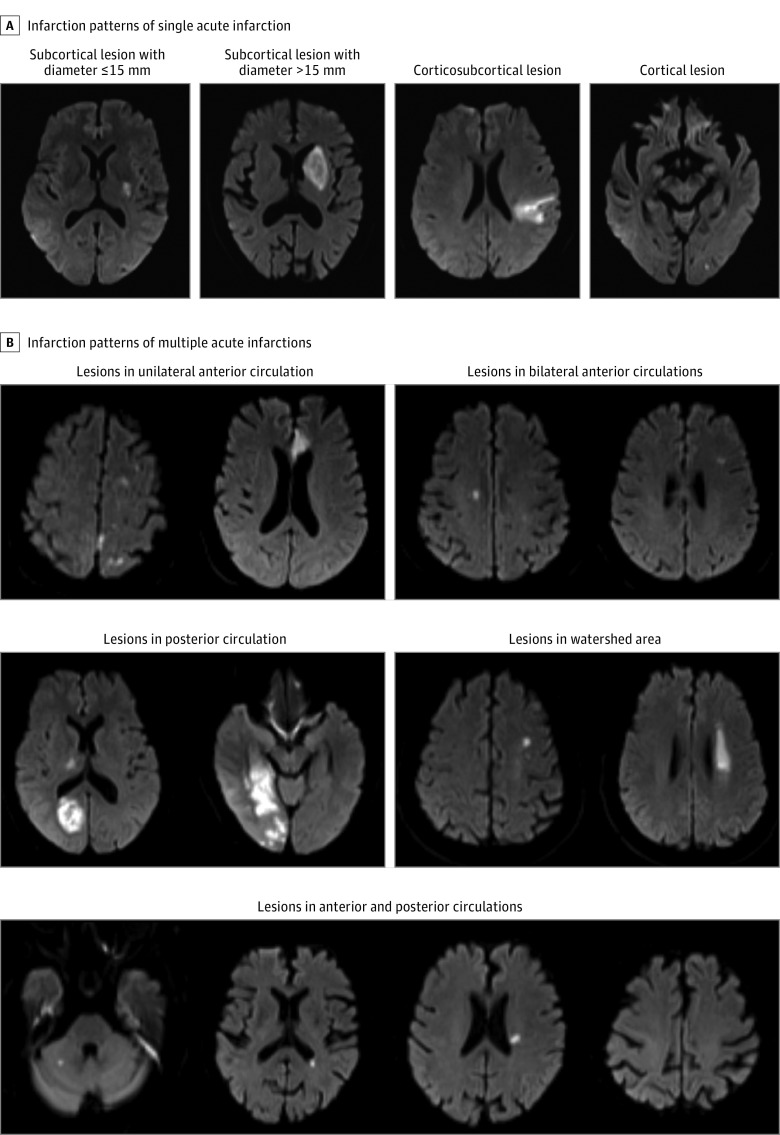
Magnetic resonance images were collected from individual centers in digital format and reviewed centrally by 2 readers (J.J. and X.Z.) blinded to the patients’ baseline and outcome information. Patients were grouped into 3 categories according to the infarction patterns (NAI, SAI, and MAIs) (Figure 1). Acute infarctions were diagnosed with hyperintense lesions on DWI and classified as MAIs or SAI according to infarction number.6 Uninterrupted lesions visible in contiguous territories were considered SAI, and more than 1 lesion topographically distinct (separated in space or discrete on contiguous slices) was defined as MAIs, according to previous DWI studies.6,9 Any disagreements were resolved by a third reader (L.L.). The overall interrater agreement for the infarction patterns classification has a κ value of 0.87 (95% CI, 0.85-0.91). Single acute infarctions were further classified as lacunar infarctions (subcortical lesion with diameter ≤ 15 mm) and nonlacunar infarctions. Multiple acute infarctions were further classified as infarctions in 1 vascular territory and infarctions in more than 1 vascular territory.
Figure 1. Infarction Patterns of Multiple Acute Infarctions and Single Acute Infarction.
Etiology Classification
All patients were classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification24 as previously described.6 Patients with cardioembolism, systemic disease (hypercoagulable states, hematologic disorders, etc) were excluded from the CHANCE trial, so there were no patients with cardioembolism or other determined pathogenesis subtype. There were only 3 TOAST subtypes (large-artery atherosclerosis [LAA], small-artery occlusion, and stroke of undetermined pathogenesis subtype) identified in this study. Subtype classification was based on patient characteristics combined with the results of 1 or more diagnostic tests, including brain MRI as well as imaging of extracranial arteries (carotid ultrasonography or computed tomographic angiography) and intracranial arteries (magnetic resonance angiography). Transient ischemic attacks without LAA or acute infarction were classified as stroke of undetermined pathogenesis subtype. All imaging data were collected at Beijing Tiantan Hospital, where 2 neurologists reviewed the clinical features and diagnostic test results before determining the subtype classifications.
Efficacy and Safety Outcomes
The efficacy and safety outcomes in this analysis were similar with those of the CHANCE trial. The primary efficacy outcome was stroke recurrence (ischemic or hemorrhagic) at 3 months. Ischemic stroke was defined as an acute focal infarction of the brain or retina with 1 of the following: sudden onset of a new focal neurological deficit lasting fewer than 24 hours with clinical or imaging evidence of infarction, or rapid worsening of an existing focal neurological deficit lasting 24 hours or more, with imaging evidence of new ischemic changes clearly distinct from the index ischemic event. Hemorrhagic stroke was defined as acute extravasation of blood into the brain parenchyma or subarachnoid space with associated neurological symptoms. Secondary efficacy outcomes included a new clinical vascular event at 3 months (ischemic stroke, hemorrhagic stroke, myocardial infarction, or vascular death), analyzed as a composite outcome and individual outcomes.
The primary safety outcome was moderate to severe bleeding at 3 months, as per the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries definition.25 A severe bleeding event was defined as fatal or intracranial hemorrhage, or other hemorrhage causing hemodynamic compromise requiring treatment. A moderate bleeding event was defined as bleeding requiring blood transfusion. Other safety outcomes also included any bleeding event. We also explored the efficacy and safety outcomes at 12 months.
Statistical Analysis
Baseline patient characteristics were compared among those with NAI, SAI, or MAIs on brain imaging. We used χ2 test for categorical variables; 1-way analysis of variance or Kruskal-Wallis test were adopted for continuous variables. We used multivariable Cox proportional hazards regression to examine the infarction pattern × treatment interaction effect. If the interaction term was significant, then the treatment was evaluated for the efficacy safety outcomes in patients with different infarction patterns. Confounding factors were selected if there were statistical differences by univariate analysis at baseline (including demographics, medical history, time to randomization, medications, and TOAST classification). Cumulative event curves were constructed for the primary outcome and selected secondary outcomes by the Kaplan-Meier method. All tests were 2-sided, and P < .05 was considered statistically significant. All statistical analyses were performed with SAS statistical software, version 9.4 (SAS Institute Inc).
Results
Patient Demographics and Baseline Characteristics
From October 1, 2009, to July 30, 2012, a total of 5170 patients with acute minor stroke and high-risk TIA were recruited to the CHANCE trial. Among them, 1342 patients from 45 sites were included in the imaging substudy. After excluding 137 patients without T1-weighted imaging, 4 patients without T2-weighted imaging, 23 patients without DWI, and 89 patients without 3-dimensional time-of-flight magnetic resonance angiography, 1089 patients (81.1%) remained with all the required sequences and were included in the current analysis. Compared with patients without MRI examinations, the patients included in this analysis were slightly older and more likely to have lower body mass index, longer time to randomization, reduced ischemic stroke history, and more cases with minor stroke as a qualifying event (eTable 1 in the Supplement).
Among the 1089 patients, the mean (SD) age was 63.1 (10.7) years and 731 patients (65%) were men. A total of 281 patients (25.8%) had MAIs, 553 patients (50.8%) had SAI, and 255 patients (23.4%) had NAI on DWI. Table 1 shows the demographic and clinical characteristics of patients with different infarction patterns. Patients with MAIs were older, more likely to be smokers, more likely categorized as having LAA, and more likely to have a history of myocardial infarction and/or congestive heart failure. Patients with SAI had a higher National Institutes of Health Stroke Scale score and longer time to randomization. Patients with NAI were more likely to have a history of TIA and/or angina. The 2 treatment groups were well balanced according to their baseline patient characteristics (eTable 2 in the Supplement). The demographic and clinical characteristics of patients with different treatments over various infarction patterns are shown in Table 2. Both TIA and minor stroke were also classified according to TOAST classification. For 24-hour TIA definition, there were 86 patients (32.5%) with TIA and positive DWI lesions, including 46 patients with SAI and 40 patients with MAIs (eTable 3 in the Supplement).
Table 1. Baseline Characteristics of Patients With Various Infarction Patterns in the Current Subgroup.
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| No Acute Infarction (n = 255) |
Single Acute Infarction (n = 553) |
Multiple Acute Infarctions (n = 281) |
||
| Age, mean (SD), y | 64.1 (10.7) | 62.1 (10.5) | 64.3 (10.9) | .004 |
| Male | 154 (6.4) | 366 (66.2) | 193 (68.7) | .12 |
| BMI, mean (SD) | 24.4 (3.2) | 24.5 (3.1) | 24.2 (3.4) | .68 |
| Current or previous smoking | 88 (34.5) | 241 (43.6) | 129 (45.9) | .02 |
| NIHSS score, median (IQR) | 0 (0-2) | 2 (1-3) | 2 (1-3) | <.001 |
| Medical history | ||||
| Ischemic stroke | 43 (16.9) | 90 (16.3) | 54 (19.2) | .56 |
| TIA | 14 (5.5) | 9 (1.6) | 9 (3.2) | .01 |
| Myocardial infarction | 0 | 10 (1.8) | 9 (3.2) | .02 |
| Angina | 11 (4.3) | 9 (1.6) | 9 (3.2) | .07 |
| Congestive heart failure | 5 (2.0) | 4 (0.7) | 10 (3.6) | .01 |
| Known atrial fibrillation or flutter | 12 (4.7) | 14 (2.5) | 11 (3.9) | .24 |
| Valvular heart disease | 1 (0.4) | 1 (0.2) | 2 (0.7) | .49 |
| Hypertension | 174 (68.2) | 352 (63.7) | 184 (65.5) | .44 |
| Diabetes | 46 (18.0) | 112 (2.3) | 69 (24.6) | .16 |
| Hypercholesterolemia | 40 (15.7) | 65 (11.8) | 32 (11.4) | .23 |
| Time to randomization, h | ||||
| <12 | 145 (56.9) | 248 (44.9) | 145 (51.6) | .005 |
| ≥12 | 110 (43.1) | 305 (55.2) | 136 (48.4) | |
| Large-artery atherosclerosis | 97 (38.0) | 201 (36.3) | 183 (65.1) | <.001 |
| TOAST classification | ||||
| Large-artery atherosclerosis | 99 (38.8) | 209 (37.8) | 183 (65.1) | <.001 |
| Small-artery occlusion | 0 | 254 (45.9) | 0 | |
| Undetermined cause | 156 (61.2) | 90 (16.3) | 98 (34.9) | |
| Medications | ||||
| Antihypertensive | 84 (48.6) | 178 (51.2) | 79 (42.9) | .20 |
| Antidiabetic | 17 (37.0) | 54 (48.2) | 30 (43.5) | .42 |
| Lipid-lowering | 21 (53.9) | 39 (6.9) | 20 (62.5) | .71 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; NIHSS, National Institute of Health Stroke Scale; TIA, transient ischemic attack; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Table 2. Baseline Characteristics of Patients With Different Treatment in Different Infarction Patterns.
| Characteristic | No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No Infarction | Single Acute Infarction | Multiple Acute Infarctions | |||||||
| Placebo Plus Aspirin (n = 141) |
Clopidogrel Plus Aspirin (n = 114) |
P Value | Placebo Plus Aspirin (n = 284) |
Clopidogrel Plus Aspirin (n = 269) |
P Value | Placebo Plus Aspirin (n = 133) |
Clopidogrel Plus Aspirin (n = 148) |
P Value | |
| Age, mean (SD), y | 64.0 (10.7) | 64.1 (10.8) | .83 | 61.3 (10.5) | 63.0 (10.5) | .11 | 64.9 (10.7) | 63.8 (11.1) | .41 |
| Male | 91 (64.5) | 63 (55.3) | .13 | 190 (66.9) | 176 (65.4) | .71 | 89 (66.9) | 104 (70.3) | .55 |
| BMI, mean (SD) | 24.5 (3.0) | 24.4 (3.4) | .90 | 24.5 (3.2) | 24.5 (3.1) | .78 | 24.3 (3.40) | 24.1 (3.4) | .66 |
| Current or previous smoking | 51 (36.2) | 37 (32.5) | .54 | 118 (41.5) | 123 (45.7) | .32 | 62 (46.6) | 67 (45.3) | .82 |
| NIHSS score, median (IQR) | 0 (0-1) | 0 (0-2) | .48 | 2 (1-3) | 2 (1-3) | .73 | 2 (1-3) | 2 (1-3) | .97 |
| Medical history | |||||||||
| Ischemic stroke | 24 (17.0) | 19 (16.7) | .94 | 44 (15.5) | 46 (17.1) | .61 | 27 (20.3) | 27 (18.2) | .66 |
| TIA | 10 (7.1) | 4 (3.5) | .21 | 4 (1.4) | 5 (1.9) | .93 | 4 (3.0) | 5 (3.4) | >.99 |
| Myocardial infarction | 0 | 0 | 2 (0.7) | 8 (3.0) | .09 | 5 (3.8) | 4 (2.7) | .87 | |
| Angina | 3 (2.1) | 8 (7.0) | .11 | 4 (1.4) | 5 (1.9) | .94 | 3 (2.3) | 6 (4.1) | .61 |
| Congestive heart failure | 2 (1.4) | 3 (2.6) | .81 | 1 (0.4) | 3 (1.1) | .58 | 6 (4.5) | 4 (2.7) | .62 |
| Valvular heart disease | 0 | 1 (0.9) | .45 | 1 (0.4) | 0 | >.99 | 2 (1.5) | 0 | .22 |
| Hypertension | 99 (70.2) | 75 (65.8) | .45 | 184 (64.8) | 168 (62.5) | .57 | 77 (57.9) | 107 (72.3) | .01 |
| Diabetes | 24 (17.0) | 22 (19.3) | .64 | 53 (18.7) | 59 (21.9) | .34 | 32 (24.1) | 37 (25.0) | .86 |
| Hypercholesterolemia | 21 (14.9) | 19 (16.7) | .70 | 30 (1.6) | 35 (13.0) | .37 | 17 (12.8) | 15 (1.1) | .49 |
| Time to randomization, h | |||||||||
| <12 | 77 (54.6) | 68 (59.6) | .42 | 115 (4.5) | 133 (49.4) | .03 | 66 (49.6) | 79 (53.4) | .53 |
| ≥12 | 64 (45.4) | 46 (40.4) | 169 (59.5) | 136 (50.6) | 67 (50.4) | 69 (46.6) | |||
| Large-artery atherosclerosis | 55 (39.0) | 42 (36.8) | .72 | 110 (38.7) | 91 (33.8) | .23 | 85 (63.9) | 98 (66.2) | .69 |
| TOAST classification | |||||||||
| Large-artery atherosclerosis | 55 (39.0) | 44 (38.6) | .95 | 112 (39.4) | 97 (36.1) | .69 | 85 (63.9) | 98 (66.2) | .69 |
| Small-artery occlusion | 0 | 0 | 126 (44.4) | 128 (47.6) | 0 | 0 | |||
| Undetermined cause | 86 (61.0) | 70 (61.4) | 46 (16.2) | 44 (16.4) | 48 (36.1) | 50 (33.8) | |||
| Medications | |||||||||
| Antihypertensive | 50 (50.5) | 34 (46.0) | .55 | 91 (49.5) | 87 (53.1) | .50 | 40 (52.0) | 39 (36.5) | .04 |
| Antidiabetic | 8 (33.3) | 9 (40.9) | .59 | 23 (43.4) | 31 (52.5) | .33 | 12 (37.5) | 18 (48.7) | .35 |
| Lipid-lowering | 10 (47.6) | 11 (61.1) | .40 | 17 (56.7) | 22 (64.7) | .51 | 10 (58.8) | 10 (66.7) | .65 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; NIHSS, National Institute of Health stroke scale; TIA, transient ischemic attack; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Efficacy Outcomes
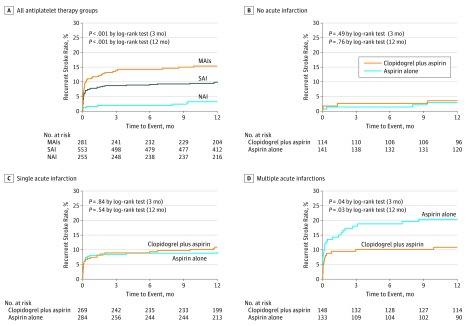
Overall, the rate of recurrent stroke was 8.5% (93 patients) at 3 months in this study population. The risk of recurrent stroke was 14.2%, 8.7%, and 2.0% in patients with MAIs, SAI, and NAI, respectively, at 3-month follow-up. Patients with MAIs (hazard ratio [HR], 5.8; 95% CI, 2.2-15.1; P < .001) and SAI (HR, 3.9; 95% CI, 1.5-10.5; P = .007) were independently associated with stroke recurrence compared with NAI. This effect remained even after adjustment for potential confounding factors, which showed statistical differences among groups of infarction patterns by univariate analysis, regardless of treatment (P < .01). Figure 2A shows Kaplan-Meier curves describing the time to event for the primary efficacy outcome according to different infarction patterns. The same results were observed at the 12-month follow-up. When compared with patients with SAI, patients with MAIs had higher stroke recurrence at 3 months (HR, 1.7; 95% CI, 1.1-2.6; P = .02) without adjustment. However, there was no statistical difference between MAIs and SAI at 3 months after adjustment (HR, 1.4; 95% CI, 0.9-2.3; P = .13). Stroke recurrence at 3-month follow-up among NAI and different infarction patterns of SAI and MAIs are shown in eTable 4 in the Supplement.
Figure 2. Stroke Recurrence Rates in Different Infarction Patterns for Patients With Transient Ischemic Attack and Minor Stroke.
A, Kaplan-Meier curves showing the time to event for the primary efficacy outcome according to various infarction patterns (multiple acute infarctions [MAIs], single acute infarction [SAI], and no acute infarction [NAI]); B-D, Kaplan-Meier curves showing the time to event for the primary efficacy outcome in various antiplatelet therapy groups among distinct infarction patterns (MAIs, SAI, and NAI).
Kaplan-Meier curves in Figure 2B through D show the time to event for the primary efficacy outcome in different infarction patterns of patients with TIA or minor stroke. Stroke was recurrent in 15 (10.1%) and 25 (18.8%) of patients with MAI administered clopidogrel plus aspirin and aspirin alone, respectively (HR, 0.5; 95% CI, 0.3-0.96; P = .04). A significant difference remained even after adjustment for potential confounding factors (age, current or previous smoking, National Institutes of Health Stroke Scale score, a history of TIA, a history of myocardial infarction, a history of congestive heart failure, time to randomization, TOAST classification, and lipid-lowering treatment). Confounding factors were selected when there were statistical differences among groups of infarction patterns or among different treatments in different infarction patterns by univariate analysis. Among the 553 patients with SAI, stroke recurrence rates were 8.9% (24 patients) and 8.5% (24 patients) in patients receiving clopidogrel plus aspirin and aspirin alone, respectively (HR, 1.1; 95% CI, 0.6-2.0; P = .71). Among the 255 patients with NAI, stroke recurrence rates were 2.6% (3 patients) and 1.4% (2 patients) for patients receiving clopidogrel plus aspirin and aspirin alone, respectively (HR, 1.7; 95% CI, 0.3-11.1; P = .56). There was a significant interaction effect with treatment × infarction patterns (P = .04). The same finding was obtained for secondary efficacy outcomes (ischemic stroke, hemorrhagic stroke, myocardial infarction, or vascular death) (Table 3). The same results were observed within the 12-month follow-up as well (eTable 5 in the Supplement). We did not observed a significant infarction number × with or without LAA × treatment interaction effect (P = .41), although our data suggest that dual antiplatelet therapy may be more efficacious mainly in patients with MAI and LAA (eTable 6 in the Supplement).
Table 3. Efficacy and Safety Outcomes of Patients With Different Antiplatelet Therapies Among Various Infarction Patterns at 3-Month Follow-up.
| No Acute Infarction | Single Acute Infarction | Multiple Acute Infarctions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Plus Aspirin, No. (%) | Clopidogrel Plus Aspirin, No. (%) | Adjusted HR (95% CI)a | P Value | Placebo Plus Aspirin, No. (%) | Clopidogrel Plus Aspirin, No. (%) | Adjusted HR (95% CI)a | P Value | Placebo Plus Aspirin, No. (%) | Clopidogrel Plus Aspirin, No. (%) | Adjusted HR (95% CI)a | P Value | |
| Outcome | ||||||||||||
| Stroke | 2 (1.4) | 3 (2.6) | 1.7 (0.3-11.1) | .56 | 24 (8.5) | 24 (8.9) | 1.1 (0.6-2.0) | .71 | 25 (18.8) | 15 (10.1) | 0.5 (0.3-.96) | .04 |
| Combined outcome | 3 (2.1) | 3 (2.6) | 1.3 (0.2-7.3) | .74 | 24 (8.5) | 24 (8.9) | 1.1 (0.6-2.0) | .71 | 26 (19.6) | 15 (10.1) | 0.5 (0.3-.92) | .03 |
| Ischemic stroke | 2 (1.4) | 3 (2.6) | 1.7 (0.3-11.1) | .56 | 24 (8.5) | 24 (8.9) | 1.1 (0.6-2.0) | .71 | 25 (18.8) | 15 (10.1) | 0.5 (0.3-.96) | .04 |
| Hemorrhagic stroke | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Myocardial infarction | 1 (0.7) | 0 | 0 | 0 | 1 (0.75) | 0 | ||||||
| Death from cardiovascular causes | 0 | 0 | 0 | 0 | 1 (0.75) | 0 | ||||||
| Death from any cause | 0 | 0 | 0 | 0 | 1 (0.75) | 1 (0.7) | 1.6 (0.1-38.5) | .76 | ||||
| TIA | 9 (6.4) | 5 (4.4) | 0.9 (0.3-2.8) | .85 | 4 (1.4) | 1 (0.4) | 0.3 (0.03-2.8) | .29 | 3 (2.26) | 3 (2.0) | 0.7 (0.1-3.9) | .72 |
| Safety outcome | ||||||||||||
| Moderate to severe bleedingb | 1 (.7) | 0 | 0 | 0 | 1 (0.75) | 0 | ||||||
| Any bleeding | 3 (2.1) | 2 (1.8) | 0.9 (0.1-5.2) | .87 | 5 (1.8) | 6 (2.2) | 1.1 (0.3-3.7) | .87 | 1 (.8) | 6 (4.1) | 9.6 (0.97-95.4) | .05 |
Abbreviations: HR, hazard ratio; TIA, Transient ischemic attack.
Adjusted for age, current or previous smoking, National Institute of Health stroke scale score, medical history of TIA, medical history of myocardial infarction, medical history of congestive heart failure, time to randomization, antihypertensive treatment, and the Trial of Org 10172 in Acute Stroke Treatment classification.
Bleeding events were defined according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries criteria.
Safety Outcomes
There were 2 moderate to severe bleeding cases (0.2%) in this substudy at the 3-month follow-up, including 1 patient with NAI in the placebo plus aspirin group and 1 patient with MAIs in the placebo plus aspirin group. There was no intracerebral hemorrhage in any of the groups. No increased moderate to severe bleeding risk was observed in the clopidogrel plus aspirin group compared with patients who were administered aspirin alone. A total of 23 patients (2.1%) showed any level of bleeding after 3 months, including 5, 11, and 7 patients with NAI, SAI, and MAIs, respectively. The same results were observed after 12-month follow-up (eTable 5 in the Supplement). The risk of any bleeding was similar in patients with SAI or NAI between 2 treatment groups (Table 3).
Discussion
In this prespecified imaging substudy of the CHANCE trial, we found that infarction patterns could be used to efficiently stratify the risk of recurrent stroke within 3 months of noncardioembolic TIA or minor ischemic stroke. Approximately 50% of risk reduction for recurrent stroke was observed in patients with MAIs administered clopidogrel plus aspirin compared with those treated with aspirin alone, and this finding was not observed in patients with SAI or NAI. Meanwhile, no increased risk of moderate to severe bleeding was observed in each infarction pattern group. However, after dual antiplatelet treatment, patients with MAIs still had a risk of stroke recurrence as high as those with SAI.
Imaging parameters have received attention as ways to predict recurrent stroke after TIA or ischemic stroke,6,9,23,26 and some studies suggest neuroimaging parameters may have better predictive value for stroke recurrence than clinical scores in patients with TIA or minor stroke.26,27 TIA.org, a worldwide registry of TIA and minor stroke, showed that patients with MAIs have higher risk of stroke recurrence than those with SAI or NAI, indicating that acute infarction patterns based on MRI could be used to efficiently stratify the risk of recurrent stroke. In our study, we observed a similar association.
Previous studies indicated that MAIs are usually related to LAA, cardioembolism, and cryptogenic disease according to the TOAST classification.11,12 Evidence exists that the pathogenesis of MAIs is likely caused by the embolism from heart or major extracranial or intracranial vessels.11,12,28,29,30 The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis18 and Clopidogrel Plus Aspirin vs Aspirin Alone for Reducing Embolisation in Patients With Acute Symptomatic Cerebral Or Carotid Artery Stenosis17 studies showed that dual antiplatelet therapy with clopidogrel plus aspirin was more effective than aspirin alone in reducing microembolic signals on transcranial Doppler ultrasonography in patients with TIA and ischemic stroke due to artery-to-artery embolism. Furthermore, the Aortic Arch Related Cerebral Hazard Trial31 showed a potential benefit in dual antiplatelet treatment compared with warfarin in patients with embolism of the thoracic aorta. In our study, all patients had noncardioembolic TIA or ischemic minor stroke and 183 patients (65.1%) with MAIs had LAA according to the TOAST classification. Artery-to-artery embolism was the most common stroke mechanism in patients with LAA.32 Therefore, the findings could explain the significant reduction of stroke risk in patients with MAIs. Although embolism still remains a possible cause,11,12 most patients with SAI had a single subcortical infarction of a small-vessel occlusion with underlying vascular changes such as lipohyalinosis or fibrinoid necrosis of small perforating arteries.13,14 In this substudy population, there were 254 patients (45.9%) with small-vessel occlusion according to the TOAST classification. The SPS3 trial indicated that dual antiplatelet treatment did not reduce the risk of ischemic stroke but rather significantly increased the risk of bleeding and death in patients with lacunar stroke.19 This may partially explain our findings in patients with SAI.
We found that patients with MAIs and NAI had higher rates of recurrent stroke at 3 months than those with noncardioembolic TIA or minor ischemic stroke, which was consistent with previous findings.6 Most importantly, patients with MAIs received the most pronounced clinical benefit from dual antiplatelet therapy in this study population; therefore, from a clinical practice point of view, our results suggest that patients with MAIs should receive early and dual antiplatelet treatment. Moreover, even if an absolute stroke risk reduction of 8.7% was observed after dual antiplatelet treatment, patients with MAIs had a similar risk of stroke at 3 months (10.1% in dual antiplatelet treatment groups) compared with those with SAI (8.9% and 8.5% in dual and single antiplatelet treatment groups, respectively) and had higher risk of stroke than patients with NAI (2.6% and 1.4% in dual and single antiplatelet treatment groups, respectively). Therefore, these findings indicated that MAIs may be a target for more intensive antithrombotic therapy in future studies. Analysis of the Triple Antiplatelets for Reducing Dependency After Ischaemic Stroke trial might help determine the overall safety and efficacy of short-term intensive antiplatelet therapy in the early phase after ischemic stroke or TIA, in comparison with treatment guidelines.33,34 Stroke mechanisms in patients with LAA (especially intracranial artery stenosis) could be occlusion of a single penetrating artery to produce a small subcortical lacuna-like infarction and an artery-to-artery embolism with MAIs.32 Our data suggest that patients with specific stroke etiology (LAA) and specific stroke pathogenesis (artery-to-artery embolism) may be the sweet spot of dual antiplatelet treatment; this hypothesis should be confirmed in the further studies.
Limitations
The study has limitations. Only 45 of 119 sites participated in the imaging study, resulting in 1089 patients (21.1% of the CHANCE population) completing MRI scans and being included in the final analysis. Stroke recurrence occurred in 7.9% of patients in the clopidogrel plus aspirin group, as compared with 9.1% of those in the aspirin alone group, differing from results in the CHANCE study. Therefore, the findings from this imaging substudy may not be generalizable to the overall CHANCE population. Although patients with a presumed cardiac source of embolism were excluded, it was possible that some with cardioembolic TIA and minor ischemic stroke were included since long-term cardiac monitoring was not performed during hospitalization in this study; this would lead to TOAST misclassification. In addition, the Chinese population has an above-average frequency of CYP2C19 loss-of-function alleles35 and intracranial atherosclerotic stenosis.36 All patients in the CHANCE trial were Chinese, which may limit the generalizability of the findings to other populations. There may have been some TIA mimics in this study, which could lead to selection bias.
Conclusions
Infarction patterns could efficiently stratify the risk of recurrent stroke within 3 months of noncardioembolic TIA or minor ischemic stroke. Patients with MAIs received the most pronounced clinical benefit from dual antiplatelet therapy without increasing the risk of moderate to severe bleeding. However, even after dual antiplatelet treatment, patients with MAIs still had a risk of stroke recurrence as high as those with SAI.
eTable 1. Baseline Characteristics of Patients in CHANCE trial included in the Current Subgroup Analysis or Not
eTable 2. Baseline Characteristics of Patients With Different Treatment in the Current Subgroup Analysis
eTable 3. TOAST Classification of Minor Stroke and TIA Respectively
eTable 4. Different Infarction Patterns and Stroke Recurrence at 3-Month Follow-up in TIA and Minor Stroke
eTable 5. Efficacy and Safety of Different Antiplatelet Therapies Among Various Infarction Patterns at 12-Month Follow-up
eTable 6. Efficacy Outcome of Different Antiplatelet Therapies in Patients With Different Infarction Numbers With or Without Large-Artery Atherosclerosis at 3-Month Follow-up
References
- 1.von Weitzel-Mudersbach P, Andersen G, Hundborg HH, Johnsen SP. Transient ischemic attack and minor stroke are the most common manifestations of acute cerebrovascular disease: a prospective, population-based study—the Aarhus TIA study. Neuroepidemiology. 2013;40(1):50-55. [DOI] [PubMed] [Google Scholar]
- 2.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167(22):2417-2422. [DOI] [PubMed] [Google Scholar]
- 3.Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study . Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328(7435):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284(22):2901-2906. [DOI] [PubMed] [Google Scholar]
- 5.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6(12):1063-1072. [DOI] [PubMed] [Google Scholar]
- 6.Amarenco P, Lavallée PC, Labreuche J, et al. ; TIAregistry.org Investigators . One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374(16):1533-1542. [DOI] [PubMed] [Google Scholar]
- 7.Baird AE, Lövblad KO, Schlaug G, Edelman RR, Warach S. Multiple acute stroke syndrome: marker of embolic disease? Neurology. 2000;54(3):674-678. [DOI] [PubMed] [Google Scholar]
- 8.Kang DW, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol. 2003;54(1):66-74. [DOI] [PubMed] [Google Scholar]
- 9.Wen HM, Lam WW, Rainer T, et al. Multiple acute cerebral infarcts on diffusion-weighted imaging and risk of recurrent stroke. Neurology. 2004;63(7):1317-1319. [DOI] [PubMed] [Google Scholar]
- 10.Hart RG, Diener HC, Coutts SB, et al. ; Cryptogenic Stroke/ESUS International Working Group . Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429-438. [DOI] [PubMed] [Google Scholar]
- 11.Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol. 2003;60(12):1730-1734. [DOI] [PubMed] [Google Scholar]
- 12.Wessels T, Wessels C, Ellsiepen A, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am J Neuroradiol. 2006;27(1):35-39. [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology. 1965;15:774-784. [DOI] [PubMed] [Google Scholar]
- 14.Miller Fisher C. Lacunar infarcts—a review. Cerebrovasc Dis. 1991;1(6):311-320.7048128 [Google Scholar]
- 15.Wang Y, Johnston SC; CHANCE Investigators . Rationale and design of a randomized, double-blind trial comparing the effects of a 3-month clopidogrel-aspirin regimen versus aspirin alone for the treatment of high-risk patients with acute nondisabling cerebrovascular event. Am Heart J. 2010;160(3):380-386, e1. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wang Y, Zhao X, et al. ; CHANCE Investigators . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11-19.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23803136&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Wong KS, Chen C, Fu J, et al. ; CLAIR Study Investigators . Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9(5):489-497. [DOI] [PubMed] [Google Scholar]
- 18.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111(17):2233-2240. [DOI] [PubMed] [Google Scholar]
- 19.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA; SPS3 Investigators . Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benavente OR, Pearce LA, Bazan C, et al. ; SPS3 Investigators . Clinical-MRI correlations in a multiethnic cohort with recent lacunar stroke: the SPS3 trial. Int J Stroke. 2014;9(8):1057-1064. [DOI] [PubMed] [Google Scholar]
- 21.Asdaghi N, Pearce LA, Nakajima M, et al. ; SPS3 Investigators . Clinical correlates of infarct shape and volume in lacunar strokes: the Secondary Prevention of Small Subcortical Strokes Trial. Stroke. 2014;45(10):2952-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Wong KS, Leng X, et al. ; CHANCE Investigators . Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of CHANCE. Neurology. 2015;85(13):1154-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Y, Meng X, Jing J, et al. ; CHANCE Investigators . Association of multiple infarctions and ICAS with outcomes of minor stroke and TIA. Neurology. 2017;88(11):1081-1088. [DOI] [PubMed] [Google Scholar]
- 24.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. ; TOAST Investigators . Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke. 1993;24(1):35-41. [DOI] [PubMed] [Google Scholar]
- 25.GUSTO Investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329(10):673-682.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8204123&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 26.Yaghi S, Rostanski SK, Boehme AK, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol. 2016;73(5):572-578.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26998948&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasr DM, Brown RD Jr. The challenges of stroke prediction scores. JAMA Neurol. 2016;73(5):510-511.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26999800&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 28.Bonati LH, Lyrer PA, Wetzel SG, Steck AJ, Engelter ST. Diffusion weighted imaging, apparent diffusion coefficient maps and stroke etiology. J Neurol. 2005;252(11):1387-1393. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Kobayashi S, Matui R, Yamaguchi S, Yamashita K. The differences of clinical parameters between small multiple ischemic lesions and single lesion detected by diffusion-weighted MRI. Acta Neurol Scand. 2002;106(1):24-29. [DOI] [PubMed] [Google Scholar]
- 30.Ferro JM. Patterns of ischaemic cerebral diseases. J Neurol. 2004;251(1):1-10. [DOI] [PubMed] [Google Scholar]
- 31.Amarenco P, Davis S, Jones EF, et al. ; Aortic Arch Related Cerebral Hazard Trial Investigators . Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45(5):1248-1257. [DOI] [PubMed] [Google Scholar]
- 32.Wong KS, Gao S, Chan YL, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52(1):74-81. [DOI] [PubMed] [Google Scholar]
- 33.Bath PM, Robson K, Woodhouse LJ, Sprigg N, Dineen R, Pocock S; TARDIS Trialists . Statistical analysis plan for the ‘Triple Antiplatelets for Reducing Dependency After Ischaemic Stroke’ (TARDIS) Trial. Int J Stroke. 2015;10(3):449-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan K, Beridze M, Christensen H, et al. ; TARDIS Trial Investigators . Safety and efficacy of intensive vs. guideline antiplatelet therapy in high-risk patients with recent ischemic stroke or transient ischemic attack: rationale and design of the Triple Antiplatelets for Reducing Dependency After Ischaemic Stroke (TARDIS) Trial (ISRCTN47823388). Int J Stroke. 2015;10(7):1159-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhao X, Lin J, et al. ; CHANCE Investigators . Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316(1):70-78. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Zhao X, Liu L, et al. ; CICAS Study Group . Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45(3):663-669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics of Patients in CHANCE trial included in the Current Subgroup Analysis or Not
eTable 2. Baseline Characteristics of Patients With Different Treatment in the Current Subgroup Analysis
eTable 3. TOAST Classification of Minor Stroke and TIA Respectively
eTable 4. Different Infarction Patterns and Stroke Recurrence at 3-Month Follow-up in TIA and Minor Stroke
eTable 5. Efficacy and Safety of Different Antiplatelet Therapies Among Various Infarction Patterns at 12-Month Follow-up
eTable 6. Efficacy Outcome of Different Antiplatelet Therapies in Patients With Different Infarction Numbers With or Without Large-Artery Atherosclerosis at 3-Month Follow-up