This randomized clinical trial assesses whether the administration of high-dose intravenous corticosteroid is superior to a bioequivalent oral corticosteroid dose for the treatment of patients with acute optic neuritis.
Key Points
Question
Is high-dose intravenous corticosteroid superior to a bioequivalent oral corticosteroid dose for the treatment of acute optic neuritis?
Findings
In this single-blind randomized clinical trial, data were analyzed from 45 participants with acute optic neuritis who received daily treatment for 3 days of high-dose intravenous methylprednisolone sodium succinate (1000 mg) or oral prednisone (1250 mg). No significant treatment group differences were found at 1 or 6 months of recovery on visual evoked potential P100 latency, best-corrected visual acuity, or low-contrast (1.25% and 2.5%) best-corrected visual acuity.
Meaning
Use of an oral high-dose corticosteroid is as effective as a high-dose intravenous corticosteroid for treatment of acute optic neuritis.
Abstract
Importance
Intravenous (IV) administration of corticosteroids is the standard of care in the treatment of acute optic neuritis. However, it is uncertain whether a bioequivalent dose of corticosteroid administered orally, which may be more cost-efficient and convenient for patients, is as effective as IV administration in the treatment of acute optic neuritis.
Objective
To determine whether recovery of vision following treatment of acute optic neuritis with a high-dose IV corticosteroid is superior to that with a bioequivalent dose of an oral corticosteroid.
Design, Setting, and Participants
This single-blind (participants unblinded) randomized clinical trial with 6-month follow-up was conducted at a single tertiary care center in London, Ontario, Canada. Participants were enrolled from March 2012 to May 2015, with the last participant’s final visit occurring November 2015. Patients 18 to 64 years of age presenting within 14 days of acute optic neuritis onset, without any recovery at time of randomization and without history of optic neuritis in the same eye, were screened. Inclusion criteria included best-corrected visual acuity (BCVA) of 20/40 or worse and corticosteroids deemed required by treating physician. In total, 89 participants were screened; 64 were eligible, but 9 declined to participate. Thus, 55 participants were enrolled and randomized. Primary analysis was unadjusted and according to the intention-to-treat principle.
Interventions
Participants were randomized 1:1 to the IV methylprednisolone sodium succinate (1000-mg) or oral prednisone (1250-mg) group.
Main Outcomes and Measures
Primary outcome was recovery of the latency of the P100 component of the visual evoked potential at 6 months. Secondary outcomes were the P100 latency at 1 month and BCVA as assessed with Early Treatment Diabetic Retinopathy Study letter scores on the alphabet chart and scores on low-contrast letters at 1 and 6 months.
Results
Of 55 randomized participants, the final analyzed cohort comprised 23 participants in the IV and 22 in the oral treatment groups. The mean (SD) age of the cohort was 34.6 (9.5) years, and there were 28 women (62.2%). At 6 months’ recovery, P100 latency in the IV group improved by 62.9 milliseconds (from a mean [SD] of 181.9 [53.6] to 119.0 [16.5] milliseconds), and the oral group improved by 66.7 milliseconds (from a mean [SD] of 200.5 [67.2] to 133.8 [31.5] milliseconds), with no significant difference between groups (P = .07). Similarly, no significant group difference was found in the mean P100 latency recovery at 1 month. For BCVA, recovery between the groups did not reach statistical significance at 1 month or 6 months. In addition, improvements in low-contrast (1.25% and 2.5%) BCVA were not significantly different between treatment groups at 1 or 6 months’ recovery.
Conclusions and Relevance
This study finds that bioequivalent doses of oral corticosteroids may be used as an alternative to IV corticosteroids to treat acute optic neuritis.
Trial Registration
clinicaltrials.gov Identifier: NCT01524250
Introduction
Acute demyelinating optic neuritis (ON), an inflammatory disorder causing painful monocular visual loss, has an annual incidence in the United States of 6.4 per 100 000 persons.1 It is common in persons with multiple sclerosis (MS), with a lifetime prevalence of 50%. Corticosteroids are traditionally used for treatment of acute demyelinating events, including ON. High-dose (≥1 g) corticosteroids administered intravenously became the standard of practice after the landmark Optic Neuritis Treatment Trial (ONTT),2 which compared 3 different interventions: high-dose intravenous (IV) methylprednisolone (1 g daily for 3 days), low-dose oral prednisone (1 mg/kg daily for 14 days), and oral placebo; there was no IV placebo arm. In the ONTT, the IV group had a higher rate of visual recovery. In addition, there was a higher risk of recurrent ON in the oral prednisone group than in either the IV or oral placebo groups. However, the treatments were not pharmacologically equivalent. Since the publication of the ONTT, studies have shown that a high-dose oral corticosteroid and high-dose IV methylprednisolone are bioequivalent,3 have similar effects on magnetic resonance imaging (MRI) outcomes,4,5 and have similar clinical effects on MS relapse.4,6,7 Furthermore, the American Academy of Neurology guidelines published in 2000 state that although 1 mg/kg/d of oral prednisone is ineffective, higher doses of oral or IV methylprednisolone may hasten recovery of visual function in patients with ON.8 Yet IV corticosteroid administration remains the recommended treatment of ON, as indicated in recent Cochrane reviews9 and other reviews making recommendations for the treatment of acute ON.10,11,12 In addition, a 2009 study found that only 47% of Canadian MS specialists prefer to administer IV corticosteroids for MS relapse.13 Thus, the objective of the present clinical trial was to assess whether IV corticosteroids were superior to bioequivalent doses of oral corticosteroids for the treatment of ON by examining the recovery of optic nerve function after acute demyelinating ON.
Methods
Trial Design and Randomization
This single-blind randomized clinical trial compared optic nerve function recovery in patients with ON who were treated with bioequivalent high doses of IV and oral corticosteroids and were followed up for 6 months. The trial protocol is available in the Supplement. Using a computer-based random number generator, participants were randomly assigned to either IV or oral treatment. Prior to randomization, only the study coordinator was not blinded to the treatment assignment. After randomization, participants were unblinded, whereas all assessors remained blinded. Participants were assessed 3 times: at baseline prior to treatment and at 1 and 6 months after corticosteroid treatment. To ensure that assessors remained blinded, all baseline assessments were performed prior to treatment administration, and participants were reminded at all visits of the blinding of treatment assignment. This study was approved by the Western University Health Sciences research ethics board. Participants provided written informed consent prior to participation.
Study Population
From March 2012 through May 2015, patients presenting with unilateral acute demyelinating ON who, in the opinion of their treating clinician, warranted treatment were recruited. To ensure treatment was recommended based on clinical judgment and was not influenced by potential enrollment in the trial, prospective participants were contacted by the study coordinator (D.B.) only after the decision to use corticosteroids had been made by the treating clinician (who was not necessarily involved in the trial). Optic neuritis could have presented as a first demyelinating event (clinically isolated syndrome) or in a patient with a previous diagnosis of clinically isolated syndrome or MS; however, it had to have been the first presentation of ON in the currently affected eye. Antimyelin oligodendrocyte glycoprotein and antineuromyelitis optica antibody titers were not routinely checked at the first presentation of ON.
Participants aged 18 to 64 years were recruited from outpatient clinics at London Health Sciences Center and St Joseph’s Health Care Center in London, Ontario, Canada, including neurologic, ophthalmologic, neuro-ophthalmologic, and MS clinics. Posters were displayed in these clinics, and quarterly letters regarding study recruitment were sent to the neurologists, optometrists, ophthalmologists, and neuro-ophthalmologists at these centers and at the clinics within the catchment area of these tertiary care clinics.
Participants were included only if (1) treatment initiation occurred within 14 days of symptom onset, (2) the best-corrected visual acuity (BCVA) on the Snellen eye chart of the affected eye was 20/40 or worse, and (3) there was no reported spontaneous recovery because corticosteroid treatment has not been shown to confer improvement in this group.2 The use of BCVA eliminated decreased vision due to refractive error. Participants were excluded if they had previously experienced ON in the same eye, received corticosteroids in the previous 30 days, or had another medical condition that might affect visual outcomes, such as, but not limited to, diabetic retinopathy, glaucoma, or cataracts. Participants were removed from the study if they required a second treatment of high-dose corticosteroids anytime during the study or if a diagnosis other than ON was established.
Intervention
Participants were randomized to either methylprednisolone sodium succinate (1000 mg, IV) daily for 3 days or oral prednisone (1250 mg) daily for 3 days. The oral dose was based on previous evidence of bioequivalence between 1250 mg of oral prednisone and 1000 mg of IV methylprednisolone in persons with MS.3 Intravenous treatment was administered either entirely in a hospital outpatient infusion center or, when possible, in a hospital outpatient infusion center for the first dose and at home for subsequent doses. Participants randomized to the oral group were provided 75 tablets of 50 mg of prednisone (25 tablets daily) to consume at home. Compliance with this oral regimen has been previously shown to be very high (compliance rate, 98%).14 No taper was used after the 3-day treatment because previous research indicates tapering has no effect on short- or long-term outcomes.15
Study Measures
The primary end point was recovery of the visual evoked potential (VEP) P100 component latency in the affected eye assessed 6 months after corticosteroid treatment. Secondary end points were high-contrast BCVA and contrast sensitivity, specifically low-contrast BCVA, 1 and 6 months after treatment, and the recovery of P100 latency 1 month after treatment.
Visual Evoked Potential
The primary measure was recovery of the VEP P100 latency because P100 latencies are a reliable measure of the functional integrity of the visual pathway, correlating with visual acuity and recovery of visual fields in patients with ON.16,17 In addition, studies have shown an increase in the P100 amplitude, suggesting improved conduction, approximately 30 days after onset of symptoms,18 closely mirroring the clinical recovery noted in ON.19 The sensitivity of a prolonged P100 latency is as high as 88% for severe ON, 68% for mild or moderate ON.16 The P100 latency, along with low-contrast BCVA, is considered to be a sensitive test for clinical and subclinical demyelinating lesions of the optic nerve.20 The P100 peak is the most prominent VEP waveform, has little within-participant or interrater variation, and is used as the primary outcome when interpreting VEPs.21
Visual evoked potentials were recorded with Teca Synergy equipment (Viasys Healthcare) using the pattern reversal VEP, a checkerboard stimulus.21 To ensure consistency in technique, the same technician (C.D.) performed all 3 assessments (baseline and 1 and 6 months) on each participant whenever possible. Recording of the VEPs was performed following a standard protocol.21,22
Best-Corrected Visual Acuity
Best-corrected visual acuity was measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart (14 lines having 5 numbers each for a total of 70 responses), which is considered the criterion standard for ophthalmologic clinical trials of visual acuity outcomes.23,24 The BCVA was assessed by a neuro-ophthalmologist (J.A.F.) and was measured in each eye individually under standardized lighting conditions following standard protocols.24 Participants wore corrective lenses as needed. The participants started reading at a row in which they thought they could read all 5 letters. They continued reading down the chart until they could not read all 5 letters in a given row. Testing was conducted at a distance of 2 m from the ETDRS alphabet chart.
Low-Contrast BCVA
Low-contrast BCVA was measured, under conditions identical to ETDRS testing, with low-contrast Sloan letter charts, which are valid and reliable for assessing low-contrast visual acuity in persons with MS.25,26 Each chart is similar to the chart used in the ETDRS, except the letters are gray and consist of different levels of contrast, ranging from 0.6% to 100%.26 Each chart was scored separately for each eye, and the number of total letters read on each chart was scored to a maximum of 70.25,27 Similar to previous clinical trials,28,29 we used the scores determined at 1.25% and 2.5% contrast levels as secondary outcomes.
Adverse Events
After 1 week of treatment, participants were contacted by telephone by the study coordinator (D.B.), who was unblinded to the treatment allocation, to assess adverse events (AEs). All AEs were reported to the lead study investigator (S.A.M.) in a manner that maintained blinding. The AEs were managed by the lead study investigator on a case-by-case basis, as per standard of practice in MS clinics in Canada.13
Statistical Analysis
The sample size was based on the normal distribution of the VEP P100 mean (SD) latency of 100 (5) milliseconds.16,30,31,32 The upper limit of the normal distribution is commonly defined as 2 SDs above the mean,16,30,32 or 110 milliseconds. The estimated clinically significant difference between the 2 groups was hypothesized to be 1 SD. Based on a 1:1 randomization, a probability (power) of 0.8, and an α of .05, 38 participants were needed. Assuming a potential dropout rate of 20% before the third follow-up visit, we planned to recruit a total of 46 participants.
Participants’ baseline characteristics of the 2 treatment groups were compared using an unpaired, 2-tailed t test and 2-tailed χ2 analyses as appropriate. To compare longitudinal changes on visual outcomes across groups at 1 and 6 months after treatment, we used an analysis of covariance with the baseline measures as covariates to assess the significance of between-group differences at 1 and 6 months. Analyses were performed using SPSS, version 23 (SPSS Inc). A 2-sided P < .05 was considered statistically significant.
Results
The first participant was enrolled and randomized in March 2012, and the last participant was enrolled and randomized in May 2015, with this last participant’s final visit in November 2015. Although the original recruitment goal was 46 participants, after the 46th participant was randomized, it was noted that the final number of participants completing the study would be less than 38 owing to dropouts or removal from the study. Thus, recruitment continued until 55 participants were enrolled and randomized to ensure enough power for the statistical analyses.
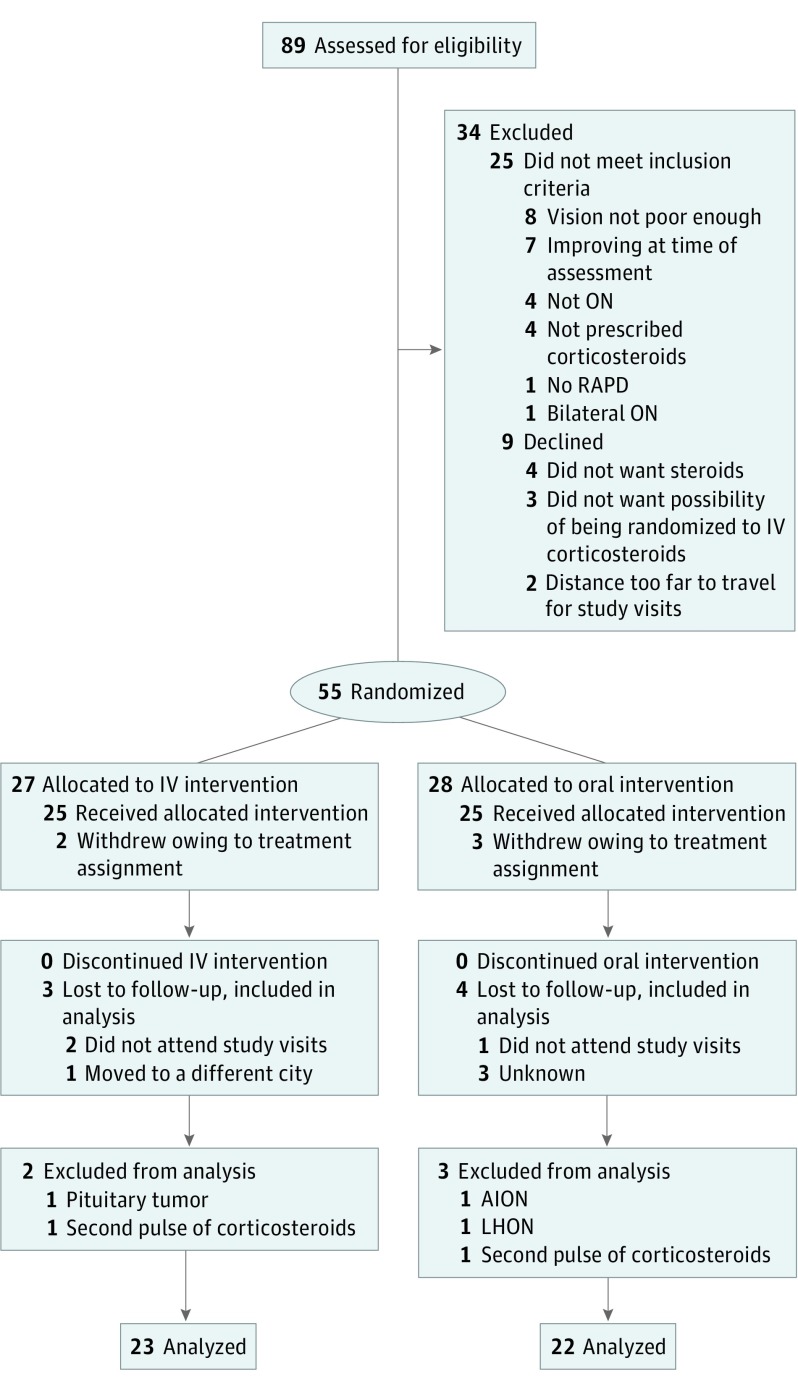
In total, 89 participants with new-onset ON were referred for potential participation in this trial. Twenty-five participants did not meet the inclusion criteria, and 9 declined to participate. Thus, 55 participants were enrolled and randomized. Subsequently, 2 participants from the IV group and 3 from the oral group withdrew because they were opposed to their treatment assignment and thus did not receive any treatment in the study. These participants were not included in any analysis because the first dose had not been administered. Consequently, 25 participants received IV treatment, and 25 participants received oral treatment (Figure 1).
Figure 1. Flowchart of Participant Enrollment and Retention.
AION indicates nonarteritic anterior ischemic optic neuropathy; IV, intravenous; LHON, Leber hereditary optic neuropathy; ON, optic neuritis; and RAPD, relative afferent pupillary effect.
In the IV group, 2 participants were removed because 1 participant with a known MS diagnosis had another relapse and required another course of corticosteroid and the other participant was found to have a pituitary tumor that had been previously missed on an MRI scan as the cause of vision loss (in retrospect). In the oral group, 2 participants were removed as the result of ON misdiagnoses (nonarteritic anterior ischemic optic neuropathy and Leber hereditary optic neuropathy) by the referring physicians. A third participant was removed after she required a second course of corticosteroid administration. She subsequently developed ON in the other eye and later received a diagnosis of acute disseminated encephalomyelitis. Thus, 23 participants in the IV group and 22 participants in the oral group were included in the analysis (Figure 1). In the IV group, 1 participant moved midstudy and could no longer attend study visits, and 2 other participants were lost to follow-up after the baseline assessment. In the oral group, 4 participants were lost to follow-up after the baseline assessment. All available data were included in the analysis.
The mean (SD) age of our final entire cohort was 34.6 (9.5) years, with 28 women (62.2%) and 17 men (37.8%). In the final cohort, 29 participants (64.4%) presented with a first demyelinating event, whereas 13 (28.9%) had a previous diagnosis of MS and 3 (6.7%) had a previous diagnosis of clinically isolated syndrome. There were no significant differences between the 2 groups in baseline demographic characteristics (Table 1). The median baseline BCVA as assessed using the Snellen eye chart (inclusion criteria) in the affected eye at presentation was 20/100 (range, 20/40 to no light perception [NLP]). The mean (SD) baseline VEP P100 latency was 191.0 (60.6) milliseconds, indicating substantial slowing. The median baseline ETDRS BCVA was 20/100 (range, 20/16 to NLP). Fifteen participants (33.3%) presented with visual acuity of counting fingers or worse. The results of BCVA testing at a letter contrast of 2.5% indicated that participants had a median score of 0, meaning no numbers could be seen, with a range of 0 to 59; 40 participants (88.9%) could not see any numbers at this level. For BCVA testing at a lower letter contrast of 1.25%, the median score was also 0 (range, 0-40); 42 participants (93.3%) could not see any numbers at this level. There were no significant differences in any of these baseline visual characteristics between the oral and IV treatment groups (Table 2).
Table 1. Demographic Characteristics of the 45 Study Participants.
| Characteristic | Participants, No. (%) | P Value | |
|---|---|---|---|
| IV Group (n = 23) | Oral Group (n = 22) | ||
| Age, mean (SD), y | 33.1 (10.1) | 30.1 (8.8) | .31a |
| Female | 14 (60.9) | 14 (63.6) | .85b |
| White | 20 (90.0) | 20 (90.9) | .51b |
| Presentation | |||
| RRMS | 7 (30.4) | 6 (27.3) | .19b |
| CIS | 0 (0.0) | 3 (13.6) | |
| First demyelinating event | 16 (69.6) | 13 (59.1) | |
| Left eye affected | 12 (52.2) | 15 (68.2) | .27b |
Abbreviations: CIS, clinically isolated syndrome; IV, intravenous; RRMS, relapsing-remitting multiple sclerosis.
Two-tailed t test.
Two-tailed χ2 test.
Table 2. Baseline Visual Characteristics of the Affected Eye.
| Characteristic | IV Group (n = 23) | Oral Group (n = 22) | P Value |
|---|---|---|---|
| VEP P100 latency, mean (SD), ms | 181.9 (53.6) | 200.5 (67.2) | .31a |
| ETDRS BCVA, median (range) | 20/100 (20/16 to NLP) | 20/160 (20/16 to NLP) | .41b |
| CF or worse, No. (%) | 5 (21.7) | 10 (45.5) | |
| LCBCVA, 2.5%, median (range) | 0 (0-59) | 0 (0-10) | .39b |
| With 0/70, No. (%) | 20 (87.0) | 20 (90.9) | |
| LCBCVA, 1.25%, median (range) | 0 (0-40) | 0 (0-5) | .34b |
| With 0/70, No. (%) | 21 (91.3) | 21 (95.5) |
Abbreviations: BCVA, best-corrected visual acuity; CF, counting fingers; ETDRS, Early Treatment Diabetic Retinopathy Study; LCBCVA, low-contrast best-corrected visual acuity; NLP, no light perception; and VEP, visual evoked potential.
Two-tailed t test.
Two-tailed χ2 test.
VEP P100 Latency
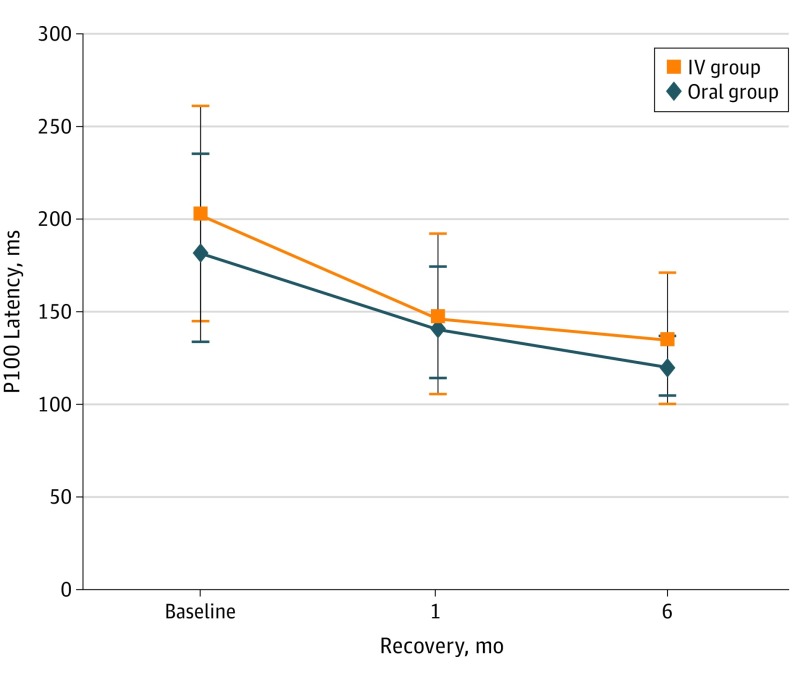
At 6 months of recovery, the mean (SD) P100 latency in the IV group decreased from 181.9 (53.6) milliseconds to 119.0 (16.5) milliseconds, an improvement of 62.9 milliseconds (Figure 2). The P100 latency in the group receiving oral administration decreased from a mean (SD) of 200.5 (67.2) milliseconds to 133.8 (31.5) milliseconds, a mean improvement of 66.7 milliseconds. This improvement was not significantly different between the 2 groups (P = .07). Similarly, at 1 month of recovery, there was no significant difference in the P100 latency between groups: the IV group improved by 41.8 milliseconds to 140.1 (28.5) milliseconds, and the oral improved by 55.1 milliseconds to 145.4 (41.5) milliseconds (P = .75).
Figure 2. Visual Evoked Potential P100 Latency Before and After Treatment With Bioequivalent Oral and Intravenous (IV) Corticosteroids.
Error bars indicate SD.
BCVA Assessed With ETDRS
The BCVA in the affected eye at 1 month of recovery was not significantly different between the oral (median, 20/36; range, 20/12.5 to NLP) and IV (median, 20/20; range, 20/10 to NLP) groups (P = .06). Similarly, BCVA at 6 months of recovery was not significantly different between the oral (median, 20/32; range, 20/12.5 to 20/400) and IV (median, 20/20; range 20/12.5 to 20/400) groups (P = .30).
BCVA Assessed at 2.5% Contrast
At 1 month of recovery, the BCVA in the affected eye as assessed at a low letter contrast of 2.5% in the oral group (median, 0; range, 0-40) was not significantly different from that in the IV group (median, 15; range 0-45) (P = .58). Similarly, the low-contrast BCVA at 6 months of recovery in the oral group (median, 10; range, 0-50) was not significantly different from that in the IV group (median, 20; range, 0-35) (P = .33).
BCVA Assessed at 1.25% Contrast
At 1 month of recovery, the BCVA in the affected eye as assessed at a letter contrast of 1.25% in the oral group (median, 0; range, 0-50) was not significantly different from that in the IV group (median, 5; range, 0-55) (P = .85). Similarly, no significant group difference was detected at 6 months of recovery (orally treated: median, 5; range, 0-45; IV treated: median, 15; range, 0-45; P = .33).
Adverse Events
Although 25 participants experienced at least 1 AE, the number of AEs between the 2 treatment groups was not significantly different (11 in the IV group and 14 in the oral group; χ21 = 1.226; n = 43; P = .27). In addition, the most frequently reported AEs were not significantly different between the 2 groups: gastrointestinal (5 in the IV group and 8 in the oral group; χ21 = 1.203; n = 43; P = .27), insomnia (4 in the IV group and 6 in the oral group; χ21 = 0.650; n = 43; P = .42), and tiredness or fatigue (5 in the IV group and 1 in the oral group; χ21 = 2.888; n = 43; P = .09).
Discussion
This single-blind randomized clinical trial indicates that the administration of a high-dose IV corticosteroid was not superior to the administration of a bioequivalent oral dose for the treatment of acute ON.
Optic neuritis is a common presentation in MS, occurring in 50% of persons with relapsing-remitting MS, and it is the presenting demyelinating event in 15% to 20% of all MS cases.33,34 Previous studies have shown that recovery of vision is expected to start within 30 days of symptom onset, corresponding to the resolution of optic nerve inflammation.19 Recovery of color vision and subjective visual improvement have been noted as early as 3 weeks but can be accelerated to as soon as 1 week with corticosteroid administration,35 although most recovery occurs within 6 months.36
Corticosteroids have been used to treat acute demyelinating events for many years. Corticosteroids reduce the breakdown of the blood-brain barrier, as indicated by a reduction in the intensity, or complete resolution, of gadolinium-enhanced lesions on MRI scans, leading to accelerated recovery from acute relapse.37 On the basis of the ONTT results, IV administration became the standard of practice for treating ON;2 however, the active treatments used in the ONTT were not bioequivalent. The IV group received 1000 mg of methylprednisolone daily for 3 days followed by an 11-day oral taper, whereas the prednisone group received a much lower daily dose: 1 mg/kg daily for 14 days. Subsequent studies using MRI resolution of gadolinium-enhanced lesions as the main outcome indicated a better response to high-dose (≥1.0 g) oral corticosteroids, supporting the ONTT findings that high-dose corticosteroid treatment is the standard of care, but still did not address whether the route of medication delivery played a role.4 A large multicenter trial in France (COPOUSEP)7 compared recovery of relapse treated with 1000 mg of oral or IV methylprednisolone with the primary end point examined at 28 days. Similar to the results of our study, they reported that oral administration was not inferior to IV administration.
The present study supports the use of either IV or oral administration for the treatment of ON or MS relapse in general. However, oral medication may be more convenient, minimizing travel to an infusion center, especially for those residing in rural locations. In addition, our previous study showed that oral administration is preferred by persons with MS.14 The use of oral administration is also more cost-effective. A Canadian study reported the cost of a 4-day IV treatment to be approximately CaD $715 (approximately US $580).38 By contrast, a 4-day course of 1250 mg of oral prednisone (at CaD $0.20 [US $0.16] per 50-mg tablet) costs CaD $20 (approximately US $16). In the United States, oral corticosteroids are more cost-effective because IV infusions in the United States are estimated to cost US $800 per hour.39 Data from the National Clinical Guideline Centre 2014 publication on MS in the United Kingdom stated that there were “no relevant economic evaluations comparing steroids with placebo or comparing (IV) with oral steroids.”40(p534) However, data were not provided on the cost of administration in different settings. The cost of 100 mg of oral prednisone for 5 days is £121 (approximately US $170), whereas 1000 mg of IV prednisolone for 5 days costs £87 (approximately US $125), in addition to the daily cost of clinic (£560 or US $796) or home (£213-355 or approximately US $300-500) administration.41,42 The group who published the COPOUSEP study also reported a budget impact analysis examining the annual cost of oral or IV treatment of relapses for all newly diagnosed cases of MS; they concluded that the use of oral corticosteroids would save €5 681 832 (approximately US $7 050 470) in the first year alone.43 Data from an academic neurological center in Buenos Aires, Argentina (the FLENI Institute) also indicate a cost benefit: US $337 for IV methylprednisolone vs US $30 for equivalent oral prednisone daily (Jorge Correale, MD, the FLENI Institute, written communication, October 2017).
Strengths and Limitations
The present study has several strengths and unique aspects. First, we examined objective measures of recovery, with VEP P100 latency and low-contrast BCVA, which can detect more subtle differences than BCVA alone. Second, we focused specifically on ON, rather than MS relapses in general, similar to the original ONTT. Finally, our primary end point was recovery at 6 months, while the COPOUSEP study used a primary end point at 28 days.7
However, the present study does not address other important clinical issues, namely, who will benefit from corticosteroid treatment and the ideal timing for initiation of therapy. Research to date has not provided any evidence that treatment of ON with high-dose corticosteroids affects long-term visual outcomes.
The present study has some additional limitations. There may have been a referral bias; if the treating physician decided that IV (or oral) corticosteroids were more appropriate, the participant would not have been screened for our study. Second, most referrals for this study were received from within the London Health Sciences Centre, London, Ontario, Canada; thus, there may be differences between ON assessed at an academic tertiary care center and ON assessed at other clinics. Finally, we did not use optical coherence tomography (OCT) in this study. Baseline OCT images may have allowed for the detection of subclinical axonal attrition in the optic nerves, a known phenomenon in demyelinating disease that may limit the degree of expected visual recovery.44 We assumed that any participants with subclinical optic neuropathy at baseline would have been distributed equally between treatment arms of our study during randomization, but OCT would have verified this. Follow-up OCT images could have been useful in showing whether there was a differential effect of IV vs oral corticosteroids on retinal nerve fiber layer loss and may have allowed for correlations between optic nerve function (VEP) and structure (retinal nerve fiber layer) by treatment modality. Previous research by Balcer and Frohman,27 however, indicated that low-contrast BCVA, which was used in the present study, strongly correlates with OCT.
Conclusions
Bioequivalent doses of oral and IV corticosteroids are equally viable treatment options for acute ON. However, oral prednisone has the advantages of being less expensive and more convenient to access and administer and is preferred by patients to IV methylprednisolone.45,46
Trial protocol
References
- 1.Percy AK, Nobrega FT, Kurland LT. Optic neuritis and multiple sclerosis: an epidemiologic study. Arch Ophthalmol. 1972;87(2):135-139. [DOI] [PubMed] [Google Scholar]
- 2.Beck RW, Cleary PA, Anderson MM Jr, et al. ; The Optic Neuritis Study Group . A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326(9):581-588. [DOI] [PubMed] [Google Scholar]
- 3.Morrow SA, Stoian CA, Dmitrovic J, Chan SC, Metz LM. The bioavailability of IV methylprednisolone and oral prednisone in multiple sclerosis. Neurology. 2004;63(6):1079-1080. [DOI] [PubMed] [Google Scholar]
- 4.Oliveri RL, Valentino P, Russo C, et al. . Randomized trial comparing two different high doses of methylprednisolone in MS: a clinical and MRI study. Neurology. 1998;50(6):1833-1836. [DOI] [PubMed] [Google Scholar]
- 5.Martinelli V, Rocca MA, Annovazzi P, et al. . A short-term randomized MRI study of high-dose oral vs intravenous methylprednisolone in MS. Neurology. 2009;73(22):1842-1848. [DOI] [PubMed] [Google Scholar]
- 6.Burton JM, O’Connor PW, Hohol M, Beyene J. Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev. 2009;(3):CD006921. [DOI] [PubMed] [Google Scholar]
- 7.Le Page E, Veillard D, Laplaud DA, et al. ; COPOUSEP investigators; West Network for Excellence in Neuroscience . Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974-981. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman DI, Trobe JD, Eggenberger ER, Whitaker JN. Practice parameter: the role of corticosteroids in the management of acute monosymptomatic optic neuritis: report of the quality standards subcommittee of the American Academy of Neurology. Am J Ophthalmol. 2000;130(4):541. [DOI] [PubMed] [Google Scholar]
- 9.Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. 2015;(8):CD001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodin DS. Glucocorticoid treatment of multiple sclerosis. Handb Clin Neurol. 2014;122:455-464. [DOI] [PubMed] [Google Scholar]
- 11.Mackay DD. Should patients with optic neuritis be treated with steroids? Curr Opin Ophthalmol. 2015;26(6):439-444. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Cambrodí RJ, Gómez-Hurtado Cubillana A, Merino-Suárez ML, Piñero-Llorens DP, Laria-Ochaita C. Optic neuritis in pediatric population: a review in current tendencies of diagnosis and management. J Optom. 2014;7(3):125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow SA, Metz LM, Kremenchutzky M. High dose oral steroids commonly used to treat relapses in Canadian MS clinics. Can J Neurol Sci. 2009;36(2):213-215. [DOI] [PubMed] [Google Scholar]
- 14.Morrow SA, McEwan L, Alikhani K, Hyson C, Kremenchutzky M. MS patients report excellent compliance with oral prednisone for acute relapses. Can J Neurol Sci. 2012;39(3):352-354. [DOI] [PubMed] [Google Scholar]
- 15.Perumal JS, Caon C, Hreha S, et al. . Oral prednisone taper following intravenous steroids fails to improve disability or recovery from relapses in multiple sclerosis. Eur J Neurol. 2008;15(7):677-680. [DOI] [PubMed] [Google Scholar]
- 16.Naismith RT, Tutlam NT, Xu J, et al. . Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology. 2009;73(1):46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trauzettel-Klosinski S, Diener HC, Dietz K, Zrenner E. The effect of oral prednisolone on visual evoked potential latencies in acute optic neuritis monitored in a prospective, randomized, controlled study. Doc Ophthalmol. 1995-1996;91(2):165-179. [DOI] [PubMed] [Google Scholar]
- 18.Youl BD, Turano G, Miller DH, et al. . The pathophysiology of acute optic neuritis: an association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991;114(pt 6):2437-2450. [DOI] [PubMed] [Google Scholar]
- 19.Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis: experience of the Optic Neuritis Treatment Trial. Ophthalmology. 1994;101(11):1771-1778. [DOI] [PubMed] [Google Scholar]
- 20.van Diemen HA, Lanting P, Koetsier JC, Strijers RL, van Walbeek HK, Polman CH. Evaluation of the visual system in multiple sclerosis: a comparative study of diagnostic tests. Clin Neurol Neurosurg. 1992;94(3):191-195. [DOI] [PubMed] [Google Scholar]
- 21.Odom JV, Bach M, Brigell M, et al. . ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol. 2010;120(1):111-119. [DOI] [PubMed] [Google Scholar]
- 22.American Clinical Neurophysiology Society Guideline 5: guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 2006;23(2):107-110. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of Snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans Am Ophthalmol Soc. 2009;107:311-324. [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris FL III, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103(1):181-182. [DOI] [PubMed] [Google Scholar]
- 25.Balcer LJ, Baier ML, Cohen JA, et al. . Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology. 2003;61(10):1367-1373. [DOI] [PubMed] [Google Scholar]
- 26.Balcer LJ, Baier ML, Pelak VS, et al. . New low-contrast vision charts: reliability and test characteristics in patients with multiple sclerosis. Mult Scler. 2000;6(3):163-171. [DOI] [PubMed] [Google Scholar]
- 27.Balcer LJ, Frohman EM. Evaluating loss of visual function in multiple sclerosis as measured by low-contrast letter acuity. Neurology. 2010;74(suppl 3):S16-S23. [DOI] [PubMed] [Google Scholar]
- 28.Cohen JA, Cutter GR, Fischer JS, et al. ; IMPACT Investigators . Benefit of interferon β-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59(5):679-687. [DOI] [PubMed] [Google Scholar]
- 29.Balcer LJ, Galetta SL, Calabresi PA, et al. . Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68(16):1299-1304. [DOI] [PubMed] [Google Scholar]
- 30.Della Sala S, Comi G, Martinelli V, Somazzi L, Wilkins AJ. The rapid assessment of visual dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1987;50(7):840-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews WB, Small DG, Small M, Pountney E. Pattern reversal evoked visual potential in the diagnosis of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1977;40(10):1009-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sisto D, Trojano M, Vetrugno M, Trabucco T, Iliceto G, Sborgia C. Subclinical visual involvement in multiple sclerosis: a study by MRI, VEPs, frequency-doubling perimetry, standard perimetry, and contrast sensitivity. Invest Ophthalmol Vis Sci. 2005;46(4):1264-1268. [DOI] [PubMed] [Google Scholar]
- 33.Frohman EM, Frohman TC, Zee DS, McColl R, Galetta S. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol. 2005;4(2):111-121. [DOI] [PubMed] [Google Scholar]
- 34.Foroozan R, Buono LM, Savino PJ, Sergott RC. Acute demyelinating optic neuritis. Curr Opin Ophthalmol. 2002;13(6):375-380. [DOI] [PubMed] [Google Scholar]
- 35.Sellebjerg F, Nielsen HS, Frederiksen JL, Olesen J. A randomized, controlled trial of oral high-dose methylprednisolone in acute optic neuritis. Neurology. 1999;52(7):1479-1484. [DOI] [PubMed] [Google Scholar]
- 36.Kolappan M, Henderson AP, Jenkins TM, et al. . Assessing structure and function of the afferent visual pathway in multiple sclerosis and associated optic neuritis. J Neurol. 2009;256(3):305-319. [DOI] [PubMed] [Google Scholar]
- 37.Miller DH, Thompson AJ, Morrissey SP, et al. . High dose steroids in acute relapses of multiple sclerosis: MRI evidence for a possible mechanism of therapeutic effect. J Neurol Neurosurg Psychiatry. 1992;55(6):450-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robson LS, Bain C, Beck S, Guthrie S, Coyte PC, O’Connor P. Cost analysis of methylprednisolone treatment of multiple sclerosis patients. Can J Neurol Sci. 1998;25(3):222-229. [DOI] [PubMed] [Google Scholar]
- 39.Kister I, Corboy JR. Reducing costs while enhancing quality of care in MS. Neurology. 2016;87(15):1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minden SL, Feinstein A, Kalb RC, et al. ; Guideline Development Subcommittee of the American Academy of Neurology . Evidence-based guideline: assessment and management of psychiatric disorders in individuals with MS: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(2):174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chataway J, Porter B, Riazi A, et al. . Home versus outpatient administration of intravenous steroids for multiple-sclerosis relapses: a randomised controlled trial. Lancet Neurol. 2006;5(7):565-571. [DOI] [PubMed] [Google Scholar]
- 42.Multiple sclerosis: management of multiple sclerosis in primary and secondary care: clinical guideline 186. National Clinical Guideline Centre. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0068954/pdf/PubMedHealth_PMH0068954.pdf. Published 2014. Accessed October 26, 2017.
- 43.Veillard D, Legrand P, Michel M, Le Page E. Multiple sclerosis relapses: budget impact analysis of oral high-dose corticosteroids [abstract P399]. European Committee for Treatment and Research of Multiple Sclerosis Online Library. October 26, 2017.
- 44.Talman LS, Bisker ER, Sackel DJ, et al. . Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tse FL, Welling PG. Relative bioavailability of prednisone and prednisolone in man. J Pharm Pharmacol. 1979;31(7):492-493. [DOI] [PubMed] [Google Scholar]
- 46.International Journal of MS Care Abstracts From the 19th annual meeting of the Consortium of Multiple Sclerosis Centers. Summer 2005;7(2):45-76. http://ijmsc.org/doi/abs/10.7224/1537-2073-7.2.45?code=cmsc-site. Accessed October 26, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol