Key Points
Question
In patients with advanced or metastatic urothelial carcinoma, does atezolizumab therapy remain safe and provide clinical benefit over the long term?
Findings
In this cohort of 95 evaluable patients treated with atezolizumab in a phase 1 clinical trial, most treatment-related adverse events occurred within the first year of treatment, and no serious treatment-related adverse events were observed thereafter. After a median follow-up of 3 years, sustained responses were observed, with long-term survival seen for a subset of patients.
Meaning
Single-agent atezolizumab was well tolerated, resulting in prolonged efficacy over an extended study period in patients with metastatic urothelial carcinoma.
This cohort study of an ongoing phase 1 trial reports long-term clinical outcomes with atezolizumab therapy for patients with metastatic urothelial carcinoma.
Abstract
Importance
Atezolizumab (anti–programmed death ligand 1) has demonstrated safety and activity in advanced and metastatic urothelial carcinoma, but its long-term clinical profile remains unknown.
Objective
To report long-term clinical outcomes with atezolizumab therapy for patients with metastatic urothelial carcinoma.
Design, Setting, and Participants
Patients were enrolled in an expansion cohort of an ongoing, open-label, phase 1 study. Median follow-up was 37.8 months (range, >0.7 to 44.4 months). Enrollment occurred between March 2013 and August 2015 at US and European academic medical centers. Eligible patients had measurable disease per Response Evaluation Criteria in Solid Tumors version 1.1, Eastern Cooperative Oncology Group performance status 0 to 1, and a representative tumor sample. Programmed death ligand 1 expression on immune cells was assessed (VENTANA SP142 assay).
Interventions
Atezolizumab was given intravenously every 3 weeks until unacceptable toxic effects, protocol nonadherence, or loss of clinical benefit.
Main Outcomes and Measures
Primary outcome was safety. Secondary outcomes included objective response rate, duration of response, and progression-free survival. Response and overall survival were assessed in key baseline subgroups.
Results
Ninety-five patients were evaluable (72 [76%] male; median age, 66 years [range, 36-89 years]). Forty-five (47%) received atezolizumab as third-line therapy or greater. Nine patients (9%) had a grade 3 to 4 treatment-related adverse event, mostly within the first treatment year; no serious related adverse events were observed thereafter. One patient (1%) discontinued treatment due to a related event. No treatment-related deaths occurred. Responses occurred in 26% (95% CI, 18%-36%) of patients. Median duration of response was 22.1 months (range, 2.8 to >41.0 months), and median progression-free survival was 2.7 months (95% CI, 1.4-4.3 months). Median overall survival was 10.1 months (95% CI, 7.3-17.0 months); 3-year OS rate was 27% (95% CI, 17%-36%). Response occurred in 40% (95% CI, 26%-55%; n = 40) and 11% (95% CI, 4%-25%; n = 44) of patients with programmed death ligand 1 expression of at least 5% tumor-infiltrating immune cells (IC2/3) or less than 5% (IC0/1), respectively. Median overall survival in patients with IC2/3 and IC0/1 was 14.6 months (95% CI, 9.0 months to not estimable) and 7.6 months (95% CI, 4.7 to 13.9 months), respectively.
Conclusions and Relevance
Atezolizumab remained well tolerated and provided durable clinical benefit to a heavily pretreated metastatic urothelial carcinoma population in this long-term study.
Trial Registration
clinicaltrials.gov Identifier: NCT01375842
Introduction
Platinum-based chemotherapy is the most commonly used first-line treatment for patients with locally advanced or metastatic urothelial carcinoma (mUC). However, progression with platinum-based chemotherapy is typical, and patients have limited treatments in this setting with historically poor outcomes. Recently, advances in cancer immunotherapy have provided more options for these patients.
Atezolizumab is a humanized, engineered monoclonal antibody that targets programmed death ligand 1 (PD-L1). Atezolizumab prevents the binding of PD-L1 to receptors programmed death 1 (PD-1) and B7.1, reinvigorating and enhancing anticancer immunity. Activation of B7.1 can potentially stimulate long-term responses through development of new immunity via priming and activation of T cells in lymph nodes. Additionally, atezolizumab leaves the PD-L2/PD-1 interaction intact. Atezolizumab has demonstrated safety and clinical benefit in a variety of cancers, including mUC. In phase 1 and 2 studies, atezolizumab demonstrated durable objective responses and good tolerability in patients with inoperable locally advanced or mUC and is approved in the United States and Europe for the treatment of both patients whose disease has progressed during or following platinum-based chemotherapy and those ineligible for cisplatin-containing chemotherapy. Atezolizumab is also approved for previously treated metastatic non–small-cell lung cancer.
The first-in-human phase 1 study, PCD4989g, tested single-agent atezolizumab in previously treated patients with solid or hematologic cancers, including an expansion cohort of patients with mUC. In the first report from this cohort, atezolizumab was well tolerated with promising clinical activity in mUC based on a minimum follow-up of 6 weeks. In the present analysis, including additional patients with mUC, we sought to determine long-term safety and efficacy outcomes following atezolizumab monotherapy. Herein, we report updated data from the ongoing phase 1 study, with a median follow-up of 37.8 months, including 3-year overall survival (OS) and safety outcomes.
Methods
Study Design
This phase 1 study (PCD4989g; NCT01375842) is investigating single-agent atezolizumab (formerly known as MPDL3280A) for the treatment of advanced or metastatic solid tumors and hematologic malignant neoplasms. The overall study design, which consists of dose escalation and expansion cohorts, was previously reported, including the mUC expansion cohort. This study was approved by local institutional review boards at all study sites and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent. The protocol is available in Supplement 1.
Patients and Treatment
Key eligibility criteria for this cohort included measurable disease per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1), Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1, and a representative tumor sample. Baseline PD-L1 expression at enrollment was evaluated using a prototype of the commercially available VENTANA SP142 immunohistochemistry assay (Ventana Medical Systems). Expression of PD-L1 was evaluated on tumor-infiltrating immune cells (IC) based on scoring levels: IC3 (≥10%), IC2 (≥5% and <10%), IC1 (≥1% and <5%), and IC0 (<1%). Enrollment was initially restricted to PD-L1–selected patients (IC2 or IC3) but subsequently extended to allow for enrollment regardless of PD-L1 status.
Patients with mUC received atezolizumab intravenously at 15 mg/kg or 1200 mg every 3 weeks. Initially, patients received atezolizumab for 16 cycles or 1 year (whichever occurred first). A subsequent protocol amendment allowed for treatment beyond 16 cycles (or 1 year) and retreatment of patients who discontinued per the original criteria. Patients were treated until disease progression per both RECIST v1.1 and immune-related response criteria, unacceptable toxic effects, or protocol nonadherence. Patients could also continue treatment past RECIST v1.1 progression until loss of clinical benefit at the investigator’s discretion.
Outcomes and Assessments
The primary objective was to evaluate the safety and tolerability of atezolizumab. Safety-evaluable patients were defined by those who received any amount of atezolizumab. Safety assessments occurred every 3 weeks, with a final evaluation approximately 90 days following the final dose. Afterward, only serious adverse events (AEs) were reported if deemed related to prior study treatment. Adverse event frequencies and severity were graded per the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0. Adverse events of special interest (AESIs) were defined as follows: conditions suggestive of autoimmune disorder; AEs of grade 3 or greater including acute infections, events suggestive of hypersensitivity, cytokine release, systemic inflammatory response, or infusion reaction syndromes, asymptomatic elevation of aspartate aminotransferase/alanine aminotransferase/total bilirubin; or AEs of grade 2 or greater including rash, pruritus, diarrhea, elevation of aspartate aminotransferase/alanine aminotransferase/total bilirubin with constitutional symptoms, hypoxia, or dyspnea. Multiple occurrences of the same event were counted once at highest grade. Annual incidences for AEs deemed treatment related and serious per the investigator were summarized.
Key secondary end points presented in this analysis include RECIST v1.1 objective response rate, duration of response (DOR), and progression-free survival (PFS). The efficacy-evaluable population was the same as the safety-evaluable population, and objective response–evaluable patients must have had measurable disease at baseline (RECIST v1.1). Confirmed objective responses were assessed by the investigator using RECIST v1.1. Radiological assessments were performed every 6 weeks for the first 24 weeks and every 12 weeks thereafter. Tumors were assessed at the next scheduled visit and then every 6 weeks in patients who continued treatment after disease progression. Tumors were assessed every 12 weeks in patients who went into follow-up after an initial 16 cycles (or 1 year) of treatment and in patients who restarted treatment. For clinical activity evaluated by PD-L1 status, patients were grouped as IC2/3 and IC0/1. Overall survival was investigated as an exploratory objective. Overall survival follow-up information was collected from clinic visits, telephone calls, and/or review of patient medical records and/or public data approximately every 3 months until death or loss to follow-up. Median OS was also evaluated by baseline characteristics.
Statistical Analyses
Objective response rate and corresponding 95% confidence intervals (CIs) were calculated using the Clopper-Pearson method. The DOR (time from first occurrence of a documented response to disease progression or death from any cause), PFS, and OS were assessed by the Kaplan-Meier method. The OS and PFS were calculated from time of first dose of atezolizumab. For OS, patients who were alive or lost to follow-up as of the clinical cutoff date were censored at the last known date they were alive. The 95% CI for the median OS was estimated using the Brookmeyer-Crowley method. Milestone rates for PFS and OS were estimated using the Kaplan-Meier method, with 95% CIs calculated using the Greenwood formula.
Results
Patient Characteristics
Four hundred twenty-six patients were screened, of whom 95 met all eligibility criteria and were included in this analysis. Patients were enrolled into this cohort from March 2013 to August 2015; approximately 40% were initially enrolled based on a requirement for evidence of PD-L1 expression (IC2/3 status), and 60% were enrolled regardless of PD-L1 expression levels. The clinical cutoff date of this analysis was December 31, 2016, and 95 patients with mUC were evaluable for safety and clinical activity.
Patient demographic and baseline clinical characteristics for the safety-evaluable population are presented in eTable 1 in Supplement 2. Of this population, 53% (50 of 95) had IC2/3 status, and 46% (44 of 95) had IC0/1 status. In general, most patients were heavily pretreated, with 68 (72%) receiving prior platinum-based chemotherapy in the metastatic setting, and 45 (47%) receiving atezolizumab as the third or greater line of therapy. During this study, patients received atezolizumab for a median of 3 months (range, 0 to 44 months) and median of 5 doses (range, 1 to 57 doses). Eighty-six patients (91%) received atezolizumab at 15 mg/kg, and 9 received atezolizumab at a flat 1200-mg dose. The median follow-up duration was 37.8 months (range, >0.7 to 44.4 months) in all patients, 38.2 months (range, >0.7 to 44.4 months) in patients with IC2/3 tumors, and 29.2 months (range, >0.7 to 38.7 months) in patients with IC0/1 tumors. At data cutoff, 23 patients (24%) had been treated for at least 1 year, with 14 patients (15%) continuing treatment and 23 (24%) remaining in the study.
Safety
Overall, atezolizumab was well tolerated in patients with mUC. Adverse events observed regardless of attribution occurred in 93 patients (98%), with 50 (53%) experiencing grade 3 to 4 AEs of any cause. All-grade treatment-related AEs (TRAEs) were observed in 64 patients (67%), with the majority experiencing grade 1 to 2 events. Grade 3 to 4 TRAEs were observed in 9 patients (9%) (Table 1). Only 1 patient had a grade 4 TRAE (increased γ-glutamyltransferase level). The most common any-grade treatment-related toxic effects were fatigue, asthenia, decreased appetite, and pruritus (Table 1). No treatment-related deaths occurred. Treatment-related AEs leading to dose modification (or interruption) or to discontinuation were observed in 5 (5%) and 1 (1%) patients, respectively. Atezolizumab was also tolerable in patients 65 years or older (eTable 2 in Supplement 2). Adverse events of special interest regardless of attribution occurred in 37 (39%) patients, with all-grade AESIs in more than 10%, including rash in 12 patients (13%) and increased aspartate aminotransferase level in 11 patients (12%). Eight patients (8%) had a grade 3 AESI, and no grade 4 AESIs occurred.
Table 1. Treatment-Related Adverse Events (AEs).
| Treatment-Related AE | Events, No. (%) (N = 95) | |
|---|---|---|
| Any Grade in ≥3% of Patients | Grades 3-4 in ≥1% of Patients | |
| Any AE | 64 (67) | 9 (9) |
| Fatigue | 17 (18) | 0 |
| Asthenia | 13 (14) | 2 (2) |
| Decreased appetite | 12 (13) | 0 |
| Pruritus | 12 (13) | 0 |
| Nausea | 11 (12) | 0 |
| Rash | 8 (8) | 0 |
| Diarrhea | 7 (7) | 0 |
| Pyrexia | 7 (7) | 0 |
| Arthralgia | 6 (6) | 0 |
| Hypothyroidism | 5 (5) | 0 |
| Alanine aminotransferase level increased | 4 (4) | 1 (1) |
| Anemia | 4 (4) | 1 (1) |
| Aspartate aminotransferase level increased | 4 (4) | 2 (2) |
| Dry skin | 4 (4) | 0 |
| Influenza-like illness | 4 (4) | 0 |
| Myalgia | 4 (4) | 0 |
| Chills | 3 (3) | 0 |
| Cough | 3 (3) | 0 |
| Dysgeusia | 3 (3) | 0 |
| Lethargy | 3 (3) | 0 |
| Thrombocytopenia | 3 (3) | 1 (1) |
| Blood phosphorus level decreased | 2 (2) | 1 (1) |
| γ-Glutamyltransferase level increased | 2 (2) | 1 (1) |
| Lymphopenia | 2 (2) | 1 (1) |
| Maculopapular rash | 2 (2) | 1 (1) |
| Neutropenia | 1 (1) | 1 (1) |
Safety was also evaluated as a function of follow-up duration (Table 2). Most TRAEs occurred within the first year of treatment, with the incidence of AEs beyond year 1 reduced substantially compared with that in year 1 (15 [41%] vs 63 [66%], respectively). Further decline was observed with every additional year of follow-up (13 [35%], 7 [28%], and 1 [8%] in years 2, 3, and 4, respectively). No treatment-related serious AEs occurred beyond year 1 (Table 2). Beyond year 1, most TRAEs were grade 1 or 2, the most common of which, pruritus, occurred in 3 patients (8%) (eTable 3 in Supplement 2); 2 grade 3 TRAEs were seen (maculopapular rash and neutropenia) (eTable 3 in Supplement 2).
Table 2. Patterns of Treatment-Related Adverse Event (TRAE) Occurrence.
| Time Following Initiation of Atezolizumab Treatmenta | TRAEs, No. (%) | |||
|---|---|---|---|---|
| All | Serious | |||
| Any Grade | Grades 3-4 | Any Grade | Grades 3-4 | |
| Within year 1 (N = 95) | 63 (66) | 7 (7) | 5 (5) | 0 |
| Beyond year 1 (n = 37) | 15 (41) | 2 (5) | 0 | 0 |
| Year 2 (n = 37) | 13 (35) | 2 (5) | 0 | 0 |
| Year 3 (n = 25) | 7 (28) | 0 | 0 | 0 |
| Year 4 (n = 13) | 1 (8) | 0 | 0 | 0 |
Values in parentheses indicate the number of patients evaluable for safety at the beginning of each period.
Clinical Activity
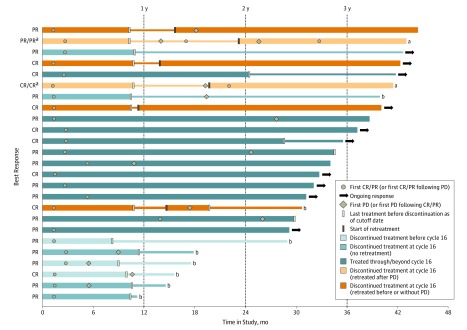
Objective responses were observed in 25 of 95 patients (26%; 95% CI, 18% to 36%), including 9 patients with complete responses (Table 3). Of these 9 patients, 8 had tumors with PD-L1 IC2/3 status, and 1 had a tumor with PD-L1 IC0/1 status. Eighteen patients experienced stable disease, of which 9 each had IC2/3 or IC0/1 tumors. The median DOR was 22.1 months (range, 2.8 to >41.0 months) in all patients, 18.0 months (range, 2.8 to >41.0 months) in the IC2/3 subgroup, and 27.6 months (range, 6.2 to >34.3 months) in the IC0/1 subgroup. Ten of 25 responders (40%) had ongoing responses at the time of clinical cutoff. Long-term responses were seen in a variety of patients, including those who discontinued therapy (Figure 1): 11 of 25 responders discontinued treatment at cycle 16 in accordance with an early version of the protocol. Among them, 2 patients restarted treatment after disease progression, and 4 restarted treatment while their disease was in response. As of the data cutoff, the longest ongoing response duration, approximately 41 months, occurred in a patient in the latter group (interim off-treatment period, approximately 2.5 months). This patient initially experienced a partial response and achieved a complete response after reinitiation of atezolizumab therapy. Of the 5 patients who did not resume treatment, only 1 was still in response as of the clinical cutoff, with an ongoing response duration of approximately 40 months. The first confirmed response in this patient occurred during cycle 4 of atezolizumab, and at the last tumor assessment, the patient was off treatment for approximately 31 months.
Table 3. Objective Response Rates to Atezolizumab Treatment and Duration of Response by Programmed Death Ligand 1 Immunohistochemical Status.
| Parameter | IC0/1 (n = 44) | IC2/3 (n = 50) | All Patients (N = 95)a |
|---|---|---|---|
| Objective response rate (95% CI)b | 11 (4 to 25) | 40 (26 to 55) | 26 (18 to 36) |
| Best overall response, No. (%) | |||
| Complete response | 1 (2) | 8 (16) | 9 (10) |
| Partial response | 4 (9) | 12 (24) | 16 (17) |
| Stable disease | 9 (21) | 9 (18) | 18 (19) |
| Progressive disease | 24 (55) | 17 (34) | 42 (44) |
| No assessmentc | 6 (14) | 4 (8) | 10 (11) |
| Duration of response, mo (range) | 27.6 (6.2 to >34.3) | 18.0 (2.8 to >41.0) | 22.1 (2.8 to >41.0) |
Abbreviation: IC, tumor-infiltrating immune cells.
Includes 1 patient with unknown programmed death ligand 1 IC immunohistochemical status.
Confirmed Response Evaluation Criteria in Solid Tumors version 1.1 objective responses assessed by investigator.
Refers to missing or unevaluable response status.
Figure 1. Duration of Treatment and Response in Patients With Metastatic Urothelial Cancer Treated With Atezolizumab.
Time in study is plotted for patients with confirmed, investigator-assessed Response Evaluation Criteria in Solid Tumors version 1.1 responses. Bar color indicates treatment or retreatment status, and symbols, response assessments. Thin bars indicate off-treatment periods. Ongoing response indicates no observation of progressive disease (PD) or death. CR indicates complete response; and PR, partial response.
a Subsequent, postretreatment response calculated after rebaselining of target lesion diameters at time of retreatment. No PD or death following subsequent response.
b Patient is deceased.
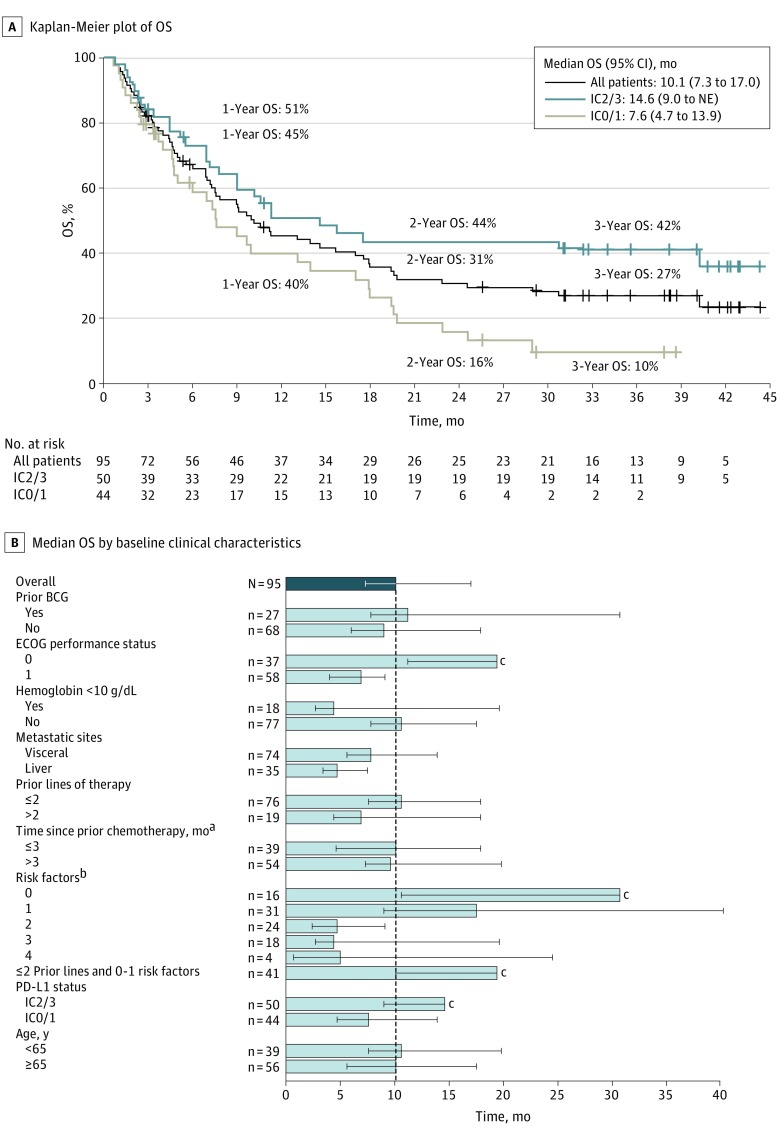
Median PFS in all 95 patients was 2.7 months (95% CI, 1.4-4.3 months); in 50 patients with IC2/3 status, the median PFS was 5.5 months (95% CI, 2.7-10.8 months), and in 44 patients with IC0/1 status, the median PFS was 1.4 months (95% CI, 1.3-2.7 months) (eFigure in Supplement 2). At the time of clinical cutoff, 64 (67%) patients had died (28 patients for IC2/3, 35 patients for IC0/1, and 1 patient for IC unknown subgroup). The median OS (Figure 2A) in all patients was 10.1 months (95% CI, 7.3-17.0 months). The IC2/3 and IC0/1 subgroup median OS was 14.6 months (95% CI, 9.0 months to not estimable) and 7.6 months (95% CI, 4.7-13.9 months), respectively. Among all patients, the 1-, 2-, and 3-year survival rates were 45% (95% CI, 35%-56%), 31% (95% CI, 21%-41%), and 27% (95% CI, 17%-36%), respectively. The corresponding OS rates in patients with PD-L1 IC2/3 tumors were 51% (95% CI, 36%-65%), 44% (95% CI, 29%-58%), and 42% (95% CI, 27%-56%); in the IC0/1 subgroup, these rates were 40% (95% CI, 25%-56%), 16% (95% CI, 4%-28%), and 10% (95% CI, 0%-20%) (Figure 2A).
Figure 2. Overall Survival (OS) by Programmed Death Ligand 1 (PD-L1) Status and Key Clinical Subgroups.
A, Kaplan-Meier estimates of OS in all patients and based on PD-L1 status on tumor-infiltrating immune cells (ICs). Plus signs indicate censoring. One patient with unknown PD-L1 IC immunohistochemical status is included in the all-patient curve. B, Median OS by baseline characteristics. Error bars indicate 95% CIs; BCG, bacille Calmette-Guérin; ECOG, Eastern Cooperative Oncology Group; and NE, not estimable.
aIndicates time from last prior chemotherapy to first administration of atezolizumab.
bIndicates baseline ECOG performance status 1 or greater, baseline liver metastasis, baseline hemoglobin level less than 10 g/dL (to convert to grams per liter, multiply by 10.0), and time since prior chemotherapy 3 months or less.
cThe upper 95% CI is NE.
In an exploratory subgroup analysis, we also evaluated median OS following atezolizumab therapy in key clinical subgroups. A numerically longer median OS was observed in patients with ECOG PS 0 or PD-L1 IC2/3. Assessment of OS by established risk factors at baseline (ECOG ≥1, liver metastasis, hemoglobin level <10 g/dL [to convert to grams per liter, multiply by 10.0], and time since prior chemotherapy ≤3 months) also revealed numerically longer OS in patients with fewer risk factors (Figure 2B). Notably, when the OS analysis was restricted to patients with only 0 to 2 prior lines of therapy (excluding more heavily pretreated patients) who had no or only 1 risk factor (n = 41), the median OS was 19.4 months (95% CI, 10.1 months to not estimable) (Figure 2B). When analyzed by age, median OS was similar in patients younger than 65 years (10.6 months; 95% CI, 7.6-19.8 months) and 65 years or older (10.1 months; 95% CI, 5.6-17.5 months) (Figure 2B).
Discussion
Effective therapies for mUC are needed, given the approximately 5% 5-year OS rate for US patients with mUC. While platinum-based therapy is an established first-line treatment, no consensus exists for treatment of patients with more advanced disease. Treatment options include taxanes, or in European countries, vinflunine. Recently, checkpoint inhibitors have succeeded chemotherapy in this setting, leading to several regulatory approvals. Attractive aspects of these agents are the durability of response and comparatively low rate of AEs. The initial report on the phase 1 study cohort of atezolizumab in mUC reported good tolerability and preliminary clinical activity in mUC. In this long-term follow-up analysis, we assessed whether toxic effects remained manageable and responses durable, and whether these results translated into long-term survival benefit.
We demonstrated that atezolizumab monotherapy was tolerable in patients with mUC with extended treatment. Within the first year of treatment, only 7% of patients experienced a grade 3 to 4 TRAE. The more than 50% reduction in all-grade TRAE incidence over the safety follow-up duration (66% in year 1 vs 8% in year 4), decline in grade 3 to 4 TRAEs from year 1 to 2, and absence of treatment-related deaths confirm these findings. The safety profile was consistent with our previous report and with subsequent phase 2 reports on atezolizumab monotherapy in pretreated patients with mUC. Together with the low rates of TRAEs leading to dose modifications or discontinuation and rates of grade 3 AESIs, these results provide further evidence of long-term tolerability.
In our initial report, DOR and OS data were immature, with medians nonestimable. In this update, atezolizumab treatment resulted in prolonged clinical benefit to patients with mUC (Figures 1 and 2 and eFigure in Supplement 2). Notably here, 40% of responders had ongoing responses at the last tumor assessment as of clinical cutoff, and long-term responses were seen even in patients who transiently or permanently discontinued therapy. The all-patient median DOR was 22.1 months, and several patients were in response for approximately 40 months as of data cutoff (Figure 1). These results compare favorably with a prior vinflunine report (median DOR, 7.4 months) and with estimable DOR data for patients with mUC treated with checkpoint inhibitors.
In this analysis, 46%, 31%, and 27% of patients were alive after 1, 2, and 3 years, respectively. With a median survival follow-up of approximately 38 months, the median OS was 10.1 months, and poor prognostic factors did not preclude long-term survival (Figure 2). Many patients were heavily pretreated; yet median survival in the third-line setting or beyond was comparable with that of the overall cohort. Similar OS results were observed in the phase 2 IMvigor210 study, wherein no major trends in survival outcomes were observed in more heavily pretreated patients vs the overall cohort. The median OS reported in the present study is also consistent with the 10.3-month duration reported with pembrolizumab in the phase 3 KEYNOTE-045 study. Furthermore, when compared with the phase 3 IMvigor211 study of atezolizumab vs chemotherapy, 12-month OS rates reported herein (IC2/3, 51%; all patients, 45%) are generally consistent with the atezolizumab arm (IC2/3, 46%; intent-to-treat population, 39%), although the phase 1 median OS (IC2/3, 14.6; all patients, 10.1 months) was slightly longer than in IMvigor211 (IC2/3, 11.1 months; intent-to-treat, 8.6 months), and atezolizumab did not significantly improve OS over chemotherapy in the IC2/3 population of IMvigor211. However, shorter follow-up and differences in enrollment criteria for the aforementioned studies limit direct comparisons with this phase 1 study. Most notably, both phase 3 checkpoint inhibitor studies in platinum-treated patients with mUC restricted the maximum prior lines of therapy to 2, whereas the phase 1 study permitted broader pretreatment (eg, approximately 20% of patients received pembrolizumab in the third line vs 47% who received atezolizumab in the third line or beyond in this study). Additionally, in IMvigor210, IMvigor211, and KEYNOTE-045, patients were enrolled regardless of PD-L1 status, while this phase 1 study contains a higher prevalence of patients with PD-L1 IC2/3 status than would a PD-L1–unselected population. Notwithstanding these population differences, data from this study suggest that a subset of patients treated with atezolizumab can attain long-term survival.
To our knowledge, the present analysis reports safety and clinical outcomes in patients with mUC based on the longest median follow-up for an anti–PD-L1/PD-1 checkpoint inhibitor to date. This long-term analysis of an mUC population allows for further insights into reductions in TRAEs after the first year of treatment in addition to the durable responses and survival observed in many patients.
Limitations
Limitations in the present study include its single arm, relatively limited sample size, and changes in initial PD-L1 selection criteria for patient enrollment—which resulted in a higher proportion of patients with PD-L1 IC2/3 status and slightly longer median follow-up in these patients (approximately 33 months) than in those with IC0/1 tumors (approximately 29 months). Still, clinical benefit exceeding historical controls (eg, DOR and long-term OS) was observed regardless of PD-L1 IC status. Furthermore, our data are generally in agreement with results from the IMvigor210 and IMvigor211 trials, and also appear consistent with data reported for patients treated with other checkpoint inhibitors.
Conclusions
In conclusion, with an extended median follow-up of more than 3 years, this analysis demonstrates continued tolerability of atezolizumab in the mUC cohort of this phase 1 study. Adverse effects were generally manageable, with no emergent safety concerns seen. Encouraging clinical benefit, including durability of response and long-term survival, were observed in this heavily pretreated mUC population. Collectively, clinical results reported here compare well with published results for other checkpoint inhibitors or chemotherapy and support a continuing role for atezolizumab in altering treatment paradigms and outcomes in patients with mUC. Ongoing studies of single-agent atezolizumab and combination therapies in a variety of settings will be required to fully delineate and confirm the clinical correlates of response and survival in patients with urothelial cancer.
Trial Protocol
eTable 1. Baseline Demographics and Clinical Characteristics of Patients With mUC (N = 95)
eTable 2. Summary of AEs in Patients < 65 Years and ≥ 65 Years
eTable 3. Treatment-related AEs Occurring Beyond 1 Year Following Initiation of Atezolizumab
eFigure. Progression-Free Survival
References
- 1.Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A; ESMO Guidelines Working Group . Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii40-iii48. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer. V5.2017. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed September 7, 2017.
- 3.Tecentriq (atezolizumab) [package insert]. South San Francisco, CA: Genentech, Inc; 2017.
- 4.Opdivo (nivolumab) [package insert]. New York, NY: Bristol-Myers Squibb; 2017.
- 5.Keytruda (pembrolizumab) [package insert]. Whitehouse Station, NJ; 2017.
- 6.Imfinzi (durvalumab) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2017.
- 7.Bavencio (avelumab) [package insert]. New York, NY: EMD Serono, Inc, and Pfizer Inc; 2017.
- 8.Herbst RS, Soria JC, Kowanetz M, et al. . Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1-10. [DOI] [PubMed] [Google Scholar]
- 10.McDermott DF, Sosman JA, Sznol M, et al. . Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34(8):833-842. [DOI] [PubMed] [Google Scholar]
- 11.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powles T, Eder JP, Fine GD, et al. . MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg JE, Hoffman-Censits J, Powles T, et al. . Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balar AV, Galsky MD, Rosenberg JE, et al. ; IMvigor210 Study Group . Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tecentriq (atezolizumab) [package insert]. Welwyn Garden City, United Kingdom: Roche Registration Ltd; 2017.
- 16.Fehrenbacher L, Spira A, Ballinger M, et al. ; POPLAR Study Group . Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. [DOI] [PubMed] [Google Scholar]
- 17.Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators . Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results Program Cancer stat facts: bladder cancer. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed April 28, 2017.
- 19.Bellmunt J, Théodore C, Demkov T, et al. . Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454-4461. [DOI] [PubMed] [Google Scholar]
- 20.Massard C, Gordon MS, Sharma S, et al. . Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P, Callahan MK, Bono P, et al. . Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apolo AB, Infante JR, Balmanoukian A, et al. . Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P, Retz M, Siefker-Radtke A, et al. . Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322. [DOI] [PubMed] [Google Scholar]
- 24.Plimack ER, Bellmunt J, Gupta S, et al. . Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212-220. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Gracia JL, Loriot Y, Rosenberg JE, et al. . Atezolizumab (atezo) in platinum-treated locally advanced or metastatic urothelial carcinoma (mUC): outcomes by prior therapy. 2017;35(suppl 6S):abstr 323. [Google Scholar]
- 26.Powles T, Loriot Y, Durán I, et al. IMvigor211: a phase III randomized study examining atezolizumab versus chemotherapy for platinum-treated advanced urothelial carcinoma. Paper presented at: European Association for Cancer Research–American Association for Cancer Research–Italian Cancer Society Special Conference 2017; June 24–27, 2017; Florence, Italy. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Demographics and Clinical Characteristics of Patients With mUC (N = 95)
eTable 2. Summary of AEs in Patients < 65 Years and ≥ 65 Years
eTable 3. Treatment-related AEs Occurring Beyond 1 Year Following Initiation of Atezolizumab
eFigure. Progression-Free Survival