Key Points
Question
What is the efficacy of first-line avelumab treatment in patients with distant metastatic Merkel cell carcinoma?
Findings
In a preplanned interim analysis of a phase 2 study in 39 patients with metastatic Merkel cell carcinoma, avelumab treatment resulted in a confirmed objective response rate of 62.1% (95% CI, 42.3%-79.3%); 16 of 18 responses (88.9%) occurred by the first planned postbaseline assessment. Grade 3 treatment-related adverse events occurred in 8 of 39 patients (20.5%), with no treatment-related deaths.
Meaning
First-line avelumab monotherapy in patients with metastatic Merkel cell carcinoma was associated with early responses, high response rates, and a manageable safety profile.
Abstract
Importance
Merkel cell carcinoma (MCC) is an aggressive skin cancer that is associated with poor survival outcomes in patients with distant metastatic disease. Results of part A of the JAVELIN Merkel 200 trial (avelumab in patients with Merkel cell carcinoma) showed that avelumab, an anti–programmed cell death ligand 1 (PD-L1) antibody, demonstrated efficacy in second-line or later treatment of patients with metastatic MCC (mMCC).
Objective
To evaluate the efficacy and safety of avelumab as first-line treatment for patients with distant mMCC.
Design, Setting, and Participants
JAVELIN Merkel 200 part B is an international, multicenter, single-arm, open-label clinical trial of first-line avelumab monotherapy. Eligible patients were adults with mMCC who had not received prior systemic treatment for metastatic disease. Patients were not selected for PD-L1 expression or Merkel cell polyomavirus status. Data were collected from April 15, 2016, to March 24, 2017, and enrollment is ongoing.
Interventions
Patients received avelumab, 10 mg/kg, by 1-hour intravenous infusion every 2 weeks until confirmed disease progression, unacceptable toxic effects, or withdrawal occurred.
Main Outcomes and Measures
Tumor status was assessed every 6 weeks and evaluated by independent review committee per Response Evaluation Criteria in Solid Tumors version 1.1. The primary end point was durable response, defined as an objective response with a duration of at least 6 months. Secondary end points include best overall response, duration of response, progression-free survival, safety, and tolerability.
Results
As of March 24, 2017, 39 patients were enrolled (30 men and 9 women; median age, 75 years [range, 47-88 years]), with a median follow-up of 5.1 months (range, 0.3-11.3 months). In a preplanned analysis, efficacy was assessed in 29 patients with at least 3 months of follow-up; the confirmed objective response rate was 62.1% (95% CI, 42.3%-79.3%), with 14 of 18 responses (77.8%) ongoing at the time of analysis. In responding patients, the estimated proportion with duration of response of at least 3 months was 93% (95% CI, 61%-99%); duration of response of at least 6 months, 83% (95% CI, 46%-96%). First-line avelumab treatment was generally well tolerated, and no treatment-related deaths or grade 4 adverse events occurred.
Conclusions and Relevance
High rates of response to first-line avelumab therapy in patients with distant mMCC build on previously reported antitumor activity after second-line or later treatment, and maturing progression-free survival data suggest that responses are durable. These data further support avelumab’s approval in the United States and European Union and use as a standard-of-care treatment for mMCC.
Trial Registration
clinicaltrials.gov Identifier: NCT02155647
This preplanned interim analysis of a single-arm clinical trial evaluates the efficacy and safety of avelumab as first-line treatment for patients with distant metastatic Merkel cell carcinoma.
Introduction
Metastatic Merkel cell carcinoma (mMCC) is a rare, aggressive skin cancer with poor survival.1 Merkel cell carcinoma (MCC) is associated with Merkel cell polyomavirus infection, UV light exposure, and advanced age; additional risk factors include fair skin and male sex.1,2 Despite MCC being a chemosensitive disease, durable responses are rare.3 In retrospective studies of first-line chemotherapy in patients with mMCC, median progression-free survival (PFS) was 3.1 to 4.6 months,4,5 highlighting the need for improved treatment options. Programmed cell death ligand 1 (PD-L1) is expressed on MCC tumor cells and in the MCC tumor microenvironment, indicating a potential role for immune checkpoint inhibitors such as anti–PD-L1 and anti–programmed cell death 1 (PD-1) antibodies.6,7,8 Trials involving anti–PD-1/PD-L1 antibodies have shown clinical activity and durable responses in patients with advanced MCC.9,10,11 Based on findings from the phase 2 trial of avelumab, an IgG1 anti–PD-L1 monoclonal antibody, in patients with MCC (JAVELIN Merkel 200), avelumab became the first treatment for patients with mMCC to receive approval in the United States, the European Union, and Japan.11,12,13,14,15 Herein, we report data from an interim analysis of avelumab as first-line treatment in patients with mMCC.
Methods
In part B of the ongoing JAVELIN Merkel 200 trial (start date, April 15, 2016), eligible patients had stage IV mMCC and had not received prior systemic therapy for metastatic disease. Patients with autoimmune conditions were excluded. The primary end point was durable response, defined as an objective response with a duration of at least 6 months according to Response Evaluation Criteria in Solid Tumors version 1.1, per independent review. Secondary end points included best overall response, duration of response, PFS, and safety. PD-L1 expression and viral status will be investigated in a future analysis. Time-to-event end points were analyzed by Kaplan-Meier methods; medians were calculated with corresponding CIs using the Brookmeyer-Crowley method. Additional eligibility criteria, procedures for statistical analysis, and study design information for JAVELIN Merkel 200 have been previously reported.11 Patients provided written informed consent to enroll in the protocol with institutional review board approval from the participating centers (eTable 1 in the Supplement) and in accordance with international good clinical practice standards.
Results
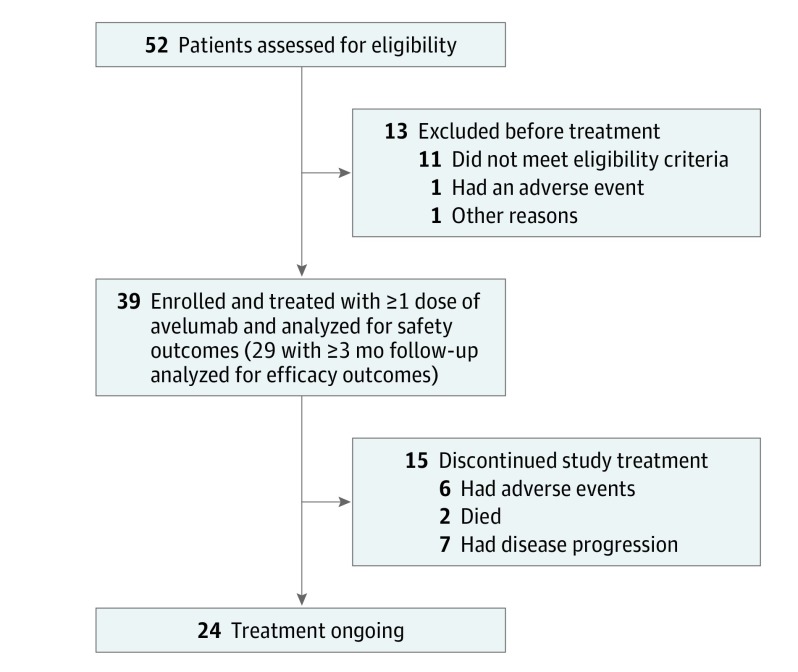
From April 15, 1016, to March 24, 2017, 39 of 112 patients initiated avelumab treatment (Figure 1), with a median follow-up of 5.1 months (range, 0.3-11.3 months). Thirty patients were men, and 9 were women, and median age was 75 years (range, 47-88 years); additional demographics are described in eTable 2 in the Supplement. Median duration of treatment was 12 weeks (range, 2.0-49.9 weeks). Treatment was ongoing in 24 patients (61.5%), and 15 (38.5%) discontinued treatment owing to disease progression (7 [17.9%]), adverse events (6 [15.4%]), or death (2 [5.1%]). In this prespecified interim analysis, safety was assessed in 39 patients who received at least 1 dose of avelumab, and efficacy was evaluated in 29 patients with at least 3 months of follow-up. Efficacy was also assessed in a subset of 14 patients with at least 6 months of follow-up.
Figure 1. Flowchart of Study Population.
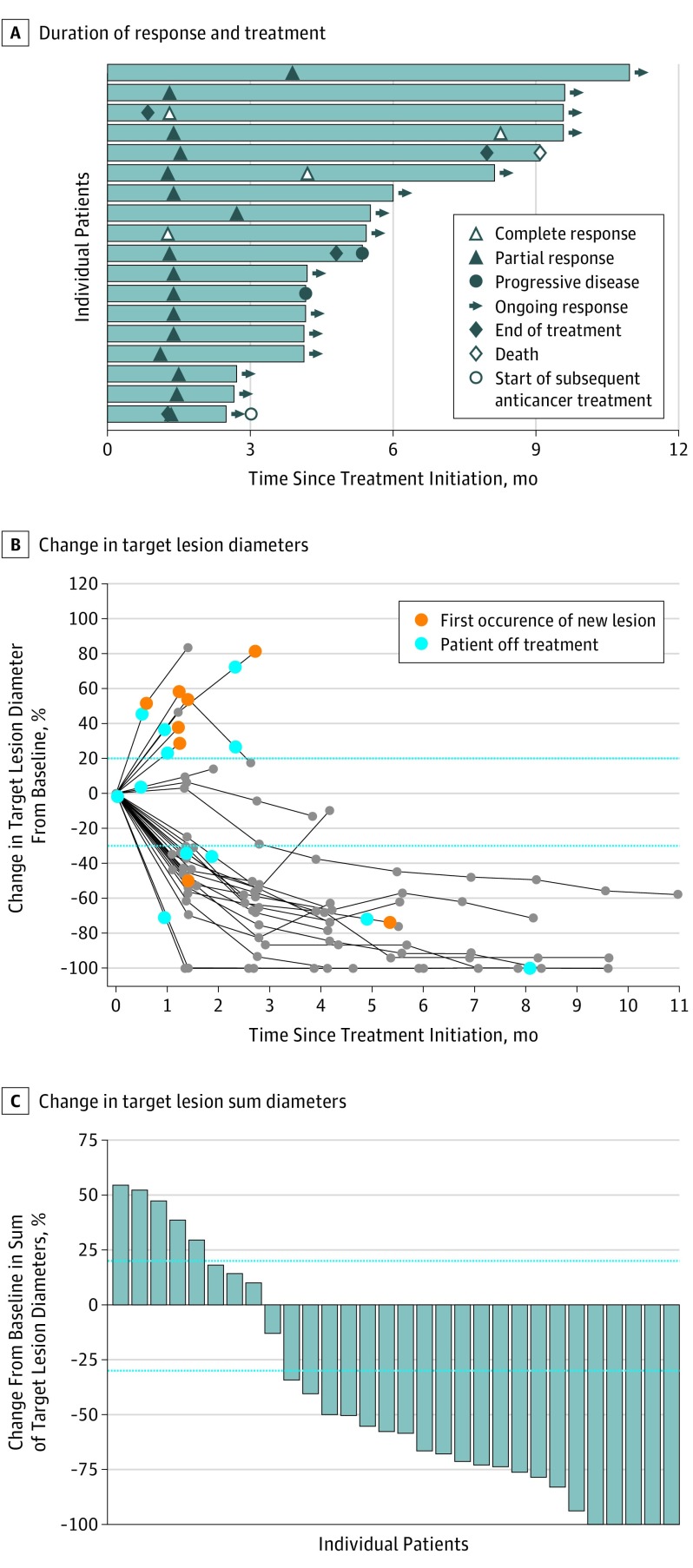
Of the 29 patients with at least 3 months of follow-up, 4 (13.8%) had a complete response and 14 (48.3%) had a partial response, resulting in a confirmed objective response rate of 62.1% (95% CI, 42.3%-79.3%) (Table); 3 additional patients (10.3%) had stable disease. Median time to response was 6.1 weeks (range, 5-17 weeks), with 16 of 18 responses (88.9%) observed at the first scheduled postbaseline assessment approximately 6 weeks from the start of treatment (Figure 2A). Among the 14 patients with at least 6 months of follow-up, the confirmed objective response rate was 71.4% (95% CI, 41.9%-91.6%). Four patients (28.6%) had a complete response, and 6 (42.9%) had a partial response; an additional patient (7.1%) had stable disease.
Table. Outcomes by RECIST Version 1.1, per Independent Review Committee Assessment.
| Outcome | Patient Follow-up Group | |
|---|---|---|
| ≥3 mo | ≥6 mo | |
| Responsea | ||
| Confirmed ORR, % (95% CI) | 62.1 (42.3-79.3) | 71.4 (41.9-91.6) |
| Confirmed BOR, No. (%) | ||
| Complete response | 4 (13.8) | 4 (28.6) |
| Partial response | 14 (48.3) | 6 (42.9) |
| Stable disease | 3 (10.3) | 1 (7.1) |
| Progressive disease | 7 (24.1) | 2 (14.3) |
| Nonevaluableb | 1 (3.4) | 1 (7.1) |
| Response durabilityc | ||
| Median DOR (95% CI), mo | NE (4.0 to NE) | NE (4.0 to NE) |
| Proportion of responses with duration ≥3 mo, % (95% CI) | 93 (61-99) | 100 (NE) |
| Proportion of responses with duration ≥6 mo, % (95% CI) | 83 (46-96) | 89 (43-98) |
Abbreviations: BOR, best overall response; DOR, duration of response; NE, not estimable; ORR, objective response rate; RECIST, Response Evaluation Criteria in Solid Tumors.
Includes 29 patients with at least 3 and 14 with at least 6 months of follow-up.
Patient died before tumor assessment due to an adverse event unrelated to treatment with avelumab.
Includes 18 patients with at least 3 and 10 with at least 6 months of follow-up.
Figure 2. Clinical Activity of Avelumab.
A, Time to and duration of response and duration of treatment were measured in 18 patients with a confirmed response within the analysis set of patients with at least 3 months of follow-up. B and C, Change in target lesion diameters and percentage of change in target lesion sum diameters from baseline in individual patients were measured in 30 patients with a follow-up tumor assessment available. Parallel broken lines represent Response Evaluation Criteria in Solid Tumors values for progressive disease (≥20% increase in the sum of diameters of target lesions) and partial response (≥30% decrease in the sum of diameters of target lesions).
Responses were ongoing at the time of analysis in 14 of 18 responders (77.8%), and the median duration of response was not estimable (range, 1.2-8.3 months). Based on Kaplan-Meier estimates, the proportion of responses with a duration of at least 3 months was 93% (95% CI, 61%-99%); those with a response duration of at least 6 months, 83% (95% CI, 49%-96%). In this still-maturing cohort, the 3-month PFS rate was 67% (95% CI, 48%-80%), and median PFS was 9.1 months (range, 1.9 to not estimable) (Table). Twenty-one of 30 patients (70%) with a follow-up tumor assessment available were reported to have at least 30% reduction in target lesions from baseline (Figure 2B-C).
Among 39 patients evaluable for safety, 28 (71.8%) had a treatment-related adverse event (TRAE), and 8 patients (20.5%) had a grade 3 TRAE (eTable 3 in the Supplement). No grade 4 TRAEs or treatment-related deaths occurred. Treatment-related infusion-related reactions occurred in 9 patients (23.1%) and were grade 3 in 1 patient (2.6%). Grade 1 immune-related adverse events occurred in 6 patients (15.4%) (eTable 4 in the Supplement). Avelumab therapy was permanently discontinued because of a TRAE in 1 patient each with cholangitis, elevated aspartate aminotransferase and alanine aminotransferase levels, paraneoplastic syndrome and gait disturbance, and paraneoplastic encephalomyelitis and polyneuropathy and in 2 patients with infusion-related reaction (6 patients [15.4%]). Of these 6 patients, 1 had a complete response ongoing at the time of analysis, 1 had a partial response assessed before the start of subsequent anticancer therapy, and 1 had stable disease before the start of subsequent anticancer therapy.
Discussion
In this prespecified interim analysis of patients with mMCC, first-line avelumab treatment was associated with early and durable responses and a manageable safety profile. The confirmed objective response rate is consistent with results of previous studies of immune checkpoint inhibitors for first-line treatment of MCC.9,10 To our knowledge, this study is the first to investigate immune checkpoint inhibitors exclusively in patients with stage IV MCC. Two anti–PD-1 antibodies have been investigated for the first-line treatment of patients with advanced MCC, including nivolumab in patients with stages II to IV MCC and pembrolizumab in patients with stages IIIB to IV MCC.9,10
Before the approval of avelumab, chemotherapy was commonly used to treat mMCC; it is now recommended for patients who are not candidates for immunotherapy.2 In a systematic literature review, first-line chemotherapy produced high initial response rates (range, 53%-61%); however, response durability was short (range, 2.8-8.0 months), and treatment was associated with considerable toxic effects.3 We report promising evidence of response durability among patients with an objective response and at least 3 months of follow-up, given that 83% of patients are estimated to have responses lasting at least 6 months. Our median PFS of 9.1 months for patients receiving first-line treatment exceeds that previously reported with chemotherapy.4,5 Our safety analysis showed that avelumab was generally tolerable. These findings suggest overall improvement of outcomes with immune checkpoint inhibitors vs chemotherapy in patients with mMCC.
Our results corroborate prior evidence from part A of the JAVELIN Merkel 200 study,11 which showed that previously treated patients with fewer prior lines of treatment experienced more favorable outcomes. JAVELIN Merkel 200 is the largest prospective clinical trial performed in stage IV distant mMCC to date and is actively enrolling participants in part B of the study. Findings from this trial led to approval of avelumab for the treatment of mMCC in the United States, the European Union, and Japan.12,13,14
Strengths and Limitations
Analysis of PFS in the present study is based on limited follow-up in some patients and may evolve as the minimum follow-up extends; longer-term follow-up will also permit meaningful analysis of overall survival. Because data with extended follow-up have not been published for nivolumab and pembrolizumab, comparisons of long-term survival outcomes, TRAEs, and economic impact with other immune checkpoint inhibitors for first-line treatment were not possible.9,10 Future analysis of first-line avelumab treatment will investigate whether biomarkers, such as PD-L1 expression or viral status, correlate with response to treatment.
Conclusions
First-line avelumab monotherapy had antitumor activity and a manageable safety profile in patients with mMCC. Maturing PFS data suggest durable responses, supporting avelumab as a standard of care in patients with mMCC.
eTable 1. JAVELIN Merkel 200 Part B Study Group
eTable 2. Patient Demographics and Baseline Characteristics
eTable 3. Incidence of Treatment-Related Adverse Events
eTable 4. Immune-Related Adverse Events
References
- 1.Schadendorf D, Lebbé C, Zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:-. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Merkel Cell Carcinoma, version 1. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf. Updated 2017. Accessed October 19, 2017. [DOI] [PMC free article] [PubMed]
- 3.Nghiem P, Kaufman HL, Bharmal M, Mahnke L, Phatak H, Becker JC. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017;13(14):1263-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowey CL, Mahnke L, Espirito J, Helwig C, Oksen D, Bharmal M. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13(19):1699-1710. [DOI] [PubMed] [Google Scholar]
- 5.Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5(9):2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipson EJ, Vincent JG, Loyo M, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1(1):54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schadendorf D, Nghiem P, Bhatia S, et al. Immune evasion mechanisms and immune checkpoint inhibition in advanced Merkel cell carcinoma. Oncoimmunology.2017;6(10):e1338237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terheyden P, Becker JC. New developments in the biology and the treatment of metastatic Merkel cell carcinoma. Curr Opin Oncol. 2017;29(3):221-226. [DOI] [PubMed] [Google Scholar]
- 9.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Bhatia S, Hollebecque A, et al. Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): efficacy and safety in Merkel cell carcinoma (MCC). Cancer Res. 2017;77(13 suppl):Abstract CT074. [Google Scholar]
- 11.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bavencio (avelumab) injection [package insert]. Darmstadt, Germany: Merck KGaA; 2017.
- 13.European Commission approves Bavencio (avelumab) for metastatic Merkel cell carcinoma. Darmstadt, Germany: Merck KGaA and Pfizer Inc; September 21, 2017. https://www.pfizer.com/news/press-release/press-release-detail/european_commission_approves_bavencio_avelumab_for_metastatic_merkel_cell_carcinoma. Accessed October 8, 2017.
- 14.Bavencio (avelumab) approved for Merkel cell carcinoma in Japan. Darmstadt, Germany: Merck KGaA and Pfizer Inc; September 21, 2017. https://www.pfizer.com/news/press-release/press-release-detail/bavencio_avelumab_approved_for_merkel_cell_carcinoma_in_japan. Accessed October 8, 2017.
- 15.Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. JAVELIN Merkel 200 Part B Study Group
eTable 2. Patient Demographics and Baseline Characteristics
eTable 3. Incidence of Treatment-Related Adverse Events
eTable 4. Immune-Related Adverse Events