Abstract
Importance
Patients with hepatocellular carcinoma showing macroscopic vascular invasion have a poor prognosis. Sorafenib is the sole treatment option for these patients, with unsatisfactory response and survival benefit. Combined treatment with transarterial chemoembolization (TACE) plus external beam radiotherapy (RT) has shown promising results for these patients in observational studies.
Objective
To evaluate the efficacy and safety of TACE plus RT compared with sorafenib for patients with hepatocellular carcinoma and macroscopic vascular invasion.
Design, Setting, and Participants
In this randomized, open-label clinical trial conducted at an academic tertiary care center between July 1, 2013, and October 31, 2016, 90 treatment-naive patients with liver-confined hepatocellular carcinoma showing macroscopic vascular invasion were randomly assigned to receive sorafenib (400 mg twice daily; 45 participants [the sorafenib group]) or TACE (every 6 weeks) plus RT (within 3 weeks after the first TACE, maximum 45 Gy with the fraction size of 2.5 to 3 Gy; 45 participants [the TACE-RT group]).
Main Outcomes and Measures
The primary end point was the 12-week progression-free survival rate by intention-to-treat analysis. Radiologic response was assessed by independent review according to the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1). Treatment crossover was permitted after confirming disease progression.
Results
Of the 90 patients (median age, 55 years; range, 33-82 years), 77 were men and 13 were women. All patients had portal vein invasion of hepatocellular carcinoma and Child-Pugh class A liver function. The median maximal tumor diameter was 9.7 cm. Most patients (71 [78.9%]) had multiple lesions. At week 12, the progression-free survival rate was significantly higher in the TACE-RT group than the sorafenib group (86.7% vs 34.3%; P < .001). The TACE-RT group showed a significantly higher radiologic response rate than the sorafenib group at 24 weeks (15 [33.3%] vs 1 [2.2%]; P < .001), a significantly longer median time to progression (31.0 vs 11.7 weeks; P < .001), and significantly longer overall survival (55.0 vs 43.0 weeks; P = .04). Curative surgical resection was conducted for 5 patients (11.1%) in the TACE-RT group owing to downstaging. No patients in the TACE-RT group discontinued treatment owing to hepatic decompensation.
Conclusions and Relevance
For patients with advanced hepatocellular carcinoma showing macroscopic vascular invasion, first-line treatment with TACE plus RT was well tolerated and provided an improved progression-free survival, objective response rate, time to progression, and overall survival compared with sorafenib treatment.
Trial Registration
clinicaltrials.gov Identifier: NCT01901692
This randomized clinical trial evaluates the efficacy and safety of transarterial chemoembolization plus external beam radiotherapy compared with sorafenib in patients with hepatocellular carcinoma and macroscopic vascular invasion.
Key Points
Question
Does combined local treatment of transarterial chemoembolization plus external beam radiotherapy improve survival of patients with hepatocellular carcinoma showing macroscopic vascular invasion compared with sorafenib, the current standard systemic treatment?
Findings
In this randomized clinical trial of 90 patients, patients receiving transarterial chemoembolization plus external beam radiotherapy had a significantly higher 12-week progression-free survival rate and a significantly longer median overall survival time compared with patients receiving sorafenib.
Meaning
For patients with locally advanced hepatocellular carcinoma showing macroscopic vascular invasion, combined treatment with transarterial chemoembolization plus external beam radiotherapy may represent a new treatment paradigm, providing improved patient survival compared with sorafenib.
Introduction
Liver cancer is the second leading cause of cancer death worldwide,1 with hepatocellular carcinoma (HCC) accounting for about 90% of all primary liver cancers.2 By 2030, the annual number of new liver cancer cases worldwide is predicted to increase 35% from 2005 (when the number of new cases was 708 536), especially in Western Europe and North America.3,4
About half of patients with HCC receive a diagnosis when the cancer is at a locally advanced stage, often with macroscopic vascular invasion (MVI) bearing a poor prognosis with an expected median survival time of only 2 to 5 months without treatment.5,6,7,8 Sorafenib is the only evidence-based treatment option for this patient group. However, in a pooled analysis of 2 pivotal phase 3 trials, sorafenib prolonged the median survival of these patients by only 47 days compared with placebo.8,9,10
With recent technological advances, external beam radiotherapy (RT) could be considered an alternative treatment option for patients with HCC.11 Recent early-phase trials have demonstrated that RT can be safely and effectively delivered to locally advanced HCC, leading to sustained local control and survival rates higher than in historical controls.12,13,14 The combined treatment of transarterial chemoembolization (TACE) and RT has shown promising radiologic response rates and improved overall survival with HCC and MVI in observational studies.15,16,17 This randomized clinical trial assesses the efficacy and safety of TACE plus RT compared with sorafenib for patients with HCC and MVI.
Methods
Study Design and Participants
This study was a randomized, single-center, open-label clinical trial conducted at Asan Medical Center, Seoul, Republic of Korea. The trial protocol is available in Supplement 1. Eligible patients were aged 20 years or older and had a primary diagnosis of HCC with MVI. No patients had received previous therapy for HCC. Hepatocellular carcinoma was diagnosed by histologic findings and/or the American Association for the Study of Liver Diseases criteria.18 The presence of MVI was assessed by 4-phase dynamic computed tomography (CT) using the following criteria: an intraluminal filling defect adjacent to the primary tumor in a portal and/or hepatic vein, an enhancement of the filling defect on the arterial phase, and a washout on the portal or delayed phases. Patients with HCC invading at least the first- or second-branch portal vein, with preserved unilateral portal blood flow, were included. All patients provided written informed consent before enrollment. This study was approved by the Asan Medical Center institutional review board.
Eligibility criteria also included an Eastern Cooperative Oncology Group performance status score of 0 or 1; Child-Pugh class A liver function; no extrahepatic metastasis detected on abdominal CT scan, chest CT scan, and whole-body bone scan; and adequate hematologic, hepatic, and renal function (hemoglobin level ≥8.5 g/dL [to convert to grams per liter, multiply by 10.0], absolute neutrophil count ≥750/µL [to convert to ×109/L, multiply by 0.001], platelet count ≥30 × 103/µL [to convert to ×109/L, multiply by 1.0], international normalized ratio ≤1.5, albumin level ≥2.8 g/dL [to convert to grams per liter, multiply by 10.0], total bilirubin level ≤3 mg/dL [to convert to micromoles per liter, multiply by 17.104], alanine aminotransferase level <10 times the upper limit of normal, and serum creatinine level ≤1.5 mg/dL [to convert to micromoles per liter, multiply by 88.4]). Patients were required to have at least 1 target lesion measurable in 1 dimension according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.19 Patients were excluded if they had uncontrolled ascites, hepatic encephalopathy, a prior organ transplant, an active gastric or duodenal ulcer, other uncontrolled comorbidities or malignant neoplasms, or were positive for HIV.
Randomization
Between July 1, 2013, and October 31, 2016, all eligible patients were randomly assigned to receive sorafenib or TACE plus RT in a 1:1 ratio using an interactive web response system integrated with the electronic data capture system (Medrio Inc). Randomization was conducted within 1 week after eligibility confirmation, and treatments began within 1 week thereafter.
Interventions
Sorafenib was continuously administered orally as a standard 400-mg dose twice daily (800 mg/d). Patient visits were scheduled every 2 weeks for the first 6 weeks and every 3 weeks thereafter to monitor safety and drug accountability. Dose reductions and treatment interruptions were allowed according to drug-related toxicity grade as recommended.
For the TACE procedure, after selective catheterization of the feeding artery, 2 mg/kg of cisplatin was infused as the chemotherapeutic agent. The feeding arteries were then embolized using an emulsion of 5 to 10 mL of cisplatin and iodized oil (Lipiodol Ultra-Fluide; Laboratoire Guerbet) mixture, followed by an absorbable gelatin sponge (Gelfoam; Upjohn). To minimize the risk of hepatic decompensation after TACE, gelatin sponge particle embolization was not performed according to the severity of portal blood flow impairment at the discretion of the investigator. Transarterial chemoembolization was repeated every 6 of the first 24 weeks and every 6 to 8 weeks thereafter.
External beam RT for vascular invasion began within 3 weeks after the first TACE. Gross tumor volume included vascular invasion and a 2-cm margin into the contiguous HCC at the end-expiratory phase of the CT image. Internal target volume was delineated as the sum of the individual gross tumor volumes, as defined within the gated respiration phases. Planning target volume was expanded to include a 0.7-cm margin from the internal target volume. The planned total dose to the planning target volume was 45 Gy with the fraction size of 2.5 to 3.0 Gy, using 6-, 10-, or 15-MV X-rays at 5 fractions per week with a linear accelerator (Varian Medical Systems).17 A 3-dimensional conformal RT technique was used to determine radiation ports using a planning system (Eclipse, version 10.0; Varian), and the actual beam delivery was performed with a respiratory-gated beam delivery technique.
Outcomes and Assessments
The primary end point of the study was the 12-week progression-free survival (PFS) rate. Progression was defined as progressive disease by independent radiologic review according to RECIST, version 1.1, criteria19 or death from any cause. Secondary end points were the 24-week PFS rate, 12- and 24-week radiologic response rates, 12- and 24-week treatment crossover rates, time to progression, time to treatment crossover, and overall patient survival.
The overall treatment period was divided into 6-week cycles for the efficacy and safety assessment. Tumor measurements and response evaluations were conducted by an independent radiologist (S.J.L.) based on liver dynamic CT images at screening and every 6 weeks after randomization. A secondary radiologist in the central imaging core laboratory (Asan Image Metrics) performed an independent blinded image review at the end of the study without interfering with the primary judgment concerning disease progression and treatment decision. The radiologic response rate was defined as the proportion of patients with complete response or partial response. Treatment crossover was permitted after confirming disease progression during the initially assigned treatment. Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
After completion of the 24-week initial study period, patients continued treatment and were followed up for disease progression and overall survival until August 31, 2017. Curative surgical resection was allowed for patients with partial response after 24 weeks.
Statistical Analysis
The primary data set for efficacy analyses comprised all randomized patients (intention-to-treat analysis), and the analyses were performed by comparing the originally randomized treatment groups (ie, the sorafenib and TACE-RT groups). Assuming 12-week PFS rates of 50% for the sorafenib group8,9 and 80% for the TACE-RT group17 plus a 2-sided 5% significance level, 90 patients were required to achieve 80% power based on a test for equality of proportions.
The χ2 test or the Fisher exact test were used to compare the 12- and 24-week radiologic response rates between treatment groups, as appropriate. Survival curves for time-to-event variables were determined by the Kaplan-Meier method, and the log-rank test was used for treatment comparisons. The hazard ratio for survival and its 95% CI were calculated using a Cox proportional hazards regression model.
All tests were 2-sided, and P < .05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics, version 21 (IBM Corp).
Results
Study Population
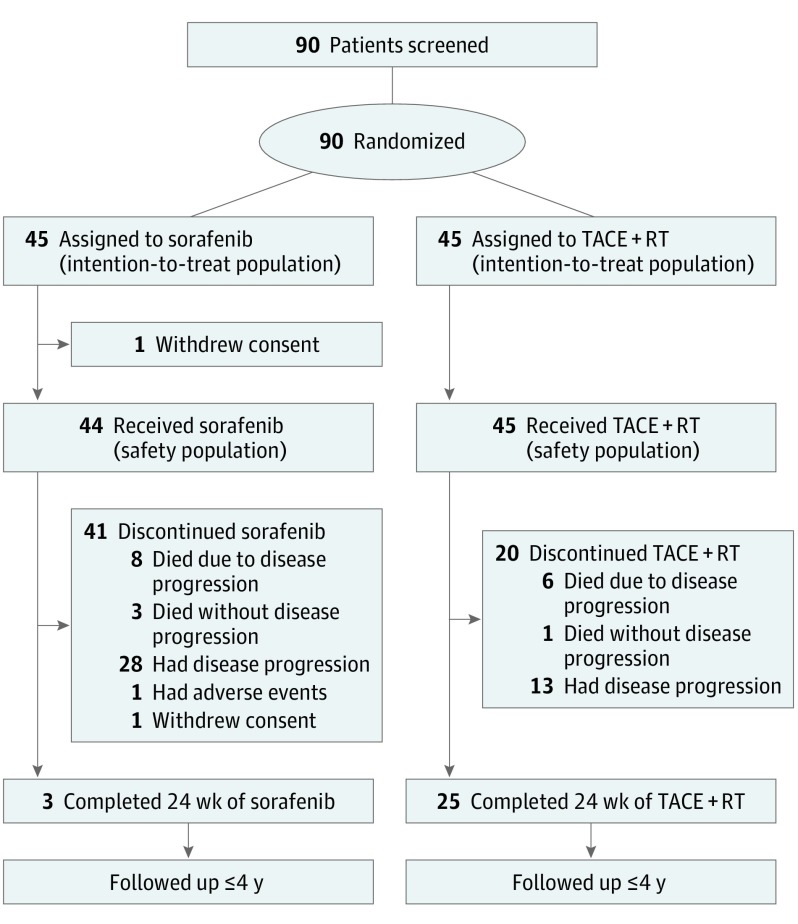
Between July 1, 2013, and October 31, 2016, 90 patients were randomly assigned to receive sorafenib (45 participants) or TACE plus RT (45 participants), and they were included in the intention-to-treat analysis (Figure 1). Baseline characteristics were well balanced between the sorafenib and TACE-RT groups (Table 1 and eTable 1 in Supplement 2). All patients had Child-Pugh class A liver function and an Eastern Cooperative Oncology Group performance status of 0 or 1. Chronic hepatitis B virus infection was the predominant cause of liver disease (76 [84.4%]). The median maximal tumor diameter was 9.7 cm (interquartile range, 7.1-12.2 cm). Most patients had multiple lesions (71 [78.9%]). Unilateral portal vein invasion was observed in 53 patients (58.9%), and 37 patients (41.1%) had tumor invasion to multiple vessels. No patients had extrahepatic spread.
Figure 1. Patient Flow Diagram.
TACE + RT indicates transarterial chemoembolization plus external beam radiotherapy.
Table 1. Baseline Characteristics of the Patients.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Total (N = 90) | Sorafenib Group (n = 45) | TACE-RT Group (n = 45) | |
| Age, median (range), y | 55 (33-82) | 55 (33-82) | 55 (42-77) |
| Sex | |||
| Male | 77 (85.6) | 39 (86.7) | 38 (84.4) |
| Female | 13 (14.4) | 6 (13.3) | 7 (15.6) |
| Child-Pugh class A | 90 (100) | 45 (100) | 45 (100) |
| Albumin, median (IQR), g/dL | 3.5 (3.3-3.8) | 3.5 (3.3-3.8) | 3.5 (3.3-3.9) |
| Total bilirubin, median (IQR), mg/dL | 0.7 (0.6-1.0) | 0.7 (0.6-1.0) | 0.7 (0.6-1.0) |
| ECOG performance status | |||
| 0 | 41 (45.6) | 22 (48.9) | 19 (42.2) |
| 1 | 49 (54.4) | 23 (51.1) | 26 (57.8) |
| Cause of disease | |||
| Hepatitis B virus infection | 76 (84.4) | 40 (88.9) | 36 (80.0) |
| Hepatitis C virus infection | 1 (1.1) | 0 | 1 (2.2) |
| Other | 13 (14.4) | 5 (11.1) | 8 (17.8) |
| Tumor size, maximum, median (IQR), cm | 9.7 (7.1-12.2) | 9.6 (7.0-11.8) | 9.8 (8.0-12.8) |
| Tumor, No. | |||
| Single | 19 (21.1) | 13 (28.9) | 6 (13.3) |
| Multiple | 71 (78.9) | 32 (71.1) | 39 (86.7) |
| Tumor extent | |||
| Unilobar involvement | 48 (53.3) | 27 (60.0) | 21 (46.7) |
| Bilobar involvement | 42 (46.7) | 18 (40.0) | 24 (53.3) |
| Level of vascular invasion | |||
| Unilateral PV | 53 (58.9) | 27 (60.0) | 26 (57.8) |
| Bilateral or main PV | 33 (36.7) | 16 (35.6) | 17 (37.8) |
| Unilateral PV + HV or IVC | 2 (2.2) | 1 (2.2) | 1 (2.2) |
| Bilateral PV + HV or IVC | 2 (2.2) | 1 (2.2) | 1 (2.2) |
| Bile duct invasion | |||
| No | 86 (95.6) | 45 (100) | 41 (91.1) |
| Yes | 4 (4.4) | 0 | 4 (8.9) |
| α-Fetoprotein, median (IQR), ng/mL | 832 (65-37 525) | 667 (36-51 138) | 1496 (78-37 736) |
| PIVKA-II, median (IQR), mAU/mL | 2346 (142-15 333) | 2426 (111-13 775) | 2266 (150-16 339) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HV, hepatic vein; IQR, interquartile range; IVC, inferior vena cava; PIVKA-II, protein induced by vitamin K absence or antagonist; PV, portal vein; RT, external beam radiotherapy; TACE, transarterial chemoembolization.
SI conversion factors: To convert albumin to grams per liter, multiply by 10.0; to convert bilirubin to micromoles per liter, multiply by 17.104; and to convert α-fetoprotein to micrograms per liter, multiply by 1.0.
PFS and Response Rates at 24 Weeks
At week 12, a total of 5 patients had died and 27 patients had radiologic disease progression in the sorafenib group, while none had died and 9 had radiologic disease progression in the TACE-RT group. The 12-week PFS rate was significantly higher in the TACE-RT group than in the sorafenib group (86.7% vs 34.3%; P < .001) (Table 2 and Figure 2A). At 12 weeks, a radiologic response was achieved in 2 patients (4.4%) in the sorafenib group and 13 patients (28.9%) in the TACE-RT group (P = .002). After confirmation of radiologic disease progression, 20 patients (48.9% by Kaplan-Meier estimation) in the sorafenib group switched their treatment to TACE plus RT within 12 weeks, while no patients in the TACE-RT group switched their treatment to sorafenib (P < .001).
Table 2. Disease Responses in the Intention-to-Treat Populationa.
| Outcome | Sorafenib Group (n = 45)b | TACE-RT Group (n = 45)b | P Valuec |
|---|---|---|---|
| Radiologic responses at 12 wk, No. (%) | |||
| Overall response rated | 2 (4.4) | 13 (28.9) | .002 |
| Disease control ratee | 12 (26.7) | 36 (80.0) | <.001 |
| Complete response | 0 | 0 | |
| Partial response | 2 (4.4) | 13 (28.9) | |
| Stable disease | 10 (22.2) | 23 (51.1) | |
| Progressive disease | 27 (60.0) | 9 (20.0) | |
| Intrahepatic | 22 (48.9) | 1 (2.2) | |
| Extrahepatic | 0 | 5 (11.1) | |
| Both intrahepatic and extrahepatic | 5 (11.1) | 3 (6.7) | |
| Not evaluablef | 6 (13.3) | 0 | |
| Progression-free survival rate at 12 wk, % (95% CI)g | 34.3 (22.6-52.2) | 86.7 (77.3-97.2) | <.001 |
| Treatment crossover rate at 12 wk, % (95% CI)g | 48.9 (30.9-62.3) | 0 | <.001 |
| Radiologic responses at 24 wk, No. (%) | |||
| Overall response rated | 1 (2.2) | 15 (33.3) | <.001 |
| Disease control ratee | 3 (6.7) | 25 (55.6) | <.001 |
| Complete response | 0 | 0 | |
| Partial response | 1 (2.2) | 15 (33.3) | |
| Stable disease | 2 (4.4) | 10 (22.2) | |
| Progressive disease | 36 (80.0) | 19 (42.2) | |
| Intrahepatic | 24 (53.3) | 5 (11.1) | |
| Extrahepatic | 0 | 6 (13.3) | |
| Both intrahepatic and extrahepatic | 12 (26.7) | 8 (17.8) | |
| Not evaluablef | 6 (13.3) | 1 (2.2) | |
| Progression-free survival rate at 24 wk, % (95% CI)g | 7.4 (2.5-21.8) | 55.6 (42.8-72.1) | <.001 |
| Treatment crossover rate at 24 wk, % (95% CI)g | 90.7 (73.1-96.8) | 23.0 (8.6-35.1) | <.001 |
Abbreviations: RT, external beam radiotherapy; TACE, transarterial chemoembolization.
The level of response was measured according to RECIST (Response Evaluation Criteria in Solid Tumors, version 1.1) criteria by independent radiologic review.
All patients who were randomized were included in the analyses.
By χ2 test or Fisher exact test, as appropriate, for radiologic responses; and by log-rank test for progression-free survival rate and treatment crossover rate.
Overall response rate, defined as the proportion of patients who had complete response or partial response.
Disease control rate, defined as the proportion of patients who had complete response, partial response, or stable disease.
Owing to death without radiologic disease progression or early study termination by withdrawal or adverse event.
By Kaplan-Meier analysis.
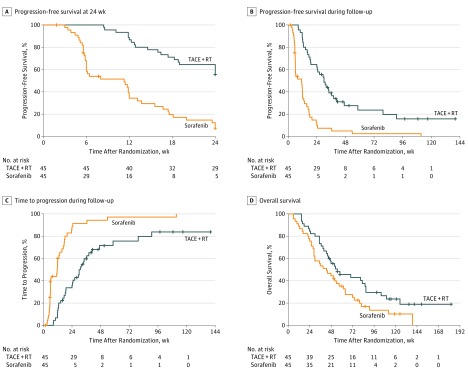
Figure 2. Estimated Survival Outcomes.
A, Progression-free survival at 24 weeks. Progression-free survival rate at 12 weeks: transarterial chemoembolization plus external beam radiotherapy (TACE + RT), 86.7% (95% CI, 77.3%-97.2%); sorafenib, 34.3% (95% CI, 22.6%-52.2%) (hazard ratio [HR], 0.21; 95% CI, 0.12-0.37; log-rank P < .001). B, Median progression-free survival during follow-up (TACE + RT, 30.0 weeks; 95% CI, 22.9-37.1 weeks; sorafenib, 11.3 weeks; 95% CI, 4.4-18.2 weeks) (HR, 0.27; 95% CI, 0.17-0.44; log-rank P < .001). C, Median time to progression during follow-up (TACE + RT, 31.0 weeks; 95% CI, 23.2-38.8 weeks; sorafenib, 11.7 weeks; 95% CI, 5.5-17.9 weeks) (HR, 0.28; 95% CI, 0.17-0.46; log-rank P < .001). D, Median overall survival (TACE + RT, 55.0 weeks; 95% CI, 28.9-81.2 weeks; sorafenib, 43.0 weeks; 95% CI, 25.9-60.1 weeks) (HR, 0.61; 95% CI, 0.38-0.98; log-rank P = .04).
At week 24, a total of 11 patients had died and 36 patients had radiologic disease progression in the sorafenib group, while 7 had died and 19 had radiologic disease progression in the TACE-RT group. The 24-week PFS rate was significantly higher in the TACE-RT group than in the sorafenib group (55.6% vs 7.4%; P < .001) (Table 2 and Figure 2A and B). A radiologic response was observed in 1 patient (2.2%) in the sorafenib group and 15 patients (33.3%) in the TACE-RT group (P < .001). In the sorafenib group, 34 patients (90.7% by Kaplan-Meier estimation) switched their treatment to TACE plus RT owing to disease progression within 24 weeks, while 9 (23.0%) in the TACE-RT group switched their treatment to sorafenib (P < .001) (Table 2 and eTable 2 in Supplement 2).
The discordance rate between the primary and secondary radiologic review was 4.8% (4 of 83; P = .13) (eTables 3 and 4 in Supplement 2), indicating low interrater variability with no effect on patient outcomes (eFigure 1 in Supplement 2). α-Fetoprotein levels decreased significantly at 24 weeks in the TACE-RT group only (eTable 5 and eFigure 2 in Supplement 2). Regardless of the extent of vascular invasion, those in the TACE-RT group showed significantly better median 24-week PFS than those in the sorafenib group (patients with HCC and unilateral portal vein invasion, 29.3 weeks [95% CI, 13.9-44.7] vs 11.7 weeks [95% CI, 4.9-18.6]; P < .001; patients with HCC and multiple vascular invasion, 30.0 weeks [95% CI, 20.1-40.0] vs 8.0 weeks [95% CI, 0.9-15.1] for both; P = .003; eFigure 3A and B in Supplement 2).
Time to Disease Progression
During a maximum of 140 weeks of follow-up, disease progression occurred in 39 patients in the sorafenib group and 33 patients in the TACE-RT group (100% in the sorafenib group vs 83.8% in the TACE-RT group by Kaplan-Meier estimation; P < .001 (Figure 2C). The median time to progression was significantly longer in the TACE-RT group than in the sorafenib group (31.0 vs 11.7 weeks; hazard ratio, 0.28; 95% CI, 0.17-0.46; P < .001) (Table 3).
Table 3. Survival Outcomes.
| Outcome | Median Time (95% CI), wk | HR (95% CI)a | P Value | |
|---|---|---|---|---|
| Sorafenib Group (n = 45) | TACE-RT Group (n = 45) | |||
| Progression-free survival | 11.3 (4.4-18.2) | 30.0 (22.9-37.1) | 0.27 (0.17-0.44) | <.001 |
| Time to radiologic progression | 11.7 (5.5-17.9) | 31.0 (23.2-38.8) | 0.28 (0.17-0.46) | <.001 |
| Time to treatment crossover | 13.0 (7.4-18.6) | 53.0 (45.2-60.8) | 0.21 (0.12-0.36) | <.001 |
| Overall survivalb | 43.0 (25.9-60.1) | 55.0 (28.9-81.2) | 0.61 (0.38-0.98) | .04 |
Abbreviations: HR, hazard ratio; RT, external beam radiotherapy; TACE, transarterial chemoembolization.
Cox proportional hazards regression model for the TACE-RT group with the sorafenib group as a reference.
The overall survival rate at 48 weeks was 44.4% in the sorafenib group and 55.4% in the TACE-RT group; the overall survival rate at 96 weeks was 13.6% in the sorafenib group and 29.5% in the TACE-RT group.
Overall Survival
The median overall survival was significantly longer in the TACE-RT group than in the sorafenib group (55.0 vs 43.0 weeks; hazard ratio, 0.61; 95% CI, 0.38-0.98; P = .04) (Table 3 and Figure 2D). The 48-week survival rates were 55.4% in the TACE-RT group and 44.4% in the sorafenib group.
By the last follow-up, 18 patients were alive (6 in the sorafenib group and 12 in the TACE-RT group). All living patients in the sorafenib group had switched their treatment to TACE plus RT owing to disease progression. No patients continued to receive sorafenib treatment.
Curative surgical resection could be performed on 5 patients (11.1%) only in the TACE-RT group between 27 and 40 weeks owing to downstaging; 1 patient died at 51 weeks, and 4 were alive at the last follow-up, with an overall survival time of 119 to 149 weeks. A representative case is shown in eFigure 4 in Supplement 2.
Safety
One patient assigned to the sorafenib group withdrew consent after randomization and did not receive treatment. Therefore, the safety analysis population comprised 89 patients who received the assigned treatments.
The overall incidences of adverse events were 93.2% in the sorafenib group (41 of 44 patients) and 91.1% in the TACE-RT group (41 of 45 patients) (eTables 6 and 7 in Supplement 2). Serious adverse events were reported for 5 patients in each group. A patient in the sorafenib group discontinued treatment because of severe mucositis. In the TACE-RT group, a transient grade 3 elevation in total bilirubin level was observed in 1 patient; however, this patient’s treatment was discontinued owing to lung metastasis.
The actual mean daily dose of sorafenib was 739 mg. Overall, 14 of 44 patients (31.8%) in the sorafenib group required dose modifications for adverse events (eTable 8 in Supplement 2). In the TACE-RT group, for the first TACE, full-dose chemoembolization with cisplatin, iodized oil, and gelatin sponge cubes was performed for 29 patients (64.4%), and chemoembolization without gelatin sponge infusion was performed for 16 patients (35.6%) who had severely reduced portal blood flow. The median number of TACE procedures was 4 (interquartile range, 3-4) during the 24-week study period. All patients in the TACE-RT group received RT within 3 weeks after first TACE, with a median dose of 40 Gy (interquartile range, 30-45 Gy) in 2.5- to 3-Gy doses per fraction.
Discussion
In this randomized clinical trial of patients with HCC showing MVI compared with sorafenib treatment, the combined TACE plus RT treatment was associated with a significantly higher rate of PFS (86.7% vs 34.3% at 12 weeks), a significantly higher radiologic response rate (33.3% vs 2.2% at 24 weeks), a markedly longer median time to progression (31.0 vs 11.7 weeks), and a significantly longer overall survival (55.0 vs 43.0 weeks). The results of this study represent a significant advance in addressing an urgent unmet need in treating patients with advanced HCC. All recent phase 3 trials assessing first-line systemic therapies for patients with advanced HCC have failed to improve overall patient survival compared with sorafenib treatment.20,21 Our study may also be significant in that it is one of the few randomized clinical trials conducted specifically for patients with HCC showing MVI; previous trials for patients with advanced HCC included patients showing MVI as a subgroup.8,9,10
Macroscopic vascular invasion is a prognostic factor for poorer overall survival among patients with HCC.6,7,8 Even with sorafenib treatment, patients with HCC are prone to rapid progression of tumors.8,9,10 In our study, the median time to disease progression was only 11.7 weeks with sorafenib, which was shorter than that of patients with advanced HCC treated with sorafenib in previous phase 3 trials (5.5 months in the SHARP [Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol] trial10 and 2.8 months in the Asia-Pacific trial9). Nevertheless, the median overall patient survival in the sorafenib group in our study (43 weeks) was similar to that in the SHARP trial (10.7 months)10 and longer than that in the Asia-Pacific trial (6.5 months).9 The fact that most of the patients in the sorafenib group switched their treatment to TACE plus RT after identifying disease progression (median, 13 weeks; 34 participants [90.7%] in 24 weeks) may explain these findings. To ensure that patients were not disadvantaged by participation, treatment crossover was allowed with disease progression. We reasoned that the high rate of treatment crossover might obscure any benefit of the initially assigned treatment if overall survival was chosen as the primary end point. Hence, we thought that PFS was the most appropriate primary end point. Nonetheless, overall survival in the TACE-RT group was significantly longer than in the sorafenib group, suggesting that it would be better to begin treatment with TACE plus RT than with sorafenib.
Tumor invasion of portal veins not only promotes intrahepatic tumor spread but also rapidly decreases blood supply to the liver, causing an abrupt increase in portal pressure, resulting in rapid deterioration of liver function and increased risk of portal hypertensive complications.17 This, in turn, may limit further treatment options. Because of the rapidity of HCC tumor thrombus progression, quick reduction in tumor thrombus volume is important to facilitate subsequent treatment of the primary tumor. However, sorafenib is only likely to delay tumor progression, and the incidence of objective responses is very low (2%-3.3%).9,10 Transarterial chemoembolization alone may also have limited efficacy in reducing the tumor thrombus.15,22
Although MVI is a route of distant tumor spread, intrahepatic vascular invasion is a local disease. A potent locoregional treatment may be better than systemic therapy before the diagnosis of distant metastasis. Owing to the advent of CT-based treatment planning, 3-dimensional conformal liver irradiation has become a feasible and safe technique for advanced HCC, allowing safe delivery of high tumoricidal doses of radiation that conform tightly to the tumor, with minimal risk of radiation-induced liver disease (<5%).11,12,14 One of the primary indications for RT is the MVI of HCC. Radiotherapy has been shown to have objective response rates ranging from 39% to 62% in patients with HCC and MVI.12,23,24,25,26,27 Despite high local control rates by RT alone, failure outside the radiation field provides the rationale for combining regional or systemic treatments with RT. Especially, TACE is a proven treatment for locally advanced HCC, and TACE and RT may complement each other; focal field RT targeting the MVI may relieve intravascular tumor growth and maintain portal blood flow, allowing the maintenance of liver function, limiting intrahepatic tumor spread, and thereby allowing additional TACE.25,26,28 Furthermore, as shown in our study, some patients can receive subsequent curative surgical resection owing to downstaging by TACE plus RT, enabling long-term patient survival.
Previous studies have raised caution regarding the risk of TACE-induced liver failure in patients with HCC and MVI.2 However, in our trial, no patients in the TACE-RT group discontinued treatment for adverse events. Our study may not be large enough to accurately establish the incidence of adverse events. However, considering the dismal prognosis, the superior efficacy of TACE plus RT vs sorafenib may justify its use for these patients.
Limitations
This study has potential limitations. First, the nature of the treatments under investigation did not permit blinding. As an open-label trial, this study may be prone to bias. Although radiologic progression was assessed by an independent radiologist according to the RECIST criteria, blinded assessment was not feasible owing to the different image responses after treatment with sorafenib and TACE. However, objective clinical outcomes, such as overall survival, are unlikely to be biased by unblinding.29 Second, the generalizability of our results may be limited. The predominant population (76 [84.4%]) in this study had hepatitis B virus–associated HCC; thus, the treatment strategy described may not be extrapolatable to patients with HCC not associated with hepatitis B virus.
Conclusions
This randomized clinical trial demonstrated that, for patients with advanced HCC showing MVI, the combined treatment of TACE and RT was well tolerated and provided an improved PFS, objective response rate, time to progression, and overall survival compared with sorafenib treatment. Transarterial chemoembolization plus RT also provided a chance for curative resection in some patients. Further studies are needed to confirm our findings and, given the poor overall patient survival even with TACE plus RT, to further improve patient outcome.
Trial Protocol
eTable 1. Baseline Characteristics of the Patients
eTable 2. Characteristics of Patients at Baseline vs Treatment Crossover on Disease Progression in 24 Weeks
eTable 3. Radiologic Response Evaluation Between the Primary and Secondary Radiologic Reviews in 24 Weeks
eTable 4. Details of Discordance Between Primary and Secondary Central Radiologic Reviews
eTable 5. Changes in Alpha-Fetoprotein (AFP) at Weeks 12 and 24 by Treatment Groups
eTable 6. Treatment-Emergent Adverse Events in the Safety Population
eTable 7. Adverse Event Categories in the Safety Population
eTable 8. Exposure to Sorafenib, Transarterial Chemoembolization, and Radiation in 24 Weeks
eFigure 1. Estimated Progression-Free Survival Rate in 24 Weeks by Secondary Central Radiologic Reviews
eFigure 2. Changes in Alpha-Fetoprotein (AFP) Levels in 24 Weeks by Treatment Groups
eFigure 3. Estimated Progression-Free Survival During Follow-up
eFigure 4. A Representative Case of a 52-Year-Old Man With Hepatocellular Carcinoma (HCC) With Portal Vein Tumor Thrombus (PVTT) Who Was Assigned to the Combined Transarterial Chemoembolization (TACE) Plus Radiotherapy (RT) Group
References
- 1.International Agency for Research on Cancer, World Health Organization Estimated number of deaths, both sexes, worldwide (top 10 cancer sites) in 2012. http://gco.iarc.fr/today/online-analysis-multi-bars?mode=cancer&mode_population=continents&population=900&sex=0&cancer=29&type=1&statistic=0&prevalence=0&color_palette=default. Accessed September 1, 2017.
- 2.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943. [DOI] [PubMed] [Google Scholar]
- 3.Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide [published online August 31, 2017]. Hepatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 2017;3(4):524-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim BH, Lim YS, Kim EY, et al. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus–endemic population [published online June 14, 2017]. J Gastroenterol Hepatol. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62-67. [DOI] [PubMed] [Google Scholar]
- 7.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274-1283. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67(5):999-1008. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V, et al. ; SHARP Investigators Study Group . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. [DOI] [PubMed] [Google Scholar]
- 11.Citrin DE. Recent developments in radiotherapy. N Engl J Med. 2017;377(11):1065-1075. [DOI] [PubMed] [Google Scholar]
- 12.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631-1639. [DOI] [PubMed] [Google Scholar]
- 13.Feng M, Suresh K, Schipper MJ, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 2018;4(1):40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma: an intention-to-treat analysis. J Hepatol. 2017;67(1):92-99. [DOI] [PubMed] [Google Scholar]
- 15.Koo JE, Kim JH, Lim YS, et al. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2010;78(1):180-187. [DOI] [PubMed] [Google Scholar]
- 16.Park HC, Yu JI, Cheng JC, et al. Consensus for radiotherapy in hepatocellular carcinoma from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014): current practice and future clinical trials. Liver Cancer. 2016;5(3):162-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82(5):2004-2011. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835-853. [DOI] [PubMed] [Google Scholar]
- 21.Nault J-C. The end of almost 10 years of negative RCTs in advanced hepatocellular carcinoma. Lancet. 2017;389(10064):4-6. [DOI] [PubMed] [Google Scholar]
- 22.Kim GA, Shim JH, Yoon SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26(3):320-9.e6. [DOI] [PubMed] [Google Scholar]
- 23.Huang YJ, Hsu HC, Wang CY, et al. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73(4):1155-1163. [DOI] [PubMed] [Google Scholar]
- 24.Kim DY, Park W, Lim DH, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103(11):2419-2426. [DOI] [PubMed] [Google Scholar]
- 25.Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57(1):113-119. [DOI] [PubMed] [Google Scholar]
- 26.Zeng ZC, Fan J, Tang ZY, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61(2):432-443. [DOI] [PubMed] [Google Scholar]
- 27.Toya R, Murakami R, Baba Y, et al. Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma. Radiother Oncol. 2007;84(3):266-271. [DOI] [PubMed] [Google Scholar]
- 28.Yu JI, Park JW, Park HC, et al. Clinical impact of combined transarterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: an external validation study. Radiother Oncol. 2016;118(2):408-415. [DOI] [PubMed] [Google Scholar]
- 29.Kahan BC, Cro S, Doré CJ, et al. Reducing bias in open-label trials where blinded outcome assessment is not feasible: strategies from two randomised trials. Trials. 2014;15:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Characteristics of the Patients
eTable 2. Characteristics of Patients at Baseline vs Treatment Crossover on Disease Progression in 24 Weeks
eTable 3. Radiologic Response Evaluation Between the Primary and Secondary Radiologic Reviews in 24 Weeks
eTable 4. Details of Discordance Between Primary and Secondary Central Radiologic Reviews
eTable 5. Changes in Alpha-Fetoprotein (AFP) at Weeks 12 and 24 by Treatment Groups
eTable 6. Treatment-Emergent Adverse Events in the Safety Population
eTable 7. Adverse Event Categories in the Safety Population
eTable 8. Exposure to Sorafenib, Transarterial Chemoembolization, and Radiation in 24 Weeks
eFigure 1. Estimated Progression-Free Survival Rate in 24 Weeks by Secondary Central Radiologic Reviews
eFigure 2. Changes in Alpha-Fetoprotein (AFP) Levels in 24 Weeks by Treatment Groups
eFigure 3. Estimated Progression-Free Survival During Follow-up
eFigure 4. A Representative Case of a 52-Year-Old Man With Hepatocellular Carcinoma (HCC) With Portal Vein Tumor Thrombus (PVTT) Who Was Assigned to the Combined Transarterial Chemoembolization (TACE) Plus Radiotherapy (RT) Group