Abstract
Cyclophilin A (CyPA) is involved in the pathophysiology of several inflammatory and cardiovascular diseases. To our knowledge there is no specific inhibitor targeting extracellular CyPA without affecting other extracellular cyclophilins or intracellular CyPA functions. In this study we developed an antibody-based inhibitor of extracellular CyPA and analyzed its effects in vitro and in vivo. To generate a specific antibody mice and rats were immunized with a peptide containing the EMMPRIN binding side and various antibody clones were selected and purified. At first, antibodies were tested for their binding capacity to recombinant CyPA and their functional activity, based on which the clone 8H7-mAb was chosen for further experiments. 8H7-mAb reduces CyPA-induced migration of inflammatory cells in vitro and in vivo. Furthermore 8H7-mAb revealed strong anti-thrombotic effects by inhibiting CyPA-dependent activation of platelets and thrombus formation in vitro and in vivo. Surprisingly 8H7-mAb did not influence in vivo tail bleeding time or in vitro whole blood coagulation parameters. Our study provides first evidence that antibody-based inhibition of extracellular CyPA inhibits thrombosis and thrombo-inflammation without affecting blood homeostasis. Thus, 8H7-mAb may be a promising compound for thrombi-modulation in inflammatory diseases in order to prevent organ dysfunction.
Keywords: Cyclophilin A, thrombosis, inflammation, platelets
Introduction
CyPA is a 18 kDa chaperone and contains peptidyl-prolyl cis-trans isomerase (PPIase) activity(1) that critically regulates physiological intracellular functions like protein folding, trafficking, and Ca2+ signaling(2–5). Binding of intracellular CyPA by cyclosporine A has significant immunosuppressant activity via inhibition of the NFAT-pathway (6–8).
Besides its well defined physiological role in the regulation of intracellular functions(2–5), CyPA has extracellular biological effects mainly associated with pathophysiological processes: Upon activation there is an active secretion of CyPA from several cell types, including platelets(9), macrophages(10) and vascular endothelial cells(11) into the extracellular space. In this context platelets are a major source of extracellular CyPA(9, 12). Released CyPA binds to the extracellular matrix metalloproteinase inducer (EMMPRIN; CD147) which is expressed on the plasma membrane of various cell types including monocyte/macrophages(13, 14), proliferating tumor cells(15), and platelets(16). Binding of extracellular CyPA to EMMPRIN induces monocyte adhesion, migration, and matrix metalloproteinase (MMP) activity, resulting in extracellular matrix degradation(17). Further, CyPA binding to its surface receptor EMMPRIN promotes platelet activation via the PI3 kinase/Akt pathway, change of platelet shape and secretion of proinflammatory chemokines, like SDF-1α. Therefore, at site of vascular or tissue injury where platelets rapidly accumulate and undergo release reaction proinflammatory chemokines and other proinflammatory mediators, like CyPA, are released into the extracellular space (12). This leads to high amounts of CyPA in the surrounding microenvironment which might have a significant impact on thrombosis and thrombo-inflammation and thus organ dysfunction. Platelet adhesion in vitro and in vivo is mediated by CyPA-EMMPRIN interaction and independent of intracellular CyPA(12). Beside its significant effects on platelets CyPA plays a role in inflammatory processes, like coxsackievirus B3-induced and Troponin-I-induced myocarditis(18, 19). In a mouse model of ischemia and reperfusion it has been shown that the interaction of CyPA and its receptor EMMPRIN enhances myocardial injury(20) mainly via recruitment of inflammatory cells.
Currently available CyPA inhibitors such as cyclosporine A or a synthetic small molecule NIM811 both neutralize intra- and extracellular CyPA activity(21, 22). Another cyclosporine A derivative is MM284 which neutralizes extracellular CyPA but also other extracellular cyclophilins(18, 23). So far no pharmacological strategy exists that specifically targets extracellular CyPA without affecting other extracellular cyclophilins or the intracellular function of CyPA.
Therefore, we intended to develop a neutralizing anti-CyPA monoclonal antibody directed against the active EMMRPIN receptor site which specifically targets the extracellular function of CyPA and not affecting the intracellular function.
Materials and Methods
Structure of CyPA
The structure of CyPA(24–26) (RCSB protein data bank) and pdb-files are displayed with Jmol: an open-source Java viewer for chemical structures in 3D (http://jmol.org).
Generation of a monoclonal antibody against TRIM-Cyp fusion protein
A peptide comprising amino acids 56TAKTEWLDGKHVVFGKVKEG75 of human TRIM-Cyp fusion protein was synthesized and coupled to ovalbumin (OVA, Peps4LS, Heidelberg, Germany). Lou/c rats (antibody 8H7) or mice (antibody 7C2, 5A11, 1B7, 8E6) were immunized subcutaneously and intraperitoneally with a mixture of 50 μg peptide-OVA, 5 nmol/L CPG oligonucleotides (Tib Molbiol, Berlin, Germany), 500 μl PBS and 500 μl incomplete Freund’s adjuvant. A boost without adjuvant was given six weeks after the primary injection. Monoclonal antibodies were produced by fusing antibody-secreting spleen cells with immortal myeloma cells, to create monoclonal hybridoma cell lines that express the specific antibodies in cell culture supernatants using standard procedures. Supernatants were tested in a differential ELISA with the peptide coupled to BSA and on an irrelevant peptide. Monoclonal antibodies (mAb) that reacted specifically with the TRIM-Cyp peptide were further analysed by Western Blotting. Tissue culture supernatant or purified mAb (Protein G Sepharose 4 Fast Flow, GE Healthcare, Solingen, Germany) of TAKTE 8H7-mAb (rat IgG2a) were used in this study.
Immunoblotting
Human monocytes and platelets were isolated from healthy volunteers and lysed in RIPA buffer(27) (NaCl 150 mmol/L, Tris 50 mmol/L, SDS 1%, sodium deoxycholate 0.5%, Triton X-100 1%) containing proteinase inhibitor cocktail (Roche, Mannheim, Germany). For detection of CyPA, monocyte or platelet lysate or recombinant protein were run under reduced conditions in a 15% SDS-polyacrylamide gel, and CyPA was detected by using the TAKTE-mAbs as primary antibody or commercially available anti-CyPA antibody (abcam, Cambridge, UK). Bands were detected using a Li-Cor Odyssey infrared imaging system (LI-COR, Lincoln, NE, USA).
Binding of anti-CyPA mAb (8H7) to immobilized CyPA
The binding between 8H7-mAb and CyPA was tested in a modified enzyme-linked immunoabsorbent assay (ELISA)(28). A 96 well plate was coated overnight with 200 nmol/L of CyPA and blocked overnight with 1% BSA (Applichem, St. Louis, MO, USA). On the next day 8H7-mAb or ratIgG2a (R&D Systems, Minneapolis, MN, USA) were added in concentrations of 0, 10, 50, 100 μg/ml and incubated for 3 hours at room temperature. For detection of the primary 8H7-mAb, a biotinylated secondary antibody (rabbit anti-rat, Dako, Glostrup, Denmark) was added followed by incubation with a streptavidin-HRP complex (Dako). Binding was detected with 3,3′,5,5′-tetramethylbenzidine (Serva, Heidelberg, Germany) and the reaction was stopped with 1 M H2SO4 (Carl Roth GmbH, Karlsruhe, Germany). The reaction was measured in an ELISA plate reader at 450 nm with a reference value of 570 nm.
Isolation of human monocytes and in vitro migration assay
Human monocytes were isolated as described previously(18, 20, 29). Briefly, monocytes were isolated by centrifugation of citrate-phosphate-dextrose-adenine (CPDA)-coagulated blood (S-Monovettes, Sarstedt, Nümbrecht, Germany) on a Ficoll-Paque gradient (GE Healthcare). Leukocytes were cultured overnight at 37°C, 5% CO2 in a cell culture flask in RPMI 1640 (Gibco, Carlsbad, CA, USA) supplemented with 10% FCS (Gibco) and 1% Penicillin/Streptomycin (Sigma-Aldrich, St. Louis, MO, USA). For the migration assay, non-adherent leukocytes were removed and adherent cells were trypsinised (Gibco), centrifuged, and resuspended in medium. Monocyte migration was performed in a modified 48-well Boyden chamber (NeuroProbe Inc., Geithersburg, MD, USA), using a 5 μm pore filter to separate the 2 compartments. Monocytes were added in the upper compartment and 20 μg/ml S100A9 (abcam) or 200 nmol/L CyPA (R&D Systems) or CyPA together with 20 μg/ml of the potential antibodies or rIgG2a (Invitrogen, Carlsbad, CA, USA) were added in the lower compartment. The receptor blocking antibody αCD147 and mIgG1 were added to the monocytes into the upper compartment. After 4 hours at 37°C, 5% CO2 the filter was fixed in Methanol, stained in a May-Grünwald/ Giemsa solution (Merck Millipore, Darmstadt, Germany) and the migrated cells on the filter were counted using a microscope.
Calcium measurement
Calcium measurements were performed as described elsewhere(12, 30). Briefly, isolated human platelets were loaded for 30 minutes with 5 μM Fura-2 acetoxymethylester (Invitrogen) in presence of 0.2 μg/ml Pluronic F-127 (Biotium, Fremont, CA, USA). Where indicated platelets were pre-incubated for 15 minutes with the indicated inhibitors (200 nM NIM811, 0.2 % ethanol, 20 μg/ml 8H7-mAb, 20 μg/ml rIgG2a) and were resuspended in Ca2+ free Tyrode buffer before adding the SERCA inhibitor thapsigargin (5 μM) for 10 minutes. Afterwards a surplus of 1 mM Ca2+ was added to measure store operated Ca2+ entry (SOCE). Calcium responses were measured under stirring with a spectrofluorimeter (LS 55, PerkinElmer), at alternate excitation wavelength of 340 and 380 nm (37°C). The 340/380 nm ratio values were converted into nanomolar concentrations of [Ca2+] by lysis with Triton X-100 (Sigma-Aldrich) and addition of EGTA.
Monocyte activation
Monocytes were isolated as described above. The non-adherent leukocytes were removed and the monocytes were stimulated in a 6 well plate with CyPA (200 nmol/L) in presence of 8H7-mAb and rIgG2a (both 20 μg/ml). After 24 hours the cells were fixed and the surface expression of CD11b was evaluated with an anti-CD11b antibody (R&D Systems) and measured using FACSCalibur flow cytometer (BD Biosciences).
Monocyte adhesion to endothelial cells under flow conditions
The experiment was performed as described before(31). Briefly, glass cover slips were coated with gelatin (Sigma-Aldrich) and human umbilical endothelial cells (HUVECs, CellSystems, Troisdorf, Germany) were cultured to a monolayer of cells. HUVECs were thereafter activated with TNFα (50 ng/ml, Peprotech, Rocky Hill, NJ, USA) and INFγ (20 μg/ml, Peprotech) for 4 hours. Human monocytes where stimulated with CyPA (200 nmol/L) after pre-treatment with 8H7-mAb or rIgG2a (both 20 μg/ml) for 30 minutes as indicated. Monocytes (2 × 105/ml) were perfused over the activated HUVECs using shear rates of 2000 second−1. All experiments were recorded in real time on video-CD and analyzed afterwards off-line.
CyPA-induced peritonitis model in vivo
10–12 weeks old C57Bl/6J mice (Charles River, Wilmington, MA, USA) received intraperitoneal 10 μg of recombinant CyPA and 10 μg/kg body weight 8H7-mAb or rIgG2a(32). The mice were sacrificed after 24 hours and a peritoneal lavage was performed to harvest the infiltrated cells. The cells were centrifuged and the pellet was resuspended in lysing solution (BD Biosciences, New Jersey, NJ, USA). Then the cells were stained with anti-CD3-APC, anti-Ly6G-PE and anti-F4/80-APC (all ebioscience, San Diego, CA, USA) and analyzed using FACSCalibur flow cytometer (BD Biosciences). After 24 hours blood was taken and analyzed for platelet and leukocyte count.
Positron Emission Tomography (PET) / Magnetic Resonance imaging (MR imaging)
Chelator conjugation
The 8H7 antibody was conjugated with the chelator NODAGA by incubation with 20 equivalents of p-NCS-benzyl-NODAGA (Chematech, Dijon, France) in 0.1 M HEPES pH 9 for two hours at room temperature. Excess of chelator was removed and antibody was buffered with 0.25 M sodium acetate pH 6 by successive ultrafiltration using Amicon Ultra-15 tubes with 30 kDa cut-off (Merck Millipore).
Radiolabeling
64Cu was produced as previously described(33). After buffering the activity to pH 5–6 with ammonium acetate, 19 μg of the NODAGA-conjugated antibody were added for each MBq of activity. After incubation at 42°C for one hour, complete incorporation of 64Cu (>95 %) was verified by radio-TLC with 0.1 M sodium citrate pH 5 on Polygram SIL G/UV254 plates (Macherey-Nagel, Düren , Germany), and by radio-High Performance Size Exclusion Chromatography (HPSEC) with PBS containing 0.5 mM EDTA on a BioSep SEC-s3000 column (Phenomenex, Torrance, CA, USA). Aqueous SEC 1 (Phenomenex) was used as molecular weight standard for HPSEC.
PET/MR imaging
Three healthy C57BL/6 mice were intraperitoneally injected with 8.9±0.3 MBq of 64Cu-NODAGA-8H7-mAb. Tracer distribution was analyzed using a small animal PET insert (Bruker Biospin GmbH, Ettlingen, Germany) and simultaneously, MR imaging was performed on a 7 T small animal MR tomograph (Bruker Biospin GmbH) to obtain anatomical information. During PET and MR imaging, the animals were anaesthetized with 1.5 % isoflurane in 100 % oxygen. Static (10 minutes) PET scans were acquired 1 hour and 24 hours after the injection of the PET tracer. PET data were acquired in list-mode, histogrammed in a single ten-minute frame and reconstructed using a 2D iterative ordered subset expectation maximization (OSEM2D) algorithm. PET images were normalized to each other and subsequently fused to the respective MR images. Image analysis was performed with Inveon Research Workplace software (Siemens Preclinical Solutions, Knoxville, TN, USA).
Ex vivo biodistribution analysis
After the last PET/MRI scan, mice were killed by cervical dislocation and dissected organs were analyzed for tracer uptake using a Wallac 2480 WIZARD 3″ gamma counter (Perkin Elmer) with the energy window set to 350–650 keV. Measured activities were normalized to the organ weight and calibrated to the injected dose using 64Cu standards. The results are expressed as mean values and standard deviation (mean±SD) of the percentual injected dose per g (%ID/g) of tissue.
For plasma analysis, a sample of fresh blood was cleared from cells by centrifugation, and 20 μl were analyzed by radio-HPLC as described under “Radiolabeling”.
Isolation of human platelets and FACS analysis
Human platelets were isolated from venous blood which was drawn and collected in acid-citrate-dextrose (ACD)-buffer(12, 34) Platelet rich plasma (PRP) was removed after centrifugation and added to a Tyrodes-HEPES buffer (pH 6.5; NaCl, 137 mmol/L; KCl, 2,6 mmol/L; NaHCO3, 12 mmol/L; glucose, 6 mmol/L; BSA, 1 mg/ml) and centrifuged to a pellet. The pellet was carefully resuspended in Tyrodes-buffer (pH 7.4, supplemented with 1 mmol/L CaCl2) (all reagents were obtained from Merck Millipore, AppliChem, and Sigma-Aldrich). For flow cytometry, platelets were stimulated with CyPA (200 nmol/L) and 8H7-mAb, αCD147 (Clone: UM8D6); Biomol, Hamburg, Germany) or IgG control (all 20 μg/ml) were added as indicated for 1 hour. Then the platelets were stained for P-selectin (CD62P, Beckman Coulter, Brea, CA, USA) for 30 minutes and analyzed by flow cytometry.
Monocyte-platelet-aggregates
Monocytes and platelets were isolated from CPDA coagulated blood (Sarstedt) from healthy donors. At first the PRP was collected after centrifugation and the platelets were stimulated with CyPA (200 nmol/L) and 8H7-mAb, αCD147 or IgG control for 30 minutes. Meanwhile, leukocytes were isolated over a Ficoll-Paque gradient. Then the activated platelets (1 × 107) were incubated with the isolated leukocytes (5 × 105) for 1 hour. The monocyte-platelet-aggregates were stained with anti-CD14-APC (R&D Systems) and anti-CD42b-PE (BD Biosciences) and analyzed in a flow cytometer for double positive cells.
In vitro thrombus formation with human blood
Cover slips (24 × 60 mm) were coated overnight with fibrillar type I collagen (100 μg/ml; Takeda, Osaka, Japan) and blocked with 1% BSA for at least 1 hour at room temperature as described before(12, 35). Human whole blood was drawn in CPDA-monovettes and stimulated for 1 hour with 200 nmol/L CyPA in presence or absence of 8H7-mAb or rIgG2a (both 20 μg/ml). Thereafter, 1 ml whole blood was perfused over the cover slips at shear rates of 1000 seconds−1 and 1700 seconds−1 and photo documented after the blood perfusion was stopped. The thrombus area was analyzed in photo-documented images off-line.
In vitro thrombus formation with murine blood
Cover slips (24 × 60 mm) were coated overnight with fibrillar type I collagen (Takeda, Osaka, Japan) and CyPA (200 nmol/L). Then the cover slips were blocked with 1% BSA for at least 1 hour at room temperature. Mice were intravenously injected with 8H7-mAb or rIgG2a (both 10 μg/kg body weight). After 30 minutes the blood was taken using heparin (20 Units) as anticoagulant. 1 ml whole blood was perfused over the cover slips at a shear rate of 1700 seconds−1. The thrombi were photo-documented after the blood perfusion was stopped. The thrombus area was analyzed in photo-documented images off-line.
In vivo thrombus formation in mesenteric arterioles
In vivo thrombus formation was performed as described before(12, 30, 35) using 5–7 weeks old C57Bl/6J mice. Mice were anesthetized with midazolame (5 mg/kg body weight), medetomidine (0.5 mg/kg body weight) and fentanyl (0.05 mg/kg body weight) via intraperitoneal injection. Additionally the mice were anesthetized with isoflurane via inhalation. 30 minutes before starting the experiment the mice were intravenously injected with 8H7-mAb or rIgG2a (both 10 μg/kg body weight). The mesenteric arterioles were exteriorized and the fat tissue around the arterioles was removed. To induce in vivo thrombus formation a filter paper saturated with 20% ferric(III)chloride (FeCl3) was placed in contact with an arteriole for 10 seconds. Thrombus formation was visualized with acridine orange using a fluorescent microscope (Axiovert 200, Carl Zeiss AG, Jena, Germany). The time to vessel occlusion was measured. The experiment was stopped after 40 minutes if no vessel occlusion occurred or after complete vessel occlusion for more than one minute.
Tail bleeding time and surface marker on platelets
7–9 weeks old C57Bl/6J mice were anesthetized with midazolame (5 mg/kg body weight), medetomidine (0.5 mg/kg body weight) and fentanyl (0.05 mg/kg body weight)(30) via intraperitoneal injection and 8H7-mAb or rIgG2a (both 10 μg/kg body weight) were injected intravenously 30 minutes before starting the tail bleeding time measurement. To analyze bleeding time, a 3 mm piece of the tail was cut off with a scalpel. Every 20 seconds blood drops were collected on a filter paper without touching the tail lesion until the blood flow stopped or after 20 minutes if blood flow did not stop till then. Afterwards blood was taken to measure the platelet and leukocyte count and for flow cytometric analysis. Murine blood was collected in ACD-buffer and centrifuged(12). The platelet-rich plasma (PRP) was collected and again centrifuged in Tyrode. The resulting platelet pellet was carefully resuspended in Tyrode pH 6.5 and neutralized in Tyrode pH 7.4. 1 × 106 platelets were used for each experiment and the surface expression was analyzed by flow cytometry using antibodies against CD42b (emfret analytics, Eibelstadt, Germany), CD29 (eBiosciences), CD41 (BD Biosciences), CD49b (eBiosciences), GPVI (emfret analytics) and fibrinogen-488 (Invitrogen/ThermoFisher Scientific, Waltham, MA, USA).
Platelet coagulation
The prothrombin time (PT) and the activated partial thromboplastin time (aPTT) were measured in a Start4 (Diagnostica Stago, Asnières sur Seine, France) according to the manufacturer’s protocol. Briefly, human blood was collected in Na-Citrate monovettes (Sarstedt) and centrifuged for 10 minutes at 2500g. Plasma was preincubated with 8H7-mAb or rIgG2a (both 20 μg/ml) for 10 minutes and PT and aPTT measurements were performed.
Ex vivo bleeding time
The influence of 8H7-mAb on platelets function was analyzed in a PFA-100 (Platelet Function Analyzer-100) system (Siemens Healthineers USA, Malvern, PA, USA). Citrated human whole blood was taken and added in collagen/epinephrine, collagen/ADP or P2Y disposable cartridges together with 8H7-mAb or rIgG2a (both 20 μg/ml) and the time to occlusion was measured.
Murine polytrauma and haemorrhagic shock model
The polytrauma and haemorrhagic shock model was performed as described previously(36, 37). Briefly, C57Bl/6J mice were anesthetized with intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight) and inhaled isoflurane. The blood pressure of the mice was monitored in the left femoral artery by a blood pressure transducer (Micro-Med, Tustin, CA, USA). The mice were treated intravenously 30 minutes before the induction of the polytrauma with 8H7-mAb rIgG2a (both 10 μg/kg body weight).
The induction of the polytrauma was performed in four steps. i) Soft tissue injury was performed bilaterally on lower extremities with 270 pounds per square inch (psi). ii) Bone matrix was prepared from donor mice of the same age to induce a pseudofracture. For this the tibia and femur of donor mice were crushed and resuspended in PBS. A volume of 0.15 cubic centimeters of this solution was injected bilaterally into the posterior muscles of each thigh. iii) 30% of the total blood volume (based on 80 ml blood/kg body weight) was removed within 1 minute via cardiac puncture. iv) A cut was made under the xyphoid to expose the right middle lobe of the liver. The liver was crushed four times with 80 psi. After this the laparotomy was closed and the animals were allowed to recover for 120 minutes. Then all mice were again intraperitoneally anesthetized with pentobarbital sodium (20 mg/kg body weight) and resuscitated over a 5 minutes period with ringers lactate solution (3 × shed blood volume) and observed for post-resuscitation.
After this the mice were sacrificed via cardiac puncture. The whole body of the animal was perfused with PBS followed by 2% paraformaldehyde using the site of cardiac puncture following withdrawal of blood. The liver was removed and fixed for an additional 2 hours in 2% paraformaldehyde and then switched to 30% sucrose in distilled water solution for 12 hours. The tissue was then slowly frozen in 2-methylbutane according to a standardized protocol for cryopreservation. Tissue sections (5 μm) were incubated with 2% bovine serum albumin (BSA) in PBS for 1 hour, followed by five washes with PBS containing 0.5% BSA (PBB). The samples were then incubated overnight with primary antibodies. The following primary antibodies were used for staining: anti-CD41 monoclonal antibody (2 μg/ml, BD Pharmigen, San Jose, CA, USA), anti-F4/80 (1:500, BD Pharmigen) and Alexa 488 phalloidin (Invitrogen), anti-rat secondary antibody (Cy3, 1:1000, Jackson ImmunoResearch, West Grove, PA, USA) were used for CD41 and F4/80 . Nuclear staining was carried out with bisBenzimide (20 μM; Sigma-Aldrich). Imaging was performed utilizing a Nikon A1 confocal microscope (Nikon, Melville, NY, USA). Quantification of the mean fluorescent intensities was performed using a ratio of actin(37).
Platelet aggregation after polytrauma
Blood samples were obtained 30 minutes after induction of trauma and further analyzed for platelet aggregation. Platelet aggregation induced by collagen (2 μg/ml, ChronoLog) was assessed using a ChronoLog aggregometer (Model 700). Aggregation was quantified as area under the curve (AUC) and analysis was performed using the aggrolink-8 software (ChronoLog).
Ethics statement
All animal experiments were performed according to the animal protection law of Germany and USA and were approved by local ethic committees. All efforts were made to minimize suffering.
Statistical analysis
For statistical analysis unpaired Students t-test or one-way ANOVA using Dunnett post hoc test were performed using Graphpad Prism 6.0 (Graphpad Software, La Jolla, CA, USA). Values of p<0.05 were considered as significant. If not indicated differently, all data represents mean±S.E.M.
Results
Generation and validation of a specific CyPA neutralizing antibody
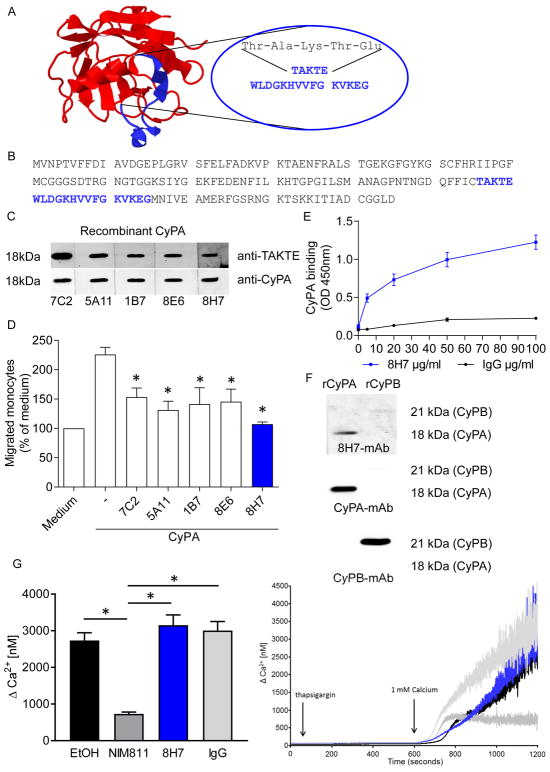
In order to specifically target a functional site of extracellular CyPA we generated a mAb which should interfere with the binding of CyPA and its receptor EMMPRIN. The postulated EMMPRIN-binding site of CyPA is characterized by an amino acid sequence Thr-Ala-Lys-Thr-Glu (TAKTE). The representation of the three-dimensional structure of CyPA is shown in Figure 1A(24, 25). For mAb generation rats or mice were immunized with a 20mer peptide containing the signal sequence (TAKTE) of the postulated EMMPRIN binding site of CyPA (Figure 1A/B). During the selection procedure we identified five specific anti-CyPA mAbs (8H7, generated in rats, 7C2, 5A11, 1B7, 8E6, generated in mice), which revealed positive immunoreactive signals in immunoblotting experiments for recombinant CyPA. Comparison was made with a commercially available anti-CyPA mAb (Figure 1C).
Figure 1. Generation and validation of a specific CyPA neutralizing antibody.
5 CyPA antibodies were induced by injection of a peptide containing the EMMPRIN-binding sequence as described in material and methods. 4 mice (7C2, 5A11, 1B7, 8E6) and 1 rat (8H7) antibodies were tested for their binding capacity to CyPA and their inhibitory function. (A) Figure shows structure of CyPA (PBD-ID: 3K0N) and the epitope of the antibody highlighted in blue. (B) The CyPA amino acid sequence (blue) indicated the suggested binding region of the TAKTE-antibodies. (C) Antibodies were tested for their binding to CyPA in a SDS page using recombinant CyPA. (D) To test the function of TAKTE-antibodies in a functional assay monocyte migration was analyzed. (n=6). * means p ≤ 0.05 vs. CyPA. (E) In a modified ELISA the binding between CyPA and 8H7-mAb / IgG control was analyzed. (F) Recombinant CyPA and recombinant CyPB were loaded on a SDS page and detected with 8H7-mAb (upper panel). 8H7-mAb could only detect CyPA but not CyPB. The band size of CyPA (18 kDa) and CyPB (21 kDa) was indicated using anti-CyPA (middle panel) and anti-CyPB (lower panel) antibodies. (G) A Ca2+ measurement was performed to analyze if the 8H7-mAb antibody interfere with intracellular calcium hemostasis. The intra- and extracellular CyPA inhibitor NIM811 (200 nmol/L) and ethanol (0.2%) were used as controls (n ≤ 5). * means p ≤ 0.05.
To analyze and screen the potential functional activity of our anti-CyPA mAbs, we analyzed the inhibitory effects in a monocyte migration assay. All anti-CyPA mAbs could significantly reduce the CyPA-induced monocyte migration (Figure 1D). Due to its binding ability to endogenous CyPA and the markedly inhibitory effect on monocyte migration we selected clone 8H7-mAb for further analysis. At first, an ELISA-based binding assay was performed. We found that 8H7-mAb bound to immobilized CyPA in a concentration-dependent manner with a half-maximal binding activity at 75.33 nM (Figure 1E). In immunoblotting analysis 8H7-mAb showed specific immunoreactive bands for recombinant CyPA but not for Cyclophilin B (CyPB) (Figure 1F). To show that 8H7-mAb is specific for extracellular CyPA, its impact on intracelllular calcium mobilization was evaluated since intracellular CyPA is involved in SOCE (store-operated calcium entry)-dependent Ca2+-signaling but not extracellular CyPA(12). As hypothesized, 8H7-mAb did not influence Ca2+-signaling, this provides evidence for its strict extracellular activity. As control the intra- and extracellular CyPA inhibitor NIM811 influenced Ca2+-signaling of platelets (G). To exclude a direct interaction with the EMMPRIN-receptor and thus other EMMPRIN-ligands we analyzed its effect in a monocyte migration towards the EMMPRIN-ligand S100A9. Notably, we observed no difference between 8H7-mAb and rIgG2a in the S100A9-induced monocyte migration (198.2±18.27 vs. 210.5±28.21; Supplemental figure 1A), which further underscores its specificity for extracellular CyPA. In summary, our data indicate that the generated CyPA-mAb specifically neutralizes extracellular CyPA and its functional effects.
In vivo pharmacokinetics after intraperitoneal injection
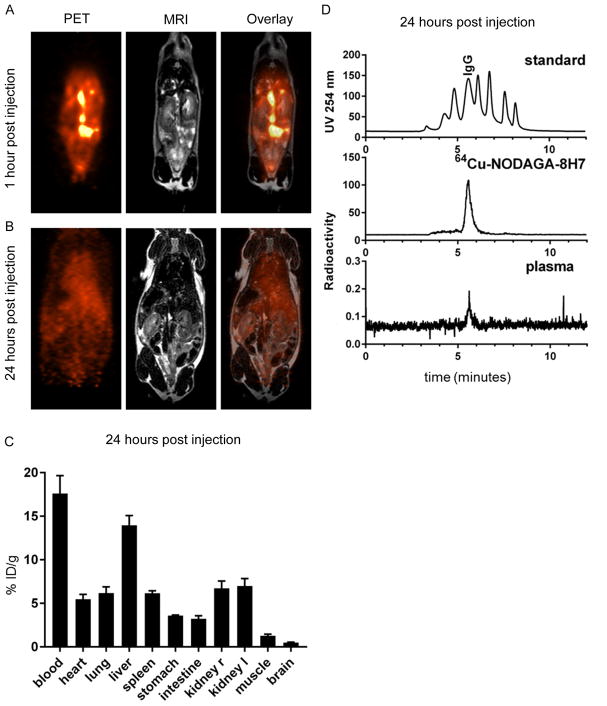
In the next step we analyzed bioavailability and distribution for further in vivo experiments. After intraperitoneal injection of radiolabeled 8H7-mAb, the antibody was distributes within the peritoneal space and successively enters the blood circulation (Figure 3). Already one hour after injection, the antibody was distributed in the peritoneum and can be found in the blood circulation (Figure 2A). After 24 hours, the antibody was cleared from the injection site and the highest concentration was found in blood (17.6±2.0 %ID/g) and liver (14.0±1.1 %ID/g, Figure 2B/C). Since accumulation in the liver is also observed in other studies involving 64Cu it might be attributed to incorporation of the isotope into superoxide dismutase when released from the antibody.(38)
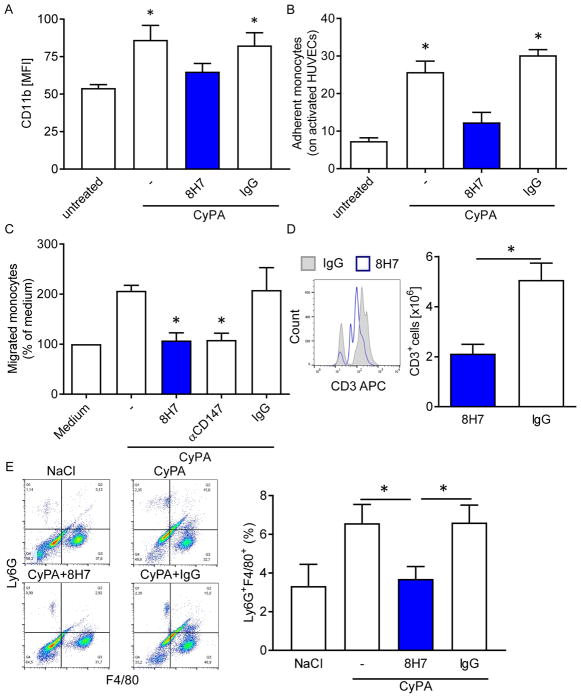
Figure 3. Inhibition of extracellular CyPA inhibits CyPA-dependent monocyte/ endothelium adhesion and migration of monocytes/macrophages in vitro and in vivo.
(A) Human monocytes were activated with 200 nmol/L CyPA with an additional treatment of 8H7-mAb or rIgG2a. The surface expression of the integrin CD11b expression was measured using flow cytometry. * p ≤ 0.05 compared to untreated. (B) Human monocytes were treated with CyPA and 8H7-mAb or IgG control as indicated and perfused over activated HUVECs under arterial shear rates. Bar graphs show mean±SEM of adherent monocytes on activated HUVECs compared to unstimulated (n ≤ 8). * means p ≤ 0.05. (C) The migration of human monocytes was performed in a modified Boyden chamber. Human monocytes were allowed to migrate towards CyPA in presence or absence of 8H7-mAb, αCD147 or IgG control. (n≤5) * means p ≤ 0.05 vs. CyPA+IgG. (D) The infiltration of CD3+ cells into the peritoneal lavage were analyzed using flow cytometry (n≤5) * means p ≤ 0.05, with representative overlays of CD3+ cells. (E) The Ly6G+F4/80+ cells in the lavage in a CyPA-induced peritonitis model were analyzed using flow cytometry (n≤8) * means p ≤ 0.05, with representative quadrat statistic for the cells in the lavage.
Figure 2. PET/MR imaging and biodistribution analysis of radiolabeled 8H7-mAb.
64Cu-NODAGA-8H7-mAb was injected i.p. and its distribution was quantified by simultaneous PET/MR imaging. (A/B) PET and MRI images of a representative animal 1 hour (A) and 24 hours (B) after i.p. injection of 64Cu-NODAGA-8H7 demonstrate systemic distribution of the antibody over time. (C) Ex vivo analysis of the organs by gamma-counting 24 hours after injection showed highest activities in blood and liver, followed by organs with good blood supply (heart, lung, spleen, kidneys). Data represent mean ± SD of three independent experiments. (D) Size standard (Vo, 669 kDa, 300 kDa, 150 kDa, 45 kDa, 17 kDa, 0.2 kDa, Vi; upper panel) and radiolabeled 8H7-mAb before injection (middle panel) are shown for reference. Representative analysis of plasma taken 24 hours after injection showed radioactivity signal corresponding to the molecular weight of 64Cu-NODAGA-8H7, demonstrating the molecular integrity of the circulating radiolabeled antibody for over 24 hours (lower panel).
Radio-HPSEC analysis of serum samples taken 24 hours post injection demonstrate that the radioactivity found in serum was bound to IgG-sized molecules, indicating that the radiolabeled 8H7-mAb antibody entered the blood stream where it was stable for at least 24 hours (Figure 2D).
Inhibition of extracellular CyPA inhibits CyPA-dependent monocyte/ endothelium adhesion and migration of monocytes/macrophages in vitro and in vivo
Next we analyzed functional activity of our antibody in the context of inflammation and recruitment of inflammatory cells. Thus, CyPA-induced monocyte activation was analyzed. Human monocytes were activated with CyPA and the change of integrin CD11b expression was measured. CyPA significantly increases the expression of CD11b compared to untreated monocytes (54.25±2.83 vs. 85.03±10.4). Treatment of monocytes with 8H7-mAb (65.53±6.96) inhibits the CyPA-induced increase of CD11b expression (Figure 3A). To evaluate whether inhibition of extracellular CyPA by 8H7-mAb modulates monocyte adhesion to an immobilized endothelial monolayer, human monocytes were perfused over TNFα/INFγ-activated human umbilical vein endothelial cells (HUVECs) under arterial shear rates. CyPA significantly enhanced monocyte adhesion to endothelial cells under flow which could be significantly inhibited in the presence of neutralizing anti-CyPA mAb 8H7 but not rIgG2a (25.75±2.88 vs. 30.21±1.5 vs. 12.34±2.64; Figure 3B). In line with this findings 8H7-mAb (107.5±5.38) and αCD147 (108.4±6.04) significantly reduced CyPA-induced migration in vitro compared to IgG (207.4±12.58); Figure 3C). Further, we analyzed whether inhibition of extracellular CyPA attenuates leukocyte migration in vivo in a novel CyPA-induced peritonitis model. Treatment of mice with 8H7-mAb significantly reduced the CyPA-dependent infiltration of CD3+ cells in the group which was treated with 8H7-mAb compared to rIgG2a (5.1±0.67 vs. 2.1±0.37; p<0.01; Figure 3D). Similarly, the percentage of infiltrated Ly6G+F4/80+ macrophages in the 8H7-mAb treated group was significantly decreased compared to rIgG2a treated group (6.61±0.9 vs. 3.7±0.64). There was no difference between NaCl and 8H7-mAb threated groups. (* means p<0.05; Figure 3E). Notably 8H7-mAb did not lead to a depletion of platelets (supplemental figure 2A) or leukocytes (supplemental figure 2B) after 24 hours of CyPA-induced peritonitis.
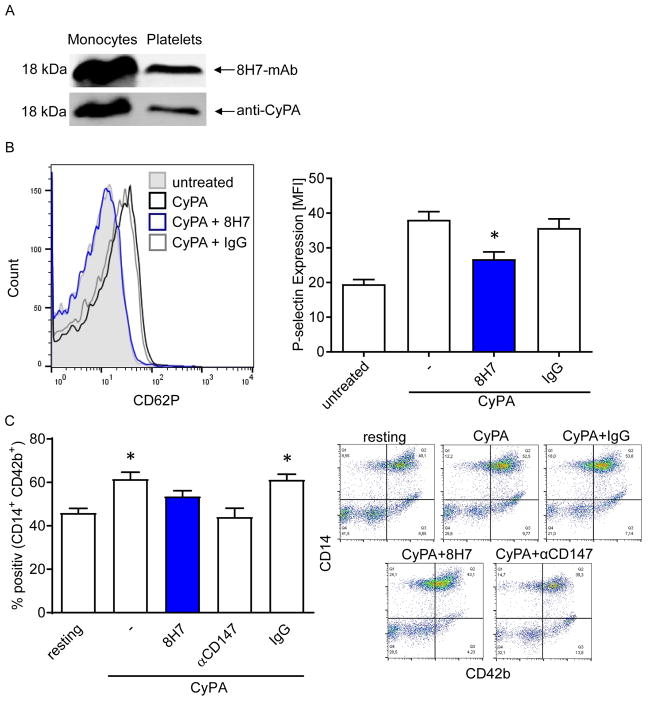
Extracellular CyPA enhances platelet-dependent thrombosis in vivo and in vitro
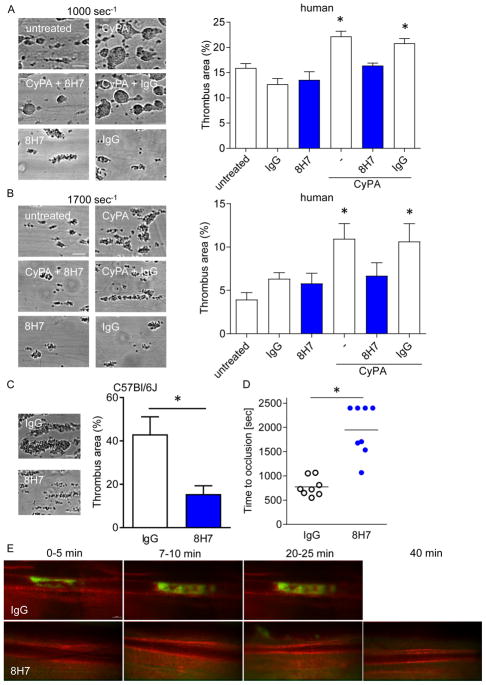
Previously, we found that CyPA is released from platelets upon activation. In turn, extracellular CyPA activates platelets via PI3 kinase. The activation of platelets followed by accumulation on injured endothelium can be inhibited by the cyclosporine A derivatives NIM811 and MM284. (12) In the present study, we found that the neutralizing anti-CyPA 8H7-mAb described herein detected endogenous platelet CyPA in immunoblotting studies (Figure 4A). Further, 8H7-mAb inhibited CyPA-induced P-selectin surface expression compared to rIgG2a (35.72±2.65 vs. 26.76±2.1), indicating that blockage of extracellular CyPA via 8H7-mAb has antithrombotic effects (Figure 4B). In the next step the effect of soluble CyPA on platelet-monocyte interaction was analyzed. CyPA-stimulated platelets showed increased formation of monocyte-platelet aggregates (MPA) compared to resting platelets (MFI: 61.49±3.19 vs. 45.9±2.15). In contrast we observed that CyPA did not increase MPA in 8H7-mAb and anti-EMMPRIN (αCD147) treated platelets (Figure 4C). Next, the effect of 8H7-mAb was tested on platelet-dependent thrombus formation under dynamic flow condition in whole blood. Under low (1000 seconds−1; Figure 5A) or high shear (1700 seconds−1; Figure 5B) conditions 8H7-mAb did not attenuate thrombus formation on immobilized collagen compared to rIgG2a (Figure 5A and B). In contrast, when recombinant CyPA was added to the whole blood, 8H7-mAb significantly reduced formation of platelet-dependent thrombi compared to rIgG2a (20.8±0.96 vs 16.4±0.51 (1000 seconds−1) and 10.64±2.0 vs 6.7±1.5 (1700 seconds−1), p<0.05) (Figure 5A and B) both under low and high shear conditions. In line with this finding we observed a difference in thrombus formation by perfusing murine 8H7-mAb or rIgG2a treated blood over CyPA and immobilized collagen under high shear rates (1700 seconds−1; 43.09±8.03 vs. 15.51±3.82; Figure 5C). Finally, administration of 8H7-mAb but not of rIgG2a inhibited in vivo thrombus formation in a FeCl3-induced thrombus formation model in mice. 8H7-mAb but not rIgG2a substantially increased the time to occlusion dramatically (32.5±3.0 vs. 12.9±1.1 minutes; p<0.01) (Figure 5D/E). This indicates that extracellular CyPA promotes platelet-dependent thrombus formation which can be neutralized by 8H7-mAb.
Figure 4. Extracellular CyPA enhances platelet-activation in vitro.
(A) 8H7-mAb detects CyPA in monocyte as well in platelet lysate (upper line). Afterwards the same membrane was incubated with an anti-CyPA antibody (lower line) to prove the specificity of 8H7-mAb. (B) 8H7-mAb significantly reduces the CyPA-dependent platelet activation. Representative overlays of the p-selectin expression and bar graphs show mean±SEM of P-selectin expression on platelets. * means p ≤ 0.05 vs. CyPA+IgG. (C) Monocyte-platelet aggregate (MPA) formation was analyzed by using double staining with CD42b (as platelets marker) and CD14 (as monocyte marker) as described in material and methods. The platelets were treated as indicated and incubated with monocytes for the MPA formation. Figure shows representative flow cytometry analysis. (n ≤ 6) * means p ≤ 0.05 vs. resting. Right panel shows representative quadrat statistic for the CD14+CD42+ double positive cells.
Figure 5. Extracellular CyPA enhances platelet-dependent thrombosis in vivo and in vitro.
Human blood was preincubated with 8H7-mAb or IgG control and stimulated with 200 nmol/L CyPA. Then the blood was perfused using a shear rate of 1000 seconds−1 (A) and 1700 seconds−1 (B) over a fibrillar type I collagen coated coverslip. (n ≤ 4) * means p ≤ 0.05 vs. untreated. (C) Murine blood was perfused over a CyPA/fibrillar type I collagen coated coverslip using a shear rate of 1700 seconds−1 after i.v. injection of 8H7-mAb or IgG control (n ≤ 8) * means p ≤ 0.05, scale bar 20 μm. 8H7-mAb reduceed thrombus formation in vitro, and as the next step the in vivo thrombus formation in mesenteric arterioles was analyzed in a FeCl3 model after administration of 8H7-mAb or IgG control (D/E). Representative pictures of the in vivo thrombus formation (E) and time to occlusion for individual mice (D). * means p ≤ 0.05, scale bar 50 μm.
Blockage of extracellular CyPA through 8H7-mAb does not affect blood hemostasis
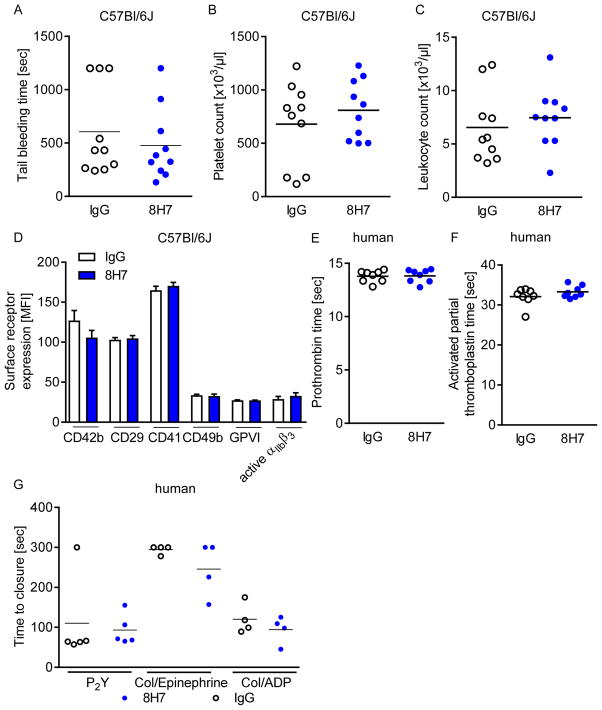
To evaluate the role of extracellular CyPA on hemostasis, tail bleeding time was assessed in mice after administration of 8H7-mAb and rIgG2a. Surprisingly, tail bleeding time was not different in mice receiving 8H7-mAb or rIgG2a (10.1±2.2 vs. 8.0±1.8 min; p=0.4) (Figure 6A). Further, there was neither any difference in platelet (679±123 vs. 810±88 ×103/μl; Figure 6B) nor leukocyte count (7.45±0.9 vs. 6.54±1.06 ×103/μl; Figure 6C) between the two groups nor in the surface expression of platelet adhesion receptors (Figure 6D). Further, no effect of 8H7-mAb was found in human whole blood on prothrombin time (Figure 6E), activated partial thromboplastin time (Figure 6F) or ex vivo bleeding time (Figure 6G). Therefore the antibody has no general effect on blood hemostasis. Besides, without an inflammatory response there is no secretion of CyPA and the antibody is only against CyPA-induced pro-thrombotic and pro-inflammatory effects. Thus the antibody has no anti-thrombotic effects in absence of CyPA.
Figure 6. Blockage of extracellular CyPA through 8H7-mAb does not affect hemostasis.
(A/B) 8H7-mAb treated mice did not exhibita prolonged tail bleeding time (A). After tail bleeding time the blood was collected and the number of platelets (B) and leukocytes (C) were estimated for both groups. (D) An in vivo treatment with 8H7-mAb did not change the surface expression of CD42b, CD29, CD41, CD49b, GPVI or the active form of αIIbβ3 on platelets compared to IgG control treated mice. (F/G) Every dot shows the response of one donor for the prothrombin time (E) and the activated partial thromboplastin time (F) (n=8). (G) PFA-100 measurement was performed with human blood. With the cartilages P2Y, Collagen/Epinephrine and Collage/ADP no difference could be detected.
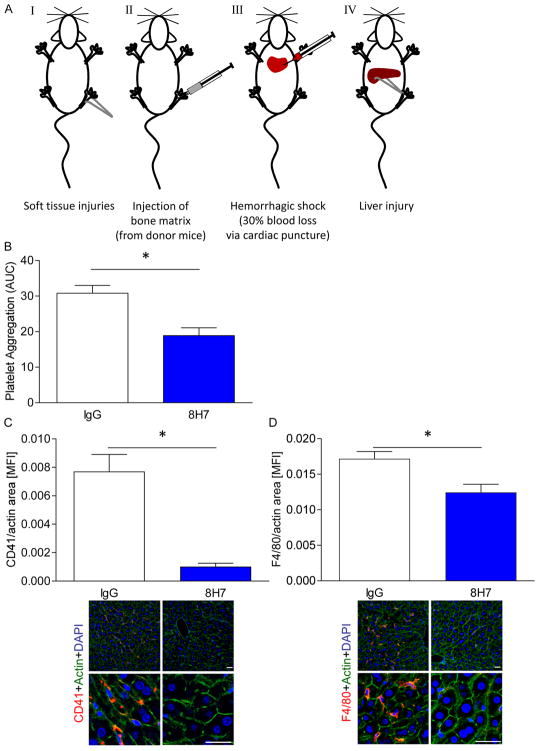
Inhibition of extracellular CyPA limits platelet accumulation and macrophage infiltration in shock liver
Recently, we found that platelets play a critical role in organ failure in shock(39). In shock patients and in mice undergoing a traumatic hemorrhagic shock platelet aggregation in the circulation and accumulation in the microvasculature of failing liver and lung is enhanced(32). In this model blood homeostasis is an important parameter beside impaired microcirculation and thrombo-inflammation. Thus we intended to finally test our inhibitor in a mouse model of trauma and hemorrhagic shock(39) (Figure 7A). Interestingly, administration of 8H7-mAb but not rIgG2a reduced platelet aggregation ex vivo in mice undergoing trauma/hemorrhagic shock (18.9±2.2 vs. 30.8±2.2 (AUC); Figure 7B). Moreover, platelet accumulation in the shock liver was markedly attenuated in mice treated with 8H7-mAb prior to induction of trauma compared to rIgG2a animals (Figure 7C). Further, infiltration of macrophages in the liver of trauma mice was significantly decreased in the presence of the 8H7-mAb compared to IgG (Figure 7D). These data indicate that extracellular CyPA plays a critical pathophysiological role in platelet-mediated thrombo-inflammation and microcirculatory arrest of organ failure in trauma/hemorrhage-induced shock.
Figure 7. Inhibition of extracellular CyPA limits platelet accumulation and macrophage infiltration in shock liver.
The trauma/hemorrhagic shock model consists of four different parts: soft tissue injuries, injection of bone matrix, hemorrhagic shock and liver injury (AI-AIV). (B) 8H7-mAb significantly reduced platelet aggregation after trauma compared to IgG control. Moreover there were also less platelet (C) and monocyte (D) infiltration into the liver tissue compared to IgG control. Scale bar 5 μm * means p ≤ 0.05 (n ≤ 3).
Discussion
The major findings of the present study are: a) extracellular CyPA promotes both in vitro and in vivo platelet-dependent thrombus formation which can be attenuated by a novel antibody-based CyPA inhibitor 8H7-mAb. b) Inhibition of extracellular CyPA through 8H7-mAb reduces monocyte/macrophage migration in vitro and in vivo. c) 8H7-mAb exhibits an excellent systemic bioavailability after intraperitoneal application and consistent molecular integrity of the antibody is observed over at least 24 hours. d) Administration of a neutralizing extracellular CyPA mAb prevents accumulation of platelets and macrophages in the failing liver in shock in vivo. The findings imply that extracellular CyPA is a critical mediator for platelet-dependent thrombosis and thrombo-inflammation and as well as organ dysfunction following shock.
Intracellular CyPA is present in all mammalian cell types and basically recognized as chaperone that regulates important intracellular function like modulating protein folding, trafficking, and cellular Ca2+ hemostasis(2–5). Addionally it is an attractive pharmacological target to control host defense in organ transplantation(8). Inhibitors of CyPA such as cyclosporine A (CsA) or its derivatives such as has been shown to inhibit the PPIase activity of CyPA, a major motif within the CyPA molecule involved in immune defense(40). Thus, tissue restricted CyPA-inhibitors are needed for the reduction of side effects mediated via intracellular CyPA.
In platelets, intracellular CyPA is a central regulator of cellular Ca2+ homeostasis(30) and involved in αIIbβ3 mediated bidirectional signaling(41). Thus, Inhibition of intracellular CyPA leads to an impaired blood homeostasis(30). We could detect no effect on general blood hemostasis by 8H7. This could be explained by the fact that only in thrombotic and inflammatory conditions CyPA is secreted. The novel antibody blocks only extracellular CyPA-induced pro-thrombotic and pro-inflammatory effects. Our data indicate that 8H7 has no anti-thrombotic effects without the presence of extracellular CyPA.
Extracellular CyPA binds to its surface receptor EMMPRIN(13, 42) that can be detected on most inflammatory cells including monocytes/macrophages and platelets(13, 16, 43). Binding of CyPA to EMMPRIN stimulates cell migration(18, 43, 44) as well as platelet activation(12). Current inhibitor strategies with cyclosporine A and synthetic molecules (NIM811) target both intracellular and extracellular function of CyPA(21, 22). Recently, we described a CsA-derivative MM284 that specifically inhibits extracellular CyPA and prevents myocardial fibrosis in autoimmune myocarditis(18). However, MM284 targets also other extracellular cyclophilins. Since the lack of specific inhibitors of the respective CyPA the specific effects of blocking selectively extracellular CyPs has not been investigated yet. Our data suggest that it is possible to reduce the pro-inflammatory and pro-thrombotic effect of CyPA by using our new developed specific anti-CyPA antibody 8H7.
Previously, it was shown that the hydrophobic pocket of CyPA is a critical binding site for the human immunodeficiency virus type 1 gag polyprotein(45). To obtain a functional CyPA mAb that likely blocks a critical binding site of CyPA, we generated mAbs directed against the amino acid sequence 56TAKTEWLDGKHVVFGKVKEG75 derived from the linear sequence of the hydrophobic pocket of CyPA. We identified the clone 8H7-mAb that specifically binds to extracellular CyPA and inhibits CyPA-dependent migration of monocytes in vitro and in vivo. However there are some limitations of our study. We cannot exclude that the antibody has non-specific immunological interactions with the Fc-part since no Fab fragment is used as control. Thus further studies have to be done with the Fab fragment only to evaluate the possible influence of the Fc-part on these results.
Platelets are a major source of extracellular CyPA and release substantial amounts upon activation(9). Extracellular CyPA binds to its surface-expressed receptor EMMPRIN on platelets and promotes platelet activation(12). Thus, we asked whether 8H7-mAb inhibits CyPA-dependent platelet activation and thrombus formation. As hypothesized, we found that 8H7-mAb significantly attenuates both ex vivo and in vivo thrombus formation, indicating an important function of extracellular CyPA in regulation of thrombosis. Findings that 8H7-mAb blocks thrombus formation under high shear rates only may indicate that CyPA requires von Willebrand factor to induce platelet adhesion(46, 47). Under normal conditions platelets are important for hemostasis and necessary to avoid blood loss at sites of vascular injury. On the other hand, platelets have important roles in inflammation and thrombosis(48). Our novel CyPA antibody 8H7-mAb is able to reduce pro-thrombotic effects without affecting normal platelet functions.
This observation was further substantiated in a trauma-mediated hemorrhagic shock model in mice. Recently, we reported that platelets are critical regulator of inflammation(49) and are involved in organ failure in shock(39). Administration of 8H7-mAb but not IgG control substantially attenuated platelet aggregation and accumulation in the damaged liver. Further, 8H7-mAb remarkably reduced infiltration of macrophage within the shocked liver and into the peritoneum after a CyPA-induced peritonitis, indicating an important role of CyPA for thrombo-inflammation. As CyPA is released in context of inflammation, targeting extracellular CyPA may now provide the opportunity to locally targeting CyPA-mediated thrombo-inflammatory pathways. Our findings that 8H7-mAb interferes with leukocyte recruitment and thrombus formation may also extent to other inflammatory diseases, given that CyPA functions as an important mediator of inflammation(18, 44) and elevated levels of CyPA have been described in inflammatory diseases like sepsis(50) or rheumatoid arthritis(51). However, the antibody 8H7-mAb is designed to interrupt the CyPA-EMMPRIN interaction. It has to keep in mind that there is a possibility that CyPA binds to unknown receptors. It can only be speculated that our antibody would influence this interaction as well.
Currently there are many recombinant antibody derived therapies in clinical practice available such as PKSC-9 inhibitors or GPIIb/IIIa antangonists (52). Thus, based on the high clinical experience with recombinant antibodies it is tempting to speculate that a medium term further development into clinical practice seems to be easier than with CsA-derived compounds.
Conclusion
Thus, our herein described strategy to target extracellular CyPA with a novel antibody 8H7-mAb may open novel therapeutic avenues for diseases that are characterized by enhanced platelet activation and thrombo-inflammation such as myocardial infarction, stroke and septic organ failure without altering blood hemostasis.
Supplementary Material
Acknowledgments
Sources of Funding
This work has been supported, in part, by grants from the Deutsche Forschungsgemeinschaft (Transregio SFB19 and the Klinische Forschergruppe KFO274), the Tuebingen Platelet Investigative Consortium (TuePIC) and the NIH (1S10OD019973-01).
The authors thank Klaudia Posavec for outstanding technical assistance and Carsten Calaminus for advice on the imaging study.
Footnotes
Disclosure
None
References
- 1.Fischer G, Wittmann-Liebold B, Lang K, et al. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337(6206):476–8. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 2.Galigniana MD, Morishima Y, Gallay PA, et al. Cyclophilin-A is bound through its peptidylprolyl isomerase domain to the cytoplasmic dynein motor protein complex. The Journal of biological chemistry. 2004;279(53):55754–9. doi: 10.1074/jbc.M406259200. [DOI] [PubMed] [Google Scholar]
- 3.Rosado JA, Pariente JA, Salido GM, et al. SERCA2b activity is regulated by cyclophilins in human platelets. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(3):419–25. doi: 10.1161/ATVBAHA.109.194530. [DOI] [PubMed] [Google Scholar]
- 4.Kern G, Kern D, Schmid FX, et al. Reassessment of the putative chaperone function of prolyl-cis/trans-isomerases. FEBS letters. 1994;348(2):145–8. doi: 10.1016/0014-5793(94)00591-5. [DOI] [PubMed] [Google Scholar]
- 5.Baker EK, Colley NJ, Zuker CS. The cyclophilin homolog NinaA functions as a chaperone, forming a stable complex in vivo with its protein target rhodopsin. The EMBO journal. 1994;13(20):4886–95. doi: 10.1002/j.1460-2075.1994.tb06816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handschumacher RE, Harding MW, Rice J, et al. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226(4674):544–7. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 7.Colgan J, Asmal M, Neagu M, et al. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21(2):189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Colgan J, Asmal M, Yu B, et al. Cyclophilin A-deficient mice are resistant to immunosuppression by cyclosporine. Journal of immunology. 2005;174(10):6030–8. doi: 10.4049/jimmunol.174.10.6030. [DOI] [PubMed] [Google Scholar]
- 9.Coppinger JA, Cagney G, Toomey S, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103(6):2096–104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 10.Sherry B, Yarlett N, Strupp A, et al. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(8):3511–5. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SH, Lessner SM, Sakurai Y, et al. Cyclophilin A as a novel biphasic mediator of endothelial activation and dysfunction. The American journal of pathology. 2004;164(5):1567–74. doi: 10.1016/S0002-9440(10)63715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seizer P, Ungern-Sternberg SN, Schonberger T, et al. Extracellular cyclophilin A activates platelets via EMMPRIN (CD147) and PI3K/Akt signaling, which promotes platelet adhesion and thrombus formation in vitro and in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2015;35(3):655–63. doi: 10.1161/ATVBAHA.114.305112. [DOI] [PubMed] [Google Scholar]
- 13.Yuan W, Ge H, He B. Pro-inflammatory activities induced by CyPA-EMMPRIN interaction in monocytes. Atherosclerosis. 2010;213(2):415–21. doi: 10.1016/j.atherosclerosis.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Kasinrerk W, Fiebiger E, Stefanova I, et al. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. Journal of immunology. 1992;149(3):847–54. [PubMed] [Google Scholar]
- 15.Chen X, Kanekura T, Kanzaki T. Expression of Basigin in human fetal, infantile and adult skin and in basal cell carcinoma. Journal of cutaneous pathology. 2001;28(4):184–90. doi: 10.1034/j.1600-0560.2001.028004184.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt R, Bultmann A, Fischel S, et al. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circulation research. 2008;102(3):302–9. doi: 10.1161/CIRCRESAHA.107.157990. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Lu N, Zhou J, et al. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology. 2008;47(9):1299–310. doi: 10.1093/rheumatology/ken225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzmann D, Bangert A, Muller AM, et al. The Novel Extracellular Cyclophilin A (CyPA) - Inhibitor MM284 Reduces Myocardial Inflammation and Remodeling in a Mouse Model of Troponin I-Induced Myocarditis. PloS one. 2015;10(4):e0124606. doi: 10.1371/journal.pone.0124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seizer P, Klingel K, Sauter M, et al. Cyclophilin A affects inflammation, virus elimination and myocardial fibrosis in coxsackievirus B3-induced myocarditis. Journal of molecular and cellular cardiology. 2012;53(1):6–14. doi: 10.1016/j.yjmcc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Seizer P, Ochmann C, Schonberger T, et al. Disrupting the EMMPRIN (CD147)-cyclophilin A interaction reduces infarct size and preserves systolic function after myocardial ischemia and reperfusion. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(6):1377–86. doi: 10.1161/ATVBAHA.111.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seizer P, Schonberger T, Schott M, et al. EMMPRIN and its ligand cyclophilin A regulate MT1-MMP, MMP-9 and M-CSF during foam cell formation. Atherosclerosis. 2010;209(1):51–7. doi: 10.1016/j.atherosclerosis.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Billich A, Hammerschmid F, Peichl P, et al. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. Journal of virology. 1995;69(4):2451–61. doi: 10.1128/jvi.69.4.2451-2461.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malesevic M, Kuhling J, Erdmann F, et al. A cyclosporin derivative discriminates between extracellular and intracellular cyclophilins. Angewandte Chemie. 2010;49(1):213–5. doi: 10.1002/anie.200904529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallen J, Spitzfaden C, Zurini MG, et al. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991;353(6341):276–9. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- 25.Ke HM, Zydowsky LD, Liu J, et al. Crystal structure of recombinant human T-cell cyclophilin A at 2. 5 A resolution. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(21):9483–7. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harding MW, Handschumacher RE, Speicher DW. Isolation and amino acid sequence of cyclophilin. The Journal of biological chemistry. 1986;261(18):8547–55. [PubMed] [Google Scholar]
- 27.Schmidt R, Bultmann A, Ungerer M, et al. Extracellular matrix metalloproteinase inducer regulates matrix metalloproteinase activity in cardiovascular cells: implications in acute myocardial infarction. Circulation. 2006;113(6):834–41. doi: 10.1161/CIRCULATIONAHA.105.568162. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler M, Elvers M, Baumer Y, et al. The bispecific SDF1-GPVI fusion protein preserves myocardial function after transient ischemia in mice. Circulation. 2012;125(5):685–96. doi: 10.1161/CIRCULATIONAHA.111.070508. [DOI] [PubMed] [Google Scholar]
- 29.May AE, Schmidt R, Bulbul BO, et al. Plasminogen and matrix metalloproteinase activation by enzymatically modified low density lipoproteins in monocytes and smooth muscle cells. Thrombosis and haemostasis. 2005;93(4):710–5. doi: 10.1160/TH04-11-0720. [DOI] [PubMed] [Google Scholar]
- 30.Elvers M, Herrmann A, Seizer P, et al. Intracellular cyclophilin A is an important Ca(2+) regulator in platelets and critically involved in arterial thrombus formation. Blood. 2012;120(6):1317–26. doi: 10.1182/blood-2011-12-398438. [DOI] [PubMed] [Google Scholar]
- 31.Seizer P, Borst O, Langer HF, et al. EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI-EMMPRIN interaction. Thrombosis and haemostasis. 2009;101(4):682–6. doi: 10.1160/th08-06-0368. [DOI] [PubMed] [Google Scholar]
- 32.Borst O, Schaub M, Walker B, et al. Pivotal role of serum- and glucocorticoid-inducible kinase 1 in vascular inflammation and atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2015;35(3):547–57. doi: 10.1161/ATVBAHA.114.304454. [DOI] [PubMed] [Google Scholar]
- 33.Rolle AM, Hasenberg M, Thornton CR, et al. ImmunoPET/MR imaging allows specific detection of Aspergillus fumigatus lung infection in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(8):E1026–33. doi: 10.1073/pnas.1518836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borst O, Munzer P, Gatidis S, et al. The inflammatory chemokine CXC motif ligand 16 triggers platelet activation and adhesion via CXC motif receptor 6-dependent phosphatidylinositide 3-kinase/Akt signaling. Circulation research. 2012;111(10):1297–307. doi: 10.1161/CIRCRESAHA.112.276444. [DOI] [PubMed] [Google Scholar]
- 35.Borst O, Schmidt EM, Munzer P, et al. The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood. 2012;119(1):251–61. doi: 10.1182/blood-2011-06-359976. [DOI] [PubMed] [Google Scholar]
- 36.Darwiche SS, Kobbe P, Pfeifer R, et al. Pseudofracture: an acute peripheral tissue trauma model. Journal of visualized experiments : JoVE. 2011;(50) doi: 10.3791/2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel S, Bodenstein R, Chen Q, et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. The Journal of clinical investigation. 2015;125(12):4638–54. doi: 10.1172/JCI81660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bass LA, Wang M, Welch MJ, et al. In vivo transchelation of copper-64 from TETA-octreotide to superoxide dismutase in rat liver. Bioconjug Chem. 2000;11(4):527–32. doi: 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]
- 39.Vogel S, Chatterjee M, Metzger K, et al. Activated platelets interfere with recruitment of mesenchymal stem cells to apoptotic cardiac cells via high mobility group box 1/Toll-like receptor 4-mediated down-regulation of hepatocyte growth factor receptor MET. The Journal of biological chemistry. 2014;289(16):11068–82. doi: 10.1074/jbc.M113.530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma S, Boerner JE, TiongYip C, et al. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with alpha interferon. Antimicrobial agents and chemotherapy. 2006;50(9):2976–82. doi: 10.1128/AAC.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Soe NN, Sowden M, et al. Cyclophilin A is an important mediator of platelet function by regulating integrin alphaIIbbeta3 bidirectional signalling. Thrombosis and haemostasis. 2014;111(5):873–82. doi: 10.1160/TH13-09-0738. [DOI] [PubMed] [Google Scholar]
- 42.Yurchenko V, Zybarth G, O’Connor M, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. The Journal of biological chemistry. 2002;277(25):22959–65. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 43.Arora K, Gwinn WM, Bower MA, et al. Extracellular cyclophilins contribute to the regulation of inflammatory responses. Journal of immunology. 2005;175(1):517–22. doi: 10.4049/jimmunol.175.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. Journal of leukocyte biology. 2007;82(3):613–8. doi: 10.1189/jlb.0506317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braaten D, Ansari H, Luban J. The hydrophobic pocket of cyclophilin is the binding site for the human immunodeficiency virus type 1 Gag polyprotein. Journal of virology. 1997;71(3):2107–13. doi: 10.1128/jvi.71.3.2107-2113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94(5):657–66. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 47.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 48.Ruggeri ZM. Platelets in atherothrombosis. Nature medicine. 2002;8(11):1227–34. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 49.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. The Journal of clinical investigation. 2005;115(12):3378–84. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tegeder I, Schumacher A, John S, et al. Elevated serum cyclophilin levels in patients with severe sepsis. Journal of clinical immunology. 1997;17(5):380–6. doi: 10.1023/a:1027364207544. [DOI] [PubMed] [Google Scholar]
- 51.Billich A, Winkler G, Aschauer H, et al. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. The Journal of experimental medicine. 1997;185(5):975–80. doi: 10.1084/jem.185.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. Journal of the American College of Cardiology. 2014;63(23):2531–40. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.