This population-based cohort study assesses the risk of serotonin syndrome associated with concomitant use of triptans and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants.
Key Points
Questions
What is the risk of serotonin syndrome associated with concomitant use of triptans and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants and how did the use of these drugs change after the 2006 US Food and Drug administration warning about this risk?
Findings
In this data registry study of 47 968 patients prescribed triptans, the incidence of serotonin syndrome was 0 to 4 cases per 10 000 person-years of exposure to coprescription of triptans and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants. The proportion of patients who were coprescribed these drugs ranged from 21% to 29% and remained stable before and after the warning.
Meaning
Serotonin syndrome was rare in patients who were coprescribed triptans and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants; those with coexisting affective disorders and migraine need not forgo management of one condition to treat the other.
Abstract
Importance
In 2006, the US Food and Drug Administration (FDA) issued an advisory warning on the risk of serotonin syndrome with concomitant use of triptans and selective serotonin reuptake inhibitor (SSRI) or selective norepinephrine reuptake inhibitor (SNRI) antidepressants, but the true risk of serotonin syndrome in these patients remains unknown.
Objective
To assess the risk of serotonin syndrome with concomitant use of triptans and SSRI or SNRI antidepressants.
Design, Setting, and Participants
This study used electronic health record data from the Partners Research Data Registry (RPDR) to identify patients who had received an International Classification of Diseases, Ninth Revision diagnosis compatible with serotonin syndrome who had been coprescribed triptans and SSRI or SNRI antidepressants in the Greater Boston, Massachusetts, area from January 1, 2001, through December 31, 2014 (14 years). Clinical information was extracted to determine whether the case met formal diagnostic criteria and had coprescription within a calendar year. Both conservative and broad case definitions were used to better characterize the spectrum of risk. Data analysis was performed from November 23, 2016, to July 15, 2017.
Main Outcomes and Measures
Incidence of serotonin syndrome.
Results
The RPDR search revealed 47 968 (±3) unique patients who were prescribed triptans during the 14-year period of the study. A total of 19 017 (±3) patients were coprescribed triptans and antidepressants during the study, with a total of 30 928 person-years of exposure. Serotonin syndrome was suspected in 17 patients. Only 2 patients were classified as having definite serotonin syndrome (incidence rate, 0.6 cases per 10 000 person-years of exposure; 95% CI, 0.0-1.5). Five patients were classified as having possible serotonin syndrome (incidence rate with these 5 cases added to the 2 definite cases, 2.3 cases per 10 000 person-years of exposure; 95% CI, 0.6-3.9). The proportion of patients with triptan prescriptions who were coprescribed an SSRI or SNRI antidepressant was relatively stable during the study, ranging from 21% to 29%.
Conclusions and Relevance
The risk of serotonin syndrome associated with concomitant use of triptans and SSRIs or SNRIs was low. Coprescription of these drugs is common and did not decrease after the 2006 FDA advisory. Our results cast doubt on the validity of the FDA advisory and suggest that it should be reconsidered.
Introduction
In 2006, the US Food and Drug Administration (FDA) issued an advisory about the risk of serotonin syndrome associated with concomitant use of drugs from 2 widely prescribed medication classes: (1) selective serotonin reuptake inhibitor (SSRI) and selective norepinephrine reuptake inhibitor (SNRI) antidepressants and (2) triptan antimigraine drugs.1 Cases of serotonin syndrome associated with triptan monotherapy have also been reported.2 Serotonin syndrome is thought to result from elevated serotonin levels. It causes a constellation of features, including tachycardia, unstable blood pressure, hyperthermia, nausea, vomiting, and diarrhea. Severity varies, but it can be fatal.3 The FDA advisory was based on a small number of case reports. Doubts exist about whether these cases actually met criteria for the disorder.4,5 A position paper by the American Headache Society questioned the basis for the advisory and noted conflicting and insufficient information to discern the risk.6
Triptan antimigraine drugs and SSRI and SNRI antidepressants are widely prescribed. Depression and migraine are highly prevalent, long-lasting, disabling conditions that occur together at a frequency higher than expected by chance. In 2007 to 2008, triptans were prescribed for more than 5 million patients with migraine; other evidence suggests that 20% to 25% of triptan users are also prescribed SSRI or SNRI antidepressants.7,8 One study suggested that the FDA advisory thus applies to an estimated 1.8% of the US population, with investigators remarking that “What remains missing from the literature is documentation as to the number of cases of serotonin syndrome due to the concomitant use of triptan, and SSRI or SNRI, and the resulting consequences.”8(p 199)
Because there have been no population-based studies that link coprescription with the outcome of serotonin syndrome, the true risk remains unknown. Precise risk estimates would aid clinical decision making and would support or refute the validity of the FDA advisory. Meanwhile, the situation for physicians and patients is confusing. Pharmacy systems and other decision support systems routinely issue safety alerts when coprescription occurs. These alerts result in substantial disruption of clinical care.9 For these reasons, we conducted a study to identify patients who were coprescribed these drugs and evaluate whether any had developed serotonin syndrome and to determine whether changes occurred in coprescription after the 2006 FDA advisory.
Methods
Study Population
We used the Partners Research Patient Data Registry (RPDR) to identify patients who had received coprescriptions for triptans and SSRI or SNRI antidepressants from January 1, 2001, through December 31, 2014, and to search within that group for patients who had developed serotonin syndrome. The RPDR is a centralized clinical data warehouse that contains electronic health record information for more than 6.5 million patients seen in the Partners HealthCare Network, which provides care for approximately half of the population in the greater Boston, Massachusetts, area. Data analysis was performed from November 23, 2016, to July 15, 2017. The study was approved by the institutional review board of Brigham and Women’s Hospital, Boston, Massachusetts. The Partners Institutional Review Board determined that, as a retrospective database study, this project met criteria for exempt status; therefore, informed consent was waived.
During the period of this study, Partners HealthCare used a proprietary electronic medical record system known as the Longitudinal Medical Record. Clinicians entered dictated or typed information about patients into the Longitudinal Medical Record. In most cases, diagnoses were recorded using the International Classification of Diseases, Ninth Revision (ICD-9) codes and were written by hand on a billing sheet or indicated by circling or checking the relevant diagnosis on a list of common diagnoses. This diagnostic information was entered into the electronic billing system by billing assistants and subsequently imported into RPDR.
For data analysis, we defined coprescription as receipt of at least 1 prescription for a triptan and 1 prescription of an SNRI or SSRI antidepressant during the study period. We examined data for 2001 through 2014. The year 2001 is the first year for which the RPDR contains complete information, whereas 2014 is the most recent year for which complete information is available owing to the time it takes to import data from different sources into the RPDR. This period of 14 years also allowed evaluation of prescribing practices before and after the advisory. Our search terms included the 7 triptans available during the study period (sumatriptan, zolmitriptan, naratriptan, rizatriptan, eletriptan, almotriptan, or frovatriptan) and all the SNRI and SSRI agents included in the FDA advisory (citalopram, fluvoxamine, escitalopram, paroxetine, fluoxetine, sertraline, duloxetine, or venlafaxine).
We used the RPDR Query Tool to identify patients with coprescriptions of these agents. The Query Tool allows users to identify patients with specific combinations of demographic information, diagnoses, or other variables. To minimize the chance that searches will be inappropriately used to identify a particular patient, general RPDR searches return obfuscated numbers reported to an accuracy of ±3. For example, if the total number of patients who satisfy the criteria of a search query is 124, the RPDR results might indicate that 123 (±3) patients met the criteria. We conducted a search in the entire study period for all patients who had received at least 1 prescription for a triptan. We then searched within that group for patients who had also received at least 1 prescription for an SSRI or SNRI during that same period.
Within this population, we searched for cases of potential serotonin syndrome using the search words serotonin syndrome. Serotonin syndrome does not have its own ICD-9 code but is included in the broader category of “other extrapyramidal diseases and abnormal movement disorders” (ICD-9 code 333.99), referred to hereafter as extrapyramidal syndrome (EPS); our search identified cases associated with this broad category code. We made the conservative assumption that all these diagnoses might represent serotonin syndrome and obtained medical records for all patients in the coprescription population who had an ICD-9 diagnosis of EPS.
Because the symptoms of serotonin syndrome overlap with those of neuroleptic malignant syndrome, we conducted an additional search of the RPDR for unique patients who had received a neuroleptic malignant syndrome diagnosis (ICD-9 code 333.92) between 2001 and 2014 and had also received at least 1 prescription for a triptan during this period. We found that only 4 (±3) patients (range, 1-7 patients) satisfied these criteria. This small subset of patients with a neuroleptic malignant syndrome diagnosis was likely included in the broader group of patients retrieved by our more general diagnostic query. If they were not included, such a small number would not significantly alter our findings.
Reliability and Validity of Diagnosis
We received institutional review board approval to inspect individual medical records for this patient subgroup. One of us (Y.O.) used a standardized data abstraction form to record patient demographic characteristics, medical history, dates, doses and types of medications, and physical examination, laboratory, and imaging information, which were entered into an Excel spreadsheet. Information about medications was obtained from several sources within the patient record, including lists of medications prescribed by Partners HealthCare Network clinicians, as well as medications mentioned in clinical notes on the date of the event that, in some cases, were probably prescribed by non–Partners HealthCare Network clinicians. Then, based on information recorded in the medical record, the patient was determined (by Y.O.) to meet the Sternbach criteria or the Hunter Area Toxicology Service diagnostic criteria for serotonin syndrome (Box). All of us (Y.O., P.R., and E.L.) then met and reviewed each case again to verify the accuracy of data abstraction and application of diagnostic criteria. Differences were resolved by consensus.
Box. Sternbach and Hunter Criteria for Serotonin Syndrome.
Sternbach Criteria (Adapted From Sternbach10)
Addition or increased dose of serotonergic agent
No neuroleptic treatment started or dosage increased before onset of symptoms
-
At least 3 of the following:
Agitation
Tremor
Myoclonus
Hyperreflexia
Diaphoresis
Shivering
Diarrhea
Incoordination
Fever
Hunter Criteria (Adapted from Dunkley et al11)
-
One or more of the following in the presence of a serotonergic drug:
Spontaneous clonus
Inducible clonus and agitation or diaphoresis
Ocular clonus and agitation or diaphoresis
Tremor and hyperreflexia
Hypertonia
Temperature >38°C and ocular clonus or inducible clonus
Statistical Analysis
We calculated the proportion of patients receiving coprescriptions who received a diagnosis of serotonin syndrome. We also calculated the proportion of patients with a triptan prescription who also received a prescription for an SSRI or SNRI antidepressant. We determined the overall incidence rate for serotonin syndrome among patients receiving coprescriptions by dividing the number of new cases of serotonin syndrome by the person-years at risk during the study period. Because direct calculation of person-time was not feasible, we assumed that patients were at risk of developing serotonin syndrome during the full calendar year in which they received prescriptions for both an SSRI or SNRI antidepressant and a triptan.
To explore the effect of diagnostic uncertainty on risk estimates, we used 2 different case definitions to calculate the incidence rate. The first was a conservative definition in which the numerator included only cases that fulfilled diagnostic criteria for serotonin syndrome in patients receiving coprescriptions during the year of the event and for whom medical record information indicated ingestion of a triptan in close temporal relation to the event (definite cases). The second was a liberal and more inclusive definition in which the numerator included all patients receiving coprescriptions during the year of the event. This definition included cases in which serotonin syndrome was suspected but did not meet diagnostic criteria, information was insufficient to apply diagnostic criteria, or triptan ingestion did not occur in temporal relation to the event (possible cases). For all incidence rates, we calculated a 95% CI. All calculations were performed using OpenEpi, version 3, open source calculator software (http://www.openepi.com/PersonTime1/PersonTime1.htm and http://www.openepi.com/Proportion/Proportion.htm).
Results
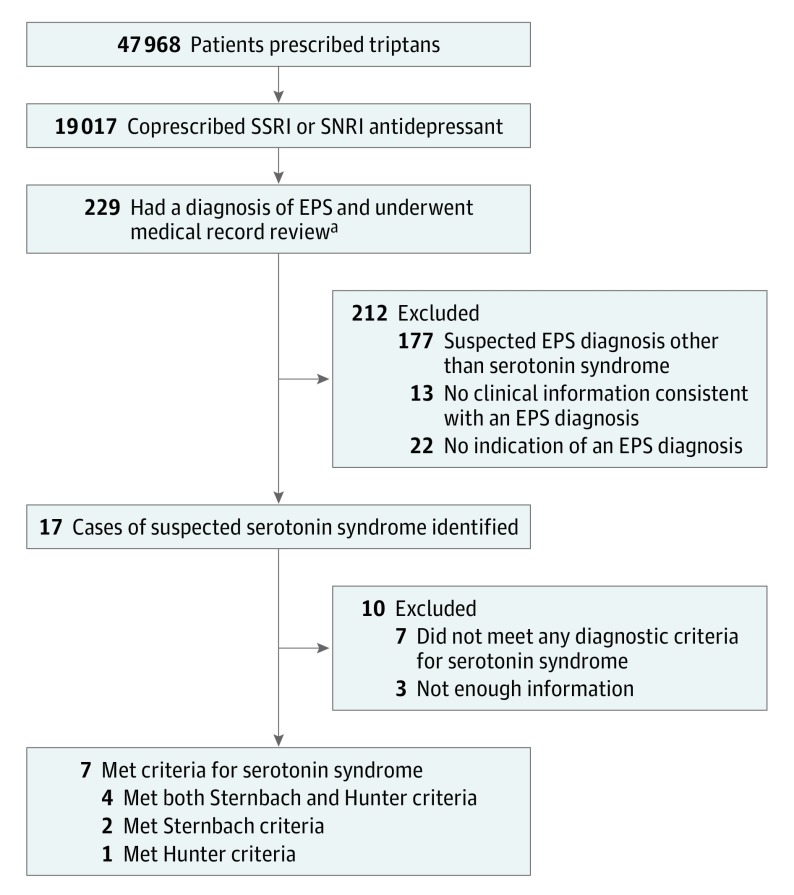
Figure 1 shows the flow of patients through the study. The RPDR search revealed 47 968 (±3) unique patients who were prescribed triptans during the 14-year period of the study. A total of 19 017 (±3) of these patients also received a prescription for an SSRI or SNRI antidepressant, of whom 229 (0.01%) had a diagnosis of EPS at some point. Review of individual medical records revealed that serotonin syndrome had been clinically suspected in 17 of these 229 patients. Suspected diagnoses in the other 212 patients included restless legs syndrome (n = 109), periodic limb movement disorder of sleep diagnosed by polysomnography (n = 47), akathisia attributed to dopaminergic drugs (n = 7), dystonia (n = 4), tremor (n = 3), nocturnal myoclonus (n = 2), poststroke spasticity (n = 2), and other abnormal movements (n = 3). In 13 cases, medical record review identified no clinical information compatible with any EPS diagnosis, and we assumed that the clinical encounter had been incorrectly coded. In 22 cases, we could find no indication that an EPS diagnosis had been made.
Figure 1. Flow of Patients Through the Study (2001 to 2014).
SNRI indicates selective norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
aSee the Study Population subsection of the Methods section for an explanation of extrapyramidal syndrome.
Of the 17 cases in which medical record review confirmed a clinical suspicion of serotonin syndrome, 4 met both the Sternbach and Hunter criteria, 2 satisfied the Sternbach criteria only, and 1 met the Hunter criteria only. Triptans had been used in close temporal relation to the development of symptoms that met the criteria for serotonin syndrome in only 2 of these cases. In both cases, however, some symptoms began before triptan ingestion. Detailed clinical information for each case is given in the eTable in the Supplement (cases 7 and 17).
Of importance, of these 17 cases, only 7 occurred during a year in which coprescription of triptans and SSRI or SNRI antidepressants was documented in the Longitudinal Medical Record medication list or in the corresponding clinical notes that we searched manually. Only these cases are included in our calculations of incidence rate given in the Table.
Table. Incidence of Serotonin Syndrome in Patients Receiving Triptans and Selective Serotonin Reuptake Inhibitors or Selective Norepinephrine Reuptake Inhibitorsa.
| Year | No. of Patients Receiving a Triptan Prescriptionb | No. (%) Exposed to Coprescription [95% CI] | No. of Definite Cases/Total No. of Casesb |
|---|---|---|---|
| 2001 | 1444 | 717 (49.6) [47.1-52.2] | 0/0 |
| 2002 | 2347 | 503 (21.4) [19.8-23.1] | 0/0 |
| 2003 | 2827 | 647 (22.9) [21.3-24.4] | 0/0 |
| 2004 | 3615 | 889 (24.6) [23.2-26.0] | 0/0 |
| 2005 | 4767 | 1230 (25.8) [24.6-27.1] | 0/0 |
| 2006 (FDA advisory) | 6941 | 1827 (26.3) [25.3-27.4] | 0/0 |
| 2007 | 8284 | 2163 (26.1) [25.2-27.1] | 0/0 |
| 2008 | 9132 | 2244 (24.6) [23.7-25.5] | 0/1 |
| 2009 | 9737 | 2326 (23.9) [23.1- 24.7] | 0/0 |
| 2010 | 10 288 | 2433 (23.6) [22.8-24.5] | 0/1 |
| 2011 | 11 566 | 2729 (23.6) [22.8-24.4] | 0/2 |
| 2012 | 14 397 | 3665 (25.5) [24.8-26.2] | 1/1 |
| 2013 | 16 833 | 4645 (27.6) [26.9-28.3] | 1/1 |
| 2014 | 17 353 | 4910 (28.3) [27.6-28.9] | 0/1 |
| Total | 119 531 | 30 928 (25.9) [25.6-26.1] | 2/7 |
Abbreviation: FDA, US Food and Drug Administration.
The incidence rate per 10 000 person-years was 0.6 (95% CI, 0-1.5) for definite cases and 2.3 (95% CI, 0.6-3.9) for total cases.
The total number of cases included definite and possible cases. Definite cases were those that met diagnostic criteria for serotonin syndrome with documented coprescriptions during the year of the event. Possible cases were those in which serotonin syndrome was suspected but did not meet diagnostic criteria, had insufficient information to apply diagnostic criteria, or in which triptan ingestion did not occur in temporal relation to the event but had documented coprescriptions during the year of the event.
The aforementioned data allow estimation of the risk of serotonin syndrome among patients who are coprescribed triptans and SSRI or SNRI antidepressants. A calculation of incidence rate gives a better estimate of the risk in relation to the total time of exposure to coprescription. Using a strict, conservative case definition gives a point estimate of 2 definite cases per 30 928 person-years or 0.6 cases per 10 000 person-years (95% CI, 0-1.5).
However, assuming that serotonin syndrome occurred in all patients in whom it was clinically suspected and with documented coprescription gives a point estimate of 7 cases, both definite and possible, per 30 928 person-years of exposure or 2.3 cases per 10 000 person-years (95% CI, 0.6-3.9). Thus, our estimates suggest that the incidence of serotonin syndrome among patients coprescribed triptans and SSRI or SNRI antidepressants ranged from 0 to 4 cases per 10 000 person-years of exposure.
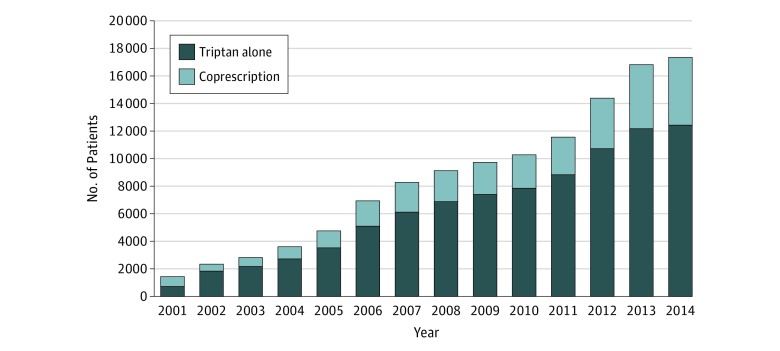
The number of patients receiving triptan prescriptions increased steadily during the study period (Figure 2). Except in 2001, the proportion of patients who were coprescribed an SSRI or SNRI antidepressant remained stable, with a mean of 26% (range, 21%-29%).
Figure 2. Number of Patients by Year Prescribed Triptan Alone or Coprescribed Triptan With a Selective Serotonin Reuptake Inhibitor (SSRI) or Selection Norepinephrine Reuptake Inhibitor (SNRI) .
Discussion
Serotonin syndrome is rare in patients who are coprescribed antidepressants and triptans. In this large population-based study with more than 30 000 person-years of exposure to coprescription of these drugs, we found no cases of life-threatening serotonin syndrome and no cases in which triptan use was unequivocally implicated as a cause. Careful medical record review identified only 2 credible cases of serotonin syndrome. These cases occurred in patients who satisfied diagnostic criteria for serotonin syndrome and ingested a triptan in temporal association with symptoms. Our findings are consistent with other smaller studies4,12 that found a low risk of serotonin syndrome associated with coprescription. In one such study,12 the incidence was estimated to be less than 0.03%, lower than the estimated incidence of serotonin syndrome attributable to SSRIs alone.
Our results provide additional reasons to be skeptical that triptans increase the risk of serotonin syndrome beyond the risk already associated with SSRI and SNRIs alone. SSRI and SNRI antidepressants are more firmly established as a cause of serotonin syndrome. In one study,13 serotonin syndrome was diagnosed in 14% of patients with SSRI overdose. In patients taking the SSRI antidepressant nefazodone, the incidence of serotonin syndrome was estimated to be 0.5 to 0.9 cases per 1000 patient-months of treatment.14
In contrast, the biological plausibility of triptans as a cause of serotonin syndrome has been questioned. Evidence suggests that serotonin syndrome is mediated by serotonin 2A receptors, with possible involvement of serotonin 1A receptors. Triptans, however, are serotonin agonists with high affinity at serotonin 1B and 1D receptors and only low affinity for serotonin 1A receptors.15,16
Most of the 17 patients in our study with possible serotonin syndrome were taking a large number of other medications. It seems possible that symptoms were attributable to the effects of those other drugs and not triptans. Acute dystonic reactions, akathisia, or drug-induced tremors are not rare in patients who are receiving treatment for migraine that includes phenothiazines or neuroleptic drugs for migraine-associated nausea or pain. Less common neuroleptic-related problems, such as drug-induced parkinsonism or neuroleptic malignant syndrome, also can occur. All these conditions produce symptoms that can be confused with serotonin syndrome. In patients with comorbid mood disorders who are taking SSRI or SNRI antidepressants daily, those drugs alone are a more plausible cause of serotonin syndrome than intermittent use of triptans.
We noted no change in prescribing patterns during the study. After the 2006 advisory, there was no decrease in triptan prescriptions and no major change in the proportion of patients receiving coprescriptions. Instead, during the study, the number of patients receiving a triptan prescription increased 12-fold, whereas the number receiving coprescriptions increased almost 6-fold (Table). Despite these increases, there was no corresponding increase in the incidence of serotonin syndrome. Approximately one-quarter of patients treated with triptans received coprescriptions for SSRI or SNRI antidepressants, a figure that is consistent with the estimate of a previous study12 in which the prevalence of coprescription was 21%.
Strengths and Limitations
Our study has a number of strengths. We had access to high-quality information about a large population of patients during a long period. We performed a careful review of the clinical documentation in all possible cases of serotonin syndrome. To evaluate the effect of diagnostic uncertainty on risk estimates and provide a credible estimate of the range of risk, we performed our calculations using an inclusive and strict definition of possible cases.
Our study also has a number of limitations. The quality of medical documentation was highly variable. We often had to infer diagnostic criteria based on imprecise descriptions of symptoms or physical examination findings. We cannot exclude the possibility that clinicians who did not recognize serotonin syndrome might have used other diagnostic codes that were not included in our search. We encountered several cases in which diagnostic codes did not match the clinical data in a specific medical record, suggesting that coding errors had occurred. We also might have missed milder cases of serotonin syndrome that were not brought to medical attention or cases in which symptoms resolved by the time patients were seen. Most triptans have a short half-life of several hours, and the symptoms of serotonin syndrome might thus be short-lived. In formulating diagnostic criteria for the disorder, both the Sternbach criteria and the Hunter Area Toxicology Service criteria recognized that serotonin syndrome exists on a spectrum of severity and is not always easily diagnosed.10,11,17
It is possible that some patients were prescribed triptans or SSRI and SNRI medications outside the Partners HealthCare Network or via telephone prescriptions that were not subsequently logged in our system. We think that this was unlikely to have happened frequently enough to materially alter our findings, especially because several systems exist to capture such medications in the medical record at subsequent visits.
A final important limitation is that our estimate of person-time is imprecise. It was not practical to directly calculate person-time for each patient who received coprescriptions; thus, we assumed that individuals were at risk for serotonin syndrome for the entire calendar year during which coprescription occurred. This misallocation of exposure time might result in overestimation or underestimation of risk.
Conclusions
Our study provides a quantitative estimate of the risk of serotonin syndrome. This estimate is useful for physicians and patients who want to understand the balance of benefits and harms of concomitant treatment of coexisting migraine and depression. Overall, our results are reassuring and suggest that patients with coexisting affective disorders and migraine need not forgo management of one condition to treat the other. Our results do not show major changes in prescribing patterns as a result of the FDA advisory. Taken as a whole, our data suggest that the FDA advisory should be reconsidered.
eTable. Clinical Details of 17 Cases of Clinically Suspected Serotonin Syndrome
References
- 1.Research Center for Drug Evaluation and Drug Safety Information for Healthcare Professionals Information for Healthcare Professionals: Selective Serotonin Reuptake Inhibitors (SSRIs), Selective Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), 5-Hydroxytryptamine Receptor Agonists (Triptans). https://wayback.archive-it.org/7993/20170406044818/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085845.htm. Accessed June 14, 2017.
- 2.Soldin OP, Tonning JM; Obstetric-Fetal Pharmacology Research Unit Network . Serotonin syndrome associated with triptan monotherapy. N Engl J Med. 2008;358(20):2185-2186. [DOI] [PubMed] [Google Scholar]
- 3.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120. [DOI] [PubMed] [Google Scholar]
- 4.Evans RW. The FDA alert on serotonin syndrome with combined use of SSRIs or SNRIs and triptans: an analysis of the 29 case reports. MedGenMed. 2007;9(3):48. [PMC free article] [PubMed] [Google Scholar]
- 5.Evans RW. More on serotonin syndrome associated with triptan monotherapy. N Engl J Med. 2008;359(8):870. [DOI] [PubMed] [Google Scholar]
- 6.Evans RW, Tepper SJ, Shapiro RE, Sun-Edelstein C, Tietjen GE. The FDA alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American Headache Society position paper. Headache. 2010;50(6):1089-1099. [DOI] [PubMed] [Google Scholar]
- 7.Tepper S, Allen C, Sanders D, Greene A, Boccuzzi S. Coprescription of triptans with potentially interacting medications: a cohort study involving 240,268 patients. Headache. 2003;43(1):44-48. [DOI] [PubMed] [Google Scholar]
- 8.Sclar DA, Robison LM, Castillo LV, et al. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52(2):198-203. [DOI] [PubMed] [Google Scholar]
- 9.Kogut SJ. Do triptan antimigraine medications interact with SSRI/SNRI antidepressants? what does your decision support system say? J Manag Care Pharm. 2011;17(7):547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):705-713. [DOI] [PubMed] [Google Scholar]
- 11.Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro RE, Tepper SJ. The serotonin syndrome, triptans, and the potential for drug-drug interactions. Headache. 2007;47(2):266-269. [DOI] [PubMed] [Google Scholar]
- 13.Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42(3):277-285. [DOI] [PubMed] [Google Scholar]
- 14.Mackay FJ, Dunn NR, Mann RD. Antidepressants and the serotonin syndrome in general practice. Br J Gen Pract. 1999;49(448):871-874. [PMC free article] [PubMed] [Google Scholar]
- 15.Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol. 2005;28(5):205-214. [DOI] [PubMed] [Google Scholar]
- 16.Pauwels PJ, Palmier C, Dupuis DS, Colpaert FC. Interaction of 5-HT1B/D ligands with recombinant H5-HT1A receptors: intrinsic activity and modulation by G-protein activation state. Naunyn Schmiedebergs Arch Pharmacol. 1998;357(5):490-499. [DOI] [PubMed] [Google Scholar]
- 17.Gillman PK. A review of serotonin toxicity data: implications for the mechanisms of antidepressant drug action. Biol Psychiatry. 2006;59(11):1046-1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Clinical Details of 17 Cases of Clinically Suspected Serotonin Syndrome