Key Points
Question
What is the effect of a peer navigation intervention on viral suppression of human immunodeficiency virus (HIV) among people living with HIV released from jail?
Findings
In the LINK LA randomized clinical trial of peer navigation that included 356 men and transgender women leaving Los Angeles County Jail, the adjusted probability of viral suppression among controls declined from 52% at baseline to 30%, while the LINK LA intervention group maintained viral suppression at 49% from baseline to 12 months, for a significant difference-in-difference of 22%.
Meaning
The LINK LA peer navigation intervention prevented the declines in viral suppression observed in standard care and typically seen after release from incarceration.
Abstract
Importance
Diagnosis of human immunodeficiency virus (HIV) infection, linkage and retention in care, and adherence to antiretroviral therapy are steps in the care continuum enabling consistent viral suppression for people living with HIV, extending longevity and preventing further transmission. While incarcerated, people living with HIV receive antiretroviral therapy and achieve viral suppression more consistently than after they are released. No interventions have shown sustained viral suppression after jail release.
Objective
To test the effect on viral suppression in released inmates of the manualized LINK LA (Linking Inmates to Care in Los Angeles) peer navigation intervention compared with standard transitional case management controls.
Design, Setting, and Participants
Randomized clinical trial conducted from December 2012 through October 2016 with people living with HIV being released from Los Angeles (LA) County Jail. All participants were (1) 18 years or older; (2) either men or transgender women diagnosed with HIV; (3) English speaking; (4) selected for the transitional case management program prior to enrollment; (5) residing in LA County; and (6) eligible for antiretroviral therapy.
Main Outcomes and Measures
Change in HIV viral suppression (<75 copies/mL) over a 12-month period.
Interventions
During the 12-session, 24-week LINK LA Peer Navigation intervention, trained peer navigators counseled participants on goal setting and problem solving around barriers to HIV care and adherence, starting while the participants were still in jail. After their release, they continued counseling while they accompanied participants to 2 HIV care visits, then facilitated communication with clinicians during visits.
Results
Of 356 participants randomized, 151 (42%) were black; 110 (31%) were Latino; 303 (85%) were men; 53 (15%) were transgender women; and the mean (SD) age was 39.5 (10.4) years. At 12 months, viral suppression was achieved by 62 (49.6%) of 125 participants in the peer navigation (intervention) arm compared with 45 (36.0%) of 125 in the transitional case management (control) arm, for an unadjusted treatment difference of 13.6% (95% CI, 1.34%-25.9%; P = .03). In the repeated measures, random effects, logistic model the adjusted probability of viral suppression declined from 52% at baseline to 30% among controls, while those in the peer navigation arm maintained viral suppression at 49% from baseline to 12 months, for a difference-in-difference of 22% (95% CI, 0.03-0.41; P = .02).
Conclusions and Relevance
The LINK LA peer navigation intervention was successful at preventing declines in viral suppression, typically seen after release from incarceration, compared with standard transitional case management. Future research should examine ways to strengthen the intervention to increase viral suppression above baseline levels.
Trial Registration
clinicaltrials.gov Identifier: NCT01406626
This randomized clinical trial tests the effect of the manualized LINK LA peer navigation intervention vs standard transitional case management on HIV viral suppression among released inmates with HIV.
Introduction
The human immunodeficiency virus (HIV) care continuum has emerged as the leading paradigm for controlling the HIV epidemic, as linkage to care, retention in care, and viral suppression have both individual and public health benefits. Approximately 1 in 7 HIV-positive persons pass through US corrections annually, and HIV prevalence among incarcerated persons is 3 to 5 times that of the general population. While incarcerated, people living with HIV experience highly structured environments and access to health care, including antiretroviral therapy. They often achieve viral suppression while incarcerated despite lacking HIV care before entry. After release from incarceration, many fail to link to care soon enough or long enough to sustain viral suppression. An observational study found that only 30% had filled antiretroviral prescriptions 60 days after release from Texas prisons. A recent randomized clinical trial of a multi-component intervention among HIV-positive persons leaving 2 southern state prisons showed no improvement in viral suppression at 6 months after release. There are no known randomized trials of any interventions shown to sustain viral suppression over 12 months after jail release.
We conducted a randomized clinical trial called Linking Inmates to Care in LA (Los Angeles) (or “LINK LA”), a peer navigation intervention among HIV-positive men and transgender women released from a large municipal jail system to evaluate its effects on viral suppression. The trial protocol from the application for funding is provided in Supplement 1. The concept of peer navigation is rooted in patient navigation—the direct assistance provided to help low-income, vulnerable patients find their way through complex health care systems to obtain timely diagnosis and treatment. While patient navigators may include professionals such as case managers, we adopted a model in which peer navigators were strictly lay staff members, who could be considered peers of the participants, to promote trust with this often stigmatized population.
In designing LINK LA, we hypothesized that peer navigation would improve viral suppression compared with transitional case management over 12 months. We also hypothesized that the intervention would be more effective among nonusers of substances (opiates, stimulants, and binge alcohol) than among users.
Methods
Setting
This study was conducted from December 2012 through October 2016 among inmates being released from LA County Jail, the largest jail system in the United States. With an estimated 62 000 people living with HIV, LA County is the second largest epicenter of the US epidemic after New York City. The study was approved by the University of California, Los Angeles Institutional Review Board, and all participants provided their written informed consent.
Study Design
LINK LA was a 2-group randomized trial: the intervention group participated in a 12-session, 24-week peer navigation intervention, while the control group followed the standard transitional case management protocol. The study had a preplanned target sample size of 356 participants. Assuming 30% attrition, and a final sample of 250 participants at 12 months (125 per arm), in simulations we had 80% power (α = .05) to detect a minimal difference of 17% viral suppression between arms when controls had 50% viral suppression, and a 15% minimal difference when controls had 30% viral suppression.
Eligibility and Participant Flow
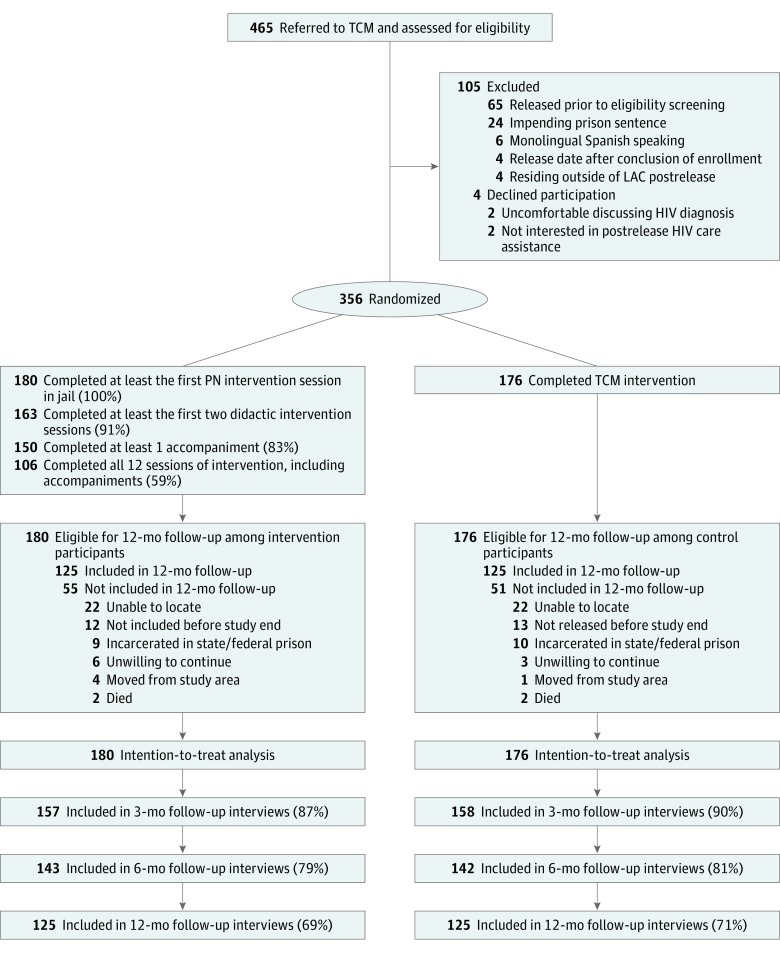
Routinely, inmates were screened at intake and referred for medical and transitional case management services if they tested positive for HIV. All participants were (1) 18 years or older; (2) either men or transgender women diagnosed with HIV; (3) English speaking; (4) selected for the transitional case management program prior to enrollment; (5) residing in LA County; and (6) eligible for antiretroviral therapy or incarcerated while undergoing antiretroviral therapy. Exclusion criteria were (1) inability to give informed consent; (2) planned transfer to prison; and/or (3) a stay in jail of less than 5 days. Of 465 potentially eligible persons, 105 ultimately were not eligible, and 4 declined. The final sample included 356 participants (Figure). All 180 participants randomized to the peer navigation intervention completed the first session in jail; 91% completed at least the first 2 didactic intervention sessions (n = 163); 83% completed at least 1 of the 2 accompaniment sessions to medical care (n = 150); and 59% completed all 12 sessions of LINK LA (n = 106), including 2 accompaniments (Figure).
Figure. Study Enrollment and Progress Flowchart for the LINK LA Trial.
HIV indicates human immunodeficiency virus; LAC, Los Angeles County; LINK LA, Linking Inmates to Care in Los Angeles; TCM, transitional case management; PN, peer navigation.
Enrollment, Randomization, and Blinding
After recruitment, staff members obtained written informed consent and conducted the baseline interview. Then, using sequentially numbered envelopes, we randomized the participants in a 1:1 ratio to the peer navigation intervention or transitional case management control group using computer-generated, randomly permuted blocks of 4 and 6 to prevent anticipation of assignment to study condition. To prevent contamination, peer navigators interacted with participants only in the intervention arm and did not share intervention materials with controls. We provided basic cell phones with text functions to all participants to arrange data collection, promote study retention, and facilitate intervention activities.
Peer Navigation Intervention
We developed the manualized LINK LA peer navigation intervention using a conceptual model adapted from social cognitive theory, as applied to the continuum of HIV care. We trained lay peer navigators to act as role models who could walk participants through the continuum steps: (1) linkage or reengagement, (2) retention, and (3) antiretroviral therapy adherence. The peer navigation intervention addresses social-environmental factors by promoting social support and trusting relationships with peer navigators and clinicians. LINK LA teaches skills to overcome social stigma and discrimination and to facilitate access to care through appointment scheduling, reminders, transportation assistance, accompaniment to medical and other health care appointments, and assistance with meeting competing subsistence needs. The intervention addresses personal factors by emphasizing the importance of retention in care and antiretroviral therapy adherence and advancing the knowledge and skills necessary for engaging these activities. The intervention supports behavioral factors related to HIV care and adherence by promoting self-efficacy, positive health expectations, and goal-setting and problem-solving behaviors.
Before the participants were released from jail, peer navigators delivered the intervention content during 1- to 2-hour sessions in person in a private conference room. After the participants were released, the navigators conducted the sessions in community settings. They initiated relationships with participants before they left jail, met them at the time of release, held sessions in private community settings, then counseled and modeled retention and adherence behaviors during accompaniment to 2 scheduled HIV medical care appointments up to 24 weeks after release. Navigators imparted the manualized content in conversational format in each session (eTable 1 in Supplement 2). During accompaniment, navigators prepared participants for clinician visits, then helped answer questions, took notes during the visit, and facilitated recommendations following visits.
Peer Navigator Selection, Training, and Supervision
Peer navigators were either black or Latino (1 black woman, 1 Latina woman, 1 Latino man, and 2 black men) and were selected for having experiences in common with incarcerated people living with HIV (such as prior incarceration, being a patient retained in HIV care, and/or prior substance abuse recovery). Peer navigators completed a comprehensive, 1-week training regimen prior to field work, using a detailed manual of operations. To address fidelity, navigators received daily monitoring, weekly supervision, and periodic auditing of records. Navigators completed a checklist documenting delivery of each session. We also held unannounced field visits to observe select intervention sessions to assure fidelity. We rated intervention fidelity on a 3-point scale with 1 representing high and 3, low fidelity. We found a mean (SD) overall fidelity rating of 1.6 (0.8).
Control Intervention
The control arm was the standard of care, which is transitional case management. All known people living with HIV in LA County Jail received transitional case management needs assessments. Transitional case management arm participants also received referrals for postrelease housing, substance abuse treatment, and HIV care from case managers. Peer navigators provided these referrals to peer navigator arm participants.
Data Collection
We conducted face-to-face interviews at baseline before release, and at 3, 6, and 12 months after release. At baseline, we collected electronic medical record data on HIV viral load. At months 3 and 12, we collected blood samples for viral load and analyzed it using RealTime HIV-1 assays (Abbott Laboratories). Participants were paid $25 for participation in the baseline interview, $50 each for 3- and 6-month interviews, and $75 for 12-month interviews plus blood samples.
Outcome Measures
The prespecified primary outcome was viral suppression, defined as undetectable viral load (<75 copies/mL). We supplemented viral load testing with data from the LA County Department of Public Health Casewatch system whenever interviewed participants were not available for testing when due. Casewatch maintains electronic record data for Ryan White–funded clinics where most (n = 313) of the participants were seen after their release. Blinded to arm, we selected viral load values closest to the scheduled data collection time points, within 30 days plus or minus scheduled time points at 3 months and 12 months after release. These values were used for 6 (2%) of the 315 3-month follow-ups and 23 (9%) of the 250 12-month follow-ups. The proportion of Casewatch values used did not differ significantly by arm.
Secondary outcome measures at each follow-up (collected using timeline follow-back) included participant-reported information on the following: linkage to HIV care after release (probability of HIV primary care visits); retention in HIV care (number of HIV primary care visits, given linkage); antiretroviral use and adherence; retention and adherence knowledge (10-item scale); physical and mental health as assessed by the 12-item short-form health survey (SF-12); and the numbers of specialty visits, mental health visits, case management visits, medication-assisted treatment visits, psychiatric hospital nights, emergency department visits, and days of substance use in the prior 30 days. We validated participant-reported visit data by electronic Casewatch visit records (eTable 2 in Supplement 2).
Analysis
We examined the effect of the peer navigation intervention on viral suppression using intention-to-treat, generalized linear mixed models for longitudinally measured data. All models included random intercepts for participants and used a logistic link for viral suppression and all binary outcomes; linear link, normal models for continuous outcomes; and zero-inflated Poisson models for HIV primary care visits (retention) and all count data outcomes. Adjusted probabilities of viral suppression were estimated at baseline, months 3 and 12 for the intervention and control arms, and differences in probabilities were estimated between arms, over time within arms, and difference-in-differences over 12 months (with 95% confidence intervals [CIs]). These models accommodate data missing at random; predictors include intervention arm, categorical time and intervention*time interactions (see the statistical eAppendix in Supplement 2). Finally, we modeled the effect of peer navigation on linkage to care as the binomial probability of at least 1 visit from baseline to 3, 6, and 12 months, by arm, and differences between arms (including 95% CIs).
To examine the heterogeneity of intervention effects, we explored potential effect modifiers of age, race/ethnicity, risk/gender group, education, income, insurance, substance use, SF-12 physical and mental health scores, and CD4 count, with all main effects, 2-way and 3-way interactions for intervention, time, and moderator predictors (including 95% CIs).
Results
Sample Characteristics
Among 465 screened inmates, 356 (78%) were eligible and randomized; 250 (70%) completed the 12-month interview (Figure). At baseline, the participants reported a mean (SD) age of 39.5 (10.4) years; 57% (n = 201) reported being men who have sex with men; 15% (n = 53), male to female transgender status; 42% (n = 150), an annual income of $10 000 or less; 51% (n = 180), CD4 counts lower than 500 cells/mm3; and 56% (n = 199) no insurance (Table 1). Recent use of substances (opiates, stimulants, and binge alcohol) was prevalent (78%; n = 277), and mean SF-12 mental health was a standard deviation below the US national norm. About half of the participants were virally suppressed (n = 184, 52%; 95% CI, 46%-57%).
Table 1. Baseline Characteristics of the Study Participants.
| Characteristicsa | Study Participants, No. (%) | P Valueb | ||
|---|---|---|---|---|
| Intervention (n = 180) |
Control (n = 176) |
Total (n = 356) |
||
| Age, y | .34 | |||
| 18-34 | 73 (41) | 64 (36) | 137 (39) | |
| 35-49 | 78 (43) | 73 (42) | 151 (42) | |
| ≥50 | 29 (16) | 39 (22) | 68 (19) | |
| Race/ethnicity | .58 | |||
| White, non-Hispanic | 47 (26) | 48 (27) | 95 (27) | |
| Black, non-Hispanic | 81 (45) | 70 (40) | 151 (42) | |
| Hispanic | 52 (29) | 58 (33) | 110 (31) | |
| HIV risk group/gender | .21 | |||
| MSM contact | 93 (52) | 108 (61) | 201 (57) | |
| Male heterosexual contact | 36 (20) | 23 (13) | 59 (17) | |
| Male to female transgender | 27 (15) | 26 (15) | 53 (15) | |
| Male IV drug use | 24 (13) | 19 (11) | 43 (12) | |
| Educational attainment (n = 355) | .15 | |||
| Less than high school | 75 (42) | 56 (32) | 131 (37) | |
| High school or GED | 47 (26) | 49 (28) | 96 (27) | |
| Some college or more | 58 (32) | 70 (40) | 128 (36) | |
| Annual household income, $ (n = 355) | .57 | |||
| ≤10 000 | 76 (42) | 74 (42) | 150 (42) | |
| 10 001-20 000 | 45 (25) | 51 (29) | 96 (27) | |
| 20 001-30 000 | 20 (11) | 18 (10) | 38 (11) | |
| 30 001-50 000 | 10 (6) | 13 (7) | 23 (7) | |
| ≥50 001 | 29 (16) | 19 (11) | 48 (14) | |
| Uninsuredc | 109 (61) | 90 (51) | 199 (56) | .07 |
| Substance use | ||||
| Binge alcohol (n = 88)d | 42 (23) | 46 (26) | 88 (25) | .54 |
| Heroin (n = 24) | 15 (8) | 9 (5) | 24 (7) | .23 |
| Crack (n = 47) | 18 (10) | 29 (17) | 47 (13) | .07 |
| Cocaine (n = 23) | 10 (6) | 13 (7) | 23 (7) | .48 |
| Methamphetamine (n = 206) | 104 (58) | 102 (58) | 206 (58) | .97 |
| Oxycodone or other opiates (n = 15) | 10 (6) | 5 (3) | 15 (4) | .20 |
| Binge alcohold and hard drug usee | 135 (75) | 142 (81) | 277 (78) | .20 |
| Other substances (n = 265)f | 131 (73) | 134 (76) | 265 (74) | .47 |
| Length of stay in jail, mean (SD), wk (n = 153) | 33 (30) | 32 (25) | 33 (27) | .87 |
| SF-12 mental health, mean (SD)g | 39 (13) | 38 (12) | 38 (12) | .31 |
| SF-12 physical health, mean (SD)g | 51 (10) | 51 (10) | 51 (10) | .97 |
| CD4 count, cells/mm3 (n = 355) | .85 | |||
| <200 | 21 (12) | 17 (10) | 38 (11) | |
| 200-349 | 25 (14) | 29 (17) | 54 (15) | |
| 350-499 | 44 (24) | 44 (25) | 88 (25) | |
| ≥500 | 90 (50) | 85 (49) | 175 (49) | |
| Virally suppressedh | 90 (50) | 94 (53) | 184 (52) | .60 |
| Antiretroviral therapy | ||||
| Ever prescribed (n = 356) | 159 (88) | 159 (90) | 318 (89) | .54 |
| Currently using (n = 318) | 147 (92) | 149 (94) | 296 (93) | .66 |
| ART adherence (VAS), mean (SD)i (n = 269) | 86 (24) | 82 (26) | 84 (25) | .36 |
| Preincarceration utilization measuresj | ||||
| HIV primary care visits (n = 350) | 27 (15) | 24 (14) | 51 (15) | .75 |
| Specialty care visits (n = 351) | 22 (12) | 26 (15) | 48 (14) | .47 |
| Mental health visits (n = 352) | 58 (33) | 54 (31) | 112 (32) | .76 |
| Case manager visits (n = 350) | 71 (40) | 63 (37) | 134 (38) | .53 |
| MAT visits (n = 352)k | 44 (25) | 46 (26) | 90 (26) | .71 |
| Psychiatric hospital nights (n = 351) | 47 (26) | 51 (29) | 98 (28) | .52 |
| ED visits (n = 351) | 87 (49) | 85 (49) | 172 (49) | .96 |
Abbreviations: ART, antiretroviral therapy; ED, emergency department; GED, general equivalency diploma; HIV, human immunodeficiency virus; IV, intravenous; MAT, medication assisted treatment; MSM, men who have sex with men; SF-12, 12-item short-form health survey; VAS, visual analogue scale.
Data are reported as the total number of participants with nonmissing data on each item, generally n = 356; however, in categories where data were missing, the total number of participants for whom data were available is reported after each category name.
Equality of the difference in proportions between study arms at baseline tested using the χ2 test.
Insured category (reference group) includes participants with private insurance (5%, n = 19), Medicaid/Medi-Cal (22%, n = 77), or other public insurance (17%, n = 61).
Binge alcohol is defined as 5 or more alcoholic drinks at a time.
Hard drugs include heroin, crack cocaine, powder cocaine, methamphetamines, and/or oxycodone or other opiates.
Other substances include marijuana, barbiturates, valium, or other sedative-hypnotics, ecstasy, LSD or other hallucinogens, and any alcohol.
SF-12 Mental Component and Physical Component Scores (T-scores, normed to US general population: mean (SD) score, 50 (10).
Defined as undetectable viral load, based on the laboratory limit of detection ≤75 copies/mL.
ART adherence is defined as the mean percentage of ART doses taken, among those prescribed and using ART, measured using a VAS.
Included are participants who reported at least 1 visit of the specified type in the 12 months prior to incarceration, measured by timeline follow-back.
MAT visits include those for prescription medications such as methadone or buprenorphine to treat addiction to drugs or alcohol.
Effects of Peer Navigation on Viral Suppression
At 12 months, 62 (49.6%) of 125 participants had achieved viral suppression in the peer navigation arm compared with 45 (36.0%) of 125 in the transitional case management arm, for an unadjusted treatment difference of 13.6% (95% CI, 1.3%-25.9%; P = .03). In the repeated measures analysis, the peer navigation arm’s adjusted probabilities of viral suppression did not change from 49% at baseline to 49% at month 12, while it declined from 52% at baseline to 30% at 12 months in the transitional case management arm (Table 2). Thus, the difference-in-difference of viral suppression probability over 12 months was 22% (95% CI, 3%-41%; P = .02).
Table 2. Effect of the LINK LA Intervention on Viral Suppressiona After Jail Release.
| Measurement Time | No./No. (Probabilityb) | Probability Difference (95% CI)c | P Valued | |
|---|---|---|---|---|
| Intervention | Control | |||
| Baseline (n = 356) | 88/180 (0.49) | 91/176 (0.52) | −0.04 (−0.18 to 0.10) | .60 |
| 3 Months (n = 315) | 82/157 (0.53) | 63/158 (0.37) | 0.16 (0.01 to 0.31) | .03 |
| Change at 3 months (95% CI)c,e | 0.04 (−0.08 to 0.17) | −0.16 (−0.28 to −0.03)f | 0.20 (0.02 to 0.38) | .02 |
| 12 Months (n = 250) | 62/125 (0.49) | 45/125 (0.30) | 0.18 (0.02 to 0.40) | .03 |
| Change at 12 months (95% CI)c,e | 0.003 (−0.130 to 0.140) | −0.22 (−0.35 to −0.09)g | 0.22 (0.03 to 0.41) | .02 |
Abbreviations: GLMM, generalized linear mixed model; LINK LA, Linking Inmates to Care in Los Angeles.
Viral suppression is measured as the probability of having an undetectable viral load (<75 copies/mL) at each follow-up session by study arm using a repeated measures, random-intercept, logistic model (a GLMM) with predictors of study arm, categorical time, and intervention × time interaction. The model accommodates data missing at random, and loss to follow-up was not different between study arms.
Probability is not necessarily equal to the numerator divided by the denominator because the probabilities are estimated from the random-intercept, logistic GLMMs.
Intervention arm value minus control arm value; probability differences are not always precise totals owing to rounding.
P value for test between intervention and control arm values at each follow-up period and difference-in-difference tested for change from baseline by study arm, based on the single random-intercept, logistic GLMM.
Follow-up interview value minus baseline value.
P < .05 for change from baseline.
P < .01 for change from baseline.
Secondary Outcomes
There was no significant difference between arms in the probability of having at least 1 postrelease HIV primary care visit (linkage) at 12 months, but the probability of linkage was greater in the peer navigation arm at 6 months (difference, 12%; 95% CI, 4%-22%; P = .01; Table 3). Among those with at least 1 visit, there was a greater increase from baseline in the number of visits per year (retention) since release in the peer navigation arm than in the control arm over 12 months (difference, 0.71; 95% CI, 0.01-1.40; P = .047) (Table 3). While the intervention improved retention and adherence knowledge at 12 months, more than 90% of participants (n = 296 of 321) reported currently using antiretroviral therapy in both arms and all follow-up periods (Table 4). Similarly, mean self-reported adherence exceeded 80% in both arms and all follow-up periods. Peer navigation arm participants reported a greater increase in number per year of mental health visits at 3 months (21.6; 95% CI, 12.2-31.0; P < .001) and 6 months (13.6; 95% CI, 7.0-20.2; P < .001), case management visits at 3 months (10.0; 95% CI, 4.3-15.8; P < .001) and 6 months (14.3; 95% CI, 9.9-18.7; P < .001), medication-assisted treatment visits at 6 months (2.4; 95% CI, 0.02-4.8; P = .048), psychiatric hospital nights at 3 months (125.3; 95% CI, 36.4-214.2; P = .01); and they had fewer emergency department visits at 3 months (−1.2; 95% CI, −2.0 to −0.3; P = .01) and 6 months (−0.5; 95% CI, −0.9 to −0.1; P = .005). Control arm participants had a greater increase in the number of specialty visits at 3 months and medication-assisted treatment visits at 12 months. There was no effect on reported substance use, although use declined substantially in both arms, particularly at 3 months, and remained lower than baseline at 12 months (eTable 3 in Supplement 2).
Table 3. Effect of the LINK LA Intervention on Linkage to and Retention in HIV Primary Care After Jail Releasea.
| Measurement Time | Intervention (95% CI) (n = 180) |
Control (95% CI) (n = 176) |
Difference (95% CI)b,c | P Valued |
|---|---|---|---|---|
| Linkage to HIV Caree | ||||
| 0-3 Months (n = 312) | 0.64 (0.57 to 0.72) | 0.63 (0.56 to 0.71) | 0.01 (−0.09 to 0.12) | .81 |
| 0-6 Months (n = 260) | 0.89 (0.84 to 0.95) | 0.77 (0.69 to 0.84) | 0.12 (0.04 to 0.22) | .01 |
| 0-12 Months (n = 220) | 0.92 (0.87 to 0.97) | 0.88 (0.82 to 0.94) | 0.04 (−0.04 to 0.12) | .32 |
| Retention in HIV Caref | ||||
| Baseline (n = 350) | 1.64 (1.29 to 2.00) | 2.26 (1.74 to 2.77) | −0.61 (−1.24 to 0.01)g | .054 |
| 3 Months (n = 312) | 3.08 (2.56 to 3.61) | 3.04 (2.52 to 3.55) | 0.04 (−0.69 to 0.77) | .90 |
| Change at 3 months (n = 307)a,c | 1.44 (0.89 to 1.98)h | 0.78 (0.18 to 1.38)g | 0.66 (−0.15 to 1.47) | .11 |
| 6 Months (n = 261) | 2.15 (1.79 to 2.50) | 2.15 (1.75 to 2.55) | −0.001 (−0.54 to 0.53) | >.99 |
| Change at 6 months (n = 256)a,c | 0.50 (0.10 to 0.90)g | −0.11 (−0.66 to 0.44) | 0.61 (−0.07 to 1.30) | .08 |
| 12 Months (n = 235) | 2.25 (1.87 to 2.64) | 2.16 (1.79 to 2.53) | 0.09 (−0.44 to 0.62) | .73 |
| Change at 12 months (n = 232)a,c | 0.61 (0.17 to 1.06)g | −0.10 (−0.63 to 0.44) | 0.71 (0.01 to 1.40)g | .047 |
Abbreviations: GLMM, generalized linear mixed model; HIV, human immunodeficiency virus; LINK LA, Linking Inmates to Care in Los Angeles; ZIP, zero-inflated Poisson.
Follow-up interview value minus baseline value.
Intervention arm estimate minus control arm estimate.
Difference and change values are not always precise totals owing to rounding.
P value for test between intervention and control arm values at each measurement time and difference-in-difference tested for change from baseline by study arm, based on a single regression model.
Probability of having HIV primary care visits from baseline (0 months) to each measurement time at 3, 6, and 12 months after release from incarceration by arm, and difference (with 95% CIs) estimated using unadjusted binomial model.
Estimated number of HIV primary care visits over the preceding 3 months, given linkage to care (at least 1 visit), using a repeated measures, random-intercept, ZIP model (a GLMM) with predictors of intervention arm, categorical time, and intervention × time interaction. The model accommodates data missing at random, and loss to follow-up was not different between study arms. ZIP models had 2 uncorrelated random effects, one for the zero-inflation component and the other for the Poisson component. All values are estimated per-12-month rate, given at least 1 visit (or hospital night) in the previous 12 months.
P < .05 for change from baseline.
P < .001 for change from baseline
Table 4. Effect of the LINK LA Intervention on Secondary Outcomes After Jail Releasea.
| Measurement Time | No./Total No.; Probability (95% CI) | Difference (95% CI)b | P Valuec | |
|---|---|---|---|---|
| Intervention | Control | |||
| Current ART Used | ||||
| Baseline (n = 321) | 147/161; 0.98 (0.96 to 1.00) | 149/160; 0.99 (0.97 to 1.00) | −0.01 (−0.03 to 0.01) | .49 |
| 3 Months (n = 315) | 128/157; 0.94 (0.90 to 0.99) | 130/158; 0.95 (0.92 to 0.99) | −0.01 (−0.06 to 0.04) | .69 |
| Change at 3 monthsb,e | −0.040 (−0.070 to 0.001) | −0.0300 (−0.0700 to −0.0002)f | −0.003 (−0.050 to 0.050) | .90 |
| 6 Months (n = 285) | 116/143; 0.94 (0.89 to 0.98) | 111/142; 0.92 (0.86 to 0.98) | 0.02 (−0.05 to 0.08) | .65 |
| Change at 6 monthsb,e | −0.0400 (−0.0800 to 0.0001)f | −0.07 (−0.12 to −0.01) | 0.02 (−0.04 to 0.09) | .49 |
| 12 Months (n = 250) | 104/125; 0.95 (0.91 to 0.99) | 107/125; 0.96 (0.93 to 0.996) | −0.01 (−0.06 to 0.03) | .60 |
| Change at 12 monthsb,e | −0.03 (−0.07 to 0.01) | −0.02 (−0.05 to 0.01) | −0.01 (−0.05 to 0.04) | .82 |
| Adherence to ARTg,h | ||||
| Baseline (n = 354) | (98) 85.7 (80.8 to 90.7) | (109) 81.6 (76.9 to 86.3) | 4.1 (−2.7 to 10.9) | .54 |
| 3 Months (n = 315) | (116) 85.1 (80.7 to 89.5) | (121) 82.6 (78.3 to 86.9) | 2.5 (−3.6 to 8.7) | .40 |
| Change at 3 monthsb,e | −0.6 (−7.3 to 6.0) | 0.95 (−5.5 to 7.4) | −1.6 (−10.8 to 7.7) | .38 |
| 6 Months (n = 285) | (110) 88.4 (84.5 to 92.3) | (101) 86.1 (82.0 to 90.1) | 2.4 (−3.3 to 8.0) | .20 |
| Change at 6 monthsb,e | 2.7 (−3.4 to 8.8) | 4.5 (−1.5 to 1.4) | −1.8 (−10.3 to 6.7) | .14 |
| 12 Months (n = 250) | (96) 86.7 (82.4 to 91.1) | (101) 85.4 (81.2 to 89.7) | 1.3 (−4.8 to 7.4) | .50 |
| Change at 12 monthsb,e | 1.0 (−5.4 to 7.4) | 3.8 (−2.3 to 9.9) | −2.8 (−11.6 to 6.1) | .20 |
| Retention and Adherence Knowledgeh,i | ||||
| Baseline (n = 354) | (176) 8.9 (8.7 to 9.0) | (176) 9.0 (8.8 to 9.1) | −0.1 (−0.3 to 0.1) | .35 |
| 3 Months (n = 315) | (158) 8.9 (8.8 to 9.1) | (158) 9.0 (8.9 to 9.2) | −0.1 (−0.3 to 0.1) | .42 |
| Change at 3 monthsb,e | 0.06 (−0.10 to 0.30) | 0.05 (−0.10 to 0.30) | 0.01 (−0.30 to 0.30) | .95 |
| 6 Months (n = 285) | (142) 9.0 (8.8 to 9.1) | (142) 9.05 (8.90 to 9.20) | −0.1 (−0.3 to 0.1) | .40 |
| Change at 6 monthsb,e | 0.08 (−0.1 to 0.3) | 0.07 (−0.10 to 0.30) | 0.01 (−0.30 to 0.30) | .95 |
| 12 Months (n = 250) | (125) 9.2 (9.0 to 9.3) | (125) 9.0 (8.8 to 9.1) | 0.20 (−0.06 to 0.40) | .14 |
| Change at 12 monthsb,e | 0.30 (0.08 to 0.5)j | −0.002 (−0.200 to 0.200) | 0.30 (0.01 to 0.60)f | .04 |
| SF-12 Physical Healthh,k | ||||
| Baseline (n = 352) | (180) 51.4 (49.9 to 53.0) | (172) 51.4 (49.9 to 53.0) | 0.02 (−2.1 to 2.2) | .99 |
| 3 Months (n = 309) | (154) 48.5 (46.8 to 50.2) | (155) 47.9 (46.2 to 49.6) | 0.5 (−1.9 to 2.9) | .67 |
| Change at 3 monthsb,e | −3.0 (−4.6 to −1.4)l | −3.5 (−5.1 to −1.9)l | 0.5 (−1.8 to 2.8) | .66 |
| 6 Months (n = 274) | (138) 48.5 (46.7 to 50.3) | (136) 48.2 (46.4 to 49.9) | 0.4 (−2.2 to 2.9) | .77 |
| Change at 6 monthsb,e | −2.9 (−4.8 to −1.0)j | −3.3 (−5.2 to −1.4)l | 0.4 (−2.3 to 3.0) | .80 |
| 12 Months (n = 248) | (124) 48.6 (46.8 to 50.5) |
(124) 46.5 (44.7 to 48.3) |
2.1 (−0.5 to 4.7) | .11 |
| Change at 12 monthsb,e | −2.8 (−4.7 to −0.9)j | −4.9 (−6.9 to −3.01)l | 2.1 (−0.6 to 4.8) | .13 |
| SF-12 Mental Healthh,k | ||||
| Baseline (n = 352) | (180) 38.9 (37.1 to 40.7) | (172) 37.6 (35.7 to 39.4) | 1.3 (−1.3 to 3.9) | .32 |
| 3 Months (n = 309) | (154) 41.0 (39.1 to 42.9) | (155) 39.9 (38.0 to 41.8) | 1.1 (−1.6 to 3.8) | .42 |
| Change at 3 monthsb,e | 2.10 (0.08 to 4.20)f | 2.3 (0.3 to 4.4)f | −0.2 (−3.1 to 2.7) | .88 |
| 6 Months (n = 274) | (138) 40.4 (38.4 to 42.4) | (136) 40.7 (38.7 to 42.7) | −0.3 (−3.1 to 2.5) | .84 |
| Change at 6 monthsb,e | 1.5 (−0.5 to 3.5) | 3.1 (1.1 to 5.1)j | −1.6 (−4.4 to 1.2) | .27 |
| 12 Months (n = 248) | (124) 40.1 (38.0 to 42.2) | (120) 41.3 (39.2 to 43.4) | −1.2 (−4.1 to 1.8) | .44 |
| Change at 12 monthsb,e | 1.3 (−0.9 to 3.4) | 3.7 (1.6 to 5.9)l | −2.5 (−5.5 to 0.6) | .11 |
| Specialty Care Visitsm | ||||
| Baseline (n = 351) | 22/178; 3.8 (2.8 to 4.9) | 26/173; 5.3 (3.3 to 7.2) | −1.4 (−3.6 to 0.7) | .19 |
| 3 Months (n = 311) | 34/156; 3.6 (2.5 to 4.7) | 31/155; 6.5 (4.0 to 8.9) | −2.8 (−5.5 to −0.2) | .04 |
| Change at 3 monthsb | −0.2 (−1.3 to 0.9) | 1.2 (−1.2 to 3.6) | −1.4 (−4.0 to 1.2) | .30 |
| 6 Months (n = 262) | 39/133; 3.6 (2.6 to 4.5) | 36/129; 4.4 (3.1 to 5.7) | −0.8 (−2.4 to 0.7) | .30 |
| Change at 6 monthsb | −0.3 (−1.1 to 0.6) | −0.9 (−2.7 to 1.0) | 0.6 (−1.4 to 2.6) | .55 |
| 12 Months (n = 235) | 38/117; 6.9 (4.6 to 9.2) | 35/118; 7.0 (4.5 to 9.5) | −0.1 (−3.4 to 3.3) | .97 |
| Change at 12 monthsb | 3.1 (1.3 to 5.0)l | 1.7 (−0.7 to 4.2) | 1.4 (−1.7 to 4.5) | .38 |
| Mental Health Visitsn | ||||
| Baseline (n = 352) | 58/178; 10.1 (6.5 to 13.6) | 54/174; 10.2 (6.4 to 14.1) | −0.1 (−4.8 to 4.5) | .95 |
| 3 Months (n = 310) | 48/156; 31.9 (19.6 to 44.2) | 43/154; 10.4 (6.1 to 14.7) | 21.5 (9.2 to 33.8) | <.001 |
| Change at 3 monthsb | 21.8 (12.7 to 30.9)l | 0.2 (−2.3 to 2.7) | 21.6 (12.2 to 31.0)l | <.001 |
| 6 Months (n = 261) | 53/133; 24.6 (15.2 to 34.0) | 44/128; 11.1 (6.6 to 15.6) | 13.5 (3.8 to 23.1) | .01 |
| Change at 6 monthsb | 14.5 (8.3 to 20.8)l | 0.9 (−1.4 to 3.2) | 13.6 (7.0 to 20.2)l | <.001 |
| 12 Months (n = 235) | 40/117; 14.7 (9.0 to 20.4) | 40/118; 20.2 (11.8 to 28.7) | −5.5 (−14.9 to 3.8) | .25 |
| Change at 12 monthsb | 4.6 (1.6 to 7.6)j | 10.0 (4.7 to 15.3)l | −5.4 (−11.4 to 0.6) | .08 |
| Case Manager Visitso | ||||
| Baseline (n = 351) | 71/178; 9.6 (6.8 to 12.4) | 63/172; 10.9 (7.9 to 14.0) | −1.3 (−5.0 to 2.3) | .47 |
| 3 Months (n = 311) | 100/156; 25.5 (17.7 to 33.3) | 78/155; 16.9 (12.1 to 21.6) | 8.7 (0.4 to 17.0) | .04 |
| Change at 3 monthsb | 16.0 (10.6 to 21.3)l | 5.9 (3.3 to 8.6)l | 10.0 (4.3 to 15.8) | <.001 |
| 6 Months (n = 262) | 66/133; 19.7 (13.6 to 25.8) | 53/128; 6.7 (4.9 to 8.5) | 13.0 (6.9 to 19.0) | <.001 |
| Change at 6 monthsb | 10.1 (6.3 to 14.0)l | −4.2 (−6.1 to −2.3)l | 14.3 (9.9 to 18.7) | <.001 |
| 12 Months (n = 235) | 54/117; 11.3 (7.8 to 14.7) | 57/118; 10.7 (7.6 to 13.9) | 0.5 (−3.7 to 4.7) | .81 |
| Change at 12 monthsb | 1.7 (−0.1 to 3.5) | −0.2 (−2.1 to 1.7) | 1.9 (−0.8 to 4.5) | .17 |
| Medication Assisted Treatment Visitsp | ||||
| Baseline (n = 351) | 4/178; 6.9 (4.8 to 9.0) | 5/173; 4.5 (3.3 to 5.8) | 2.3 (−0.1 to 4.7) | .06 |
| 3 Months (n = 311) | 10/156; 7.5 (4.7 to 10.2) | 3/155; 4.7 (3.1 to 6.4) | 2.7 (−0.5 to 5.9) | .09 |
| Change at 3 monthsb | 0.6 (−1.5 to 2.7) | 0.2 (−1.2 to 1.6) | 0.4 (−2.1 to 2.9) | .76 |
| 6 Months (n = 262) | 11/133; 8.8 (5.4 to 13.1) | 4/129; 4.0 (2.8 to 5.2) | 4.7 (1.6 to 7.9) | .003 |
| Change at 6 monthsb | 1.6 (−0.1 to 3.3) | −0.6 (−1.6 to 0.5) | 2.40 (0.02 to 4.80)f | .05 |
| 12 Months (n = 235) | 3/117; 5.5 (3.5 to 7.5) | 9/118; 7.3 (4.7 to 9.9) | −1.8 (−5.1 to 1.5) | .28 |
| Change at 12 monthsb | −1.4 (−3.2 to 0.4) | 2.8 (0.8 to 4.7)j | −4.1 (−6.8 to −1.5)j | .002 |
| Nights Spent in Psychiatric Hospitals | ||||
| Baseline (n = 351) | 47/178; 118.6 (70.7 to 166.6) | 51/173; 95.7 (57.0 to 134.5) | 22.9 (−28.8 to 74.6) | .38 |
| 3 Months (n = 311) | 56/156; 342.2 (204.2 to 480.1) | 34/155; 194.0 (115.0 to 273.0) | 148.2 (11.5 to 284.4) | .03 |
| Change at 3 monthsb | 223.5 (132.4 to 314.7)l | 98.3 (56.2 to 140.3)l | 125.3 (36.4 to 214.2)j | .01 |
| 6 Months (n = 262) | 34/133; 254.8 (151.8 to 357.8) | 25/129; 318.9 (188.7 to 449.0) | −64.1 (−203.5 to 75.3) | .37 |
| Change at 6 monthsb | 136.1 (79.5 to 192.8)l | 223.1 (13.4 to 315.9)l | −87.0 (−180.7 to −6.7) | .07 |
| 12 Months (n = 235) | 27/117; 265.9 (157.9 to 373.8) | 25/118; 287.8 (169.9 to 405.7) | −21.9 (−155.6 to 111.7) | .08 |
| Change at 12 monthsb | 147.3 (85.3 to 209.2)l | 192.1 (111.3 to 272.9)l | −44.8 (−131.5 to 41.9) | .31 |
| Emergency Department Visitsq | ||||
| Baseline (n = 351) | 87/178; 4.1 (3.3 to 5.0) | 85/173; 2.9 (2.4 to 3.4) | 1.2 (0.4 to 2.1) | .01 |
| 3 Months (n = 311) | 47/156; 3.3 (2.6 to 4.0) | 37/155; 3.2 (2.5 to 4.0) | −0.06 (−0.8 to 0.9) | .89 |
| Change at 3 monthsb | −0.8 (−1.5 to −0.2)f | 0.30 (−0.20 to 0.90) | −1.2 (−2.0 to −0.3)j | .01 |
| 6 Months (n = 262) | 42/133; 3.8 (2.9 to 4.6) | 38/128; 4.4 (3.2 to 5.6) | −0.6 (−2.0 to 0.8) | .38 |
| Change at 6 monthsb | −0.3 (−1.0 to 0.3) | 1.5 (0.5 to 2.5)j | −1.8 (−3.1 to −0.6)j | .004 |
| 12 Months (n = 235) | 37/117; 5.0 (3.6 to 6.4) | 43/118; 3.6 (2.7 to 4.5) | 1.4 (−0.1 to 3.0) | .07 |
| Change at 12 monthsb | 0.9 (−0.1 to 1.9) | 0.70 (−0.02 to 1.40) | 0.2 (−1.0 to 1.5) | .74 |
Abbreviations: ART, antiretroviral therapy; GLMM, generalized linear mixed model; LINK LA, Linking Inmates to Care in Los Angeles; SF-12, 12-item short-form health survey; ZIP, zero-inflated Poisson.
Estimated values of each secondary outcome variable using repeated measures, random-intercept, ZIP models (for count data variables, ie, the health care services utilization variables), logistic link models (for binary, 0/1 variables), and linear link, normal GLMMs (for continuous variables) with predictors of intervention arm, categorical time, and intervention × time interaction term. The model accommodates data missing at random, and we conducted analysis to determine that loss to follow-up was not different between study arms. The ZIP models had 2 uncorrelated random effects, one for the zero-inflation component and the other for the Poisson component. All values are estimated at a per 12-month-rate, given at least 1 visit (or hospital night) in the previous 12 months.
Difference and change values are not always precise totals owing to rounding.
P value for test between intervention and control arm values at each follow-up time, and difference-in-difference tested for change from baseline by study arm, based on a single regression model.
Probability of reporting currently using (yes/no) at least 1 of a list of all available ART medications (list available at https://aidsinfo.nih.gov/drugs).
Follow-up interview value minus baseline value.
P < .05 for change from baseline.
Adherence was measured using a visual analogue scale, mean percentage from 0 to 100, where 100 indicates perfect adherence.
The first parenthetical number in this category reports the number of nonmissing observations from participants who responded.
Mean number of knowledge items (yes/no) answered correctly on a 10-item measure.
P < .01 for change from baseline.
SF-12 values are T-scores, normed relative to a US mean (SD) of 50 (10).
P < .001 for change from baseline.
Reported as mean number of office visits with physicians for specialty care, such as ophthalmologist for an eye problem.
Reported as mean number of visits to get counseling for psychological or emotional problems.
Reported as mean number of visits to case managers.
Reported as mean number of visits to a physician to get prescription medications to treat drug or alcohol problem.
Reported as mean number of visits to the emergency department.
Substance Use and Other Potential Moderators
We hypothesized that the intervention effect would be greater among non–substance users than among substance users, but the evidence did not support this. However, the intervention was most effective at 12 months among the homeless (P = .004 for the interaction) and those who were virally suppressed at baseline (P < .001 for the interaction). See eTable 4 in Supplement 2 for supporting data.
Discussion
In this study of people living with HIV released from a large metropolitan jail, the LINK LA peer navigation intervention better maintained viral suppression over 12 months than transitional case management, for a 22% adjusted difference-in-difference. No prior interventions to our knowledge have shown a sustained level of viral suppression after jail release among people living with HIV reentering the community. A randomized clinical trial comparing a motivational interviewing/care-coordination intervention with standard care found about 60% viral suppression in both arms at 6 months after release from 2 southern US prisons and temporal declines in both arms from over 85% before release. Two recently published trials of somewhat similar interventions (patient navigation, peer mentors) among people living with HIV after hospitalization did not find that viral suppression was significantly better in the intervention groups than among controls at 12 months. However, attending more patient navigation sessions was associated with improved viral suppression at 6 months in the trial of patient navigation plus financial incentives. Although the level of viral suppression did not increase from baseline in LINK LA, the peer navigation intervention was successful at preventing the declines in viral suppression usually observed after release from incarceration.
The baseline probability of 49% viral suppression sustained by LINK LA participants in the intervention arm is comparable to that observed in other studies of incarcerated people living with HIV, while suppression among controls declined to 30% at 12 months. Achievement of viral suppression is important because it promotes immune reconstitution and virtually eliminates HIV transmission. Also, the 49% 12-month viral suppression probability found in LINK LA is substantially greater than the 24% to 39% levels estimated nationally and the 30% seen among our controls. In addition, the decline in viral suppression among controls is consistent with the decline over time in adherence verified by medication event monitoring systems observed across 14 US adherence studies. The control condition also represents a fairly robust test of the LINK LA peer navigation intervention because transitional case management has been effective at improving linkage to medical and supportive services after incarceration in some studies.
The LINK LA peer navigation intervention is distinct from previously published HIV navigation interventions tested in randomized trials. First, the peers were not “matched” to participants based on demographic characteristics as in some patient navigation interventions, but rather were selected for experience relevant to postincarcerated persons. Second, LINK LA was designed more as a behavior change intervention rather than as another “wraparound” service (eg, case management), as patient navigation has been described in some literature. Third, we conducted key curriculum sessions during 2 accompaniments to HIV care, conveying important concepts in context and modeling patient behavior. One review of interventions to enhance retention in HIV care defined accompaniment as a separate kind of intervention from patient navigation. Some navigation interventions consisted of accompaniment to 1 HIV care visit. Fourth, LINK LA facilitated communication with clinicians during visits and follow through on their recommendations afterwards. Few other navigation interventions have included this component, but some work points to the critical role of the patient-clinician relationship in HIV care retention among postincarcerated persons and other persons living with HIV. Finally, previous peer mentor and peer support interventions used non–full-time staff, whereas our peer navigators were staff members paid full-time salaries with benefits, resulting in little turnover. Perhaps these differences help to explain why the LINK LA peer navigation intervention was successful, in contrast to those using other navigation models.
LINK LA peer navigation also improved self-reported retention in HIV primary care, as well as retention and adherence knowledge at 12 months, supporting the viral suppression outcome finding and suggesting a possible mechanism consistent with the conceptual model. Moreover, LINK LA improved the use of mental health care and case management in the community, while it reduced emergency department visits, further supporting the main outcome findings. LINK LA was also particularly effective for those who were homeless before incarceration. However, it did not significantly improve self-reported antiretroviral use or adherence. More than 90% of participants reported antiretroviral use, and adherence exceeded 80% at all follow-up times and both arms, suggesting possible overreporting. A somewhat similar randomized clinical trial of the effect of patient navigation and financial incentives on viral suppression among people living with HIV discharged from hospitals demonstrated a similar gap (30%-40% difference) between self-reported adherence and viral suppression proportions.
Limitations
There were several limitations to this study. Although viral load tests were conducted by laboratory assay, baseline measures were collected by jail staff rather than research staff, so we lacked control over the test conditions, and results were often reported only as detectable or undetectable, rather than number of copies per milliliter. Thus, our outcome evaluation was limited to assessing only binary viral suppression outcomes rather than change in viral load level. Since this limitation was not differential by intervention arm, it should not have biased our key findings, but it reduced our power to detect smaller changes. Approximately half of the sample had viral suppression at baseline, limiting our detection of improvement over time. Attrition also limited power to detect effects, but fortunately, study retention was 70% at 12 months; attrition was not differential by arm; and we replaced a small amount of missing data on the outcome using public health data. Finally, covariates and secondary outcomes were self-reported by the participants. Although the measure we used was previously validated, self-reported antiretroviral medication adherence is often an overestimate. In fact, more than 26% of those with detectable viral load reported 100% adherence, a level reported by 42% of the overall sample. Therefore, it might be expected that we found significant effects on viral suppression but did not find these effects for self-reported antiretroviral therapy adherence.
Conclusions
Despite these limitations, this study provides evidence of an effect of the LINK LA peer navigation intervention sustaining the level of viral suppression over 12 months, curtailing the typical decline in viral suppression observed among postincarcerated people living with HIV. While our data may generalize to other large municipal jails, future research should examine ways to further improve viral suppression among people living with HIV after release from incarceration.
Trial Protocol and Funding Application
eTable 1. LINK-LA Peer Navigation Intervention: Session Content, Timing, Duration and Locations
eTable 2. Validation of Self-Reported HIV Primary Care Visits Data by Electronic Public Health Department Records
eTable 3. The Effect of the LINK LA Intervention on Hard Substance Use after Jail Release
eTable 4. Potential Heterogeneity in the Effect of LINK LA on Viral Suppression after Jail Release
eAppendix. LINK LA Statistical Appendix
References
- 1.Giordano TP, Suarez-Almazor ME, Grimes RM. The population effectiveness of highly active antiretroviral therapy: are good drugs good enough? Curr HIV/AIDS Rep. 2005;2(4):177-183. [DOI] [PubMed] [Google Scholar]
- 2.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobias C, Cunningham WE, Cunningham CO, Pounds MB. Making the connection: the importance of engagement and retention in HIV medical care. AIDS Patient Care STDS. 2007;21(suppl 1):S3-S8. [DOI] [PubMed] [Google Scholar]
- 4.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4(11):e7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabin KM, Frey RL Jr, Horsley R, Greby SM. Characteristics and trends of newly identified HIV infections among incarcerated populations: CDC HIV voluntary counseling, testing, and referral system, 1992-1998. J Urban Health. 2001;78(2):241-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruschak LM. U.S. Department of Justice, Office of Justice Programs; Bureau of Justice Statistics. HIV in Prisons, 2006. April 22, 2008. https://www.bjs.gov/content/pub/pdf/hivp06.pdf. Accessed January 16, 2018.
- 7.Harawa NT, Amani B, Bowers JR, Sayles JN, Cunningham WE. Understanding interactions of formerly incarcerated HIV-positive men and transgender women with substance use treatment, medical, and criminal justice systems. Int J Drug Policy. 2017;Oct(48):63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draine J, Ahuja D, Altice FL, et al. . Strategies to enhance linkages between care for HIV/AIDS in jail and community settings. AIDS Care. 2011;23(3):366-377. [DOI] [PubMed] [Google Scholar]
- 9.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38(12):1754-1760. [DOI] [PubMed] [Google Scholar]
- 10.Meyer JP, Cepeda J, Wu J, Trestman RL, Altice FL, Springer SA. Optimization of human immunodeficiency virus treatment during incarceration: viral suppression at the prison gate. JAMA Intern Med. 2014;174(5):721-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapp RC, Ciomcia R, Zaller N, Draine J, Ferguson A, Cagey R. The role of jails in engaging PLWHA in care: from jail to community. AIDS Behav. 2013;17(suppl 2):S89-S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palepu A, Tyndall MW, Chan K, Wood E, Montaner JS, Hogg RS. Initiating highly active antiretroviral therapy and continuity of HIV care: the impact of incarceration and prison release on adherence and HIV treatment outcomes. Antivir Ther. 2004;9(5):713-719. [PubMed] [Google Scholar]
- 13.Baillargeon J, Giordano TP, Rich JD, et al. . Accessing antiretroviral therapy following release from prison. JAMA. 2009;301(8):848-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohl DA, Golin CE, Knight K, et al. . Randomized controlled trial of an intervention to maintain suppression of HIV viremia after prison release: the imPACT trial. J Acquir Immune Defic Syndr. 2017;75(1):81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(suppl 1):S49-S58. [DOI] [PubMed] [Google Scholar]
- 16.Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9(4):313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler T, Steakley C, Garcia AR, Kwok J, Bennett LM. Reducing disparities in the burden of cancer: the role of patient navigators. PLoS Med. 2006;3(7):e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metsch LR, Feaster DJ, Gooden L, et al. . Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. JAMA. 2016;316(2):156-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okeke NL, Ostermann J, Thielman NM. Enhancing linkage and retention in HIV care: a review of interventions for highly resourced and resource-poor settings. Curr HIV/AIDS Rep. 2014;11(4):376-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobias CR, Rajabiun S, Franks J, et al. . Peer knowledge and roles in supporting access to care and treatment. J Community Health. 2010;35(6):609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni SP. The LAC HIV epidemic and TLC+ response presentation to LAC Board of Supervisors. Los Angeles County Department of Public Health Division of HIV and STD Programs; June 5, 2012. http://publichealth.lacounty.gov/dhsp/Reports/HIV/TLCBrief4-12.pdf. Accessed January 16, 2018.
- 22.Malek M, Bazazi AR, Cox G, et al. . Implementing opt-out programs at Los Angeles county jail: a gateway to novel research and interventions. J Correct Health Care. 2011;17(1):69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Washington, DC: Department of Health and Human Services; 2011. [Google Scholar]
- 24.Gifford AL, Sengupta S. Self-management health education for chronic HIV infection. AIDS Care. 1999;11(1):115-130. [DOI] [PubMed] [Google Scholar]
- 25.Bandura A. Self-efficacy: The Exercise of Control. New York, NY: W.H. Freeman; 1997. [Google Scholar]
- 26.Huynh AK, Kinsler JJ, Cunningham WE, Sayles JN. The role of mental health in mediating the relationship between social support and optimal ART adherence. AIDS Care. 2013;25(9):1179-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsler JJ, Wong MD, Sayles JN, Davis C, Cunningham WE. The effect of perceived stigma from a health care provider on access to care among a low-income HIV-positive population. AIDS Patient Care STDS. 2007;21(8):584-592. [DOI] [PubMed] [Google Scholar]
- 28.Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24(10):1101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuster MA, Collins R, Cunningham WE, et al. . Perceived discrimination in clinical care in a nationally representative sample of HIV-infected adults receiving health care. J Gen Intern Med. 2005;20(9):807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen R, Bozzette S, Shapiro M, et al. . Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States: HCSUS Consortium: HIV cost and services utilization study. Health Serv Res. 2000;35(2):389-416. [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham WE, Hays RD, Williams KW, et al. . Access to medical care and health-related quality of life for low-income persons with symptomatic human immunodeficiency virus. Med Care. 1995;33(7):739-754. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham WE, Andersen RM, Katz MH, et al. . The impact of competing subsistence needs and barriers on access to medical care for persons with human immunodeficiency virus receiving care in the United States. Med Care. 1999;37(12):1270-1281. [DOI] [PubMed] [Google Scholar]
- 33.Casewatch Millennium [computer program]. Los Angeles, California: Automated Case Management Systems, Inc; 1987.
- 34.Midanik LT, Hines AM, Barrett DC, Paul JP, Crosby GM, Stall RD. Self-reports of alcohol use, drug use and sexual behavior: expanding the timeline follow-back technique. J Stud Alcohol. 1998;59(6):681-689. [DOI] [PubMed] [Google Scholar]
- 35.Ware J Jr, Kosinski M, Keller SDA. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. [DOI] [PubMed] [Google Scholar]
- 36.Weiss RE. Modeling Longitudinal Data. New York, NY: Springer; 2005. [Google Scholar]
- 37.Ning Li, Elashoff DA, Robbins WA, Lin Xun. A hierarchical zero-inflated log-normal model for skewed responses. Stat Methods Med Res. 2011;20(3):175-189. [DOI] [PubMed] [Google Scholar]
- 38.Giordano TP, Cully J, Amico KR, et al. . A randomized trial to test a peer mentor intervention to improve outcomes in persons hospitalized with HIV infection. Clin Infect Dis. 2016;63(5):678-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stitzer M, Matheson T, Cunningham C, et al. . Enhancing patient navigation to improve intervention session attendance and viral load suppression of persons with HIV and substance use: a secondary post hoc analysis of the Project HOPE study. Addict Sci Clin Pract. 2017;12(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson IB, Bangsberg DR, Shen J, et al. ; Multisite Adherence Collaboration on HIV 14 Investigators . Heterogeneity among studies in rates of decline of antiretroviral therapy adherence over time: results from the multisite adherence collaboration on HIV 14 study. J Acquir Immune Defic Syndr. 2013;64(5):448-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iroh PA, Mayo H, Nijhawan AE. The HIV care cascade before, during, and after incarceration: a systematic review and data synthesis. Am J Public Health. 2015;105(7):e5-e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Springer SA, Friedland GH, Doros G, Pesanti E, Altice FL. Antiretroviral treatment regimen outcomes among HIV-infected prisoners. HIV Clin Trials. 2007;8(4):205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26(7):893-896. [DOI] [PubMed] [Google Scholar]
- 45.Coviello DM, Zanis DA, Wesnoski SA, Alterman AI. The effectiveness of outreach case management in re-enrolling discharged methadone patients. Drug Alcohol Depend. 2006;85(1):56-65. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen JL, Masson CL, Delucchi K, et al. . Randomized trial of drug abuse treatment-linkage strategies. J Consult Clin Psychol. 2005;73(6):1026-1035. [DOI] [PubMed] [Google Scholar]
- 47.Rajabiun S, Mallinson RK, McCoy K, et al. . “Getting me back on track”: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDS. 2007;21(S1)(suppl 1):S20-S29. [DOI] [PubMed] [Google Scholar]
- 48.Liau A, Crepaz N, Lyles CM, et al. ; HIV/AIDS Prevention Research Synthesis (PRS) Team . Interventions to promote linkage to and utilization of HIV medical care among HIV-diagnosed persons: a qualitative systematic review, 1996-2011. AIDS Behav. 2013;17(6):1941-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. ; Antiretroviral Treatment and Access Study Group . Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423-431. [DOI] [PubMed] [Google Scholar]
- 50.Molitor F, Waltermeyer J, Mendoza M, et al. . Locating and linking to medical care HIV-positive persons without a history of care: findings from the California Bridge Project. AIDS Care. 2006;18(5):456-459. [DOI] [PubMed] [Google Scholar]
- 51.Gardner LI, Giordano TP, Marks G, et al. ; Retention in Care Study Group . Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis. 2014;59(5):725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bracken N, Hilliard C, McCuller WJ, Harawa NT. Facilitators of HIV medical care engagement among former prisoners. AIDS Educ Prev. 2015;27(6):566-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katz MH, Cunningham WE, Fleishman JA, et al. . Effect of case management on unmet needs and utilization of medical care and medications among HIV-infected persons. Ann Intern Med. 2001;135(8, pt 1):557-565. [DOI] [PubMed] [Google Scholar]
- 54.Cunningham WE, Sohler NL, Tobias C, et al. . Health services utilization for people with HIV infection: comparison of a population targeted for outreach with the U.S. population in care. Med Care. 2006;44(11):1038-1047. [DOI] [PubMed] [Google Scholar]
- 55.Simoni JM, Huh D, Wang Y, et al. . The validity of self-reported medication adherence as an outcome in clinical trials of adherence-promotion interventions: findings from the MACH14 study. AIDS Behav. 2014;18(12):2285-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5(2):74-79. [DOI] [PubMed] [Google Scholar]
- 57.Wagner GJ, Rabkin JG. Measuring medication adherence: are missed doses reported more accurately then perfect adherence? AIDS Care. 2000;12(4):405-408. [DOI] [PubMed] [Google Scholar]
- 58.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939-941. [DOI] [PubMed] [Google Scholar]
- 59.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45(3):372-379. [DOI] [PubMed] [Google Scholar]
- 60.Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95%. J Acquir Immune Defic Syndr. 2007;45(1):4-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Funding Application
eTable 1. LINK-LA Peer Navigation Intervention: Session Content, Timing, Duration and Locations
eTable 2. Validation of Self-Reported HIV Primary Care Visits Data by Electronic Public Health Department Records
eTable 3. The Effect of the LINK LA Intervention on Hard Substance Use after Jail Release
eTable 4. Potential Heterogeneity in the Effect of LINK LA on Viral Suppression after Jail Release
eAppendix. LINK LA Statistical Appendix