Key Points
Question
Is induction treatment with cetuximab plus a modified schedule of FOLFOXIRI for 4 months followed by maintenance with cetuximab or bevacizumab feasible and active for patients with RAS and BRAF wild-type metastatic colorectal cancer?
Findings
In this randomized clinical trial of 116 patients with RAS and BRAF wild-type metastatic colorectal cancer, neither of the 2 treatment arms met the primary end point of improvement in 10-month progression-free survival. Front-line induction with modified FOLFOXIRI plus cetuximab, however, appeared to be a feasible treatment and led to a response rate of 71.6%.
Meaning
Safety and activity results show that modified FOLFOXIRI plus cetuximab regimen warrants further investigation as a first-line treatment for patients with RAS and BRAF wild-type metastatic colorectal cancer.
Abstract
Importance
The combination of a triple-drug chemotherapy regimen with an anti–epidermal growth factor receptor (EGFR) agent as a first-line treatment of metastatic colorectal cancer (mCRC) showed promising activity along with safety concerns in single-arm phase 2 trials. The role of maintenance following chemotherapy and anti-EGFR and the optimal regimen to be adopted are not established.
Objectives
To evaluate the activity and safety of cetuximab plus modified FOLFOXIRI (mFOLFOXIRI) and explore the role of maintenance with cetuximab or bevacizumab in RAS and BRAF wild-type mCRC.
Design, Setting, and Participants
In a prospective, noncomparative, open-label, multicenter, randomized phase 2 trial, patients aged 18 to 75 years with unresectable, previously untreated RAS and BRAF wild-type (before amendment, KRAS wild-type) mCRC were recruited from 21 oncology units in Italy from October 19, 2011, to March 1, 2015 (followed up through May 31, 2017). In total, 323 patients were screened and 143 were randomized to 2 treatment arms to receive as a first-line induction a regimen of mFOLFOXIRI plus cetuximab followed by cetuximab (arm A) or bevacizumab (arm B) until disease progression. Primary analyses were conducted in a modified intention-to-treat population.
Interventions
mFOLFOXIRI plus cetuximab repeated every 2 weeks for up to 8 cycles, followed by maintenance with cetuximab or bevacizumab until disease progression.
Main Outcomes and Measures
The primary end point was the 10-month progression-free rate (PFR); secondary end points included progression-free and overall survival, response rate, rate of metastases resection, and adverse events.
Results
Of 143 patients randomized, 116 (81.1%) (median [interquartile range (IQR)] age, 59.5 [53-67] years; 34 [29.3%] women) had RAS and BRAF wild-type mCRC. At a median (IQR) follow-up of 44.0 (30.5-52.1) months, 10-month PFRs were 50.8% (90% CI, 39.5%-62.2%) in arm A and 40.4% (90% CI, 29.4%-52.1%) in arm B. The overall response rate was 71.6% (95% CI, 62.4%-79.5%). Main grade 3/4 adverse events were neutropenia (occurring in 36 patients [31%]), diarrhea (in 21 patients [18%]), skin toxic effects (in 18 patients [16%]), asthenia (in 11 patients [9%]), stomatitis (in 7 patients [6%]), and febrile neutropenia (in 3 patients [3%]).
Conclusions and Relevance
Although neither of the 2 arms met the primary end point, the findings indicate that a 4-month induction regimen of mFOLFOXIRI plus cetuximab is feasible and provides relevant activity results, leading to a high surgical resection rate.
Trial Registration
clinicaltrials.gov Identifier: NCT02295930
This phase 2 randomized clinical trial examines the treatment regimen of mFOLFOXIRI plus cetuximab followed by either cetuximab or bevacizumab for patients with RAS and BRAF wild-type metastatic colorectal cancer.
Introduction
The triplet FOLFOXIRI (fluorouracil, oxaliplatin, and irinotecan hydrochloride) plus bevacizumab is regarded by major guidelines as a safe and efficacious first-line therapeutic option for selected patients with metastatic colorectal cancer (mCRC).
In the past decade, different phase 2 trials investigated the combination of triple chemotherapy regimens with an anti–epidermal growth factor receptor (EGFR) monoclonal antibody (ie, cetuximab or panitumumab), achieving remarkable activity results at the price of a substantial increase in mucosal adverse events, especially diarrhea (ranging from 25% to 96%). Most of these trials included patients with mCRC, irrespective of their mutational status, or assessed only KRAS (OMIM 190070) exon 2 mutations.
In a previous phase 2 TRIP (Phase II Trial of FOLFOXIRI Plus Panitumumab as First-Line Treatment for KRAS and BRAF Wild-Type Metastatic Colorectal Cancer) study, the combination of GONO (Gruppo Oncologico Nord Ovest)–FOLFOXIRI and panitumumab was administered to a cohort of 37 patients with KRAS, NRAS (OMIM 164790), HRAS (OMIM 190020), or BRAF (OMIM 164757) wild-type mCRC. Severe gastrointestinal toxicities were reported in the first enrolled patients, leading to the amendment of the FOLFOXIRI schedule. This modification improved the toxicity profile and the treatment feasibility. Overall, 33 patients (89%) achieved RECIST (Response Evaluation Criteria In Solid Tumors) response, and 13 patients (35%) underwent secondary resection of metastases, making the modified schedule of FOLFOXIRI plus an anti-EGFR agent a combination worthy of further investigation.
The optimal duration of the upfront treatment comprising chemotherapy and a biological agent for mCRC is a debated issue. Recent trials highlight the possibility of moving from continuing the treatment until progression to developing other strategies, including off-therapy breaks or phases of deintensified therapy to spare adverse effects and to improve patients’ quality of life without compromising their clinical outcome. A bevacizumab-based maintenance therapy is regarded as a preferred option following upfront chemotherapy plus bevacizumab, but the role of maintenance after chemotherapy plus an anti-EGFR agent is not well-established, and the optimal deintensified regimen needs to be adopted. Available data from phase 2 studies suggest that administering the anti-EGFR agent as maintenance may not be inferior to continuing the combined treatment until progression and that alternating phases of chemotherapy plus an anti-EGFR agent, with periods of an anti-EGFR agent alone or treatment breaks, may be strategies of interest for further trials.
Preclinical data show that the selective pressure of EGFR inhibitors might lead to the activation of proangiogenic pathways. Based on this finding, tumors treated with anti-EGFR agents may be more sensitive to antiangiogenic agents, thus providing a sound biological rationale for investigating the potential efficacy of the sequential administration of an anti-EGFR agent and an anti–vascular endothelial growth factor agent.
From these considerations, the Modified FOLFOXIRI Plus Cetuximab, Followed by Cetuximab or Bevacizumab Maintenance, as First-Line Treatment of Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer (MACBETH) trial aimed (1) to evaluate the safety and activity of first-line induction with a modified schedule of FOLFOXIRI (mFOLFOXIRI) plus cetuximab and (2) to explore the role of maintenance with cetuximab or bevacizumab after a cetuximab-containing induction therapy in a cohort of unresectable and molecularly selected patients with mCRC.
Methods
Study Design and Patient Eligibility
The MACBETH trial was a prospective, open-label, multicenter, randomized phase 2 trial that recruited patients with mCRC from 21 hospital oncology units in Italy. Patient recruitment began on October 19, 2011, and ended on March 1, 2015. The last date of follow-up was May 31, 2017. The trial protocol is available in Supplement 1. The trial was conducted in accordance with the Declaration of Helsinki and adhered to the international Good Clinical Practice guidelines. Approval for the protocol was obtained from the local ethics committees of the 21 participating sites (eAppendix in Supplement 2). All patients provided written informed consent.
The main inclusion criteria were histologically confirmed colorectal adenocarcinoma; RAS and BRAF wild-type status centrally assessed on primary tumors or related metastases (the study was initially designed to randomize patients with KRAS codons 12, 13, and 61 wild-type tumors and was amended on October 15, 2013, to include only patients with RAS and BRAF wild-type disease); age of 18 to 75 years; Eastern Cooperative Oncology Group performance status of 0 to 2 for patients 70 years of age or younger or a performance status of 0 for patients 71 to 75 years of age; and unresectable and measurable metastatic disease according to the RECIST Guideline, version 1.1.
Study Treatments and Procedures
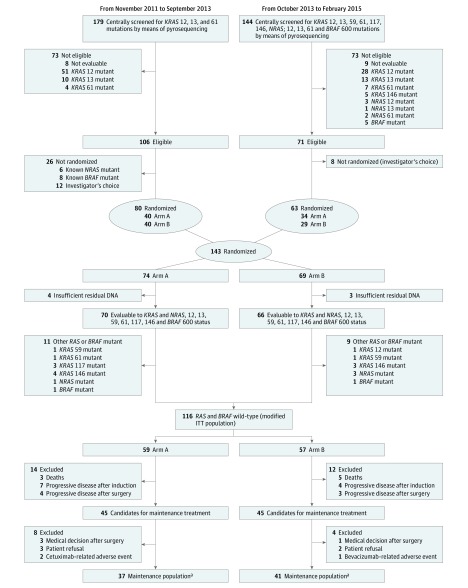
Of the 323 patients screened, 143 (44.3%) were randomly assigned in a 1:1 ratio to arm A (n = 74) or arm B (n = 69) before starting the induction phase (Figure 1). Up to 8 cycles of induction with mFOLFOXIRI plus cetuximab were planned. In the case of partial or complete response or stable disease after 8 cycles, patients were treated in the maintenance phase with cetuximab (arm A) or bevacizumab (arm B) until the tumor progressed, toxicity reached unacceptable levels, or the patient refused treatment. A centralized web-based system with a minimization algorithm stratified according to the oncology unit.
Figure 1. MACBETH Study CONSORT Diagram.
Arm A comprises patients receiving a modified schedule of fluorouracil, oxaliplatin, and irinotecan hydrochloride (mFOLFOXIRI) plus cetuximab, followed by maintenance with cetuximab. Arm B comprises patients receiving mFOLFOXIRI plus cetuximab, followed by maintenance with bevacizumab. ITT indicates intention to treat.
aPatients who received at least 1 cycle of maintenance therapy.
Patients in both arms received cetuximab (intravenous [IV] dose of 500 mg/m2 over 60 minutes) plus mFOLFOXIRI (consisting of a 130-mg/m2 IV infusion of irinotecan over 60 minutes, followed by an 85-mg/m2 IV infusion of oxaliplatin given concurrently with 200 mg/m2 of L-leucovorin calcium over 120 minutes, followed by a 2400-mg/m2 flat infusion of fluorouracil for 48 hours) every 14 days for up to 8 cycles. Maintenance treatment with cetuximab (500 mg/m2 IV over 60 minutes) was planned for arm A and bevacizumab (5 mg/kg IV over 30 minutes) was planned for arm B and was repeated every 14 days until the disease progressed, the patient refused, toxic effects became unacceptable, or patient consent was withdrawn. Granulocyte colony-stimulating factor was not recommended as a primary prophylaxis.
The assessment of response and progression was based on investigator-reported measurements, which were subsequently centrally reviewed according to RECIST version 1.1 criteria, and computed tomography was performed every 8 weeks. Adverse events were recorded and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events guidelines, version 4.0.
Unresectability was established according to the OncoSurge criteria by a multidisciplinary team comprising liver surgeons and medical oncologists from the participating oncology units. Only if disease progression occurred during the course of maintenance, it was suggested to re-treat patients with mFOLFOXIRI plus cetuximab for 4 cycles, followed by maintenance according to the randomization arm.
RAS and BRAF Status Analyses
Mutational analyses were centrally carried out on formalin-fixed, paraffin-embedded samples. Before the study amendment, KRAS codons 12, 13, and 61 mutations were assessed by a pyrosequencing approach, as previously reported. After the amendment, molecular analysis was extended to KRAS codons 59, 117, and 146; NRAS codons 12, 13, and 61; and BRAF codon 600. When the study enrollment was completed, all samples from randomized patients were reanalyzed for RAS (KRAS and NRAS codons 12, 13, 59, 61, 117, and 146) and BRAF hot spots by mass spectrometry (MassARRAY System; Agena Bioscience Inc).
Clinical Outcomes
The primary end point was the 10-month progression-free rate (PFR), defined as the proportion of patients free from disease progression 10 months after randomization out of the total number of patients included in the modified intention-to-treat (mITT) population. The secondary end points were progression-free survival (PFS), response rate, early objective response rate (the percentage of patients achieving tumor shrinkage—a ≥20% decrease in the sum of diameters of target lesions [per the RECIST guideline] at week 8 compared with baseline), depth of response (the relative change in the sum of the longest diameters of target lesions [per the RECIST guideline] at the nadir compared with baseline), overall survival (OS), and rate of metastases resection and adverse events.
Statistical Analysis
This trial was a randomized phase 2 noncomparative study, and its primary end point was the 10-month PFR. Considering that FOLFIRI (folinic acid, fluorouracil, and irinotecan) plus cetuximab achieved a median PFS of approximately 10 months (10-month PFR of 50%), mFOLFOXIRI plus cetuximab for 4 months followed by maintenance with a biologic drug until progression would have been promising if the 10-month PFR were increased from 50% to 70%. According to the Fleming single-stage design, in selecting a 10-month PFR of 0.50 in the null hypothesis and 0.70 in the alternative hypothesis, with 90% power and an α error of .05, 53 patients per arm were needed. The treatment of each arm would have been judged promising if at least 33 patients were alive and progression free at 10 months.
After the amendment (considering that before study amendment, patients bearing rare KRAS and NRAS, as well as BRAF V600E, mutations could have been included), the sample size was increased to 68 patients per arm, to finally define for the primary analysis a mITT population of at least 106 patients with RAS and BRAF wild-type disease. The primary analysis for PFS in the mITT population would have been conducted separately in each arm, when 53 patients would have been observed for 10 months.
The PFS and OS results were summarized using the Kaplan-Meier method. Hazard ratios (HRs) and corresponding 95% CIs were estimated with the Cox proportional hazards regression model. The median period of follow-up was calculated for the entire study cohort according to the reverse Kaplan-Meier method. Statistical analyses were performed using SAS, version 9.2 (SAS Institute Inc).
Results
In total, 323 patients from 21 Italian oncology units were screened, and 143 patients (44.3%) were randomized to arm A (n = 74) or arm B (n = 69). Of the 143 patients randomized, 116 patients (81.1%) had RAS and BRAF wild-type disease and were included in the mITT population: 59 patients were allocated to maintenance with cetuximab (arm A) and 57 to bevacizumab (arm B) (Figure 1). Three patients in arm B received cetuximab instead of bevacizumab so that they were included in arm A in the analysis of safety during maintenance.
Patients’ demographic and baseline characteristics were similar between arms (eTable 1 in Supplement 2), except for a higher percentage of patients in arm A with a right-sided primary. Of these patients, 82 (70.7%) were men and 34 (29.3%) were women; the median (interquartile range [IQR]) age was 59.5 (53-67) years. Altogether, 103 patients (88.8%) had an Eastern Cooperative Oncology Group performance status of 0, 98 (84.5%) presented with synchronous metastases, 54 (46.6%) had multiple metastatic sites, and 52 (44.8%) had liver-limited disease.
After the induction phase, 45 patients per arm were candidates for maintenance; 37 patients (82.2%) in arm A and 41 patients (91.1%) in arm B actually received at least 1 cycle of maintenance therapy and were included in the maintenance population (Figure 1). The median (IQR) duration of follow-up was 44.0 (30.5-52.1) months. All patients were observed at least 10 months after randomization. Thirty of the 59 patients in arm A (50.8%; 90% CI, 39.5%-62.2%) and 23 of the 57 patients in arm B (40.4%; 90% CI, 29.4%-52.1%) were alive and progression free at 10 months.
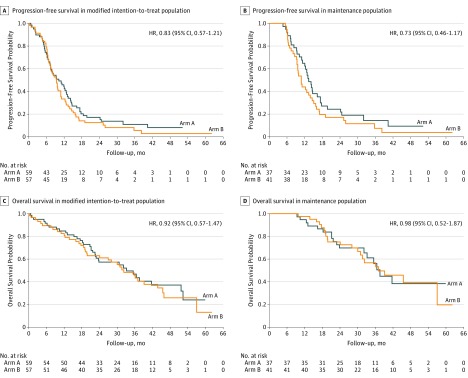
The PFS analysis was based on 107 events (92.2%)—53 in arm A and 54 in arm B—among 116 patients. The median PFS was 10.1 months (95% CI, 7.2-12.8 months) in arm A and 9.3 months (95% CI, 7.1-10.8 months) in arm B (HR, 0.83; 95% CI, 0.57-1.21) (Figure 2A). In the maintenance population (n = 78), the median PFS was 13.3 months (95% CI, 11.2-17.3 months) in arm A and 10.8 months (95% CI, 9.3-13.9 months) in arm B (HR, 0.73; 95% CI, 0.46-1.17) (Figure 2B).
Figure 2. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival, According to Treatment Arm.
Arm A comprises 59 patients receiving a modified schedule of fluorouracil, oxaliplatin, and irinotecan hydrochloride (mFOLFOXIRI) plus cetuximab followed by maintenance with cetuximab. Arm B comprises 57 patients receiving mFOLFOXIRI plus cetuximab followed by maintenance with bevacizumab. HR indicates hazard ratio; NE, not estimable.
All patients in the mITT population (n = 116) were assessed for their RECIST response. Centrally assessed activity results in the mITT population and according to randomization arm are summarized in eTable 2 in Supplement 2. Of 116 patients, 5 (4.3%) achieved complete response and 78 (67.2%) achieved partial response, for a response rate of 71.6% (95% CI, 62.4%-79.5%); 22 (19.0%) had disease stabilization, thus achieving a disease control rate of 90.5%. Of 107 evaluable patients, 81 (75.7%) achieved an early objective response rate (early tumor shrinkage), and the median (IQR) depth of response was 53.2% (37.1%-67.6%) (eTable 2 in Supplement 2); for the distribution of depth of response according to randomization arm, see eFigure in Supplement 2.
Secondary surgery for metastases was attempted with a curative intent for 45 patients (38.8%; 28 patients in arm A and 17 patients in arm B), and R0 resections were performed for 33 patients (28.4%; 21 patients in arm A and 12 patients in arm B). Among 52 patients (28 in arm A and 24 in arm B) with liver-only metastases, the R0 resection rate was 51.9% (27 patients [17 in arm A and 10 in arm B]).
The OS analysis was based on 70 events (60.3%)—34 in arm A and 36 in arm B—among 116 patients. The median OS was 33.2 months (95% CI, 22.6-51.6 months) in arm A and 32.2 months (95% CI, 19.8-43.8 months) in arm B (HR, 0.92; 95% CI, 0.57-1.47) (Figure 2C). In the maintenance population, the median OS was 37.5 months (95% CI, 32.0 to not estimable) in arm A and 37.0 months (95% CI, 30.0 to not estimable) in arm B (HR, 0.98; 95% CI, 0.52-1.87) (Figure 2D).
All patients in the mITT population were assessed for safety. With regard to induction, a total of 785 cycles with a median (IQR) of 8 (6-8) cycles per patient was administered. Treatment was delayed because of any reason in 182 cycles (23.2%) or because of any adverse event in 100 cycles (12.7%) and was administered with a reduced dose in 193 cycles (24.6%). The mean relative dose intensities were 84% for fluorouracil, 83% for irinotecan, 83% for oxaliplatin, and 82% for cetuximab.
With regard to the safety profile, diarrhea (occurring in 21 patients [18%]), skin toxic effects (in 18 patients [16%]), asthenia (in 11 patients [9%]), stomatitis (in 7 patients [6%]), anorexia (in 4 patients [3%]), and neurotoxicity (in 4 patients [3%]) were the most frequent grade 3 to 4 nonhematological adverse events (eTable 3 in Supplement 2). Grade 3 to 4 neutropenia occurred in 36 patients (31%), whereas only 3 patients (3%) experienced febrile neutropenia. Granulocyte colony-stimulating factor was administered in 93 of 785 cycles (11.8%). Serious adverse events occurred in 29 cases (25.0%), and 4 (3.4%) of them were fatal.
With regard to maintenance, a total of 965 cycles (480 in arm A and 485 in arm B) with a median (IQR) of 9 cycles (8.5 [4-16] cycles in arm A; 9 [5-14.5] cycles in arm B) per patient were administered. In the maintenance population, grade 3 to 4 adverse events were rare (eTable 3 in Supplement 2); only skin toxicity, hand-foot syndrome, and hypertension occurred in at least 3% of patients in either arm. No significant differences in adverse events were observed between arms, with the only exception of skin toxicity being more frequent in arm A than in arm B (20% vs 3%; P = .03).
Of 107 patients who experienced disease progression, 85 (79.4%) received further treatment (eTable 4 in Supplement 2). Of these 85 patients, 60 (70.6%) were re-treated (second-line treatment) with mFOLFOXIRI or modified schedules plus cetuximab, per protocol indication.
Of 35 patients with liver-limited metastases who underwent secondary surgery of metastases with curative intent during first-line treatment, 11 (31.4%) underwent further resections and/or nonsurgical locoregional treatments of metastases after disease progression.
Discussion
To our knowledge, the MACBETH trial is the largest clinical trial investigating the combination of an anti-EGFR agent with a triple-drug chemotherapy backbone. Unlike most previous studies, this study applied an extended (ie, RAS and BRAF status) and centralized molecular selection.
Neither of the 2 study arms met the primary end point of increasing the 10-month PFR from 50% (literature based) to 70%, so neither of the 2 investigated strategies appeared worthy of further investigation. Nevertheless, the trial provides other results that deserve consideration.
First, the reduction in the doses of fluorouracil and irinotecan (compared with the standard GONO-FOLFOXIRI regimen), adopted with the aim of minimizing the incidence of gastrointestinal toxicities observed in previous trials, made the combination with cetuximab safe and feasible. The occurrence of the most common adverse events, including neutropenia, diarrhea, skin toxicity, asthenia, and stomatitis, favorably compares with the safety profile of the classic GONO-FOLFOXIRI regimen with or without bevacizumab and does not differ from the toxicity reported with doublets plus an anti-EGFR agent.
Second, the activity results of mFOLFOXIRI plus cetuximab are very promising. The duration of the induction phase was shortened to 4 months, but high proportions of patients achieved a response rate of 71.6%, an early objective response rate or tumor shrinkage of 75.7%, and a clinically meaningful depth of response (53.2%), leading to remarkable secondary resectability of metastases (38.8%). Consistent results have been recently reported in the phase 2 randomized trial FOLFOXIRI With or Without Panitumumab in Metastatic Colorectal Cancer (VOLFI), which found that a modified schedule of FOLFOXIRI plus panitumumab achieved a response rate of 85.7% with a 70% resection rate in the potentially resectable cohort.
Why these activity results did not translate into similar gains in delaying tumor progression is puzzling. A potential explanation for the worse-than-expected PFS performance of both first-line strategies may be the choice of a shortened induction phase (ie, up to 8 cycles) and of a maintenance approach using a biologic agent alone that is suboptimal at least to bevacizumab. Furthermore, the lack of internal consistency between activity and efficacy results has already been reported with regimens that contained anti-EGFR agents, challenging the usefulness of PFS measures as surrogate end points of OS with this class of agents.
Third, unlike other trials conceived with the aim of studying maintenance, the MACBETH trial was not designed to formally compare different maintenance approaches. The objective of this study was to investigate 2 first-line strategies, both including a short induction of a triplet plus cetuximab followed by 2 different maintenance approaches. This objective was the rationale for randomizing patients before rather than after the induction phase. As expected, not all randomized patients actually received maintenance, and a 33% dropout rate was reported, mainly owing to secondary surgical procedures. The choice of bevacizumab as monotherapy, instead of its combination with a fluoropyrimidine, might have affected the results in arm B. However, the importance of adding a fluoropyrimidine to bevacizumab as maintenance was not established when the MACBETH trial was planned. Although it recognizes a clear separation of PFS curves favoring the continuation of cetuximab rather than the switch to bevacizumab, the MACBETH trial avoids drawing any conclusion about the best maintenance following chemotherapy plus an anti-EGFR agent.
Finally, our results confirm the feasibility of systemic treatments after disease progression when the triplet is chosen as first-line chemotherapy. A strong adherence to protocol recommendations on treatments after progression was reported; mFOLFOXIRI or modified schedules plus cetuximab were reintroduced in 70.6% of progressed patients. The reintroduction of cytotoxic agents used during the induction phase after a “full chemotherapy”–free interval is supported by a biologics rationale, but the continuation of cetuximab beyond progression did not provide convincing results in the phase 2 randomized Cetuximab Continuation After First Progression in Metastatic Colorectal Cancer (CAPRI-GOIM) study. The emergence of mechanisms of acquired resistance to anti-EGFR agents clearly challenges the potential efficacy of a sustained inhibition of EGFR that today cannot be recommended in daily practice.
Limitations
When interpreting results from the MACBETH trial, at least 3 limitations should be acknowledged: (1) the choice of the “switch maintenance” arm is clearly suboptimal, considering recent data identifying the combination of a fluoropyrimidine with bevacizumab, instead of single-agent bevacizumab, as the optimal maintenance when using the antiangiogenic; (2) the noncomparative design of the study, which prevents us from drawing any conclusions about the best maintenance approach; and (3) the choice of a PFS-related measure as primary end point, considering the poor surrogacy of PFS for OS when adopting anti-EGFR–containing regimens.
Conclusions
Neither of the two arms of the MACBETH trial met the primary endpoint of demonstrating a relevant increase in 10m-PFR against literature data with doublets plus anti-EGFR. However, a short induction with mFOLFOXIRI plus cetuximab is feasible as first-line treatment for mCRC and allows achieving impressive activity results in RAS and BRAF wild-type patients, thus emerging as an appealing treatment option especially when a rapid and consistent tumor shrinkage is required.
Based on these results, the Italian cooperative G.O.N.O. has now launched the phase III randomized TRIPLETE trial, to compare the activity of panitumumab with mFOLFOX6 or mFOLFOXIRI, both followed by maintenance with 5-fluorouracil and panitumumab until disease progression, as first-line therapy in RAS and BRAF wild-type mCRC patients.
Trial protocol
eAppendix. Participating Centers and Principle Investigators
eTable 1. Main Demographic and Clinical Characteristics of Patients in the mITT Population
eTable 2. Activity Results in the mITT Population
eTable 3. Grade ≥3 Adverse Events Occurring in at Least 3% of Patients During Induction and Maintenance
eTable 4. Treatments After Progression According to Randomization Arm
eFigure. Distribution of Deepness of Response in the mITT Population, According to Treatment Arm
References
- 1.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology—Colon Cancer; Version 1.2017. https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed September 1, 2017.
- 2.Van Cutsem E, Cervantes A, Adam R, et al. . ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386-1422. [DOI] [PubMed] [Google Scholar]
- 3.Garufi C, Torsello A, Tumolo S, et al. . Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103(10):1542-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assenat E, Desseigne F, Thezenas S, et al. . Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist. 2011;16(11):1557-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folprecht G, Hamann S, Schütte K, Trarbach T, Stoehlmacher-Williams J, Ehninger G. Dose escalating study of cetuximab and 5-FU/folinic acid (FA)/oxaliplatin/irinotecan (FOLFOXIRI) in first line therapy of patients with metastatic colorectal cancer. BMC Cancer. 2014;14:521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saridaki Z, Androulakis N, Vardakis N, et al. . A triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in KRAS wt, metastatic colorectal cancer: a pilot phase II trial. Br J Cancer. 2012;107(12):1932-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornaro L, Lonardi S, Masi G, et al. . FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. 2013;24(8):2062-2067. [DOI] [PubMed] [Google Scholar]
- 8.Stein A, Schwenke C, Folprecht G, Arnold D. Effect of application and intensity of bevacizumab-based maintenance after induction chemotherapy with bevacizumab for metastatic colorectal cancer: a meta-analysis. Clin Colorectal Cancer. 2016;15(2):e29-e39. [DOI] [PubMed] [Google Scholar]
- 9.García Alfonso P, Benavides M, Sánchez Ruiz A, et al. . Phase II study of first-line mFOLFOX plus cetuximab (C) for 8 cycles followed by mFOLFOX plus C or single agent (S/A) C as maintenance therapy in patients (P) with metastatic colorectal cancer (mCRC): the MACRO-2 trial (Spanish Cooperative Group for the Treatment of Digestive Tumours [TTD]). Ann Oncol. 2014;25(suppl 4):iv167-iv209. doi: 10.1093/annonc/mdu333.3 [DOI] [Google Scholar]
- 10.Wasan H, Meade AM, Adams R, et al. ; COIN-B investigators . Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol. 2014;15(6):631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciardiello F, Bianco R, Caputo R, et al. . Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. 2004;10(2):784-793. [DOI] [PubMed] [Google Scholar]
- 12.Bianco R, Rosa R, Damiano V, et al. . Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin Cancer Res. 2008;14(16):5069-5080. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Published May 28, 2009. September 1, 2017.
- 15.Poston GJ, Adam R, Alberts S, et al. . OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23(28):7125-7134. [DOI] [PubMed] [Google Scholar]
- 16.Arcila M, Lau C, Nafa K, Ladanyi M. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrometry genotyping. J Mol Diagn. 2011;13(1):64-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fumagalli D, Gavin PG, Taniyama Y, et al. . A rapid, sensitive, reproducible and cost-effective method for mutation profiling of colon cancer and metastatic lymph nodes. BMC Cancer. 2010;10:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cremolini C, Loupakis F, Antoniotti C, et al. . Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26(6):1188-1194. [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Köhne CH, Láng I, et al. . Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011-2019. [DOI] [PubMed] [Google Scholar]
- 20.Loupakis F, Cremolini C, Masi G, et al. . Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609-1618. [DOI] [PubMed] [Google Scholar]
- 21.Masi G, Loupakis F, Salvatore L, et al. . Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(9):845-852. [DOI] [PubMed] [Google Scholar]
- 22.Falcone A, Ricci S, Brunetti I, et al. ; Gruppo Oncologico Nord Ovest . Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670-1676. [DOI] [PubMed] [Google Scholar]
- 23.Van Cutsem E, Lenz HJ, Köhne CH, et al. . Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692-700. [DOI] [PubMed] [Google Scholar]
- 24.Douillard JY, Siena S, Cassidy J, et al. . Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25(7):1346-1355. [DOI] [PubMed] [Google Scholar]
- 25.Geissler M, Martens U, Knorrenschield R, et al. . mFOLFOXIRI + panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer m(CRC): a randomized phase II VOLFI trial of the AIO (AIO-KRK0109). Ann Oncol. 2017;28(suppl 5):159. [Google Scholar]
- 26.Heinemann V, von Weikersthal LF, Decker T, et al. . FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065-1075. [DOI] [PubMed] [Google Scholar]
- 27.Venook AP, Niedzwiecki D, Lenz HJ, et al. . Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simkens LH, van Tinteren H, May A, et al. . Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843-1852. [DOI] [PubMed] [Google Scholar]
- 29.Koeberle D, Betticher DC, von Moos R, et al. . Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: a randomized phase III non-inferiority trial (SAKK 41/06). Ann Oncol. 2015;26(4):709-714. [DOI] [PubMed] [Google Scholar]
- 30.Hegewisch-Becker S, Graeven U, Lerchenmüller CA, et al. . Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015;16(13):1355-1369. [DOI] [PubMed] [Google Scholar]
- 31.Ciardiello F, Normanno N, Martinelli E, et al. ; CAPRI-GOIM Investigators; CAPRI-GOIM investigators . Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann Oncol. 2016;27(6):1055-1061. [DOI] [PubMed] [Google Scholar]
- 32.Pietrantonio F, Vernieri C, Siravegna G, et al. . Heterogeneity of acquired resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23(10):2414-2422. [DOI] [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov. First line mFOLFOXIRI + panitumumab vs mFOLFOX + panitumumab in RAS and BRAF WT metastatic colorectal cancer patients (TRIPLETE). https://clinicaltrials.gov/ct2/show/NCT03231722. September 1, 2017.
- 34.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix. Participating Centers and Principle Investigators
eTable 1. Main Demographic and Clinical Characteristics of Patients in the mITT Population
eTable 2. Activity Results in the mITT Population
eTable 3. Grade ≥3 Adverse Events Occurring in at Least 3% of Patients During Induction and Maintenance
eTable 4. Treatments After Progression According to Randomization Arm
eFigure. Distribution of Deepness of Response in the mITT Population, According to Treatment Arm