Abstract
Importance
Patients with soft tissue sarcoma are at risk for local recurrence and distant metastases despite optimal local treatment. Preoperative anthracycline plus ifosfamide chemotherapy improves outcome in common histological subtypes.
Objective
To analyze whether the previously reported improvement in local progression-free survival by adding regional hyperthermia to neoadjuvant chemotherapy translates into improved survival.
Design, Setting, and Participants
Open-label, phase 3 randomized clinical trial to evaluate the efficacy and toxic effects of neoadjuvant chemotherapy plus regional hyperthermia. Adult patients (age ≥18 years) with localized soft tissue sarcoma (tumor ≥5 cm, French Federation Nationale des Centers de Lutte Contre le Cancer [FNCLCC] grade 2 or 3, deep) were accrued across 9 centers (6, Germany; 1, Norway; 1, Austria; 1, United States) from July 1997 to November 2006. Follow-up ended December 2014.
Interventions
After stratification for tumor presentation and site, patients were randomly assigned to either neoadjuvant chemotherapy consisting of doxorubicin, ifosfamide, and etoposide alone, or combined with regional hyperthermia.
Main Outcomes and Measures
The primary end point was local progression-free survival. Secondary end points included treatment safety and survival, with survival defined from date of randomization to death due to disease or treatment. Patients lost to follow-up were censored at the date of their last follow-up.
Results
A total of 341 patients were randomized, and 329 (median [range] age, 51 [18-70] years; 147 women, 182 men) were eligible for the intention-to-treat analysis. By December 2014, 220 patients (67%; 95% CI, 62%-72%) had experienced disease relapse, and 188 (57%; 95% CI, 52%-62%) had died. Median follow-up was 11.3 years. Compared with neoadjuvant chemotherapy alone, adding regional hyperthermia improved local progression-free survival (hazard ratio [HR], 0.65; 95% CI, 0.49-0.86; P = .002). Patients randomized to chemotherapy plus hyperthermia had prolonged survival rates compared with those randomized to neoadjuvant chemotherapy alone (HR, 0.73; 95% CI, 0.54-0.98; P = .04) with 5-year survival of 62.7% (95% CI, 55.2%-70.1%) vs 51.3% (95% CI, 43.7%-59.0%), respectively, and 10-year survival of 52.6% (95% CI, 44.7%-60.6%) vs 42.7% (95% CI, 35.0%-50.4%).
Conclusions and Relevance
Among patients with localized high-risk soft tissue sarcoma the addition of regional hyperthermia to neoadjuvant chemotherapy resulted in increased survival, as well as local progression-free survival. For patients who are candidates for neoadjuvant treatment, adding regional hyperthermia may be warranted.
Trial Registration
clinicaltrials.gov Identifier: NCT00003052
This randomized clinical trial examines whether the previously reported improvement in local progression-free survival by adding regional hyperthermia to neoadjuvant chemotherapy vs chemotherapy alone translates into improved survival in patients with soft tissue sarcoma.
Key Points
Question
Does the previously reported improvement in local progression-free survival with neoadjuvant chemotherapy plus regional hyperthermia translate into improved survival of patients with high-risk soft tissue sarcoma?
Findings
In this randomized clinical trial that included 329 eligible patients, survival was significantly improved by adding regional hyperthermia to neoadjuvant chemotherapy with an absolute difference at 5 years of 11.4% and at 10 years of 9.9% compared with neoadjuvant chemotherapy alone.
Meaning
For patients with localized high-risk soft tissue sarcoma who are candidates for neoadjuvant treatment, adding regional hyperthermia may be warranted.
Introduction
Soft tissue sarcoma accounts for less than 1% of all malignancies. According to the American Cancer Society, about 12 000 new cases per year are diagnosed in the United States, and more than 4900 people die of these tumors annually.1 Tumor size, grade, and location are the predominant prognostic factors used to define patients at high risk for local recurrence or early dissemination.2 To account for prognostic differences, site-specific nomograms have been developed for both extremity and retroperitoneal tumors.3,4 For localized tumors, surgery combined with preoperative or postoperative radiotherapy is considered the backbone of care. Regarding perioperative chemotherapy, current clinical practice guidelines recommend it as an option in patients deemed high risk.5
Heat exposure (40 °C to 43 °C) of cancer cells in preclinical studies, and hyperthermia regionally applied to patients in early randomized clinical studies, have shown synergistic activity with ionizing radiation and chemotherapy.6 For the combination of hyperthermia with chemotherapy, the study group at Munich7 was the first to demonstrate the safety and efficacy of regional hyperthermia (RHT) in patients with high-risk sarcoma. As a consequence, this study—the EORTC 62961-ESHO 95—was designed as the first randomized study that we know of to compare RHT added to neoadjuvant chemotherapy with neoadjuvant chemotherapy alone in patients undergoing surgery followed by radiotherapy whenever possible. Results for the primary end point of local progression-free survival and second end points including tumor response, survival outcome, and adverse effects accompanying therapy have been published previously.8 Herein, we present the final, long-term results with a cutoff date of December 2014.
Methods
Patients
The study details have been reported previously.8 Briefly, eligible patients were ages 18 to 70 years and had histologically proven soft tissue sarcoma with the following risk criteria: tumor diameter 5 cm or larger, French Federation Nationale des Centers de Lutte Contre le Cancer (FNCLCC) grade 2 or 3, deep to the fascia, and no evidence of distant metastases. In patients who had undergone an attempt of prior surgical resection with the result of marginal margins (tumor-free margins less than 1 cm) random allocation to treatment was allowed within 8 weeks of surgery.
The trial protocol is available in Supplement 1.
Trial Design and Logistics
EORTC 62961-ESHO 95 was a multicenter, open-label, parallel group study8 with centralized randomization to either an experimental treatment group (neoadjuvant chemotherapy plus RHT) or a control group (neoadjuvant chemotherapy alone), with a similar follow-up schedule, stratified according to site and presentation of tumor.
The trial was initiated by the European Society of Hyperthermia Oncology (ESHO), with trial coordination carried out by the Klinikum der Universität München, Munich, Germany in collaboration with the European Organisation for Research and Treatment of Cancer (EORTC) Soft-Tissue Bone Sarcoma Group (STBSG). The participating university centers were in Germany (6), Norway (1), Austria (1), and the United States (1). The study protocol was approved by the EORTC in May 1997 and by review boards of each study site. Written informed consent for all patients was obtained. External pathological review was performed by one of us (S.D.) on behalf of EORTC.
The primary objective was local progression-free survival. Among secondary end points, tumor response to induction therapy, disease-free survival, and survival were included. Tumor response was based on investigator assessment by imaging using World Health Organization (WHO) criteria for patients with measurable disease at baseline. According to the STBSG recommendation at the time of the study, a blinded review of responses was performed by board members of the STBSG. Survival was defined as the time to death due to sarcoma or its treatment with survivors being censored at the time of last follow-up. Deaths from other causes were not considered events and censored at the time of death. Patients alive without recurrence were censored on the date of last follow-up. Adverse events related to chemotherapy were graded according to Common Toxicity Criteria (CTC) of the National Cancer Institute. Toxic effects related to hyperthermia were scored according to protocol guidelines.
Randomization
Patients were randomized in a 1:1 ratio to both treatment arms. Block randomization was performed centrally at the EORTC data center with stratification according to site (extremity vs nonextremity) and presentation of tumor (primary vs recurrent vs prior surgery).
Procedures
Patients were to receive either 4 cycles of chemotherapy alone (neoadjuvant chemotherapy consisting of doxorubicin, ifosfamide, and etoposide [NACT] alone) or chemotherapy combined with RHT every 3 weeks as induction therapy followed by evaluation of tumor response. Tumor assessments included abdominal computed tomography or magnetic resonance imaging and chest radiography. Local treatment consisted of definitive surgery within 4 to 6 weeks of induction therapy, including re-resections for patients with initial inadequate surgery. For external beam radiation therapy, the dose was administered to 50.0 to 60.0 Gy (to convert Gy to rad, multiply by 100), with daily fractions of 1.8 to 2.2 Gy, and a boost up to 66.0 Gy. Within 6 weeks of local therapy, patients were to receive another 4 cycles of their allocated treatment for postinduction therapy. Patients with previous surgery had to receive the complete induction and postinduction therapy. NACT consisted of doxorubicin (50 mg/m2 over a 60-minute period on day 1), ifosfamide (1500 mg/m2 on days 1 to 4), and etoposide (125 mg/m2 on days 1 and 4). Treatment continued unless progressive disease, unacceptable toxic effects, or withdrawal from the study occurred. Regional hyperthermia (42 °C for a 60-minute period) was given concurrently with ifosfamide on day 1 and day 4 of each cycle during both induction and postinduction therapy. Quality of hyperthermia was ensured by European Society for Hyperthermic Oncology guidelines.8,9
Statistical Analysis
The accrual goal of 334 eligible patients was based on a statistical power of 80% to detect, on a 5% significance level, an improvement in local progression-free survival (median, 86 months for NACT plus RHT vs 43 months for NACT alone). An accrual period of 6 years and a follow-up time of 9 years were set. As defined in the study protocol, the final analysis required 146 distal failures. The analysis was undertaken using SAS version 9.2 (SAS Institute Inc). Survival of patients was estimated according to the Kaplan-Meier method, providing medians with 95% CIs and survival differences at specific time points. Number-needed-to-treat analysis was performed by standard procedure. Comparisons between the groups of stratified patients were performed using the log-rank test. The stratified proportional hazard Cox model was used for multivariate analysis. The subgroup effects were represented by a forest plot using the Cochrane Review Manager software version 5.3 (Cochrane Community). All P values are 2-sided and of exploratory nature except for the primary analysis. Results were considered significant at P ≤ .05. The survival-type analyses presented were based on the intention-to-treat population, which includes all eligible patients in the study who started their allocated treatment.
Results
Patients and Treatment
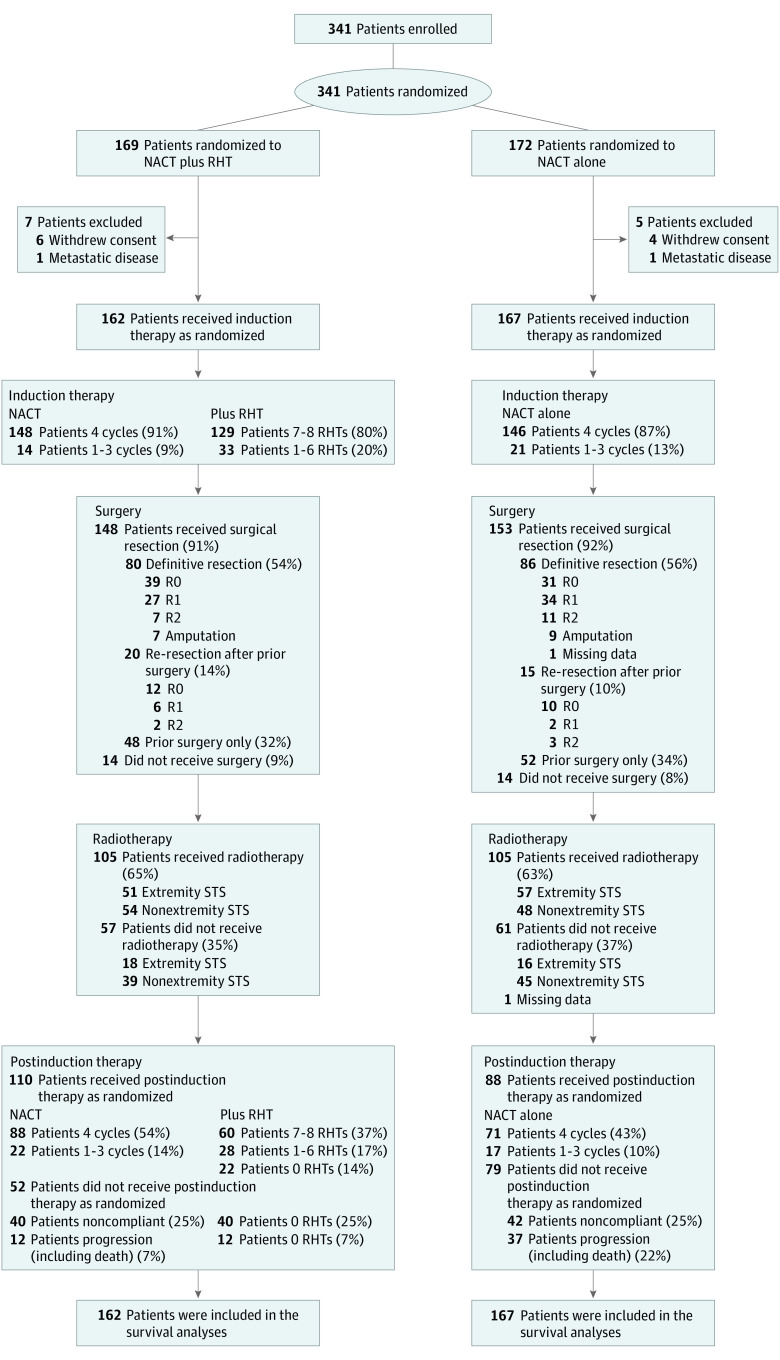
Between July 1997 and November 2006, a total of 341 patients were enrolled and underwent randomization over a 9-year period. Of these, 169 patients were assigned to the NACT plus RHT group and 172 to NACT-alone group. A total of 162 patients from the NACT plus RHT group and 167 patients from the NACT-alone group were eligible for the intention-to-treat analyses of survival end points. Seven patients of the NACT plus RHT group were excluded (6 withdrew consent and 1 had metastatic disease), and 5 patients of the NACT-alone group were excluded (4 withdrew of consent and 1 had metastatic disease) (eAppendix in Supplement 2). The major baseline characteristics of eligible patients were well-balanced across study groups (Table 1). The number of patients who received study treatment and outcomes of surgery is summarized in Figure 1. For local therapy, nearly all patients underwent surgery. About two-thirds of patients in both treatment arms underwent postoperative external beam radiotherapy; the mean (SD) doses were 53.2 (8.9) Gy vs 52.7 (9.6) Gy.
Table 1. Baseline Characteristics of Eligible Patientsa.
| Characteristic | No. (%) | |
|---|---|---|
| NACT Plus RHT (n = 162) |
NACT Alone (n = 167) |
|
| Age, y | ||
| 18-40 | 44 (27.2) | 44 (26.3) |
| 41-70 | 118 (72.8) | 123 (73.7) |
| Median (range) | 51.0 (18.0-70.0) | 52.0 (19.0-70.0) |
| Sex | ||
| Male | 91 (56.2) | 91 (54.5) |
| Female | 71 (43.8) | 76 (45.5) |
| WHO performance status | ||
| 0 | 106 (65.4) | 112 (67.1) |
| 1 | 48 (29.6) | 48 (28.7) |
| 2 | 8 (4.9) | 7 (4.2) |
| Site of tumor | ||
| Nonextremityb | 93 (57.4) | 93 (55.7) |
| Extremity | 69 (42.6) | 74 (44.3) |
| Presentation of tumor | ||
| Primary | 75 (46.3) | 82 (49.1) |
| Recurrent | 19 (11.7) | 18 (10.8) |
| Prior surgery | 68 (42.0) | 67 (40.1) |
| Size of tumor, cm | ||
| 5.0-12.0 | 93 (57.4) | 106 (63.5) |
| >12.0 | 69 (42.6) | 61 (36.5) |
| Median (range) | 11.0 (5.0-36.0) | 11.0 (5.0-40.0) |
| Histologic grade | ||
| G2 | 79 (48.8) | 74 (44.3) |
| G3 | 83 (51.2) | 93 (55.7) |
| Histologic type | ||
| Liposarcoma | 30 (18.5) | 30 (18.0) |
| Leiomyosarcoma | 25 (15.4) | 27 (16.2) |
| Synovial sarcoma | 24 (14.8) | 19 (11.4) |
| Sarcoma NOS | 33 (20.4) | 35 (21.0) |
| Other sarcomac | 37 (22.8) | 39 (23.4) |
| Not soft-tissue sarcomad | 2 (1.2) | 4 (2.4) |
| Unreviewed sarcomae | 11 (6.8) | 13 (7.8) |
Abbreviations: NACT, neoadjuvant chemotherapy consisting of doxorubicin, ifosfamide, and etoposide; NOS, not otherwise specified; RHT, regional hyperthermia; WHO, World Health Organization.
Percentages may not sum to 100 because of rounding.
Nonextremity includes retroperitoneal-visceral tumors and tumors localized in the pelvis (81%), trunk (18%), and head and neck (1%).
Angiosarcoma, rhabdomyosarcoma, fibrosarcoma, myxofibrosarcoma, nerve sheath tumor, gastrointestinal tumor, epitheloid sarcoma, alveolar soft-part sarcoma, extraskeletal myxoid chondrosarcoma, myogenic not otherwise specified, haemangiopericytoma, malignant solitary fibrous tumor, extraskeletal Ewings sarcoma, myofibrosarcoma.
Anaplastic large-cell lymphoma, solid pseudopapillary neoplasm of the pancreas, pleomorphic T-cell lymphoma, atypical Burkitt lymphoma, giant-cell tumor of tendon sheath, chondrosarcoma (not mesenchymal).
Unreviewed means that external pathological review was not performed.
Figure 1. CONSORT Flow Diagram.
Number of patients who were randomly assigned to a treatment group, and outcomes. NACT indicates neoadjuvant chemotherapy consisting of doxorubicin, ifosfamide, and etoposide; RHT, regional hyperthermia; STS, soft tissue sarcomas.
Efficacy
The database was closed in December 2014, when 220 disease relapses including 149 distant events had occurred in 329 patients: 101 disease relapses (62%; 95% CI, 55%-69%) in the NACT plus RHT group and 119 disease relapses (71%; 95% CI, 64%-78%) in the NACT-alone group with no failures in 109 patients (61 in the NACT plus RHT group [38%; 95% CI, 31%-45%] vs 48 in the NACT-alone group [29%; 95% CI, 22%-36%]). The median (interquartile range) follow-up duration was 11.3 (9.2-14.7) years.
The relative hazard for local progression or death between patients receiving NACT plus RHT or NACT alone was 0.65 (95% CI, 0.49-0.86; P = .002) with a median duration of 67.3 months vs 29.2 months (Figure 2A). The addition of RHT prolonged the median disease-free survival from 17.4 months to 33.3 months (HR for local or distant failure or death, 0.71; 95% CI, 0.55-0.93; P = .01; Figure 2B).
Figure 2. Kaplan-Meier Survival Curves.
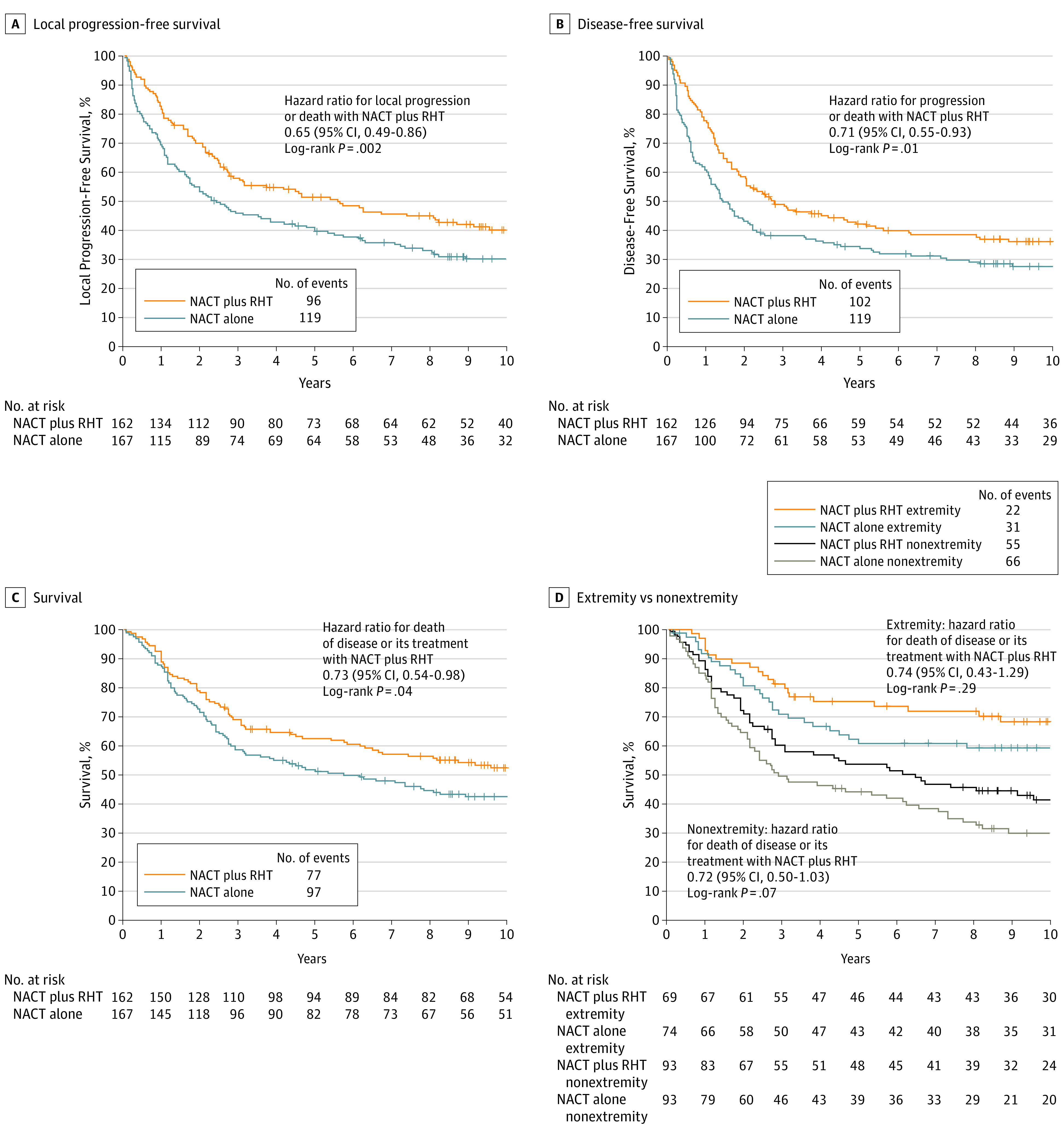
A, Median local progression free survival was 5.6 years (95% CI, 2.9-8.7) in the NACT plus RHT group compared with 2.4 years (95% CI, 1.7-4.2) in the NACT-alone group. B, Median disease-free survival was 2.8 years (95% CI, 2.0-4.9) in the NACT plus RHT group compared with 1.5 years (95% CI, 1.1-2.1) in the NACT-alone group. C, Median survival was 15.4 years (95% CI, 6.6 to >17.0 [the upper confidence limit cannot be estimated and represents the lower bound for the value to be expected]) in the NACT plus RHT group compared with 6.2 years (95% CI, 3.2-10.3) in the NACT-alone group. D, Extremity tumor–survival rates at 5 and 10 years were 75.2% and 68.3% in the NACT plus RHT group compared with 60.8% and 59.2% in the NACT-alone group. The absolute difference at 5 years was 14.4% (95% CI, 0%-29.5%) and was 9.1% (95% CI, 0%-24.7%) at 10 years. Nonextremity tumor–survival rates at 5 years and 10 years were 53.5% and 41.3% in the NACT plus RHT group compared with 44.0% and 29.9% in the NACT-alone group. The absolute difference at 5 years was 9.5% (95% CI, 0%-23.8%) and was 11.4% (95% CI, 0%-25.1%) at 10 years. NACT indicates neoadjuvant chemotherapy consisting of doxorubicin, ifosfamide, and etoposide; RHT, regional hyperthermia.
By December 2014, 188 patients (57%; 95% CI, 52%-62%) had died, and 141 patients were still alive (75 in the NACT plus RHT group and 66 in the NACT-alone group). One-hundred seventy four patients had died due to disease or treatment (77 in the NACT plus RHT group and 97 in the NACT-alone group); 5 deaths (3.1%) were attributable to treatment in the NACT plus RHT treatment group, and 2 deaths (1.2%) to treatment in the NACT-alone group. Fourteen patients had died from other causes (4, myocardial infarction; 7, second malignancy; 1, drug abuse; and 2, other reasons), of which 10 (6.2%) occurred in the NACT plus RHT group and 4 (2.4%) in the NACT-alone group.
Survival between the study groups was significantly improved in the NACT plus RHT group, with a median duration of 15.4 years compared with 6.2 years in the NACT-alone group (HR 0.73; 95% CI, 0.54-0.98; P = .04; Figure 2C). Survival rates at 5-years and 10 years were 62.7% (95% CI, 55.2%-70.1%) and 52.6% (95% CI, 44.7%-60.6%), respectively, in the NACT plus RHT group, and 51.3% (95% CI, 43.7%-59.0%) and 42.7% (95% CI, 35.0%-50.4%), respectively, in the NACT-alone group. The number of patients needed to treat to achieve the survival benefit at 5 years and 10 years were 8.8 and 10.1, respectively. By post hoc analyses, in patients with extremity tumors survival rates at 5 years and 10 years in favor of RHT were 75.2% vs 60.8% (absolute difference, 14.4%; 95% CI, 0.0%-29.5%), and 68.3% vs 59.2% (absolute difference, 9.1%; 95% CI, 0%-24.7%), respectively. In patients with nonextremity survival rates at 5 years and 10 years in favor of RHT were 53.5% vs 44% (absolute difference, 9.5%; 95% CI, 0%-23.8%) and 41.3% vs 29.9% (absolute difference, 11.4%; 95% CI 0%-25.1%), respectively (Figure 2D). The summary of treatment outcomes is provided in eTable 1 in Supplement 2.
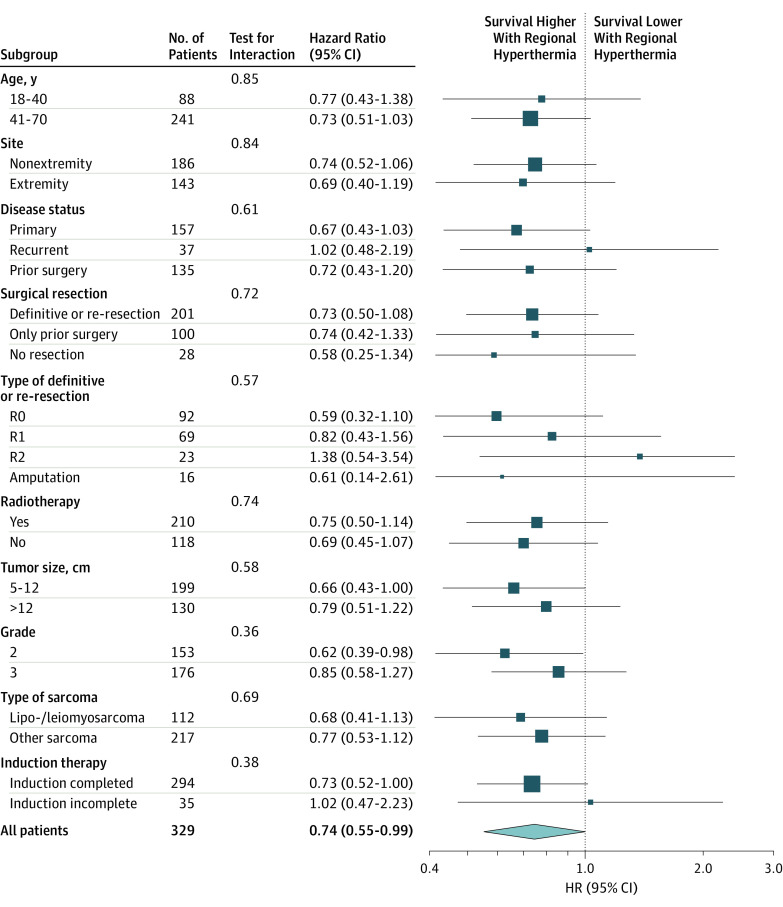
A consistently higher survival was observed with the NACT plus RHT treatment across all subgroup factors (age, site, disease status, definitive/re-resection, R0, R1, R2, amputation, prior surgery, no resection, radiotherapy, size, grade, and histologic subtype), with no major treatment and subgroup interaction (Figure 3). The univariate and multivariate analyses showed that beside treatment, grade and tumor size remain the dominant prognostic factors in terms of survival (Table 2).
Figure 3. Forest Plot Survival for 329 Patients.
Analyses were univariate and not stratified according to subgroup.
Table 2. Univariate and Multivariate Analyses of Prognostic Factorsa.
| Prognostic Factor | No. | Survival | |||
|---|---|---|---|---|---|
| Univariate | Multivariable | ||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Treatment | |||||
| NACT alone | 167 | 1 [Reference] | 1 [Reference] | ||
| NACT plus RHT | 162 | 0.73 (0.54-0.98) | .04 | 0.70 (0.52-0.95) | .02 |
| Age, y | |||||
| 18-40 | 88 | 1 [Reference] | ND | ||
| 41-70 | 241 | 0.97 (0.69-1.37) | .87 | ND | |
| Sex | |||||
| Men | 182 | 1 [Reference] | ND | ||
| Women | 147 | 0.85 (0.63-1.16) | .31 | ND | |
| Grade | |||||
| G3 | 176 | 1 [Reference] | 1 [Reference] | ||
| G2 | 153 | 0.68 (0.50-0.92) | .01 | 0.69 (0.51-0.94) | .02 |
| Tumor size, cm | |||||
| >12.0 | 130 | 1 [Reference] | 1 [Reference] | ||
| 5.0-12.0 | 199 | 0.63 (0.47-0.86) | .003 | 0.62 (0.46-0.84) | .002 |
| Presentation of tumor | |||||
| Recurrent | 37 | 1 [Reference] | ND | ||
| Primary | 157 | 0.62 (0.40-0.95) | .03 | ND | |
| Prior surgery | 135 | 0.42 (0.27-0.67) | <.001 | ND | |
| Site | |||||
| Nonextremity | 186 | 1 [Reference] | ND | ||
| Extremity | 143 | 0.45 (0.33-0.63) | <.001 | ND | |
Abbreviations: HR, hazard ratio; NACT neoadjuvant doxorubicin, ifosfamide, and etoposide chemotherapy; ND, no data; RHT, regional hyperthermia.
The analyses of subgroups (treatment, age, sex, grade, tumor size) were prespecified and stratified to tumor presentation and site. The univariate HR estimates for the stratification variables (tumor presentation and site) are given; HRs for stratification variables in multivariate analyses cannot be calculated.
Considering the effect of further salvage treatment, the survival from local progression to the time of death (HR, 1.02; 95% CI, 0.69-1.52; P = .90) or from distant metastasis to the time of death (HR, 1.06; 95% CI, 0.74-1.50; P = .77), comparing both treatment groups, showed no statistical difference.
Discussion
That we know of, EORTC 62961-ESHO 95 was the first phase 3 randomized trial in soft tissue sarcoma research that investigated the effects of neoadjuvant chemotherapy combined with RHT.
The main result was that with a median follow-up of more than 11 years neoadjuvant chemotherapy combined with RHT lead to a 27% improvement in survival, with a statistically significant absolute 11.4% improvement in the 5-year survival rate (62.7% vs 51.3%) and a 9.9% improvement in the 10-year survival rate (52.6% vs 42.7%), compared with neoadjuvant chemotherapy alone. The treatment effect was robust and consistent among all prespecified risk factors and stratification criteria. Owing to the fact that our study comprises a 20-year data set that included an older age group between 41 to 70 years that represented more than 70% of the patients, there was an increasing risk of death from natural causes unrelated to sarcoma. Therefore, the survival benefit has been analyzed as death of disease or its treatment so to be not confounded by the occurrence of disease-unrelated deaths.10 In extremity and nonextremity tumors, the hazard for death or its treatment was equally pronounced, but the study was not powered for these subgroups. Because of the larger subgroup of nonextremity tumors, the survival effect is most likely driven by downsizing and prevention of early progression of these tumors because local failure is the leading cause of death in patients with abdominal and/or retroperitoneal tumors.4 The positive impact of RHT of completely resected tumors in this subgroup has been previously reported.11
A puzzling observation in the study was the delayed divergence of the survival curves after treatment completion (Figure 2C). The same observation was made recently in 2 other randomized studies12,13 of soft tissue sarcoma testing eribulin as second-line therapy and olaratumab as first-line therapy. The delayed improvement of survival was discussed to be related to effects of further salvage therapies which seemed not to be the case in our study. Similar to these multitargeting agents, RHT also affects different targets encompassing DNA repair, microenvironment, and immunity.14,15,16 Our results fit to the early action–late benefit model of immunotherapy trials, where the therapeutic effects are exerted prior to the curve divergence. The survival curves will not separate until the time when corresponding control patients (who did not receive RHT) experience disease relapse and die.17
The multidisciplinary approach included the best possible local treatment. Surgery as the backbone of care was performed in almost all patients. Postoperative external beam radiotherapy was equally limited in one-third of patients owing to the risk of functional restrictions or adjacent organs at risk. The number of patients who received radiotherapy with R0 or R1 resected tumors were well-balanced. For local progression-free survival, radiotherapy after R0 resection had no effect, whereas after R1 resection the positive effect seen in both treatment arms was comparable (eTable 2 and eTable 3 in Supplement 2). Today, more advanced techniques involving image-guided radiotherapy may improve both tolerance and effectiveness.18,19 Results of using preoperative or postoperative external beam radiotherapy in the neoadjuvant setting from nonrandomized studies in extremity tumors, as well as results expected from the recently completed randomized STRASS trial, should be the basis for future trials with the addition of RHT.20,21,22 Noncompliance and the rate of early dropouts were higher than expected from our previous experience in a phase 2 study.23 However, the number of patients with progressive disease or death prior to postinduction chemotherapy was higher in the NACT-alone group, thereby reducing the number of candidates for postinduction chemotherapy (OR, 3.4; 95% CI, 1.5-7.9; P = .003).
There are only a few trials in the neoadjuvant setting, and some with a similar parallel group design of chemotherapy. A small, phase 2 trial randomized 134 patients with heterogeneous risk criteria to doxorubicin (50 mg/m2) plus ifosfamide (5 g/m2) given for 5 cycles or to local treatment. The study was stopped owing to low accrual and no evidence that neoadjuvant chemotherapy improved survival.24
Using isolated limb perfusion under hyperthermic conditions as induction therapy in 231 patients who were all candidates for functional or anatomic amputation, the limb salvage rate was 81%, but 5-year overall survival was only 42% and poorest in patients with large tumors (P = .01) and with leiomyosarcoma (P = .001).25
The benefit of preoperative systemic chemotherapy in high-risk patients is supported by the results of the Italian Sarcoma Intergroup (ISG) and the Spanish Sarcoma-Intergroup trial. Designed as a noninferiority trial, 328 patients were randomized to 3 cycles of preoperative epirubicin (120 mg/m2) plus ifosfamide (9 g/m2) chemotherapy with or without 2 further cycles postoperatively.26 The 5-year overall survival rate was 70% in both treatment arms, and these results were similar to the published results of the Italian Sarcoma-Intergroup adjuvant trials, which demonstrated improved overall survival rates at 5 years of 66% and 70%, respectively, while the 5-year survival rates of the control arms were significantly lower (46% and 47%, respectively).27,28 A recent update confirmed the noninferiority of the preoperative 3 cycles with a 10-year overall survival of 61% (95% CI, 56%-67%) for the entire group of patients.29 The results apply predominantly to extremity tumors. With this restriction, the results of the EORTC 62961-ESHO 95 control arm for patients with extremity tumors who were treated with the 3-drug NACT regimen alone as comparator showed a 10-year survival rate of 59%, which was similar to the results of the Intergroup trial (61%).29 In addition, the 5-year survival rate after neoadjuvant chemotherapy alone was also much better than the 5-year overall survival rates after local treatment (surgery plus radiation) in the Italian adjuvant trials.29 Taking together, survival of patients with high-risk extremity tumors who were treated in our control arm without RHT was almost identical to those receiving short, full-dose preoperative chemotherapy, and was further improved adding RHT by almost 10%.
Therefore, these results reinforced the significance of the additional benefit by RHT because they were not confounded by an insufficient efficacy of the chemotherapy regimen. The survival benefit was also observed in patients with less favorable, abdominal-retroperitoneal tumors, and was even more pronounced in grade 2 tumors, due to yet unknown mechanisms. This observation is surprising because only high-grade tumors are supposed to be chemosensitive as supported by the retrospective analysis of the French Sarcoma Group30 showing that grade 2 tumors did not benefit from adjuvant chemotherapy in contrast to grade 3 tumors. Because distinct histotypes were thought to be more sensitive to specific cytotoxic drugs, the most recent ISG trial randomized 287 patients to their standard of preoperative epirubicin plus ifosfamide chemotherapy, or to 1 of 5 histologically tailored chemotherapy regimens.31 The study was stopped because the experimental arm showed a significantly lower relapse-free and overall survival. In EORTC 62961-ESHO 95, improved survival by RHT was seen in L-sarcoma, as well as in all other high-grade histological subtypes.
That we know of, EORTC 62961-ESHO 95 is still the first randomized trial to be carried out and completed comparing systemic chemotherapy with or without RHT in a high-risk patient population. As such, we should not exclude the potential therapeutic benefits RHT may also have in solid tumors other than soft tissue sarcoma. To test this further, a multicenter, randomized phase 3 trial in resected pancreatic cancer is ongoing (NCT01077427). We have also been conditioned to discount observational studies, and practice changes are only made based on results from randomized trials.32 However, in the rare subset of pediatric, malignant nontesticular germ-cell tumors, a phase 2 study adding RHT to salvage chemotherapy has demonstrated outcome benefits almost similar to first-line treatment.33 Therefore, there is an urgent need to raise more interest in this treatment modality by oncologists in dedicated centers.
Limitations
The EORTC 62961-ESHO 95 trial showed a significant improvement in survival in patients receiving neoadjuvant chemotherapy combined with regional hyperthermia. However, the study design was not powered enough to show the statistical evidence for all subgroups (eg, extremity vs abdominal and/or retroperitoneal sarcomas). For patients who were treated in combination with regional hyperthermia, completion of induction therapy was significant for survival, however only two-thirds of these patients received post-induction therapy. Therefore, the required number of post-induction therapy cycles, for the overall survival benefit, remained open.
Conclusions
The EORTC 62961-ESHO 95 study provides robust evidence that among patients with localized high-risk soft tissue sarcoma, the use of RHT added to neoadjuvant chemotherapy resulted in increased survival, as well as local progression-free survival. For patients who are candidates for neoadjuvant treatment, adding RHT may be warranted.
Trial Protocol.
eAppendix. Reasons patients were excluded from the study
eTable 1. Summary of efficacy measures in the Intention-to-Treat population
eTable 2. Distribution of radiotherapy between R0 and R1 resected patients
eTable 3. Effect of radiotherapy in different treatment groups (R0, R1, R0+R1) on local progression-free survival
References
- 1.American Cancer Society . Cancer Facts and Figures 2017. 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed July 13, 2017.
- 2.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701-711. [DOI] [PubMed] [Google Scholar]
- 3.Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17(5):671-680. [DOI] [PubMed] [Google Scholar]
- 4.Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31(13):1649-1655. [DOI] [PubMed] [Google Scholar]
- 5.Group ESESNW; ESMO/European Sarcoma Network Working Group . Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii102-iii112. [DOI] [PubMed] [Google Scholar]
- 6.Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487-497. [DOI] [PubMed] [Google Scholar]
- 7.Issels RD, Prenninger SW, Nagele A, et al. Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: a phase II study. J Clin Oncol. 1990;8(11):1818-1829. [DOI] [PubMed] [Google Scholar]
- 8.Issels RD, Lindner LH, Verweij J, et al. ; European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC-STBSG); European Society for Hyperthermic Oncology (ESHO) . Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11(6):561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagendijk JJ, Van Rhoon GC, Hornsleth SN, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia. 1998;14(2):125-133. [DOI] [PubMed] [Google Scholar]
- 10.Mariotto AB, Noone AM, Howlader N, et al. Cancer survival: an overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr. 2014;2014(49):145-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angele MK, Albertsmeier M, Prix NJ, et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: a subgroup analysis of a randomized phase-III multicenter study. Ann Surg. 2014;260(5):749-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629-1637. [DOI] [PubMed] [Google Scholar]
- 13.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell SN, Kachnic LA. Homologous recombination research is heating up and ready for therapy. Proc Natl Acad Sci U S A. 2011;108(24):9731-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182(3):1449-1459. [DOI] [PubMed] [Google Scholar]
- 16.Issels R, Kampmann E, Kanaar R, Lindner LH. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: translation into clinical application. Int J Hyperthermia. 2016;32(1):89-95. [DOI] [PubMed] [Google Scholar]
- 17.Thorén FB, Anderson H, Strannegård Ö. Late divergence of survival curves in cancer immunotherapy trials: interpretation and implications. Cancer Immunol Immunother. 2013;62(10):1547-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folkert MR, Singer S, Brennan MF, et al. Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J Clin Oncol. 2014;32(29):3236-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paumier A, Le Péchoux C, Beaudré A, et al. IMRT or conformal radiotherapy for adjuvant treatment of retroperitoneal sarcoma? Radiother Oncol. 2011;99(1):73-78. [DOI] [PubMed] [Google Scholar]
- 20.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56(4):1117-1127. [DOI] [PubMed] [Google Scholar]
- 21.Mullen JT, Kobayashi W, Wang JJ, et al. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large, extremity soft tissue sarcomas. Cancer. 2012;118(15):3758-3765. [DOI] [PubMed] [Google Scholar]
- 22.Roeder F, Lehner B, Schmitt T, et al. Excellent local control with IOERT and postoperative EBRT in high grade extremity sarcoma: results from a subgroup analysis of a prospective trial. BMC Cancer. 2014;14:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Issels RD, Abdel-Rahman S, Wendtner C, et al. Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk adult soft-tissue sarcomas (STS) of adults: long-term results of a phase II study. Eur J Cancer. 2001;37(13):1599-1608. [DOI] [PubMed] [Google Scholar]
- 24.Gortzak E, Azzarelli A, Buesa J, et al. ; E.O.R.T.C. Soft Tissue Bone Sarcoma Group and the National Cancer Institute of Canada Clinical Trials Group/Canadian Sarcoma Group . A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37(9):1096-1103. [DOI] [PubMed] [Google Scholar]
- 25.Deroose JP, Eggermont AM, van Geel AN, et al. Long-term results of tumor necrosis factor alpha- and melphalan-based isolated limb perfusion in locally advanced extremity soft tissue sarcomas. J Clin Oncol. 2011;29(30):4036-4044. [DOI] [PubMed] [Google Scholar]
- 26.Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. 2012;30(8):850-856. [DOI] [PubMed] [Google Scholar]
- 27.Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19(5):1238-1247. [DOI] [PubMed] [Google Scholar]
- 28.Petrioli R, Coratti A, Correale P, et al. Adjuvant epirubicin with or without Ifosfamide for adult soft-tissue sarcoma. Am J Clin Oncol. 2002;25(5):468-473. [DOI] [PubMed] [Google Scholar]
- 29.Gronchi A, Stacchiotti S, Verderio P, et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann Oncol. 2016;27(12):2283-2288. [DOI] [PubMed] [Google Scholar]
- 30.Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol. 2010;21(12):2436-2441. [DOI] [PubMed] [Google Scholar]
- 31.Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812-822. [DOI] [PubMed] [Google Scholar]
- 32.Brade AM, Dawson LA. To RCT or Not to RCT: How to Change Practice for Rare Cancers? J Clin Oncol. 2016;34(3):203-204. [DOI] [PubMed] [Google Scholar]
- 33.Wessalowski R, Schneider DT, Mils O, et al. ; MAKEI study group . Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: an open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol. 2013;14(9):843-852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eAppendix. Reasons patients were excluded from the study
eTable 1. Summary of efficacy measures in the Intention-to-Treat population
eTable 2. Distribution of radiotherapy between R0 and R1 resected patients
eTable 3. Effect of radiotherapy in different treatment groups (R0, R1, R0+R1) on local progression-free survival