Key Points
Question
Several studies have shown an association of prompt treatment with status epilepticus duration, but what is the association with other outcome measures, such as death?
Findings
In this multicenter observational study of 218 children with refractory convulsive status epilepticus, a delay in first-line benzodiazepine treatment was associated with death, the need for continuous infusions, longer convulsive duration, and more frequent hypotension.
Meaning
These findings may change the perception of acute seizure and status epilepticus treatment, tentatively converting it into an extremely time-sensitive emergency that is similar to stroke or other cardiovascular events.
Abstract
Importance
Treatment delay for seizures can lead to longer seizure duration. Whether treatment delay is associated with major adverse outcomes, such as death, remains unknown.
Objective
To evaluate whether untimely first-line benzodiazepine treatment is associated with unfavorable short-term outcomes.
Design, Setting, and Participants
This multicenter, observational, prospective cohort study included 218 pediatric patients admitted between June 1, 2011, and July 7, 2016, into the 11 tertiary hospitals in the United States within the Pediatric Status Epilepticus Research Group. Patients, ranging in age from 1 month to 21 years, with refractory convulsive status epilepticus (RCSE) that did not stop after the administration of at least 2 antiseizure medications were included. Patients were divided into 2 cohorts: those who received the first-line benzodiazepine treatment in less than 10 minutes and those who received it 10 or more minutes after seizure onset (untimely). Data were collected and analyzed from June 1, 2011, to July 7, 2016.
Main Outcomes and Measures
The primary outcome was death during the related hospital admission. The secondary outcome was the need for continuous infusion for seizure termination. Multivariate analysis of mortality controlled for structural cause, febrile RCSE, age, and previous neurological history (including previous RCSE events). Use of continuous infusions was additionally adjusted for generalized RCSE, continuous RCSE, and 5 or more administrations of antiseizure medication.
Results
A total of 218 patients were included, among whom 116 (53.2%) were male and the median (interquartile range) age was 4.0 (1.2-9.6) years. The RCSE started in the prehospital setting for 139 patients (63.8%). Seventy-four patients (33.9%) received their first-line benzodiazepine treatment in less than 10 minutes, and 144 (66.1%) received untimely first-line benzodiazepine treatment. Multivariate analysis showed that patients who received untimely first-line benzodiazepine treatment had higher odds of death (adjusted odds ratio [AOR], 11.0; 95% CI, 1.43 to ∞; P = .02), had greater odds of receiving continuous infusion (AOR, 1.8; 95% CI, 1.01-3.36; P = .047), had longer convulsive seizure duration (AOR, 2.6; 95% CI, 1.38-4.88; P = .003), and had more frequent hypotension (AOR 2.3; 95% CI, 1.16-4.63; P = .02). In addition, the timing of the first-line benzodiazepine treatment was correlated with the timing of the second-line (95% CI, 0.64-0.95; P < .001) and third-line antiseizure medications (95% CI, 0.25-0.78; P < .001).
Conclusions and Relevance
Among pediatric patients with RCSE, an untimely first-line benzodiazepine treatment is independently associated with a higher frequency of death, use of continuous infusions, longer convulsion duration, and more frequent hypotension. Results of this study raise the question as to whether poor outcomes could, in part, be prevented by earlier administration of treatment.
This cohort study examines the association between delay in initiation of first-line benzodiazepine treatment and short-term outcomes for children hospitalized with refractory convulsive status epilepticus.
Introduction
Status epilepticus (SE) consists of a prolonged, self-sustaining seizure or repeated seizures without return to baseline neurological function. It is one of the most frequent life-threatening emergencies affecting approximately 17 to 23 per 100 000 children in the United States and Europe. The short-term mortality ranges from 3% to 9%, and the long-term mortality rate is 7%. Survivors of SE often have long-term sequelae, including cognitive and neurodevelopmental impairment, epilepsy, and recurrent SE.
Current SE treatment protocols suggest a timely and stepwise progression of treatment. Protocols recommend the first-line treatment—a benzodiazepine—to be administered within 5 to 10 minutes of seizure onset. This first-line treatment is usually followed by the second-line treatment—the administration of a nonbenzodiazepine antiseizure medication (ASM)—10 to 20 minutes later. If SE persists, the third-line treatment—anesthetic agents via continuous infusion—may be started within 30 to 70 minutes of seizure onset. The rationale is to stop seizures quickly, thereby reducing the risk for brain damage and other medical complications. Different studies have shown that seizures usually last less than 10 minutes, and it is unlikely that they stop spontaneously after that threshold. Animal models and clinical studies have demonstrated a progressive diminished efficacy of benzodiazepine with prolonged seizures, and untimely treatment leads to longer convulsion duration. However, previous data have indicated that the treatment of SE is often delayed. To date, the main SE outcome predictors include patient age, SE cause, and SE duration, with SE duration being most often the only modifiable risk factor through timely ASM management. Although it is known that time to treatment is independently associated with SE duration, it is not known if untimely treatment affects other outcome measures, such as death.
To address this gap in knowledge, we aimed to study the association between untimely first-line benzodiazepine treatment and short-term outcome in children with refractory convulsive status epilepticus (RCSE). We hypothesized that patients with RCSE have worse short-term outcomes if they receive the first-line benzodiazepine treatment 10 or more minutes after seizure onset.
Methods
Study Design
This multicenter, observational, prospective cohort study is part of the Pediatric Status Epilepticus Research Group, a consortium of 11 tertiary pediatric hospitals in the United States. Data on time to treatment of a subgroup of initial cases in this cohort have been previously reported. Data were collected with a standardized data acquisition tool and entered into an electronic database. Prehospital data, including time to treatment and SE onset, were prospectively obtained from patients’ families and corroborated with emergency medical services (EMS) reports and medical records. The inhospital information was obtained from hospital records and inpatient medical teams. In the case of missing or incomplete data, we prospectively corroborated data with the families, medical teams, and objective sources. In the case of small differences of timing, we chose the time with stronger and more precise evidence and excluded the patient if the timing was unclear. The research protocol was approved by the institutional review boards of all 11 participating institutions: Boston Children’s Hospital, The Children’s Hospital of Philadelphia, Cincinnati Children’s Hospital Medical Center, The University of Virginia Health System, Children's National Health System, Children’s Hospital Colorado, Northwestern University Feinberg School of Medicine, Duke University Medical Center, Baylor College of Medicine, Phoenix Children’s Hospital, and Mayo Clinic. All participants gave written informed consent. Data were collected and analyzed from June 1, 2011, to July 7, 2016.
Patients
Although the Pediatric Status Epilepticus Research Group centers usually follow published guidelines on SE treatment, there is no common management protocol, and decisions were made based on clinical judgment by the individual treating teams, permitting this comparative effectiveness approach.
The inclusion criteria were (1) hospital admission into 1 of the consortium’s 11 tertiary pediatric hospitals in the United States for an SE episode during hospital admission between June 1, 2011, and July 7, 2016; (2) age from 1 month to 21 years at the time of admission; (3) focal or generalized convulsive epileptic seizures at onset; and (4) failure of treatment with 2 or more ASMs to terminate SE or the initiation of a continuous infusion for seizure control. In cases in which a patient had more than 1 SE episode during the study period, only the first episode was analyzed. Of the 268 potentially eligible patients, 218 (81.3%) met the inclusion criteria.
The exclusion criteria were (1) nonconvulsive SE detected on electroencephalogram without convulsive seizures at onset, (2) nonconvulsive SE with motor manifestations limited to infrequent myoclonic jerks, (3) unknown time to first-line benzodiazepine treatment, (4) psychogenic nonepileptic seizure, or (5) incomplete clinical information regarding ASM administration.
Variables
We divided the cohort into 2 groups: (1) patients who received the first-line benzodiazepine treatment in less than 10 minutes and (2) patients who received the first-line benzodiazepine treatment 10 or more minutes after seizure onset (untimely first-line benzodiazepine treatment). The primary outcome was death during the hospital admission. The secondary outcome was the need for continuous infusion for seizure termination. We also looked at tertiary outcomes, including baseline neurological function at hospital discharge (as determined by previous neurological examinations or history obtained from families), convulsive duration, intensive care unit duration, hypotension, and other medical complications during hospitalization (not included in other variables). We reported race and ethnicity as classified by the investigators, with the categories defined by the investigators. Race and ethnicity were reported as descriptive demographic variables.
Statistical Analysis
We used descriptive statistics to summarize demographic and clinical characteristics. Continuous variables were expressed as median and interquartile ranges (IQRs) because evaluation of the data distribution did not fit a normal distribution, and we therefore used nonparametric analyses. Initial bivariate analyses for binary outcome variables were performed using the Fisher exact test. To test the association of the untimely first-line benzodiazepine treatment with our primary outcome, we performed an exact logistic regression adjusting for age, structural cause, febrile RCSE, and no previous neurological history (including previous RCSE events). One of the outcome’s bins had a zero, which precluded the use of standard logistic regression. Exact logistic regression allows for the construction of logistic regression models in which the sample size is too small and/or some of the cells have zero observations in the data. The model parameter estimates are based on the permutational distributions of sufficient statistics rather than asymptotic statistics. In such scenarios, the maximum likelihood–based approaches fail to perform satisfactorily. The secondary and tertiary outcomes were more frequent; thus, we performed a logistic regression adjusting for age, structural cause, febrile RCSE, no previous neurological history, generalized SE, continuous RCSE, and more than the median number of ASMs (≥5). We selected the variables on the basis of prior scientific knowledge that prevents multiple testing and reduces the number of false negatives. The number of variables was limited to avoid overfitting of models that had rare events. Because the data were highly skewed, we used linear regression with log transformation to calculate the association between time to first-line benzodiazepine treatment and duration of convulsive SE. We compared times to benzodiazepine and nonbenzodiazepine ASMs between groups using the Wilcoxon rank sum test. Times were calculated from seizure onset. The Wilcoxon signed rank test was used to compare treatment timing with protocol recommendations. The 2-sided α value was set at 0.05. All statistical analyses were performed with Stata, version 13 (StataCorp LLC).
Results
Patients
A total of 218 patients were included, with a median (IQR) age of 4.0 (1.2-9.6) years, and 116 (53.2%) were male. Seventy-four patients (33.9%) received the first-line benzodiazepine treatment in less than 10 minutes, and 144 patients (66.1%) received untimely first-line benzodiazepine treatment. One hundred ninety-seven patients (90.4%) had an electroencephalogram during the admission for RCSE. The RCSE started in the prehospital setting for 139 patients (63.8%). Demographics and clinical features are summarized in Table 1.
Table 1. Demographic and Clinical Characteristics.
| Characteristic | Patients, No. (%) |
|---|---|
| All patients | 218 |
| Male sex | 116 (53.2) |
| Age, median (IQR), y | 4.0 (1.2-9.6) |
| RCSE onset setting | |
| Prehospital RCSE onset | 139 (63.8) |
| Inhospital RCSE onset | 79 (36.2) |
| Race | |
| White | 130 (59.6) |
| Black or African American | 48 (22.0) |
| Arabic | 8 (3.7) |
| Asian | 8 (3.7) |
| Native Hawaiian or Pacific Islander | 1 (0.5) |
| Not reported | 8 (3.7) |
| Unknown | 15 (6.9) |
| Ethnicity | |
| Not Hispanic or Latino | 159 (72.9) |
| Hispanic or Latino | 38 (17.4) |
| Not reported | 13 (5.9) |
| Unknown | 8 (3.7) |
| Type of RCSEa | |
| Intermittent RCSE | 144 (66.1) |
| Continuous RCSE | 74 (33.9) |
| Status epilepticus cause | |
| Structural | 62 (28.4) |
| Genetic | 32 (14.7) |
| Metabolic | 12 (5.5) |
| Other | 32 (14.7) |
| Unknown | 80 (36.7) |
| Past medical conditionb | |
| Developmental delay | 108 (49.5) |
| Epilepsy | 112 (51.4) |
| Status epilepticus | 47 (21.6) |
| Febrile seizure | 25 (11.5) |
| Cerebral palsy | 22 (10.1) |
| None | 74 (33.9) |
Abbreviations: IQR, interquartile range; RCSE, refractory convulsive status epilepticus.
Intermittent RCSE indicates that the patient presents with multiple seizures and does not return to baseline; continuous RCSE indicates ongoing seizure.
The numbers may sum up higher than the total, as some patients may present with more than 1 category.
Untimely Treatment and Death
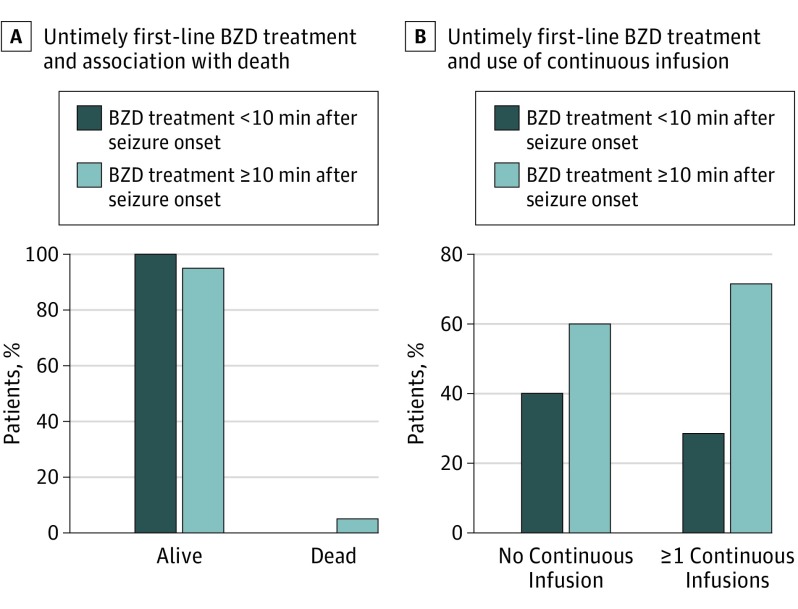
Seven patients (3.2%) died prior to hospital discharge, and all 7 received untimely first-line benzodiazepine treatment (Figure 1A). Multivariate analysis showed that those who received untimely first-line benzodiazepine treatment had higher odds of death after adjusting for known confounders (adjusted odds ratio [AOR], 11.0; 95% CI, 1.43 to ∞; P = .02) (Table 2). Five (71.4%) of 7 patients who died had prehospital SE onset (eTable 1 in the Supplement).
Figure 1. Untimely First-line Benzodiazepine (BZD) Treatment and Association With Outcome.
A, All 7 patients who died prior to hospital discharge received untimely first-line BZD treatment (≥10 minutes). B, Of the 112 patients (51.4%) who received a continuous infusion as treatment for refractory convulsive status epilepticus, 80 (71.4%) received untimely first-line BZD treatment.
Table 2. Untimely First-line Benzodiazepine Treatment and Death, Adjusted for Confoundersa.
| Variable | Outcome Proportion, No. (%) (N = 218) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Received Untimely Treatment, No. (%) | P Value | AOR (95% CI) | P Value | ||
| Death | 7 (3.2) | 7 (100) | .98 | 11.0 (1.43 to ∞) | .02 |
| Structural cause | 62 (28.4) | 38 (61.3) | .35 | 0.1 (0 to 0.80) | .03 |
| Febrile RCSE | 45 (20.6) | 39 (86.7) | .001 | 0.05 (0 to 0.45) | .006 |
| Older than the median age of 4 y | 109 (50.0) | 69 (63.3) | .48 | 0.2 (0.02 to 1.61) | .16 |
| No previous neurology history | 78 (35.8) | 53 (67.9) | .77 | 11.3 (1.52 to 145.02) | .02 |
Abbreviations: AOR, adjusted odds ratio; RCSE, refractory convulsive status epilepticus.
The results of AORs for multivariate analysis using an exact logistic regression model, with untimely first-line benzodiazepine treatment (≥10 minutes after seizure onset) as the predictor and death as the primary outcome, adjusted for structural cause, febrile RCSE, age, and no neurological history. The main finding is that, after controlling for structural etiology, febrile RCSE, age, and no neurological history, we found that the AOR of death was 11 for patients with untimely first-line benzodiazepine treatment compared with patients with first-line benzodiazepine treatment in less than 10 minutes after seizure onset.
Untimely Treatment and Use of Continuous Infusion
One hundred twelve patients (51.4% [112 of 218]) received a continuous infusion, and of these 112 patients, 80 (71.4%) received untimely first-line benzodiazepine treatment (P = .09) (Figure 1B). After adjusting for confounders, we found that the untimely treatment group was more likely to receive 1 or more continuous infusions (AOR, 1.8; 95% CI, 1.01-3.36; P = .047) (Table 3). Continuous infusions included midazolam hydrochloride (91 [81.3%]), propofol (7 [6.3%]), pentobarbital sodium (4 [3.6%]), and other (10 [8.9%]).
Table 3. Untimely First-line Benzodiazepine Treatment and Use of Continuous Infusionsa.
| Variable | Outcome Proportion, No. (%) (N = 218) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Received Untimely Treatment, No. (%) | P Value | AOR | SE (95% CI) | P Value | ||
| ≥1 Continuous infusions | 112 (51.4) | 80 (71.4) | .09 | 1.8 | 0.6 (1.01-3.36) | .047 |
| Age, median (IQR), y | 4 (1-10) | 3.7 (1-10) | .32 | 1.1 | 0.0 (0.99-1.12) | .08 |
| Structural cause | 62 (28.4) | 38 (61.3) | .35 | 1.0 | 0.3 (0.54-1.94) | .93 |
| Febrile RCSE | 45 (20.6) | 39 (86.7) | .001 | 0.6 | 0.3 (0.29-1.36) | .23 |
| No previous neurological history | 78 (35.8) | 53 (67.8) | .77 | 1.5 | 0.5 (0.83-2.78) | .18 |
| Generalized RCSE | 151 (69.3) | 100 (66.2) | >.99 | 2.3 | 0.7 (1.21-4.20) | .01 |
| Continuous RCSE | 74 (33.9) | 50 (67.6) | .77 | 0.9 | 0.3 (0.50-1.66) | .77 |
| ≥5 ASMs | 150 (68.8) | 95 (63.3) | .22 | 1.0 | 0.3 (0.52-1.74) | .87 |
Abbreviations: AOR, adjusted odds ratio; ASMs, antiseizure medications; IQR, interquartile range; RCSE, refractory convulsive status epilepticus.
The results of AORs for multivariate analysis using a logistic regression model, with untimely first-line benzodiazepine treatment (≥10 minutes after seizure onset) as the predictor and use of continuous infusions as the main outcome, adjusted for age, structural cause, febrile RCSE, no neurological history, generalized RCSE, continuous RCSE, and use of 5 or more ASMs. The main finding is that, after controlling for age, structural cause, febrile RCSE, no neurological history, generalized RCSE, continuous RCSE, and use of 5 or more ASMs, we found that the AOR of needing continuous infusion(s) was 1.8 for patients with untimely first-line benzodiazepine treatment compared with patients who received first-line benzodiazepine treatment in less than 10 minutes after seizure onset.
Untimely Treatment and Tertiary Outcomes
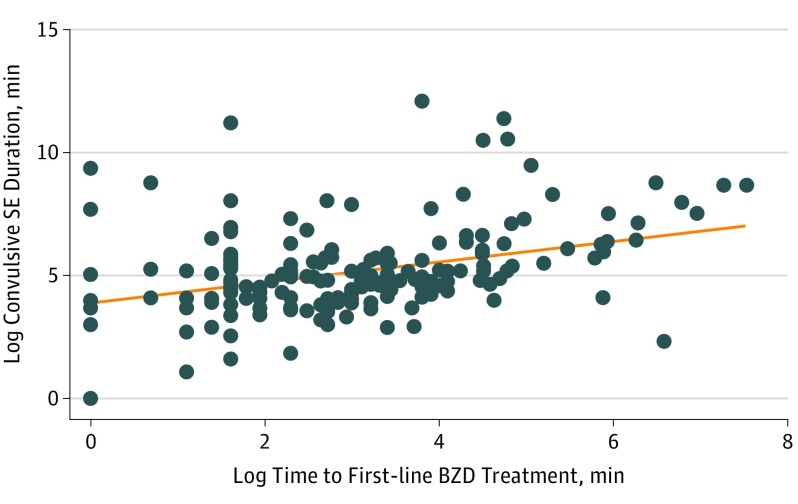
The median (IQR) convulsion duration was 48.5 minutes longer for patients who received untimely first-line benzodiazepine treatment than for those who received it in less than 10 minutes (91 [50-200] minutes vs 139.5 [60-360] minutes; P = .03]). Multivariate analysis showed that the untimely treatment group, compared with the group treated in less than 10 minutes, had greater odds of having a longer-than-the-median convulsive duration (AOR, 2.6; 95% CI, 1.38-4.88; P = .003) and hypotension (AOR, 2.3; 95% CI, 1.16-4.63; P = .02) (Table 4). For every minute in log time to treatment, the log duration of SE increased by 0.32 log minutes (95% CI, 0.21-0.44; P < .001) (Figure 2). The outcomes stratified by RCSE onset are detailed in eTables 2 and 3 in the Supplement.
Table 4. Untimely First-line Benzodiazepine and Other Clinical Outcomesa.
| Variable | Outcome Proportion, No. (%) (N = 218) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Received Untimely Treatment, No. (%) | P Value | AOR | SE (95% CI) | P Value | ||
| More than the median convulsive duration (2 h) | 119 (54.6) | 87 (73.1) | .02 | 2.6 | 0.8 (1.38-4.88) | .003 |
| Decline in baseline function at hospital dischargeb | 47 (21.6) | 33 (70.2) | .49 | 1.8 | 0.7 (0.81-3.80) | .15 |
| ICU stay more than the median duration (4 d) | 112 (51.4) | 73 (65.2) | .89 | 1.1 | 0.4 (0.61-2.09) | .66 |
| Hypotension | 64 (29.4) | 49 (76.6) | .04 | 2.3 | 0.8 (1.16-4.63) | .02 |
| Other medical complications | 58 (26.6) | 39 (67.2) | .87 | 1.2 | 0.4 (0.62-2.36) | .63 |
Abbreviations: AOR, adjusted odds ratio; ICU, intensive care unit; RCSE, refractory convulsive status epilepticus.
The results of AORs for multivariable analysis using logistic regression models, with untimely first-line benzodiazepine treatment (≥10 minutes after seizure onset) as the predictor and different tertiary clinical outcomes. Each outcome was adjusted for age, structural etiology, febrile RCSE, no neurological history, generalized RCSE, continuous RCSE, and use of 5 or more ASMs.
Not able to do the same tasks as before at hospital discharge.
Figure 2. Association Between Log Time to First-line Benzodiazepine (BZD) Treatment and Log Convulsive Status Epilepticus (SE) Duration.
The x-axis represents the time to the first-line BZD treatment in minutes in a log transformation, and the y-axis represents the duration of the convulsive SE duration in minutes in log transformation. A linear association exists between log time to the first-line BZD treatment and the log convulsive SE duration (95% CI, 0.21-0.44; P < .001).
Time to Treatment
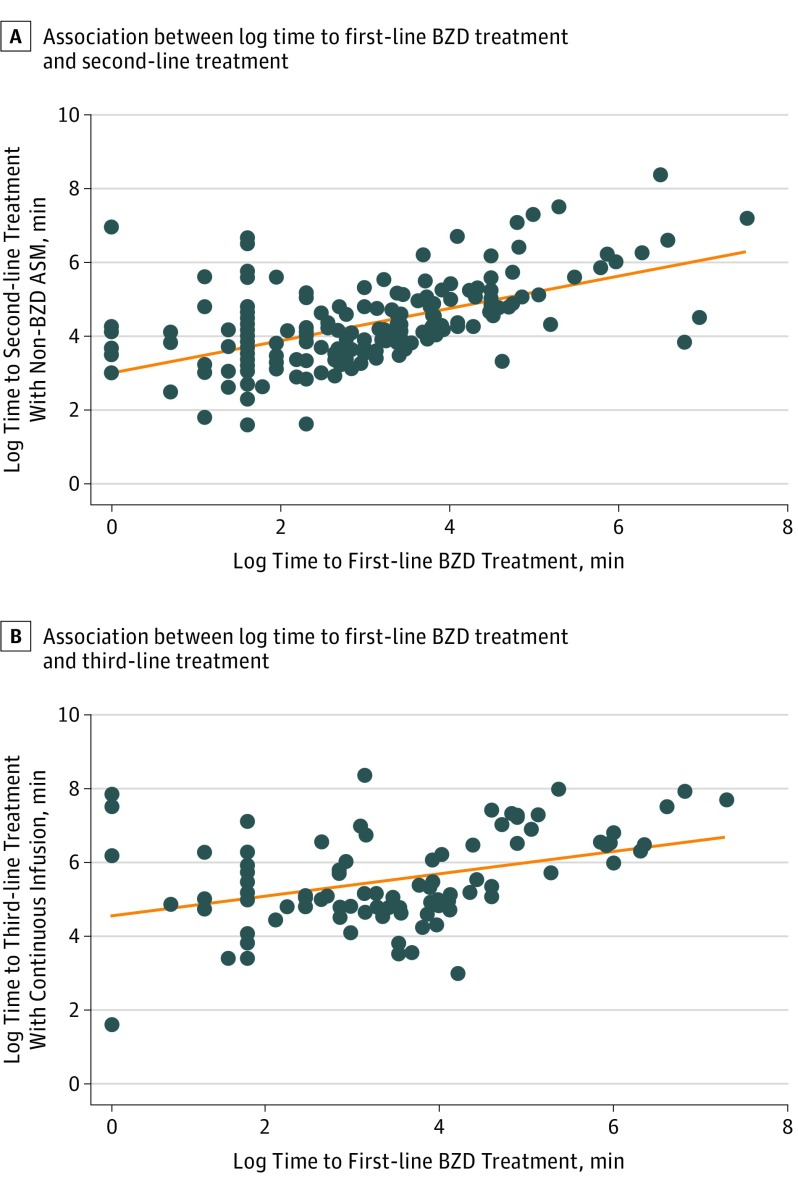
The timing of the first-line benzodiazepine treatment was correlated with the time to the second-line treatment with nonbenzodiazepine ASM; with every 1 minute of delay, the first second-line nonbenzodiazepine ASM was administered 0.8 log minutes later (95% CI, 0.64-0.95; P < .001) (Figure 3A). The timing of the first-line benzodiazepine treatment was also correlated with the time to the third-line treatment with continuous infusion (0.5 log minutes later per minute of delay; 95% CI, 0.25-0.78; P < .001) (Figure 3B). The timing to medical assistance and treatment are summarized in eTable 4 in the Supplement. The median (IQR) time to first-line benzodiazepine treatment was 17 (5-45) minutes, and it was longer for the prehospital group than for the inhospital group (25 [10-60] minutes vs 8 [4-24] minutes; P < .001). The second and third administrations of benzodiazepine (when given) also took longer for those with prehospital SE onset compared with inhospital onset (median [IQR] time, 43.5 [22-96] vs 20.5 [10-45] minutes for second administration of benzodiazepine [P = .001]; median [IQR] time, 65 [45-149] vs 40 [22-189] minutes for third administration of benzodiazepine [P = .04]). The median (IQR) time to first administration of nonbenzodiazepine ASM was 63 (33-150) minutes; it was longer for the prehospital group than for the inhospital group (82 [45-170] minutes vs 40 [22-74] minutes; P < .001) and also longer than guideline recommendations (vs 20 minutes; P < .001). Nonbenzodiazepine ASM included fosphenytoin sodium (104 [49.5%]), levetiracetam (63 [30.0%]), phenytoin sodium (18 [8.6%]), phenobarbital (16 [7.6%]), valproic acid (5 [2.4%]), and others (4 [1.9%]). The second administration of the nonbenzodiazepine ASM also took longer for patients with prehospital SE onset than for patients with inhospital onset (median [IQR] time, 131 [75.5-330] minutes vs 74 [40-206] minutes; P = .002).
Figure 3. Association Between Log Time to First-line Benzodiazepine (BZD) Treatment and Second- and Third-line Treatments.
A, The x-axis represents the time to the first-line BZD treatment in minutes in a log transformation, and the y-axis represents the time to the second-line treatment with non-BZD antiseizure medication (ASM) in minutes in log transformation. A linear association exists between log time to the first-line BZD treatment and log time to the second-line treatment with non-BZD ASM; with every 1 minute change in a log scale of the first-line BZD treatment, the second-line treatment was administered 0.8 log minutes later (95% CI, 0.64-0.95; P < .001). B, The x-axis represents the time to the first-line BZD treatment in minutes in a log transformation, and the y-axis represents the time to the third-line treatment with continuous infusion in minutes also in log transformation. A linear association exists between log time to the first-line BZD treatment and log time to the third-line treatment; with every log minute change of the first-line BZD treatment, the third-line treatment was administered 0.5 log minutes later (95% CI, 0.25-0.78; P < .001).
Discussion
After multivariate analysis, we found that patients who received untimely first-line benzodiazepine treatment died more frequently, received continuous infusions more often, and had longer convulsive SE duration and more frequent hypotension during the admission. In addition, the time to treatment with benzodiazepine and the time to treatment with nonbenzodiazepine ASM were more frequently delayed for the prehospital group compared with the inhospital group, and the time to the first-line benzodiazepine treatment was correlated with the timing of the second- and third-line treatments, suggesting a subsequent delay in the workflow.
Time to Treatment and Death
We could not find previous reports describing an independent association between time to treatment of SE and subsequent death. However, a longer duration of seizures is associated with a higher frequency of death, and prompt ASM treatment can shorten SE duration. A study including 140 adults with SE did not find a difference in time to treatment between a survivor group and a nonsurvivor group (median [IQR] time, 3 [0-90] minutes vs 15 [0-60] minutes). Our study is different in that we included solely pediatric refractory patients and enrolled an overall larger number of children and young adults. Of note, in our study, all patients who died received untimely first-line benzodiazepine treatment; after adjusting for known confounders, we found that this group had an OR of 11 for death.
Preclinical studies have suggested that the development of benzodiazepine pharmacoresistance during SE is the result of an internalization of gamma 2 subunit-containing, benzodiazepine-sensitive γ-aminobutyric acid type A receptors during prolonged seizures. In this study, the first-line benzodiazepine treatment did not terminate seizures, and this later (ie, untimely) benzodiazepine treatment was associated with death. A possible explanation for this finding is that faster first-line benzodiazepine treatment may be a marker for a more aggressive treatment and workflow, in general, and we accordingly detected a linear correlation between the timing of the different treatment lines. All of these aspects may contribute to improved outcomes with faster treatment, always taking into account the cause of the SE.
Time to Treatment and Need of Continuous Infusions
Our data show that patients who received untimely first-line benzodiazepine treatment had greater chances to require continuous infusions, but the data do not distinguish the extent to which subsequent treatments after the administration of first-line agents affected the outcome. Limited evidence exists on the choices of nonbenzodiazepine ASM and on the use of continuous infusions. The established SE trial is evaluating the efficacy of nonbenzodiazepine ASM but not yet of continuous infusions. Previous studies have shown that delayed or inadequate treatment leads to increased refractoriness of SE and to greater chances of requiring second-line treatment. A study involving 126 adults showed that patients who received third-line medications had worse outcomes. This result could be due to the dual consequences of longer seizure duration and exposure to multiple medications with varying adverse outcomes, and those who need continuous infusions could reflect a more refractory group of patients. Our data raise the question of whether the use of continuous infusions could, in part, be prevented by the early administration of first-line treatment.
Time to Treatment and SE Duration
Several studies have shown a relationship between time to treatment and duration of SE. A series of 240 SE episodes in children demonstrated that, for every minute of delay until hospital arrival, patients had a 5% higher risk of having an episode longer than 1 hour. Another study of 120 adults with SE showed that treatment initiated in less than 30 minutes was associated with an 80% response rate, whereas less than 40% of patients responded when treated more than 2 hours after the episode. Our data support these results because patients who received the first-line benzodiazepine treatment in less than 10 minutes had more chances of having a shorter SE episode, and the time to benzodiazepine treatment was linearly associated with the duration of SE.
Opportunities for Improvement in Care
Delay in the treatment of RCSE is independently associated with adverse outcomes. Therefore, SE should be considered a time-sensitive emergency, such as a stroke or other cardiovascular events. Our data set is not designed for detailed factor analysis; however, further work is needed to delineate the aspects contributing to delay. At this point, we hypothesize that several factors could improve outcomes, such as further attention toward faster detection, recognition, and treatment of SE; implementation of seizure action plans; and improved availability and use of rescue medications for patients with known seizures, as well as improved education for families and EMS. The timing of the first-line benzodiazepine treatment is significantly slower in the prehospital setting; therefore, this study could fuel prehospital and quality improvement interventions that could change SE outcomes.
Time to Treatment in SE Treatment Guidelines
To date, exact time thresholds are mostly based on expert opinion. Most SE treatment guidelines recommend benzodiazepine initiation within 5 to 10 minutes from SE onset. Furthermore, seizures normally last less than 10 minutes, and it is unlikely that they stop spontaneously after that time frame. Based on these studies, we decided to evaluate the under-10-minute threshold because 5 minutes is the time when treatment is usually started. In our study, the median (IQR) time to treatment was 17 (5-45) minutes, with a median (IQR) prehospital time of 25 (10-60) minutes and a median (IQR) inhospital time of 8 (4-24) minutes. These results showed that most inhospital patients were already being treated in less than 10 minutes. This under-10-minute goal is realistic for the inhospital setting and for prehospital patients with previous SE or epilepsy; therefore, we recommend the under-10-minute threshold. However, this time frame may be more difficult to achieve for patients with new-onset SE, with longer EMS response times, or in states and institutions where first responders are not authorized to treat.
Strengths and Limitations
This work was a multicenter study using 6 years of prospective data, providing a large but selected population of children with RCSE. Many of the available studies on SE treatment and outcome are based on adult populations, and this cohort permitted us to fill some gaps in the literature. The large sample size allowed us to study both common and rare outcomes and to demonstrate new associations that will affect the treatment of SE. It is not completely clear if benzodiazepine doses are associated with outcomes in SE; because no difference was found in lower doses of first-line benzodiazepine treatment between the groups (P = .19), we did not include the lower dose in the multivariable model. The data on time to treatment were highly skewed, and we therefore used log transformation, which is the standard technique for time-to-event outcomes.
The distribution of time to treatment and death is difficult to characterize because of the small number of events. Hence, we analyzed the outcome of death as a binary outcome using exact logistic regression and adjusted for predictor variables. Exact logistic regression was used owing to the small number of observations. Because one of the cells (benzodiazepine treatment in <10 minutes) had zero deaths, the 95% CI became infinite, but it will decrease when the outcome becomes present in both groups. The small number of deaths limited the number of confounders that we could include in the model without overfitting. We corrected for neurological history, including previous RCSE events. We could not adjust for selected confounders, such as distance between the patient’s home and the center. Although our hypothesis was based on time to treatment and not RCSE-onset setting, we also stratified for inhospital and prehospital RCSE onset. The secondary outcome remained significant after stratification, but the number of the primary outcome in each subgroup did not have enough power for the differences to be statistically significant. We also cannot rule out additional unknown identifiable or nonidentifiable confounders that may have influenced mortality and outcomes, which may have not been detected by our comparative effectiveness approach and would need a randomized control trial.
Therefore, these results need to be interpreted in the setting of data acquisition, including inherent selection and information bias. The episodes that occur in the community and stop spontaneously or with prehospital treatment and do not reach a hospital were not included in this study. In addition, we included patients whose treatment with at least 2 ASMs failed; therefore, these results apply to patients with RCSE. Receiving prolonged status therapy and continuous infusions may be a poor prognostic factor for outcome, possibly owing to confounding by indication. The times to treatment in the prehospital setting were based on information provided by families. We corroborated the information with EMS reports and medical records when available; therefore, we did the best that we could in this study, which was set up to corroborate treatment times.
Conclusions
For pediatric patients with RCSE, the untimely administration of the first-line benzodiazepine treatment (ie, ≥10 minutes after seizure onset) is associated with adverse short-term outcomes, including a higher frequency of death, more frequent use of continuous infusions, longer convulsive seizure duration, and more frequent hypotension. In addition, the timing of the first-line benzodiazepine treatment is associated with the time to treatment of the second- and third-line medications.
eTable 1. Summary of Cases Who Died During RCSE Hospitalization
eTable 2. Untimely First Benzodiazepine (≥10 Minutes) and Clinical Outcomes Stratified by Prehospital RCSE Onset (N = 139)
eTable 3. Untimely First Benzodiazepine (≥10 Minutes) and Clinical Outcomes Stratified by Inhospital RCSE Onset (N = 79)
eTable 4. Time from RCSE Onset to Treatment in the Prehospital and Inhospital Setting
References
- 1.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22(4):489-501. [DOI] [PubMed] [Google Scholar]
- 2.Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC; NLSTEPSS Collaborative Group . Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368(9531):222-229. [DOI] [PubMed] [Google Scholar]
- 3.DeLorenzo RJ, Hauser WA, Towne AR, et al. . A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46(4):1029-1035. [DOI] [PubMed] [Google Scholar]
- 4.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology. 1998;50(3):735-741. [DOI] [PubMed] [Google Scholar]
- 5.Chin RF, Neville BG, Scott RC. A systematic review of the epidemiology of status epilepticus. Eur J Neurol. 2004;11(12):800-810. [DOI] [PubMed] [Google Scholar]
- 6.Maegaki Y, Kurozawa Y, Tamasaki A, et al. ; Status Epilepticus Study Group . Early predictors of status epilepticus-associated mortality and morbidity in children. Brain Dev. 2015;37(5):478-486. [DOI] [PubMed] [Google Scholar]
- 7.Maytal J, Shinnar S, Moshé SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83(3):323-331. [PubMed] [Google Scholar]
- 8.Raspall-Chaure M, Chin RF, Neville BG, Scott RC. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol. 2006;5(9):769-779. [DOI] [PubMed] [Google Scholar]
- 9.Brophy GM, Bell R, Claassen J, et al. ; Neurocritical Care Society Status Epilepticus Guideline Writing Committee . Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3-23. [DOI] [PubMed] [Google Scholar]
- 10.Wilkes R, Tasker RC. Pediatric intensive care treatment of uncontrolled status epilepticus. Crit Care Clin. 2013;29(2):239-257. [DOI] [PubMed] [Google Scholar]
- 11.Glauser T, Shinnar S, Gloss D, et al. . Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenssen S, Gracely EJ, Sperling MR. How long do most seizures last? a systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia. 2006;47(9):1499-1503. [DOI] [PubMed] [Google Scholar]
- 13.Afra P, Jouny CC, Bergey GK. Duration of complex partial seizures: an intracranial EEG study. Epilepsia. 2008;49(4):677-684. [DOI] [PubMed] [Google Scholar]
- 14.Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol. 2001;49(5):659-664. [PubMed] [Google Scholar]
- 15.Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814(1-2):179-185. [DOI] [PubMed] [Google Scholar]
- 16.Goodkin HP, Liu X, Holmes GL. Diazepam terminates brief but not prolonged seizures in young, naïve rats. Epilepsia. 2003;44(8):1109-1112. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson K, Kälviäinen R. Pharmacologic management of convulsive status epilepticus in childhood. Expert Rev Neurother. 2005;5(6):777-783. [DOI] [PubMed] [Google Scholar]
- 18.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59(2):205-210. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez Fernández I, Abend NS, Agadi S, et al. ; Pediatric Status Epilepticus Research Group (pSERG) . Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology. 2015;84(23):2304-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahin M, Menache CC, Holmes GL, Riviello JJ. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42(11):1461-1467. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez Fernández I, Abend NS, Agadi S, et al. ; Pediatric Status Epilepticus Research Group (pSERG) . Gaps and opportunities in refractory status epilepticus research in children: a multi-center approach by the Pediatric Status Epilepticus Research Group (pSERG). Seizure. 2014;23(2):87-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta CR, Patel NR. Exact logistic regression: theory and examples. Stat Med. 1995;14(19):2143-2160. [DOI] [PubMed] [Google Scholar]
- 23.Hirji KF. Exact Analysis of Discrete Data. New York: Taylor and Francis; 2006. [Google Scholar]
- 24.DeLorenzo RJ, Garnett LK, Towne AR, et al. . Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia. 1999;40(2):164-169. [DOI] [PubMed] [Google Scholar]
- 25.Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long-term mortality after a first episode of status epilepticus. Neurology. 2002;58(4):537-541. [DOI] [PubMed] [Google Scholar]
- 26.Legriel S, Mourvillier B, Bele N, et al. . Outcomes in 140 critically ill patients with status epilepticus. Intensive Care Med. 2008;34(3):476-480. [DOI] [PubMed] [Google Scholar]
- 27.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17(19):7532-7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodkin HP, Kapur J. The impact of diazepam’s discovery on the treatment and understanding of status epilepticus. Epilepsia. 2009;50(9):2011-2018. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez Fernández I, Loddenkemper T. Subunit composition of neurotransmitter receptors in the immature and in the epileptic brain. Biomed Res Int. 2014;2014:301950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleck T, Cock H, Chamberlain J, et al. . The established status epilepticus trial 2013. Epilepsia. 2013;54(suppl 6):89-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aranda A, Foucart G, Ducassé JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia. 2010;51(10):2159-2167. [DOI] [PubMed] [Google Scholar]
- 32.Kowalski RG, Ziai WC, Rees RN, et al. . Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med. 2012;40(9):2677-2684. [DOI] [PubMed] [Google Scholar]
- 33.Madžar D, Geyer A, Knappe RU, et al. . Association of seizure duration and outcome in refractory status epilepticus. J Neurol. 2016;263(3):485-491. [DOI] [PubMed] [Google Scholar]
- 34.Alldredge BK, Wall DB, Ferriero DM. Effect of prehospital treatment on the outcome of status epilepticus in children. Pediatr Neurol. 1995;12(3):213-216. [DOI] [PubMed] [Google Scholar]
- 35.Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study [published correction appears in Lancet Neurol. 2008;7(9):771]. Lancet Neurol. 2008;7(8):696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43(3, pt 1):483-488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of Cases Who Died During RCSE Hospitalization
eTable 2. Untimely First Benzodiazepine (≥10 Minutes) and Clinical Outcomes Stratified by Prehospital RCSE Onset (N = 139)
eTable 3. Untimely First Benzodiazepine (≥10 Minutes) and Clinical Outcomes Stratified by Inhospital RCSE Onset (N = 79)
eTable 4. Time from RCSE Onset to Treatment in the Prehospital and Inhospital Setting