This analysis of data from a phase 1/2 study evaluates potential cases of isocitrate dehydrogenase differentiation syndrome among patients treated with enasidenib for relapsed/refractory acute myeloid leukemia.
Key Points
Question
What are the signs, symptoms, and appropriate approaches to management of isocitrate dehydrogenase differentiation syndrome, an unexpected event observed in the first clinical study of enasidenib mesylate, recently approved for relapsed/refractory mutant IDH2 acute myeloid leukemia?
Findings
In a phase 1/2 clinical trial of 281 patients with relapsed/refractory acute myeloid leukemia treated with enasidenib, possible or probable isocitrate dehydrogenase differentiation syndrome was identified in 33 patients and had recognizable signs and symptoms, including dyspnea, unexplained fever, pulmonary infiltrates, and hypoxia. These signs and symptoms could occur months after initiation of enasidenib treatment and could mimic symptoms seen during treatment and progression of myeloid neoplasms; prompt intervention with systemic corticosteroid therapy was an effective management approach.
Meaning
As use of mutant isocitrate dehydrogenase inhibitors increases, awareness of the potential for isocitrate dehydrogenase differentiation syndrome is imperative so that patients can be promptly and effectively treated.
Abstract
Importance
Enasidenib mesylate, a mutant isocitrate dehydrogenase 2 (IDH2) protein inhibitor that promotes differentiation of leukemic myeloblasts, was recently approved by the US Food and Drug Administration for use in relapsed/refractory (R/R) mutant IDH2 acute myeloid leukemia (AML). During the first study of enasidenib in humans, a minority of patients with advanced myeloid neoplasms experienced unexpected signs/symptoms of a differentiation syndrome (DS), a potentially lethal entity.
Objective
To characterize IDH-inhibitor–associated DS (IDH-DS) and its effective management.
Design, Setting, and Participants
Using data obtained from a multicenter, open-label, pivotal phase 1/2 study of enasidenib, a differentiation syndrome review committee retrospectively evaluated potential cases of IDH-DS in enasidenib-treated patients with R/R AML. Data were collected between August 27, 2013, and October 14, 2016. The committee identified and agreed on signs and symptoms characteristic of IDH-DS and developed an algorithm for identification and treatment. Among 281 patients with R/R AML enrolled in the trial, the committee identified 72 patients for review based on investigator-reported cases of DS (n = 33) or reported adverse events or signs and symptoms characteristic of IDH-DS.
Interventions
Treatment with enasidenib at a dosage of 50 to 650 mg/d was evaluated during the dose-escalation phase, and a dosage of 100 mg/d was used in the phase 1 expansion and phase 2, all in continual 28-day cycles.
Main Outcomes and Measures
Unexpected adverse events of IDH-DS during the phase 1/2 study.
Results
Thirty-three of the 281 patients (11.7%) were identified as having possible or probable IDH-DS. Median age of those 33 patients was 70 years (range, 38-80 years); 20 (60.6%) were male. The most frequent manifestations were dyspnea, fever, pulmonary infiltrates, and hypoxia. Median time to onset was 30 days (range, 7-129 days). Patients who experienced IDH-DS were less likely to have less than 20% bone marrow blasts (6% vs 22%, P = .04) and more likely to have undergone fewer previous anticancer regimens (median, 1.0 [range, 1-4] vs 2.0 [range, 1-14], P = .05) at study entry than those who did not. Thirteen patients (39.4%) had concomitant leukocytosis. Isocitrate dehydrogenase differentiation syndrome was effectively managed with systemic corticosteroids. The enasidenib regimen was interrupted for 15 patients (45.5%), but permanent discontinuation of treatment was not required.
Conclusions and Relevance
Isocitrate dehydrogenase differentiation syndrome is a recognizable and potentially lethal clinical entity, occurring in approximately 12% of enasidenib-treated patients with mutant-IDH2 R/R AML. It requires prompt recognition and management. As use of mutant IDH inhibitors increases, these findings and recommendations are increasingly germane to care of patients with mutant-IDH neoplasms.
Trial Registration
clinicaltrials.gov Identifier: NCT01915498
Introduction
Genetic and epigenetic alterations that block cellular differentiation underlie the development of myeloid neoplasms.1 Agents that induce resumption of differentiation may be clinically beneficial; however, drug-induced differentiation of leukemic cells can be accompanied by a potentially serious condition, differentiation syndrome (DS), as seen during treatment of acute promyelocytic leukemia.2,3,4,5 Proliferation of differentiated leukemic cells can alter cytokine balance, leading to tissue damage and inflammation.4 Signs and symptoms of DS include unexplained fever, weight gain, respiratory symptoms, and pleural or pericardial effusions.4
Enasidenib mesylate (AG-221; IDHIFA; Celgene Corporation) is a first-in-class, oral inhibitor of mutant isocitrate dehydrogenase 2 (mIDH2) proteins. Enasidenib was approved by the US Food and Drug Administration (FDA) in August 2017 for treatment of adult patients with mutant IDH2 (OMIM 147650) (mIDH2) relapsed/refractory acute myeloid leukemia (R/R AML). Mutant IDH2 proteins neomorphically catalyze the oncometabolite, (R)-2-hydroxyglutarate ([R]-2HG).6 High (R)-2HG concentrations promote hypermethylation, altered gene expression, and blocked differentiation of hematopoietic cells.7 Enasidenib suppresses 2HG and induces differentiation of mIDH2 leukemic cells, producing fully functioning neutrophils that retain the IDH2 mutation.8,9 Patients who respond to treatment with enasidenib exhibit evidence of hematopoietic recovery, typically without intervening bone marrow aplasia.3,8
In the first human, phase 1/2 study of enasidenib in patients with mIDH2 malignant hematologic neoplasms, investigators reported adverse events reminiscent of those seen in DS. Similar events have been reported for ivosidenib (AG-120), a mutant IDH1 protein inhibitor.10 These signs and symptoms of mutant-IDH inhibitor–associated DS (termed IDH differentiation syndrome [IDH-DS])11 are nonspecific and can resemble other clinical conditions common in AML. To characterize IDH-DS and establish practicable diagnostic and therapeutic recommendations, an independent differentiation syndrome review committee (DSRC) retrospectively evaluated potential cases of IDH-DS among patients with R/R AML enrolled in the aforementioned study.
Methods
This was a multicenter, open-label, pivotal phase 1/2 study. Study design, conduct, eligibility criteria, and clinical response and safety assessments in the phase 1 dose-escalation and expansion study portions are reported elsewhere.3 Phase 2 enrollment was limited to adult patients with R/R AML, all of whom received 100 mg of enasidenib once daily. This study was conducted in accordance with the Declaration of Helsinki.12 All patients provided written informed consent. The institutional review boards that approved the study can be found in the eAppendix in the Supplement.
The DSRC independently reviewed cases of investigator-reported DS and those with adverse events suggestive of IDH-DS (eTable 1 in the Supplement). Isocitrate dehydrogenase differentiation syndrome was considered when characteristic signs and symptoms occurred in the absence of significant secondary causes, with concurrent evidence of cellular differentiation and proliferation in the blood or bone marrow, and with subsequent clinical response to corticosteroid therapy. Hematologic response was assessed per International Working Group 2003 AML criteria.13 Baseline characteristics of patients who did or did not experience IDH-DS were summarized and compared using the Wilcoxon Mann-Whitney test (continuous variables) or Fisher exact test (categorical variables). P values are 2-sided, with a significance level of .05. Statistics were performed using SAS 9.2 statistical software (SAS Institute Inc).
Results
Two hundred eighty-one patients with R/R AML participated between August 27, 2013, and data cutoff (October 14, 2016). The DSRC reviewed 72 suspected cases of IDH-DS and, of them, identified 33 patients (11.7% of all patients with R/R AML) as having experienced possible or probable IDH-DS. The median age of patients with possible or probable IDH-DS was 70 years (range, 38-80 years). Twenty of the 33 (60.6%) were male. Twenty-five patients had 1 IDH-DS episode, 6 had 2 episodes, and 2 experienced 3 episodes. Reasons for rejecting an IDH-DS diagnosis included alternative causes for symptoms, lack of clinical evidence of cellular differentiation, lack of response to corticosteroid therapy, and clear evidence of disease progression as a more likely explanation for the constellation of clinical signs and/or symptoms.
Baseline characteristics were generally comparable between patients who experienced IDH-DS and those who did not (eTable 2 in the Supplement). Patients with IDH-DS were significantly less likely to have had less than 20% bone marrow blasts (6% vs 22%, P = .04) and more likely to have undergone a lower median number of previous antileukemic regimens (median, 1.0 [range, 1-4] vs 2.0 [range, 1-14], P = .05). Higher peripheral blast counts (median 31% [range, 0%-88%] vs 13% [range, 0%-98%], P = .05) and greater proportion of those with lactate dehydrogenase levels above 1 × upper limit of normal (51% vs 36%, P = .09) were noted for patients with IDH-DS, but these variables did not reach statistical significance. In multivariate analysis, only a lesser median number of previous antileukemic therapies was significantly associated with IDH-DS (odds ratio, 0.66; 95% CI, 0.44-1.00; P = .05).
Median time from beginning of enasidenib treatment to IDH-DS onset was 30 days (range, 7-129 days); onset at day 129 occurred for 1 patient after a treatment interruption of approximately 1 month. The most frequent clinical manifestations of IDH-DS were dyspnea, culture-negative fever, pulmonary infiltrates, and hypoxia (Table 1). Ten patients required intensive care unit admission for related symptoms. Leukocytosis occurred concurrently in 13 patients. No death was attributed to IDH-DS. Clinical and laboratory profiles for 2 illustrative cases in patients who experienced IDH-DS are described in eFigures 1 and 2 in the Supplement.
Table 1. Frequency of Signs and Symptoms Consistent With IDH-DSa.
| Sign or Symptom | Patients With IDH-DS, No. (%) (n = 33)b |
|---|---|
| Dyspnea | 28 (85) |
| Unexplained fever (body temperature of 38.0°C for 2 d) | 26 (79) |
| Pulmonary infiltrates | 24 (73) |
| Hypoxia | 19 (58) |
| Acute kidney injury (CTCAE grade ≥2) | 14 (42) |
| Pleural effusion | 14 (42) |
| Bone pain or arthralgia | 9 (27) |
| Lymphadenopathy | 8 (24) |
| Rash | 8 (24) |
| Disseminated intravascular coagulopathy | 7 (21) |
| Edema or weight gain of >5 kg from screening | 7 (21) |
| Pericardial effusion | 5 (15) |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events14; IDH-DS, isocitrate dehydrogenase differentiation syndrome.
Signs and symptoms included in this table are based on retrospective differentiation syndrome review committee review of clinical records.
Patients may have had multiple symptoms.
Dosages greater and less than 100 mg/d were evaluated in small patient cohorts during dose escalation. Events related to IDH-DS occurred in none of 19 patients with R/R AML receiving enasidenib dosages less than 100 mg/d, 26 of 214 patients (12.1%) receiving 100 mg/d, and 7 of 48 patients (14.6%) receiving greater than 100 mg/d. Frequency of IDH-DS was not statistically different among these groups.
IDH-DS Management
Twenty-eight of the 33 patients with IDH-DS (84.8%) received symptomatic treatment with corticosteroids. For 20 patients with available data, median duration of corticosteroid therapy for IDH-DS was 12 days (range, 4-43 days). Eleven of 13 patients with concomitant leukocytosis received hydroxyurea (median, 15 days [range, 4-71 days]). The enasidenib regimen was interrupted for 15 patients (45.5%) until IDH-DS symptoms improved. Two patients (6.1%) had dosage reduced, but none discontinued enasidenib therapy permanently because of IDH-DS.
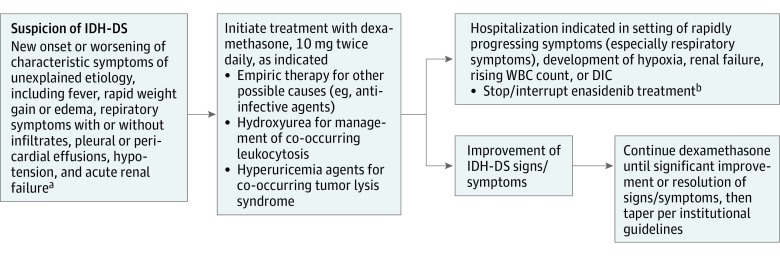
Isocitrate dehydrogenase differentiation syndrome management guidelines have been incorporated into an amended protocol. At first suspicion of IDH-DS, dexamethasone, 10 mg twice daily, is recommended until signs and symptoms are significantly improved (Figure).
Figure. Differentiation Syndrome Review Committee Amended Protocol for Isocitrate Dehydrogenase Differentiation Syndrome (IDH-DS) Diagnosis and Management.
DIC indicates disseminated intravascular coagulation; WBC, white blood cells.
aTypical onset is between 7 to 10 days and 5 months from start of enasidenib treatment or reinitiation of enasidenib after prolonged treatment interruption.
bOwing to the long half-life of enasidenib, treatment may not immediately reverse symptoms of IDH-DS.
Efficacy
Overall response rate for patients with IDH-DS was 45.5% (15 of 33 patients) and for patients without IDH-DS, 37.5% (93 of 248) (Table 2). Complete remission or complete remission with incomplete platelet or neutrophil count recovery was experienced by 36.4% of patients (12 of 33) in the IDH-DS group and 26.2% (65 of 248) in the non–IDH-DS group (P = .22). Disease remained stable in approximately half of the patients in each group.
Table 2. Response Among Patients With and Without IDH-DS.
| Patient Response | IDH-DS, No. (%) (n = 33)a | No IDH-DS, No. (%) (n = 248) |
|---|---|---|
| Overall responseb | 15 (45.5) | 93 (37.5) |
| CR | 6 (18.2) | 49 (19.8) |
| CRi/CRp | 6 (18.2) | 16 (6.5) |
| PR | 2 (6.1) | 14 (5.7) |
| MLFS | 1 (3.0) | 14 (5.7) |
| Stable diseasec | 16 (48.5) | 121 (48.8) |
| Disease progression | 1 (3.0) | 14 (5.7) |
Abbreviations: CR, complete remission; CRi, CR with incomplete hematologic recovery; CRp, CR with incomplete platelet recovery; IDH-DS, isocitrate dehydrogenase differentiation syndrome; MLFS, morphologic leukemia-free state; PR, partial remission; R/R AML, relapsed/refractory acute myeloid leukemia.
One patient was not evaluable for response; the patient developed IDH-DS on the ninth study day and discontinued treatment before a response assessment.
Investigator-reported overall response rate included CR, CRi/CRp, PR, or MLFS.
No international working group–defined response with no evidence of disease progression, sustained for 56 consecutive days.
Discussion
Isocitrate dehydrogenase differentiation syndrome is a newly defined clinical entity in patients with mIDH2 AML receiving enasidenib, likely owing to the drug’s mechanism of action, promoting differentiation of myeloblasts into mature cells.8 No single pathognomonic clinical sign or laboratory result is diagnostic of IDH-DS. A constellation of findings supports the diagnosis, which can be challenging because signs and symptoms can mimic those of leukemic progression or other acute comorbidities. Diagnosis should be suspected on exclusion of other potential causes of characteristic symptoms.
Our results showed that IDH-DS was significantly associated with fewer previous anticancer therapies and nonsignificantly associated with higher baseline peripheral blast counts (P = .05) and lactate dehydrogenase levels (P = .09), both laboratory markers suggestive of cellular turnover. Why fewer preceding therapies might increase risk is unclear. This IDH-DS study population is small; further study is needed to determine whether reliable biomarkers of the potential to develop IDH-DS exist.
The rate of IDH-DS with enasidenib (11.7%) was lower than DS rates reported with all-trans retinoic acid or arsenic trioxide in acute promyelocytic leukemia .5,15 Unlike all-trans retinoic acid– or arsenic trioxide–induced DS, which have a typical onset of approximately 7 to 12 days from treatment initiation),4 IDH-DS onset can occur from 7 days to 5 months after initiation of enasidenib treatment. In addition, IDH-DS can occur after enasidenib therapy interruption or dose escalation. Onset appears to correspond to enasidenib-induced myeloid cell differentiation and maturation, similar to the “neutrophil surge” associated with DS in patients treated with FLT3 (FMS-like tyrosine kinase 3) inhibitors, such as quizartinib.2
Although potentially life-threatening, IDH-DS can be effectively managed. When alternative secondary causes for characteristic symptoms are excluded or do not respond to treatment, or if symptoms worsen within 48 hours of initiating treatment for a different suspected cause, the event should be managed as potential IDH-DS (Figure). For concomitant leukocytosis, cytoreduction per local standard practice is recommended.
Approximately half of patients with IDH-DS received enasidenib therapy without interruption. If associated with severe pulmonary symptoms and/or renal dysfunction lasting more than 48 hours after corticosteroid treatment initiation, enasidenib therapy should be withheld until symptoms improve. Symptoms may not resolve immediately. Given the prolonged half-life of enasidenib (approximately 137 hours),16 drug treatment interruption is not an alternative to corticosteroid use.
Limitations
The studied population was relatively small, and larger studies are needed. In addition, because IDH-DS was not initially anticipated, the protocol did not define proactive monitoring for it; however, IDH-DS monitoring is now part of clinical protocols for enasidenib studies.
Conclusions
As use of mutant-IDH inhibitors increases, these recommendations are increasingly germane to care of patients with mutant-IDH hematologic neoplasms.
eAppendix. List of IECs/IRBs for AG221-C-001
eTable 1. MedDRA Terms Searched in the Safety Database for Assessment of Potential IDH-DS
eTable 2. Baseline Characteristics
eFigure 1. Case 1: Clinical and Laboratory Profiles for a Responding Patient With Enasidenib-Induced IDH-DS
eFigure 2. Case 2: Clinical and Laboratory Profiles for a Patient With Enasidenib-Induced IDH-DS With Sustained Stable Disease
References
- 1.Korn C, Méndez-Ferrer S. Myeloid malignancies and microenvironment. Blood. 2017;129(7):811-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sexauer A, Perl A, Yang X, et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120(20):4205-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montesinos P, Sanz MA. The differentiation syndrome in patients with acute promyelocytic leukemia: experience of the pethema group and review of the literature. Mediterr J Hematol Infect Dis. 2011;3(1):e2011059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tallman MS, Andersen JW, Schiffer CA, et al. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95(1):90-95. [PubMed] [Google Scholar]
- 6.Losman JA, Kaelin WG Jr. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev. 2013;27(8):836-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen K, Travins J, Wang F, et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7(5):478-493. [DOI] [PubMed] [Google Scholar]
- 9.Amatangelo MD, Quek L, Shih A, et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130(6):732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birendra KC, DiNardo CD. Evidence for clinical differentiation and differentiation syndrome in patients with acute myeloid leukemia and IDH1 mutations treated with the targeted mutant IDH1 inhibitor, AG-120. Clin Lymphoma Myeloma Leuk. 2016;16(8):460-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medical Dictionary for Regulatory Activities. https://www.meddra.org/. Accessed November 8, 2017.
- 12.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Bennett JM, Kopecky KJ, et al. ; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia . Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia [published correction in J Clin Oncol. 2004;22(3):576]. J Clin Oncol. 2003;21(24):4642-4649. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health and National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE), v4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Updated June 14, 2010. Accessed November 17, 2017.
- 15.Camacho LH, Soignet SL, Chanel S, et al. Leukocytosis and the retinoic acid syndrome in patients with acute promyelocytic leukemia treated with arsenic trioxide. J Clin Oncol. 2000;18(13):2620-2625. [DOI] [PubMed] [Google Scholar]
- 16.Fan B, Chen Y, Wang F, et al. Pharmacokinetic/pharmacodynamic (PK/PD) evaluation of AG-221, a potent mutant IDH2 inhibitor, from a phase 1 trial of patients with IDH2 mutation-positive hematologic malignancies [abstract 379]. Haematologica. 2015;100(suppl 1):379. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. List of IECs/IRBs for AG221-C-001
eTable 1. MedDRA Terms Searched in the Safety Database for Assessment of Potential IDH-DS
eTable 2. Baseline Characteristics
eFigure 1. Case 1: Clinical and Laboratory Profiles for a Responding Patient With Enasidenib-Induced IDH-DS
eFigure 2. Case 2: Clinical and Laboratory Profiles for a Patient With Enasidenib-Induced IDH-DS With Sustained Stable Disease