Key Points
Question
Do anesthesia and surgery lead to changes in levels of neurofilament light and tau, 2 biomarkers of neurological injury now detectable in blood?
Findings
In this study, mean plasma neurofilament light increased postoperatively to a maximal increase at 48 hours. Tau levels also increased significantly from baseline with a peak increase at 6 hour postoperatively, after which they declined but still remained elevated for at least 48 hours.
Meaning
These findings indicate that general anesthesia and surgery exert neurotoxicity on the central nervous system, which has implications for both neurological outcomes and for the mechanism of anesthesia.
This study assesses neuronal injury by measuring plasma levels of neurofilament light and tau biomarkers in a series of timed collections in the 48 hours after anesthesia and surgery.
Abstract
Importance
Anesthesia and surgery are believed to act on the central nervous system by a fully reversible mechanism innocuous to nerve cells. Evidence that neurological sequelae may follow would challenge this belief and would thereby suggest a need to reassess theories of the mechanism of anesthetic action or the response of the central nervous system to surgery.
Objective
To measure 2 biomarkers of neurological injury (neurofilament light and tau) in plasma in a series of timed collections before and after anesthesia and surgery.
Design, Setting, and Participants
These 2 related observational studies (CAPACITY and ARCADIAN) recruited patients 60 years and older who were undergoing general anesthesia for surgeries performed within a tertiary hospital. Blood samples were taken immediately before surgical anesthesia was administered and then sequentially after surgery at 30-minute, 6-hour, 24-hour, and 48-hour intervals. Sampling took place from January 2014 to August 2015. Data analysis took place from October 2016 to February 2017.
Main Outcomes and Measures
Plasma neurofilament light and tau.
Results
A total of 30 patients were enrolled (13 from the CAPACITY study and 17 from the ARCADIAN study). The mean (SD) age was 69.1 (7.0) years, and 18 members (59%) of the participant group were female; 22 (73%) were undergoing joint arthroplasty. Mean neurofilament light increased at each measurement from a combined baseline mean (SD) of 22.3 (20.4) pg/mL to a maximal combined mean (SD) level of 35.1 (28.7) pg/mL, a maximum increase of 67% (95% CI, 45%-89%; P < .001), at 48 hours postoperatively. The level of tau increased significantly from baseline at every measurement, from a combined baseline mean (SD) of 3.1 (1.3) pg/mL to a maximal combined mean (SD) of 10.8 (9.5) pg/mL, a peak increase of 257% (95% CI, 154%-361%; P < .001), at 6 hours postoperatively. After 6 hours, the mean level began to return to baseline but remained elevated after 48 hours.
Conclusions and Relevance
Neurofilament light is a specific marker of axonal injury and has been shown to indicate neuronal damage in a number of diseases. Tau proteins are an integral component of axonal integrity, and increased tau indicates neuronal damage. The increases in both neurofilament light and tau over 48 hours after surgery suggest that general anesthesia and surgery may be associated with neuronal damage in the short term. Further investigations will be required to study any association with clinical outcomes. These preliminary findings demand that we question the prevailing assumption that anesthesia and surgery are innocuous, transient, and without injurious changes to the central nervous system.
Introduction
Since the first public demonstration of general anesthesia in 1846, it has generally been believed that the state of general anesthesia is temporary, reversible, and nondamaging to the central nervous system (CNS). This belief stemmed from the empirical observation that most patients rapidly recovered their mental faculties after anesthesia and surgery. Consequently, theories of the mechanism of anesthesia have universally encompassed processes that are fully reversible and do not damage the CNS. These theories have evolved over time and have ranged from the purely physicochemical (eg, the Overton-Meyer theory1), to current theories that evoke receptor mechanisms involving γ-aminobutyric acid activation,2 N-methyl-d-aspartate receptor blockade, and changes in neural connectivity.1,2,3 Regardless of the specific mechanisms, all these theories imply the belief that general anesthesia does not cause neuronal damage but rather acts on the CNS by a fully reversible mechanism.
Our implicit faith in the safety of anesthesia and surgery on the CNS has been at odds with some clinical observations, however. Cognitive decline after anesthesia and surgery, particularly in elderly patients, is now well recognized, with an incidence 3 months after surgery of approximately 10%.4,5 Research into postoperative cognitive decline has focused on causes external to the CNS, including physiological perturbations such as hypoxemia, hypotension, cardiopulmonary bypass,6 or neuroinflammation.7 Primary neurological damage by anesthesia or surgery in humans has rarely been seriously entertained.
However, neuronal proteins are released to plasma in response to acute neuronal injury (eg, acute brain trauma and concussion), as well as acute ischemia, where the degree of increase correlates with severity of injury and clinical outcome.8,9,10 This is analogous to the release of the troponins after myocardial infarction, which correlates with degree of damage to myocardial cells.11 As substudies of 2 clinical studies investigating cognition after anesthesia and surgery, we used highly sensitive assays in blood to measure neurofilament light (NFL) and tau, 2 biomarkers of neurological injury, in participants in the first 48 hours after surgical incision as a means of assessing neuronal injury induced by anesthesia and surgery.
Methods
Data reported here were derived from blood samples obtained from participants in 2 observational clinical studies approved by St Vincent’s Hospital Melbourne Human Research Ethics Committee. Written informed consent was obtained from all participants.
The first study is the Cerebrospinal Fluid and Preclinical Alzheimer Cognitive Trajectory (CAPACITY) study (ACTRN12612000493842), and the second is the Assessment and Review of Cognition, Alzheimer Disease, and Inflammation in Elderly Patients After Hospital Intervention (ARCADIAN) Study (ACTRN12615001070527). Both studies recruited patients 60 years and older who were undergoing surgery with general anesthesia that consisted of either a volatile agent (sevoflurane or desflurane) or intravenous anesthesia (propofol). The general anesthesia type was chosen according to the preference of the anesthesiologist. For hip and knee arthroplasty, general anesthesia was usually administered in combination with spinal anesthesia (bupivacaine). Patients undergoing knee arthroplasty may have also received femoral nerve or sciatic nerve blocks (ropivicaine).
The CAPACITY study initially recruited 59 participants commencing in June 2013. However, blood sampling was only introduced from January 2014 through August 2014,12 and therefore samples were taken from 13 of the 59 participants (22%). Data from these participants are included in this study.
The ARCADIAN study commenced in May 2015. By August 2015, 17 participants had undergone sequential blood sampling. Combining these participants with those of the CAPACITY trial resulted in a total cohort size of 30 people.
Blood for both studies was sampled preoperatively and at 6 hours, 24 hours, and 48 hours after surgical incision; an additional sample was taken at 30 minutes after surgery for the participants in the ARCADIAN study. The identical blood sampling times in adults of similar age undergoing general anesthesia and surgery provided an opportunity to pool blood assay results.
Blood samples (approximately 10 mL) were taken and stored on ice in vacutainer tubes (Becton Dickinson) containing the anticoagulant EDTA. Within 1 hour, these tubes were centrifuged at 4°C for 10 minutes at 4000 revolutions per minute, and then 500 μL aliquots were pipetted into Eppendorf tubes (Becton Dickinson). Samples were stored at −80°C before being transported by medical courier on dry ice at −80°C to the Clinical Neurochemistry Laboratory at Sahlgrenska University Hospital, Mölndal, Sweden, for analysis.
Plasma tau concentrations were measured using a commercial kit developed for the single molecule array (Simoa) platform (Quanterix).13 This assay uses antibodies against N-terminal tau sequences and is also likely to measure all forms of tau, irrespective of posttranslational modifications. Plasma NFL concentration was measured using an in-house assay on the Simoa platform.14
Because the assays were relatively new, different lots may give different absolute concentrations. All measurements for each study were performed in 1 round using 1 batch of reagents by board-certified laboratory technicians blinded to clinical information. Intraassay coefficients of variation were less than 10% for all measurements, indicating that relative changes are valid to compare. Therefore, for the analysis of change over time, the percentage of change from baseline in each individual was used.
The ARCADIAN and CAPACITY studies included follow-up for cognitive testing up to 18 months after surgery. Because analysis of these data is still undergoing for the 2 studies, no data on cognition are included in this analysis.
Statistical Analysis
Group comparisons were made using independent t tests for continuous variables, the Pearson correlation coefficient for continuous variables, and the χ2 or Fisher exact test for dichotomous data. All hypothesis testing was 2-tailed. A P value of less than .05 was used to indicate significance. Generalized linear models were used for multivariable analysis. Tests were performed using Stata, version 14.0 (StataCorp). The data were initially reviewed in August 2016, and full statistical analysis was done in October 2016 and finalized in December 2016 and February 2017.
Results
For the CAPACITY study, 690 patients were screened, resulting in 59 participants enrolled, of whom 13 underwent sequential blood sampling. For the ARCADIAN study, 1117 patients were screened to enroll the first 17 participants for this investigation. The mean (SD) age was 69.1 (7.0) years, and 18 of 30 patients (59%) were female. In total, there were 30 study patients, of whom 23 underwent major lower limb surgery with general anesthesia and spinal and/or perineural blockade (17 hip arthroplasty, 5 knee arthroplasty, and 1 vascular surgery), and 7 underwent major cavity surgery (6 cardiac surgery and 1 esophagogastrectomy with an epidural). General anesthesia was induced with propofol and maintained with either volatile anesthesia (sevoflurane in 10 patients and desflurane in 1 patient) or propofol infusion (19 patients). Fentanyl was used to supplement the anesthesia in all patients.
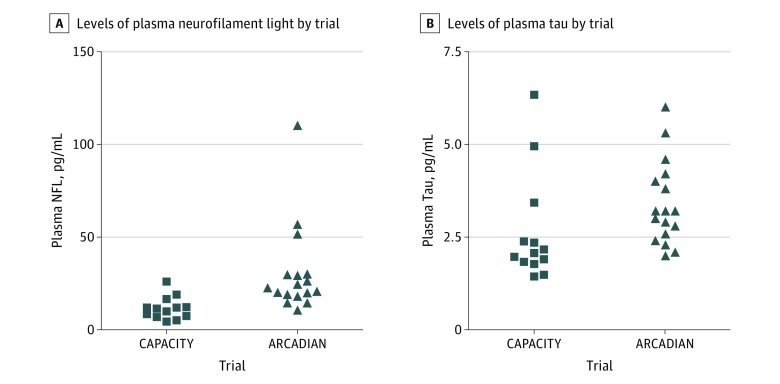
The mean plasma NFL values were significantly different between the 2 study groups at baseline (Table). The baseline values for both NFL and tau are shown in Figure 1. Blood samples were analyzed at 30 minutes (16 of 17 ARCADIAN participants; 94%), 6 hours (28 of 30; 93%), 24 hours (29 of 30; 97%), and 48 hours (26 of 30; 87%) (Table).
Table. Plasma Levels of Neurofilament Light and Tau Over Time, Stratified by Study.
| Time After Surgical Incision, h | CAPACITY Study | ARCADIAN Study | Combined Mean (SD), pg/mL | P Valuea | ||
|---|---|---|---|---|---|---|
| Mean (SD), pg/mL | Patients, No. (%) (n = 13) | Mean (SD), pg/mL | Patients, No. (%) (n = 17) | |||
| Neurofilament Light | ||||||
| 0 (baseline) | 11.7 (6.0) | 13 (100) | 30.4 (23.9) | 17 (100) | 22.3 (20.4) | .01 |
| 0.5 | NAb | NA | 29.5 (23.5) | 16 (94) | NA | NA |
| 6 | 17.2 (8.2) | 12 (92) | 35.6 (27.6) | 16 (94) | 27.7 (23.2) | .03 |
| 24 | 16.7 (5.1) | 13 (100) | 44.0 (25.9) | 16 (94) | 31.8 (23.7) | <.001 |
| 48 | 15.5 (4.1) | 12 (92) | 51.9 (30.1) | 14 (82) | 35.1 (28.7) | <.001 |
| Tau | ||||||
| 0 (baseline) | 2.6 (1.5) | 13 (100) | 3.4 (1.1) | 17 (100) | 3.1 (1.3) | .10 |
| 0.5 | NAb | NA | 7.0 (3.9) | 16 (94) | NA | NA |
| 6 | 7.5 (6.4) | 12 (92) | 13.2 (10.8) | 16 (94) | 10.8 (9.5) | .01 |
| 24 | 3.1 (1.9) | 13 (100) | 6.9 (4.2) | 16 (94) | 5.2 (3.8) | .01 |
| 48 | 3.0 (2.0) | 12 (92) | 5.0 (2.0) | 14 (82) | 4.1 (2.2) | .02 |
Abbreviations: ARCADIAN, Assessment and Review of Cognition, Alzheimer Disease, and Inflammation in Elderly Patients After Hospital Intervention; CAPACITY, Cerebrospinal Fluid and Preclinical Alzheimer Cognitive Trajectory; NA, not applicable.
P values compare the 2 study groups.
By design, blood samples were not taken 30 minutes after surgical incision in the CAPACITY study.
Figure 1. Baseline Values for Neurofilament Light (NFL) and Tau.
A and B, ARCADIAN stands for Assessment and Review of Cognition, Alzheimer Disease, and Inflammation in Elderly Patients After Hospital Intervention, and CAPACITY stands for Cerebrospinal Fluid and Preclinical Alzheimer Cognitive Trajectory.
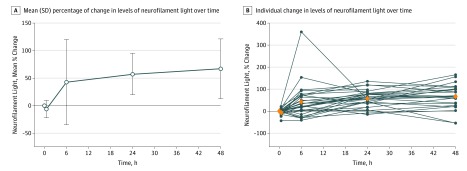
Mean plasma NFL levels increased at each measurement from baseline, from a combined baseline mean (SD) of 22.3 (20.4) pg/mL to a maximal combined mean (SD) of 35.1 (28.7) pg/mL, an increase of 67% (95% CI, 45%-89%; P < .001), at 48 hours postoperatively (Figure 2). The changes from baseline were a combined mean change of −6.4% at 30 minutes (95% CI, 43%-21%; P = .20; mean [SD], 29.5 [23.5] pg/mL) and combined mean increases of 43% at 6 hours (95% CI, −41% to 360%; P = .04; mean [SD], 227.7 [23.2] pg/mL); 57% at 24 hours (95% CI, −15% to 136%; P < .001; mean [SD], 31.8 [23.7] pg/mL); and 67% at 48 hours postoperatively (95% CI, −55% to 165%; P < .001; mean [SD], 35.1 [28.7] pg/mL). Generalized estimating equation modeling showed a significant association between increasing NFL levels and postoperative time course (β coefficient, 1.17; P < .01).
Figure 2. Change From Baseline Levels of Plasma Neurofilament Light Over Time.
A, The line indicates the mean, and the error bars indicate the standard deviation. B, Blue dots indicate individual values, and orange diamonds indicate mean values.
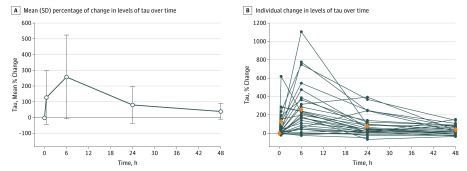
Mean plasma tau levels increased at each point from baseline, from a combined baseline mean (SD) of 3.1 (1.3) pg/mL to a maximal combined mean (SD) of 10.8 (9.5) pg/mL, an increase of 257% (95% CI, 154%-361%; P < .001), at 6 hours postoperatively (Figure 3). The changes from baseline were combined mean increases of 127% at 30 minutes (95% CI, 37%-217%; P=.01; mean [SD], 7.0 [3.9] pg/mL); 257% at 6 hours (95% CI, 154%-361%; P < .001; mean [SD], 10.8 [9.5] pg/mL); 80% at 24 hours (95% CI, 36%-124%, P < .001; mean [SD], 5.2 [3.8] pg/mL); and 39% at 48 hours (95% CI, 19%-60%; P < .001; mean [SD], 4.1 [2.2] pg/mL). Tau levels began to decline to baseline after 6 hours, but remained elevated after 48 hours.
Figure 3. Change From Baseline Levels of Tau Over Time.
A, The line indicates the mean, and the error bars indicate the standard deviation. B, Blue dots indicate individual values, and orange diamonds indicate mean values.
Discussion
Neurofilament light, a component of the axonal cytoskeleton, is a sensitive marker of CNS axonal injury, and cerebrospinal fluid levels have been shown to predict severity of neuronal injury in a number of neurodegenerative diseases,15 including multiple sclerosis, HIV infection, and Alzheimer disease. Tau proteins are an integral component of neuronal microtubules and contribute to axonal integrity. Hyperphosphorylated tau is the hallmark of several neurodegenerative disorders (tauopathies)16 and is reflective of abnormal tau metabolism leading to tau microfilament tangles and neuronal damage or death. The advent of the Simoa technique has recently allowed assays of NFL18 and tau17 in blood, obviating the need for lumbar punctures and facilitating repeat assays in the perioperative period.
We found a significant and rapid increase in plasma NFL and tau in response to anesthesia and surgery, regardless of the anesthetic technique or type of surgery. Levels of NFL continued to increase at 48 hours after surgical incision, but levels of tau peaked at 6 hours. The pathophysiological background for the different incremental time courses for tau, which had a rapid increase peaking at 6 hours, and NFL, which showed a slower profile with a continuous rise through the last 48 hours sample, is unclear. It is likely owing to differential mechanisms of release from damaged neurons, different routes of clearance from the brain to the blood (via either cerebrospinal fluid or the lymphatic system), different metabolism, or different degradation in the bloodstream, or combinations thereof. Nevertheless, these differing incremental time courses for tau and NFL closely match what has been found in other studies with repeated blood sampling in patients with acute brain injuries.8,9
Plasma levels of NFL reflect injured and/or degenerating neurons and correlate closely with cerebrospinal fluid levels.19 In contrast to chronic neurodegenerative disease, the NFL levels described here reflect an acute response to the precipitating event of anesthesia and surgery, which may be more analogous to acute traumatic brain injury.9 After traumatic brain injury, plasma NFL continues to increase for up to 2 weeks.9,20
Changes in plasma tau provide further confirmatory evidence of a response in the brain. Plasma tau increased more rapidly than plasma NFL and then began to decrease over the subsequent 48 hours.
These findings come at a time when there is concern about the neurological impact of general anesthesia and surgery in both very young patients21,22 and elderly patients.23 In young patients, this concern is mostly based on animal studies, which have suggested a variety of possible mechanisms.24 In elderly patients, there is little information on structural changes associated with general anesthesia and surgery.24 Animal studies suggest that outcomes of anesthesia use may mimic Alzheimer disease in the members of the older population who are vulnerable.25,26 This was also an assumption when increases in levels of tau in cerebrospinal fluid were found after general anesthesia and surgery.27,28 The current study suggests that the increase in tau may not reflect Alzheimer disease processes, but rather the neuronal injury associated with the release of NFL from axonal damage.
Limitations
Although anesthesia has historically been seen as the main culprit causing cognitive decline because its target organ is the brain, anesthesia and surgery invariably accompany each other in clinical practice. This makes it difficult to separate any potential injurious outcomes of surgery (eg, stress or inflammation) from any potential damaging outcomes of anesthesia by itself.
One may speculate that clinical cognitive decline may not become apparent until a critical reserve of neurons or synapses have been depleted or functionally impaired in a process analogous to renal or hepatic failure. In this regard, we await the cognitive assessments of participants in the trials described herein.
We strongly encourage others to repeat these assays to confirm or refute these findings. Additionally, and more urgently, these neurological biomarkers should be assessed in children in order to help resolve the current controversy surrounding neurological outcomes in infants.22
Conclusions
This finding is important for our understanding of the impact of general anesthesia and surgery on the brain. For more than 170 years, anesthesia and surgery have been considered to act by a reversible mechanism that does not injure the brain. The increases in plasma NFL and tau identified in this study indicate that this may not be the case. The measurement of an increase in neurological biomarkers after anesthesia and surgery suggests a neuronal injury has taken place.
References
- 1.Pocock G, Richards CD. Cellular mechanisms in general anaesthesia. Br J Anaesth. 1991;66(1):116-128. [DOI] [PubMed] [Google Scholar]
- 2.Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147(suppl 1):S72-S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert DG. Mechanisms of action of general anaesthetic drugs. Anaesth Intensive Care Med. 2017;18(7):344-346. [Google Scholar]
- 4.Moller JT, Cluitmans P, Rasmussen LS, et al. ; International Study of Post-Operative Cognitive Dysfunction . Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. Lancet. 1998;351(9106):857-861. [DOI] [PubMed] [Google Scholar]
- 5.Paredes S, Cortínez L, Contreras V, Silbert B. Post-operative cognitive dysfunction at 3 months in adults after non-cardiac surgery: a qualitative systematic review. Acta Anaesthesiol Scand. 2016;60(8):1043-1058. [DOI] [PubMed] [Google Scholar]
- 6.Hogue CW Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103(1):21-37. [DOI] [PubMed] [Google Scholar]
- 7.van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67(3):280-293. [DOI] [PubMed] [Google Scholar]
- 8.Randall J, Mörtberg E, Provuncher GK, et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. 2013;84(3):351-356. [DOI] [PubMed] [Google Scholar]
- 9.Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6:36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88(19):1788-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westermann D, Neumann JT, Sörensen NA, Blankenberg S. High-sensitivity assays for troponin in patients with cardiac disease. Nat Rev Cardiol. 2017;14(8):472-483. [DOI] [PubMed] [Google Scholar]
- 12.Evered L, Silbert B, Scott DA, Ames D, Maruff P, Blennow K. Cerebrospinal fluid biomarker for Alzheimer disease predicts postoperative cognitive dysfunction. Anesthesiology. 2016;124(2):353-361. [DOI] [PubMed] [Google Scholar]
- 13.Mattsson N, Zetterberg H, Janelidze S, et al. ; ADNI Investigators . Plasma tau in Alzheimer disease. Neurology. 2016;87(17):1827-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohrer JD, Woollacott IO, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87(13):1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zetterberg H. Neurofilament light: a dynamic cross-disease fluid biomarker for neurodegeneration. Neuron. 2016;91(1):1-3. [DOI] [PubMed] [Google Scholar]
- 16.Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12(6):609-622. [DOI] [PubMed] [Google Scholar]
- 17.Andreasson U, Blennow K, Zetterberg H. Update on ultrasensitive technologies to facilitate research on blood biomarkers for central nervous system disorders. Alzheimers Dement (Amst). 2016;3:98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2015;3:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas JC, Karydas A, Bang J, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol. 2016;3(3):216-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Nimer F, Thelin E, Nyström H, et al. Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS One. 2015;10(7):e0132177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rappaport BA, Suresh S, Hertz S, Evers AS, Orser BA. Anesthetic neurotoxicity—clinical implications of animal models. N Engl J Med. 2015;372(9):796-797. [DOI] [PubMed] [Google Scholar]
- 22.Andropoulos DB, Greene MF. Anesthesia and developing brains—implications of the FDA warning. N Engl J Med. 2017;376(10):905-907. [DOI] [PubMed] [Google Scholar]
- 23.Strøm C, Rasmussen LS, Sieber FE. Should general anaesthesia be avoided in the elderly? Anaesthesia. 2014;69(suppl 1):35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17(11):705-717. [DOI] [PubMed] [Google Scholar]
- 25.Xie Z, Dong Y, Maeda U, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104(5):988-994. [DOI] [PubMed] [Google Scholar]
- 26.Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB. Alzheimer’s disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth. 2006;97(4):445-452. [DOI] [PubMed] [Google Scholar]
- 27.Berger M, Nadler JW, Friedman A, et al. ; MAD-PIA trial team . The effect of propofol versus isoflurane anesthesia on human cerebrospinal fluid markers of Alzheimer’s disease: results of a randomized trial. J Alzheimers Dis. 2016;52(4):1299-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011;115(4):727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]