Abstract
Androgen deprivation therapy (ADT) remains the cornerstone of management for patients with metastatic prostate cancer (PC). Although the toxicities of ADT are well established, there is increasing controversy surrounding the association between ADT use and cognitive dysfunction, and some evidence suggesting an increased risk for dementia. We conducted a literature search to identify pertinent clinical studies in this field. This general review outlines the key findings and discusses the relative strengths and weaknesses when drawing conclusions on the risk of cognitive dysfunction or dementia with ADT use.
Keywords: Prostate Cancer, Androgen Deprivation, Cognitive Dysfunction, Dementia, Alzheimer’s Dementia
Over the past two decades, widespread use of the prostate-specific antigen (PSA) test and subsequent biopsy has contributed to a significant increase in the diagnosis of prostate cancer (PC) (1). Correspondingly, androgen deprivation therapy (ADT) use has increased almost 10-fold with half of all men with PC receiving ADT at some stage after diagnosis (2, 3). Historically, ADT was used primarily for those patients with metastatic disease, achieving disease control for an average of 18-24 months. Despite the ultimate transition to castration-resistant disease, ADT can slow disease progression and these men usually remain on therapy for life. In addition to prolonging median survival, ADT can improve or prevent cancer related symptoms. For example, pain, pathological fracture or spinal cord compression risks secondary to bony metastatic disease can be reduced by ADT thus limiting disability and helping to optimize a patient’s quality of life.
Contemporary literature has also demonstrated an overall survival benefit with ADT given in conjunction with radiotherapy in the localized setting, either as primary therapy or salvage therapy post prostatectomy (4–7). Collectively, this broadening of indications for ADT use in PC means, on average, that patients will receive ADT earlier and for a longer total time over their disease course. Therefore, physiological sequelae should be even more apparent. The toxicity profile of ADT can include obesity, anemia, loss of bone mineral density, muscle atrophy, gynecomastia and mood changes, and might also include an increased risk of diabetes, hypertension and myocardial infarction (8).
Cognitive dysfunction and risk of dementia have drawn an increased research focus in their association with ADT. Cognitive dysfunction here refers to changes in cognition potentially related to ADT and its effect on brain function. Dementia is a clinical syndrome involving progressive deterioration of intellectual function with multiple sub-types. Depending on the etiology, cognitive dysfunction associated with dementia can span various cognitive domains, most often memory, language, reasoning, decision making, visuospatial function, attention and orientation. In the United States, 1 in 9 people over 65 years are living with Alzheimer’s Disease (AD), which accounts for 60-80% of dementia cases and is the fifth leading cause of death among older adults (9). Considering half of all PC diagnoses occur in men over 65 years old, potential cognitive effects of treatment that may accelerate cognitive aging, as well as potentially increased risk of dementia, require careful consideration in an already increased-risk population. This general review will examine and critique the key findings from clinical studies designed to investigate the association of ADT with cognitive dysfunction or dementia.
The Neurophysiological Role of Androgens
Dihydrotestosterone (DHT) is a potent metabolite of testosterone, binding with higher affinity to the androgen receptor (AR), which functions as a transcription factor. AR messenger RNA is known to be expressed in the prefrontal cortex, parietal lobe and hippocampus (Figure 1)- brain regions critical for memory and higher order cognitive function (10). Several animal studies also support the notion that testosterone and its metabolites play significant roles in maintaining cognitive function. In rodents, there is an observed decrease in synaptic spine density of the CA1 region of the hippocampus (the region supporting anterograde memory) upon androgen withdrawal (11, 12). Levels of beta-amyloid protein, the neuropathological hallmark of AD, increase in rodents who have undergone gonadectomy, but revert with DHT supplementation (13). Androgen blockade in rodents expressing the apoE4 allele (a known human AD risk factor) impairs memory performance (14). Male estrogen (formed via testosterone aromatization) also binds to sites in the hippocampus and prefrontal cortex, increasing serotonergic and cholinergic activity, which maintains neural circuitry and alters lipoprotein levels, to help decrease the risk of cerebral ischemia. Its withdrawal in clinical studies is associated with impaired memory (15, 16).
Figure 1.
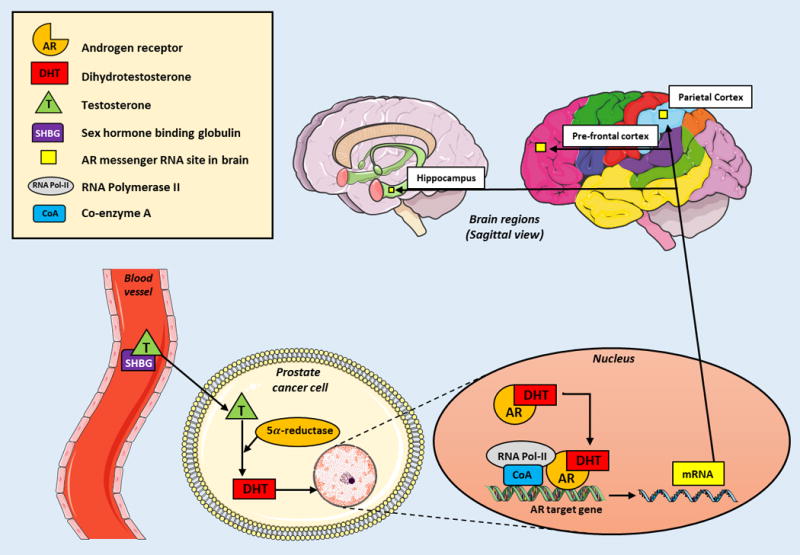
Formation of androgen receptor mRNA and its expression in higher order cognitive brain centers
Impact of Age-Related Decline in Testosterone and Supplementation on Cognitive Function
In humans, it is well known that both serum and brain levels of testosterone decrease with age (17–20). Compared to controls, both serum and brain testosterone levels are lower in men with a clinical diagnosis of AD, the latter confirmed at autopsy (21, 22).
Compared to the abrupt cessation of sex hormone production in women during menopause, the testosterone level decline in men is slower with age, with a 0.4-1.2% decrease per year beginning in the third or fourth decade (23). Sex-hormone binding globulin (SHBG) levels increase by approximately 1.2% per year, with the free testosterone index (FTI: the ratio of serum testosterone to SHBG) decreasing up to 50% (24, 25). Collectively, this gives rise to the term ‘andropause’, with male hypogonadism (defined by FTI criteria) increasing in prevalence from 9% in their fifties to 91% in their eighties (18).
Correlative studies undertaken to demonstrate a positive association between testosterone and cognitive functioning found a wider influence in men extending to other cognitive domains (26, 27). Cherrier et al has reported several placebo-controlled studies examining healthy, older men who were given supplementary testosterone, finding higher performance in spatial tasks and possibly verbal memory, although the latter is postulated to be dependent on the aromatization of testosterone to estradiol (28–30).
Studies have also reported an association between low levels of testosterone and AD risk in men. As part of the Baltimore Longitudinal Study of Aging, 574 men were assessed at multiple time points for a mean of 19.1 years (range, 4-37 years) (31). AD diagnosis was inversely associated with FTI, with a 26% decreased risk of AD for each 10nmol/nmol increase in FTI. Lower free testosterone levels also predated the clinical diagnosis of AD by up to 5-10 years. Similar observations were reported in the OPTIMA study (Oxford Project to Investigate Memory and Ageing) which examined 112 men with AD and compared gonadotropin, SHBG, total and free testosterone levels with 98 age-matched controls (32). Significantly higher levels of LH and FSH in patients with AD was postulated to, in part, explain those patient’s lower free T level, with FSH found to be an independent predictor for AD. At this point, however, a direct causal role of testosterone for increased risk of dementia has not been elucidated.
Neuroimaging in the Setting of ADT
Functional magnetic resonance imaging (fMRI) provides a non-invasive method to assess brain activity during cognitive tasks as well the integrity of regional functional connectivity at rest.
In a pilot study, 5 patients with biochemically recurrent PC after definitive therapy eligible for ADT underwent neuroimaging with blood oxygen level dependent (BOLD) functional MRI (fMRI) prior to treatment and after 9 months of ADT (33). These imaging techniques were undertaken while performing 3 cognitive tasks (encoding, recognition in spatial memory and mental rotation) to determine whether ADT affected neurovascular responses. These patients were compared to 7 matched healthy control patients who underwent the same imaging at matched time intervals. After 9 months, patients receiving ADT had reduced task-related BOLD-fMRI activation in the right parieto-occipital region, for tasks involving manipulation and spatial information.
An exploratory case-control study compared 12 localized PC patients after definitive therapy who received 6 months of ADT with 12 matched controls (34). Performance on the ‘N-back’ task, a behavioral paradigm widely used to assess working memory, was indistinguishable between the 2 groups across assessment time points at baseline and 6-months. However, voxel based morphometry (VBM), used to detect differences in the composition of brain tissue and its association with behavioral and cognitive measures, showed significantly decreased gray matter volumes after 6 months in the primary motor, frontopolar and dorsolateral prefrontal cortices of the ADT group only (p<0.05). Despite the small sample size and relatively short duration of observation, these hypothesis-generating preliminary findings warrant expansion to investigate whether the functional and structural differences associated with ADT worsen with additional time and perhaps are a harbinger of cognitive decline.
Clinical Data: Cognitive Effects of ADT in Prostate Cancer
There have been numerous prospective studies examining the impact of ADT on cognitive function (Table 1), which demonstrate conflicting results (35–42). Favoring a relationship between ADT and cognitive decline is a study by Green et al., where 82 men with extra-glandular PC were randomized in a 4-arm design to either surveillance or various forms ADT (35). Twenty four of the 50 men randomized to continuous ADT demonstrated significant declines in either verbal memory or executive function at their six-month assessments. No patients randomized to close monitoring showed cognitive decline. Despite consistent findings with longer follow-up and expansion to include a healthy control group, limitations exist. Spatial memory testing, a cognitive domain with widely reported sensitivity to both ADT use and testosterone supplementation (28, 30, 33, 36, 38, 43–46), was omitted. More than 20% of patients did not complete all cognitive tasks and there were group differences in baseline IQ and education.
Table 1.
Selected Prospective Studies Examining Cognitive Changes with ADT use in Prostate Cancer
| Study Parameter | Clinical Study First Author (Year) | |||||||
|---|---|---|---|---|---|---|---|---|
| Green (2002) | Cherrier (2003) | Salminen (2003) | Salminen (2004) | Jenkins (2005) | Yang (2015) | Ailbhai (2016) | ||
| Design | Randomized | Yes | No | No | No | No | No | No |
| Disease state | Advanced | BCR | Localized | Localized | Localized | Localized | Localized or BCR | |
| ADT group | Number of patients | 65 | 19 | 25 | 25 | 32 | 43 | 77 |
| Duration of treatment, mos | 6 | 9 | 12 | 12 | 3 to 5 | 6 | 29e | |
| Washout period, mos | X | 3 | X | X | 4 to 6 | X | X | |
| LHRHa | Leuprolide/goserelina | Leuprolide | N/S | Leuprolide | Goserelin | Goserelin | N/S | |
| Anti-androgen | Cyproterone acetatea | Flutamide | Flutamide | Flutamide | Cyproterone acetate | Bicalutamide | N/S | |
| Mean age, years | 73 | 65 | 64 | 65 | 68 | 69 | 69 | |
| Mean education, years | 9.1 | 16 | 8.9 | 8.5 | Xc | 10.8 | 16 | |
| Cognitive assessment timepoints after BL, mos | 6 | 9, 12 | 6, 12 | 6, 12 | 3, 9 | N/S | 6, 12, 24, 36 | |
| Control group | Healthy, n | X | 15 | 52b | X | 18 | 40 | 82 |
| PC patient- no treatment, n | 15a | X | X | X | X | 35 | 82 | |
| Cognitive Assessmentd | Parameter affected in ADT group (change from BL) | Verbal memory (↓6) | Spatial ability (↓9) | Immediate object recall (↑6) | Visuomotor speed (↓12) | Working memory (↓9) | Memory (recognition) (↓N/S) | None |
| Executive function (↓6) | Verbal memory (↑12) | Delayed object recall (↑6,12) | Delayed object recall (↑12) | Visuospatial (↓3, 9) | Short term memory (↓N/S) | |||
| Basic speed test (↓12) | Verbal Fluency (↓3) | Executive function (↓N/S) | ||||||
| Concentrated attention (↓6) | EBPM (↓N/S) | |||||||
| Sustained attention (↓12) | ||||||||
| Recognition speed (↓12) | ||||||||
ADT=androgen deprivation therapy; BL=baseline; BCR=biochemical recurrence; LHRHa=lutenising hormone releasing hormone agonist; X=not applicable; N/S=not specified; n=number; mos=months; wks=weeks; ↓=significant dis-improvement, ↑=significant improvement
4-arm randomized study of LHRH agonists (Leuprolide or goserelin), cyproterone acetate, or close monitoring;
BL assesment only in control group;
Full scale intelligence quotient recorded;
Superscript denotes follow-up month of significant change in cognitive parameter;
Median duration of ADT use
Additionally, supporting the relationship between ADT and declining cognition, Jenkins et al. evaluated localized PC patients undergoing neoadjuvant ADT, with cognitive assessments at baseline, 3-5 months (ADT completion) and 9 months after stopping therapy (36). Forty seven percent of patients in the ADT group declined on at least one cognitive task when compared to matched controls- exhibiting lower performance on spatial ability and spatial memory tasks, although interestingly, performance recovered at the 9-month post-treatment cessation assessment.
Salminen et al. also reported slower visuomotor skills and reaction times in various attentional domains in 26 men receiving ADT for 12 months, without a control group (37). With the addition of controls in a follow-up study with baseline assessment only, the ADT group demonstrated improvements in object recall and semantic memory, likely due to practice effects from repeated administrations (38).
Additionally, Yang et al undertook a cross-sectional study comparing neuropsychological parameters for 3 groups: PC patients on ADT, PC patients not on ADT and healthy controls (39). Notably for this trial, prospective memory (PM) was examined, specifically, time-based PM (e.g. remembering to call a friend in hour) and event-based PM (e.g. remembering to purchase fruits when passing a fruit stand). ADT subjects had significantly lower scores in the event-based PM (EBPM) but not time-based PM test, when compared to the non-ADT and healthy control groups. Given that the pre-frontal cortex is a known higher cognitive functioning center, this finding complements earlier studies which demonstrated PET/CT activity in this region with EBPM tasks (47), and conversely, deficits in EBPM in patients with pre-frontal cortex injuries (48). With respect to PC, this finding is congruent with the previously discussed study finding decreased gray matter volumes after ADT use in the primary motor, frontopolar and dorsolateral prefrontal cortices (34).
By contrast, several studies failed to demonstrate a relationship between ADT and cognition. In a small study, Cherrier et al. examined ADT-treated patients with baseline, 9-month (treatment cessation) and 12-month cognitive assessments (40). Compared to healthy controls and except for a decline on a measure of spatial ability (mental rotation)— the ADT group at 9 months showed no decline in cognitive function.
More importantly, one of the largest and best controlled matched cohort studies compared men with PC from time of ADT initiation, with PC patients not receiving ADT and healthy controls (41). Neurocognitive testing was undertaken at baseline, 6 months and 12 months. Cumulative drop-out rates were low (10% and 12% at 6 and 12 months respectively). No consistent evidence of deleterious cognitive effects of ADT over 12 months were found. Minor weaknesses included the lack of specificity for type of ADT received and the allowance of baseline cognitive assessment up to four weeks after starting ADT. The size of this study helps to underscore its negative findings, made more robust in a recent publication from the same group, updating cognitive outcomes on the same patient cohorts, with an extended follow-up of 36 months (42). Again, no significant declines in cognitive function among ADT users were found. No prior studies had examined the impact of long term ADT use beyond 1 year.
The studies discussed above have methodological deficits, not limited to size, observation or volunteer selection bias. Irrespective of the validity or reliability of cognitive testing, practice effects– the influence of prior experience taking a test on re-taking the same test- are an important confounder which can aggregate over time, concealing subtle cognitive changes. This finding is apparent in all studies examining this patient population and extends to healthy comparator individuals ≥70 years of age, evident in testing up to 2 years apart (49).
Clinical Data: Use of ADT and the Risk of Dementia
Propelling the public concerns about an association between ADT use and dementia was a recent single institution, retrospective study undertaken at Stanford University (Table 2) (50). Information collected through a previously validated text-processing method was gathered on patients between 1994-2013 to include exposed (received ADT) and non-exposed patients with PC. The start of follow-up was from the initiation of ADT in the exposed group. As the non-exposed group had no similar definitive start date, the time of follow up was a synthetic one. The follow-up period began on the date of the PC diagnosis plus the exposed group’s median time from diagnosis to ADT use. The latter is an important definition as it may have introduced immortal time bias, as some ADT users may have started ADT years after their PC diagnosis, giving the non-users a longer average follow-up which inflates the denominator of the rate, overestimating the hazard ratio (51).
Table 2.
Observational Studies Examining Incident Dementia with ADT use in Prostate Cancer
| Study Parameter | Clinical Study First Author (Year) | ||||
|---|---|---|---|---|---|
| Nead (2016) | Kao (2016) | Chung (2016) | Khosrow-Khavar (2017) | ||
| Design | Country of patient population | USA | Taiwan | Taiwan | United Kingdom |
| Years of PC diagnoses | 1994–2013 | 2001–2008 | 2001–2008 | 1988–2015 | |
| Method of capture of incident dementia | Text-Processing | Diagnostic Coding | Diagnostic Coding | Diagnostic Coding | |
| Type(s) of dementia captured | All | All | AD only | All | |
| Mean follow-up, years | 3.4 | 5 | 5 | 4.3 | |
| PC disease states excluded | None | Recurrent PC | Recurrent PC | De-novo metastatic PC | |
| Patient Demographics | Minimum age, years | ≥18 | ≥40 | ≥40 | ≥40 |
| Total number of PC patients (%) | 9272 | 1314 | 1335 | 30,903 | |
| ADT group | 1826 (20) | 755 (57) | 768 (58) | 17994 (58) | |
| Non-ADT group | 7446 (80) | 559 (43) | 567 (42) | 12909 (42) | |
| Mean age at cohort entry, years (SD) | |||||
| ADT group | 69.9 (11.0) | 74.2 (7.9) | 74.2 (8.0) | 72.8 (8.3) | |
| Non-ADT group | 66.2 (10.8) | 69.3 (10.1) | 69.5 (10.2) | 68.7 (9.0) | |
| Median duration of ADT use, years (IQR) | NS | NS | NS | 2.3 (1.2-4.0) | |
| Non-PC comparison group (number of patients) | X | X | Yes (4005) | X | |
| Dementia Incidence | Total incident dementia diagnoses (Incidence rate) | 314 | NS (2.12*) | NS (2.69**) | 799 (6.0**) |
| ADT group | NS | NS (2.35) | NS (3.43) | 524 (7.4) | |
| Non-ADT group | NS | NS (1.85) | NS (1.70) | 275 (4.4) | |
| Alzheimer’s Dementia diagnosis (% total) | NS | NS | NA | 293 (36.7) | |
| Other Dementia diagnosis (% total) | NS | NS | NA | 506 (63.3) | |
PC=prostate cancer; ADT=androgen deprivation therapy; SD=standard deviation; IQR=interquartile range; AD= Alzheimer’s dementia; NA=not applicable; NS=not specified
Crude incidence measured in per 100 person-years;
Crude incidence measured in per 1000 person-years
Of the 9272 patients meeting inclusion criteria, 1826 (19.7%) received ADT. A total of 314 incident cases of dementia were diagnosed during a median follow-up of 3.4 years. Kaplan Meier analyses demonstrated a significantly lower cumulative probability of remaining dementia free in the propensity score matched and unmatched cohorts, with a 4.4% absolute increased dementia risk in ADT users (3.5% in non-users vs 7.9% in users). This statistically significant association persisted with exclusion of patients receiving chemotherapy and patients with incident dementia diagnosed within the first 2 years of follow-up. Stratification by duration of ADT use demonstrated the highest dementia risk in patients on ADT >12 months. In addition to being a retrospective, single institution study, this study was not adequately powered to analyze dementia risk by type of ADT, nor could the disease state for ADT use be characterized. The informatics based approach to this study was previously validated in a report on surveillance of adverse events associated with pharmacotherapy, in which free text notes of both medication and adverse events were analyzed (52). That report allowed that some level of accuracy would be lost in accurately identifying relatively unambiguous and publicly observable events (treatment and adverse outcomes)- deemed an acceptable compromise in exchange for the power of larger datasets.
Two studies from Taiwan comprised the first population-based studies exploring the relationship between ADT use and dementia risk (Table 2) (53, 54). Data for both studies was retrieved from the Longitudinal Health Insurance Database, which consists of medical claims and registry files for 1 million individuals randomly sampled from the Taiwan National Health Insurance program. Patients with new PC diagnoses (by ICD-9-CM) were selected from records of ambulatory care visits or hospitalizations. The ‘index date’- the time point from which incident dementia diagnoses were recorded- differed between the 2 groups, which was the time of first receipt of ADT for the treatment group and time of PC diagnosis for the non-ADT group. All patients in both studies were followed for 5 years. Kao et al tracked patients for an incident diagnosis of dementia of any type, reporting a non-significant difference in HR for dementia between ADT and non-ADT groups (adjusted HR = 1.21, 95% CI = 0.82–1.78). Chung et al followed patients only for AD and included a 4005-patient healthy control group. Notably, Chung et al only included those patients who had both an AD diagnosis and receipt of a prescription for acetylcholinesterase inhibitor. No significant difference in the HR for AD between PC groups was observed (adjusted HR = 1.76, 95% CI = 0.55-5.62) or when PC groups were compared with the non-PC group. Despite the longer follow-up period than the prior study by Nead and colleagues, limitations were evident in both analyses. Stage and grade of PC were not captured, nor were other potential confounders such as family history of AD, smoking history, alcohol consumption and educational level.
In a recently published large UK population-based retrospective study- the use of ADT was not associated with an increased risk of dementia (Table 2) (51). Using the world’s largest primary care database (700 practices)- the Clinical Practice Research Datalink (CPRD) retrieved incident diagnoses of dementia- by Read Code Classification- among 30,903 eligible patients. Each patient’s dementia subtype was recorded by the general practitioner- a method shown to have high validity (55–57).
Unique to this trial was the use of time-dependent exposure definition to eliminate immortal time bias. Cohort entry corresponded to the incident PC diagnosis, but the start of follow-up could only begin 1 year after diagnosis of PC, to account for potential delays in a diagnosis of dementia due to its insidious presentation. As a result, patient with dementia diagnoses made in the 12-month interval after diagnosis were excluded. Exposure to ADT was lagged by an additional 1 year to account for a minimal latency period and to minimize detection bias.
Seventeen thousand nine hundred ninety-four patients (58.2%) used ADT for a median duration of 2.3 years. Seven hundred ninety-nine patients were diagnosed with dementia, giving a crude incidence of 6.0 per 1000 person-years, similar to that reported for men in the general population older than 70 years of age. Two hundred ninety-three patients (36.7%) had AD and 506 patients (63.3%) had other dementias. Compared with non-use, the use of ADT was not associated with an overall increased risk of dementia (7.4 v 4.4 per 1000 person-years, respectively; adjusted HR 1.02; 95% CI 0.87 to 1.19). Dementia risk did not vary by cumulative duration or ADT type. Furthermore, primary analyses were repeated with lengthening of the exposure-lag period to 2 and 3 years, which did not change the HRs.
Limitations still exist with this analysis however. It is likely that diagnoses were made by practitioners with varying levels of expertise in neurodegenerative conditions, and still less expertise in distinguishing sub-types of dementia. The direct effects of ADT on cognition or the fatigue mediated by ADT- may lead practitioners to misinterpret these cognitive changes and incorrectly identify them as the prodromal phase of an incipient dementia. While the authors acknowledge that there may be some decrease in diagnostic accuracy in relying on clinical diagnoses rather than autopsy results, they reference two studies that find adequate sensitivity and specificity in relying on clinical diagnosis (58, 59). In both studies, however, clinical diagnoses were made either by neurologists in an Alzheimer Disease Research Center using well-defined and standardized qualitative criteria for diagnosis (NINDS-ADRDA) or in a specialized neurological unit that included formal, objective neuropsychological assessment.
Should further work enable a more robust association of ADT with risk of dementia, a natural follow-on question would be whether an intermittent approach to ADT administration would lower the risk of AD. A recent secondary analysis of the SWOG 9346 clinical trial, which randomized patients to intermittent or continuous ADT after a PSA nadir to <4ng/ml following 7 months induction ADT- compared the difference in dementia incidence during follow-up between these 2 groups (60). This analysis of US-based patients used a novel linkage that matched the SWOG clinical records to Medicare claims data. Patients were classified as having dementia if they had any hospital claim or at least 2 physician/outpatient claims at least 30 days apart- the latter undertaken to reduce misclassification bias. Dementia codes were established prior to the analysis using Healthcare Common Procedure Coding System and International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes. Results found a statistically non-significant difference in cumulative incidence of dementia in those patients receiving continuous ADT compared to intermittent ADT (8% vs 4; HR, 1.98; P = .07%). The type of dementia was not specified. These results need to be cautiously however, interpreted given the low number of events reported, and the lack of power intrinsic to an analysis of a subset of patients.
Conclusion
Median survival rates for PC clinical states continue to improve, which equates to a larger population of older cancer survivors. ADT has been a crucial part of the improvements in cancer outcomes across the spectrum of the disease, based on sound prospective, well-controlled and adequately powered phase III studies. This progress comes with the caveat that adverse effects of contemporary cancer therapies will increase in prevalence and relevance. A discussion of the relationship between ADT and cognitive decline and dementia should be part of the discussion of the merits and drawbacks of initiating ADT in a given clinical context, with consideration of the patient’s disease risk, comorbidities, and expected longevity. At this point, the specific risk factors for cognitive decline and dementia, physiologic correlates, effects of ADT scheduling (continuous versus intermittent) or the global impact of mood or fatigue on cognition are not fully elucidated. For many viable patients who face high risks of death or disability from disease, the possibility of neurocognitive risks is acceptable.
Such risk/benefit discussions should include the quality of the supporting data for these risks. For dementia, given the difficulties of prospectively assessing the association with ADT, studies rely on retrospective, remote identification of dementia based on textual analysis and surveillance of diagnostic codes- both of which will involve trade-offs of diagnostic accuracy for large datasets. While recent population-based analyses are laudable for their clear methodology and objectives, the ambiguity between studies is concerning with respect to concluding how to appropriately inform patients prior to therapy. For cognitive decline, although authors offer a biologically-sound rationale for the development of cognitive impairment on ADT, many of the studies are small, methodologically flawed, but hypothesis provoking. They do not form a body of work that can independently support a conclusion or change in practice.
Until prospective, randomized controlled studies with close periodic follow-up are completed, we recommend that physicians discuss the possible – though not certain – association of ADT and cognitive impairment prior to the initiation of ADT with their patients, and to separate this discussion from those toxicities that are well documented. Once ADT is started, these patients can be assessed during follow-up for temporal neurocognitive changes, with early specialist referral should cognitive impairment emerge.
Condensed Abstract.
While contemporary retrospective studies assessing cognitive dysfunction or dementia risk in men receiving androgen deprivation therapy for prostate cancer are methodologically improved, limitations still exist which preclude any practice-changing conclusions. Until prospective, randomized controlled trials are completed to address this, risk/benefit discussions with patients regarding androgen deprivation therapy should include the quality of the supporting data for any potential cognitive dysfunction or dementia risk.
Acknowledgments
Funding: This research was funded through the NIH P30 Cancer Center Support Grant (P30 CA008748).
Footnotes
Financial disclosures: None
References
- 1.Barry MJ, Delorenzo MA, Walker-Corkery ES, Lucas FL, Wennberg DC. The rising prevalence of androgen deprivation among older American men since the advent of prostate specific antigen testing: a population based cohort study. BJU international. 2006;98(5):973–8. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrass B, Thurairaja R, Persad R. More should be done to prevent the harmful effects of long term androgen ablation therapy in prostate cancer. BJU international. 2004;93(9):1175–6. doi: 10.1111/j.1464-410X.2004.04888.x. [DOI] [PubMed] [Google Scholar]
- 3.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103(8):1615–24. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. The New England journal of medicine. 2009;360(24):2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 5.Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. The Lancet Oncology. 2016;17(6):747–56. doi: 10.1016/S1470-2045(16)00111-X. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Journal of Clinical oncology. 2008;26(15):2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 7.Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. The New England journal of medicine. 2017;376(5):417–28. doi: 10.1056/NEJMoa1607529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating NL, O’malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. Journal of Clinical Oncology. 2006;24(27):4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 9.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s & Dementia. 2013;9(1):63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Finley S, Kritzer M. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. Developmental Neurobiology. 1999;40(4):446–57. [PubMed] [Google Scholar]
- 11.Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. Journal of Neuroscience. 2003;23(5):1588–92. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leranth C, Prange-Kiel J, Frick KM, Horvath TL. Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cerebral cortex. 2004;14(5):503–10. doi: 10.1093/cercor/bhh012. [DOI] [PubMed] [Google Scholar]
- 13.Ramsden M, Nyborg A, Murphy M, et al. Androgens modulate β amyloid levels in male rat brain. Journal of neurochemistry. 2003;87(4):1052–5. doi: 10.1046/j.1471-4159.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- 14.Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. Journal of Neuroscience. 2002;22(12):5204–9. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Annals of the New York Academy of Sciences. 2005;1052(1):182–97. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 16.Maki PM, Sundermann E. Hormone therapy and cognitive function. Human reproduction update. 2009;15(6):667–81. doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of Clinical Endocrinology & Metabolism. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 18.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. The Journal of Clinical Endocrinology & Metabolism. 2001;86(2):724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 19.Morley JE, Kaiser FE, Perry HM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–3. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 20.Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. Jama. 2004;292(12):1431–2. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- 21.Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer’s disease. Neuroendocrinology Letters. 2001;22(3):163–8. [PubMed] [Google Scholar]
- 22.Mathias J, Lucas L. Cognitive predictors of unsafe driving in older drivers: a meta-analysis. International psychogeriatrics. 2009;21(4):637–53. doi: 10.1017/S1041610209009119. [DOI] [PubMed] [Google Scholar]
- 23.Juul A, Skakkebæk NE. Androgens and the ageing male. Human Reproduction Update. 2002;8(5):423–33. doi: 10.1093/humupd/8.5.423. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocrine reviews. 2005;26(6):833–76. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 25.Lamberts SW, van den Beld AW, Van Der Lely A-J. The endocrinology of aging. Science. 1997;278(5337):419–24. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 26.Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. The Journal of Clinical Endocrinology & Metabolism. 1999;84(10):3681–5. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 27.Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. Journal of the American Geriatrics Society. 2002;50(4):707–12. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 28.Cherrier M, Matsumoto A, Amory J, et al. The role of aromatization in testosterone supplementation effects on cognition in older men. Neurology. 2005;64(2):290–6. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- 29.Cherrier M, Matsumoto A, Amory J, et al. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32(1):72–9. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherrier MM, Asthana S, Plymate S, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57(1):80–8. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- 31.Zonderman AB. Predicting Alzheimer’s disease in the Baltimore longitudinal study of aging. Journal of geriatric psychiatry and neurology. 2005;18(4):192–5. doi: 10.1177/0891988705281863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogervorst E, Bandelow S, Combrinck M, Smith A. Low free testosterone is an independent risk factor for Alzheimer’s disease. Experimental gerontology. 2004;39(11):1633–9. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Cherrier MM, Borghesani PR, Shelton AL, Higano CS. Changes in neuronal activation patterns in response to androgen deprivation therapy: a pilot study. BMC cancer. 2010;10(1):1. doi: 10.1186/1471-2407-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao HH, Hu S, Ide JS, et al. Effects of androgen deprivation on cerebral morphometry in prostate cancer patients–an exploratory study. PloS one. 2013;8(8):e72032. doi: 10.1371/journal.pone.0072032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green HJ, Pakenham K, Headley B, et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone releasing hormone analogues and cyproterone acetate: A randomized controlled trial. BJU international. 2002;90(4):427–32. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU international. 2005;96(1):48–53. doi: 10.1111/j.1464-410X.2005.05565.x. [DOI] [PubMed] [Google Scholar]
- 37.Salminen EK, Portin RI, Koskinen A, Helenius H, Nurmi M. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clinical Cancer Research. 2004;10(22):7575–82. doi: 10.1158/1078-0432.CCR-04-0750. [DOI] [PubMed] [Google Scholar]
- 38.Salminen E, Portin R, Korpela J, et al. Androgen deprivation and cognition in prostate cancer. British journal of cancer. 2003;89(6):971–6. doi: 10.1038/sj.bjc.6601235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Zhong F, Qiu J, Cheng H, Wang K. Dissociation of event based prospective memory and time based prospective memory in patients with prostate cancer receiving androgen deprivation therapy: a neuropsychological study. European journal of cancer care. 2015;24(2):198–204. doi: 10.1111/ecc.12299. [DOI] [PubMed] [Google Scholar]
- 40.Cherrier M, Rose A, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. The Journal of urology. 2003;170(5):1808–11. doi: 10.1097/01.ju.0000091640.59812.83. [DOI] [PubMed] [Google Scholar]
- 41.Alibhai SM, Breunis H, Timilshina N, et al. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. Journal of Clinical Oncology. 2010;28(34):5030–7. doi: 10.1200/JCO.2010.30.8742. [DOI] [PubMed] [Google Scholar]
- 42.Alibhai SMH, Timilshina N, Duff-Canning S, et al. Effects of long-term androgen deprivation therapy on cognitive function over 36 months in men with prostate cancer. Cancer. 2017;123(2):237–44. doi: 10.1002/cncr.30320. [DOI] [PubMed] [Google Scholar]
- 43.Cherrier M, Aubin S, Higano C. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non metastatic prostate cancer. Psycho Oncology. 2009;18(3):237–47. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. Journal of Andrology. 2003;24(4):568–76. doi: 10.1002/j.1939-4640.2003.tb02708.x. [DOI] [PubMed] [Google Scholar]
- 45.Cherrier MM, Matsumoto A, Amory J, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64(12):2063–8. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 46.Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behavioral neuroscience. 1994;108(2):325. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- 47.Okuda J, Fujii T, Ohtake H, et al. Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time- and event-based prospective memory. International journal of psychophysiology. 2007;64(3):233–46. doi: 10.1016/j.ijpsycho.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Cheng HD, Wang K, Xi CH, Niu CS, Fu XM. Prefrontal cortex involvement in the event-based prospective memory: evidence from patients with lesions in the prefrontal cortex. Brain injury. 2008;22(9):697–704. doi: 10.1080/02699050802263035. [DOI] [PubMed] [Google Scholar]
- 49.Lamar M, Resnick SM, Zonderman AB. Longitudinal changes in verbal memory in older adults Distinguishing the effects of age from repeat testing. Neurology. 2003;60(1):82–6. doi: 10.1212/wnl.60.1.82. [DOI] [PubMed] [Google Scholar]
- 50.Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH. Association between androgen deprivation therapy and risk of dementia. JAMA oncology. 2017;3(1):49–55. doi: 10.1001/jamaoncol.2016.3662. [DOI] [PubMed] [Google Scholar]
- 51.Khosrow-Khavar F, Rej S, Yin H, Aprikian A, Azoulay L. Androgen Deprivation Therapy and the Risk of Dementia in Patients With Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(2):201–7. doi: 10.1200/JCO.2016.69.6203. [DOI] [PubMed] [Google Scholar]
- 52.Lependu P, Iyer SV, Bauer-Mehren A, et al. Pharmacovigilance using Clinical Text. AMIA Joint Summits on Translational Science proceedings AMIA Summit on Translational Science. 2013;2013:109. [PMC free article] [PubMed] [Google Scholar]
- 53.Chung S, Lin H, Tsai M, Kao L, Huang C, Chen KC. Androgen deprivation therapy did not increase the risk of Alzheimer’s and Parkinson’s disease in patients with prostate cancer. Andrology. 2016;4(3):481–5. doi: 10.1111/andr.12187. [DOI] [PubMed] [Google Scholar]
- 54.Kao L-T, Lin H-C, Chung S-D, Huang C-Y. No increased risk of dementia in patients receiving androgen deprivation therapy for prostate cancer: a 5-year follow-up study. Asian journal of andrology. 2017;19(4):414. doi: 10.4103/1008-682X.179528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Disease & Associated Disorders. 2005;19(2):91–4. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 56.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. The Lancet. 2000;356(9242):1627–31. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 57.Seshadri S, Zornberg GL, Derby LE, Myers MW, Jick H, Drachman DA. Postmenopausal estrogen replacement therapy and the risk of Alzheimer disease. Archives of neurology. 2001;58(3):435–40. doi: 10.1001/archneur.58.3.435. [DOI] [PubMed] [Google Scholar]
- 58.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. Journal of neuropathology and experimental neurology. 2012;71(4):266–73. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snowden JS, Thompson JC, Stopford CL, et al. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134(Pt 9):2478–92. doi: 10.1093/brain/awr189. [DOI] [PubMed] [Google Scholar]
- 60.Hershman DL, Unger JM, Wright JD, et al. Adverse health events following intermittent and continuous androgen deprivation in patients with metastatic prostate cancer. JAMA oncology. 2016;2(4):453–61. doi: 10.1001/jamaoncol.2015.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]


