Summary
These guidelines for the treatment of persons who have or are at risk for sexually transmitted diseases (STDs) were updated by CDC after consultation with a group of professionals knowledgeable in the field of STDs who met in Atlanta on April 30–May 2, 2013. The information in this report updates the Sexually Transmitted Diseases Treatment Guidelines, 2010 (MMWR Recomm Rep 2010;59 [No. RR–12]). These updated guidelines discuss 1) alternative treatment regimens for Neisseria gonorrhoeae; 2) the use of nucleic acid amplification tests for the diagnosis of trichomoniasis; 3) alternative treatment options for genital warts; 4) the role of Mycoplasma genitalium in urethritis/cervicitis and treatment-related implications; 5) updated HPV vaccine recommendations and counseling messages; 6) the management of persons who are transgender; 7) annual testing for hepatitis C in persons with HIV infection; 8) updated recommendations for diagnostic evaluation of urethritis; and 9) retesting to detect repeat infection. Physicians and other health-care providers can use these guidelines to assist in the prevention and treatment of STDs.
Introduction
The term sexually transmitted diseases (STDs) refers to a variety of clinical syndromes and infections caused by pathogens that can be acquired and transmitted through sexual activity. Physicians and other health-care providers play a critical role in preventing and treating STDs. These guidelines for the treatment of STDs are intended to assist with that effort. Although these guidelines emphasize treatment, prevention strategies and diagnostic recommendations also are discussed.
This document updates CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010 (1). These recommendations should be regarded as a source of clinical guidance rather than prescriptive standards; health-care providers should always consider the clinical circumstances of each person in the context of local disease prevalence. These guidelines are applicable to any patient-care setting that serves persons at risk for STDs, including family-planning clinics, HIV-care clinics, correctional health-care settings, private physicians’ offices, Federally Qualified Health Centers (FQHCs), and other primary-care facilities. These guidelines focus on treatment and counseling and do not address other community services and interventions that are essential to STD/HIV prevention efforts.
Methods
These guidelines were developed by CDC staff and an independent workgroup for which members were selected on the basis of their expertise in the clinical management of STDs. Members of the multidisciplinary workgroup included representatives from federal, state, and local health departments; public- and private-sector clinical providers; clinical and basic science researchers; and numerous professional organizations. All workgroup members disclosed potential conflicts of interest; several members of the workgroup acknowledged receiving financial support for clinical research from commercial companies. All potential conflicts of interest are listed at the end of the workgroup member section.
In 2012, CDC staff and workgroup members were charged with identifying key questions regarding treatment and clinical management that were not addressed in the 2010 STD Treatment Guidelines (1). To answer these questions and synthesize new information available since publication of the 2010 Guidelines, workgroup members collaborated with CDC staff to conduct a systematic literature review using an extensive MEDLINE database evidence-based approach (e.g., using published abstracts and peer-reviewed journal articles). These reviews also focused on four principal outcomes of STD therapy for each individual disease or infection: 1) treatment of infection based on microbiologic eradication; 2) alleviation of signs and symptoms; 3) prevention of sequelae; 4) prevention of transmission, including advantages such as cost-effectiveness and other advantages (e.g., single-dose formulations and directly observed therapy) and disadvantages (e.g., side effects) of specific regimens. The outcome of the literature review informed development of background materials, including tables of evidence from peer-reviewed publications summarizing the type of study (e.g., randomized controlled trial or case series), study population and setting, treatments or other interventions, outcome measures assessed, reported findings, and weaknesses and biases in study design and analysis.
In April 2013, the workgroup’s research was presented at an in-person meeting of the multidisciplinary workgroup members. Each key question was discussed, and pertinent publications were reviewed in terms of strengths, weaknesses, and relevance. The workgroup evaluated the quality of evidence, provided answers to the key questions, and rated the recommendations based on the United Services Preventive Services Task Forces (USPSTF) modified rating system (http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm). The discussion culminated in a proposal of recommendations to be adopted for consideration by CDC. (More detailed description of the key questions, search terms, and systematic search and review process is available at http://www.cdc.gov/std/tg2015/evidence.htm). Following the April meeting, the literature was searched periodically by CDC staff to identify subsequently published articles warranting consideration by the workgroup either through e-mail or conference calls.
CDC developed draft recommendations based on the workgroup’s proposal. To ensure development of evidence-based recommendations, a second independent panel of public health and clinical experts reviewed the draft recommendations. The recommendations for STD screening during pregnancy, cervical cancer screening, and HPV vaccination were developed after CDC staff reviewed the published recommendations from other professional organizations, including the American College of Obstetricians and Gynecologists (ACOG), USPSTF, American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and the Advisory Committee on Immunization Practices (ACIP) as part of the initial review process. The sections on hepatitis B virus (HBV) and hepatitis A virus (HAV) infections are based on previously published recommendations (2–4).
Throughout this report, the evidence used as the basis for specific recommendations is discussed briefly. More comprehensive, annotated discussions of such evidence will appear in background papers that will be available in a supplement issue of the journal Clinical Infectious Diseases after publication of these treatment guidelines. When more than one therapeutic regimen is recommended, the recommendations are listed alphabetically unless prioritized based on efficacy, tolerance, or costs. For infections with more than one recommended regimen, listed regimens have similar efficacy and similar rates of intolerance or toxicity unless otherwise specified. Recommended regimens should be used primarily; alternative regimens can be considered in instances of notable drug allergy or other medical contraindications to the recommended regimens.
Clinical Prevention Guidance
The prevention and control of STDs are based on the following five major strategies (5):
accurate risk assessment and education and counseling of persons at risk on ways to avoid STDs through changes in sexual behaviors and use of recommended prevention services;
pre-exposure vaccination of persons at risk for vaccine-preventable STDs;
identification of asymptomatically infected persons and persons with symptoms associated with STDs;
effective diagnosis, treatment, counseling, and follow up of infected persons; and
evaluation, treatment, and counseling of sex partners of persons who are infected with an STD.
STD/HIV Risk Assessment
Primary prevention of STDs includes performing an assessment of behavioral risk (i.e., assessing the sexual behaviors that may place persons at risk for infection) as well as biologic risk (i.e., testing for risk markers for HIV acquisition or transmission). As part of the clinical encounter, health-care providers should routinely obtain sexual histories from their patients and address risk reduction as indicated in this report. Guidance for obtaining a sexual history is available on the CDC Division of STD Prevention resource page (http://www.cdc.gov/std/treatment/resources.htm) and in the curriculum provided by CDC’s STD/HIV Prevention Training Centers (http://nnptc.org/clinical-ptcs). Effective interviewing and counseling skills characterized by respect, compassion, and a nonjudgmental attitude toward all patients are essential to obtaining a thorough sexual history and delivering effective prevention messages. Effective techniques for facilitating rapport with patients include the use of 1) open-ended questions (e.g., “Tell me about any new sex partners you’ve had since your last visit,” and “What has your experience with using condoms been like?”); 2) understandable, nonjudgmental language (“Are your sex partners men, women, or both?” “Have you ever had a sore or scab on your penis?”); and 3) normalizing language (“Some of my patients have difficulty using a condom with every sex act. How is it for you?”). The “Five P’s” approach to obtaining a sexual history is one strategy for eliciting information concerning five key areas of interest (Box 1). For additional information about gaining cultural competency when working with certain populations (e.g., gay, bisexual, or other men who have sex with men [MSM], women who have sex with women [WSW], or transgender men and women) see MSM, WSW and Transgender Men and Women.
BOX 1. The Five P’s: Partners, Practices, Prevention of Pregnancy, Protection from STDs, and Past History of STDs.
- Partners
- “Do you have sex with men, women, or both?”
- “In the past 2 months, how many partners have you had sex with?”
- “In the past 12 months, how many partners have you had sex with?”
- “Is it possible that any of your sex partners in the past 12 months had sex with someone else while they were still in a sexual relationship with you?”
- Practices
- “To understand your risks for STDs, I need to understand the kind of sex you have had recently.”
- “Have you had vaginal sex, meaning ‘penis in vagina sex’?” If yes, “Do you use condoms: never, sometimes, or always?”
- “Have you had anal sex, meaning ‘penis in rectum/anus sex’?” If yes, “Do you use condoms: never, sometimes, or always?”
- “Have you had oral sex, meaning ‘mouth on penis/vagina’?”
- For condom answers:
- If “never”: “Why don’t you use condoms?”
- If “sometimes”: “In what situations (or with whom) do you use condoms?”
- Prevention of pregnancy
- “What are you doing to prevent pregnancy?”
- Protection from STDs
- “What do you do to protect yourself from STDs and HIV?”
- Past history of STDs
- “Have you ever had an STD?”
- “Have any of your partners had an STD?”
Additional questions to identify HIV and viral hepatitis risk include:
“Have you or any of your partners ever injected drugs?”
“Have your or any of your partners exchanged money or drugs for sex?”
“Is there anything else about your sexual practices that I need to know about?”
In addition to obtaining a behavioral risk assessment, a comprehensive STD/HIV risk assessment should include STD screening, because STDs are biologic markers of risk, particularly for HIV acquisition and transmission among some MSM. STD screening is an essential and underutilized component of an STD/HIV risk assessment in most clinical settings. Persons seeking treatment or evaluation for a particular STD should be screened for HIV and other STDs as indicated by community prevalence and individual risk factors (see prevention section and sections on chlamydia, gonorrhea, and syphilis). Persons should be informed about all the STDs for which they are being tested and notified about tests for common STDs (e.g., genital herpes and human papillomavirus [HPV]) that are available but not being performed. Efforts should be made to ensure that all persons receive care regardless of individual circumstances (e.g., ability to pay, citizenship or immigration status, language spoken, or specific sex practices).
STD/HIV Prevention Counseling
After obtaining a sexual history from their patients, all providers should encourage risk reduction by providing prevention counseling. Prevention counseling is most effective if provided in a nonjudgmental and empathetic manner appropriate to the patient’s culture, language, gender, sexual orientation, age, and developmental level. Prevention counseling for STD/HIV should be offered to all sexually active adolescents and to all adults who have received an STD diagnosis, have had an STD in the past year, or have multiple sexual partners.
USPSTF recommends high-intensity behavioral counseling for all sexually active adolescents and for adults at increased risk for STDs and HIV (6,7). Such interactive counseling, which can be resource intensive, is directed at a person’s risk, the situations in which risk occurs, and the use of personalized goal-setting strategies. One such approach, known as client-centered STD/HIV prevention counseling, involves tailoring a discussion of risk reduction to the individual situation. While one large study in STD clinics (Project RESPECT) demonstrated that this approach was associated with lower acquisition of curable STDs (e.g., trichomoniasis, chlamydia, gonorrhea, and syphilis) (8), another study conducted 10 years later in the same settings but different contexts (Project AWARE) did not replicate this result (9). Briefer provider-delivered prevention messages have been shown to be feasible and to decrease subsequent STDs in HIV primary-care settings (10). Other approaches use motivational interviewing to move clients toward achievable risk-reduction goals. Client-centered counseling and motivational interviewing can be used effectively by clinicians and staff trained in these approaches. CDC provides additional information on these and other effective behavioral interventions at http://effectiveinterventions.org. Training in client-centered counseling is available through the CDC STD/HIV National Network of Prevention Training Centers (http://nnptc.org).
In addition to one-on-one STD/HIV prevention counseling, videos and large-group presentations can provide explicit information concerning STDs and reducing disease transmission (e.g., how to use condoms correctly and the importance of routine screening). Group-based strategies have been effective in reducing the occurrence of STDs among persons at risk, including those attending STD clinics (11).
Because the incidence of some STDs, notably syphilis, is higher in persons with HIV infection, the use of client-centered STD counseling for persons with HIV infection continues to be strongly encouraged by public health agencies and other health organizations. A recent federal guideline recommends that clinical and nonclinical providers assess an individual’s behavioral and biologic risks for acquiring or transmitting STD and HIV, including having sex without condoms, recent STDs, and partners recently treated for STDs. This guideline also recommends that clinical and nonclinical providers offer or make referral for 1) regular screening for several STDs, 2) onsite STD treatment when indicated, and 3) risk-reduction interventions tailored to the individual’s risks (12). Brief risk-reduction counseling delivered by medical providers during HIV primary-care visits coupled with routine STD screening has been shown to reduce STD incidence in persons with HIV infection (10). Several other specific methods have been designed for the HIV care setting (http://effectiveinterventions.org) (13–15).
Prevention Methods
Pre-exposure Vaccination
Pre-exposure vaccination is one of the most effective methods for preventing transmission of human papillomavirus (HPV), HAV, and HBV. HPV vaccination is recommended routinely for boys and girls aged 11 or 12 years and can be administered beginning at 9 years of age. Either bivalent, quadrivalent, or 9-valent HPV vaccine is recommended for females, whereas quadrivalent vaccine or 9-valent vaccine is recommended for males (16) http://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/hpv.html. Vaccination is recommended through age 26 years for all females and through age 21 years for all males that have not received any or all of the vaccine doses. For persons with HIV infection and for MSM, vaccination is recommended through age 26 years (16). Further details regarding HPV vaccination are available in another section of this document (see HPV Vaccine), at http://www.cdc.gov/std/hpv, and at http://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/hpv.html.
Hepatitis B vaccination is recommended for all unvaccinated, uninfected persons being evaluated or treated for an STD (3,4). In addition, hepatitis A and B vaccines are recommended for MSM, injection-drug users (IDUs), persons with chronic liver disease (CLD), and persons with HIV infection who have not yet been infected with one or both types of hepatitis virus (3,4,17). Details regarding hepatitis A and B vaccination are available at http://www.cdc.gov/hepatitis.
Abstinence and Reduction of Number of Sex Partners
The most reliable way to avoid transmission of STDs is to abstain from oral, vaginal, and anal sex or to be in a long-term, mutually monogamous relationship with a partner known to be uninfected. For persons who are being treated for an STD other than HIV (or whose partners are undergoing treatment), counseling that encourages abstinence from sexual intercourse until completion of the entire course of medication is crucial. A recent trial conducted among women on the effectiveness of counseling messages demonstrated that women whose sexual partners have used condoms may benefit from a hierarchical message that includes condoms, whereas women without such experience might benefit more from an abstinence-only message (18). A more comprehensive discussion of abstinence and other sexual practices than can help persons reduce their risk for STDs is available in Contraceptive Technology, 20th Edition (19).
Male Condoms
When used consistently and correctly, male latex condoms are highly effective in preventing the sexual transmission of HIV infection. In heterosexual HIV serodiscordant relationships (i.e., those involving one infected and one uninfected partner) in which condoms were consistently used, HIV-negative partners were 80% less likely to become infected with HIV compared with persons in similar relationships in which condoms were not used (20,21). Moreover, studies demonstrate that consistent condom use reduces the risk for other STDs, including chlamydia, gonorrhea, and trichomoniasis (22–24). By limiting lower genital tract infections, condoms also might reduce the risk of developing pelvic inflammatory disease (PID) in women (25). In addition, consistent and correct use of latex condoms reduces the risk for HPV infection and HPV-associated diseases, genital herpes, hepatitis B, syphilis, and chancroid when the infected area or site of potential exposure is covered (26–32).
Condoms are regulated as medical devices and are subject to random sampling and testing by the U.S. Food and Drug Administration (FDA). Each latex condom manufactured in the United States is tested electronically for holes before packaging. Rate of condom breakage during sexual intercourse and withdrawal is approximately two broken condoms per 100 condoms used in the United States. Rates of breakage and slippage may be slightly higher during anal intercourse (33,34). The failure of condoms to protect against STD or unintended pregnancy usually results from inconsistent or incorrect use rather than condom breakage (35). Users should check the expiration or manufacture date on the box or individual package. Latex condoms should not be used beyond their expiration date or more than 5 years after the manufacturing date. Male condoms made of materials other than latex are available in the United States and can be classified in two general categories: 1) polyurethane and other synthetic and 2) natural membrane.
Polyurethane male condoms provide comparable protection against STDs/HIV and pregnancy to that of latex condoms (19,24). These can be substituted for latex condoms by persons with latex allergy, are generally more resistant to deterioration, and are compatible with use of both oil-based and water-based lubricants. The effectiveness of other synthetic male condoms to prevent sexually transmitted infections has not been extensively studied, and FDA-labeling restricts their recommended use to latex-sensitive or allergic persons. Natural membrane condoms (frequently called “natural skin” condoms or [incorrectly] “lambskin” condoms) are made from lamb cecum and can have pores up to 1,500 nm in diameter. Although these pores do not allow the passage of sperm, they are more than 10 times the diameter of HIV and more than 25 times that of HBV. Moreover, laboratory studies demonstrate that sexual transmission of viruses, including hepatitis B, herpes simplex, and HIV, can occur with natural membrane condoms (19). While natural membrane condoms are recommended for pregnancy prevention, they are not recommended for prevention of STDs and HIV.
Providers should advise that condoms must be used consistently and correctly to be effective in preventing STDs and HIV infection; providing instructions about the correct use of condoms can be useful. Communicating the following recommendations can help ensure that patients use male condoms correctly:
Use a new condom with each sex act (i.e., oral, vaginal, and anal).
Carefully handle the condom to avoid damaging it with fingernails, teeth, or other sharp objects.
Put the condom on after the penis is erect and before any genital, oral, or anal contact with the partner.
Use only water-based lubricants (e.g., K-Y Jelly, Astroglide, AquaLube, and glycerin) with latex condoms. Oil-based lubricants (e.g., petroleum jelly, shortening, mineral oil, massage oils, body lotions, and cooking oil) can weaken latex and should not be used; however, oil-based lubricants can generally be used with synthetic condoms.
Ensure adequate lubrication during vaginal and anal sex, which might require the use of exogenous water-based lubricants.
To prevent the condom from slipping off, hold the condom firmly against the base of the penis during withdrawal, and withdraw while the penis is still erect.
Additional information about male condoms is available at http://www.cdc.gov/condomeffectiveness/index.html.
Female Condoms
Several condoms for females are globally available, including the FC2 Female Condom, Reddy condom, Cupid female condom, and Woman’s condom (36). Use of female condoms can provide protection from acquisition and transmission of STDs, although data are limited (36). Although female condoms are more costly compared with male condoms, they offer the advantage of being a female-controlled STD/HIV prevention method, and the newer versions may be acceptable to both men and women. Although the female condom also has been used during receptive anal intercourse, efficacy associated with this practice remains unknown (37). Additional information about the female condom is available at http://www.ashasexualhealth.org/sexual-health/all-about-condoms/female-condoms.
Cervical Diaphragms
In observational studies, diaphragm use has been demonstrated to protect against cervical gonorrhea, chlamydia, and trichomoniasis (38). However, a trial examining the effect of a diaphragm plus lubricant on HIV acquisition among women in Africa showed no additional protective effect when compared with the use of male condoms alone. Likewise, no difference by study arm in the rate of acquisition of chlamydia, gonorrhea, or herpes occurred (39,40). Diaphragms should not be relied on as the sole source of protection against HIV or other STDs.
Topical Microbicides and Spermicides
Nonspecific topical microbicides are ineffective for preventing HIV (41–45). Spermicides containing N-9 might disrupt genital or rectal epithelium and have been associated with an increased risk for HIV infection. Condoms with N-9 are no more effective than condoms without N-9; therefore, N-9 alone or in a condom is not recommended for STD or HIV prevention (41). N-9 use has also been associated with an increased risk for bacterial urinary tract infections in women (46,47). No proven topical antiretroviral agents exist for the prevention of HIV, though trials are underway to evaluate several candidates for vaginal and rectal microbicides using tenofovir and other antiretroviral drugs.
Nonbarrier Contraception, Surgical Sterilization, and Hysterectomy
Contraceptive methods that are not mechanical barriers offer no protection against HIV or other STDs. Sexually active women who use hormonal contraception (i.e., oral contraceptives, patch, ring, implants, injectables, or intrauterine hormonal methods), have nonhormonal intrauterine devices (IUDs), have been surgically sterilized, or have had hysterectomies should be counseled to use condoms to reduce the risk for STDs, including HIV infection. Women who take oral contraceptives and are prescribed certain antimicrobials should be counseled about potential interactions (19).
Whether hormonal contraception raises a woman’s risk for acquiring HIV or another STD is unclear. A systematic review of epidemiologic evidence found that most studies showed no association between use of oral contraceptives and HIV acquisition among women. Studies examining the association between progestin-only injectables and HIV acquisition have had mixed results; some studies show a higher risk of acquisition among women using depo-medroxyprogesterone acetate (DMPA), while other studies do not (48). The World Health Organization (WHO) and CDC reviewed the evidence on hormonal contraception and HIV acquisition and concluded that data are insufficient to recommend that women modify their hormonal contraceptive practices, but that women using progestin-only injectables should be strongly advised to also use condoms as an HIV prevention strategy (49,50).
Male Circumcision
Male circumcision reduces the risk for HIV and some STDs in heterosexual men. Three randomized, controlled trials performed in regions of sub-Saharan Africa where generalized HIV epidemics involving predominantly heterosexual transmission were occurring demonstrated that male circumcision reduced the risk for HIV acquisition among men by 50%–60% (51–53). In these trials, circumcision was also protective against other STDs, including high-risk genital HPV infection and genital herpes (54–56). Follow up studies have demonstrated sustained benefit of circumcision for HIV prevention (57) and that the effect is not mediated solely through a reduction in herpes simplex virus type 2 (HSV-2) infection or genital ulcer disease (58).
WHO and the Joint United Nations Programme on HIV/AIDS (UNAIDS) have recommended that male circumcision efforts be scaled up as an effective intervention for the prevention of heterosexually acquired HIV infection (59). These organizations also recommend that countries with hyperendemic and generalized HIV epidemics and low prevalence of male circumcision expand access to safe male circumcision services within the context of ensuring universal access to comprehensive HIV prevention, treatment, care, and support. In the United States, the American Academy of Pediatrics (AAP) recommends that newborn male circumcision be available to families that desire it, as the benefits of the procedure, including prevention of penile cancers, urinary tract infections, genital ulcer disease, and HIV outweigh the risks (60). ACOG has also endorsed the AAP’s policy statement (60). In light of these benefits, the American Urological Association states that male circumcision should be considered an option for risk reduction, among other strategies (61).
No definitive data exist to determine whether male circumcision reduces HIV acquisition in MSM, although one randomized trial is ongoing in China (62). A review found a modest protective effect among men who were the insertive partner for anal intercourse, but the evidence was rated as poor. Further higher quality studies are needed to confirm any potential benefit of male circumcision for this population (62).
Emergency Contraception
Unprotected intercourse exposes women to risks for STDs and unplanned pregnancy. Providers managing such women should offer counseling about the option of emergency contraception (EC) if pregnancy is not desired. The options for EC in the United States include the copper IUD and emergency contraceptive pills (ECPs) (63). ECPs are available in the following formulations: ulipristal acetate in a single dose (30 mg), levonorgestrel in a single dose (1.5 mg) or as a split dose (0.75 mg each taken 12 hours apart), or combined estrogen and progestin (Yuzpe regimen). Some ECPs can be obtained over the counter; ECPs can also be provided through advance prescription or supply from providers (64,65). Emergency insertion of a copper IUD up to 5 days after sex can reduce pregnancy risk by more than 99% (66). ECPs are most efficacious when initiated as soon as possible after unprotected sex but have some efficacy up to 5 days later. ECPs are ineffective (but not harmful) if the woman is already pregnant (67). A 2012 Cochrane review summarized the efficacy, safety, and convenience of various methods of emergency contraception (67). More information about EC is available in the 20th edition of Contraceptive Technology (19) or http://www.arhp.org/topics/emergency-contraception.
Postexposure Prophylaxis for HIV and STD
Guidelines for the use of postexposure prophylaxis (PEP) aimed at preventing HIV infection and other STDs as a result of sexual exposure are discussed in another section of this report (see Sexual Assault and STDs). Genital hygiene methods (e.g., vaginal washing and douching) after sexual exposure are ineffective in protecting against HIV and STDs and might increase the risk for bacterial vaginosis (BV), some STDs, and HIV infection (68).
Antiretroviral Treatment of Persons with HIV Infection to Prevent HIV Infection in Partners
The randomized controlled trial HPTN 052 demonstrated that in HIV serodiscordant, heterosexual couples, HIV antiretroviral therapy in the infected partner decreases the risk for transmission to the uninfected partner by 96% (69). Therefore, antiretroviral therapy not only is beneficial to the health of persons with HIV infection, but also reduces the risk for continued transmission. For these reasons, treatment should be offered to all persons with HIV infection. Detailed guidance for prescribing antiretroviral regimens can be found in the U.S. Department of Health and Human Services’ HIV treatment guidelines at http://aidsinfo.nih.gov/guidelines (70).
HSV Treatment of Persons with HIV and HSV Infections to Prevent HIV Infection in Uninfected Partners
Providing HSV treatment to persons co-infected with HIV and HSV has not been demonstrated to be beneficial in reducing HIV acquisition in uninfected partners. A large randomized, controlled trial evaluated 3,408 serodiscordant heterosexual couples enrolled at 14 Africa sites in which the partner with HIV infection was also seropositive for HSV-2. The co-infected partner was randomized to receive either placebo or acyclovir 400-mg twice per day, and the primary outcome was HIV transmission to the uninfected partner. Use of acyclovir had no effect on HIV transmission (71). These findings are consistent with those from a previous trial that found no benefit of acyclovir in preventing HIV-1 acquisition in persons who were seropositive for HSV-2 (72).
Preexposure Prophylaxis for HIV
Certain large, randomized, placebo-controlled trials examining daily oral antiretroviral preexposure prophylaxis (PrEP) with a fixed-dose combination of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) have demonstrated safety (73) and a substantial reduction in the rate of HIV acquisition for MSM (74), HIV-discordant heterosexual couples (75), and heterosexual men and women recruited as individuals (76). In addition, one clinical trial involving IDUs (77) and one involving heterosexual HIV-discordant couples (75) demonstrated substantial efficacy and safety of daily oral PrEP with TDF alone when combined with repeated condom provision, sexual risk-reduction counseling, and the diagnosis and treatment of STDs. High adherence to oral PrEP with TDF alone or in a fixed-dose combination with FTC was strongly associated with protection from infection. Data suggest that when administered orally, levels of TDF are lower in vaginal tissue than rectal tissue, potentially explaining why high levels of adherence were needed to yield benefits among women in these trials (78). Despite initial concerns about PrEP fostering antiretroviral resistance among persons who become infected, standard tests employed in these studies detected emergence of resistance only in persons inadvertently started on PrEP during acute HIV infection, not in persons who were initially uninfected but later became infected while taking PrEP medication (79).
The U.S. Public Health Service (USPHS) has issued recommendations on the basis of these trial results and the FDA approval of an indication for the use of TDF/FTC for PrEP. USPHS recommends that clinicians evaluate HIV-negative men and women who are sexually active or injecting illicit drugs and consider PrEP as a prevention option for persons whose sexual or injection behaviors and epidemiologic context place them at substantial risk for acquiring HIV infection. Comprehensive guidance for the use of daily PrEP to reduce the risk for acquiring HIV infection can be found at http://www.cdc.gov/hiv/prevention/research/prep/index.html.
HIV Seroadaptation Strategies
Seroadaptive strategies for HIV prevention have largely originated within communities of MSM. They are predicated on knowledge of self and partner HIV-infection status. One specific seroadaptive practice is serosorting, which includes limiting anal sex without a condom to partners with the same HIV status as their own, or choosing to selectively use condoms only with HIV serodiscordant partners. Another practice among serodiscordant couples is seropositioning, in which the person with HIV infection is the receptive partner for anal intercourse. Observational studies have consistently found that serosorting confers greater risk of HIV infection than consistent condom use, but is lower risk compared with anal intercourse without a condom and without serosorting (80–82). Serosorting practices have been associated with increased risk of STDs including chlamydia and gonorrhea (83,84).
Serosorting is not recommended for the following reasons: 1) too many MSM who have HIV do not know they are infected because they have not been tested for HIV recently, 2) men’s assumptions about the HIV status of their partners might be wrong, and 3) some men with HIV infection might not disclose or may misrepresent their HIV status. All of these factors increase the risk that serosorting could lead to HIV infection. Additional information is available at http://www.cdc.gov/msmhealth/serosorting.htm or http://www.who.int/hiv/pub/guidelines/msm_guidelines2011/en.
Retesting After Treatment to Detect Repeat Infections
Retesting several months after diagnosis of chlamydia, gonorrhea, or trichomoniasis can detect repeat infection and potentially can be used to enhance population-based prevention (85,86). Any person who tests positive for chlamydia or gonorrhea, along with women who test positive for trichomonas, should be rescreened 3 months after treatment. Any person who receives a syphilis diagnosis should undergo follow-up serologic syphilis testing per current recommendations (see Syphilis). Further details on retesting can be found in the specific sections on chlamydia, gonorrhea, syphilis, and trichomonas within this report.
Partner Services
The term “partner services” refers to a continuum of clinical evaluation, counseling, diagnostic testing, and treatment designed to increase the number of infected persons brought to treatment and to disrupt transmission networks. This continuum includes efforts undertaken by health departments, medical providers, and patients themselves. The term “public health partner services” refers to efforts by public health departments to identify the sex- and needle-sharing partners of infected persons to assure their medical evaluation and treatment.
Clinicians can provide partner services by counseling infected persons and providing them with written information and medication to give to their partners (if recommended and allowable by state law), directly evaluating and treating sex partners, and cooperating with state and local health departments. Clinicians’ efforts to ensure the treatment of a patient’s sex partners can reduce the risk for reinfection and potentially diminish transmission of STDs (87). Therefore, clinicians should encourage all persons with STDs to notify their sex partners and urge them to seek medical evaluation and treatment. Timespent counseling patients on the importance of notifying partners is associated with improved notification outcomes (88). When possible, clinicians should advise persons to bring their primary sex partner along with them when returning for treatment and should concurrently treat both persons. Although this approach can be effective for a main partner (89,90), it might not be feasible approach for additional sex partners. Some evidence suggests that providing patients with written information to share with sex partners can increase rates of partner treatment (87).
The types and comprehensiveness of public health partner services and the specific STDs for which they are offered vary by public health agency and the geographic burden of STDs. In most areas of the United States, health departments routinely attempt to provide partner services to all persons with early syphilis (primary, secondary, and early latent syphilis) and persons with a new diagnosis of HIV infection. It is also recommended that health departments provide partner services for persons who might have cephalosporin-resistant gonorrhea. In contrast, relatively few U.S. health departments routinely provide partner services to persons with gonorrhea, chlamydial infection, trichomonas, or other STDs (91). Clinicians should familiarize themselves with public health practices in their area, but in most instances, providers should understand that responsibility for ensuring the treatment of partners of persons with STDs other than syphilis and HIV rests with the diagnosing provider and the patient.
Many health departments now use the internet to notify the sex partners of persons with STDs (92), especially MSM and in cases where no other identifying information is available (http://www.ncsddc.org/Internet_Guidelines). Clinical providers are unlikely to participate directly in internet partner notification. Internet sites allowing patients to send anonymous e-mail or text messages advising partners of their exposure to an STD are operational in some areas; anonymous notification via the internet is considered better than no notification at all and might be an option in some instances. However, because the extent to which these sites affect partner notification and treatment is uncertain, patients should be encouraged either to notify their partners in person or by telephone, personal e-mail, or text message; alternatively, patients can authorize a medical provider or public health professional to do so.
Expedited Partner Therapy
Expedited Partner Therapy (EPT), also termed patient-delivered partner therapy (PDPT), is the clinical practice of treating the sex partners of persons who receive chlamydia or gonorrhea diagnoses by providing medications or prescriptions to the patient. Patients then provide partners with these therapies without the health-care provider having examined the partner (see http://www.cdc.gov/std/ept). Unless prohibited by law or other regulations, medical providers should routinely offer EPT to heterosexual patients with chlamydia or gonorrhea infection when the provider cannot confidently ensure that all of a patient’s sex partners from the prior 60 days will be treated. If the patient has not had sex in the 60 days before diagnosis, providers should attempt to treat a patient’s most recent sex partner. EPT is legal in most states. However, providers should visit http://www.cdc.gov/std/ept to obtain updated information for their state. Providing patients with appropriately packaged medication is the preferred approach to PDPT because data on the efficacy of PDPT using prescriptions is limited and many persons do not fill the prescriptions given to them by a sex partner. Medication or prescriptions provided for PDPT should be accompanied by treatment instructions, appropriate warnings about taking medications (if the partner is pregnant or has an allergy to the medication), general health counseling, and a statement advising that partners seek medical evaluation for any symptoms of STD, particularly PID.
The evidence supporting PDPT is based on three U.S. clinical trials involving heterosexual men and women with chlamydia or gonorrhea (93–95). All three trials reported that more partners were treated when patients were offered PDPT: two reported statistically significant declines in the rate of reinfection and one observed a lower risk of persistent or recurrent infection that was statistically nonsignificant. A fourth trial in the United Kingdom did not demonstrate a difference in the risk of reinfection or in the numbers of partners treated between persons offered PDPT and those advised to notify their sex partners (96).
U.S. trials and a meta-analysis of PDPT revealed that the magnitude of reduction in reinfection of index case-patients compared with patient referral differed according to the STD and the sex of the index case-patient (87,93–95). However, across trials, reductions in chlamydia prevalence at follow-up were approximately 20%; reductions in gonorrhea at follow-up were approximately 50%. Existing data suggest that PDPT also might have a role in partner management for trichomoniasis; however, no single partner management intervention has been shown to be more effective than any other in reducing trichomoniasis reinfection rates (97,98). No data support use of PDPT in the routine management of patients with syphilis. Data on the use of PDPT for gonorrhea or chlamydial infection among MSM are limited (99,100). Published studies suggest that >5% of MSM without a previous HIV diagnosis have a new diagnosis of HIV infection when evaluated as partners of patients with gonorrhea or chlamydial infection (101,102). As a result, PDPT should not be used routinely in MSM. All persons who receive bacterial STD diagnoses and their sex partners, particularly MSM, should be tested for HIV infection.
Reporting and Confidentiality
The accurate and timely reporting of STDs is integral to public health efforts to assess morbidity trends, allocate limited resources, and assist local health authorities in partner notification and treatment. STD/HIV and acquired immunodeficiency syndrome (AIDS) cases should be reported in accordance with state and local statutory requirements. Syphilis (including congenital syphilis), gonorrhea, chlamydia, chancroid, HIV infection, and AIDS are reportable diseases in every state. Because the requirements for reporting other STDs differ by state, clinicians should be familiar with the reporting requirements applicable within their jurisdictions.
Reporting can be provider- or laboratory-based or both. Clinicians who are unsure of state and local reporting requirements should seek advice from state or local health department STD programs. STDs and HIV reports are kept strictly confidential. In most jurisdictions, such reports are protected by statute or regulation. Before conducting a follow-up of a positive STD-test result, public health professionals should consult the patient’s health-care provider if possible to verify the diagnosis and determine the treatments being received.
Special Populations
Pregnant Women
Intrauterine or perinatally transmitted STDs can have severely debilitating effects on pregnant women, their partners, and their fetuses. All pregnant women and their sex partners should be asked about STDs, counseled about the possibility of perinatal infections, and provided access to screening and treatment, if needed.
Recommendations to screen pregnant women for STDs are based on disease severity and sequelae, prevalence in the population, costs, medico-legal considerations (e.g., state laws), and other factors. The screening recommendations in this report are generally broader (i.e., more pregnant women will be screened for more STDs than would by following other screening recommendations) and are consistent with other CDC guidelines.
Recommended Screening Tests
All pregnant women in the United States should be screened for HIV infection at the first prenatal visit, even if they have been previously tested (103,104). Screening should be conducted after the woman is notified of the need to be screened for HIV as part of the routine panel of prenatal tests, unless she declines (i.e., opt-out screening). For women who decline HIV testing, providers should address their objections, and when appropriate, continue to encourage testing. Women who decline testing because they have had a previous negative HIV test should be informed of the importance of retesting during each pregnancy. Testing pregnant women and treating those who are infected are vital not only to maintain the health of the woman, but to reduce perinatal transmission of HIV through available antiretroviral and obstetrical interventions. Retesting in the third trimester (preferably before 36 weeks’ gestation) is recommended for women at high risk for acquiring HIV infection (e.g., women who use illicit drugs, have STDs during pregnancy, have multiple sex partners during pregnancy, live in areas with high HIV prevalence, or have partners with HIV infection). Rapid HIV screening should be performed on any woman in labor who has not been screened for HIV during pregnancy unless she declines. If a rapid HIV test result is positive in these women, antiretroviral prophylaxis should be administered without waiting for the results of the confirmatory test (105).
A serologic test for syphilis should be performed for all pregnant women at the first prenatal visit (106). When access to prenatal care is not optimal, rapid plasma reagin (RPR) card test screening (and treatment, if that test is reactive) should be performed at the time that a pregnancy is confirmed. Women who are at high risk for syphilis or live in areas of high syphilis morbidity should be screened again early in the third trimester (at approximately 28 weeks’ gestation) and at delivery. Some states require all women to be screened at delivery. Neonates should not be discharged from the hospital unless the syphilis serologic status of the mother has been determined at least one time during pregnancy and preferably again at delivery if at risk. Any woman who delivers a stillborn infant should be tested for syphilis.
All pregnant women should be routinely tested for hepatitis B surface antigen (HBsAg) at the first prenatal visit even if they have been previously vaccinated or tested (107). Women who were not screened prenatally those who engage in behaviors that put them at high risk for infection (e.g., having had more than one sex partner in the previous 6 months, evaluation or treatment for an STD, recent or current injection-drug use, and an HBsAg-positive sex partner) and those with clinical hepatitis should be retested at the time of admission to the hospital for delivery. Pregnant women at risk for HBV infection also should be vaccinated. To avoid misinterpreting a transient positive HBsAg result during the 21 days after vaccination, HBsAg testing should be performed before vaccine administration. All laboratories that conduct HBsAg tests should test initially reactive specimens with a licensed neutralizing confirmatory test. When pregnant women are tested for HBsAg at the time of admission for delivery, shortened testing protocols can be used, and initially reactive results should prompt expedited administration of immunoprophylaxis to neonates (107). Pregnant women who are HBsAg positive should be reported to the local or state health department to ensure that they are entered into a case-management system and that timely and appropriate prophylaxis is provided to their infants. Information concerning the pregnant woman’s HBsAg status should be provided to the hospital in which delivery is planned and to the health-care provider who will care for the newborn. In addition, household and sex contacts of women who are HBsAg positive should be vaccinated. Women who are HBsAg positive should be provided with, or referred for, appropriate counseling and medical management.
All pregnant women aged <25 years and older women at increased risk for infection (e.g., those who have a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection) should be routinely screened for Chlamydia trachomatis at the first prenatal visit (108). Women aged <25 years and those at increased risk for chlamydia also should be retested during the third trimester to prevent maternal postnatal complications and chlamydial infection in the neonate. Pregnant women found to have chlamydial infection should have a test-of-cure to document chlamydial eradication (preferably by nucleic acid amplification testing [NAAT]) 3–4 weeks after treatment and then retested within 3 months. Screening during the first trimester might prevent the adverse effects of chlamydia during pregnancy, but evidence for such screening is lacking.
All pregnant women aged <25 years and older women at increased risk for gonorrhea (e.g., those with a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection) should be screened for N. gonorrhoeae at the first prenatal visit (108). Additional risk factors for gonorrhea include inconsistent condom use among persons not in mutually monogamous relationships, previous or coexisting sexually transmitted infection, and exchanging sex for money or drugs. Clinicians should consider the communities they serve and might choose to consult local public health authorities for guidance on identifying groups that are at increased risk. Gonococcal infection, in particular, is concentrated in specific geographic locations and communities. Women found to have gonococcal infection should be treated immediately and retested within 3 months. Pregnant women who remain at high risk for gonococcal infection also should be retested during the third trimester to prevent maternal postnatal complications and gonococcal infection in the neonate.
All pregnant women at risk for HCV infection should be screened for hepatitis C antibodies at the first prenatal visit. The most important risk factor for HCV infection is past or current injection drug use (109). Additional risk factors include having had a blood transfusion before July 1992, receipt of an unregulated tattoo, having been on long-term hemodialysis, intranasal drug use, and other percutaneous exposures. No established treatment regimen exists for pregnant women infected with HCV. However, all women with HCV infection should receive appropriate counseling and supportive care as needed (see Hepatitis C, Prevention). No vaccine is available to prevent HCV transmission.
Pregnant women should undergo a Papanicolau (Pap) test at the same frequency as nonpregnant women, although recommendations for management of abnormal Pap tests in pregnancy differ (110).
Other Tests
Evidence does not support routine screening for BV in asymptomatic pregnant women at high risk for preterm delivery (111). Symptomatic women should be evaluated and treated (see Bacterial Vaginosis).
Evidence does not support routine screening for Trichomonas vaginalis in asymptomatic pregnant women. Women who report symptoms should be evaluated and treated appropriately (see Trichomonas).
Evidence does not support routine HSV-2 serologic screening among asymptomatic pregnant women. However, type-specific serologic tests might be useful for identifying pregnant women at risk for HSV infection and guiding counseling regarding the risk for acquiring genital herpes during pregnancy. In the absence of lesions during the third trimester, routine serial cultures for HSV are not indicated for women in the third trimester who have a history of recurrent genital herpes.
For a more detailed discussion of STD screening and treatment among pregnant women, refer to the following references: Screening for HIV in Pregnant Women: Systematic Review to Update the 2005 U.S. Preventive Services Task Force Recommendation (103); Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement (104); ACOG/AAP Guidelines for Perinatal Care (112); Rapid HIV Antibody Testing During Labor and Delivery for Women of Unknown HIV Status: A Practical Guide and Model Protocol (113); Viral Hepatitis in Pregnancy (114); Hepatitis B Virus: A Comprehensive Strategy for Eliminating Transmission in the United States — Recommendations of the Immunization Practices Advisory Committee (ACIP) (4); Screening for Chlamydia and Gonorrhea: U.S. Preventive Services Task Force Recommendation Statement (108); Canadian guidelines on sexually transmitted infections (115); USPSTF recommendations for STI screening (116); and Screening for Bacterial Vaginosis in Pregnancy to Prevent Preterm Delivery: U.S. Preventive Services Task Force Recommendation Statement (111).
Adolescents
In the United States, prevalence rates of many sexually acquired infections are highest among adolescents and young adults (117,118). For example, the reported rates of chlamydia and gonorrhea are highest among females during their adolescent and young adult years, and many persons acquire HPV infection at this time.
Persons who initiate sex early in adolescence are at higher risk for STDs, along with adolescents residing in detention facilities, those who use injection drugs, adolescents attending STD clinics, and young men who have sex with men (YMSM). Factors contributing to this increased risk during adolescence include having multiple sexual partners concurrently, having sequential sexual partnerships of limited duration, failing to use barrier protection consistently and correctly, having increased biologic susceptibility to infection, and facing multiple obstacles to accessing health care (118).
All 50 states and the District of Columbia explicitly allow minors to consent for their own health services for STDs. No state requires parental consent for STD care, although some states restrict a minor’s ability to provide consent on the basis of age or type of service (i.e., prevention, diagnosis, or treatment only). No state requires that providers notify parents that an adolescent minor has received STD services, except in limited or unusual circumstances. However, many states authorize parental notification of a minor’s receipt of STD services, even where the minor can legally provide his or her own consent to the service (http://www.guttmacher.org/statecenter/spibs/spib_OMCL.pdf; http://www.cahl.org/state-minor-consent-laws-a-summary-third-edition). Protecting confidentiality for such care, particularly for adolescents enrolled in private health insurance plans, presents multiple problems. After a claim has been reported, many states mandate that health plans provide a written statement to the beneficiary indicating the service performed, the charges covered, what the insurer allows, and the amount for which the patient is responsible (i.e., explanation of benefit [EOB]) (119). In addition, federal laws obligate notices to beneficiaries when claims are denied, including alerting beneficiaries who need to pay for care until the allowable deductible is reached. For STD detection- and treatment-related care, an EOB or medical bill that is received by a parent might disclose services provided and list STD laboratory tests performed or treatment given.
Despite the high rates of infections documented in the adolescent population, providers frequently fail to inquire about sexual behaviors, assess STD risks, provide risk-reduction counseling, and ultimately, screen for asymptomatic infections during clinical encounters. Discussions concerning sexual behavior should be appropriate for the patient’s developmental level and should be aimed at identifying risk behaviors (e.g., multiple partners; unprotected oral, anal, or vaginal sex; and drug-use behaviors). Careful, nonjudgmental, and thorough counseling is particularly vital for adolescents who might not feel comfortable acknowledging their engagement in behaviors that place them at high risk for STDs.
Screening Recommendations
Routine laboratory screening for common STDs is indicated for sexually active adolescents. The following screening recommendations summarize published federal agency and medical professional organizations’ clinical guidelines for sexually active adolescents.
Routine screening for C. trachomatis on an annual basis is recommended for all sexually active females aged <25 years (108). Evidence is insufficient to recommend routine screening for C. trachomatis in sexually active young men based on efficacy and cost-effectiveness. However, screening of sexually active young males should be considered in clinical settings serving populations of young males with a high prevalence of chlamydia (e.g., adolescent clinics, correctional facilities, and STD clinics) and should be offered to YMSM (see Special Populations, MSM) (120,121).
Routine screening for N. gonorrhoeae on an annual basis is recommended for all sexually active females <25 years of age (108). Gonococcal infection is concentrated in specific geographic locations and communities. Clinicians should consider the communities they serve and might choose to consult local public health authorities for guidance on identifying groups that are at increased risk. Screening should be offered to YMSM (see MSM section).
HIV screening should be discussed and offered to all adolescents. Frequency of repeat screenings of those who are at risk for HIV infection should be based on level of risk (122,123). Persons who test positive for HIV should receive prevention counseling and referral to care before leaving the testing site.
The routine screening of adolescents who are asymptomatic for certain STDs (e.g., syphilis, trichomoniasis, BV, HSV, HPV, HAV, and HBV) is not generally recommended. However, YMSM and pregnant adolescent females should be screened for syphilis.
Guidelines from USPSTF, ACOG, and ACS recommend that cervical cancer screening begin at age 21 years (124–126). This recommendation is based on the low incidence of cervical cancer and limited utility of screening for cervical cancer in adolescents (127).
Primary Prevention Recommendations
Primary prevention and anticipatory guidance to recognize symptoms and behaviors associated with STDs are strategies that can be incorporated into any or all types of healthcare visits for adolescents and young adults. The following recommendations for primary prevention of STDs (i.e., vaccination and counseling) are based on published federal agency and medical professional organizations’ clinical guidelines for sexually active adolescents and young adults.
The HPV vaccine, bivalent, quadrivalent, or 9-valent, is recommended routinely for females aged 11 and 12 years and can be administered beginning at 9 years of age (16) http://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/hpv.html. Vaccination is also recommended for females aged 13–26 years who have not yet received all doses or completed the vaccine series. The quadrivalent or 9-valent HPV vaccine is recommended routinely for males aged 11 and 12 years and also can be administered beginning at 9 years of age (16). Vaccination with quadrivalent or the 9-valent HPV vaccine is recommended for males aged 13–21 years who have not yet received all doses or completed the vaccine series, although males aged 22–26 years also can be vaccinated (16). For persons with HIV infection and for MSM, vaccination is recommended through age 26. HPV vaccination has not been associated with a change in perceptions about risks posed by sexual behavior (128).
The HBV vaccination series is recommended for all adolescents and young adults who have not previously received the hepatitis B vaccine (3,4).
The HAV vaccination series should be offered to adolescents and young adults who have not previously received the HAV vaccine series.
Information regarding HIV infection, testing, transmission, and implications of infection should be regarded as an essential component of the anticipatory guidance provided to all adolescents and young adults as part of health care (122).
Health-care providers who care for adolescents and young adults should integrate sexuality education into clinical practice. Providers should counsel adolescents about the sexual behaviors that are associated with risk for acquiring STDs and educate patients regarding evidence-based prevention strategies, all of which include a discussion about abstinence and other risk-reduction behaviors (e.g., consistent and correct condom use and reduction in the number of sex partners). Interactive counseling approaches, such as high-intensity behavioral counseling (HIBC) and motivational interviewing, are effective STD/HIV prevention strategies. USPSTF recommends high-intensity behavioral counseling for all sexually active adolescents (7) to prevent sexually transmitted infections.* Educational materials (e.g., handouts, pamphlets, and videos) can reinforce office-based educational efforts.
Children
Management of children who have STDs requires close cooperation between clinicians, laboratorians, and child-protection authorities. Official investigations, when indicated, should be initiated promptly. Certain diseases (e.g., gonorrhea, syphilis, and chlamydia), if acquired after the neonatal period, strongly suggest sexual contact. For other diseases (e.g., HPV infections and vaginitis), the association with sexual contact is not as clear (see Sexual Assault and STDs).
Persons in Correctional Facilities
Multiple studies have demonstrated that persons entering correctional facilities have high rates of STDs (including HIV) and viral hepatitis (http://www.cdc.gov/hepatitis/Settings/corrections.htm), especially those aged ≤35 years (118). Incarcerated persons are more likely to have low socioeconomic status, live in urban areas, and be ethnic and racial minorities. Risk behaviors for contracting STDs (e.g., having unprotected sex; having multiple sexual partners; using drugs and alcohol; and engaging in commercial, survival, or coerced sex) are common among incarcerated populations. Before incarceration, many have had limited access to medical care.
Although no comprehensive national guidelines regarding STD care and management have been developed for correctional populations, growing evidence demonstrates the utility of expanded STD screening and treatment services in correctional settings. For example, in jurisdictions with comprehensive, targeted jail screening, more chlamydial infections among females (and males if screened) are detected and subsequently treated in the correctional setting than any other single reporting source (118,129) and might represent the majority of reported cases in certain jurisdictions (130).
Both men and women ≤35 years of age in juvenile and adult detention facilities have been reported to have higher rates of chlamydia (131) and gonorrhea (118) than their nonincarcerated counterparts in the community, and across many studies, rates have been consistently higher among women than men. Syphilis seroprevalence rates, which can indicate previous or current infection, are considerably higher among adult men and women than in adolescents, consistent with the overall national syphilis trends (132). Detection and treatment of early syphilis in correctional facilities might impact rates of transmission (133).
In short-term facilities, including jails and juvenile detention facilities that commonly house entrants for <1 year, up to half of entrants are released back in the community within 48 hours. As a result, treatment completion rates for those screened for STDs and who receive STD diagnoses in short-term facilities might not be optimal. However, because of the mobility of incarcerated populations in and out of the community, the impact of screening in correctional facilities on the prevalence of infections among detainees and subsequent transmission in the community after release might be considerable (134). Moreover, treatment completion rates of ≥95% can be achieved by offering screening at or shortly after intake, facilitating earlier receipt of test results; follow-up of untreated persons can be conducted through public health outreach (130).
Universal screening for chlamydia and gonorrhea in women ≤35 years entering juvenile and adult correctional facilities has been a long-standing recommendation. However, no such recommendation existed for men until 2006, when CDC convened a consultation on male chlamydia screening (121) that resulted in recommendations to screen men <30 years for chlamydia at intake into jails.
Whereas several studies have shown a high prevalence of trichomonas among incarcerated persons, none have demonstrated the impact of trichomonas screening in correctional facilities (135–137). Women who report vaginal discharge should be evaluated and treated appropriately.
Chlamydia and Gonorrhea Screening
Women ≤35 and men <30 years in correctional facilities should be screened for chlamydia and gonorrhea. Chlamydia and gonorrhea screening should be conducted at intake.
Syphilis Screening
Universal screening should be conducted on the basis of the local area and institutional prevalence of early (primary, secondary, and early latent) infectious syphilis. Correctional facilities should stay apprised of syphilis prevalence as it changes over time.
Men Who Have Sex with Men
The term “men who have sex with men” (MSM) describes a heterogeneous group of men who have varied behaviors, identities, and health-care needs (138). Some MSM are at high risk for HIV infection and other viral and bacterial STDs because MSM may practice anal sex, and the rectal mucosa is uniquely susceptible to certain STD pathogens. In addition, multiple sex partners, substance use, and sexual network dynamics of MSM increase risk for HIV and STDs in this population. The frequency of unsafe sexual practices and the reported rates of bacterial STDs and incident HIV infection declined substantially in MSM from the 1980s through the mid-1990s. However, since that time, increased rates of early syphilis (primary, secondary, or early latent), gonorrhea, and chlamydial infection and higher rates of sexual risk behaviors have been documented among MSM in the United States and virtually all industrialized countries.
Approximately two thirds of the cases of primary and secondary syphilis diagnoses in the United States are in MSM, particularly those in ethnic minority groups (118,139,140). Increased syphilis screening in MSM demonstrated a doubling of early syphilis detection; however, 71% of the syphilis diagnoses occurred when the patient sought care for symptoms (141). Acute HIV infection has been associated with a recent or concurrent STD, including syphilis, among men at a municipal STD clinic (142) and in the multisite iPrex study (143), and several studies have demonstrated that early syphilis is associated with HIV infection among MSM (144,145). Factors associated with increases in syphilis among MSM have included substance abuse (e.g., methamphetamine), having multiple anonymous partners, and seeking sex partners through the internet (146,147). One study found that 5.9% of MSM had repeat primary or secondary syphilis infection within 2 years of an initial infection; factors associated with repeat syphilis infection were HIV infection, black race, and having ≥10 recent sexual partners (148). Because of this risk for repeat infection, these data suggest that prevention efforts should include follow up serologic testing.
Gonococcal infection in MSM has been associated with similar risk factors, including having multiple anonymous partners and abuse of substances, particularly crystal methamphetamine (149). Rectal gonococcal rates are increasing among MSM with HIV infection, underscoring the importance of obtaining an accurate, current sexual history and asking about correlates of increased risk (e.g., anonymous sex and substance use) (150). Insertive oral sex has been associated with urethral gonorrhea acquisition (151,152); the prevalence of pharyngeal gonorrhea and pharyngeal chlamydia has been demonstrated to be 7.3% and 2.3%, respectively (153). In a multicity study, rectal gonorrhea and rectal chlamydia prevalence rates among MSM were 5.4% and 8.9%, respectively (154). Rectal gonorrhea and chlamydia infections, especially those that are recurrent, have been associated with increased risk for HIV seroconversion among MSM (155,156). MSM with new HIV infection diagnoses are more likely than HIV-uninfected MSM to receive a diagnosis of asymptomatic gonorrhea (25.9% versus 10.9%, p<0.001) and chlamydia (18.5% vs 7.8%, p<0.001) (157). Thus, rectal gonorrhea and chlamydia screening in MSM might be a cost-effective intervention in certain urban settings (158).
MSM remain at disproportionate risk for HIV acquisition and transmission in the United States, particularly those who are black or Hispanic. Factors that increase the risk for HIV infection in MSM include either receptive or insertive anal sex without a condom, having another STD, having sex with anonymous partners without a condom, and using methamphetamines or drugs that enhance sexual performance (159).
Substantial numbers of MSM remain unaware of their serostatus (up to 44% in one recent survey of young men in minority populations) (160). Unfortunately, many men are not asked about STD-related risks, including the gender of sex partners. Even if gender of sex partners is ascertained, many MSM, including those with HIV infection, are neither asked about risky sexual behaviors nor provided with routine STD testing (especially at anatomic sites of exposure for gonorrhea or chlamydia), often because of the discomfort associated with these discussions (161–163). Clinicians should routinely ask sexually active MSM about symptoms consistent with common STDs, including urethral discharge, dysuria, genital and perianal ulcers, regional lymphadenopathy, skin rash, and anorectal symptoms consistent with proctitis (e.g., discharge and pain on defecation or during anal intercourse) and then perform appropriate diagnostic testing. In addition, providers should offer evidence-based counseling on safer sex using interventions that have been demonstrated to decrease STD incidence in clinical-care settings (10).
Clinicians should be familiar with local resources available to assist MSM with syphilis and HIV partner services as well as HIV linkage and retention in care. In addition, interventions promoting behavior change also might be appropriate. In recent years, medical educational materials have been developed in print (164) and through electronic media (http://www.lgbthealtheducation.org) to increase primary-care provider knowledge and cultural competency regarding the diagnosis and management of STDs and other clinical conditions in the lesbian, gay, bisexual, and transgender populations. Electronic media is also an important tool for disseminating and collecting information to and from MSM. Because many MSM meet partners online and seek health information from websites, increased use of the internet for STD prevention might be warranted. MSM are amenable to receiving HIV and STD risk-reduction messages online (165) and willing to respond to requests for partner identification from public health authorities through the internet (166).
The following screening tests should be performed at least annually for sexually active MSM, including those with HIV infection.
HIV serology if HIV status is unknown or negative and the patient himself or his sex partner(s) has had more than one sex partner since most recent HIV test.
Syphilis serology to establish whether persons with reactive tests have untreated syphilis, have partially treated syphilis, are manifesting a slow serologic response to appropriate prior therapy, or are serofast.
A test for urethral infection† with N. gonorrhoeae and C. trachomatis in men who have had insertive intercourse§ during the preceding year (testing of the urine using NAAT† is the preferred approach).
A test for rectal infection† with N. gonorrhoeae and C. trachomatis in men who have had receptive anal intercourse§ during the preceding year (NAAT of a rectal specimen is the preferred approach).
A test for pharyngeal infection† with N. gonorrhoeae in men who have had receptive oral intercourse§ during the preceding year (NAAT of a pharyngeal specimen is the preferred approach). Testing for C. trachomatis pharyngeal infection is not recommended.
MSM with HIV infection are also at risk for STDs. Data from a study of 557 adults with HIV infection receiving primary care in four U.S. cities demonstrate that 13% had STD at study enrollment, and 7% had incident STD at 6 months; among MSM with HIV infection, STD incidence was 20% (10). Excluding trichomoniasis, 94% of incident STDs were diagnosed in MSM. All MSM with HIV infection entering care should be screened for gonorrhea and chlamydia at appropriate anatomic sites of exposure, as well as for syphilis (17). The frequency of follow-up testing might be dictated by subsequent behavior; screening is recommended annually, at a minimum, to include syphilis serologic testing and chlamydia and gonorrhea screening at exposed anatomic sites (138). STD screening rates in HIV clinics have been suboptimal. In one study involving eight U.S. cities, although syphilis testing was provided to most MSM with HIV infection, <10% were screened for extra-genitourinary gonorrhea or chlamydia, and <20% provided the urine or urethral specimens needed for testing (162). More frequent STD screening (i.e., for syphilis, gonorrhea, and chlamydia) at 3–6-month intervals is indicated for MSM, including those with HIV infection if risk behaviors persist or if they or their sexual partners have multiple partners. Evaluation for HSV-2 infection with type-specific serologic tests also can be considered if infection status is unknown in persons with previously undiagnosed genital tract infection.
HPV infection and HPV-associated conditions (e.g., anogenital warts and anal squamous intraepithelial lesions) are highly prevalent among MSM. The quadrivalent vaccine is recommended routinely for MSM through age 26 years (16,167,168); the efficacy of this vaccine in preventing HPV associated diseases in men aged >26 years is unknown.
Data are insufficient to recommend routine anal-cancer screening with anal cytology in persons with HIV infection or HIV-negative MSM. More evidence is needed concerning the natural history of anal intraepithelial neoplasia, the best screening methods and target populations, safety of and response to treatments, and other programmatic considerations before screening can be routinely recommended. However, some clinical centers perform anal cytology to screen for anal cancer among high-risk populations (e.g., persons with HIV infection and MSM), followed by high-resolution anoscopy for those with abnormal cytologic results (e.g., ASC-US).
All MSM should be tested for HBsAg to detect chronic HBV infection. Prompt identification of chronic infection with HBV is essential to ensure necessary care and services to prevent transmission to others (169). Screening among past or current drug users should include HCV and HBV testing. Vaccination against hepatitis A and B is recommended for all MSM in whom previous infection or vaccination cannot be documented (2,3). Preimmunization serologic testing might be considered to reduce the cost of vaccinating MSM who are already immune to these infections, but this testing should not delay vaccination. Vaccinating persons who are immune to HAV or HBV infection because of previous infection or vaccination does not increase the risk for vaccine-related adverse events (see Hepatitis A and Hepatitis B).
Sexual transmission of HCV can occur, especially among MSM with HIV infection (see Emerging Issues, Hepatitis C). Serologic screening for HCV is recommended at initial evaluation of persons with newly diagnosed HIV infection. Because of accumulating evidence of acute HCV infection acquisition among persons with HIV infection (especially MSM with HIV infection [170–175]) and because regular screening for HCV infection is cost effective (176,177), MSM with HIV infection should be regularly screened for HCV. Screening should be performed at least yearly and more frequently depending on specific circumstances (e.g., local HCV prevalence and incidence, high-risk sexual behavior, and concomitant ulcerative STDs or STD-related proctitis). Screening should be performed using HCV antibody assays followed by HCV RNA testing for those with a positive antibody result (178).
Women Who Have Sex with Women
Women who have sex with women (WSW) are a diverse group with variations in sexual identity, sexual behaviors, sexual practices, and risk behaviors. Recent studies indicate that some WSW, particularly adolescents and young women as well as women with both male and female partners, might be at increased risk for STDs and HIV based on reported risk behaviors (179–183). Certain studies have highlighted the wide diversity of sexual practices and examined use of protective/risk reduction strategies among populations of WSW (184–186). Use of barrier protection with female partners (gloves during digital-genital sex, condoms with sex toys, and latex or plastic barriers [also known as dental dams for oral-genital sex]) was infrequent in all studies. Despite this, few comprehensive and reliable resources of sexual health information for WSW are available (187).
Few data are available on the risk for STDs conferred by sex between women, but transmission risk probably varies by the specific STD and sexual practice (e.g., oral-genital sex; vaginal or anal sex using hands, fingers, or penetrative sex items; and oral-anal sex) (188,189). Practices involving digital-vaginal or digital-anal contact, particularly with shared penetrative sex items, present a possible means for transmission of infected cervicovaginal or anal secretions. This possibility is most directly supported by reports of shared trichomonas infections (190,191) and by concordant drug resistance genotype testing and phylogenetic linkage analysis identifying HIV transmitted sexually between women (192,193). Most self-identified WSW (53%–97%) have had sex with men in the past and might continue this practice, with 5%–28% of WSW reporting male partners within the past year (189,194–196).
HPV, which can be transmitted through skin-to-skin contact, is common among WSW, and sexual transmission of HPV likely occurs between female sex partners (197–199). HPV DNA has been detected through polymerase chain reaction (PCR)-based methods from the cervix, vagina, and vulva in 13%–30% of WSW (197,198). Among WSW who reported never having had a male sexual partner, 26% had antibodies to HPV-16, and 42% had antibodies to HPV-6 (197). High- and low-grade squamous intraepithelial lesions (SIL) have been detected on Pap tests in WSW who reported no previous sex with men (197,198,200,201). WSW are at risk for acquiring HPV from both their female partners and from current or prior male partners, and thus are at risk for cervical cancer. Therefore, routine cervical cancer screening should be offered to all women, regardless of sexual orientation or sexual practices, and women should be offered HPV vaccine as per current guidelines (16).
Genital transmission of HSV-2 between female sex partners is inefficient, but can occur. A U.S. population-based survey among women aged 18–59 years demonstrated an HSV-2 seroprevalence of 30% among women reporting same-sex partners in the past year, 36% among women reporting samesex partners in their lifetime, and 24% among women reporting no lifetime same-sex behavior (195). HSV-2 seroprevalence among women self-identifying as “homosexual or lesbian” was 8%, similar to a prior clinic-based study of WSW (195,196). The relatively frequent practice of orogenital sex among WSW might place them at higher risk for genital infection with HSV-1, a hypothesis supported by the recognized association between HSV-1 seropositivity and previous number of female partners among WSW. Thus, sexual transmission of HSV-1 and HSV-2 can occur between female sex partners. This information should be communicated to women as part of a larger sexual health counseling and evaluation effort.
Less is known regarding transmission of bacterial STDs between female partners. Transmission of syphilis between female sex partners, probably through oral sex, has been reported. Although the rate of transmission of C. trachomatis between women is unknown, infection also might be acquired from past or current male partners. More recent data suggests that C. trachomatis infection among WSW might be more common than previously believed (179,202). Reports of same-sex behavior in women should not deter providers from offering and providing screening for STDs, including chlamydia, according to current guidelines.
BV is common among women in general and even more so among women with female partners (203,204). Sexual behaviors that facilitate the transfer of vaginal fluid and bacteria between partners may be involved in the pathogenesis of BV. A study including monogamous couples demonstrated that female sex partners frequently share identical genital Lactobacillus strains (205). Within a community-based cohort of WSW, extravaginal (i.e., oral and rectal) reservoirs of BV-associated bacteria were a risk factor for incident BV (206). Several new studies have examined the impact of specific sexual practices on the vaginal microflora (207–209) and on recurrent (210) or incident (211,212) BV among WSW and non-WSW. These studies have continued to support, though have not proven, the hypothesis that sexual behaviors, specific BV-associated bacteria, and possibly exchange of vaginal or extravaginal microbiota (e.g., oral bacterial communities) between partners might be involved in the pathogenesis of BV in WSW.
Although BV is common in WSW, routine screening for BV is not recommended. Results of a randomized trial using a behavioral intervention to reduce persistent BV among WSW through reduced sharing of vaginal fluid on hands or sex toys has been published (213). Although women randomized to the intervention were 50% less likely to report receptive digitalvaginal contact without gloves than controls and reported sharing sex toys infrequently, these women had no reduction in persistent BV at 1 month post-treatment and no reduction in incident episodes of recurrent BV. To date, no reported trials have examined the potential benefits of treating female partners of women with BV; thus, no recommendation can be made regarding partner therapy in WSW. Increasing awareness of signs and symptoms of BV in women and encouraging healthy sexual practices (e.g., avoiding shared sex toys, cleaning shared sex toys, and barrier use) might benefit women and their partners. WSW are at risk for acquiring bacterial, viral, and protozoal STDs from current and prior partners, both male and female. WSW should not be presumed to be at low or no risk for STDs based on sexual orientation. Report of same sex behavior in women should not deter providers from considering and performing screening for STDs and cervical cancer according to current guidelines. Effective screening requires that care providers and their female patients engage in a comprehensive and open discussion of sexual and behavioral risks that extends beyond sexual identity.
Transgender Men and Women
Persons who are transgender identify with a sex that differs from that they were assigned at birth. Transgender women (“trans-women” or “transgender male to female”) identify as women but were born with male anatomy. Similarly, transgender men (also referred to as “trans-men” or “transgender female to male”) identify as men but were born with female anatomy. However, transgender persons might use different and often fluid terminology to refer to themselves through their life course. Gender identity is independent from sexual orientation. Persons who are transgender might have sex with men, women, or both and consider themselves to be heterosexual, gay, lesbian, or bisexual. Prevalence studies of transgender persons in the overall population have been limited and often based on small convenience samples.
Transgender Women
A systematic review of studies of HIV among transgender women suggests that the prevalence of HIV in the United States is 27.7% among all transgender women and 56.3% among black transgender women (214). Data also suggests high rates of HIV among transgender women globally (215). Bacterial STD prevalence varies among transgender women, but is based largely on convenience samples. Providers caring for transgender women should have knowledge of their patients’ current anatomy and patterns of sexual behavior before counseling them about STD and HIV prevention (216). Most transgender women have not undergone genital affirmation surgery and may retain a functional penis (217–219); in this instance, they might engage in insertive oral, vaginal, or anal sex with men and women.
Transgender Men
The few studies of HIV prevalence and incidence in transgender men suggest that although some transgender men engage in risky behaviors, they have a lower prevalence of HIV than transgender women (220). Providers should consider the anatomic diversity among transgender men, because many still have a vagina and cervix and are at risk for bacterial STDs, cervical HPV, and cervical cancer (221).
Recommendations
Clinicians should assess STD- and HIV-related risks for their transgender patients based on current anatomy and sexual behaviors. Because of the diversity of transgender persons regarding surgical affirming procedures, hormone use, and their patterns of sexual behavior, providers must remain aware of symptoms consistent with common STDs and screen for asymptomatic STDs on the basis of behavioral history and sexual practices.
Emerging Issues
Hepatitis C
HCV infection is the most common chronic bloodborne infection in the United States, with an estimated 2.7 million persons living with chronic infection (222). HCV is not efficiently transmitted through sex (170,223). Studies of HCV transmission between heterosexual or homosexual couples have yielded mixed results, but generally have found either no or very minimally increased rates of HCV infection in partners of persons with HCV infection compared with those whose partners are not HCV-infected (223–230). However, data indicate that sexual transmission of HCV can occur, especially among persons with HIV infection. Increasing incidence of acute HCV infection among MSM with HIV infection has been reported in New York City (231,232) and Boston (175,177), along with multiple European cities (233–235). These men usually engage in high-risk and traumatic sexual practices and might have concurrent genital ulcerative disease or STD-related proctitis (233,235). Other common practices associated with new cases of HCV infection include group sex and use of cocaine and other nonintravenous drugs during sex. Certain studies have revealed that risk increases commensurate with increasing numbers of sex partners among heterosexual persons with HIV infection (225,226,236–238) and MSM (239–242), especially if their partners are also coinfected with HIV (234,235,239–243).
Persons newly infected with HCV typically are either asymptomatic or have a mild clinical illness. HCV RNA can be detected in blood within 1–3 weeks after exposure. The average time from exposure to antibody to HCV (anti-HCV) seroconversion is 8–9 weeks, and anti-HCV can be detected in >97% of persons by 6 months after exposure. Chronic HCV infection develops in 70%–85% of HCV-infected persons; 60%–70% of chronically infected persons develop evidence of active liver disease. Most infected persons remain unaware of their infection because they are not clinically ill. However, infected persons serve as a source of transmission to others and are at risk for CLD and other HCV-related chronic diseases decades after infection.
HCV is primarily transmitted parenterally, usually through shared drug-injection needles and paraphernalia. HCV also can be transmitted through exposures in health-care settings as a consequence of inadequate infection-control practices (244). Transmission following receipt of blood, tissues, and organs from donors with HCV infection has occurred only rarely since 1992, when routine screening of these donated products was mandated in the United States. Tattoos applied in regulated settings have not been associated with HCV transmission, although those obtained in unregulated settings have been linked to such transmission (224). Occupational and perinatal exposures also can result in transmission of HCV, but such transmission is uncommon.
Acute hepatitis C is a reportable condition in 49 states, and matching viral hepatitis and HIV surveillance registries can facilitate early detection of social networks of HCV transmission among MSM with HIV infection. Suspected clusters of acute HCV infection should be reported to the appropriate public health authorities.
HCV screening is recommended by CDC and USPSTF for all persons born during 1945–1965 and others based on their risk for infection or on a recognized exposure, including past or current injection drug use, receiving a blood transfusion before 1992, long-term hemodialysis, being born to a mother with HCV infection, intranasal drug use, receipt of an unregulated tattoo, and other percutaneous exposures (109,224,245).
Diagnosis
Testing for HCV infection should include use of an FDA-cleared test for antibody to HCV (i.e., immunoassay, EIA, or enhanced chemiluminescence immunoassay and, if recommended, a supplemental antibody test) followed by NAAT to detect HCV RNA for those with a positive antibody result (178). Persons with HIV infection with low CD4-positive cell count might require further testing by NAAT because of the potential for a false-negative antibody assay.
Persons determined to be anti-HCV positive should be evaluated (by referral or consultation, if appropriate) for the presence of acute infection; presence, severity, or development of CLD; and eligibility for treatment. Nucleic acid testing, including reverse transcriptase polymerase chain reaction (RT-PCR) to detect HCV RNA, is necessary to confirm the diagnosis of current HCV infection, and testing of liver function (alanine aminotransferase level) provides biochemical evidence of CLD.
Treatment
Providers should consult with specialists knowledgeable about management of hepatitis C infection. Further, they can consult existing guidelines to learn about the latest advances in the management of hepatitis C (http://www.hcvguidelines.org).
Management of Sex Partners
Because incident HCV has not been demonstrated to occur in heterosexual couples followed over time (223,227–229), condom use might not be necessary in such circumstances. Persons with HCV infection with one long-term, steady sex partner do not need to change their sexual practices. However, they should discuss the low but present risk for transmission with their partner and discuss the need for testing (170,245). Heterosexuals and MSM with HCV infection and more than one partner, especially those with concurrent HIV infection, should protect their partners against HCV and HIV acquisition by using male latex condoms (231,234,235). Partners of persons with HCV and HIV infection should be tested for HCV and HIV, if not known to be infected.
Other Management Considerations
All persons with HCV for whom HIV and HBV infection status is unknown should be tested for these infections. Those who have HIV or HBV should be referred for or provided with appropriate care and treatment.
Prevention
Reducing the burden of HCV infection and disease in the United States requires implementation of both primary and secondary prevention activities. Primary prevention reduces or eliminates HCV transmission, whereas secondary prevention activities are aimed at reducing CLD and other chronic diseases in persons with HCV infection by first identifying them and then providing medical management and antiviral therapy, if appropriate. No vaccine for hepatitis C is available, and prophylaxis with immune globulin is not effective in preventing HCV infection after exposure.
Persons with HCV infection should be provided information regarding how to protect their liver from further harm (i.e., hepatotoxic agents); for instance, persons with HCV infection should be advised to avoid drinking alcohol and taking any new medicines (including over-the-counter and herbal medications) without checking with their clinician. In addition, a determination for the need of hepatitis A and B vaccination should be made; persons who are not immune should be vaccinated.
To reduce the risk for transmission to others, persons with HCV infection should be advised 1) not to donate blood, body organs, other tissue, or semen; 2) not to share any personal items that might have blood on them (e.g., toothbrushes and razors); and 3) to cover cuts and sores on the skin to keep the virus from spreading by blood or secretions. Women with HCV infection do not need to avoid pregnancy or breastfeeding.
Persons who use or inject drugs should be counseled about the importance of stopping drug-use behaviors and provided with assistance to enter and complete substance-abuse treatment (including relapse prevention). Persons who continue to inject drugs despite counseling should be encouraged to take the following additional steps to reduce personal and public health risks:
never reuse or share syringes, water, or drug preparation equipment;
only use syringes obtained from a reliable source (e.g., pharmacies);
use a new, sterile syringe to prepare and inject drugs;
if possible, use sterile water to prepare drugs; otherwise, use clean water from a reliable source (e.g., fresh tap water);
use a new or disinfected container (i.e., cooker) and a new filter (i.e., cotton) to prepare drugs;
clean the injection site before injection with a new alcohol swab; and
safely dispose of syringes after one use.
Postexposure Follow-Up
No postexposure prophylaxis has been demonstrated to be effective against HCV. HCV testing is recommended for health-care workers after percutaneous or permucosal exposures to HCV-positive blood. Children born to women with HCV infection also should be tested for HCV. Prompt identification of acute infection is important, because outcomes are improved when treatment is initiated early in the course of illness.
Special Considerations
Pregnancy
Routine screening for HCV infection is not recommended for all pregnant women. Pregnant women with a known risk factor for HCV infection should be offered screening. Although the rate for transmission is highly variable, up to six of every 100 infants born to HCV-infected women become infected; this infection occurs predominantly during or near delivery, and no treatment or delivery method—such as caesarian section—has been demonstrated to decrease this risk (246). However, the risk is increased by the presence of maternal HCV viremia at delivery and is two- to threefold greater if the woman is coinfected with HIV. HCV has not been shown to be transmitted through breast milk, although mothers with HCV infection should consider abstaining from breastfeeding if their nipples are cracked or bleeding. Infants born to mothers with HCV infection should be tested for HCV infection; because maternal antibody is present for the first 18 months of life and before the infant mounts an immunologic response, nucleic acid testing is recommended (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6218a5.htm?s_cid=mm6218a5_w).
HIV Infection
All persons with HIV infection should undergo serologic screening for HCV at initial evaluation (17,247). Providers should be aware of the likelihood that MSM with HIV infection will acquire HCV after initial screening. Because of accumulating evidence of acute HCV infection acquisition in persons with HIV infection, especially MSM, and cost-effectiveness of regular screening (176,177), periodic HCV screening should be considered (170–175). For persons with HIV infection, HCV screening with HCV antibody assays can be considered at least yearly in those at high risk for infection and more frequently depending on specific circumstances (e.g., community HCV prevalence and incidence, high-risk sexual behavior, and concomitant ulcerative STDs and STD-related proctitis). Indirect testing (e.g., ALT) is not recommended for detecting incident HCV infections because such testing, especially if performed once a year, can miss many persons who have reverted after acute HCV infection to a normal ALT level at the time of testing (175,177). Conversely, ALT can be elevated by antiretroviral and other medications, alcohol, and toxins. If ALT levels are being monitored, persons with HIV infection who experience new and unexplained increases in ALT should be tested for acute HCV infection and evaluated for possible medication toxicity or excessive alcohol use.
Continued unprotected sexual contact between partners with HIV infection can facilitate spread of HCV, as the virus can be recovered from the semen of men with HIV (248). Specific prevention practices (e.g., barrier precautions that limit contact with body fluids during sexual contact with other MSM) should be discussed.
Because a minimal percentage of persons with HIV infection fail to develop HCV antibodies, HCV RNA testing should be performed in persons with unexplained liver disease who are anti-HCV negative. The course of liver disease is more rapid in HIV/HCV coinfected persons, and the risk for cirrhosis is nearly twice that of persons with HCV infection alone. Coinfected persons receiving HIV antiviral regimens are now being treated for HCV after their CD4+ cell counts increase, optimizing their immune response.
Mycoplasma genitalium
M. genitalium was first identified in the early 1980s (249) and has become recognized as a cause of male urethritis, responsible for approximately 15%–20% of nongonococcal urethritis (NGU) cases, 20%–25% of nonchlamydial NGU, and approximately 30% of persistent or recurrent urethritis (250). In most settings, it is more common than N. gonorrhoeae but less common than C. trachomatis. While M. genitalium is often the sole pathogen detected, coinfection with C. trachomatis is not uncommon in selected areas (251–253).
Although strong and consistent evidence has linked M. genitalium to urethritis in men, it remains unknown whether this infection can cause male infertility or other male anogenital tract disease syndromes. The organism has been detected in men with epididymitis in a limited number of cases, but this has not been extensively investigated. Similarly, M. genitalium has been found in the rectum, but detection is infrequently accompanied by rectal symptoms, and its presence does not appear to cause a syndrome of clinical proctitis.
The pathogenic role of M. genitalium is less definitive in women than it is in men. M. genitalium can be found in the vagina, cervix, and endometrium and, like chlamydial and gonococcal infections, M. genitalium infections in women are commonly asymptomatic. M. genitalium can be detected in 10%–30% of women with clinical cervicitis, and most (253–259) studies have found that this organism is more common among women with cervicitis than those without this syndrome (251,260,261).
M. genitalium is found in the cervix and/or endometrium of women with PID more often than in women without PID (262–271), and endosalpingitis develops in nonhuman primates after inoculation with M. genitalium, suggesting that this organism can cause PID. M. genitalium has been detected in 2%–22% of PID cases (median: 10%) depending on the setting, but the frequency with which M. genitalium-infected women experience PID has been under studied. Although one study in Sweden reported a substantial increase in risk for postabortal PID among women with M. genitalium (262), the proportion of M. genitalium-positive women who subsequently experienced PID in two other studies was relatively low (<5%) (272,273), and evidence from serologic studies assessing the association of PID with antibody to M. genitalium is inconsistent. Overall, evidence suggests that M. genitalium can cause PID, but that this occurs less frequently than it does with C. trachomatis (271,273).
A few seroepidemologic studies have found that women with tubal factor infertility are more likely to have antibodies to M. genitalium than fertile women, suggesting that this organism might cause female infertility. However, more research is needed. On the basis of certain reports, M. genitalium was uncommonly identified in women who experience adverse pregnancy outcomes, but was associated with increased risk for preterm delivery in one U.S. and another Peruvian study (274,275). Data are scarce regarding M. genitalium and ectopic pregnancy.
Diagnostic Considerations
M. genitalium is a slow-growing organism. Culture can take up to 6 months, and only a few laboratories in the world are able to recover clinical isolates. Therefore, NAAT is the preferred method for M. genitalium detection. In research settings, M. genitalium is diagnosed by NAAT testing of urine, urethral, vaginal, and cervical swabs and through endometrial biopsies, typically using in-house PCR or assays intended for research use only. NAAT tests (polymerase chain reaction or transcription mediated amplification) for M. genitalium are available in some large medical centers and commercial laboratories, but there is no diagnostic test for M. genitalium that is cleared by the FDA for use in the United States. In the absence of validated tests, M. genitalium should be suspected in cases of persistent or recurrent urethritis and may be considered in persistent or recurrent cases of cervicitis and PID.
Treatment
M. genitalium lacks a cell wall, and thus antibiotics targeting cell-wall biosynthesis (e.g., beta-lactams including penicillins and cephalosporins) are ineffective against this organism. Given the diagnostic challenges, treatment of most M. genitalium infections will occur in the context of syndromic management for urethritis, cervicitis, and PID.
Urethritis and Cervicitis
The 7-day doxycycline regimen recommended for treatment of urethritis is largely ineffective against M. genitalium with a median cure rate of approximately 31% (276–278). The 1-g single dose of azithromycin was significantly more effective against M. genitalium than doxycycline in two randomized urethritis treatment trials (276,277) and is preferred over doxycycline. However, resistance to azithromycin appears to be rapidly emerging. The median cure rate for both men and women is approximately 85%, but was only 40% in the most recent trial (278). Persons with treatment failures after the 1-g azithromycin regimen frequently have macrolide-resistant strains, suggesting that single-dose azithromycin therapy might select for resistance. A longer course of azithromycin (an initial 500-mg dose followed by 250 mg daily for 4 days) might be marginally superior to the single dose regimen (279–281). However, in some settings, approximately 50% of all M. genitalium infections are caused by organisms that are already resistant to azithromycin (282), and persons who do not respond to the 1-g azithromycin regimen generally do not benefit from retreatment with the extended dose regimen. Moxifloxacin (400 mg daily × 7, 10 or 14 days) has been successfully used to treat M. genitalium in men and women with previous treatment failures, with cure rates of 100% in initial reports (280,283). However, moxifloxacin has been used in only a few cases, and the drug has not been tested in clinical trials. Although generally considered effective, studies in Japan, Australia, and the United States have reported moxifloxacin treatment failures after the 7 day regimen (284–287).
PID
Recommended PID treatment regimens are based on antibiotics that are not effective against M. genitalium. Therefore, clinicians might consider M. genitalium in cases that do not respond to therapy within 7–10 days. Where validated M. genitalium testing is available, clinicians might test women with PID for M. genitalium. When M. genitalium is detected, a regimen of moxifloxacin 400 mg/day for 14 days has been effective in eradicating the organism (288). Nevertheless, no data have been published that assess the benefits of testing women with PID for M. genitalium, and the importance of directing treatment against this organism is currently unknown.
Follow-up
In settings where validated M. genitalium testing is available, persons with persistent urethritis, cervicitis, or PID accompanied by persistent detection of M. genitalium might be treated with moxifloxacin. However, routine tests-of-cure in asymptomatic persons are not recommended.
Management of Sex Partners
Sex partners should be managed according to guidelines for patients with nongonococcal urethritis (NGU), cervicitis, and PID. In settings with access to validated M. genitalium tests, partner testing and treatment of identified infections might be considered.
Special Considerations
HIV Infection
Persons who have an M. genitalium infection and HIV infection should receive the same treatment regimen as those who are HIV negative. Treatment of most M. genitalium infections will occur in the context of syndromic management for urethritis, cervicitis, and PID (See Mycoplasma genitalium, Treatment).
HIV Infection: Detection, Counseling, and Referral
HIV infection typically begins with a brief acute retroviral syndrome, transitions to a multi-year chronic illness that progressively depletes CD4 T-lymphocytes critical for maintenance of effective immune function, and ends with symptomatic, life-threatening immunodeficiency. This late stage of infection, known as acquired immunodeficiency syndrome (AIDS), develops over months to years with an estimated median time of approximately 11 years (289). In the absence of treatment, virtually all persons with AIDS will die from AIDS-related causes; however with antiretroviral therapy, persons provided early effective treatment can expect to live a near normal lifespan (290–292). Early diagnosis of HIV infection and linkage to care are essential not only for the patients’ own health but also to reduce the risk for transmitting HIV to others. As of March 2012, U.S. guidelines recommend all persons with HIV infection diagnoses be offered effective antiretroviral therapy (70).
As of 2011, approximately 16% of the estimated 1.2 million persons with HIV infection in the United States are unaware of their infection (http://www.cdc.gov/hiv/pdf/2011_Monitoring_HIV_Indicators_HSSR_FINAL.pdf). Knowledge of HIV-infection status has important clinical implications, because HIV infection alters the immune system and thereby affects the diagnosis, evaluation, treatment, and follow-up of some other STDs. Diagnosing HIV infection during the acute phase of disease is particularly important (see Acute HIV Infection). Persons with acute HIV infection are highly infectious, because HIV concentrations are extremely high in plasma and genital secretions following initial infection (293–296). However, tests for HIV antibodies are often negative during this phase of infection, causing persons to mistakenly believe they are uninfected and unknowingly continue to engage in behaviors associated with HIV transmission. Of persons with acute HIV infection, 50%–90% are symptomatic, many of whom seek medical care (297,298). Because persons with no HIV-associated symptoms might present for assessment or treatment of a concomitantly acquired STD, providers serving persons at risk for STDs are in a position to diagnose HIV infection in persons during the acute phase of infection.
Despite the availability of effective antiretroviral therapy, many cases of HIV infection continue to be diagnosed at advanced stages, as evidenced by low CD4 cell counts. Nationally, the proportion of patients who receive AIDS diagnoses at or within 12 months of their HIV diagnosis in 2010 was 32% (299). Since 2006, CDC has recommended efforts to increase HIV testing by streamlining the consent process and expanding opt-out testing to all health-care settings, including those serving persons at risk for STDs (122). HIV testing facilitates early diagnosis, which reduces the spread of disease, extends life expectancy, and reduces costs of care. However, rates of testing remain low: CDC estimates that in 2008, only 45% of adults aged 18–64 years had ever been tested (300), and that during 2006–2009 approximately 41% of persons with newly diagnosed HIV infection had never been previously tested (301).
Comprehensive HIV treatment services are usually not available in facilities focusing primarily on STD treatment (e.g., STD clinics). In such settings, patients with a new diagnosis of HIV infection or those with an existing diagnosis of HIV infection who are not engaged in regular on-going care should be linked promptly to a health-care provider or facility experienced in caring for HIV-infected patients (70). Providers working in STD clinics should be knowledgeable about the treatment options available in their communities, educate HIV-infected persons about their illness, and link these patients to HIV-related care and support services. Provision of care also should include behavioral and psychosocial services, especially for alcohol and drug addiction and for mental health problems.
A detailed discussion of the complex issues required for the management of HIV infection is beyond the scope of this report; however this information is available elsewhere (17,70,247). These HIV care and management resources are updated frequently, and the most current versions are available online (see URLs accompanying each reference). These resources provide additional information about the diagnosis, medical management, and counseling of persons with HIV infection, referral for support services, and management of sex and injection-drug partners in STD-treatment facilities. In addition, subsequent sections of this report briefly discuss HIV infection during pregnancy and among infants and children.
Detection of HIV Infection: Screening
All persons who seek evaluation and treatment for STDs should be screened for HIV infection. Screening should be routine, regardless of whether the patient reports any specific behavioral risks for HIV infection. Persons at high risk for HIV infection with early syphilis, gonorrhea, or chlamydia should be screened at the time of the STD diagnosis, even if an HIV test was recently performed. Some STDs, especially rectal gonorrhea and syphilis, are a risk marker for HIV acquisition (142,145,156).
CDC recommends HIV screening for patients aged 13–64 years in all health-care settings (122). Persons should be notified that testing will be performed, but retain the option to decline or defer testing (an opt-out approach) (302). Consent for HIV screening should be incorporated into the general informed consent for medical care in the same manner as other screening or diagnostic tests. A separate consent form for HIV testing is not recommended.
Providing prevention counseling in conjunction with HIV diagnostic testing or as part of HIV screening programs should not be required in health-care settings. However, some persons might be more likely to think about HIV and consider their risk-related behavior when undergoing an HIV test. HIV testing presents providers with an opportunity to conduct HIV/STD prevention counseling and communicate risk-reduction messages.
Diagnosing HIV Infection
HIV infection can be diagnosed by serologic tests that detect antibodies against HIV-1 and HIV-2 and by virologic tests that detect HIV antigens or ribonucleic acid (RNA). Testing begins with a sensitive screening test, usually an antigen/antibody combination or antibody immunoassay (IA). Available serologic tests are both highly sensitive and specific and can detect all known subtypes of HIV-1. Most can also detect HIV-2 and uncommon variants of HIV-1 (e.g., group O and group N). Rapid HIV tests enable clinicians to make a preliminary diagnosis of HIV infection within 30 minutes. However, most rapid antibody assays become reactive later than conventional laboratory-based antibody or combination antigen/antibody serologic assays, and thus can produce negative results in recently infected persons.
The recommended diagnostic algorithm for HIV infection consists of a laboratory-based immunoassay, which if repeatedly reactive is followed by a supplemental test (e.g., an HIV-1/HIV-2 antibody differentiation assay, Western blot, or indirect immunofluorescence assay). However, available HIV laboratory antigen/antibody immunoassays detect HIV infection earlier than these supplemental tests. Therefore, during very early stages of HIV infection, discordant HIV test results (reactive immunoassay results with negative supplemental test results) have been erroneously interpreted as negative (303). This problem is minimized by use of a combination HIV-1/HIV-2 antigen-antibody (Ag/Ab) immunoassay which if reactive is followed by an HIV-1/HIV-2 antibody differentiation assay (304). This algorithm confers an additional advantage, as it can detect HIV-2 antibodies after the initial immunoassay. Although HIV-2 is uncommon in the United States, accurate identification is important because monitoring and therapy for HIV-2 differs from that for HIV-1 (305). RNA testing is performed on all specimens with reactive immunoassay but negative supplemental antibody test results to determine whether the discordance represents acute HIV infection.
The following are specific recommendations that apply to testing for HIV infection.
HIV screening is recommended for all persons who seek evaluation or treatment for STDs. This testing should be performed at the time of STD diagnosis (e.g., early syphilis, gonorrhea, and chlamydia) in populations at high risk for HIV infection.
HIV testing must be voluntary and free from coercion. Patients must not be tested without their knowledge.
Opt-out HIV screening (notifying the patient that an HIV test will be performed, unless the patient declines) is recommended in all health-care settings.
Specific signed consent for HIV testing should not be required. General informed consent for medical care is considered sufficient to encompass informed consent for HIV testing.
Use of Ag/Ab combination tests is encouraged unless persons are unlikely to receive their HIV test results.
Preliminary positive screening tests for HIV infection must be followed by additional testing to definitively establish the diagnosis.
Providers should be alert to the possibility of acute HIV infection and perform an antigen/antibody immunoassay or HIV RNA in conjunction with an antibody test. Persons suspected of recently acquired HIV infection should be referred immediately to an HIV clinical-care provider.
Acute HIV Infection
Health-care providers should be knowledgeable about the symptoms and signs of acute retroviral syndrome, which develops in 50%–90% of persons within the first few weeks after they become infected with HIV (298). Acute retroviral syndrome is characterized by nonspecific symptoms, including fever, malaise, lymphadenopathy and skin rash. Suspicion of acute retroviral syndrome should prompt urgent assessment with an antigen/antibody immunoassay or HIV RNA in conjunction with an antibody test. If the immunoassay is negative or indeterminate, then testing for HIV RNA should follow. Clinicians should not assume that a laboratory report of a negative HIV antibody test result indicates that the necessary RNA screening for acute HIV infection has been conducted. Further, HIV home-testing kits only detect HIV antibodies and therefore will not detect acute HIV infection.
Persons with acute HIV infection are highly infectious because the concentration of virus in plasma and genital secretions is extremely elevated during this stage of infection (294,306). Antiretroviral therapy during acute HIV infection is recommended, because it substantially reduces infectiousness to others, improves laboratory markers of disease, may decrease severity of acute disease, lowers viral set-point, reduces the size of the viral reservoir, decreases rate of viral mutation by suppressing replication, and preserves immune function (70). Persons who receive an acute HIV infection diagnosis should be referred immediately to an HIV clinical-care provider, provided prevention counseling (e.g., advised to reduce number of partners and to use condoms correctly and consistently), and screened for STDs. Information should be provided on the availability of postexposure prophylaxis for sexual and needle-sharing partners not known to have HIV infection if the most recent contact was within the 72 hours preceding HIV diagnosis (http://www.cdc.gov/hiv).
After Establishing a New HIV Diagnosis
Persons with newly diagnosed HIV infection should be informed about 1) the importance of promptly initiating medical care for their own health and to reduce further transmission of HIV, 2) the effectiveness of HIV treatments, and 3) what to expect as they enter medical care for HIV infection (70). They should be linked promptly to a health-care provider or facility experienced in caring for patients with HIV. Persons with symptoms or signs that suggest advanced HIV infection (e.g., fever, weight loss, diarrhea, cough, shortness of breath, and oral candidiasis) should be immediately evaluated or referred for evaluation. Persons experiencing psychologic distress should be referred accordingly (see Counseling for Persons with HIV Infection and Referral to Support Services). Detailed and regularly updated recommendation for the initial management of persons with HIV infection can be found elsewhere (17,70,247).
Counseling for Persons with HIV Infection and Referral to Support Services
Providers should expect persons with HIV infection to be distressed when first informed of a positive test result. Such persons face multiple major adaptive challenges, including coping with the reactions of others to a stigmatizing illness, developing and adopting strategies for maintaining physical and emotional health, initiating changes in behavior to prevent HIV transmission to others, and reducing the risk for acquiring additional STDs. Many persons will require assistance with making reproductive choices, gaining access to health services, and coping with changes in personal relationships. Therefore, behavioral and psychosocial services are an integral part of health care for persons with HIV infection.
Persons testing positive for HIV infection have unique needs. Some require referral for specific behavioral interventions (e.g., a substance abuse program), mental health disorders (e.g., depression), and emotional distress, while others require assistance with securing and maintaining employment and housing. Women should be counseled or appropriately referred regarding reproductive choices and contraceptive options, and persons with multiple psychosocial problems might be candidates for comprehensive risk-reduction counseling and other support services.
The following are specific recommendations for HIV counseling and linkage to services that should be offered to patients before they leave the testing site.
Persons who test positive for HIV should be counseled, either on-site or through referral, concerning the behavioral, psychosocial, and medical implications of HIV infection.
Health-care providers should assess the need for immediate medical care and psychosocial support.
Providers should link persons with newly diagnosed HIV infection to services provided by health-care personnel experienced in the management of HIV infection. Additional services that might be needed include substance abuse counseling and treatment, treatment for mental health disorders or emotional distress, reproductive counseling, risk-reduction counseling, and case management. Providers should follow up to ensure that patients have received services for any identified needs.
Persons with HIV infection should be educated about the importance of ongoing medical care and what to expect from these services.
Several successful, innovative interventions to assist persons with HIV infection reduce the possibility of transmission to others have been developed for diverse at-risk populations, and these can be locally replicated or adapted (12,15,307–310). Involvement of nongovernment and community-based organizations might complement such efforts in the clinical setting.
Management of Sex Partners and Injection-Drug Partners
Clinicians providing services to persons with HIV infection should determine whether any partners should be notified concerning possible exposure to HIV (122,311). In the context of HIV management, “partner” includes sex partners and persons with whom syringes or other injection equipment is shared. Partner notification is an important component of disease management, because early diagnosis and treatment of HIV infection reduces risk for HIV transmission, decreases individual morbidity and mortality risk, and provides the opportunity to modify risk behaviors. Partner notification for HIV infection should be confidential. Specific guidance regarding spousal notification varies by jurisdiction. Detailed recommendations concerning identification, notification, diagnosis, and treatment of exposed partners are available in CDC’s Recommendations for Partner Services Programs for HIV Infection, Syphilis, Gonorrhea, and Chlamydial Infections (See Partner Services) (311).
The following are specific recommendations for implementing partner-notification procedures:
Health-care providers should inform persons with HIV infection about partner services including processes, benefits, and risks.
Persons with HIV infection should be encouraged to notify their partners and to refer them for counseling and testing.
Health-care providers should assist in the partner-notification process, either directly or by referral to health department partner-notification programs, which might attempt to contact them.
If persons with HIV infection are unwilling to notify their partners or cannot ensure their partners will seek counseling, HIV care staff or health department personnel should use confidential partner notification procedures. Health department staff are trained to employ public health investigation strategies to confidentially locate persons who are hard to reach, whereas most clinical providers do not have the time or expertise to conduct this type of partner notification.
Partners who have been reached and are not known to have HIV infection should be offered postexposure prophylaxis with combination antiretrovirals if they were exposed to genital secretions or blood of a partner with HIV infection though sex or injection-drug use within the preceding 72 hours (312).
STD Testing During HIV Care
At the initial HIV care visit, providers should test all sexually active persons with HIV infection for curable STDs (e.g., syphilis, gonorrhea, and chlamydia) and perform testing at least annually during the course of HIV care (12). Specific testing includes syphilis serology and NAAT for N. gonorrhoeae and C. trachomatis at the anatomic site of exposure, as the preferred approach. Women with HIV infection should also be screened for trichomonas at the initial visit and annually thereafter. Women should be screened for cervical cancer precursor lesions by cervical Pap tests per existing guidelines (247).
More frequent screening for curable STDs might be appropriate depending on individual risk behaviors and the local epidemiology of STDs. Many STDs are asymptomatic, and their diagnosis might indicate risk behavior that should prompt referral for partner services and prevention counseling (10). Pathogen-specific sections of this document provide more detailed information on screening, testing, and treatment.
Special Considerations
Pregnancy
All pregnant women should be tested for HIV infection during the first prenatal visit. A second test during the third trimester, preferably at <36 weeks’ gestation, should be considered for all pregnant women and is recommended for those known to be at high risk for acquiring HIV, those who receive health care in jurisdictions with elevated incidence of HIV or AIDS among women, and women seen in clinical settings in which prenatal screening identifies at least one pregnant women with HIV infection per 1,000 women screened (122). Diagnostic algorithms for HIV infection in pregnant women are not different than those for nonpregnant women (See Diagnosis, HIV Infection). Pregnant women should be informed about being tested for HIV as part of the panel of prenatal tests (103,122); for women who decline, providers should address concerns that pose obstacles to testing and encourage testing at subsequent prenatal visits. Women who decline testing because they have had a previous negative HIV test result should be informed about the importance of retesting during each pregnancy. Women with no prenatal care should be tested for HIV at the time of delivery.
Testing pregnant women is important not only because knowledge of infection status can help maintain the health of the woman, but because it enables receipt of interventions (i.e., antiretroviral and obstetrical) that can substantially reduce the risk for perinatal transmission of HIV. After a pregnant woman has been identified as having HIV infection, she should be educated about the benefits of antiretroviral treatment for her health and for reducing the risk for transmission to her infant. In the absence of antiretroviral treatment, a mother’s risk of transmitting HIV to her neonate is approximately 30% but can be reduced to <2% through antiretroviral treatment, obstetrical interventions (i.e., elective cesarean section at 38 weeks of pregnancy), and breastfeeding avoidance (105). Pregnant women who have HIV infection should be linked to an HIV care provider and given appropriate antenatal and postpartum treatment and advice. Detailed and regularly updated recommendations for the initial management of persons with HIV infection and pregnancy are available in existing guidance at http://aidsinfo.nih.gov/guidelines.
HIV Infection Among Neonates, Infants, and Children
Diagnosis of HIV infection in a pregnant woman indicates the need to evaluate and manage the HIV-exposed neonate and consider whether the woman’s other children might be infected. Detailed recommendations regarding diagnosis and management of HIV in neonates and children of mothers with HIV infection are beyond the scope of this report and can be found at http://aidsinfo.nih.gov/guidelines. Exposed neonates and children with HIV infection should be referred to physicians with such expertise.
Diseases Characterized by Genital, Anal, or Perianal Ulcers
In the United States, most young, sexually active patients who have genital, anal, or perianal ulcers have either genital herpes or syphilis. The frequency of each condition differs by geographic area and population; however, genital herpes is the most prevalent of these diseases. More than one etiologic agent (e.g., herpes and syphilis) can be present in a genital, anal, or perianal ulcer. Less common infectious causes of genital, anal, or perianal ulcers include chancroid and donovanosis. Genital herpes, syphilis, and chancroid have been associated with an increased risk for HIV acquisition and transmission. Genital, anal, or perianal lesions can also be associated with infectious as well as noninfectious conditions that are not sexually transmitted (e.g., yeast, trauma, carcinoma, aphthae, fixed drug eruption, and psoriasis).
A diagnosis based only on medical history and physical examination frequently is inaccurate. Therefore, all persons who have genital, anal, or perianal ulcers should be evaluated; in settings where chancroid is prevalent, a test for Haemophilus ducreyi also should be performed. Specific evaluation of genital, anal, or perianal ulcers includes 1) syphilis serology, darkfield examination, or PCR testing if available; 2) culture or PCR testing for genital herpes; and 3) serologic testing for type-specific HSV antibody.
No FDA-cleared PCR test to diagnose syphilis is available in the United States, but two FDA-cleared PCR tests are available for the diagnosis of HSV-1 and HSV-2 in genital specimens. Some clinical laboratories have developed their own syphilis and HSV PCR tests and have conducted Clinical Laboratory Improvement Amendment (CLIA) verification studies in genital specimens. Type-specific serology for HSV-2 might be helpful in identifying persons with genital herpes (see Genital Herpes, Type-Specific Serologic Tests). In addition, biopsy of ulcers can help identify the cause of ulcers that are unusual or that do not respond to initial therapy. HIV testing should be performed on all persons with genital, anal, or perianal ulcers not known to have HIV infection (see Diagnostic Considerations, sections on Syphilis, Chancroid, and Genital Herpes Simplex Virus).
Because early treatment decreases the possibility of transmission, public health standards require health-care providers to presumptively treat any patient with a suspected case of infectious syphilis at the initial visit, even before test results are available. Presumptive treatment of a patient with a suspected first episode of genital herpes also is recommended, because successful treatment depends on prompt initiation of therapy. The clinician should choose the presumptive treatment on the basis of clinical presentation (i.e., HSV lesions begin as vesicles and primary syphilis as a papule) and epidemiologic circumstances (e.g., high incidence of disease among populations and communities and travel history). For example, syphilis is so common in MSM that any man who has sex with men presenting with a genital ulcer should be presumptively treated for syphilis at the initial visit after syphilis and HSV tests are performed. After a complete diagnostic evaluation, at least 25% of patients who have genital ulcers have no laboratory-confirmed diagnosis (313).
Chancroid
The prevalence of chancroid has declined in the United States (118). When infection does occur, it is usually associated with sporadic outbreaks. Worldwide, chancroid appears to have declined as well, although infection might still occur in some regions of Africa and the Caribbean. Like genital herpes and syphilis, chancroid is a risk factor in the transmission and acquisition of HIV infection (314).
Diagnostic Considerations
A definitive diagnosis of chancroid requires the identification of H. ducreyi on special culture media that is not widely available from commercial sources; even when these media are used, sensitivity is <80% (315). No FDA-cleared PCR test for H. ducreyi is available in the United States, but such testing can be performed by clinical laboratories that have developed their own PCR test and have conducted CLIA verification studies in genital specimens.
The combination of a painful genital ulcer and tender suppurative inguinal adenopathy suggests the diagnosis of chancroid (316). For both clinical and surveillance purposes, a probable diagnosis of chancroid can be made if all of the following criteria are met: 1) the patient has one or more painful genital ulcers; 2) the clinical presentation, appearance of genital ulcers and, if present, regional lymphadenopathy are typical for chancroid; 3) the patient has no evidence of T. pallidum infection by darkfield examination of ulcer exudate or by a serologic test for syphilis performed at least 7 days after onset of ulcers; and 4) an HSV PCR test or HSV culture performed on the ulcer exudate is negative.
Treatment
Successful treatment for chancroid cures the infection, resolves the clinical symptoms, and prevents transmission to others. In advanced cases, scarring can result despite successful therapy.
| Recommended Regimens |
|
|
| Azithromycin 1 g orally in a single dose |
| OR |
| Ceftriaxone 250 mg IM in a single dose |
| OR |
| Ciprofloxacin 500 mg orally twice a day for 3 days |
| OR |
| Erythromycin base 500 mg orally three times a day for 7 days |
|
|
Azithromycin and ceftriaxone offer the advantage of singledose therapy. Worldwide, several isolates with intermediate resistance to either ciprofloxacin or erythromycin have been reported. However, because cultures are not routinely performed, data are limited regarding the current prevalence of antimicrobial resistance.
Other Management Considerations
Men who are uncircumcised and patients with HIV infection do not respond as well to treatment as persons who are circumcised or HIV-negative. Patients should be tested for HIV infection at the time chancroid is diagnosed. If the initial test results were negative, a serologic test for syphilis and HIV infection should be performed 3 months after the diagnosis of chancroid.
Follow-Up
Patients should be re-examined 3–7 days after initiation of therapy. If treatment is successful, ulcers usually improve symptomatically within 3 days and objectively within 7 days after therapy. If no clinical improvement is evident, the clinician must consider whether 1) the diagnosis is correct, 2) the patient is coinfected with another STD, 3) the patient is infected with HIV, 4) the treatment was not used as instructed, or 5) the H. ducreyi strain causing the infection is resistant to the prescribed antimicrobial. The time required for complete healing depends on the size of the ulcer; large ulcers might require >2 weeks. In addition, healing is slower for some uncircumcised men who have ulcers under the foreskin. Clinical resolution of fluctuant lymphadenopathy is slower than that of ulcers and might require needle aspiration or incision and drainage, despite otherwise successful therapy. Although needle aspiration of buboes is a simpler procedure, incision and drainage might be preferred because of reduced need for subsequent drainage procedures.
Management of Sex Partners
Regardless of whether symptoms of the disease are present, sex partners of patients who have chancroid should be examined and treated if they had sexual contact with the patient during the 10 days preceding the patient’s onset of symptoms.
Special Considerations
Pregnancy
Data suggest ciprofloxacin presents a low risk to the fetus during pregnancy, with a potential for toxicity during breastfeeding (317). Alternate drugs should be used during pregnancy and lactation. No adverse effects of chancroid on pregnancy outcome have been reported.
HIV Infection
Persons with HIV infection who have chancroid should be monitored closely because they are more likely to experience treatment failure and to have ulcers that heal slowly. Persons with HIV infection might require repeated or longer courses of therapy, and treatment failures can occur with any regimen. Data are limited concerning the therapeutic efficacy of the recommended single-dose azithromycin and ceftriaxone regimens in persons with HIV infection.
Genital HSV Infections
Genital herpes is a chronic, life-long viral infection. Tw o types of HSV can cause genital herpes: HSV-1 and HSV-2. Most cases of recurrent genital herpes are caused by HSV-2, and approximately 50 million persons in the United States are infected with this type of genital herpes (318). However, an increasing proportion of anogenital herpetic infections have been attributed to HSV-1 infection, which is especially prominent among young women and MSM (319–321).
Most persons infected with HSV-2 have not had the condition diagnosed. Many such persons have mild or unrecognized infections but shed virus intermittently in the anogenital area. As a result, most genital herpes infections are transmitted by persons unaware that they have the infection or who are asymptomatic when transmission occurs. Management of genital HSV should address the chronic nature of the disease rather than focusing solely on treatment of acute episodes of genital lesions.
Diagnostic Considerations
The clinical diagnosis of genital herpes can be difficult, because the painful multiple vesicular or ulcerative lesions typically associated with HSV are absent in many infected persons. Recurrences and subclinical shedding are much more frequent for genital HSV-2 infection than for genital HSV-1 infection (322,323). A patient’s prognosis and the type of counseling needed depend on the type of genital herpes (HSV-1 or HSV-2) causing the infection; therefore, the clinical diagnosis of genital herpes should be confirmed by type-specific laboratory testing (321,324). Both type-specific virologic and type-specific serologic tests for HSV should be available in clinical settings that provide care to persons with or at risk for STDs. Persons with genital herpes should be tested for HIV infection.
Virologic Tests
Cell culture and PCR are the preferred HSV tests for persons who seek medical treatment for genital ulcers or other mucocutaneous lesions. The sensitivity of viral culture is low, especially for recurrent lesions, and declines rapidly as lesions begin to heal. Nucleic acid amplification methods, including PCR assays for HSV DNA, are more sensitive and are increasingly available (325–327). PCR is the test of choice for diagnosing HSV infections affecting the central nervous system and systemic infections (e.g., meningitis, encephalitis, and neonatal herpes). Viral culture isolates and PCR amplicons should be typed to determine which type of HSV is causing the infection. Failure to detect HSV by culture or PCR, especially in the absence of active lesions, does not indicate an absence of HSV infection because viral shedding is intermittent. Cytologic detection of cellular changes associated with HSV infection is an insensitive and nonspecific method of diagnosing genital lesions (i.e., Tzanck preparation) and therefore should not be relied on. Although a direct immunofluorescence (IF) assay using fluorescein-labeled monoclonal antibodies is also available to detect HSV antigen from genital specimens, this assay lacks sensitivity (328).
Type-Specific Serologic Tests
Both type-specific and type-common antibodies to HSV develop during the first several weeks after infection and persist indefinitely. Accurate type-specific HSV serologic assays are based on the HSV-specific glycoprotein G2 (HSV-2) and glycoprotein G1 (HSV-1). Providers should only request type-specific glycoprotein G (gG)-based serologic assays when serology is performed for their patients (329–331).
Both laboratory-based assays and point-of-care tests that provide results for HSV-2 antibodies from capillary blood or serum during a clinic visit are available. The sensitivities of these glycoprotein G type-specific tests for the detection of HSV-2 antibody vary from 80%–98%; false-negative results might be more frequent at early stages of infection (330,332,333). The most commonly used test, HerpeSelect HSV-2 Elisa might be falsely positive at low index values (1.1–3.5) (334–336). Such low values should be confirmed with another test, such as Biokit or the Western blot (337). The HerpeSelect HSV-2 Immunoblot should not be used for confirmation, because it uses the same antigen as the HSV-2 Elisa. Repeat testing is indicated if recent acquisition of genital herpes is suspected. The HerpeSelect HSV-1 Elisa is insensitive for detection of HSV-1 antibody. IgM testing for HSV 1 or HSV-2 is not useful, because IgM tests are not type-specific and might be positive during recurrent genital or oral episodes of herpes (337).
Because nearly all HSV-2 infections are sexually acquired, the presence of type-specific HSV-2 antibody implies anogenital infection. In this instance, education and counseling appropriate for persons with genital HSV infections should be provided. The presence of HSV-1 antibody alone is more difficult to interpret. Many persons with HSV-1 antibody have oral HSV infection acquired during childhood, which might be asymptomatic. However, acquisition of genital HSV-1 is increasing, and genital HSV-1 also can be asymptomatic (318–321,338). Lack of symptoms in a person who is HSV-1 seropositive does not distinguish anogenital from orolabial or cutaneous infection, and regardless of site of infection, these persons remain at risk for acquiring HSV-2.
Type-specific HSV serologic assays might be useful in the following scenarios: 1) recurrent genital symptoms or atypical symptoms with negative HSV PCR or culture; 2) clinical diagnosis of genital herpes without laboratory confirmation; and 3) a patient whose partner has genital herpes. HSV serologic testing should be considered for persons presenting for an STD evaluation (especially for those persons with multiple sex partners), persons with HIV infection, and MSM at increased risk for HIV acquisition. Screening for HSV-1 and HSV-2 in the general population is not indicated.
Management of Genital Herpes
Antiviral chemotherapy offers clinical benefits to most symptomatic patients and is the mainstay of management. Counseling regarding the natural history of genital herpes, sexual and perinatal transmission, and methods to reduce transmission is integral to clinical management.
Systemic antiviral drugs can partially control the signs and symptoms of genital herpes when used to treat first clinical and recurrent episodes or when used as daily suppressive therapy. However, these drugs neither eradicate latent virus nor affect the risk, frequency, or severity of recurrences after the drug is discontinued. Randomized trials have indicated that three antiviral medications provide clinical benefit for genital herpes: acyclovir, valacyclovir, and famciclovir (339–347). Valacyclovir is the valine ester of acyclovir and has enhanced absorption after oral administration. Famciclovir also has high oral bioavailability. Topical therapy with antiviral drugs offers minimal clinical benefit and is discouraged.
First Clinical Episode of Genital Herpes
Newly acquired genital herpes can cause a prolonged clinical illness with severe genital ulcerations and neurologic involvement. Even persons with first-episode herpes who have mild clinical manifestations initially can develop severe or prolonged symptoms. Therefore, all patients with first episodes of genital herpes should receive antiviral therapy.
| Recommended Regimens* |
|
|
| Acyclovir 400 mg orally three times a day for 7–10 days |
| OR |
| Acyclovir 200 mg orally five times a day for 7–10 days |
| OR |
| Valacyclovir 1 g orally twice a day for 7–10 days |
| OR |
| Famciclovir 250 mg orally three times a day for 7–10 days |
|
|
| *Treatment can be extended if healing is incomplete after 10 days of therapy. |
Established HSV-2 Infection
Almost all persons with symptomatic first-episode genital HSV-2 infection subsequently experience recurrent episodes of genital lesions; recurrences are less frequent after initial genital HSV-1 infection. Intermittent asymptomatic shedding occurs in persons with genital HSV-2 infection, even in those with longstanding or clinically silent infection. Antiviral therapy for recurrent genital herpes can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to ameliorate or shorten the duration of lesions. Some persons, including those with mild or infrequent recurrent outbreaks, benefit from antiviral therapy; therefore, options for treatment should be discussed. Many persons prefer suppressive therapy, which has the additional advantage of decreasing the risk for genital HSV-2 transmission to susceptible partners (348,349).
Suppressive Therapy for Recurrent Genital Herpes
Suppressive therapy reduces the frequency of genital herpes recurrences by 70%–80% in patients who have frequent recurrences (345–348); many persons receiving such therapy report having experienced no symptomatic outbreaks. Treatment also is effective in patients with less frequent recurrences. Safety and efficacy have been documented among patients receiving daily therapy with acyclovir for as long as 6 years and with valacyclovir or famciclovir for 1 year (350,351). Quality of life is improved in many patients with frequent recurrences who receive suppressive therapy rather than episodic treatment (352).
The frequency of genital herpes recurrences diminishes over time in many persons, potentially resulting in psychological adjustment to the disease. Therefore, periodically during suppressive treatment (e.g., once a year), providers should discuss the need to continue therapy. However, neither treatment discontinuation nor laboratory monitoring in a healthy person is necessary.
Treatment with valacyclovir 500 mg daily decreases the rate of HSV-2 transmission in discordant, heterosexual couples in which the source partner has a history of genital HSV-2 infection (349). Such couples should be encouraged to consider suppressive antiviral therapy as part of a strategy to prevent transmission, in addition to consistent condom use and avoidance of sexual activity during recurrences. Suppressive antiviral therapy also is likely to reduce transmission when used by persons who have multiple partners (including MSM) and by those who are HSV-2 seropositive without a history of genital herpes.
| Recommended Regimens |
|
|
| Acyclovir 400 mg orally twice a day |
| OR |
| Valacyclovir 500 mg orally once a day* |
| OR |
| Valacyclovir 1 g orally once a day |
| OR |
| Famiciclovir 250 mg orally twice a day |
|
|
| *Valacyclovir 500 mg once a day might be less effective than other valacyclovir or acyclovir dosing regimens in persons who have very frequent recurrences (i.e., ≥10 episodes per year). |
Acyclovir, famciclovir, and valacyclovir appear equally effective for episodic treatment of genital herpes (342–346), but famciclovir appears somewhat less effective for suppression of viral shedding (353). Ease of administration and cost also are important considerations for prolonged treatment.
Episodic Therapy for Recurrent Genital Herpes
Effective episodic treatment of recurrent herpes requires initiation of therapy within 1 day of lesion onset or during the prodrome that precedes some outbreaks. The patient should be provided with a supply of drug or a prescription for the medication with instructions to initiate treatment immediately when symptoms begin.
| Recommended Regimens |
|
|
| Acyclovir 400 mg orally three times a day for 5 days |
| OR |
| Acyclovir 800 mg orally twice a day for 5 days |
| OR |
| Acyclovir 800 mg orally three times a day for 2 days |
| OR |
| Valacyclovir 500 mg orally twice a day for 3 days |
| OR |
| Valacyclovir 1 g orally once a day for 5 days |
| OR |
| Famciclovir 125 mg orally twice daily for 5 days |
| OR |
| Famciclovir 1 gram orally twice daily for 1 day |
| OR |
| Famciclovir 500 mg once, followed by 250 mg twice daily for 2 days |
|
|
Severe Disease
Intravenous (IV) acyclovir therapy should be provided for patients who have severe HSV disease or complications that necessitate hospitalization (e.g., disseminated infection, pneumonitis, or hepatitis) or CNS complications (e.g., meningoencephalitis). The recommended regimen is acyclovir 5–10 mg/kg IV every 8 hours for 2–7 days or until clinical improvement is observed, followed by oral antiviral therapy to complete at least 10 days of total therapy. HSV encephalitis requires 21 days of intravenous therapy. Impaired renal function warrants an adjustment in acyclovir dosage.
Counseling
Counseling of infected persons and their sex partners is critical to the management of genital herpes. The goals of counseling include helping patients cope with the infection and preventing sexual and perinatal transmission. Although initial counseling can be provided at the first visit, many patients benefit from learning about the chronic aspects of the disease after the acute illness subsides. Multiple resources, including websites (http://www.ashasexualhealth.org) and printed materials, are available to assist patients, their partners, and clinicians who become involved in counseling (354,355).
Although the psychological effect of a serologic diagnosis of HSV-2 infection in a person with asymptomatic or unrecognized genital herpes appears minimal and transient (356,357), some HSV-infected persons might express anxiety concerning genital herpes that does not reflect the actual clinical severity of their disease; the psychological effect of HSV infection can be substantial. Common concerns regarding genital herpes include the severity of initial clinical manifestations, recurrent episodes, sexual relationships and transmission to sex partners, and ability to bear healthy children. The misconception that HSV causes cancer should be dispelled.
The following topics should be discussed when counseling persons with genital HSV infection:
the natural history of the disease, with emphasis on the potential for recurrent episodes, asymptomatic viral shedding, and the attendant risks of sexual transmission;
the effectiveness of suppressive therapy for persons experiencing a first episode of genital herpes in preventing symptomatic recurrent episodes;
use of episodic therapy to shorten the duration of recurrent episodes;
importance of informing current sex partners about genital herpes and informing future partners before initiating a sexual relationship;
potential for sexual transmission of HSV to occur during asymptomatic periods (asymptomatic viral shedding is more frequent in genital HSV-2 infection than genital HSV-1 infection and is most frequent during the first 12 months after acquiring HSV-2);
importance of abstaining from sexual activity with uninfected partners when lesions or prodromal symptoms are present;
effectiveness of daily use of valacyclovir in reducing risk for transmission of HSV-2, and the lack of effectiveness of episodic or suppressive therapy in persons with HIV and HSV infection in reducing risk for transmission to partners who might be at risk for HSV-2 acquisition;
effectiveness of male latex condoms, which when used consistently and correctly can reduce (but not eliminate) the risk for genital herpes transmission (27,358,359);
HSV infection in the absence of symptoms (type-specific serologic testing of the asymptomatic partners of persons with genital herpes is recommended to determine whether such partners are already HSV seropositive or whether risk for acquiring HSV exists);
risk for neonatal HSV infection; and
increased risk for HIV acquisition among HSV-2 seropositive persons who are exposed to HIV (suppressive antiviral therapy does not reduce the increased risk for HIV acquisition associated with HSV-2 infection) (75,347).
Asymptomatic persons who receive a diagnosis of HSV-2 infection by type-specific serologic testing should receive the same counseling messages as persons with symptomatic infection. In addition, such persons should be educated about the clinical manifestations of genital herpes.
Pregnant women and women of childbearing age who have genital herpes should inform the providers who care for them during pregnancy and those who will care for their newborn infant about their infection. More detailed counseling messages are described in Special Considerations, Genital Herpes in Pregnancy.
Management of Sex Partners
The sex partners of persons who have genital herpes can benefit from evaluation and counseling. Symptomatic sex partners should be evaluated and treated in the same manner as patients who have genital herpes. Asymptomatic sex partners of patients who have genital herpes should be questioned concerning histories of genital lesions and offered type-specific serologic testing for HSV infection.
Special Considerations
Allergy, Intolerance, and Adverse Reactions
Allergic and other adverse reactions to oral acyclovir, valacyclovir, and famciclovir are rare. Desensitization to acyclovir has been described (360).
HIV Infection
Immunocompromised patients can have prolonged or severe episodes of genital, perianal, or oral herpes. Lesions caused by HSV are common among persons with HIV infection and might be severe, painful, and atypical. HSV shedding is increased in persons with HIV infection. Whereas antiretroviral therapy reduces the severity and frequency of symptomatic genital herpes, frequent subclinical shedding still occurs (361,362). Clinical manifestations of genital herpes might worsen during immune reconstitution early after initiation of antiretroviral therapy.
Suppressive or episodic therapy with oral antiviral agents is effective in decreasing the clinical manifestations of HSV among persons with HIV infection (363–365). HSV type-specific serologic testing can be offered to persons with HIV infection during their initial evaluation if infection status is unknown, and suppressive antiviral therapy can be considered in those who have HSV-2 infection. Suppressive anti-HSV therapy in persons with HIV infection does not reduce the risk for either HIV transmission or HSV-2 transmission to susceptible sex partners (71,366).
| Recommended Regimens for Daily Suppressive Therapy in Persons with HIV |
|
|
| Acyclovir 400–800 mg orally twice to three times a day |
| OR |
| Valacyclovir 500 mg orally twice a day |
| OR |
| Famciclovir 500 mg orally twice a day |
|
|
| Recommended Regimens for Episodic Infection in Persons with HIV |
|
|
| Acyclovir 400 mg orally three times a day for 5–10 days |
| OR |
| Valacyclovir 1 g orally twice a day for 5–10 days |
| OR |
| Famciclovir 500 mg orally twice a day for 5–10 days |
|
|
For severe HSV disease, initiating therapy with acyclovir 5–10 mg/kg IV every 8 hours might be necessary.
Antiviral-resistant HSV
If lesions persist or recur in a patient receiving antiviral treatment, HSV resistance should be suspected and a viral isolate obtained for sensitivity testing (367). Such persons should be managed in consultation with an infectious-disease specialist, and alternate therapy should be administered. All acyclovir-resistant strains are also resistant to valacyclovir, and most are resistant to famciclovir. Foscarnet (40–80 mg/kg IV every 8 hours until clinical resolution is attained) is often effective for treatment of acyclovir-resistant genital herpes (368,369). Intravenous cidofovir 5 mg/kg once weekly might also be effective. Imiquimod is a topical alternative (370), as is topical cidofovir gel 1%; however, cidofovir must be compounded at a pharmacy (371). These topical preparations should be applied to the lesions once daily for 5 consecutive days.
Clinical management of antiviral resistance remains challenging among persons with HIV infection, necessitating other preventative approaches. However, experience with another group of immunocompromised persons (hematopoietic stem-cell recipients) demonstrated that persons receiving daily suppressive antiviral therapy were less likely to develop acyclovir-resistant HSV compared with those who received episodic therapy for outbreaks (372).
Genital Herpes in Pregnancy
Most mothers of newborns who acquire neonatal herpes lack histories of clinically evident genital herpes (373,374). The risk for transmission to the neonate from an infected mother is high (30%–50%) among women who acquire genital herpes near the time of delivery and low (<1%) among women with prenatal histories of recurrent herpes or who acquire genital HSV during the first half of pregnancy (375,376).
Prevention of neonatal herpes depends both on preventing acquisition of genital HSV infection during late pregnancy and avoiding exposure of the neonate to herpetic lesions and viral shedding during delivery. Because the risk for herpes is highest in newborn infants of women who acquire genital HSV during late pregnancy, these women should be managed in consultation with maternal-fetal medicine and infectious-disease specialists.
Women without known genital herpes should be counseled to abstain from vaginal intercourse during the third trimester with partners known or suspected of having genital herpes. In addition, pregnant women without known orolabial herpes should be advised to abstain from receptive oral sex during the third trimester with partners known or suspected to have orolabial herpes. Type-specific serologic tests may be useful for identifying pregnant women at risk for HSV infection and guiding counseling regarding the risk for acquiring genital herpes during pregnancy. For example, such testing could be offered to women with no history of genital herpes whose sex partner has HSV infection. However, the effectiveness of antiviral therapy to decrease the risk for HSV transmission to pregnant women by infected partners has not been studied. Routine HSV-2 serologic screening of pregnant women is not recommended.
All pregnant women should be asked whether they have a history of genital herpes. At the onset of labor, all women should be questioned carefully about symptoms of genital herpes, including prodromal symptoms, and all women should be examined carefully for herpetic lesions. Women without symptoms or signs of genital herpes or its prodrome can deliver vaginally. Although cesarean delivery does not completely eliminate the risk for HSV transmission to the neonate, women with recurrent genital herpetic lesions at the onset of labor should deliver by cesarean delivery to reduce the risk for neonatal HSV infection.
Many infants are exposed to acyclovir each year, and no adverse effects in the fetus or newborn attributable to the use of this drug during pregnancy have been reported. Acyclovir can be safely used to treat women in all stages of pregnancy, along with those who are breastfeeding (317,377). Although data regarding prenatal exposure to valacyclovir and famciclovir are limited, data from animal trials suggest these drugs also pose a low risk in pregnant women. Acyclovir can be administered orally to pregnant women with first-episode genital herpes or recurrent herpes and should be administered IV to pregnant women with severe HSV infection. Suppressive acyclovir treatment late in pregnancy reduces the frequency of cesarean delivery among women who have recurrent genital herpes by diminishing the frequency of recurrences at term (378–380). However, such treatment may not protect against transmission to neonates in all cases (381). No data support use of antiviral therapy among HSV-seropositive women without a history of genital herpes.
| Recommended regimen for suppressive therapy of pregnant women with recurrent genital herpes* |
|
|
| Acyclovir 400 mg orally three times a day |
| OR |
| Valacyclovir 500 mg orally twice a day |
|
|
| *Treatment recommended starting at 36 weeks of gestation. (Source: American College of Obstetricians and Gynecologists. Clinical management guidelines for obstetrician-gynecologists. Management of herpes in pregnancy. ACOG Practice Bulletin No. 82. Obstet Gynecol 2007;109:1489–98.) |
Neonatal Herpes
Newborn infants exposed to HSV during birth, as documented by maternal virologic testing of maternal lesions at delivery or presumed by observation of maternal lesions, should be followed carefully in consultation with a pediatric infectious-disease specialist. Guidance is available on management of neonates who are delivered vaginally in the presence of maternal genital HSV lesions (382).
Surveillance cultures or PCR of mucosal surfaces of the neonate to detect HSV infection might be considered before the development of clinical signs of neonatal herpes to guide initiation of treatment. In addition, administration of acyclovir might be considered for neonates born to women who acquired HSV near term because the risk for neonatal herpes is high for these infants. All infants who have neonatal herpes should be promptly evaluated and treated with systemic acyclovir. The recommended regimen for infants treated for known or suspected neonatal herpes is acyclovir 20 mg/kg IV every 8 hours for 14 days if disease is limited to the skin and mucous membranes, or for 21 days for disseminated disease and that involving the central nervous system.
Granuloma Inguinale (Donovanosis)
Granuloma inguinale is a genital ulcerative disease caused by the intracellular gram-negative bacterium Klebsiella granulomatis (formerly known as Calymmatobacterium granulomatis). The disease occurs rarely in the United States, although it is endemic in some tropical and developing areas, including India; Papua, New Guinea; the Caribbean; central Australia; and southern Africa (383–385). Clinically, the disease is commonly characterized as painless, slowly progressive ulcerative lesions on the genitals or perineum without regional lymphadenopathy; subcutaneous granulomas (pseudobuboes) also might occur. The lesions are highly vascular (i.e., beefy red appearance) and bleed. Extragenital infection can occur with extension of infection to the pelvis, or it can disseminate to intra-abdominal organs, bones, or the mouth. The lesions also can develop secondary bacterial infection and can coexist with other sexually transmitted pathogens.
Diagnostic Considerations
The causative organism of granuloma inguinale is difficult to culture, and diagnosis requires visualization of dark-staining Donovan bodies on tissue crush preparation or biopsy. No FDA-cleared molecular tests for the detection of K. granulomatis DNA exist, but such an assay might be useful when undertaken by laboratories that have conducted a CLIA verification study.
Treatment
Several antimicrobial regimens have been effective, but only a limited number of controlled trials have been published (383). Treatment has been shown to halt progression of lesions, and healing typically proceeds inward from the ulcer margins; prolonged therapy is usually required to permit granulation and re-epithelialization of the ulcers. Relapse can occur 6–18 months after apparently effective therapy.
| Recommended Regimen |
|
|
| Azithromycin 1 g orally once per week or 500 mg daily for at least 3 weeks and until all lesions have completely healed |
|
|
| Alternative Regimens |
|
|
| Doxycycline 100 mg orally twice a day for at least 3 weeks and until all lesions have completely healed |
| OR |
| Ciprofloxacin 750 mg orally twice a day for at least 3 weeks and until all lesions have completely healed |
| OR |
| Erythromycin base 500 mg orally four times a day for at least 3 weeks and until all lesions have completely healed |
| OR |
| Trimethoprim-sulfamethoxazole one double-strength (160 mg/800 mg) tablet orally twice a day for at least 3 weeks and until all lesions have completely healed |
|
|
The addition of another antibiotic to these regimens can be considered if improvement is not evident within the first few days of therapy. Addition of an aminoglycoside to these regimens is an option (gentamicin 1 mg/kg IV every 8 hours).
Other Management Considerations
Persons should be followed clinically until signs and symptoms have resolved. All persons who receive a diagnosis of granuloma inguinale should be tested for HIV.
Follow-up
Patients should be followed clinically until signs and symptoms resolve.
Management of Sex Partners
Persons who have had sexual contact with a patient who has granuloma inguinale within the 60 days before onset of the patient’s symptoms should be examined and offered therapy. However, the value of empiric therapy in the absence of clinical signs and symptoms has not been established.
Special Considerations
Pregnancy
Doxycycline should be avoided in the second and third trimester of pregnancy because of the risk for discoloration of teeth and bones, but is compatible with breastfeeding (317). Data suggest that ciprofloxacin presents a low risk to the fetus during pregnancy (317). Sulfonamides are associated with rare but serious kernicterus in those with G6PD deficiency and should be avoided in third trimester and during breastfeeding (317). For these reasons, pregnant and lactating women should be treated with a macrolide regimen (erythromycin or azithromycin). The addition of an aminoglycoside (gentamicin 1 mg/kg IV every 8 hours) can be considered if improvement is not evident within the first few days of therapy.
HIV Infection
Persons with both granuloma inguinale and HIV infection should receive the same regimens as those who do not have HIV infection. The addition of an aminoglycoside (gentamicin 1 mg/kg IV every 8 hours) can be considered if improvement is not evident within the first few days of therapy.
Lymphogranuloma Venereum
Lymphogranuloma venereum (LGV) is caused by C. trachomatis serovars L1, L2, or L3 (386,387). The most common clinical manifestation of LGV among heterosexuals is tender inguinal and/or femoral lymphadenopathy that is typically unilateral. A self-limited genital ulcer or papule sometimes occurs at the site of inoculation. However, by the time patients seek care, the lesions have often disappeared. Rectal exposure in women or MSM can result in proctocolitis mimicking inflammatory bowel disease, and clinical findings may include mucoid and/or hemorrhagic rectal discharge, anal pain, constipation, fever, and/or tenesmus (388,389). Outbreaks of LGV protocolitis have been reported among MSM (390–392). LGV can be an invasive, systemic infection, and if it is not treated early, LGV proctocolitis can lead to chronic colorectal fistulas and strictures; reactive arthropathy has also been reported. However, reports indicate that rectal LGV can be asymptomatic (393). Persons with genital and colorectal LGV lesions can also develop secondary bacterial infection or can be coinfected with other sexually and nonsexually transmitted pathogens.
Diagnostic Considerations
Diagnosis is based on clinical suspicion, epidemiologic information, and the exclusion of other etiologies for proctocolitis, inguinal lymphadenopathy, or genital or rectal ulcers. Genital lesions, rectal specimens, and lymph node specimens (i.e., lesion swab or bubo aspirate) can be tested for C. trachomatis by culture, direct immunofluorescence, or nucleic acid detection (394). NAATs for C. trachomatis perform well on rectal specimens, but are not FDA-cleared for this purpose. Many laboratories have performed the CLIA validation studies needed to provide results from rectal specimens for clinical management. MSM presenting with protocolitis should be tested for chlamydia; NAAT performed on rectal specimens is the preferred approach to testing.
Additional molecular procedures (e.g., PCR-based genotyping) can be used to differentiate LGV from non-LGV C. trachomatis in rectal specimens. However, they are not widely available, and results are not available in a timeframe that would influence clinical management.
Chlamydia serology (complement fixation titers ≥1:64 or microimmunofluorescence titers >1:256) might support the diagnosis of LGV in the appropriate clinical context. Comparative data between types of serologic tests are lacking, and the diagnostic utility of these older serologic methods has not been established. Serologic test interpretation for LGV is not standardized, tests have not been validated for clinical proctitis presentations, and C. trachomatis serovar-specific serologic tests are not widely available.
Treatment
At the time of the initial visit (before diagnostic tests for chlamydia are available), persons with a clinical syndrome consistent with LGV, including proctocolitis or genital ulcer disease with lymphadenopathy, should be presumptively treated for LGV. As required by state law, these cases should be reported to the health department.
Treatment cures infection and prevents ongoing tissue damage, although tissue reaction to the infection can result in scarring. Buboes might require aspiration through intact skin or incision and drainage to prevent the formation of inguinal/femoral ulcerations.
| Recommended Regimen |
|
|
| Doxycycline 100 mg orally twice a day for 21 days |
|
|
| Alternative Regimen |
|
|
| Erythromycin base 500 mg orally four times a day for 21 days |
|
|
Although clinical data are lacking, azithromycin 1 g orally once weekly for 3 weeks is probably effective based on its chlamydial antimicrobial activity. Fluoroquinolone-based treatments also might be effective, but the optimal duration of treatment has not been evaluated.
Other Management Considerations
Patients should be followed clinically until signs and symptoms have resolved. Persons who receive an LGV diagnosis should be tested for other STDs, especially HIV, gonorrhea, and syphilis. Those who test positive for another infection should be referred for or provided with appropriate care and treatment.
Follow-up
Patients should be followed clinically until signs and symptoms resolve.
Management of Sex Partners
Persons who have had sexual contact with a patient who has LGV within the 60 days before onset of the patient’s symptoms should be examined and tested for urethral, cervical, or rectal chlamydial infection depending on anatomic site of exposure. They should be presumptively treated with a chlamydia regimen (azithromycin 1 g orally single dose or doxycycline 100 mg orally twice a day for 7 days).
Special Considerations
Pregnancy
Pregnant and lactating women should be treated with erythromycin. Doxycycline should be avoided in the second and third trimester of pregnancy because of risk for discoloration of teeth and bones, but is compatible with breastfeeding (317). Azithromycin might prove useful for treatment of LGV in pregnancy, but no published data are available regarding an effective dose and duration of treatment.
HIV Infection
Persons with both LGV and HIV infection should receive the same regimens as those who are HIV negative. Prolonged therapy might be required, and delay in resolution of symptoms might occur.
Syphilis
Syphilis is a systemic disease caused by Treponema pallidum. The disease has been divided into stages based on clinical findings, helping to guide treatment and follow-up. Persons who have syphilis might seek treatment for signs or symptoms of primary syphilis infection (i.e., ulcers or chancre at the infection site), secondary syphilis (i.e., manifestations that include, but are not limited to, skin rash, mucocutaneous lesions, and lymphadenopathy), or tertiary syphilis (i.e., cardiac, gummatous lesions, tabes dorsalis, and general paresis). Latent infections (i.e., those lacking clinical manifestations) are detected by serologic testing. Latent syphilis acquired within the preceding year is referred to as early latent syphilis; all other cases of latent syphilis are late latent syphilis or syphilis of unknown duration. T. pallidum can infect the central nervous system and result in neurosyphilis, which can occur at any stage of syphilis. Early neurologic clinical manifestations (i.e., cranial nerve dysfunction, meningitis, stroke, acute altered mental status, and auditory or ophthalmic abnormalities) are usually present within the first few months or years of infection. Late neurologic manifestations (i.e., tabes dorsalis and general paresis) occur 10–30 years after infection.
Diagnostic Considerations
Darkfield examinations and tests to detect T. pallidum directly from lesion exudate or tissue are the definitive methods for diagnosing early syphilis (395). Although no T. pallidum detection tests are commercially available, some laboratories provide locally developed and validated PCR tests for the detection of T. pallidum DNA. A presumptive diagnosis of syphilis requires use of two tests: a nontreponemal test (i.e., Venereal Disease Research Laboratory [VDRL] or Rapid Plasma Reagin [RPR]) and a treponemal test (i.e., fluorescent treponemal antibody absorbed [FTA-ABS] tests, the T. pallidum passive particle agglutination [TP-PA] assay, various enzyme immunoassays [EIAs], chemiluminescence immunoassays, immunoblots, or rapid treponemal assays). Although many treponemal-based tests are commercially available, only a few are approved for use in the United States. Use of only one type of serologic test is insufficient for diagnosis and can result in false-negative results in persons tested during primary syphilis and false-positive results in persons without syphilis. False-positive nontreponemal test results can be associated with various medical conditions and factors unrelated to syphilis, including other infections (e.g., HIV), autoimmune conditions, immunizations, pregnancy, injection-drug use, and older age (395,396). Therefore, persons with a reactive nontreponemal test should always receive a treponemal test to confirm the diagnosis of syphilis.
Nontreponemal test antibody titers might correlate with disease activity and are used to follow treatment response. Results should be reported quantitatively. A fourfold change in titer, equivalent to a change of two dilutions (e.g., from 1:16 to 1:4 or from 1:8 to 1:32), is considered necessary to demonstrate a clinically significant difference between two nontreponemal test results obtained using the same serologic test. Sequential serologic tests in individual patients should be performed using the same testing method (VDRL or RPR), preferably by the same laboratory. The VDRL and RPR are equally valid assays, but quantitative results from the two tests cannot be compared directly because RPR titers frequently are slightly higher than VDRL titers. Nontreponemal test titers usually decline after treatment and might become nonreactive with time; however, in some persons, nontreponemal antibodies can persist for a long period of time, a response referred to as the “serofast reaction.” Most patients who have reactive treponemal tests will have reactive tests for the remainder of their lives, regardless of treatment or disease activity. However, 15%–25% of patients treated during the primary stage revert to being serologically nonreactive after 2–3 years (397). Treponemal antibody titers do not predict treatment response and therefore should not be used for this purpose.
Some clinical laboratories are screening samples using treponemal tests, typically by EIA or chemiluminescence immunoassays (398,399). This reverse screening algorithm for syphilis testing can identify persons previously treated for syphilis, those with untreated or incompletely treated syphilis, and persons with false-positive results that can occur with a low likelihood of infection. Persons with a positive treponemal screening test should have a standard nontreponemal test with titer performed reflexively by the laboratory to guide patient management decisions. If the nontreponemal test is negative, the laboratory should perform a different treponemal test (preferably one based on different antigens than the original test) to confirm the results of the initial test. If a second treponemal test is positive, persons with a history of previous treatment will require no further management unless sexual history suggests likelihood of re-exposure. In this instance, a repeat nontreponemal test in 2–4 weeks is recommended to evaluate for early infection. Those without a history of treatment for syphilis should be offered treatment. Unless history or results of a physical examination suggest a recent infection, previously untreated persons should be treated for late latent syphilis. If the second treponemal test is negative and the epidemiologic risk and clinical probability for syphilis are low, further evaluation or treatment is not indicated. Two studies demonstrate that high quantitative index values from treponemal EIA/CIA tests correlate with TPPA positivity; however, the range of optical density values varies among different treponemal immunoassays, and the clinical significance of these findings warrant further investigation (400,401).
For most persons with HIV infection, serologic tests are accurate and reliable for diagnosing syphilis and following a patient’s response to treatment. However, atypical nontreponemal serologic test results (i.e., unusually high, unusually low, or fluctuating titers) might occur regardless of HIV-infection status. When serologic tests do not correspond with clinical findings suggestive of early syphilis, presumptive treatment is recommended for persons with risk factors for syphilis, and use of other tests (e.g., biopsy and PCR) should be considered.
Further testing is warranted for persons with clinical signs of neurosyphilis (e.g., cranial nerve dysfunction, auditory or ophthalmic abnormalities, meningitis, stroke, acute or chronic altered mental status, and loss of vibration sense). Laboratory testing is helpful in supporting the diagnosis of neurosyphilis; however, no single test can be used to diagnose neurosyphilis in all instances. The diagnosis of neurosyphilis depends on a combination of cerebrospinal fluid (CSF) tests (CSF cell count or protein and a reactive CSF-VDRL) in the presence of reactive serologic test results and neurologic signs and symptoms. CSF laboratory abnormalities are common in persons with early syphilis and are of unknown significance in the absence of neurologic signs or symptoms (402). CSF-VDRL is highly specific but insensitive. In a person with neurologic signs or symptoms, a reactive CSF-VDRL (in the absence of blood contamination) is considered diagnostic of neurosyphilis. When CSF-VDRL is negative despite the presence of clinical signs of neurosyphilis, reactive serologic test results, and abnormal CSF cell count and/or protein, neurosyphilis should be considered. In this instance, additional evaluation using FTA-ABS testing on CSF may be warranted. The CSF FTA-ABS test is less specific for neurosyphilis than the CSF-VDRL but is highly sensitive. Neurosyphilis is highly unlikely with a negative CSF FTA-ABS test, especially among persons with nonspecific neurologic signs and symptoms (403).
Among persons with HIV infection, CSF leukocyte count usually is elevated (>5 white blood cell count [WBC]/mm3). Using a higher cutoff (>20 WBC/mm3) might improve the specificity of neurosyphilis diagnosis (404).
Treatment
Penicillin G, administered parenterally, is the preferred drug for treating persons in all stages of syphilis. The preparation used (i.e., benzathine, aqueous procaine, or aqueous crystalline), dosage, and length of treatment depend on the stage and clinical manifestations of the disease. Treatment for late latent syphilis and tertiary syphilis require a longer duration of therapy, because organisms theoretically might be dividing more slowly (the validity of this rationale has not been assessed). Longer treatment duration is required for persons with latent syphilis of unknown duration to ensure that those who did not acquire syphilis within the preceding year are adequately treated.
Selection of the appropriate penicillin preparation is important, because T. pallidum can reside in sequestered sites (e.g., the CNS and aqueous humor) that are poorly accessed by some forms of penicillin. Combinations of benzathine penicillin, procaine penicillin, and oral penicillin preparations are not considered appropriate for the treatment of syphilis. Reports have indicated that practitioners have inadvertently prescribed combination benzathine-procaine penicillin (Bicillin C-R) instead of the standard benzathine penicillin product (Bicillin L-A) widely used in the United States. Practitioners, pharmacists, and purchasing agents should be aware of the similar names of these two products to avoid using the inappropriate combination therapy agent for treating syphilis (405).
The effectiveness of penicillin for the treatment of syphilis was well established through clinical experience even before the value of randomized controlled clinical trials was recognized. Therefore, nearly all recommendations for the treatment of syphilis are based not only on clinical trials and observational studies, but many decades of clinical experience.
Special Considerations
Pregnancy
Parenteral penicillin G is the only therapy with documented efficacy for syphilis during pregnancy. Pregnant women with syphilis in any stage who report penicillin allergy should be desensitized and treated with penicillin (see Management of Persons Who Have a History of Penicillin Allergy).
Jarisch-Herxheimer Reaction
The Jarisch-Herxheimer reaction is an acute febrile reaction frequently accompanied by headache, myalgia, fever, and other symptoms that can occur within the first 24 hours after the initiation of any therapy for syphilis. Patients should be informed about this possible adverse reaction and how to manage it if it occurs. The Jarisch-Herxheimer reaction occurs most frequently among persons who have early syphilis, presumably because bacterial burdens are higher during these stages. Antipyretics can be used to manage symptoms, but they have not been proven to prevent this reaction. The Jarisch-Herxheimer reaction might induce early labor or cause fetal distress in pregnant women, but this should not prevent or delay therapy (see Syphilis During Pregnancy).
Management of Sex Partners
Sexual transmission of T. pallidum is thought to occur only when mucocutaneous syphilitic lesions are present. Such manifestations are uncommon after the first year of infection. Persons exposed sexually to a person who has primary, secondary, or early latent syphilis should be evaluated clinically and serologically and treated according to the following recommendations:
Persons who have had sexual contact with a person who receives a diagnosis of primary, secondary, or early latent syphilis within 90 days preceding the diagnosis should be treated presumptively for early syphilis, even if serologic test results are negative.
Persons who have had sexual contact with a person who receives a diagnosis of primary, secondary, or early latent syphilis >90 days before the diagnosis should be treated presumptively for early syphilis if serologic test results are not immediately available and the opportunity for follow-up is uncertain. If serologic tests are negative, no treatment is needed. If serologic tests are positive, treatment should be based on clinical and serologic evaluation and stage of syphilis.
In some areas or populations with high rates of syphilis, health departments recommend notification and presumptive treatment of sex partners of persons with late latent syphilis who have high nontreponemal serologic test titers (i.e., >1:32), because high titers might be indicative of early syphilis. These partners should be managed as if the index case had early syphilis.
Long-term sex partners of persons who have late latent syphilis should be evaluated clinically and serologically for syphilis and treated on the basis of the evaluation’s findings.
The following sex partners of persons with syphilis are considered at risk for infection and should be confidentially notified of the exposure and need for evaluation: partners who have had sexual contact within 1) 3 months plus the duration of symptoms for persons who receive a diagnosis of primary syphilis, 2) 6 months plus duration of symptoms for those with secondary syphilis, and 3) 1 year for persons with early latent syphilis.
Primary and Secondary Syphilis
Treatment
Parenteral penicillin G has been used effectively to achieve clinical resolution (i.e., the healing of lesions and prevention of sexual transmission) and to prevent late sequelae. However, no comparative trials have been conducted to guide the selection of an optimal penicillin regimen. Substantially fewer data are available for nonpenicillin regimens.
| Recommended Regimen for Adults* |
|
|
| Benzathine penicillin G 2.4 million units IM in a single dose |
|
|
| *Recommendations for treating syphilis in persons with HIV infection and pregnant women are discussed elsewhere in this report (see Syphilis among Persons with HIV infection and Syphilis during Pregnancy). |
Available data demonstrate that use of additional doses of benzathine penicillin G, amoxicillin, or other antibiotics do not enhance efficacy when used to treat primary and secondary syphilis, regardless of HIV status (406,407).
| Recommended Regimen for Infants and Children |
|
|
| Benzathine penicillin G 50,000 units/kg IM, up to the adult dose of 2.4 million units in a single dose |
|
|
Infants and children aged ≥1 month who receive a diagnosis of syphilis should have birth and maternal medical records reviewed to assess whether they have congenital or acquired syphilis (see Congenital Syphilis). Infants and children aged ≥1 month with primary and secondary syphilis should be managed by a pediatric infectious-disease specialist and evaluated for sexual abuse (e.g., through consultation with child-protection services) (see Sexual Assault or Abuse of Children).
Other Management Considerations
All persons who have primary and secondary syphilis should be tested for HIV infection. In geographic areas in which the prevalence of HIV is high, persons who have primary or secondary syphilis should be retested for acute HIV in 3 months if the first HIV test result was negative.
Persons who have syphilis and symptoms or signs suggesting neurologic disease (e.g., cranial nerve dysfunction, meningitis, stroke, and hearing loss) or ophthalmic disease (e.g., uveitis, iritis, neuroretinitis, and optic neuritis) should have an evaluation that includes CSF analysis, ocular slit-lamp ophthalmologic examination, and otologic examination. Treatment should be guided by the results of this evaluation.
Invasion of CSF by T. pallidum accompanied by CSF laboratory abnormalities is common among adults who have primary or secondary syphilis (402). In the absence of clinical neurologic findings, no evidence supports variation from the recommended treatment regimen for primary and secondary syphilis. Symptomatic neurosyphilis develops in only a limited number of persons after treatment with the penicillin regimens recommended for primary and secondary syphilis. Therefore, unless clinical signs or symptoms of neurologic or ophthalmic involvement are present, routine CSF analysis is not recommended for persons who have primary or secondary syphilis.
Follow-Up
Clinical and serologic evaluation should be performed at 6 and 12 months after treatment; more frequent evaluation might be prudent if follow-up is uncertain or if repeat infection is a concern. Serologic response (i.e., titer) should be compared with the titer at the time of treatment. However, assessing serologic response to treatment can be difficult, and definitive criteria for cure or failure have not been well established. In addition, nontreponemal test titers might decline more slowly for persons previously treated for syphilis (408,409).
Persons who have signs or symptoms that persist or recur and those with at least a fourfold increase in nontreponemal test titer persisting for >2 weeks likely experienced treatment failure or were re-infected. These persons should be retreated and reevaluated for HIV infection. Because treatment failure usually cannot be reliably distinguished from reinfection with T. pallidum, a CSF analysis also should be performed; treatment should be guided by CSF findings.
Failure of nontreponemal test titers to decline fourfold within 6–12 months after therapy for primary or secondary syphilis might be indicative of treatment failure. However, clinical trial data have demonstrated that 15%–20% of persons with primary and secondary syphilis treated with the recommended therapy will not achieve the fourfold decline in nontreponemal titer used to define response at 1 year after treatment (406,409). Serologic response to treatment appears to be associated with several factors, including the person’s stage of syphilis (earlier stages are more likely to decline fourfold and become negative) and initial nontreponemal antibody titers (lower titers are less likely to decline fourfold than higher titers) (409). Optimal management of persons who have less than a fourfold decline in titers after treatment of syphilis is unclear. At a minimum, these persons should receive additional clinical and serologic follow-up and be evaluated for HIV infection. If additional follow-up cannot be ensured, retreatment is recommended. Because treatment failure might be the result of unrecognized CNS infection, CSF examination can be considered in such situations.
For retreatment, weekly injections of benzathine penicillin G 2.4 million units IM for 3 weeks is recommended, unless CSF examination indicates that neurosyphilis is present (see Neurosyphilis). Serologic titers might not decline despite a negative CSF examination and a repeated course of therapy (410). In these circumstances, although the need for additional therapy or repeated CSF examinations is unclear, it is not generally recommended.
Management of Sex Partners
See Syphilis, Management of Sex Partners.
Special Considerations
Penicillin Allergy
Data to support use of alternatives to penicillin in the treatment of primary and secondary syphilis are limited. However, several therapies might be effective in nonpregnant, penicillin-allergic persons who have primary or secondary syphilis. Regimens of doxycycline 100 mg orally twice daily for 14 days (411,412) and tetracycline (500 mg four times daily for 14 days) have been used for many years. Compliance is likely to be better with doxycycline than tetracycline, because tetracycline can cause gastrointestinal side effects and requires more frequent dosing. Although limited clinical studies, along with biologic and pharmacologic evidence, suggest that ceftriaxone (1–2 g daily either IM or IV for 10–14 days) is effective for treating primary and secondary syphilis, the optimal dose and duration of ceftriaxone therapy have not been defined (413). Azithromycin as a single 2 g oral dose has been effective for treating primary and secondary syphilis in some populations (414–416). However, T. pallidum chromosomal mutations associated with azithromycin and other macrolide resistance and treatment failures have been documented in multiple geographical areas in the United States (417–419). Accordingly, azithromycin should not be used as first-line treatment for syphilis and should be used with caution only when treatment with penicillin or doxycycline is not feasible. Azithromycin should not be used in MSM, persons with HIV, or pregnant women. Careful clinical and serologic follow-up of persons receiving any alternative therapies is essential.
Persons with a penicillin allergy whose compliance with therapy or follow-up cannot be ensured should be desensitized and treated with benzathine penicillin. Skin testing for penicillin allergy might be useful in some circumstances in which the reagents and expertise are available to perform the test adequately (see Management of Persons Who Have a History of Penicillin Allergy).
Pregnancy
Pregnant women with primary or secondary syphilis who are allergic to penicillin should be desensitized and treated with penicillin. For more information, see Management of Persons Who Have a History of Penicillin Allergy and Syphilis During Pregnancy.
HIV Infection
Persons with HIV infection who have primary or secondary syphilis should be treated as those without HIV infection. For more information on treatment and management, see Syphilis in Persons with HIV infection.
Latent Syphilis
Latent syphilis is defined as syphilis characterized by seroreactivity without other evidence of primary, secondary, or tertiary disease. Persons who have latent syphilis and who acquired syphilis during the preceding year are classified as having early latent syphilis, a subset of latent syphilis. Persons can receive a diagnosis of early latent syphilis if, during the year preceding the diagnosis, they had 1) a documented seroconversion or a sustained (>2 week) fourfold or greater increase in nontreponemal test titers; 2) unequivocal symptoms of primary or secondary syphilis; or 3) a sex partner documented to have primary, secondary, or early latent syphilis. In addition, for persons with reactive nontreponemal and treponemal tests whose only possible exposure occurred during the previous 12 months, early latent syphilis can be assumed. In the absence of these conditions, an asymptomatic person should be considered to have latent syphilis. Nontreponemal serologic titers usually are higher early in the course of syphilis infection. However, early latent syphilis cannot be reliably diagnosed solely on the basis of nontreponemal titers. All persons with latent syphilis should have careful examination of all accessible mucosal surfaces (i.e., the oral cavity, perianal area, perineum and vagina in women, and underneath the foreskin in uncircumcised men) to evaluate for mucosal lesions.
Treatment
Because latent syphilis is not transmitted sexually, the objective of treating persons in this stage of disease is to prevent complications and transmission from a pregnant woman to her fetus. Although clinical experience supports the effectiveness of penicillin in achieving this goal, limited evidence is available to guide choice of specific regimens or duration. Available data demonstrate that additional doses of benzathine penicillin G, amoxicillin, or other antibiotics in early latent syphilis do not enhance efficacy, regardless of HIV infection (406,407).
| Recommended Regimens for Adults* |
|
|
| Early Latent Syphilis |
| Benzathine penicillin G 2.4 million units IM in a single dose |
| Late Latent Syphilis or Latent Syphilis of Unknown Duration |
| Benzathine penicillin G 7.2 million units total, administered as 3 doses of 2.4 million units IM each at 1-week intervals |
|
|
| *Recommendations for treating syphilis in persons with HIV infection and pregnant women are discussed elsewhere in this report (see Syphilis in Persons with HIV infection and Syphilis during Pregnancy). |
| Recommended Regimens for Infants and Children |
|
|
| Early Latent Syphilis |
| Benzathine penicillin G 50,000 units/kg IM, up to the adult dose of 2.4 million units in a single dose |
| Late Latent Syphilis |
| Benzathine penicillin G 50,000 units/kg IM, up to the adult dose of 2.4 million units, administered as 3 doses at 1-week intervals (total 150,000 units/kg up to the adult total dose of 7.2 million units) |
|
|
Infants and children aged ≥1 month diagnosed with latent syphilis should be managed by a pediatric infectious-disease specialist and receive a CSF examination. In addition, birth and maternal medical records should be reviewed to assess whether these infants and children have congenital or acquired syphilis. For those with congenital syphilis, treatment should be undertaken as described in the congenital syphilis section in this document. Those with acquired latent syphilis should be evaluated for sexual abuse (e.g., through consultation with child protection services) (see Sexual Assault or Abuse of Children). These regimens are for penicillin nonallergic children who have acquired syphilis and who have normal CSF examination results.
Other Management Considerations
All persons who have latent syphilis should be tested for HIV infection. Persons who receive a diagnosis of latent syphilis and have neurologic signs and symptoms (e.g., cognitive dysfunction, motor or sensory deficits, ophthalmic or auditory symptoms, cranial nerve palsies, and symptoms or signs of meningitis or stroke) should be evaluated for neurosyphilis (see Neurosyphilis).
If a person misses a dose of penicillin in a course of weekly therapy for latent syphilis, the appropriate course of action is unclear. Clinical experience suggests that an interval of 10–14 days between doses of benzathine penicillin for latent syphilis might be acceptable before restarting the sequence of injections (i.e., if dose 1 is given on day 0, dose 2 is administered between days 10 and 14). Pharmacologic considerations suggest that an interval of 7–9 days between doses, if feasible, might be more optimal (420–422). Missed doses are not acceptable for pregnant women receiving therapy for latent syphilis (423). Pregnant women who miss any dose of therapy must repeat the full course of therapy.
Follow-Up
Quantitative nontreponemal serologic tests should be repeated at 6, 12, and 24 months. A CSF examination should be performed if 1) a sustained (>2 weeks) fourfold increase or greater in titer is observed, 2) an initially high titer (≥1:32) fails to decline at least fourfold within 12–24 months of therapy, or 3) signs or symptoms attributable to syphilis develop. In such circumstances, patients with CSF abnormalities should be treated for neurosyphilis. If the CSF examination is negative, retreatment for latent syphilis should be administered. Serologic titers might fail to decline despite a negative CSF examination and a repeated course of therapy, especially if the initial nontreponemal titer is low (<1:8); in these circumstances, the need for additional therapy or repeated CSF examinations is unclear but is generally not recommended. Serologic and clinical monitoring should be offered along with a reevaluation for HIV infection.
Management of Sex Partners
See Syphilis, Management of Sex Partners.
Special Considerations
Penicillin Allergy
The effectiveness of alternatives to penicillin in the treatment of latent syphilis has not been well documented. Nonpregnant patients allergic to penicillin who have clearly defined early latent syphilis should respond to antibiotics recommended as alternatives to penicillin for the treatment of primary and secondary syphilis (see Primary and Secondary Syphilis, Treatment). The only acceptable alternatives for the treatment of latent syphilis are doxycycline (100 mg orally twice daily) or tetracycline (500 mg orally four times daily), each for 28 days. The efficacy of these alternative regimens in persons with HIV infection has not been well studied. These therapies should be used only in conjunction with close serologic and clinical follow-up, especially in persons with HIV infection. On the basis of biologic plausibility and pharmacologic properties, ceftriaxone might be effective for treating latent syphilis. However, the optimal dose and duration of ceftriaxone therapy have not been defined; treatment decisions should be discussed in consultation with a specialist. Persons with a penicillin allergy whose compliance with therapy or follow-up cannot be ensured should be desensitized and treated with benzathine penicillin. Skin testing for penicillin allergy might be useful in some circumstances in which the reagents and expertise are available to perform the test adequately (see Management of Persons Who Have a History of Penicillin Allergy).
Pregnancy
Pregnant women who are allergic to penicillin should be desensitized and treated with penicillin. For more information, see Management of Persons Who Have a History of Penicillin Allergy and Syphilis during Pregnancy.
HIV Infection
Persons with HIV infection with latent syphilis should be treated as persons who do not have HIV infection. For more information on treatment and management of latent syphilis, see Syphilis in Persons with HIV Infection.
Tertiary Syphilis
Tertiary syphilis refers to gummas and cardiovascular syphilis but not to neurosyphilis. Guidelines for all forms of neurosyphilis (e.g., early or late neurosyphilis) are discussed elsewhere in these recommendations (see Neurosyphilis). Persons who are not allergic to penicillin and have no evidence of neurosyphilis should be treated with the following regimen.
| Recommended Regimen |
|
|
| Tertiary Syphilis with Normal CSF Examination |
| Benzathine penicillin G 7.2 million units total, administered as 3 doses of 2.4 million units IM each at 1-week intervals |
|
|
Other Management Considerations
All persons who have tertiary syphilis should be tested for HIV infection and should receive a CSF examination before therapy is initiated. Persons with CSF abnormalities should be treated with a neurosyphilis regimen. Some providers treat all persons who have cardiovascular syphilis with a neurosyphilis regimen. These persons should be managed in consultation with an infectious-disease specialist. Limited information is available concerning clinical response and follow-up of persons who have tertiary syphilis.
Management of Sex Partners
See Syphilis, Management of Sex Partners.
Special Considerations
Penicillin Allergy
Providers should ask patients about known allergies to penicillin. Any person allergic to penicillin should be treated in consultation with an infectious-disease specialist.
Pregnancy
Pregnant women who are allergic to penicillin should be desensitized and treated with penicillin. For more information, see Management of Persons Who Have a History of Penicillin Allergy and Syphilis during Pregnancy.
HIV Infection
Persons with HIV infection who have tertiary syphilis should be treated as described for persons without HIV infection. For more information on the management of tertiary syphilis in persons with HIV infection, see Syphilis in Persons with HIV Infection.
Neurosyphilis
Treatment
CNS involvement can occur during any stage of syphilis, and CSF laboratory abnormalities are common in persons with early syphilis, even in the absence of clinical neurologic findings. No evidence exists to support variation from recommended treatment for syphilis at any stage for persons without clinical neurologic findings, with the exception of tertiary syphilis. If clinical evidence of neurologic involvement is observed (e.g., cognitive dysfunction, motor or sensory deficits, ophthalmic or auditory symptoms, cranial nerve palsies, and symptoms or signs of meningitis or stroke), a CSF examination should be performed.
Syphilitic uveitis or other ocular manifestations (e.g., neuroretinitis and optic neuritis) can be associated with neurosyphilis. A CSF examination should be performed in all instances of ocular syphilis, even in the absence of clinical neurologic findings. Ocular syphilis should be managed in collaboration with an ophthalmologist and according to the treatment and other recommendations for neurosyphilis, even if a CSF examination is normal. In instances of ocular syphilis and abnormal CSF test results, follow-up CSF examinations should be performed to assess treatment response.
| Recommended Regimen |
|
|
| Neurosyphilis and Ocular Syphilis |
| Aqueous crystalline penicillin G 18–24 million units per day, administered as 3–4 million units IV every 4 hours or continuous infusion, for 10–14 days |
|
|
If compliance with therapy can be ensured, the following alternative regimen might be considered.
| Alternative Regimen |
|
|
| Procaine penicillin G 2.4 million units IM once daily |
| PLUS |
| Probenecid 500 mg orally four times a day, both for 10–14 days |
|
|
The durations of the recommended and alternative regimens for neurosyphilis are shorter than the duration of the regimen used for latent syphilis. Therefore, benzathine penicillin, 2.4 million units IM once per week for up to 3 weeks, can be considered after completion of these neurosyphilis treatment regimens to provide a comparable total duration of therapy.
Other Management Considerations
The following are other considerations in the management of persons who have neurosyphilis:
All persons who have neurosyphilis should be tested for HIV
Although systemic steroids are used frequently as adjunctive therapy for otologic syphilis, such drugs have not been proven to be beneficial.
Follow-Up
If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. Follow-up CSF examinations also can be used to evaluate changes in the CSF-VDRL or CSF protein after therapy; however, changes in these two parameters occur more slowly than cell counts, and persistent abnormalities might be less important (424,425). Leukocyte count is a sensitive measure of the effectiveness of therapy. If the cell count has not decreased after 6 months, or if the CSF cell count or protein is not normal after 2 years, retreatment should be considered. Limited data suggest that in immunocompetent persons and persons with HIV infection on highly active antiretroviral therapy, normalization of the serum RPR titer predicts normalization of CSF parameters following neurosyphilis treatment (425).
Management of Sex Partners
See Syphilis, Management of Sex Partners.
Special Considerations
Penicillin Allergy
Limited data suggest that ceftriaxone 2 g daily either IM or IV for 10–14 days can be used as an alternative treatment for persons with neurosyphilis (426,427). Cross-sensitivity between ceftriaxone and penicillin can occur, but the risk for penicillin cross-reactivity between third-generation cephalosporins is negligible (428–431) (see Management of Persons Who Have a History of Penicillin Allergy). If concern exists regarding the safety of ceftriaxone for a patient with neurosyphilis, skin testing should be performed (if available) to confirm penicillin allergy and, if necessary, penicillin desensitization in consultation with a specialist is recommended. Other regimens have not been adequately evaluated for treatment of neurosyphilis.
Pregnancy
Pregnant women who are allergic to penicillin should be desensitized and treated with penicillin. For more information, see Syphilis during Pregnancy.
HIV Infection
Persons with HIV infection who have neurosyphilis should be treated as described for persons without HIV infection. For more information on neurosyphilis, see Syphilis in Persons with HIV infection.
Persons with HIV Infection
Diagnostic Considerations
Interpretation of treponemal and nontreponemal serologic tests for persons with HIV infection is the same as for the HIV-uninfected patient. Although rare, unusual serologic responses have been observed among persons with HIV infection who have syphilis; although most reports have involved post-treatment serologic titers that were higher than expected (high serofast) or fluctuated, false-negative serologic test results and delayed appearance of seroreactivity have also been reported (432).
When clinical findings are suggestive of syphilis but serologic tests are nonreactive or their interpretation is unclear, alternative tests (e.g., biopsy of a lesion, darkfield examination, and PCR of lesion material) might be useful for diagnosis. Neurosyphilis should be considered in the differential diagnosis of neurologic signs and symptoms in persons with HIV infection.
Treatment
Persons with HIV infection who have early syphilis might be at increased risk for neurologic complications (433) and might have higher rates of serologic treatment failure with recommended regimens. The magnitude of these risks is not defined precisely, but is likely small. Although long-term (>1 year) comparative data are lacking, no treatment regimens for syphilis have been demonstrated to be more effective in preventing neurosyphilis in persons with HIV infection than the syphilis regimens recommended for persons without HIV infection (406). Careful follow-up after therapy is essential. The use of antiretroviral therapy as per current guidelines might improve clinical outcomes in persons with HIV infection and syphilis (425,434,435).
Primary and Secondary Syphilis among Persons with HIV Infection
| Recommended Regimen |
|
|
| Benzathine penicillin G, 2.4 million units IM in a single dose |
|
|
Available data demonstrate that additional doses of benzathine penicillin G, amoxicillin, or other antibiotics in primary and secondary syphilis do not result in enhanced efficacy (406,407).
Other Management Considerations
Most persons with HIV infection respond appropriately to the recommended benzathine penicillin treatment regimen for primary and secondary syphilis. CSF abnormalities (e.g., mononuclear pleocytosis and elevated protein levels) are common in persons with HIV infection, even in those without syphilis. The clinical and prognostic significance of such CSF laboratory abnormalities in persons with primary and secondary syphilis who lack neurologic symptoms is unknown. Certain studies have demonstrated that among persons with HIV infection and syphilis, CSF abnormalities are associated with a CD4 count of ≤350 cells/mL and/or an RPR titer of ≥1:32 (404,436,437); however, CSF examination has not been associated with improved clinical outcomes in the absence of neurologic signs and symptoms. All persons with HIV infection and syphilis should have a careful neurologic exam (425,434,435).
Follow-Up
Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy; those who meet the criteria for treatment failure (i.e., signs or symptoms that persist or recur or persons who have a sustained [>2 weeks] fourfold increase or greater in titer) should be managed in the same manner as HIV-negative patients (i.e., a CSF examination and retreatment guided by CSF findings). In addition, CSF examination and retreatment can be considered for persons whose nontreponemal test titers do not decrease fourfold within 12–24 months of therapy. If CSF examination is normal, treatment with benzathine penicillin G administered as 2.4 million units IM each at weekly intervals for 3 weeks is recommended. Serologic titers might not decline despite a negative CSF examination and a repeated course of therapy (410). In these circumstances, the need for additional therapy or repeated CSF examinations is unclear, but is not generally recommended. Serologic and clinical monitoring should be provided.
Management of Sex Partners
See Syphilis, Management of Sex Partners.
Special Considerations
Penicillin Allergy
Persons with HIV infection who are penicillin-allergic and have primary or secondary syphilis should be managed according to the recommendations for penicillin-allergic, HIV-negative persons. Persons with penicillin allergy whose compliance with therapy or follow-up cannot be ensured should be desensitized and treated with penicillin (see Management of Persons Who Have a History of Penicillin Allergy). The use of alternatives to penicillin has not been well studied in persons with HIV infection; azithromycin is not recommended in persons with HIV infection and primary and secondary syphilis. Alternative therapies should be used only in conjunction with close serologic and clinical follow-up.
Latent Syphilis among Persons with HIV Infection
| Recommended Regimen for Early Latent Syphilis |
|
|
| Benzathine penicillin G, 2.4 million units IM in a single dose |
|
|
| Recommended Regimen for Late Latent Syphilis |
|
|
| Benzathine penicillin G, at weekly doses of 2.4 million units for 3 weeks |
|
|
Other Management Considerations
All persons with HIV infection and syphilis should undergo a careful neurologic examination; those with neurologic symptoms or signs should undergo immediate CSF examination. In the absence of neurologic symptoms, CSF examination has not been associated with improved clinical outcomes and therefore is not recommended.
Follow-Up
Patients should be evaluated clinically and serologically at 6, 12, 18, and 24 months after therapy. If, at any time, clinical symptoms develop or a sustained (>2 weeks) fourfold or greater rise in nontreponemal titers occurs, a CSF examination should be performed and treatment administered accordingly. If the nontreponemal titer does not decline fourfold after 24 months, CSF examination can be considered and treatment administered accordingly, although initial low titers (<1:8) might not decline. Even after retreatment, serologic titers might fail to decline. In these circumstances, the need for repeated CSF examination or additional therapy is unclear but is generally not recommended. Serologic and clinical monitoring should be provided.
Management of Sex Partners
See Syphilis, Management of Sex Partners.
Special Considerations
Penicillin Allergy
The efficacy of alternative nonpenicillin regimens in persons with HIV infection has not been well studied, and these therapies should be used only in conjunction with close serologic and clinical follow-up. Patients with penicillin allergy whose compliance with therapy or follow-up cannot be ensured should be desensitized and treated with penicillin (See Management of Persons Who Have a History of Penicillin Allergy).
Neurosyphilis Among Persons with HIV Infection
All persons with HIV infection and syphilis should receive a careful neurologic examination. Persons with HIV infection and neurosyphilis should be treated according to the recommendations for HIV-negative persons with neurosyphilis (See Neurosyphilis).
Follow Up
Persons with HIV infection and neurosyphilis should be managed according to the recommendations for HIV-negative persons with neurosyphilis (see Neurosyphilis). Limited data suggest that changes in CSF parameters might occur more slowly in persons with HIV infection, especially those with more advanced immunosuppression (424,434).
Management of Sex Partners
See Syphilis, Management of Sex Partners.
Special Considerations
Penicillin Allergy
Persons with HIV infection who are penicillin-allergic and have neurosyphilis should be managed according to the recommendations for penicillin-allergic, HIV-negative patients with neurosyphilis (See Neurosyphilis). Several small observational studies conducted in persons with HIV infection with neurosyphilis suggest that ceftriaxone 1–2 g IV daily for 10–14 days might be effective as an alternate agent (438–440). The possibility of cross-sensitivity between ceftriaxone and penicillin exists, but the risk of penicillin cross-reactivity between third-generation cephalosporins is negligible (428–431) (see Management of Persons Who Have a History of Penicillin Allergy). If concern exists regarding the safety of ceftriaxone for a person with HIV infection and neurosyphilis, skin testing should be performed (if available) to confirm penicillin allergy and, if necessary, penicillin desensitization in consultation with a specialist is recommended. Other regimens have not been adequately evaluated for treatment of neurosyphilis.
Syphilis During Pregnancy
All women should be screened serologically for syphilis early in pregnancy (106). Most states mandate screening at the first prenatal visit for all women (441). In populations in which receipt of prenatal care is not optimal, RPR test screening and treatment (if the RPR test is reactive) should be performed at the time pregnancy is confirmed (442). Antepartum screening by nontreponemal antibody testing is typical, but treponemal antibody testing is being used in some settings. Pregnant women with reactive treponemal screening tests should have additional quantitative nontreponemal testing, because titers are essential for monitoring treatment response. For communities and populations in which the prevalence of syphilis is high and for women at high risk for infection, serologic testing should also be performed twice during the third trimester: once at 28–32 weeks’ gestation and again at delivery. Any woman who has a fetal death after 20 weeks’ gestation should be tested for syphilis. No mother or neonate should leave the hospital without maternal serologic status having been documented at least once during pregnancy, and if the mother is considered high risk, documented at delivery.
Diagnostic Considerations
Seropositive pregnant women should be considered infected unless an adequate treatment history is documented clearly in the medical records and sequential serologic antibody titers have declined appropriately for the stage of syphilis. In general, the risk for antepartum fetal infection or congenital syphilis at delivery is related to the stage of syphilis during pregnancy, with the highest risk occurring with the primary and secondary stage. Quantitative maternal nontreponemal titer, especially if >1:8, might be a marker of early infection and bacteremia. However, risk for fetal infection is still significant in pregnant women with late latent syphilis and low titers. Pregnant women with stable, serofast low antibody titers who have previously been treated for syphilis might not require additional treatment; however, rising or persistently high antibody titers might indicate reinfection or treatment failure, and treatment should be considered.
If a treponemal test (e.g., EIA or CIA) is used for antepartum syphilis screening, all positive EIA/CIA tests should be reflexed to a quantitative nontreponemal test (RPR or VDRL). If the nontreponemal test is negative, then the results are considered discrepant and a second treponemal test (TP-PA preferred) should be performed, preferably on the same specimen. If the second treponemal test is positive, current or past syphilis infection can be confirmed. For women with a history of adequately treated syphilis who do not have ongoing risk, no further treatment is necessary. Women without a history of treatment should be staged and treated accordingly with a recommended penicillin regimen. If the second treponemal test is negative, the positive EIA/CIA is more likely to represent a false-positive test result in low-risk women with no history of treated syphilis (400). If the woman is at low risk for syphilis, lacks signs or symptoms of primary syphilis, has a partner with no clinical or serologic evidence of syphilis, and is likely to follow up, repeat serologic testing within 4 weeks can be considered to determine whether the EIA/CIA remains positive or if the RPR/VDRL or the TP-PA becomes positive. If both the RPR and TP-PA remain negative, no further treatment is necessary. If follow-up is not possible, women without a history of treated syphilis should be treated according to the stage of syphilis.
Treatment
Penicillin G is the only known effective antimicrobial for preventing maternal transmission to the fetus and treating fetal infection (443). Evidence is insufficient to determine optimal, recommended penicillin regimens (444).
| Recommended Regimen |
|
|
| Pregnant women should be treated with the penicillin regimen appropriate for their stage of infection. |
|
|
Other Management Considerations
Some evidence suggests that additional therapy is beneficial for pregnant women. For women who have primary, secondary, or early latent syphilis, a second dose of benzathine penicillin 2.4 million units IM can be administered 1 week after the initial dose (445–447).
When syphilis is diagnosed during the second half of pregnancy, management should include a sonographic fetal evaluation for congenital syphilis. However, this evaluation should not delay therapy. Sonographic signs of fetal or placental syphilis (i.e., hepatomegaly, ascites, hydrops, fetal anemia, or a thickened placenta) indicate a greater risk for fetal treatment failure (448); cases accompanied by these signs should be managed in consultation with obstetric specialists. Evidence is insufficient to recommend specific regimens for these situations.
Women treated for syphilis during the second half of pregnancy are at risk for premature labor and/or fetal distress if the treatment precipitates the Jarisch-Herxheimer reaction (449). These women should be advised to seek obstetric attention after treatment if they notice any fever, contractions, or decrease in fetal movements. Stillbirth is a rare complication of treatment, but concern for this complication should not delay necessary treatment. No data are available to suggest that corticosteroid treatment alters the risk for treatment-related complications in pregnancy.
Missed doses are not acceptable for pregnant women receiving therapy for late latent syphilis (423). Pregnant women who miss any dose of therapy must repeat the full course of therapy.
All women who have syphilis should be offered testing for HIV infection.
Follow-Up
Coordinated prenatal care and treatment are vital. At a minimum, serologic titers should be repeated at 28–32 weeks’ gestation and at delivery. Serologic titers can be checked monthly in women at high risk for reinfection or in geographic areas in which the prevalence of syphilis is high. Providers should ensure that the clinical and antibody responses are appropriate for the patient’s stage of disease, although most women will deliver before their serologic response to treatment can be assessed definitively. Inadequate maternal treatment is likely if delivery occurs within 30 days of therapy, clinical signs of infection are present at delivery, or the maternal antibody titer at delivery is fourfold higher than the pretreatment titer.
Management of Sex Partners
See Syphilis, Management of Sex Partners.
Special Considerations
Penicillin Allergy
No proven alternatives to penicillin are available for treatment of syphilis during pregnancy. Pregnant women who have a history of penicillin allergy should be desensitized and treated with penicillin. Skin testing or oral graded penicillin dose challenge might be helpful in identifying women at risk for acute allergic reactions (see Management of Persons Who Have a History of Penicillin Allergy).
Tetracycline and doxycycline are contraindicated in the second and third trimester of pregnancy (317) Erythromycin and azithromycin should not be used, because neither reliably cures maternal infection or treats an infected fetus (444). Data are insufficient to recommend ceftriaxone for treatment of maternal infection and prevention of congenital syphilis.
HIV Infection
Placental inflammation from congenital infection might increase the risk for perinatal transmission of HIV. All women with HIV infection should be evaluated for syphilis and receive a penicillin regimen appropriate for the stage of infection. Data are insufficient to recommend any alternative regimens for pregnant women with HIV infection (see Syphilis Among Persons with HIV infection).
Congenital Syphilis
Effective prevention and detection of congenital syphilis depends on the identification of syphilis in pregnant women and, therefore, on the routine serologic screening of pregnant women during the first prenatal visit. Additional testing at 28 weeks’ gestation and again at delivery is warranted for women who are at increased risk or live in communities with increased prevalence of syphilis infection (442,450). Moreover, as part of the management of pregnant women who have syphilis, information concerning ongoing risk behaviors and treatment of sex partners should be obtained to assess the risk for reinfection. Routine screening of newborn sera or umbilical cord blood is not recommended, as diagnosis at this time does not prevent symptomatic congenital syphilis in some newborns. No mother or newborn infant should leave the hospital without maternal serologic status having been documented at least once during pregnancy, and preferably again at delivery if at risk.
Evaluation and Treatment of Neonates (Infants Aged <30 Days)
The diagnosis of congenital syphilis can be difficult, as maternal nontreponemal and treponemal IgG antibodies can be transferred through the placenta to the fetus, complicating the interpretation of reactive serologic tests for syphilis in neonates. Therefore, treatment decisions frequently must be made on the basis of 1) identification of syphilis in the mother; 2) adequacy of maternal treatment; 3) presence of clinical, laboratory, or radiographic evidence of syphilis in the neonate; and 4) comparison of maternal (at delivery) and neonatal nontreponemal serologic titers using the same test, preferably conducted by the same laboratory. Any neonate at risk for congenital syphilis should receive a full evaluation and testing for HIV infection.
All neonates born to mothers who have reactive nontreponemal and treponemal test results should be evaluated with a quantitative nontreponemal serologic test (RPR or VDRL) performed on the neonate’s serum, because umbilical cord blood can become contaminated with maternal blood and yield a false-positive result, and Wharton’s jelly within the umbilical cord can yield a false-negative result. Conducting a treponemal test (i.e., TP-PA, FTA-ABS, EIA, or CIA) on neonatal serum is not recommended because it is difficult to interpret. No commercially available immunoglobulin (IgM) test can be recommended.
All neonates born to women who have reactive serologic tests for syphilis should be examined thoroughly for evidence of congenital syphilis (e.g., nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of an extremity). Pathologic examination of the placenta or umbilical cord using specific staining (e.g., silver) or a T. pallidum PCR test using a CLIA-validated test should be considered; DFA-TP reagents are not available. Darkfield microscopic examination or PCR testing of suspicious lesions or body fluids (e.g., bullous rash and nasal discharge) also should be performed. In addition to these tests, for stillborn infants, skeletal survey demonstrating typical osseous lesions might aid in the diagnosis of congenital syphilis.
The following scenarios describe the congenital syphilis evaluation and treatment of neonates born to women who have reactive serologic tests for syphilis during pregnancy. Maternal history of infection with T. pallidum and treatment for syphilis must be considered when evaluating and treating the neonate for congenital syphilis in most scenarios, except when congenital syphilis is proven or highly probable (See Scenario 1).
Scenario 1: Proven or highly probable congenital syphilis
Any neonate with:
-
an abnormal physical examination that is consistent with congenital syphilis;
OR
-
a serum quantitative nontreponemal serologic titer that is fourfold higher than the mother’s titer;¶
OR
a positive darkfield test or PCR of lesions or body fluid(s).
Recommended Evaluation
CSF analysis for VDRL, cell count, and protein**
Complete blood count (CBC) and differential and platelet count
Other tests as clinically indicated (e.g., long-bone radiographs, chest radiograph, liver-function tests, neuroimaging, ophthalmologic examination, and auditory brain stem response).
| Recommended Regimens |
|
|
| Aqueous crystalline penicillin G 100,000–150,000 units/kg/day, administered as 50,000 units/kg/dose IV every 12 hours during the first 7 days of life and every 8 hours thereafter for a total of 10 days |
| OR |
| Procaine penicillin G 50,000 units/kg/dose IM in a single daily dose for 10 days |
|
|
If more than 1 day of therapy is missed, the entire course should be restarted. Data are insufficient regarding the use of other antimicrobial agents (e.g., ampicillin). When possible, a full 10-day course of penicillin is preferred, even if ampicillin was initially provided for possible sepsis. The use of agents other than penicillin requires close serologic follow-up to assess adequacy of therapy.
Scenario 2: Possible Congenital Syphilis
Any neonate who has a normal physical examination and a serum quantitative nontreponemal serologic titer equal to or less than fourfold the maternal titer and one of the following:
-
mother was not treated, inadequately treated, or has no documentation of having received treatment;
OR
-
mother was treated with erythromycin or a regimen other than those recommended in these guidelines (i.e., a nonpenicillin G regimen);††
OR
mother received recommended treatment <4 weeks before delivery.
Recommended Evaluation
CSF analysis for VDRL, cell count, and protein**
CBC, differential, and platelet count
Long-bone radiographs
A complete evaluation is not necessary if 10 days of parenteral therapy is administered, although such evaluations might be useful. For instance, a lumbar puncture might document CSF abnormalities that would prompt close follow-up. Other tests (e.g., CBC, platelet count, and bone radiographs) can be performed to further support a diagnosis of congenital syphilis.
| Recommended Regimens |
|
|
| Aqueous crystalline penicillin G 100,000–150,000 units/kg/day, administered as 50,000 units/kg/dose IV every 12 hours during the first 7 days of life and every 8 hours thereafter for a total of 10 days |
| OR |
| Procaine penicillin G 50,000 units/kg/dose IM in a single daily dose for 10 days |
| OR |
| Benzathine penicillin G 50,000 units/kg/dose IM in a single dose |
|
|
Before using the single-dose benzathine penicillin G regimen, the complete evaluation (i.e., CSF examination, long-bone radiographs, and CBC with platelets) must be normal, and follow-up must be certain. If any part of the infant’s evaluation is abnormal or not performed, if the CSF analysis is uninterpretable because of contamination with blood, or if follow-up is uncertain, a 10-day course of penicillin G is required. If the neonate’s nontreponemal test is nonreactive and the provider determines that the mother’s risk of untreated syphilis is low, treatment of the neonate with a single IM dose of benzathine penicillin G 50,000 units/kg for possible incubating syphilis can be considered without an evaluation.
Neonates born to mothers with untreated early syphilis at the time of delivery are at increased risk for congenital syphilis, and the 10-day course of penicillin G may be considered even if the complete evaluation is normal and follow-up is certain.
Scenario 3: Congenital Syphilis less likely
Any neonate who has a normal physical examination and a serum quantitative nontreponemal serologic titer equal to or less than fourfold the maternal titer and both of the following are true:
mother was treated during pregnancy, treatment was appropriate for the stage of infection, and treatment was administered >4 weeks before delivery and
mother has no evidence of reinfection or relapse.
Recommended Evaluation
No evaluation is recommended.
| Recommended Regimen |
|
|
| Benzathine penicillin G 50,000 units/kg/dose IM in a single dose* |
|
|
| *Another approach involves not treating the infant, but rather providing close serologic follow-up every 2–3 months for 6 months for infants whose mother’s nontreponemal titers decreased at least fourfold after appropriate therapy for early syphilis or remained stable for low-titer, latent syphilis (e.g., VDRL <1:2; RPR <1:4). |
Scenario 4: Congenital Syphilis unlikely
Any neonate who has a normal physical examination and a serum quantitative nontreponemal serologic titer equal to or less than fourfold the maternal titer and both of the following are true:
mother’s treatment was adequate before pregnancy and
mother’s nontreponemal serologic titer remained low and stable (i.e., serofast) before and during pregnancy and at delivery (VDRL <1:2; RPR <1:4).
Recommended Evaluation
No evaluation is recommended.
| Recommended Regimen |
|
|
| No treatment is required, but infants with reactive nontreponemal tests should be followed serologically to ensure the nontreponemal test returns to negative (see Follow-Up). Benzathine penicillin G 50,000 units/kg as a single IM injection might be considered, particularly if follow-up is uncertain and the neonate has a reactive nontreponemal test. |
|
|
Follow-Up
All neonates with reactive nontreponemal tests should receive careful follow-up examinations and serologic testing (i.e., a nontreponemal test) every 2–3 months until the test becomes nonreactive. In the neonate who was not treated because congenital syphilis was considered less likely or unlikely, nontreponemal antibody titers should decline by age 3 months and be nonreactive by age 6 months, indicating that the reactive test result was caused by passive transfer of maternal IgG antibody. At 6 months, if the nontreponemal test is nonreactive, no further evaluation or treatment is needed; if the nontreponemal test is still reactive, the infant is likely to be infected and should be treated. Treated neonates that exhibit persistent nontreponemal test titers by 6–12 months should be re-evaluated through CSF examination and managed in consultation with an expert. Retreatment with a 10-day course of a penicillin G regimen may be indicated. Neonates with a negative nontreponemal test at birth and whose mothers were seroreactive at delivery should be retested at 3 months to rule out serologically negative incubating congenital syphilis at the time of birth. Treponemal tests should not be used to evaluate treatment response because the results are qualitative and passive transfer of maternal IgG treponemal antibody might persist for at least 15 months.
Neonates whose initial CSF evaluations are abnormal should undergo a repeat lumbar puncture approximately every 6 months until the results are normal. A reactive CSF Venereal Disease Research Laboratory (VDRL) test or abnormal CSF indices that persist and cannot be attributed to other ongoing illness requires retreatment for possible neurosyphilis and should be managed in consultation with an expert.
Special Considerations
Penicillin Allergy
Infants and children who require treatment for congenital syphilis but who have a history of penicillin allergy or develop an allergic reaction presumed secondary to penicillin should be desensitized and then treated with penicillin (see Management of Persons with a History of Penicillin Allergy). Skin testing remains unavailable for infants and children because the procedure has not been standardized for this age group. Data are insufficient regarding the use of other antimicrobial agents (e.g., ceftriaxone) for congenital syphilis in infants and children. If a nonpenicillin G agent is used, close clinical, serologic, and CSF follow-up is required in consultation with an expert.
Penicillin Shortage
During periods when the availability of aqueous crystalline penicillin G is compromised, the following is recommended (see http://www.cdc.gov/std/treatment/drugnotices/penicilling.htm).
-
For neonates with clinical evidence of congenital syphilis (Scenario 1), check local sources for aqueous crystalline penicillin G (potassium or sodium). If IV penicillin G is limited, substitute some or all daily doses with procaine penicillin G (50,000 U/kg/dose IM a day in a single daily dose for 10 days).
If aqueous or procaine penicillin G is not available, ceftriaxone (in doses appropriate for birthweight) can be considered with careful clinical and serologic follow-up and in consultation with an expert, as evidence is insufficient to support the use of ceftriaxone for the treatment of congenital syphilis. Management might include a repeat CSF examination at age 6 months if the initial examination was abnormal. Ceftriaxone must be used with caution in infants with jaundice.
-
For neonates without any clinical evidence of congenital syphilis (Scenario 2 and Scenario 3), use
-
procaine penicillin G, 50,000 U/kg/dose IM a day in a single dose for 10 daysOR
- benzathine penicillin G, 50,000 U/kg IM as a single dose.
If any part of the evaluation for congenital syphilis is abnormal or was not performed, CSF examination is not interpretable, or follow-up is uncertain, procaine penicillin G is recommended. A single dose of ceftriaxone is inadequate therapy.
-
For premature infants who have no clinical evidence of congenital syphilis (Scenario 2 and Scenario 3) and might not tolerate IM injections because of decreased muscle mass, IV ceftriaxone can be considered with careful clinical and serologic follow-up and in consultation with an expert. Ceftriaxone dosing must be adjusted according to birthweight.
HIV Infection
Evidence is insufficient to determine whether neonates who have congenital syphilis and HIV or whose mothers have HIV infection require different therapy or clinical management than is recommended for all neonates. All neonates with congenital syphilis and HIV infection should be managed similarly as neonates with congenital syphilis who do not have HIV infection.
Evaluation and Treatment of Infants and Children with Congenital Syphilis
Infants and children aged ≥1 month who are identified as having reactive serologic tests for syphilis should be examined thoroughly and have maternal serology and records reviewed to assess whether they have congenital or acquired syphilis (see Primary and Secondary Syphilis and Latent Syphilis, Sexual Assault or Abuse of Children). Any infant or child at risk for congenital syphilis should receive a full evaluation and testing for HIV infection.
Recommended Evaluation
CSF analysis for VDRL, cell count, and protein
CBC, differential, and platelet count
Other tests as clinically indicated (e.g., long-bone radiographs, chest radiograph, liver function tests, abdominal ultrasound, ophthalmologic examination, neuroimaging, and auditory brain-stem response)
| Recommended Regimen |
|
|
| Aqueous crystalline penicillin G 200,000–300,000 units/kg/day IV, administered as 50,000 units/kg every 4–6 hours for 10 days |
|
|
If the infant or child has no clinical manifestations of congenital syphilis and the evaluation (including the CSF examination) is normal, treatment with up to 3 weekly doses of benzathine penicillin G, 50,000 U/kg IM can be considered. A single dose of benzathine penicillin G 50,000 units/kg IM up to the adult dose of 2.4 million units in a single dose can be considered after the 10-day course of IV aqueous penicillin to provide more comparable duration of treatment in those who have no clinical manifestations and normal CSF. All of the above treatment regimens also would be adequate for children who might have other treponemal infections.
Follow-Up
Careful follow-up examinations and serologic testing (i.e., a nontreponemal test) of infants and children treated for congenital syphilis after the neonatal period (30 days of age) should be performed every 3 months until the test becomes nonreactive or the titer has decreased fourfold. The serologic response after therapy might be slower for infants and children than neonates. If these titers increase at any point for more than 2 weeks or do not decrease fourfold after 12–18 months, the infant or child should be evaluated (e.g., through CSF examination), treated with a 10-day course of parenteral penicillin G, and managed in consultation with an expert. Treponemal tests should not be used to evaluate treatment response, because the results are qualitative and persist after treatment; further, passive transfer of maternal IgG treponemal antibody might persist for at least 15 months after delivery.
Infants or children whose initial CSF evaluations are abnormal should undergo a repeat lumbar puncture approximately every 6 months until the results are normal. After 2 years of follow-up, a reactive CSF VDRL test or abnormal CSF indices that persists and cannot be attributed to other ongoing illness requires retreatment for possible neurosyphilis and should be managed in consultation with an expert.
Special Considerations
Penicillin Allergy
Infants and children who require treatment for congenital syphilis but who have a history of penicillin allergy or develop an allergic reaction presumed secondary to penicillin should be desensitized and treated with penicillin (see Management of Persons with a History of Penicillin Allergy). Skin testing remains unavailable for infants and children because the procedure has not been standardized for this age group. Data are insufficient regarding the use of other antimicrobial agents (e.g., ceftriaxone) for congenital syphilis in infants and children. If a nonpenicillin G agent is used, close clinical, serologic, and CSF follow-up is required in consultation with an expert.
Penicillin Shortage
During periods when the availability of penicillin G is compromised, management options are similar to options for the neonate (see Evaluation and treatment of infants during the first month of life).
For infants and children with clinical evidence of congenital syphilis, procaine penicillin G (50,000 U/kg/dose IM up to the adult dose of 2.4 million units a day in a single daily dose for 10 days) is recommended. A single dose of benzathine penicillin G 50,000 units/kg IM up to the adult dose of 2.4 million units in a single dose can be considered after the 10-day course of procaine penicillin. If procaine or benzathine penicillin G is not available, ceftriaxone (in doses appropriate for age and weight) can be considered with careful clinical and serologic follow-up. Infants and children receiving ceftriaxone should be managed in consultation with an expert, as evidence is insufcient to support the use of ceftriaxone for the treatment of congenital syphilis in infants or children. For infants aged ≥30 days, use 75 mg/kg IV/IM of ceftriaxone a day in a single daily dose for 10–14 days (dose adjustment might be necessary based on current weight). For children, the dose should be 100 mg/kg of ceftriaxone a day in a single daily dose.
- For infants and children without any clinical evidence of infection (see Scenario 2 and Scenario 3), use
- procaine penicillin G, 50,000 U/kg/dose IM a day in a single dose for 10 days or
- benzathine penicillin G, 50,000 U/kg IM as a single dose.
If any part of the evaluation for congenital syphilis is abnormal or not performed, CSF examination is not interpretable, or follow-up is uncertain, procaine penicillin G is recommended.
HIV Infection
Evidence is insufficient to determine whether infants and children who have congenital syphilis and HIV or whose mothers have HIV infection require different therapy or clinical management than is recommended for all infants and children. All infants and children with congenital syphilis and HIV infection should be managed like infants and children without HIV infection.
Management of Persons Who Have a History of Penicillin Allergy
No proven alternatives to penicillin are available for treating neurosyphilis, congenital syphilis, or syphilis in pregnant women. Penicillin also is recommended, whenever possible, for persons with HIV infection. The prevalence of reported penicillin allergy in the United States is about 8%–10% (451–453) and might be higher in hospitalized persons (454). The prevalence of reported penicillin allergy in developing countries is unknown; however, limited data suggest that penicillin is one of the most frequently reported allergies in some developing countries (455,456). Of persons reporting penicillin allergy, 10%–15% have a positive skin test suggestive of a penicillin allergy; these persons are at risk for an immunoglobulin E (IgE)-mediated allergic response to penicillin such as urticaria, angioedema, or anaphylaxis (i.e., upper airway obstruction, bronchospasm, or hypotension) (428–430,457,458). Re-administration of penicillin to patients with a history of IgE-mediated hypersensitivity reactions can cause severe, immediate reactions. Because anaphylactic reactions to penicillin can be fatal, every effort should be made to avoid administering penicillin to penicillin-allergic persons, unless they undergo induction of drug tolerance (also referred to as “desensitization”) to temporarily eliminate IgE-mediated hypersensitivity. However, many persons with a reported history of penicillin allergy likely have had other types of adverse drug reactions or have lost their sensitivity to penicillin over time and can safely be treated with penicillin.
Penicillin skin testing with the major and minor determinants of penicillin can reliably identify persons at high risk for IgE-mediated reactions to penicillin (458,459). Although the testing reagents are easily generated, only the major determinant (benzylpenicilloyl poly-L-lysine [Pre-Pen]) and penicillin G have been available commercially. These two tests identify an estimated 90%–99% of the allergic patients. However, because skin testing without the minor determinants would still fail to identify 1%–10% of allergic persons and because serious or fatal reactions can occur among these minor-determinant–positive persons, caution should be exercised when the full battery of skin-test reagents is not available (Box 2) (457–460). Manufacturers are working on a minor determinant mixture, but at the time of publication, no such product has been cleared by FDA for use in the United States. Penicillin skin testing has been used in a variety of settings to improve antibiotic use (453,461–463).
BOX 2. Skin-test reagents for identifying persons at risk for adverse reactions to penicillin.
Major Determinant
Benzylpenicilloyl poly-L-lysine (PrePen) (AllerQuest, Plainville Connecticut) (6 × 10–5M)
Minor Determinant Precursors*
Benzylpenicillin G (10–2M, 3.3 mg/mL, 10,000 units/mL)
Benzylpenicilloate (10–2M, 3.3 mg/mL)
Benzylpenicilloate (or penicilloyl propylamine) (10–2M, 3.3 mg/mL)
Positive Control
Commercial histamine for intradermal skin testing (1.0 mg/mL)
Negative Control
Diluent (usually saline) or allergen diluent
Source: Adapted from Saxon A, Beall GN, Rohr AS, Adelman DC. Immediate hypersensitivity reactions to beta-lactam antibiotics. Ann Intern Med 1987;107:204–15.
*Aged penicillin is not an adequate source of minor determinants. Penicillin G should either be freshly prepared or come from a fresh-frozen source.
Some studies have reported cross-reactivity rates as high as 10% among persons with a history of penicillin allergy who take cephalosporins. However, more recent studies indicate a lower rate (<2.5%) of cross reactivity between these drugs (428–431,464). Risk is highest with first-generation cephalosporins and cephalosporins that have similar R-group side chains to specific penicillins (465,466). The risk for penicillin cross-reactivity between most second-generation (cefoxitin) and all third generation cephalosporins (cefixime and ceftriaxone) is negligible (428–431); cefoxitin, cefixime, and ceftriaxone do not have an R group side chain similar to penicillin G.
Recommendations
Persons with a history of severe non-IgE-mediated reactions (e.g., Stevens-Johnson syndrome, toxic epidermal necrolysis, interstitial nephritis, and hemolytic anemia) are not candidates for skin testing or challenge and should avoid penicillins indefinitely. If the full battery of skin-test reagents is available, including both major and minor determinants (see Penicillin Allergy Skin Testing), persons who report a history of penicillin reaction and who are skin-test negative can receive conventional penicillin therapy. Persons with positive skin test results should be desensitized before initiating treatment.
If the full battery of skin-test reagents, including the minor determinants, is not available, skin testing should be conducted using the major determinant (Pre-Pen) and penicillin G. Those persons who have positive test results should be desensitized. For persons with negative skin tests, a subsequent observed challenge to the penicillin of choice is recommended. In addition, for persons with a history of severe or recent suspected IgE-mediated reactions to penicillin with negative skin testing, the penicillin of choice should be given by graded challenge. If the major determinant is not available for skin testing, all persons with a history suggesting IgE-mediated reactions to penicillin (e.g., anaphylaxis, angioedema, bronchospasm, or urticaria) should be desensitized in a hospital setting. In persons with reactions not likely to be IgE-mediated, outpatient-monitored graded challenges can be considered.
Penicillin Allergy Skin Testing
Persons at high risk for anaphylaxis, including those who 1) have a history of penicillin-related anaphylaxis or other IgE-mediated reactions, asthma, or other diseases that would make anaphylaxis more dangerous or 2) are being treated with beta-adrenergic blocking agents should be tested with 100-fold dilutions of the full-strength skin-test reagents before being tested with full-strength reagents. In these situations, testing should be performed in a monitored setting in which treatment for an anaphylactic reaction is available. If possible, antihistamines (e.g., chlorpheniramine maleate, fexofenadine, diphenhydramine HCL, and hydroxyzine) should not have been taken within the 5 days before skin testing.
Procedures
Dilute the antigens in saline either 100-fold for preliminary testing (if the patient has had a IgE-mediated reaction to penicillin) or 10-fold (if the patient has had another type of immediate, generalized reaction to penicillin within the preceding year). Pre-Pen is provided full-strength (6 × 10–5 meq penicilloyl) in a single dose ampoule. Penicillin G is diluted to 10,000 IU/ml in saline and aliquoted in sterile vials that remain stable for at least 6 months if frozen.
Epicutaneous (Prick) Tests
Duplicate drops of skin-test reagent are placed on the volar surface of the forearm. The underlying epidermis is pierced with a 26-gauge needle without drawing blood. An epicutaneous test is positive if the average wheal diameter after 15 minutes is ≥4 mm larger than that of negative controls; otherwise, the test is negative. The histamine controls should be positive to ensure that results are not falsely negative because of the effect of antihistaminic drugs.
Intradermal Test
If epicutaneous tests are negative, duplicate 0.02-mL intradermal injections of negative control and antigen solutions are made into the volar surface of the forearm by using a 26-or 27-gauge needle on a syringe. The margins of the wheals induced by the injections should be marked with a ball point pen. An intradermal test is positive if the average wheal diameter 15 minutes after injection is >2 mm larger than the initial wheal size and also is >2 mm larger than the negative histamine controls. Otherwise, the tests are negative. If the duplicates are discordant, a second set of duplicate tests can be used to resolve the ambiguity.
Desensitization
Persons who have a positive skin test to one of the penicillin determinants can be desensitized (Table 1). This is a straightforward, relatively safe procedure that can be performed orally or intravenously. Modified protocols might be considered based on an individual’s symptoms, drug of choice, and route of administration (467–469). Although the two approaches have not been compared, oral desensitization is regarded as safer and easier to perform. Desensitization should occur in a hospital setting because serious IgE-mediated allergic reactions can occur; the procedure can usually be completed in approximately 4–12 hours, after which time the first dose of penicillin is administered. After desensitization, penicillin should be maintained continuously for the duration of the course of therapy. Once the course is completed, if penicillin is required in the future, the desensitization procedure should be repeated.
TABLE 1.
Oral desensitization protocol for persons with a positive skin test*
| Penicillin V suspension dose† | Amount§ (units/mL) | mL | Units | Cumulative dose (units) |
|---|---|---|---|---|
| 1 | 1,000 | 0.1 | 100 | 100 |
| 2 | 1,000 | 0.2 | 200 | 300 |
| 3 | 1,000 | 0.4 | 400 | 700 |
| 4 | 1,000 | 0.8 | 800 | 1,500 |
| 5 | 1,000 | 1.6 | 1,600 | 3,100 |
| 6 | 1,000 | 3.2 | 3,200 | 6,300 |
| 7 | 1,000 | 6.4 | 6,400 | 12,700 |
| 8 | 10,000 | 1.2 | 12,000 | 24,700 |
| 9 | 10,000 | 2.4 | 24,000 | 48,700 |
| 10 | 10,000 | 4.8 | 48,000 | 96,700 |
| 11 | 80,000 | 1.0 | 80,000 | 176,700 |
| 12 | 80,000 | 2.0 | 160,000 | 336,700 |
| 13 | 80,000 | 4.0 | 320,000 | 656,700 |
| 14 | 80,000 | 8.0 | 640,000 | 1,296,700 |
Source: Wendel GO, Jr, Stark BJ, Jamison RB, Melina RD, Sullivan TJ. Penicillin allergy and desensitization in serious infections during pregnancy. N Engl J Med 1985;312:1229–32.
Observation period was 30 minutes before parenteral administration of penicillin.
Interval between doses, 15–30 minutes; elapsed time, 4–8 hours; cumulative dose, 1.3 million units.
The specific amount of drug was diluted in approximately 30 mL of water and then administered orally.
Diseases Characterized by Urethritis and Cervicitis
Urethritis
Urethritis, as characterized by urethral inflammation, can result from infectious and noninfectious conditions. Symptoms, if present, include dysuria; urethral pruritis; and mucoid, mucopurulent, or purulent discharge. Signs of urethral discharge on examination can also be present in persons without symptoms. Although N. gonorrhoeae and C. trachomatis are well established as clinically important infectious causes of urethritis, Mycoplasma genitalium has also been associated with urethritis and, less commonly, prostatitis (470–474). If point-of-care diagnostic tools (e.g., Gram, methylene blue [MB] or gentian violet [GV] stain microscopy, first void urine with microscopy, and leukocyte esterase) are not available, drug regimens effective against both gonorrhea and chlamydia should be administered. Further testing to determine the specific etiology is recommended to prevent complications, re-infection, and transmission because a specific diagnosis might improve treatment compliance, delivery of risk reduction interventions, and partner notification. Both chlamydia and gonorrhea are reportable to health departments. NAATs are preferred for the detection of C. trachomatis and N. gonorrhoeae, and urine is the preferred specimen in males (394). NAAT-based tests for the diagnosis of T. vaginalis in men have not been cleared by FDA; however, some laboratories have performed the CLIA-compliant validation studies (475) needed to provide such testing.
Etiology
Several organisms can cause infectious urethritis. The presence of Gram-negative intracellular diplococci (GNID) or MB/GV purple intracellular diplococci on urethral smear is indicative of presumed gonorrhea infection, which is frequently accompanied by chlamydial infection. NGU, which is diagnosed when microscopy of urethral secretions indicates inflammation without GNID or MB/GV purple intracellular diplococci, is caused by C. trachomatis in 15%–40% of cases; however, prevalence varies by age group, with a lower burden of disease occurring among older men (476). Documentation of chlamydial infection as the etiology of NGU is essential because of the need for partner referral for evaluation and treatment to prevent complications of chlamydia, especially in female partners. Complications of C. trachomatis-associated NGU among males include epididymitis, prostatitis, and reactive arthritis.
M. genitalium, which can be sexually transmitted, is associated with symptoms of urethritis as well as urethral inflammation and accounts for 15%–25% of NGU cases in the United States (470–473). However, FDA-cleared diagnostic tests for M. genitalium are not available.
T. vaginalis can cause NGU in heterosexual men, but the prevalence varies substantially by region of the United States and within specific subpopulations. In some instances, NGU can be acquired by fellatio (i.e., oral penile contact), sometimes because of specific pathogens such as HSV, Epstein Barr Virus, and adenovirus (476); data supporting other Mycoplasma species and Ureaplasma as etiologic agents are inconsistent. Diagnostic and treatment procedures for these organisms are reserved for situations in which these infections are suspected (e.g., contact with trichomoniasis, urethral lesions, or severe dysuria and meatitis, which might suggest genital herpes) or when NGU is not responsive to recommended therapy. Enteric bacteria have been identified as an uncommon cause of NGU and might be associated with insertive anal intercourse (476). The importance of NGU not caused by defined pathogens is uncertain; neither complications (e.g., urethral stricture and epididymitis) nor adverse outcomes in sex partners have been identified in these cases.
Diagnostic Considerations
Clinicians should attempt to obtain objective evidence of urethral inflammation. However, if point-of-care diagnostic tests (e.g., Gram, MB or GV, or Gram stain microscopy) are not available, all men should be tested by NAAT and treated with drug regimens effective against both gonorrhea and chlamydia.
In the setting of compatible symptoms, urethritis can be documented on the basis of any of the following signs or laboratory tests:
Mucoid, mucopurulent, or purulent discharge on examination.
Gram stain of urethral secretions demonstrating ≥2 WBC per oil immersion field (477). The Gram stain is a point-of-care diagnostic test for evaluating urethritis that is highly sensitive and specific for documenting both urethritis and the presence or absence of gonococcal infection. MB/GV stain of urethral secretions is an alternative point-of-care diagnostic test with performance characteristics similar to Gram stain; thus, the cutoff number for WBC per oil immersion field should be the same (478). Presumed gonococcal infection is established by documenting the presence of WBC containing GNID in Gram stain or intracellular purple diplococci in MB/GV smears; men should be presumptively treated and managed accordingly for gonorrhea (GC) infection (see Gonococcal Infections).
Positive leukocyte esterase test on first-void urine or microscopic examination of sediment from a spun first-void urine demonstrating ≥10 WBC per high power field.
In settings where Gram stain or MB/GV smear is available, men who meet criteria for urethritis (microscopy of urethral secretions with ≥2 WBC per oil immersion field and no intracellular gram negative or purple diplococci) should receive NAAT testing for C. trachomatis and N. gonorrhoeae and can be managed as recommended (see Nongonococcal Urethritis). Men evaluated in settings in which Gram stain or MB/GV smear is not available (i.e., gonococcal infection cannot be ruled out at the point of care) who meet at least one criterion for urethritis (i.e., urethral discharge, positive LE test on first void urine, or microscopic exam of first void urine sediment with ≥10 WBC per hfp) should be tested by NAAT and treated with regimens effective against gonorrhea and chlamydia.
If symptoms are present but no evidence of urethral inflammation is present, NAAT testing for C. trachomatis and N. gonorrhoeae might identify infections (479). If the results demonstrate infection with these pathogens, the appropriate treatment should be given and sex partners referred for evaluation and treatment. If none of these clinical criteria are present, empiric treatment of symptomatic men is recommended only for those men at high risk for infection who are unlikely to return for a follow-up evaluation or test results. Such men should be treated with drug regimens effective against gonorrhea and chlamydia.
Nongonococcal Urethritis
Diagnostic Considerations
NGU is a nonspecific diagnosis that can have many infectious etiologies. NGU is confirmed in symptomatic men when staining of urethral secretions indicates inflammation without Gram negative or purple diplococci. All men who have confirmed NGU should be tested for chlamydia and gonorrhea even if point-of-care tests are negative for evidence of GC. NAATs for chlamydia and gonorrhea are recommended because of their high sensitivity and specificity; a specific diagnosis can potentially reduce complications, re-infection, and transmission (394). Testing for T. vaginalis should be considered in areas or populations of high prevalence.
Treatment
Presumptive treatment should be initiated at the time of NGU diagnosis. Azithromycin and doxycycline are highly effective for chlamydial urethritis. NGU associated with M. genitalium currently responds better to azithromycin than doxycycline, although azithromycin efficacy might be declining (See Mycoplasma genitalium).
| Recommended Regimens |
|
|
| Azithromycin 1 g orally in a single dose |
| OR |
| Doxycycline 100 mg orally twice a day for 7 days |
|
|
| Alternative Regimens |
|
|
| Erythromycin base 500 mg orally four times a day for 7 days |
| OR |
| Erythromycin ethylsuccinate 800 mg orally four times a day for 7 days |
| OR |
| Levofloxacin 500 mg orally once daily for 7 days |
| OR |
| Ofloxacin 300 mg orally twice a day for 7 days |
|
|
As a directly observed treatment, single-dose regimens might be associated with higher rates of compliance over other regimens. To maximize compliance with recommended therapies, medications should be dispensed onsite in the clinic, and regardless of the number of doses involved in the regimen, the first should be directly observed.
Other Management Considerations
To minimize transmission and reinfection, men treated for NGU should be instructed to abstain from sexual intercourse until they and their partner(s) have been adequately treated (i.e., for 7 days after single-dose therapy or until completion of a 7-day regimen and symptoms resolved). Men who receive a diagnosis of NGU should be tested for HIV and syphilis.
Follow-Up
Men should be provided results of the testing obtained as part of the NGU evaluation, and those with a specific diagnosis of chlamydia, gonorrhea, or trichomonas should be offered partner services and instructed to return 3 months after treatment for repeat testing because of high rates of reinfection, regardless of whether their sex partners were treated (480,481) (see Chlamydia, Follow-Up and Gonorrhea, Follow-Up).
If symptoms persist or recur after completion of therapy, men should be instructed to return for re-evaluation. Symptoms alone, without documentation of signs or laboratory evidence of urethral inflammation, are not a sufficient basis for retreatment. Providers should be alert to the possible diagnosis of chronic prostatitis/chronic pelvic pain syndrome in men experiencing persistent perineal, penile, or pelvic pain or discomfort, voiding symptoms, pain during or after ejaculation, or new-onset premature ejaculation lasting for >3 months. Men with persistent pain should be referred to a urologist.
Management of Sex Partners
All sex partners of men with NGU within the preceding 60 days should be referred for evaluation, testing, and presumptive treatment with a drug regimen effective against chlamydia. EPT is an alternative approach to treating female partners for CT in the absence of signs and symptoms of PID (95). If N. gonorrhea or T. vaginalis is documented, all partners should be evaluated and treated according to the management section for their respective pathogen. To avoid reinfection, sex partners should abstain from sexual intercourse until they and their partner(s) are adequately treated.
Persistent and Recurrent NGU
The objective diagnosis of persistent or recurrent NGU should be made before considering additional antimicrobial therapy. In men who have persistent symptoms after treatment without objective signs of urethral inflammation, the value of extending the duration of antimicrobials has not been demonstrated. Men who have persistent or recurrent NGU can be retreated with the initial regimen if they did not comply with the treatment regimen or were re-exposed to an untreated sex partner.
Recent studies have shown that the most common cause of persistent or recurrent NGU is M. genitalium, especially following doxycycline therapy (277,278). Azithromycin 1 g orally in a single dose should be administered to men initially treated with doxycycline. Certain observational studies have shown that moxifloxacin 400 mg orally once daily for 7 days is highly effective against M. genitalium. Therefore, men who fail a regimen of azithromycin should be retreated with moxifloxacin 400 mg orally once daily for 7 days. Higher doses of azithromycin have not been found to be effective for M. genitalium in cases of azithromycin failure (280).
T. vaginalis is also known to cause urethritis in men who have sex with women. Although no NAAT for T. vaginalis detection in men has been FDA-cleared in the United States, several large reference laboratories have performed the necessary CLIA validation of a urine-based T. vaginalis NAAT for men for clinical use. Trichomonas NAAT testing is more sensitive than culture (475). In areas where T. vaginalis is prevalent, men who have sex with women and have persistent or recurrent urethritis should be presumptively treated with metronidazole 2 g orally in a single dose or tinidazole 2 g orally in a single dose; their partners should be referred for evaluation and appropriate treatment. Persons with persistent or recurrent NGU after presumptive treatment for M. genitalium or T. vaginalis should be referred to a urologist.
Special Considerations
HIV Infection
NGU might facilitate HIV transmission. Persons with NGU and HIV should receive the same treatment regimen as those who are HIV negative.
Cervicitis
Two major diagnostic signs characterize cervicitis: 1) a purulent or mucopurulent endocervical exudate visible in the endocervical canal or on an endocervical swab specimen (commonly referred to as mucopurulent cervicitis) and 2) sustained endocervical bleeding easily induced by gentle passage of a cotton swab through the cervical os. Either or both signs might be present. Cervicitis frequently is asymptomatic, but some women complain of an abnormal vaginal discharge and intermenstrual vaginal bleeding (e.g., after sexual intercourse). A finding of leukorrhea (>10 WBC per high-power field on microscopic examination of vaginal fluid) has been associated with chlamydial and gonococcal infection of the cervix. In the absence of the major diagnostic signs of inflammatory vaginitis, leukorrhea might be a sensitive indicator of cervical inflammation with a high negative predictive value (i.e., cervicitis is unlikely in the absence of leucorrhea) (482,483). The criterion of using an increased number of WBCs on endocervical Gram stain in the diagnosis of cervicitis has not been standardized and therefore is not helpful. In addition, it has a low positive-predictive value (PPV) for infection with C. trachomatis and N. gonorrhoeae and is not available in most clinical settings. Finally, although the presence of gram negative intracellular diplococci on Gram stain of endocervical fluid may be specific for the diagnosis of gonococcal cervical infection when evaluated by an experienced laboratorian, it is not a sensitive indicator of infection.
Etiology
When an etiologic organism is isolated in the presence of cervicitis, it is typically C. trachomatis or N. gonorrhoeae. Cervicitis also can accompany trichomoniasis and genital herpes (especially primary HSV-2 infection). However, in most cases of cervicitis, no organism is isolated, especially in women at relatively low risk for recent acquisition of these STDs (e.g., women aged >30 years) (484). Limited data indicate that infection with M. genitalium or BV and frequent douching might cause cervicitis (257–259,261,265,485–487). For reasons that are unclear, cervicitis can persist despite repeated courses of antimicrobial therapy. Because most persistent cases of cervicitis are not caused by recurrent or reinfection with C. trachomatis or N. gonorrhoeae, other factors (e.g., persistent abnormality of vaginal flora, douching [or exposure to other types of chemical irritants], or idiopathic inflammation in the zone of ectopy) might be involved.
Diagnostic Considerations
Because cervicitis might be a sign of upper-genital–tract infection (endometritis), women with a new episode of cervicitis should be assessed for signs of PID and should be tested for C. trachomatis and for N. gonorrhoeae with NAAT; such testing can be performed on either vaginal, cervical, or urine samples (394) (see Chlamydia and Gonorrhea Diagnostic Considerations). Women with cervicitis also should be evaluated for the presence of BV and trichomoniasis, and if these are detected, they should be treated. Because the sensitivity of microscopy to detect T. vaginalis is relatively low (approximately 50%), symptomatic women with cervicitis and negative microscopy for trichomonads should receive further testing (i.e., culture, NAAT or other FDA approved diagnostic test) (see Trichomoniasis, Diagnosis). A finding of >10 WBC per high power field in vaginal fluid, in the absence of trichomoniasis, might indicate endocervical inflammation caused specifically by C. trachomatis or N. gonorrhoeae (488,489). Although HSV-2 infection has been associated with cervicitis, the utility of specific testing (i.e., PCR, culture or serologic testing) for HSV-2 is unknown. FDA-cleared diagnostic tests for M. genitalium are not available.
Treatment
Several factors should affect the decision to provide presumptive therapy for cervicitis. Presumptive treatment with antimicrobials for C. trachomatis and N. gonorrhoeae should be provided for women at increased risk (e.g., those aged <25 years and those with a new sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection), especially if follow-up cannot be ensured or if testing with NAAT is not possible. Trichomoniasis and BV should also be treated if detected (see Bacterial Vaginosis and Trichomoniasis). For women at lower risk of STDs, deferring treatment until results of diagnostic tests are available is an option. If treatment is deferred and NAATs for C. trachomatis and N. gonorrhoeae are negative, a follow-up visit to see if the cervicitis has resolved can be considered.
| Recommended Regimens for Presumptive Treatment* |
|
|
| Azithromycin 1 g orally in a single dose |
| OR |
| Doxycycline 100 mg orally twice a day for 7 days |
|
|
| *Consider concurrent treatment for gonococcal infection if patient is at risk for gonorrhea or lives in a community where the prevalence of gonorrhea is high. |
Other Considerations
To minimize transmission and reinfection, women treated for cervicitis should be instructed to abstain from sexual intercourse until they and their partner(s) have been adequately treated (i.e., for 7 days after single-dose therapy or until completion of a 7-day regimen) and symptoms have resolved. Women who receive a diagnosis of cervicitis should be tested for HIV and syphilis.
Follow-Up
Women receiving treatment should return to their provider for a follow-up visit, allowing the provider to determine whether cervicitis has resolved. For women who are not treated, a follow-up visit gives providers an opportunity to communicate results of tests obtained as part of the cervicitis evaluation. Additional follow-up should be conducted as recommended for the infections identified. Women with a specific diagnosis of chlamydia, gonorrhea, or trichomonas should be offered partner services and instructed to return in 3 months after treatment for repeat testing because of high rates of reinfection, regardless of whether their sex partners were treated (480). If symptoms persist or recur, women should be instructed to return for re-evaluation.
Management of Sex Partners
Management of sex partners of women treated for cervicitis should be appropriate for the specific STD identified or suspected. All sex partners in the past 60 days should be referred for evaluation, testing, and presumptive treatment if chlamydia, gonorrhea, or trichomoniasis was identified or suspected in the women with cervicitis. EPT or other effective partner referral strategies (see Partner Services) are alternative approaches to treating male partners of women who have chlamydia or gonococcal infection (93–95). To avoid reinfection, sex partners should abstain from sexual intercourse until they and their partner(s) are adequately treated.
Persistent or Recurrent Cervicitis
Women with persistent or recurrent cervicitis despite having been treated should be reevaluated for possible re-exposure or treatment failure to gonorrhea or chlamydia. If relapse and/or reinfection with a specific STD have been excluded, BV is not present, and sex partners have been evaluated and treated, management options for persistent cervicitis are undefined; in addition, the utility of repeated or prolonged administration of antibiotic therapy for persistent symptomatic cervicitis remains unknown. The etiology of persistent cervicitis including the potential role of M. genitalium (490) is unclear. M. genitalium might be considered for cases of clinically significant cervicitis that persist after azithromycin or doxycycline therapy in which re-exposure to an infected partner or medical nonadherence is unlikely. In settings with validated assays, women with persistent cervicitis could be tested for M. genitalium with the decision to treat with moxifloxacin based on results of diagnostic testing (491). In treated women with persistent symptoms that are clearly attributable to cervicitis, referral to a gynecologic specialist can be considered.
Special Considerations
HIV Infection
Women with cervicitis and HIV infection should receive the same treatment regimen as those who are HIV negative. Cervicitis increases cervical HIV shedding. Treatment of cervicitis in women with HIV infection reduces HIV shedding from the cervix and might reduce HIV transmission to susceptible sex partners (492–496).
Pregnancy
Diagnosis and treatment of cervicitis in pregnant women does not differ from that in women that are not pregnant. For more information, see Cervicitis, sections on Diagnostic Considerations and Treatment.
Chlamydial Infections
Chlamydial Infections in Adolescents and Adults
Chlamydial infection is the most frequently reported infectious disease in the United States, and prevalence is highest in persons aged ≤24 years (118). Several sequelae can result from C. trachomatis infection in women, the most serious of which include PID, ectopic pregnancy, and infertility. Some women who receive a diagnosis of uncomplicated cervical infection already have subclinical upper-reproductive–tract infection.
Asymptomatic infection is common among both men and women. To detect chlamydial infections, health-care providers frequently rely on screening tests. Annual screening of all sexually active women aged <25 years is recommended, as is screening of older women at increased risk for infection (e.g., those who have a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection (108). Although CT incidence might be higher in some women aged ≥25 years in some communities, overall the largest burden of infection is among women aged <25 years.
Chlamydia screening programs have been demonstrated to reduce the rates of PID in women (497,498). Although evidence is insufficient to recommend routine screening for C. trachomatis in sexually active young men because of several factors (e.g., feasibility, efficacy, and cost-effectiveness), the screening of sexually active young men should be considered in clinical settings with a high prevalence of chlamydia (e.g., adolescent clinics, correctional facilities, and STD clinics) or in populations with high burden of infection (e.g., MSM) (108,121). Among women, the primary focus of chlamydia screening efforts should be to detect chlamydia, prevent complications, and test and treat their partners, whereas targeted chlamydia screening in men should only be considered when resources permit, prevalence is high, and such screening does not hinder chlamydia screening efforts in women (499,500). More frequent screening for some women (e.g., adolescents) or certain men (e.g., MSM) might be indicated.
Diagnostic Considerations
C. trachomatis urogenital infection can be diagnosed in women by testing first-catch urine or collecting swab specimens from the endocervix or vagina. Diagnosis of C. trachomatis urethral infection in men can be made by testing a urethral swab or first-catch urine specimen. NAATs are the most sensitive tests for these specimens and therefore are recommended for detecting C. trachomatis infection (394). NAATs that are FDA-cleared for use with vaginal swab specimens can be collected by a provider or self-collected in a clinical setting. Self-collected vaginal swab specimens are equivalent in sensitivity and specificity to those collected by a clinician using NAATs (501,502), and women find this screening strategy highly acceptable (503,504). Optimal urogenital specimen types for chlamydia screening using NAAT include first catch-urine (men) and vaginal swabs (women) (394). Rectal and oropharyngeal C. trachomatis infection in persons engaging in receptive anal or oral intercourse can be diagnosed by testing at the anatomic site of exposure. NAATs are not FDA-cleared for use with rectal or oropharyngeal swab specimens. However, NAATs have been demonstrated to have improved sensitivity and specificity compared with culture for the detection of C. trachomatis at rectal sites (505–507) and at oropharyngeal sites among men (505–508). Some laboratories have established CLIA-defined performance specifications when evaluating rectal and oropharyngeal swab specimens for C. trachomatis, thereby allowing results to be used for clinical management. Most persons with C. trachomatis detected at oropharyngeal sites do not have oropharyngeal symptoms. However, when gonorrhea testing is performed at the oropharyngeal site, chlamydia test results might be reported as well because some NAATs detect both bacteria from a single specimen. Data indicate that performance of NAATs on self-collected rectal swabs is comparable to clinician-collected rectal swabs, and this specimen collection strategy for rectal C. trachomatis screening is highly acceptable (509–511). Self-collected rectal swabs are a reasonable alternative to clinician-collected rectal swabs for C. trachomatis screening by NAAT, especially when clinicians are not available or when self collection is preferred over clinician collection. Previous evidence suggests that the liquid-based cytology specimens collected for Pap smears might be acceptable specimens for NAAT testing, although test sensitivity using these specimens might be lower than that associated with use of cervical or vaginal swab specimens (512); regardless, certain NAATs have been FDA-cleared for use on liquid-based cytology specimens.
Treatment
Treating persons infected with C. trachomatis prevents adverse reproductive health complications and continued sexual transmission, and treating their sex partners can prevent reinfection and infection of other partners. Treating pregnant women usually prevents transmission of C. trachomatis to neonates during birth. Chlamydia treatment should be provided promptly for all persons testing positive for infection; treatment delays have been associated with complications (e.g., PID) in a limited proportion of women (513).
| Recommended Regimens |
|
|
| Azithromycin 1 g orally in a single dose |
| OR |
| Doxycycline 100 mg orally twice a day for 7 days |
|
|
| Alternative Regimens |
|
|
| Erythromycin base 500 mg orally four times a day for 7 days |
| OR |
| Erythromycin ethylsuccinate 800 mg orally four times a day for 7 days |
| OR |
| Levofloxacin 500 mg orally once daily for 7 days |
| OR |
| Ofloxacin 300 mg orally twice a day for 7 days |
|
|
A meta-analysis of 12 randomized clinical trials of azithromycin versus doxycycline for the treatment of urogenital chlamydial infection demonstrated that the treatments were equally efficacious, with microbial cure rates of 97% and 98%, respectively (514). These studies were conducted primarily in populations with urethral and cervical infection in which follow-up was encouraged, adherence to a 7-day regimen was effective, and culture or EIA (rather than the more sensitive NAAT) was used for determining microbiological outcome. More recent retrospective studies have raised concern about the efficacy of azithromycin for rectal C. trachomatis infection (515,516), however, these studies have limitations, and prospective clinical trials comparing azithromycin versus doxycycline regimens for rectal C. trachomatis infection are needed.
Although the clinical significance of oropharyngeal C. trachomatis infection is unclear and routine oropharyngeal screening for CT is not recommended, available evidence suggests oropharyngeal C. trachomatis can be sexually transmitted to genital sites (152,517); therefore, detection of C. trachomatis from an oropharyngeal specimen should be treated with azithromycin or doxycycline. The efficacy of alternative antimicrobial regimens in resolving oropharyngeal chlamydia remains unknown.
In a double-blinded randomized control trial, a doxycycline delayed-release 200 mg tablet administered daily for 7 days was as effective as generic doxycycline 100 mg twice daily for 7 days for treatment of urogenital C. trachomatis infection in men and women and had a lower frequency of gastrointestinal side effects. However, this regimen is more costly than those that involve multiple daily doses (518). Delayed-release doxycycline (Doryx) 200 mg daily for 7 days might be an alternative regimen to the doxycycline 100 mg twice daily for 7 days for treatment of urogenital C. trachomatis infection. Erythromycin might be less efficacious than either azithromycin or doxycycline, mainly because of the frequent occurrence of gastrointestinal side effects that can lead to nonadherence with treatment. Levofloxacin and ofloxacin are effective treatment alternatives, but they are more expensive and offer no advantage in the dosage regimen. Other quinolones either are not reliably effective against chlamydial infection or have not been evaluated adequately.
Other Management Considerations
To maximize adherence with recommended therapies, onsite, directly observed single-dose therapy with azithromycin should always be available for persons for whom adherence with multiday dosing is a concern. In addition, for multidose regimens, the first dose should be dispensed on site and directly observed. To minimize disease transmission to sex partners, persons treated for chlamydia should be instructed to abstain from sexual intercourse for 7 days after single-dose therapy or until completion of a 7-day regimen and resolution of symptoms if present. To minimize risk for reinfection, patients also should be instructed to abstain from sexual intercourse until all of their sex partners are treated. Persons who receive a diagnosis of chlamydia should be tested for HIV, GC, and syphilis.
Follow-Up
Test-of-cure to detect therapeutic failure (i.e., repeat testing 3–4 weeks after completing therapy) is not advised for persons treated with the recommended or alterative regimens, unless therapeutic adherence is in question, symptoms persist, or reinfection is suspected. Moreover, the use of chlamydial NAATs at <3 weeks after completion of therapy is not recommended because the continued presence of nonviable organisms (394,395,519) can lead to false-positive results.
A high prevalence of C. trachomatis infection has been observed in women and men who were treated for chlamydial infection during the preceding several months (480,481,520–522). Most post-treatment infections do not result from treatment failure, but rather from reinfection caused by failure of sex partners to receive treatment or the initiation of sexual activity with a new infected partner, indicating a need for improved education and treatment of sex partners. Repeat infections confer an elevated risk for PID and other complications in women. Men and women who have been treated for chlamydia should be retested approximately 3 months after treatment, regardless of whether they believe that their sex partners were treated (480,481). If retesting at 3 months is not possible, clinicians should retest whenever persons next present for medical care in the 12-month period following initial treatment.
Management of Sex Partners
Sexual partners should be referred for evaluation, testing, and presumptive treatment if they had sexual contact with the partner during the 60 days preceding the patient’s onset of symptoms or chlamydia diagnosis. Although the exposure intervals defined for the identification of at-risk sex partners are based on limited data, the most recent sex partner should be evaluated and treated, even if the time of the last sexual contact was >60 days before symptom onset or diagnosis.
Among heterosexual patients, if health department partner management strategies (e.g., disease intervention specialists) are impractical or not available for persons with chlamydia and a provider is concerned that sex partners are unable to promptly access evaluation and treatment services, EPT should be considered as permitted by law (see Partner Services). Compared with standard patient referral of partners, this approach to therapy, which involves delivering the medication itself or a prescription, has been associated with decreased rates of persistent or recurrent chlamydia (93–95). Providers should also provide patients with written educational materials to give to their partner(s) about chlamydia in general, to include notification that partner(s) have been exposed and information about the importance of treatment. These materials also should inform partners about potential therapy-related allergies and adverse effects, along with symptoms suggestive of complications (e.g., testicular pain in men and pelvic or abdominal pain in women). EPT is not routinely recommended for MSM with chlamydia because of a high risk for coexisting infections (especially undiagnosed HIV) among their partners, and because data are limited regarding the effectiveness of this approach in reducing persistent or recurrent chlamydia among MSM. Having partners accompany patients when they return for treatment is another strategy that has been used to ensure partner treatment (See Partner Services). To avoid reinfection, sex partners should be instructed to abstain from sexual intercourse until they and their sex partners have been adequately treated (i.e., for 7 days after a single-dose regimen or after completion of a 7-day regimen) and have resolved any symptoms.
Special Considerations
Pregnancy
Doxycycline is contraindicated in the second and third trimesters of pregnancy. Human data suggest ofloxacin and levofloxacin present a low risk to the fetus during pregnancy, with a potential for toxicity during breastfeeding; however, data from animal studies raise concerns about cartilage damage to neonates (317). Thus, alternative drugs should be used to treat chlamydia in pregnancy. Clinical experience and published studies suggest that azithromycin is safe and effective (523–525). Test-of-cure to document chlamydial eradication (preferably by NAAT) 3–4 weeks after completion of therapy is recommended because severe sequelae can occur in mothers and neonates if the infection persists. In addition, all pregnant women who have chlamydial infection diagnosed should be retested 3 months after treatment. Detection of C. trachomatis infection at repeat screening during the third semester is not uncommon in adolescent and young adult women, including in those without C. trachomatis detected at the time of initial prenatal screening (526,527). Women aged <25 years and those at increased risk for chlamydia (e.g., those who have a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection) should be rescreened during the third trimester to prevent maternal postnatal complications and chlamydial infection in the infant (108).
| Recommended Regimens |
|
|
| Azithromycin 1 g orally in a single dose |
|
|
| Alternative Regimens |
|
|
| Amoxicillin 500 mg orally three times a day for 7 days |
| OR |
| Erythromycin base 500 mg orally four times a day for 7 days |
| OR |
| Erythromycin base 250 mg orally four times a day for 14 days |
| OR |
| Erythromycin ethylsuccinate 800 mg orally four times a day for 7 days |
| OR |
| Erythromycin ethylsuccinate 400 mg orally four times a day for 14 days |
|
|
Because of concerns about chlamydia persistence following exposure to penicillin-class antibiotics that has been demonstrated in animal and in vitro studies, amoxicillin is now considered an alternative therapy for C. trachomatis in pregnant women (528,529). The frequent gastrointestinal side effects associated with erythromycin can result in nonadherence with these alternative regimens. The lower dose 14-day erythromycin regimens can be considered if gastrointestinal tolerance is a concern. Erythromycin estolate is contraindicated during pregnancy because of drug-related hepatotoxicity.
HIV Infection
Persons who have chlamydia and HIV infection should receive the same treatment regimen as those who do not have HIV infection. For more information, see Chlamydia, Treatment.
Chlamydial Infections Among Neonates
Prenatal screening and treatment of pregnant women is the best method for preventing chlamydial infection among neonates. C. trachomatis infection of neonates results from perinatal exposure to the mother’s infected cervix. Although the efficacy of neonatal ocular prophylaxis with erythromycin ophthalmic ointments to prevent chlamydia ophthalmia is not clear, ocular prophylaxis with these agents prevents gonococcal ophthalmia and therefore should be administered (see Ophthalmia Neonatorum Caused by N. gonnorrhoeae).
Initial C. trachomatis neonatal infection involves the mucous membranes of the eye, oropharynx, urogenital tract, and rectum, although infection might be asymptomatic in these locations. Instead, C. trachomatis infection in neonates is most frequently recognized by conjunctivitis that develops 5–12 days after birth. C. trachomatis also can cause a subacute, afebrile pneumonia with onset at ages 1–3 months. Although C. trachomatis has been the most frequent identifiable infectious cause of ophthalmia neonatorum, neonatal chlamydial infections (including ophthalmia and pneumonia) have occurred less frequently since the institution of widespread prenatal screening and treatment of pregnant women.
Ophthalmia Neonatorum Caused by C. trachomatis
A chlamydial etiology should be considered for all infants aged ≤30 days that have conjunctivitis, especially if the mother has a history of chlamydia infection. These infants should receive evaluation and appropriate care and treatment.
Diagnostic Considerations
Sensitive and specific methods used to diagnose chlamydial ophthalmia in the neonate include both tissue culture and nonculture tests (e.g., direct fluorescence antibody [DFA] tests and NAAT). DFA is the only nonculture FDA-cleared test for the detection of chlamydia from conjunctival swabs; NAATs are not FDA-cleared for the detection of chlamydia from conjunctival swabs, and clinical laboratories must verify the procedure according to CLIA regulations. Specimens for culture isolation and nonculture tests should be obtained from the everted eyelid using a dacron-tipped swab or the swab specified by the manufacturer’s test kit; for culture and DFA, specimens must contain conjunctival cells, not exudate alone. Ocular specimens from neonates being evaluated for chlamydial conjunctivitis also should be tested for N. gonorrhoeae (see Ophthalmia Neonatorum Caused by N. gonnorrhoeae).
Treatment of Ophthalmia Neonatorum
| Recommended Regimen |
|
|
| Erythromycin base or ethylsuccinate 50 mg/kg/day orally divided into 4 doses daily for 14 days* |
|
|
| Alternative Regimen |
|
|
| Azithromycin suspension, 20 mg/kg/day orally, 1 dose daily for 3 days* |
|
|
| *An association between oral erythromycin and azithromycin and infantile hypertrophic pyloric stenosis (IHPS) has been reported in infants aged <6 weeks. Infants treated with either of these antimicrobials should be followed for signs and symptoms of IHPS. |
Although data on the use of azithromycin for the treatment of neonatal chlamydia infection are limited, available data suggest a short course of therapy might be effective (530). Topical antibiotic therapy alone is inadequate for treatment for ophthalmia neonatorum caused by chlamydia and is unnecessary when systemic treatment is administered.
Follow-Up
Because the efficacy of erythromycin treatment for ophthalmia neonatorum is approximately 80%, a second course of therapy might be required (531). Data on the efficacy of azithromycin for ophthalmia neonatorum are limited. Therefore, follow-up of infants is recommended to determine whether initial treatment was effective. The possibility of concomitant chlamydial pneumonia should be considered (see Infant Pneumonia Caused by C. trachomatis).
Management of Mothers and Their Sex Partners
Mothers of infants who have ophthalmia caused by chlamydia and the sex partners of these women should be evaluated and presumptively treated for chlamydia. For more information, see Chlamydial Infection in Adolescents and Adults.
Infant Pneumonia Caused by C. trachomatis
Chlamydia pneumonia in infants typically occurs at 1–3 months and is a subacute pneumonia. Characteristic signs of chlamydial pneumonia in infants include 1) a repetitive staccato cough with tachypnea and 2) hyperinflation and bilateral diffuse infiltrates on a chest radiograph. In addition, peripheral eosinophilia (≥400 cells/mm3) occurs frequently. Because clinical presentations differ, all infants aged 1–3 months suspected of having pneumonia (especially those whose mothers have a history of chlamydial infection) should be tested for C. trachomatis and treated if infected.
Diagnostic Considerations
Specimens for chlamydial testing should be collected from the nasopharynx. Tissue culture is the definitive standard diagnostic test for chlamydial pneumonia. Nonculture tests (e.g., DFA and NAAT) can be used. DFA is the only nonculture FDA-cleared test for the detection of C. trachomatis from nasopharyngeal specimens, but DFA of nasopharyngeal specimens has a lower sensitivity and specificity than culture. NAATs are not FDA-cleared for the detection of chlamydia from nasopharyngeal specimens, and clinical laboratories must verify the procedure according to CLIA regulations (394). Tracheal aspirates and lung biopsy specimens, if collected, should be tested for C. trachomatis.
Treatment
Because test results for chlamydia often are not available at the time that initial treatment decisions must be made, treatment for C. trachomatis pneumonia must frequently be based on clinical and radiologic findings, age of the infant (i.e., 1–3 months), and risk of chlamydia in the mother (i.e., age <25, multiple partners, and history of chlamydial infection). The results of tests for chlamydial infection assist in the management of an infant’s illness.
| Recommended Regimen |
|
|
| Erythromycin base or ethylsuccinate 50 mg/kg/day orally divided into 4 doses daily for 14 days |
|
|
| Alternative Regimen |
|
|
| Azithromycin 20 mg/kg/day orally, 1 dose daily for 3 days |
|
|
Follow-Up
Because the effectiveness of erythromycin in treating pneumonia caused by C. trachomatis is approximately 80%, a second course of therapy might be required (532). Data on the effectiveness of azithromycin in treating chlamydial pneumonia are limited. Follow-up of infants is recommended to determine whether the pneumonia has resolved, although some infants with chlamydial pneumonia continue to have abnormal pulmonary function tests later in childhood.
Management of Mothers and Their Sex Partners
Mothers of infants who have chlamydia pneumonia and the sex partners of these women should be evaluated, tested, and presumptively treated for chlamydia. For more information, see Chlamydial Infection in Adolescents and Adults.
Neonates Born to Mothers Who Have Chlamydial Infection
Neonates born to mothers who have untreated chlamydia are at high risk for infection; however, prophylactic antibiotic treatment is not indicated, as the efficacy of such treatment is unknown. Infants should be monitored to ensure appropriate treatment if symptoms develop.
Chlamydial Infections Among Infants and Children
Sexual abuse must be considered a cause of chlamydial infection in infants and children. However, perinatally transmitted C. trachomatis infection of the nasopharynx, urogenital tract, and rectum might persist for 2–3 years (see Sexual Assault or Abuse of Children).
Diagnostic Considerations
NAAT can be used for vaginal and urine specimens from girls (see Sexual Assault or Abuse of Children), although data are insufficient to recommend the use of NAAT in boys. Data also are lacking regarding use of NAAT for specimens from extragenital sites (rectum and pharynx) in boys and girls (394); other nonculture tests (e.g., DFA) are not recommended because of specificity concerns. Culture is still the preferred method for detection of urogenital C. trachomatis in boys and at extragenital sites in boys and girls.
| Recommended Regimen for Infants and Children Who Weigh <45 kg |
|
|
| Erythromycin base or ethylsuccinate 50 mg/kg/day orally divided into 4 doses daily for 14 days |
| Data are limited on the effectiveness and optimal dose of azithromycin for the treatment of chlamydial infection in infants and children who weigh <45 kg |
|
|
| Recommended Regimen for Children Who Weigh ≥45 kg but Who Are Aged <8 Years |
|
|
| Azithromycin 1 g orally in a single dose |
|
|
| Recommended Regimens for Children Aged ≥8 years |
|
|
| Azithromycin 1 g orally in a single dose |
| OR |
| Doxycycline 100 mg orally twice a day for 7 days |
|
|
Other Management Considerations
See Sexual Assault or Abuse of Children.
Follow-Up
A test-of-cure culture (repeat testing after completion of therapy) to detect therapeutic failure ensures treatment effectiveness. Therefore, a culture should be obtained at a follow-up visit approximately 2 weeks after treatment is completed.
Gonococcal Infections
Gonococcal Infections in Adolescents and Adults
In the United States, an estimated 820,000 new N. gonorrhoeae infections occur each year (533). Gonorrhea is the second most commonly reported communicable disease (118). Urethral infections caused by N. gonorrhoeae among men can produce symptoms that cause them to seek curative treatment soon enough to prevent sequelae, but often not soon enough to prevent transmission to others. Among women, gonococcal infections are commonly asymptomatic or might not produce recognizable symptoms until complications (e.g., PID) have occurred. PID can result in tubal scarring that can lead to infertility and ectopic pregnancy.
Annual screening for N. gonorrhoeae infection is recommended for all sexually active women aged <25 years and for older women at increased risk for infection (e.g., those who have a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has an STI) (108). Additional risk factors for gonorrhea include inconsistent condom use among persons who are not in mutually monogamous relationships, previous or coexisting sexually transmitted infections, and exchanging sex for money or drugs. Clinicians should consider the communities they serve and might opt to consult local public health authorities for guidance on identifying groups at increased risk. Gonococcal infection, in particular, is concentrated in specific geographic locations and communities. Subgroups of MSM are at high risk for gonorrhea infection and should be screened at sites of exposure (see MSM). Screening for gonorrhea in men and older women who are at low risk for infection is not recommended (108). A recent travel history with sexual contacts outside of the United States should be part of any gonorrhea evaluation.
Diagnostic Considerations
Specific microbiologic diagnosis of infection with N. gonorrhoeae should be performed in all persons at risk for or suspected to have gonorrhea; a specific diagnosis can potentially reduce complications, reinfections, and transmission. Culture and NAAT are available for the detection of genitourinary infection with N. gonorrhoeae (394); culture requires endocervical (women) or urethral (men) swab specimens. NAAT allows for the widest variety of FDA-cleared specimen types, including endocervical swabs, vaginal swabs, urethral swabs (men), and urine (from both men and women). However, product inserts for each NAAT manufacturer must be carefully consulted because collection methods and specimen types vary. Culture is available for detection of rectal, oropharyngeal, and conjunctival gonococcal infection, but NAAT is not FDA-cleared for use with these specimens. Some laboratories have met CLIA regulatory requirements and established performance specifications for using NAAT with rectal and oropharyngeal swab specimens that can inform clinical management. Certain NAATs that have been demonstrated to detect commensal Neisseria species might have comparable low specificity when testing oropharyngeal specimens for N. gonorrhoeae (394). The sensitivity of NAAT for the detection of N. gonorrhoeae in urogenital and nongenital anatomic sites is superior to culture, but varies by NAAT type (394,505–508). In cases of suspected or documented treatment failure, clinicians should perform both culture and antimicrobial susceptibility testing because nonculture tests cannot provide antimicrobial susceptibility results. Because N. gonorrhoeae has demanding nutritional and environmental growth requirements, optimal recovery rates are achieved when specimens are inoculated directly and when the growth medium is promptly incubated in an increased CO2 environment (394). Several non-nutritive swab transport systems are available that might maintain gonococcal viability for up to 48 hours in ambient temperatures (534–536).
Because of its high specificity (>99%) and sensitivity (>95%), a Gram stain of urethral secretions that demonstrates polymorphonuclear leukocytes with intracellular Gramnegative diplococci can be considered diagnostic for infection with N. gonorrhoeae in symptomatic men. However, because of lower sensitivity, a negative Gram stain should not be considered sufficient for ruling out infection in asymptomatic men. Detection of infection using Gram stain of endocervical, pharyngeal, and rectal specimens also is insufficient and is not recommended. MB/GV stain of urethral secretions is an alternative point-of-care diagnostic test with performance characteristics similar to Gram stain. Presumed gonococcal infection is established by documenting the presence of WBC containing intracellular purple diplococci in MB/GV smears.
Antimicrobial-Resistant N. gonorrhoeae
Gonorrhea treatment is complicated by the ability of N. gonorrhoeae to develop resistance to antimicrobials (537). In 1986, the Gonococcal Isolate Surveillance Project (GISP), a national sentinel surveillance system, was established to monitor trends in antimicrobial susceptibilities of urethral N. gonorrhoeae strains in the United States (538). The epidemiology of antimicrobial resistance guides decisions about gonococcal treatment recommendations and has evolved because of shifts in antimicrobial resistance patterns. In 2007, emergence of fluoroquinolone-resistant N. gonorrhoeae in the United States prompted CDC to cease recommending fluoroquinolones for treatment of gonorrhea, leaving cephalosporins as the only remaining class of antimicrobials available for treatment of gonorrhea in the United States (539). Reflecting concern about emerging gonococcal resistance, CDC’s 2010 STD treatment guidelines recommended dual therapy for gonorrhea with a cephalosporin plus either azithromycin or doxycycline, even if NAAT for C. trachomatis was negative at the time of treatment (1). However, during 2006–2011, the minimum concentrations of cefixime needed to inhibit in vitro growth of the N. gonorrhoeae strains circulating in the United States and many other countries increased, suggesting that the effectiveness of cefixime might be waning (118,540). In addition, treatment failures with cefixime or other oral cephalosporins have been reported in Asia (541– 544), Europe (545–549), South Africa (550), and Canada (551,552). Ceftriaxone treatment failures for pharyngeal infections have been reported in Australia (553,554), Japan (555), and Europe (556,557). As a result, CDC no longer recommends the routine use of cefixime as a first-line regimen for treatment of gonorrhea in the United States (540). In addition, U.S. gonococcal strains with elevated MICs to cefixime also are likely to be resistant to tetracyclines but susceptible to azithromycin (540). Consequently, only one regimen, dual treatment with ceftriaxone and azithromycin, is recommended for treatment of gonorrhea in the United States. CDC (http://www.cdc.gov/std/gisp) and state health departments can provide the most current information on gonococcal susceptibility.
Criteria for resistance to cefixime and ceftriaxone have not been defined by the Clinical and Laboratory Standards Institute (CLSI). However, isolates with cefixime or ceftriaxone MICs ≥0.5 μg/mL are considered to have decreased susceptibility (558). In the United States, the proportion of isolates in GISP demonstrating decreased susceptibility to ceftriaxone or cefixime has remained low; during 2013, no isolates with decreased susceptibility (MIC ≥0.5 μg/mL) to ceftriaxone or cefixime were identified (118). Because increasing MICs might predict the emergence of resistance, GISP established lower cephalosporin MIC breakpoints than those set by CLSI to provide greater sensitivity in detecting declining gonococcal susceptibility for surveillance purposes. The percentage of isolates with cefixime MICs ≥0.25 μg/mL increased from 0.1% in 2006 to 1.4% in 2011 (118,540), and declined to 0.4% in 2013 (118). The percentage of isolates with ceftriaxone MICs ≥0.125 μg/mL increased from <0.1% in 2006 to 0.4% in 2011 and decreased to 0.05% in 2013. Isolates with high-level cefixime and ceftriaxone MICs (cefixime MICs 1.5–8 μg/mL and ceftriaxone MICs 1.5–4 μg/mL) have been identified in Japan (555), France (549), and Spain (559,560). Decreased susceptibility of N. gonorrhoeae to cephalosporins and other antimicrobials is expected to continue; state and local surveillance for antimicrobial resistance is crucial for guiding local therapy recommendations (537). Although approximately 3% of all U.S. men who have gonococcal infections are sampled through GISP, surveillance by clinicians also is critical. Clinicians who diagnose N. gonorrhoeae infection in a person with suspected cephalosporin treatment failure should perform culture and antimicrobial susceptibility testing (AST) of relevant clinical specimens, consult an infectious-disease specialist for guidance in clinical management, and report the case to CDC through state and local public health authorities. Isolates should be saved and sent to CDC through local and state public health laboratory mechanisms. Health departments should prioritize notification and culture evaluation for sexual partner(s) of persons with N. gonorrhoeae infection thought to be associated with cephalosporin treatment failure or persons whose isolates demonstrate decreased susceptibility to cephalosporin.
Dual Therapy for Gonococcal Infections
On the basis of experience with other microbes that have developed antimicrobial resistance rapidly, a theoretical basis exists for combination therapy using two antimicrobials with different mechanisms of action (e.g., a cephalosporin plus azithromycin) to improve treatment efficacy and potentially slow the emergence and spread of resistance to cephalosporins. Use of azithromycin as the second antimicrobial is preferred to doxycycline because of the convenience and compliance advantages of single-dose therapy and the substantially higher prevalence of gonococcal resistance to tetracycline than to azithromycin among GISP isolates, particularly in strains with elevated cefixime MICs (118,540). In addition, clinical trials have demonstrated the efficacy of azithromycin 1 g for the treatment of uncomplicated urogenital GC (561,562).
Limited data suggest that dual treatment with azithromycin might enhance treatment efficacy for pharyngeal infection when using oral cephalosporins (563,564). In addition, persons infected with N. gonorrhoeae frequently are coinfected with C. trachomatis; this finding has led to the longstanding recommendation that persons treated for gonococcal infection also be treated with a regimen that is effective against uncomplicated genital C. trachomatis infection, further supporting the use of dual therapy that includes azithromycin (565).
Uncomplicated Gonococcal Infections of the Cervix, Urethra, and Rectum
| Recommended Regimen |
|
|
| Ceftriaxone 250 mg IM in a single dose |
| PLUS |
| Azithromycin 1g orally in a single dose |
|
|
As dual therapy, ceftriaxone and azithromycin should be administered together on the same day, preferably simultaneously and under direct observation. Ceftriaxone in a single injection of 250 mg provides sustained, high bactericidal levels in the blood. Extensive clinical experience indicates that ceftriaxone is safe and effective for the treatment of uncomplicated gonorrhea at all anatomic sites, curing 99.2% of uncomplicated urogenital and anorectal and 98.9% of pharyngeal infections in clinical trials (566,567). No clinical data exist to support use of doses of ceftriaxone >250 mg.
Single-dose injectable cephalosporin regimens (other than ceftriaxone 250 mg IM) that are safe and generally effective against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime (500 mg IM), cefoxitin (2 g IM with probenecid 1 g orally), and cefotaxime (500 mg IM). None of these injectable cephalosporins offer any advantage over ceftriaxone for urogenital infection, and efficacy for pharyngeal infection is less certain (566,567). Several other antimicrobials are active against N. gonorrhoeae, but none have substantial advantages over the recommended regimen, and efficacy data (especially for pharyngeal infection) are limited.
| Alternative Regimens |
|
|
| If ceftriaxone is not available: |
| Cefixime 400 mg orally in a single dose |
| PLUS |
| Azithromycin 1 g orally in a single dose |
|
|
A 400-mg oral dose of cefixime should only be considered as an alternative cephalosporin regimen because it does not provide as high, nor as sustained, bactericidal blood levels as a 250-mg dose of ceftriaxone; further, it demonstrates limited efficacy for treatment of pharyngeal gonorrhea (92.3% cure; 95% confidence interval [CI] = 74.9%–99.1%); in older clinical studies, cefixime cured 97.5% of uncomplicated urogenital and anorectal gonococcal infections (95% CI = 95.4%–99.8%) (566,567). The increase in the prevalence of isolates obtained through GISP with elevated cefixime MICs might indicate early stages of development of clinically significant gonococcal resistance to cephalosporins. CDC anticipates that rising cefixime MICs soon will result in declining effectiveness of cefixime for the treatment of urogenital gonorrhea. Furthermore, as cefixime becomes less effective, continued used of cefixime might hasten the development of resistance to ceftriaxone, a safe, well-tolerated, injectable cephalosporin and the last antimicrobial known to be highly effective in a single dose for treatment of gonorrhea at all anatomic sites of infection. Other oral cephalosporins (e.g., cefpodoxime and cefuroxime) are not recommended because of inferior efficacy and less favorable pharmacodynamics (566,568).
Because of the prevalence of tetracycline resistance among GISP isolates, particularly those with elevated cefixime MICs (118), the use of azithromycin as the second antimicrobial is preferred. However, in the case of azithromycin allergy, doxycycline (100 mg orally twice a day for 7 days) can be used in place of azithromycin as an alternative second antimicrobial when used in combination with ceftriaxone or cefixime.
In a recent clinical trial, dual treatment of uncomplicated, urogenital gonorrhea with single doses of oral gemifloxacin 320 mg plus oral azithromycin 2 g was associated with cure rates of 99.5% (lower one-sided 95% CI bound = 97.6%), and dual treatment with single doses of intramuscular gentamicin 240 mg plus oral azithromycin 2 g cured 100% of cases (lower one-sided 95% CI bound = 98.5%) (569). This trial was not powered to provide reliable estimates of the efficacy of these regimens for treatment of rectal or pharyngeal infection, but both regimens cured the few extragenital infections among study participants. Either of these regimens might be considered as alternative treatment options in the presence of cephalosporin allergy. However, gastrointestinal adverse events might limit their use: 7.7% of patients treated with gemifloxacin plus azithromycin and 3.3% of patients treated with gentamicin plus azithromycin vomited within 1 hour of medication administration, necessitating retreatment with a ceftriaxone and azithromycin.
Spectinomycin, which is useful in persons who cannot tolerate cephalosporins, is expensive, has poor efficacy against pharyngeal infection (51.8%; 95% CI = 38.7%–64.9%) (566), and is not being produced in the United States (570). However, it has been effective in clinical trials, curing 98.2% of uncomplicated urogenital and anorectal gonococcal infections (566). When available, spectinomycin is an effective alternative for the treatment of urogenital and anorectal infection.
Monotherapy with azithromycin 2 g orally as a single dose has been demonstrated to be 99.2% effective against uncomplicated urogenital gonorrhea (95% CI = 97.3%–99.9%) (567). However, monotherapy is no longer recommended because of concerns over the ease with which N. gonorrhoeae can develop resistance to macrolides, and because several studies have documented azithromycin treatment failures (546,571–574). Strains of N. gonorrhoeae circulating in the United States are not adequately susceptible to penicillins, tetracyclines, and older macrolides (e.g., erythromycin), and thus use of these antimicrobials cannot be recommended.
Uncomplicated Gonococcal Infections of the Pharynx
Most gonococcal infections of the pharynx are asymptomatic and can be relatively common in some populations (505,506,575,576). Gonococcal infections of the pharynx are more difficult to eradicate than are infections at urogenital and anorectal sites (551). Few antimicrobial regimens, including those involving oral cephalosporins, can reliably cure >90% of gonococcal pharyngeal infections (566,567). Providers should ask their patients with urogenital or rectal GC about oral sexual exposure; if reported, patients should be treated with a regimen with acceptable efficacy against pharyngeal gonorrhea infection.
| Recommended Regimen |
|
|
| Ceftriaxone 250 mg IM in a single dose |
| PLUS |
| Azithromycin 1 g orally in a single dose |
|
|
Other Management Considerations
To maximize adherence with recommended therapies and reduce complications and transmission, medication for gonococcal infection should be provided on site and directly observed. If medications are not available when treatment is indicated, linkage to an STD treatment facility should be provided for same-day treatment. To minimize disease transmission, persons treated for gonorrhea should be instructed to abstain from sexual activity for 7 days after treatment and until all sex partners are adequately treated (7 days after receiving treatment and resolution of symptoms, if present). All persons who receive a diagnosis of gonorrhea should be tested for other STDs, including chlamydia, syphilis, and HIV.
Follow-Up
A test-of-cure is not needed for persons who receive a diagnosis of uncomplicated urogenital or rectal gonorrhea who are treated with any of the recommended or alternative regimens; however, any person with pharyngeal gonorrhea who is treated with an alternative regimen should return 14 days after treatment for a test-of-cure using either culture or NAAT. If the NAAT is positive, effort should be made to perform a confirmatory culture before retreatment. All positive cultures for test-of-cure should undergo antimicrobial susceptibility testing.
Symptoms that persist after treatment should be evaluated by culture for N. gonorrhoeae (with or without simultaneous NAAT), and any gonococci isolated should be tested for antimicrobial susceptibility. Persistent urethritis, cervicitis, or proctitis also might be caused by other organisms (see Urethritis, Cervicitis, and Proctitis sections).
A high prevalence of N. gonorrhoeae infection has been observed among men and women previously treated for gonorrhea (86,480,481,577). Rather than signaling treatment failure, most of these infections result from reinfection caused by failure of sex partners to receive treatment or the initiation of sexual activity with a new infected partner, indicating a need for improved patient education and treatment of sex partners. Men or women who have been treated for gonorrhea should be retested 3 months after treatment regardless of whether they believe their sex partners were treated. If retesting at 3 months is not possible, clinicians should retest whenever persons next present for medical care within 12 months following initial treatment.
Management of Sex Partners
Recent sex partners (i.e., persons having sexual contact with the infected patient within the 60 days preceding onset of symptoms or gonorrhea diagnosis) should be referred for evaluation, testing, and presumptive dual treatment. If the patient’s last potential sexual exposure was >60 days before onset of symptoms or diagnosis, the most recent sex partner should be treated. To avoid reinfection, sex partners should be instructed to abstain from unprotected sexual intercourse for 7 days after they and their sexual partner(s) have completed treatment and after resolution of symptoms, if present.
For heterosexual men and women with gonorrhea for whom health department partner-management strategies are impractical or unavailable and whose providers are concerned about partners’ access to prompt clinical evaluation and treatment, EPT with cefixime 400 mg and azithromycin 1 g can be delivered to the partner by the patient, a disease investigation specialist, or a collaborating pharmacy as permitted by law (see Partner Services). With this approach, provision of medication must be accompanied by written materials (93,95) to educate partners about their exposure to gonorrhea, the importance of therapy, and when to seek clinical evaluation for adverse reactions or complications. Educational materials for female partners should include information about the importance of seeking medical evaluation for PID (especially if symptomatic); undertreatment of PID in female partners and missed opportunities to diagnose other STDs in women are of concern. EPT should not be considered a routine partner management strategy in MSM with gonorrhea because of a high risk for coexisting infections (especially HIV infection) and because no data exist on efficacy in this population.
Special Considerations
Allergy, Intolerance, and Adverse Reactions
Allergic reactions to first-generation cephalosporins occur in <2.5% of persons with a history of penicillin allergy and are uncommon with third-generation cephalosporins (e.g., ceftriaxone and cefixime) (428,430,464). Use of ceftriaxone or cefixime is contraindicated in persons with a history of an IgE-mediated penicillin allergy (e.g., anaphylaxis, Stevens Johnson syndrome, and toxic epidermal necrolysis) (428,431). Data are limited regarding alternative regimens for treating gonorrhea among persons who have either a cephalosporin or IgE-mediated penicillin allergy. Potential therapeutic options are dual treatment with single doses of oral gemifloxacin 320 mg plus oral azithromycin 2 g or dual treatment with single doses of intramuscular gentamicin 240 mg plus oral azithromycin 2 g (569). Spectinomycin for treatment of urogenital and anorectal gonorrhea can be considered when available. Providers treating persons with cephalosporin or IgE-mediated penicillin allergy should consult an infectious-disease specialist.
Pregnancy
Pregnant women infected with N. gonorrhoeae should be treated with dual therapy consisting of ceftriaxone 250 mg in a single IM dose and azithromycin 1 g orally as a single dose. When cephalosporin allergy or other considerations preclude treatment with this regimen and spectinomycin is not available, consultation with an infectious-disease specialist is recommended.
HIV Infection
Persons who have gonorrhea and HIV infection should receive the same treatment regimen as those who are HIV negative. For more information, see appropriate treatment sections under Gonoccocal Infections.
Suspected Cephalosporin Treatment Failure
Cephalosporin treatment failure is the persistence of N. gonorrhoeae infection despite appropriate cephalosporin treatment and is indicative of infection with cephalosporin-resistant gonorrhea in persons whose partners were adequately treated and whose risk for reinfection is low. Suspected treatment failure has been reported among persons receiving oral and injectable cephalosporins (541–557,578). Treatment failure should be considered in 1) persons whose symptoms do not resolve within 3–5 days after appropriate treatment and report no sexual contact during the post-treatment follow-up period and 2) persons with a positive test-of-cure (i.e., positive culture ≥72 hours or positive NAAT ≥7 days after receiving recommended treatment) when no sexual contact is reported during the post-treatment follow-up period (579). Treatment failure should also be considered in persons who have a positive culture on test-of-cure (if obtained) if there is evidence of decreased susceptibility to cephalosporins on antimicrobial susceptibility testing, regardless of whether sexual contact is reported during the post-treatment follow-up period.
Most suspected treatment failures in the United States are likely to be re-infections rather than actual treatment failures (86,480,481,577). However, in cases where reinfection is unlikely and treatment failure is suspected, before retreatment, relevant clinical specimens should be obtained for culture (preferably with simultaneous NAAT) and antimicrobial susceptibility testing if N. gonorrhoeae is isolated. Phenotypic antimicrobial susceptibility testing should be performed using disk diffusion, Etest (BioMérieux, Durham, NC), or agar dilution. Data are limited on the use of DNA amplification and sequencing for detection of genetic mutations associated with gonococcal antimicrobial resistance. All isolates of suspected treatment failures should be sent to CDC for antimicrobial susceptibility testing by agar dilution; local laboratories should store isolates for possible further testing if needed. Testing and/or storage of specimens or isolates should be facilitated by the state or local health department according to local public health protocol.
For persons with suspected cephalosporin treatment failure, the treating clinician should consult an infectious-disease specialist, an STD/HIV Prevention Training Center clinical expert (http://www.nnptc.org), the local or state health department STD program, or CDC (telephone: 404-639-8659) for advice on obtaining cultures, antimicrobial susceptibility testing, and treatment. Suspected treatment failure should be reported to CDC through the local or state health department within 24 hours of diagnosis.
Suspected treatment failures first should be retreated routinely with the recommended regimen (ceftriaxone 250 mg IM plus azithromycin 1 g orally), because reinfections are more likely than actual treatment failures. However, in situations with a higher likelihood of treatment failure than reinfection, relevant clinical specimens should be obtained for culture (preferably with simultaneous NAAT) and antimicrobial susceptibility testing performed before retreatment. Dual treatment with single doses of oral gemifloxacin 320 mg plus oral azithromycin 2 g or dual treatment with single doses of intramuscular gentamicin 240 mg plus oral azithromycin 2 g can be considered, particularly when isolates are found to have elevated cephalosporin MICs (569). Persons with suspected treatment failure after treatment with the alternative regimen (cefixime and azithromycin) should be treated with ceftriaxone 250 mg as a single IM dose and azithromycin 2 g orally as a single dose. A test-of-cure at relevant clinical sites should be obtained 7–14 days after retreatment; culture is the recommended test, preferably with simultaneous NAAT and antimicrobial susceptibility testing of N. gonorrhoeae if isolated. Clinicians should ensure that the patient’s sex partners from the preceding 60 days are evaluated promptly with culture and presumptively treated using the same regimen used for the patient.
Gonococcal Conjunctivitis
In the only published study (conducted in 1989) of the treatment of gonococcal conjunctivitis among adults, all 12 study participants responded to a single 1 g IM injection of ceftriaxone (580). On the basis of experience with other microbes that have developed antimicrobial resistance rapidly, a theoretical basis exists for combination therapy using two antimicrobials with different mechanisms of action (e.g., a cephalosporin plus azithromycin) to improve treatment efficacy and potentially slow the emergence and spread of resistance to cephalosporins. Because gonococcal conjunctivitis is uncommon and data on treatment of gonococcal conjunctivitis in adults are limited, consultation with an infectious-disease specialist should be considered.
| Recommended Regimen |
|
|
| Ceftriaxone 1 g IM in a single dose |
| PLUS |
| Azithromycin 1 g orally in a single dose |
|
|
Consider one-time lavage of the infected eye with saline solution.
Management of Sex Partners
Patients should be instructed to refer their sex partners for evaluation and treatment. For more information, see Gonococcal Infections, Management of Sex Partners.
Disseminated Gonococcal Infection
Disseminated gonococcal infection (DGI) frequently results in petechial or pustular acral skin lesions, asymmetric polyarthralgia, tenosynovitis, or oligoarticular septic arthritis (581). The infection is complicated occasionally by perihepatitis and rarely by endocarditis or meningitis. Some strains of N. gonorrhoeae that cause DGI can cause minimal genital inflammation. If DGI is suspected, NAAT or culture specimens from urogenital and extragenital sites, as applicable, should be collected and processed in addition to specimens from disseminated sites of infection (e.g., skin, synovial fluid, blood, and the CNS). All N. gonorrhoeae isolates should be tested for antimicrobial susceptibility.
Hospitalization and consultation with an infectious-disease specialist are recommended for initial therapy, especially for persons who might not comply with treatment, have an uncertain diagnosis, or have purulent synovial effusions or other complications. Examination for clinical evidence of endocarditis and meningitis should be performed.
Treatment of Arthritis and Arthritis-Dermatitis Syndrome
| Recommended Regimen |
|
|
| Ceftriaxone 1 g IM or IV every 24 hours |
| PLUS |
| Azithromycin 1 g orally in a single dose |
|
|
| Alternative Regimens |
|
|
| Cefotaxime 1 g IV every 8 hours |
| OR |
| Ceftizoxime 1 g IV every 8 hours |
| PLUS |
| Azithromycin 1 g orally in a single dose |
|
|
When treating for the arthritis-dermatitis syndrome, the provider can switch to an oral agent guided by antimicrobial susceptibility testing 24–48 hours after substantial clinical improvement, for a total treatment course of at least 7 days.
Treatment of Gonococcal Meningitis and Endocarditis
| Recommended Regimen |
|
|
| Ceftriaxone 1–2 g IV every 12–24 hours |
| PLUS |
| Azithromycin 1 g orally in a single dose |
|
|
No recent studies have been published on the treatment of DGI. The duration of treatment of DGI has not been systematically studied and should be determined in consultation with an infectious-disease specialist. Treatment for DGI should be guided by the results of antimicrobial susceptibility testing. Pending antimicrobial susceptibility results, treatment decisions should be made on the basis of clinical presentation. Therapy for meningitis should be continued with recommended parenteral therapy for 10–14 days. Parenteral antimicrobial therapy for endocarditis should be administered for at least 4 weeks.
Management of Sex Partners
Gonococcal infection frequently is asymptomatic in sex partners of persons who have DGI. Providers should instruct patients to refer partners with whom they have had sexual contact in the past 60 days for evaluation, testing, and presumptive treatment (see Gonococcal Infection, Management of Sex Partners).
Gonococcal Infections Among Neonates
Prenatal screening and treatment of pregnant women is the best method for preventing GC infection among neonates. Gonococcal infection among neonates results from perinatal exposure to the mother’s infected cervix. It is usually an acute illness that manifests 2–5 days after birth. The prevalence of infection among infants depends on the prevalence of infection among pregnant women, whether pregnant women are screened and treated for gonorrhea, and whether newborns receive ophthalmia prophylaxis. The most severe manifestations of N. gonorrhoeae infection in newborns are ophthalmia neonatorum and sepsis, which can include arthritis and meningitis. Less severe manifestations include rhinitis, vaginitis, urethritis, and infection at sites of fetal monitoring.
Ophthalmia Neonatorum Prophylaxis
To prevent gonococcal ophthalmia neonatorum, a prophylactic agent should be instilled into both eyes of all newborn infants; this procedure is required by law in most states. Ocular prophylaxis is warranted because it can prevent sight-threatening gonococcal ophthalmia, has an excellent safety record, is easy to administer, and is inexpensive. The recommended prophylactic regimen prevents gonococcal ophthalmia; however, its efficacy for prevention of chlamydial ophthalmia is less clear, and it does not eliminate nasopharyngeal colonization by C. trachomatis.
| Recommended Regimen |
|
|
| Erythromycin (0.5%) ophthalmic ointment in each eye in a single application at birth |
|
|
This preparation should be instilled into both eyes of all neonates as soon as possible after delivery, regardless of whether they are delivered vaginally or by cesarean section. Ideally, ointment should be applied using single-use tubes or ampules rather than multiple-use tubes. If prophylaxis is delayed (i.e., not administered in the delivery room), a monitoring system should be established to ensure that all infants receive prophylaxis.
Erythromycin is the only antibiotic ointment recommended for use in neonates. Silver nitrate and tetracycline ophthalmic ointment is no longer manufactured in the United States, bacitracin is not effective, and povidone iodine has not been studied adequately (582,583). Gentamicin ophthalmic ointment has been associated with severe ocular reactions in neonates and should not be used for ocular prophylaxis (584,585). If erythromycin ointment is not available, infants at risk for exposure to N. gonorrhoeae (especially those born to a mother at risk for gonococcal infection or with no prenatal care) can be administered ceftriaxone 25–50 mg/kg IV or IM, not to exceed 125 mg in a single dose (586).
N. gonorrhoeae causes ophthalmia neonatorum relatively infrequently in the United States (587). However, identifying and treating this infection is especially important, because ophthalmia neonatorum can result in perforation of the globe of the eye and blindness (588).
Diagnostic Considerations
Infants at increased risk for gonococcal ophthalmia include those who did not receive ophthalmia prophylaxis and whose mothers had no prenatal care or have a history of STDs or substance abuse. Gonococcal ophthalmia is strongly suspected when intracellular gram-negative diplococci are identified on Gram stain of conjunctival exudate, justifying presumptive treatment for gonorrhea after appropriate cultures and antimicrobial susceptibility testing for N. gonorrhoeae are performed. Presumptive treatment for N. gonorrhoeae might be indicated for newborns at increased risk for gonococcal ophthalmia who have increased WBCs (but not intracellular gram negative diplococci) in a Gram-stained smear of conjunctival exudate. Nongonococcal causes of neonatal ophthalmia include Moraxella catarrhalis and other Neisseria species, organisms that are indistinguishable from N. gonorrhoeae on Gram-stained smear but can be differentiated in the microbiology laboratory.
Treatment
| Recommended Regimen |
|
|
| Ceftriaxone 25–50 mg/kg IV or IM in a single dose, not to exceed 125 mg |
|
|
One dose of ceftriaxone is adequate therapy for gonococcal conjunctivitis. Ceftriaxone should be administered cautiously to hyperbilirubinemic infants, especially those born prematurely. No data exist on the use of dual therapy for the treatment of gonococcal ophthalmia. Topical antibiotic therapy alone is inadequate and unnecessary if systemic treatment is administered.
Other Management Considerations
Appropriate chlamydial testing should be done simultaneously from the inverted eyelid specimen (see Ophthalmia Neonatorum Caused by C. trachomatis). Infants who have gonococcal ophthalmia should be evaluated for signs of disseminated infection (e.g., sepsis, arthritis, and meningitis). Infants who have gonococcal ophthalmia should be managed in consultation with an infectious-disease specialist.
Follow-up
Infants who have ophthalmia neonatorum should be managed in consultation with an infectious-disease specialist.
Management of Mothers and Their Sex Partners
Mothers of infants with ophthalmia neonatorum caused by N. gonorrhoeae should be evaluated, tested, and presumptively treated for gonorrhea, along with their sex partner(s). For more information, see Gonococcal Infections in Adolescents and Adults.
DGI and Gonococcal Scalp Abscesses in Neonates
DGI might present as sepsis, arthritis, or meningitis and is a rare complication of neonatal gonococcal infection. Localized gonococcal infection of the scalp can result from fetal monitoring through scalp electrodes. Detection of gonococcal infection in neonates who have sepsis, arthritis, meningitis, or scalp abscesses requires cultures of blood, CSF, and joint aspirate. Specimens obtained from the conjunctiva, vagina, oropharynx, and rectum are useful for identifying the primary site(s) of infection. Antimicrobial susceptibility testing of all isolates should be performed. Positive Gram-stained smears of exudate, CSF, or joint aspirate provide a presumptive basis for initiating treatment for N. gonorrhoeae.
| Recommended Regimens |
|
|
| Ceftriaxone 25–50 mg/kg/day IV or IM in a single daily dose for 7 days, with a duration of 10–14 days if meningitis is documented |
| OR |
| Cefotaxime 25 mg/kg IV or IM every 12 hours for 7 days, with a duration of 10–14 days if meningitis is documented |
|
|
Ceftriaxone should be administered cautiously to hyperbilirubinemic infants, especially those born prematurely. No data exist on the use of dual therapy for the treatment of DGI or gonococcal scalp abscesses.
Other Management Considerations
Appropriate chlamydial testing should be done simultaneously in neonates with gonococcal infection. For more information, see Chlamydia Infection in Neonates. Infants who have DGI should be managed in consultation with an infectious-disease specialist.
Management of Mothers and Their Sex Partners
Mothers of infants who have DGI or scalp abscesses caused by N. gonorrhoeae should be evaluated, tested, and presumptively treated for gonorrhea, along with their sex partner(s). For more information, see Gonococcal Infections in Adolescents and Adults.
Neonates Born to Mothers Who Have Gonococcal Infection
Neonates born to mothers who have untreated gonorrhea are at high risk for infection. Neonates should be tested for gonorrhea at exposed sites and treated presumptively for gonorrhea as recommended in these guidelines. No data exist on the use of dual therapy to treat neonates born to mothers who have gonococcal infection.
| Recommended Regimen in the Absence of Signs of Gonococcal Infection |
|
|
| Ceftriaxone 25–50 mg/kg IV or IM in a single dose, not to exceed 125 mg |
|
|
Other Management Considerations
Appropriate chlamydial testing should be done simultaneously in neonates with gonococcal infection. For more information, see Chlamydia Infection in Neonates. Follow-up examination is not required.
Management of Mothers and Their Sex Partners
Mothers who have gonorrhea and their sex partners should be evaluated, tested, and presumptively treated for gonorrhea. For more information, see Gonococcal Infections.
Gonococcal Infections Among Infants and Children
Sexual abuse is the most frequent cause of gonococcal infection in infants and children (see Sexual Assault or Abuse of Children). For preadolescent girls, vaginitis is the most common manifestation of this infection; gonococcal-associated PID after vaginal infection can be less common in preadolescents than adults. Among sexually abused children, anorectal and pharyngeal infections with N. gonorrhoeae are frequently asymptomatic.
Diagnostic Considerations
NAAT can be used to test vaginal and urine specimens from girls (see Sexual Assault or Abuse of Children), although data are insufficient to recommend the use of these tests in boys and from extragenital sites (rectum and pharynx) in boys and girls (394). Culture remains the preferred method for diagnosing boys and for detecting infection in specimens obtained from extragenital sites regardless of gender (394). Gram stains are inadequate for evaluating prepubertal children for gonorrhea and should not be used to diagnose or exclude gonorrhea. If evidence of disseminated gonococcal infection exists, gonorrhea culture and antimicrobial susceptibility testing should be obtained from relevant clinical sites (see DGI).
| Recommended Regimen for Infants and Children Who Weigh ≤45 kg and Who Have Uncomplicated Gonococcal Vulvovaginitis, Cervicitis, Urethritis, Pharyngitis, or Proctitis |
|
|
| Ceftriaxone 25–50 mg/kg IV or IM in a single dose, not to exceed 125 mg IM |
|
|
| Recommended Regimen for Children Who Weigh >45 kg and Who Have Uncomplicated Gonococcal Vulvovaginitis, Cervicitis, Urethritis, Pharyngitis, or Proctitis |
|
|
| Treat with one of the regimens recommended for adults (see Gonococcal Infections) |
|
|
| Recommended Regimen for Children Who Weigh ≤45 kg and Who Have Bacteremia or Arthritis |
|
|
| Ceftriaxone 50 mg/kg (maximum dose: 1 g) IM or IV in a single dose daily for 7 days |
|
|
| Recommended Regimen for Children Who Weigh >45 kg and Who Have Bacteremia or Arthritis |
|
|
| Ceftriaxone 1 g IM or IV in a single dose daily every 24 hours for 7 days |
|
|
No data exist regarding the use of dual therapy for treating children with gonococcal infection.
Other Management Considerations
Follow-up cultures are unnecessary. Only parenteral cephalosporins (i.e., ceftriaxone) are recommended for use in children. All children found to have gonococcal infections should be tested for C. trachomatis, syphilis, and HIV. For a discussion of concerns regarding sexual assault, see Sexual Assault or Abuse of Children.
Diseases Characterized by Vaginal Discharge
Most women will have a vaginal infection, characterized by discharge, itching, or odor, during their lifetime. With the availability of complementary and alternative therapies and over-the-counter medications for candidiasis, many symptomatic women seek these products before or in addition to an evaluation by a medical provider.
Obtaining a medical history alone has been shown to be insufficient for accurate diagnosis of vaginitis and can lead to the inappropriate administration of medication. Therefore, a careful history, examination, and laboratory testing to determine the etiology of vaginal symptoms are warranted. Information on sexual behaviors and practices, gender of sex partners, menses, vaginal hygiene practices (e.g., douching), and self-treatment with medications should be elicited. The three diseases most frequently associated with vaginal discharge are BV (replacement of the vaginal flora by an overgrowth of anaerobic bacteria including Prevotella sp., Mobiluncus sp., G. vaginalis, Ureaplasma, Mycoplasma, and numerous fastidious or uncultivated anaerobes), T. vaginalis, and candidiasis. Cervicitis can also cause an abnormal discharge. Although vulvovaginal candidiasis (VVC) is usually not transmitted sexually, it is included in this section because it is frequently diagnosed in women who have vaginal symptoms or are being evaluated for STDs.
Various diagnostic methods are available to identify the etiology of an abnormal vaginal discharge. Clinical laboratory testing can identify the cause of vaginitis in most women and is discussed in detail in the sections of this report dedicated to each condition. In the clinician’s office, the cause of vaginal symptoms might be determined by pH, a potassium hydroxide (KOH) test, and microscopic examination of fresh samples of the discharge. The pH of the vaginal secretions can be determined by narrow-range pH paper; an elevated pH (i.e., ≥4.5) is common with BV or trichomoniasis. Because pH testing is not highly specific, discharge should be further examined microscopically by first diluting one sample in one or two drops of 0.9% normal saline solution on one slide and a second sample in 10% KOH solution (samples that emit an amine odor immediately upon application of KOH suggest BV or trichomoniasis). Coverslips are then placed on the slides, and they are examined under a microscope at low and high power.
The saline-solution specimen might show motile trichomonads or “clue cells” (i.e., epithelial cells with borders obscured by small bacteria), which are characteristic of BV. The KOH specimen typically is used to identify hyphae or blastospores seen with candidiasis. However, the absence of trichomonads in saline or fungal elements in KOH samples does not rule out these infections, because the sensitivity of microscopy is approximately 50% compared with NAAT (trichomoniasis) or culture (yeast) (475). The presence of WBCs without evidence of trichomonads or yeast may also suggest cervicitis (see Cervicitis).
In settings where pH paper, KOH, and microscopy are not available, alternative commercially available point-of-care tests or clinical laboratory testing can be used to diagnose vaginitis. The presence of objective signs of vulvar inflammation in the absence of vaginal pathogens after laboratory testing suggests the possibility of mechanical, chemical, allergic, or other noninfectious causes of vulvovaginal signs or symptoms. In patients with persistent symptoms and no clear etiology, referral to a specialist may be helpful.
Bacterial Vaginosis
BV is a polymicrobial clinical syndrome resulting from replacement of the normal hydrogen peroxide producing Lactobacillus sp. in the vagina with high concentrations of anaerobic bacteria (e.g., Prevotella sp. and Mobiluncus sp.), G. vaginalis, Ureaplasma, Mycoplasma, and numerous fastidious or uncultivated anaerobes. Some women experience transient vaginal microbial changes, whereas others experience them for longer intervals of time. Among women presenting for care, BV is the most prevalent cause of vaginal discharge or malodor; however, in a nationally representative survey, most women with BV were asymptomatic (203).
BV is associated with having multiple male or female partners, a new sex partner, douching, lack of condom use, and lack of vaginal lactobacilli; women who have never been sexually active are rarely affected (589). The cause of the microbial alteration that precipitates BV is not fully understood, and whether BV results from acquisition of a single sexually transmitted pathogen is not known. Nonetheless, women with BV are at increased risk for the acquisition of some STDs (e.g., HIV, N. gonorrhoeae, C. trachomatis, and HSV-2), complications after gynecologic surgery, complications of pregnancy, and recurrence of BV (590–593). BV also increases the risk for HIV transmission to male sex partners (594). Although BV-associated bacteria can be found in the male genitalia, treatment of male sex partners has not been beneficial in preventing the recurrence of BV (595).
Diagnostic Considerations
BV can be diagnosed by the use of clinical criteria (i.e., Amsel’s Diagnostic Criteria) (596) or Gram stain. A Gram stain (considered the gold standard laboratory method for diagnosing BV) is used to determine the relative concentration of lactobacilli (i.e., long Gram-positive rods), Gram-negative and Gram-variable rods and cocci (i.e., G vaginalis, Prevotella, Porphyromonas, and peptostreptococci), and curved Gram-negative rods (i.e., Mobiluncus) characteristic of BV. Clinical criteria require three of the following symptoms or signs:
homogeneous, thin, white discharge that smoothly coats the vaginal walls;
clue cells (e.g., vaginal epithelial cells studded with adherent coccoobacilli) on microscopic examination;
pH of vaginal fluid >4.5; or
a fishy odor of vaginal discharge before or after addition of 10% KOH (i.e., the whiff test).
Detection of three of these criteria has been correlated with results by Gram stain (597). Other tests, including Affirm VP III (Becton Dickinson, Sparks, MD), a DNA hybridization probe test for high concentrations of G vaginalis, and the OSOM BV Blue test (Sekisui Diagnostics, Framingham, MA), which detects vaginal fluid sialidase activity, have acceptable performance characteristics compared with Gram stain. Although a prolineaminopeptidase card test is available for the detection of elevated pH and trimethylamine, it has low sensitivity and specificity and therefore is not recommended. PCR has been used in research settings for the detection of a variety of organisms associated with BV, but evaluation of its clinical utility is still underway. Detection of specific organisms might be predictive of BV by PCR (598,599). Additional validation is needed before these tests can be recommended to diagnose BV. Culture of G vaginalis is not recommended as a diagnostic tool because it is not specific. Cervical Pap tests have no clinical utility for the diagnosis of BV because of their low sensitivity and specificity.
Treatment
Treatment is recommended for women with symptoms. The established benefits of therapy in nonpregnant women are to relieve vaginal symptoms and signs of infection. Other potential benefits to treatment include reduction in the risk for acquiring C. trachomatis, N. gonorrhoeae, T. vaginalis, HIV, and herpes simplex type 2 (592,593,600).
| Recommended Regimens |
|
|
| Metronidazole 500 mg orally twice a day for 7 days |
| OR |
| Metronidazole gel 0.75%, one full applicator (5 g) intravaginally, once a day for 5 days |
| OR |
| Clindamycin cream 2%, one full applicator (5 g) intravaginally at bedtime for 7 days |
|
|
Alcohol consumption should be avoided during treatment with nitroimidazoles. To reduce the possibility of a disulfiram-like reaction, abstinence from alcohol use should continue for 24 hours after completion of metronidazole. Clindamycin cream is oil-based and might weaken latex condoms and diaphragms for 5 days after use (refer to clindamycin product labeling for additional information).
Women should be advised to refrain from sexual activity or use condoms consistently and correctly during the treatment regimen. Douching might increase the risk for relapse, and no data support the use of douching for treatment or relief of symptoms.
| Alternative Regimens |
|
|
| Tinidazole 2 g orally once daily for 2 days |
| OR |
| Tinidazole 1 g orally once daily for 5 days |
| OR |
| Clindamycin 300 mg orally twice daily for 7 days |
| OR |
| Clindamycin ovules 100 mg intravaginally once at bedtime for 3 days* |
|
|
| *Clindamycin ovules use an oleaginous base that might weaken latex or rubber products (e.g., condoms and vaginal contraceptive diaphragms). Use of such products within 72 hours following treatment with clindamycin ovules is not recommended. |
Alcohol consumption should be avoided during treatment with nitroimidazoles. To reduce the possibility of a disulfiram-like reaction, abstinence from alcohol use should continue for 72 hours after completion of tinidazole.
Alternative regimens include several tinidazole regimens (601) or clindamycin (oral or intravaginal) (602). An additional regimen includes metronidazole (750-mg extended release tablets orally once daily for 7 days); however, data on the performance of this alternative regimen are limited.
Certain studies have evaluated the clinical and microbiologic efficacy of using intravaginal lactobacillus formulations to treat BV and restore normal flora (603–607). Overall, no studies support the addition of any available lactobacillus formulations or probiotic as an adjunctive or replacement therapy in women with BV. Further research efforts to determine the role of these regimens in BV treatment and prevention are ongoing.
Other Management Considerations
All women with BV should be tested for HIV and other STDs.
Follow-Up
Follow-up visits are unnecessary if symptoms resolve. Because persistent or recurrent BV is common, women should be advised to return for evaluation if symptoms recur. Detection of certain BV-associated organisms has been associated with antimicrobial resistance and might be predictive of risk for subsequent treatment failure (608–613). Limited data are available regarding optimal management strategies for women with persistent or recurrent BV. Using a different recommended treatment regimen can be considered in women who have a recurrence; however, retreatment with the same recommended regimen is an acceptable approach for treating persistent or recurrent BV after the first occurrence (614). For women with multiple recurrences after completion of a recommended regimen, 0.75% metronidazole gel twice weekly for 4–6 months has been shown to reduce recurrences, although this benefit might not persist when suppressive therapy is discontinued (615). Limited data suggest that an oral nitroimidazole (metronidazole or tinidazole 500 mg twice daily for 7 days) followed by intravaginal boric acid 600 mg daily for 21 days and then suppressive 0.75% metronidazole gel twice weekly for 4–6 months for those women in remission might be an option for women with recurrent BV (616). Monthly oral metronidazole 2 g administered with fluconazole 150 mg has also been evaluated as suppressive therapy; this regimen reduced the incidence of BV and promoted colonization with normal vaginal flora (617).
Management of Sex Partners
Data from clinical trials indicate that a woman’s response to therapy and the likelihood of relapse or recurrence are not affected by treatment of her sex partner(s) (595). Therefore, routine treatment of sex partners is not recommended.
Special Considerations
Allergy, Intolerance, or Adverse Reactions
Intravaginal clindamycin cream is preferred in case of allergy or intolerance to metronidazole or tinidazole. Intravaginal metronidazole gel can be considered for women who are not allergic to metronidazole but do not tolerate oral metronidazole. It is advised to avoid consuming alcohol during treatment with nitroimidazoles. To reduce the possibility of a disulfiram-like reaction, abstinence from alcohol use should continue for 24 hours after completion of metronidazole or 72 hours after completion of tinidazole.
Pregnancy
Treatment is recommended for all symptomatic pregnant women. Studies have been undertaken to determine the efficacy of BV treatment among this population, including two trials demonstrating that metronidazole was efficacious during pregnancy using the 250-mg regimen (618,619); however, metronidazole administered at 500 mg twice daily can be used. One trial involving a limited number of participants revealed treatment with oral metronidazole 500 mg twice daily to be equally effective as metronidazole gel, with cure rates of 70% using Amsel criteria to define cure (620). Another trial demonstrated a cure rate of 85% using Gram-stain criteria after treatment with oral clindamycin (621). Multiple studies and meta-analyses have failed to demonstrate an association between metronidazole use during pregnancy and teratogenic or mutagenic effects in newborns (622,623). Although older studies indicated a possible link between use of vaginal clindamycin during pregnancy and adverse outcomes for the newborn, newer data demonstrate that this treatment approach is safe for pregnant women (624). Because oral therapy has not been shown to be superior to topical therapy for treating symptomatic BV in effecting cure or preventing adverse outcomes of pregnancy, symptomatic pregnant women can be treated with either of the oral or vaginal regimens recommended for nonpregnant women. Although adverse pregnancy outcomes, including premature rupture of membranes, preterm labor, preterm birth, intra-amniotic infection, and postpartum endometritis have been associated with symptomatic BV in some observational studies, treatment of BV in pregnant women can reduce the signs and symptoms of vaginal infection. A meta-analysis has concluded that no antibiotic regimen prevented preterm birth (early or late) in women with BV (symptomatic or asymptomatic). However, in one study, oral BV therapy reduced the risk for late miscarriage, and in two additional studies, such therapy decreased adverse outcomes in the neonate (625).
Treatment of asymptomatic BV among pregnant women who are at high risk for preterm delivery (i.e., those with a previous preterm birth) has been evaluated by several studies, which have yielded mixed results. Seven trials have evaluated treatment of pregnant women with asymptomatic BV at high risk for preterm delivery: one showed harm (626), two showed no benefit (627,628), and four demonstrated benefit (618,619,629,630).
Similarly, data are inconsistent regarding whether treatment of asymptomatic BV among pregnant women who are at low risk for preterm delivery reduces adverse outcomes of pregnancy. One trial demonstrated a 40% reduction in spontaneous preterm birth among women using oral clindamycin during weeks 13–22 of gestation (630). Several additional trials have shown that intravaginal clindamycin given at an average gestation of >20 weeks did not reduce likelihood of preterm birth (628,631–633). Therefore, evidence is insufficient to recommend routine screening for BV in asymptomatic pregnant women at high or low risk for preterm delivery for the prevention of preterm birth (111).
Although metronidazole crosses the placenta, no evidence of teratogenicity or mutagenic effects in infants has been found in multiple cross-sectional and cohort studies of pregnant women (634). Data suggest that metronidazole therapy poses low risk in pregnancy (317).
Metronidazole is secreted in breast milk. With maternal oral therapy, breastfed infants receive metronidazole in doses that are less than those used to treat infections in infants, although the active metabolite adds to the total infant exposure. Plasma levels of the drug and metabolite are measurable, but remain less than maternal plasma levels (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT). Although several reported case series found no evidence of metronidazole-associated adverse effects in breastfed infants, some clinicians advise deferring breastfeeding for 12–24 hours following maternal treatment with a single 2 g dose of metronidazole (635). Lower doses produce a lower concentration in breast milk and are considered compatible with breastfeeding (636,637). Data from studies of human subjects are limited regarding the use of tinidazole in pregnancy; however, animal data suggest that such therapy poses moderate risk. Thus tinidazole should be avoided during pregnancy (317).
HIV Infection
BV appears to recur with higher frequency in women who have HIV infection (638). Women with HIV who have BV should receive the same treatment regimen as those who do not have HIV infection.
Trichomoniasis
Trichomoniasis is the most prevalent nonviral sexually transmitted infection in the United States, affecting an estimated 3.7 million persons (533). Health disparities persist in the epidemiology of T. vaginalis infection in the United States: 13% of black women are affected compared with 1.8% of non-Hispanic white women (639). T. vaginalis infection affects >11% of women aged ≥40 years (640), and particularly high prevalence has been detected among STD clinic patients (641) (26% of symptomatic women and 6.5% asymptomatic women tested) and incarcerated persons (9%–32% of incarcerated women [135,136,640,642,643] and 2%–9% of incarcerated men) (136,137,644,645). The prevalence of trichomoniasis in MSM is low (646,647).
Some infected men have symptoms of urethritis, epididymitis, or prostatitis, and some infected women have vaginal discharge that might be diffuse, malodorous, or yellow-green with or without vulvar irritation. However, most infected persons (70%–85%) have minimal or no symptoms, and untreated infections might last for months to years (86,639,648,649). Although partners might be unaware of their infection, it is readily passed between sex partners during penile-vaginal sex (650). Among persons who are sexually active, the best way to prevent trichomoniasis is through consistent and correct use of condoms during all penile-vaginal sexual encounters (22). Partners of men who have been circumcised might have a somewhat reduced risk of T. vaginalis infection (56,651). Douching is not recommended because it might increase the risk for vaginal infections, including trichomoniasis (652).
T. vaginalis infection is associated with two- to threefold increased risk for HIV acquisition (653–656), preterm birth, and other adverse pregnancy outcomes among pregnant women. Among women with HIV infection, T. vaginalis infection is associated with increased risk for PID (657–659). Routine screening of asymptomatic women with HIV infection for T. vaginalis is recommended because of the adverse events associated with asymptomatic trichomoniasis and HIV infection.
Diagnostic testing for T. vaginalis should be performed in women seeking care for vaginal discharge. Screening might be considered for persons receiving care in high-prevalence settings (e.g., STD clinics and correctional facilities) and for asymptomatic persons at high risk for infection (e.g., persons with multiple sex partners, exchanging sex for payment, illicit drug use, or a history of STD). However, data are lacking on whether screening and treatment for asymptomatic trichomoniasis in high prevalence settings or persons at high risk can reduce any adverse health events and health disparities or reduce community burden of infection. Decisions about screening might be informed by local epidemiology of T. vaginalis infection.
Whether the rectum can be a reservoir for T. vaginalis infection is unclear; data are needed to clarify whether this occasional finding might reflect recent depositing contamination in up to 5% of persons reporting recent receptive anal sex (660,661). Further, the efficacy, benefit, and cost-effectiveness of rectal screening are unknown; therefore, rectal testing for T. vaginalis is not recommended. Similarly, oral testing for T. vaginalis is not recommended because of a lack of evidence for oral infections. T. vaginalis infection is not a nationally notifiable condition in the United States (118,662).
Diagnostic Considerations
The use of highly sensitive and specific tests is recommended for detecting T. vaginalis. Among women, NAAT is highly sensitive, often detecting three to five times more T. vaginalis infections than wet-mount microscopy, a method with poor sensitivity (51%–65%) (663,664). The APTIMA T. vaginalis assay (Hologic Gen-Probe, San Diego, CA) is FDA-cleared for detection of T. vaginalis from vaginal, endocervical, or urine specimens from women. This assay detects RNA by transcription-mediated amplification with a clinical sensitivity of 95.3%–100% and specificity of 95.2%–100% (665,666). Among women, vaginal swab and urine have up to 100% concordance (663). As analyte-specific reagents, this assay can be used with urine or urethral swabs from men if validated per CLIA regulations. The sale, distribution, and use of analyte-specific reagents are allowed under 21 C.F.R. 809.30 pertaining to in vitro diagnostic products for human use. For T. vaginalis diagnosis in men, the sensitivity of self-collected penile-meatal swabs was higher than that of urine in one study (80% and 39%, respectively) (667). The BD Probe Tec TV Qx Amplified DNA Assay (Becton Dickinson, Franklin Lakes, New Jersey) is FDA-cleared for detection of T. vaginalis from endocervical, vaginal, or urine specimens from women. Although it might be feasible to perform these tests on the same specimen used for chlamydia and gonorrhea screening, the epidemiology of trichomoniasis is distinct and should not be overlooked in older adults.
Other FDA-cleared tests to detect T. vaginalis in vaginal secretions include the OSOM Trichomonas Rapid Test (Sekisui Diagnostics, Framingham, MA), an antigen-detection test using immunochromatographic capillary flow dipstick technology that can be performed at the point of care, and the Affirm VP III (Becton Dickinson, Sparks, MD), a DNA hybridization probe test that evaluates for T. vaginalis, G. vaginalis, and Candida albicans. The results of the OSOM Trichomonas Rapid Test are available in approximately 10 minutes, with sensitivity 82%–95% and specificity 97%–100% (666,668). Self-testing might become an option, as a study of 209 young women aged 14–22 years found that >99% could correctly perform and interpret her own self-test using the OSOM assay, with a high correlation with clinician interpretation (96% agreement, κ = 0.87) (669). The results of the Affirm VP III are available within 45 minutes. Sensitivity and specificity are 63% and 99.9%, respectively, compared with culture and TMA; sensitivity might be higher among women who are symptomatic (670,671). Neither the OSOM nor the Affirm VP III test is FDA-cleared for use with specimens obtained from men.
Culture was considered the gold standard method for diagnosing T. vaginalis infection before molecular detection methods became available. Culture has a sensitivity of 75%–96% and a specificity of up to 100% (475). In women, vaginal secretions are the preferred specimen type for culture, as urine culture is less sensitive (475,672,673). In men, culture specimens require a urethral swab, urine sediment, and/or semen. To improve yield, multiple specimens from men can be used to inoculate a single culture.
The most common method for T. vaginalis diagnosis might be microscopic evaluation of wet preparations of genital secretions because of convenience and relatively low cost. Unfortunately, the sensitivity of wet mount is low (51%–65%) in vaginal specimens (475,666) and lower in specimens from men (e.g., urethral specimens, urine sediment, and semen). Clinicians using wet mounts should attempt to evaluate slides immediately because sensitivity declines as evaluation is delayed, decreasing by up to 20% within 1 hour after collection (674,675). When highly sensitive (e.g., NAAT) testing on specimens is not feasible, a testing algorithm (e.g., wet mount first, followed by NAAT if negative) can improve diagnostic sensitivity in persons with an initial negative result by wet mount (475). Although T. vaginalis may be an incidental finding on a Pap test, neither conventional nor liquid-based Pap tests are considered diagnostic tests for trichomoniasis, because false negatives and false positives can occur.
Treatment
Treatment reduces symptoms and signs of T. vaginalis infection and might reduce transmission. Likelihood of adverse outcomes in women with HIV also is reduced with T. vaginalis therapy.
| Recommended Regimen |
|
|
| Metronidazole 2 g orally in a single dose |
| OR |
| Tinidazole 2 g orally in a single dose |
|
|
| Alternative Regimen |
|
|
| Metronidazole 500 mg orally twice a day for 7 days |
|
|
Alcohol consumption should be avoided during treatment with nitroimidazoles. To reduce the possibility of a disulfiram-like reaction, abstinence from alcohol use should continue for 24 hours after completion of metronidazole or 72 hours after completion of tinidazole.
The nitroimidazoles are the only class of antimicrobial medications known to be effective against T. vaginalis infections. Of these drugs, metronidazole and tinidazole have been cleared by FDA for the oral or parenteral treatment of trichomoniasis. Tinidazole is generally more expensive, reaches higher levels in serum and the genitourinary tract, has a longer half-life than metronidazole (12.5 hours versus 7.3 hours), and has fewer gastrointestinal side effects (676–678). In randomized clinical trials, recommended metronidazole regimens have resulted in cure rates of approximately 84%–98% (679–681), and the recommended tinidazole regimen has resulted in cure rates of approximately 92%–100% (680,682–685). Randomized controlled trials comparing single 2 g doses of metronidazole and tinidazole suggest that tinidazole is equivalent or superior to metronidazole in achieving parasitologic cure and resolution of symptoms (686).
Metronidazole gel does not reach therapeutic levels in the urethra and perivaginal glands. Because it is less efficacious than oral metronidazole, it is not recommended.
Other Management Considerations
Providers should advise persons infected with T. vaginalis to abstain from sex until they and their sex partners are treated (i.e., when therapy has been completed and any symptoms have resolved). Testing for other STDs including HIV should be performed in persons infected with T. vaginalis.
Follow-up
Because of the high rate of reinfection among women treated for trichomoniasis (17% within 3 months in one study) (86), retesting for T. vaginalis is recommended for all sexually active women within 3 months following initial treatment regardless of whether they believe their sex partners were treated (see Diagnostic Considerations). Testing by nucleic acid amplification can be conducted as soon as 2 weeks after treatment (687,688). Data are insufficient to support retesting men.
Management of Sex Partners
Concurrent treatment of all sex partners is critical for symptomatic relief, microbiologic cure, and prevention of transmission and reinfections. Current partners should be referred for presumptive therapy to avoid reinfection. Partners should be advised to abstain from intercourse until they and their sex partners have been adequately treated and any symptoms have resolved. EPT might have a role in partner management for trichomoniasis (97,98,689) and can be used in states where permissible by law; however, no one partner management intervention has been shown to be superior in reducing reinfection rates. Though no definitive data exist to guide treatment for partners of persons with persistent or recurrent trichomoniasis in whom nonadherance and reinfection are unlikely, partners benefit from undergoing evaluation and receiving the same regimen as the patient (see Persistent or Recurrent Trichomoniasis).
Persistent or Recurrent Trichomoniasis
Persistent or recurrent infection caused by antimicrobial-resistant T. vaginalis or other causes should be distinguished from the possibility of reinfection from an untreated sex partner. Although most recurrent T. vaginalis infections are thought to result from reinfection, some infections might be attributed to antimicrobial resistance. Metronidazole resistance occurs in 4%–10% of cases of vaginal trichomoniasis (690,691), and tinidazole resistance in 1% (691). In general, T. vaginalis isolates have lower minimum lethal concentrations to tinidazole than metronidazole (692). Emerging nitroimidazole-resistant trichomoniasis is concerning, because few alternatives to standard therapy exist. Single-dose therapy should be avoided for treating recurrent trichomoniasis that is not likely a result of reinfection. If treatment failure has occurred with metronidazole 2 g single dose and reinfection is excluded, the patient (and their partner[s]) can be treated with metronidazole 500 mg orally twice daily for 7 days. If this regimen fails, clinicians should consider treatment with metronidazole or tinidazole at 2 g orally for 7 days. If several 1-week regimens have failed in a person who is unlikely to have nonadherence or reinfection, testing of the organism for metronidazole and tinidazole susceptibility is recommended (693). CDC has experience with susceptibility testing for nitroimidazole-resistant T. vaginalis and treatment management of infected persons and can provide assistance (telephone: 404-718-4141; website: http://www.cdc.gov/std). Higher dose tinidazole at 2–3 g for 14 days, often in combination with intravaginal tinidazole, can be considered in cases of nitroimidazole-resistant infections; however, such cases should be managed in consultation with an expert.
Alternative regimens might be effective but have not been systematically evaluated; therefore, consultation with an infectious-disease specialist is recommended. The most anecdotal experience has been with intravaginal paromomycin in combination with high-dose tinidazole (694–696); clinical improvement has been reported with other alternative regimens including intravaginal boric acid (697,698) and nitazoxanide (699). The following topically applied agents have shown minimal success (<50%) and are not recommended: intravaginal betadine (povidone-iodine), clotrimazole, acetic acid, furazolidone, gentian violet, nonoxynol-9, and potassium permanganate (700). No other topical microbicide has been shown to be effective against trichomoniasis (701).
Special Considerations
Allergy, Intolerance, and Adverse Reactions
Metronidazole and tinidazole are both nitroimidazoles. Patients with an IgE mediated-type allergy to a nitroimidazole can be managed by metronidazole desensitization according to a published regimen (702) and in consultation with a specialist.
Pregnancy
T. vaginalis infection in pregnant women is associated with adverse pregnancy outcomes, particularly premature rupture of membranes, preterm delivery, and delivery of a low birthweight infant (658,703–705). Although metronidazole treatment produces parasitologic cure, certain trials have shown no significant difference in perinatal morbidity following metronidazole treatment. One trial suggested the possibility of increased preterm delivery in women with T. vaginalis infection who received metronidazole treatment (706), yet study limitations prevented definitive conclusions regarding the risks of treatment. More recent, larger studies have shown no positive or negative association between metronidazole use during pregnancy and adverse outcomes of pregnancy (634,707–710). If treatment is considered, the recommended regimen in pregnant women is metronidazole 2 g orally in a single dose. Symptomatic pregnant women, regardless of pregnancy stage, should be tested and considered for treatment. Treatment of T. vaginalis infection can relieve symptoms of vaginal discharge in pregnant women and reduce sexual transmission to partners. Although perinatal transmission of trichomoniasis is uncommon, treatment also might prevent respiratory or genital infection of the newborn (711,712). Clinicians should counsel symptomatic pregnant women with trichomoniasis regarding the potential risks for and benefits of treatment and about the importance of partner treatment and condom use in the prevention of sexual transmission.
The benefit of routine screening for T. vaginalis in asymptomatic pregnant women has not been established. However, screening at the first prenatal visit and prompt treatment, as appropriate, are recommended for pregnant women with HIV infection, because T. vaginalis infection is a risk factor for vertical transmission of HIV (713). Pregnant women with HIV who are treated for T. vaginalis infection should be retested 3 months after treatment.
Although metronidazole crosses the placenta, data suggest that it poses a low risk to pregnant women (317). No evidence of teratogenicity or mutagenic effects in infants has been found in multiple cross-sectional and cohort studies of pregnant women (708–710,714). Women can be treated with 2 g metronidazole in a single dose at any stage of pregnancy.
Metronidazole is secreted in breast milk. With maternal oral therapy, breastfed infants receive metronidazole in doses that are lower than those used to treat infections in infants, although the active metabolite adds to the total infant exposure. Plasma levels of the drug and metabolite are measurable, but remain less than maternal plasma levels (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT). Although several reported case series found no evidence of adverse effects in infants exposed to metronidazole in breast milk, some clinicians advise deferring breastfeeding for 12–24 hours following maternal treatment with a single 2 g dose of metronidazole (635). Maternal treatment with metronidazole (400 mg three times daily for 7 days) produced a lower concentration in breast milk and was considered compatible with breastfeeding over longer periods of time (636,637).
Data from studies involving human subjects are limited regarding use of tinidazole in pregnancy; however, animal data suggest this drug poses moderate risk. Thus, tinidazole should be avoided in pregnant women, and breastfeeding should be deferred for 72 hours following a single 2-g dose of tinidazole (http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).
HIV Infection
Up to 53% of women with HIV infection also are infected with T. vaginalis (715,716). T. vaginalis infection in these women is significantly associated with PID (659), and treatment of trichomoniasis is associated with significant decreases in genital-tract HIV viral load and viral shedding (717,718). For these reasons, routine screening and prompt treatment are recommended for all women with HIV infection; screening should occur at entry to care and then at least annually thereafter. A randomized clinical trial involving women with HIV infection and T. vaginalis infection demonstrated that a single dose of metronidazole 2 g orally was less effective than 500 mg twice daily for 7 days (719). Thus, to improve cure rates, women with HIV infection who receive a diagnosis of T. vaginalis infection should be treated with metronidazole 500 mg orally twice daily for 7 days (rather than with a 2-g single dose of metronidazole). Factors that might interfere with standard single-dose treatment for trichomoniasis in these women include high rates of asymptomatic BV co-infections, use of antiretroviral therapy, changes in vaginal ecology, and impaired immunity (656,720,721).
Treatment
Treatment reduces symptoms and signs of T. vaginalis infection and might reduce transmission. Likelihood of adverse outcomes in women with HIV is also reduced with T. vaginalis therapy.
| Recommended Regimen for Women with HIV Infection |
|
|
| Metronidazole 500 mg orally twice daily for 7 days |
|
|
In women with HIV infection who receive a diagnosis of T. vaginalis infection, retesting is recommended within 3 months following initial treatment; NAAT is encouraged because of higher sensitivity of these tests. Data are insufficient to recommend routine screening, alternative treatment regimens of longer duration, or retesting in men.
Vulvovaginal Candidiasis
VVC usually is caused by C. albicans but can occasionally be caused by other Candida sp. or yeasts. Typical symptoms of VVC include pruritus, vaginal soreness, dyspareunia, external dysuria, and abnormal vaginal discharge. None of these symptoms is specific for VVC. An estimated 75% of women will have at least one episode of VVC, and 40%–45% will have two or more episodes. On the basis of clinical presentation, microbiology, host factors, and response to therapy, VVC can be classified as either uncomplicated or complicated (Box 3). Approximately 10%–20% of women will have complicated VVC, requiring special diagnostic and therapeutic considerations.
BOX 3. Classification of vulvovaginal candidiasis.
Uncomplicated VVC
-
Sporadic or infrequent VVC
AND
-
Mild-to-moderate VVC
AND
-
Likely to be Candida albicans
AND
Nonimmunocompromised women
Complicated VVC
-
Recurrent VVC
OR
-
Severe VVC
OR
-
Nonalbicans candidiasis
OR
Women with diabetes, immunocompromising conditions (e.g., HIV infection), debilitation, or immunosuppressive therapy (e.g., corticosteroids)
Abbreviation: HIV = human immunodeficiency virus; VVC = vulvovaginal candidiasis.
Uncomplicated VVC
Diagnostic Considerations
A diagnosis of Candida vaginitis is suggested clinically by the presence of external dysuria and vulvar pruritus, pain, swelling, and redness. Signs include vulvar edema, fissures, excoriations, and thick curdy vaginal discharge. The diagnosis can be made in a woman who has signs and symptoms of vaginitis when either 1) a wet preparation (saline, 10% KOH) or Gram stain of vaginal discharge demonstrates budding yeasts, hyphae, or pseudohyphae or 2) a culture or other test yields a positive result for a yeast species. Candida vaginitis is associated with a normal vaginal pH (<4.5). Use of 10% KOH in wet preparations improves the visualization of yeast and mycelia by disrupting cellular material that might obscure the yeast or pseudohyphae. Examination of a wet mount with KOH preparation should be performed for all women with symptoms or signs of VVC, and women with a positive result should be treated. For those with negative wet mounts but existing signs or symptoms, vaginal cultures for Candida should be considered. If Candida cultures cannot be performed for these women, empiric treatment can be considered. Identifying Candida by culture in the absence of symptoms or signs is not an indication for treatment, because approximately 10%–20% of women harbor Candida sp. and other yeasts in the vagina. PCR testing for yeast is not FDA-cleared; culture for yeast remains the gold standard for diagnosis. VVC can occur concomitantly with STDs. Most healthy women with uncomplicated VVC have no identifiable precipitating factors.
Treatment
Short-course topical formulations (i.e., single dose and regimens of 1–3 days) effectively treat uncomplicated VVC. The topically applied azole drugs are more effective than nystatin. Treatment with azoles results in relief of symptoms and negative cultures in 80%–90% of patients who complete therapy.
| Recommended Regimens |
|
|
| Over-the-Counter Intravaginal Agents: |
| Clotrimazole 1% cream 5 g intravaginally daily for 7–14 days |
| OR |
| Clotrimazole 2% cream 5 g intravaginally daily for 3 days |
| OR |
| Miconazole 2% cream 5 g intravaginally daily for 7 days |
| OR |
| Miconazole 4% cream 5 g intravaginally daily for 3 days |
| OR |
| Miconazole 100 mg vaginal suppository, one suppository daily for 7 days |
| OR |
| Miconazole 200 mg vaginal suppository, one suppository for 3 days |
| OR |
| Miconazole 1,200 mg vaginal suppository, one suppository for 1 day |
| OR |
| Tioconazole 6.5% ointment 5 g intravaginally in a single application |
| Prescription Intravaginal Agents: |
| Butoconazole 2% cream (single dose bioadhesive product), 5 g intravaginally in a single application |
| OR |
| Terconazole 0.4% cream 5 g intravaginally daily for 7 days |
| OR |
| Terconazole 0.8% cream 5 g intravaginally daily for 3 days |
| OR |
| Terconazole 80 mg vaginal suppository, one suppository daily for 3 days |
| Oral Agent: |
| Fluconazole 150 mg orally in a single dose |
|
|
The creams and suppositories in these regimens are oil-based and might weaken latex condoms and diaphragms. Refer to condom product labeling for further information. Intravaginal preparations of clotrimazole, miconazole, and tioconazole are available over-the-counter (OTC). Even women who have previously received a diagnosis of VVC by a clinician are not necessarily more likely to be able to diagnose themselves; therefore, any woman whose symptoms persist after using an OTC preparation or who has a recurrence of symptoms within 2 months after treatment for VVC should be clinically evaluated and tested. Unnecessary or inappropriate use of OTC preparations is common and can lead to a delay in the treatment of other vulvovaginitis etiologies, which can in turn result in adverse outcomes.
Follow-Up
Follow-up typically is not required. However, women in whom symptoms persist or recur after treatment of initial symptoms should be instructed to return for follow-up visits.
Management of Sex Partners
Uncomplicated VVC is not usually acquired through sexual intercourse; thus, data do not support treatment of sex partners. A minority of male sex partners have balanitis, characterized by erythematous areas on the glans of the penis in conjunction with pruritus or irritation. These men benefit from treatment with topical antifungal agents to relieve symptoms.
Special Considerations
Allergy, Intolerance, and Adverse Reactions
Topical agents usually cause no systemic side effects, although local burning or irritation might occur. Oral azoles occasionally cause nausea, abdominal pain, and headache. Therapy with the oral azoles has been associated rarely with abnormal elevations of liver enzymes. Clinically important interactions can occur when oral azoles agents are administered with other drugs (722).
Complicated VVC
Diagnostic Considerations
Vaginal cultures should be obtained from women with complicated VVC to confirm clinical diagnosis and identify unusual species, including nonalbicans species. C. glabrata does not form pseudohyphae or hyphae and is not easily recognized on microscopy. Although C. albicans azole resistance is possibly becoming more common in vaginal isolates (723,724), susceptibility testing is usually not warranted for individual treatment guidance.
Recurrent Vulvovaginal Candidiasis
Recurrent Vulvovaginal Candidiasis (RVVC), usually defined as four or more episodes of symptomatic VVC within 1 year, affects a small percentage of women (<5%). The pathogenesis of RVVC is poorly understood, and most women with RVVC have no apparent predisposing or underlying conditions. C. glabrata and other nonalbicans Candida species are observed in 10%–20% of women with RVVC. Conventional antimycotic therapies are not as effective against these nonalbicans species as against C. albicans.
Treatment
Each individual episode of RVVC caused by C. albicans responds well to short duration oral or topical azole therapy. However, to maintain clinical and mycologic control, some specialists recommend a longer duration of initial therapy (e.g., 7–14 days of topical therapy or a 100-mg, 150-mg, or 200-mg oral dose of fluconazole every third day for a total of 3 doses [day 1, 4, and 7]) to attempt mycologic remission before initiating a maintenance antifungal regimen.
Oral fluconazole (i.e., 100-mg, 150-mg, or 200-mg dose) weekly for 6 months is the first line maintenance regimen. If this regimen is not feasible, topical treatments used intermittently can also be considered. Suppressive maintenance therapies are effective in reducing RVVC. However, 30%–50% of women will have recurrent disease after maintenance therapy is discontinued. Symptomatic women who remain culture-positive despite maintenance therapy should be managed in consultation with a specialist.
Severe VVC
Severe vulvovaginitis (i.e., extensive vulvar erythema, edema, excoriation, and fissure formation) is associated with lower clinical response rates in patients treated with short courses of topical or oral therapy. Either 7–14 days of topical azole or 150 mg of fluconazole in two sequential oral doses (second dose 72 hours after initial dose) is recommended.
Nonalbicans VVC
Because at least 50% of women with positive cultures for nonalbicans Candida might be minimally symptomatic or have no symptoms and because successful treatment is often difficult, clinicians should make every effort to exclude other causes of vaginal symptoms in women with nonalbicans yeast (725). The optimal treatment of nonalbicans VVC remains unknown. Options include longer duration of therapy (7–14 days) with a nonfluconazole azole regimen (oral or topical) as first-line therapy. If recurrence occurs, 600 mg of boric acid in a gelatin capsule is recommended, administered vaginally once daily for 2 weeks. This regimen has clinical and mycologic eradication rates of approximately 70% (726). If symptoms recur, referral to a specialist is advised.
Management of Sex Partners
No data exist to support the treatment of sex partners of patients with complicated VVC. Therefore, no recommendation can be made.
Special Considerations
Compromised Host
Women with underlying immunodeficiency, those with poorly controlled diabetes or other immunocompromising conditions (e.g., HIV), and those receiving immunosuppression therapy (e.g., corticosteroid treatment) do not respond as well to short-term therapies. Efforts to correct modifiable conditions should be made, and more prolonged (i.e., 7–14 days) conventional treatment is necessary.
Pregnancy
VVC occurs frequently during pregnancy. Only topical azole therapies, applied for 7 days, are recommended for use among pregnant women.
HIV Infection
Vaginal Candida colonization rates among women with HIV infection are higher than among seronegative women with similar demographic and risk behavior characteristics, and the colonization rates correlate with increasing severity of immunosuppression. Symptomatic VVC is also more frequent in women with HIV infection and similarly correlates with severity of immunodeficiency. In addition, among women with HIV infection, systemic azole exposure is associated with the isolation of nonalbicans Candida species from the vagina.
On the basis of available data, therapy for uncomplicated and complicated VVC in women with HIV infection should not differ from that for seronegative women. Although long-term prophylactic therapy with fluconazole at a dose of 200 mg weekly has been effective in reducing C. albicans colonization and symptomatic VVC (727), this regimen is not recommended for women with HIV infection in the absence of complicated VVC (247). Although VVC is associated with increased HIV seroconversion in HIV-negative women and increased HIV cervicovaginal levels in women with HIV infection, the effect of treatment for VVC on HIV acquisition and transmission remains unknown.
Pelvic Inflammatory Disease
Pelvic inflammatory disease (PID) comprises a spectrum of inflammatory disorders of the upper female genital tract, including any combination of endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis (728). Sexually transmitted organisms, especially N. gonorrhoeae and C. trachomatis, are implicated in many cases. Recent studies suggest that the proportion of PID cases attributable to N. gonorrhoeae or C. trachomatis is declining; of women who received a diagnosis of acute PID, <50% test positive for either of these organisms (270,729,730). Microorganisms that comprise the vaginal flora (e.g., anaerobes, G. vaginalis, Haemophilus influenzae, enteric Gram-negative rods, and Streptococcus agalactiae) have been associated with PID (731). In addition, cytomegalovirus (CMV), M. hominis, U. urealyticum, and M. genitalium might be associated with some PID cases (264,265,267,732). Newer data suggest that M. genitalium might play a role in the pathogenesis of PID (270,487) and might be associated with milder symptoms (267), although one study failed to demonstrate a significant increase in PID following detection of M. genitalium in the lower genital tract (733). All women who receive a diagnosis of acute PID should be tested for HIV, as well as gonorrhea and chlamydia, using NAAT. The value of testing women with PID for M. genitalium is unknown, and there is no commercially available diagnostic test that has been cleared by FDA for use in the United States (see Mycoplasma genitalium).
Screening and treating sexually active women for chlamydia reduces their risk for PID (456,682). Although BV is associated with PID, whether the incidence of PID can be reduced by identifying and treating women with BV is unclear (731,734).
Diagnostic Considerations
Acute PID is difficult to diagnose because of the wide variation in symptoms and signs associated with this condition. Many women with PID have subtle or nonspecific symptoms or are asymptomatic. Delay in diagnosis and treatment probably contributes to inflammatory sequelae in the upper reproductive tract. Laparoscopy can be used to obtain a more accurate diagnosis of salpingitis and a more complete bacteriologic diagnosis. However, this diagnostic tool frequently is not readily available, and its use is not easily justifiable when symptoms are mild or vague. Moreover, laparoscopy will not detect endometritis and might not detect subtle inflammation of the fallopian tubes. Consequently, a diagnosis of PID usually is based on imprecise clinical findings (735,736).
Data indicate that a clinical diagnosis of symptomatic PID has a PPV for salpingitis of 65%–90% compared with laparoscopy (737–739). The PPV of a clinical diagnosis of acute PID depends on the epidemiologic characteristics of the population, with higher PPVs among sexually active young women (particularly adolescents), women attending STD clinics, and those who live in communities with high rates of gonorrhea or chlamydia. Regardless of PPV, no single historical, physical, or laboratory finding is both sensitive and specific for the diagnosis of acute PID. Combinations of diagnostic findings that improve either sensitivity (i.e., detect more women who have PID) or specificity (i.e., exclude more women who do not have PID) do so only at the expense of the other. For example, requiring two or more findings excludes more women who do not have PID and reduces the number of women with PID who are identified.
Many episodes of PID go unrecognized. Although some cases are asymptomatic, others are not diagnosed because the patient or the health-care provider fails to recognize the implications of mild or nonspecific symptoms or signs (e.g., abnormal bleeding, dyspareunia, and vaginal discharge). Even women with mild or asymptomatic PID might be at risk for infertility (740). Because of the difficulty of diagnosis and the potential for damage to the reproductive health of women, health-care providers should maintain a low threshold for the diagnosis of PID (729). The following recommendations for diagnosing PID are intended to help health-care providers recognize when PID should be suspected and when additional information should be obtained to increase diagnostic certainty. Diagnosis and management of other common causes of lower abdominal pain (e.g., ectopic pregnancy, acute appendicitis, ovarian cyst, and functional pain) are unlikely to be impaired by initiating antimicrobial therapy for PID.
Presumptive treatment for PID should be initiated in sexually active young women and other women at risk for STDs if they are experiencing pelvic or lower abdominal pain, if no cause for the illness other than PID can be identified, and if one or more of the following minimum clinical criteria are present on pelvic examination:
-
cervical motion tenderness
or
-
uterine tenderness
or
adnexal tenderness.
The requirement that all three minimum criteria be present before the initiation of empiric treatment could result in insufficient sensitivity for the diagnosis of PID. After deciding whether to initiate empiric treatment, clinicians should also consider the risk profile for STDs.
More elaborate diagnostic evaluation frequently is needed because incorrect diagnosis and management of PID might cause unnecessary morbidity. For example, the presence of signs of lower-genital–tract inflammation (predominance of leukocytes in vaginal secretions, cervical exudates, or cervical friability), in addition to one of the three minimum criteria, increases the specificity of the diagnosis. One or more of the following additional criteria can be used to enhance the specificity of the minimum clinical criteria and support a diagnosis of PID:
oral temperature >101°F (>38.3°C);
abnormal cervical mucopurulent discharge or cervical friability;
presence of abundant numbers of WBC on saline microscopy of vaginal fluid;
elevated erythrocyte sedimentation rate;
elevated C-reactive protein; and
laboratory documentation of cervical infection with N. gonorrhoeae or C. trachomatis.
Most women with PID have either mucopurulent cervical discharge or evidence of WBCs on a microscopic evaluation of a saline preparation of vaginal fluid (i.e., wet prep). If the cervical discharge appears normal and no WBCs are observed on the wet prep of vaginal fluid, the diagnosis of PID is unlikely, and alternative causes of pain should be considered. A wet prep of vaginal fluid also can detect the presence of concomitant infections (e.g., BV and trichomoniasis).
The most specific criteria for diagnosing PID include:
endometrial biopsy with histopathologic evidence of endometritis;
transvaginal sonography or magnetic resonance imaging techniques showing thickened, fluid-filled tubes with or without free pelvic fluid or tubo-ovarian complex, or Doppler studies suggesting pelvic infection (e.g., tubal hyperemia); or
laparoscopic findings consistent with PID.
A diagnostic evaluation that includes some of these more extensive procedures might be warranted in some cases. Endometrial biopsy is warranted in women undergoing laparoscopy who do not have visual evidence of salpingitis, because endometritis is the only sign of PID for some women.
Treatment
PID treatment regimens must provide empiric, broad spectrum coverage of likely pathogens. Several parenteral and oral antimicrobial regimens have been effective in achieving clinical and microbiologic cure in randomized clinical trials with short-term follow-up (741,742). However, only a limited number of investigations have assessed and compared these regimens with regard to elimination of infection in the endometrium and fallopian tubes or determined the incidence of long-term complications (e.g., tubal infertility and ectopic pregnancy) after antimicrobial regimens (730,735,743). The optimal treatment regimen and long-term outcome of early treatment of women with subclinical PID are unknown. All regimens used to treat PID should also be effective against N. gonorrhoeae and C. trachomatis because negative endocervical screening for these organisms does not rule out upper-reproductive–tract infection. The need to eradicate anaerobes from women who have PID has not been determined definitively. Anaerobic bacteria have been isolated from the upper-reproductive tract of women who have PID, and data from in vitro studies have revealed that some anaerobes (e.g., Bacteroides fragilis) can cause tubal and epithelial destruction. BV is present in many women who have PID (731,734). Until treatment regimens that do not cover anaerobic microbes have been demonstrated to prevent long-term sequelae (e.g., infertility and ectopic pregnancy) as successfully as the regimens that are effective against these microbes, the use of regimens with anaerobic activity should be considered. Treatment should be initiated as soon as the presumptive diagnosis has been made, because prevention of long-term sequelae is dependent on early administration of appropriate antibiotics. When selecting a treatment regimen, health-care providers should consider availability, cost, and patient acceptance (742). In women with PID of mild or moderate clinical severity, parenteral and oral regimens appear to have similar efficacy. The decision of whether hospitalization is necessary should be based on provider judgment and whether the woman meets any of the following suggested criteria:
surgical emergencies (e.g., appendicitis) cannot be excluded;
tubo-ovarian abscess;
pregnancy;
severe illness, nausea and vomiting, or high fever;
-
unable to follow or tolerate an outpatient oral regimen;
or
no clinical response to oral antimicrobial therapy.
No evidence is available to suggest that adolescents have improved outcomes from hospitalization for treatment of PID, and the clinical response to outpatient treatment is similar among younger and older women. The decision to hospitalize adolescents with acute PID should be based on the same criteria used for older women.
Parenteral Treatment
Several randomized trials have demonstrated the efficacy of parenteral regimens (734,741,742). Clinical experience should guide decisions regarding transition to oral therapy, which usually can be initiated within 24–48 hours of clinical improvement. In women with tubo-ovarian abscesses, at least 24 hours of inpatient observation is recommended.
| Recommended Parenteral Regimens |
|
|
| Cefotetan 2 g IV every 12 hours |
| PLUS |
| Doxycycline 100 mg orally or IV every 12 hours |
| OR |
| Cefoxitin 2 g IV every 6 hours |
| PLUS |
| Doxycycline 100 mg orally or IV every 12 hours |
| OR |
| Clindamycin 900 mg IV every 8 hours |
| PLUS |
| Gentamicin loading dose IV or IM (2 mg/kg), followed by a maintenance dose (1.5 mg/kg) every 8 hours. Single daily dosing (3–5 mg/kg) can be substituted. |
|
|
Because of the pain associated with intravenous infusion, doxycycline should be administered orally when possible. Oral and IV administration of doxycycline provide similar bioavailability. Although use of a single daily dose of gentamicin has not been evaluated for the treatment of PID, it is efficacious in analogous situations.
When using the parenteral cefotetan or cefoxitin regimens, oral therapy with doxycycline 100 mg twice daily can be used 24–48 hours after clinical improvement to complete the 14 days of therapy for the clindamycin/gentamicin regimen, and oral therapy with clindamycin (450 mg orally four times daily) or doxycycline (100 mg twice daily) can be used to complete the 14 days of therapy. However, when tubo-ovarian abscess is present, clindamycin (450 mg orally four times daily) or metronidazole (500 mg twice daily) should be used to complete at least 14 days of therapy with doxycycline to provide more effective anaerobic coverage than doxycycline alone.
Limited data are available to support use of other parenteral second- or third-generation cephalosporins (e.g., ceftizoxime, cefotaxime, and ceftriaxone). In addition, these cephalosporins are less active than cefotetan or cefoxitin against anaerobic bacteria.
Alternative Parenteral Regimens
Ampicillin/sulbactam plus doxycycline has been investigated in at least one clinical trial and has broad-spectrum coverage (744). Ampicillin/sulbactam plus doxycycline is effective against C. trachomatis, N. gonorrhoeae, and anaerobes in women with tubo-ovarian abscess. Another trial demonstrated high short-term clinical cure rates with azithromycin, either as monotherapy for 1 week (500 mg IV daily for 1 or 2 doses followed by 250 mg orally for 5–6 days) or combined with a 12-day course of metronidazole (745). Limited data are available to support the use of other parenteral regimens.
| Alternative Parenteral Regimen |
|
|
| Ampicillin/Sulbactam 3 g IV every 6 hours |
| PLUS |
| Doxycycline 100 mg orally or IV every 12 hours |
|
|
Intramuscular/Oral Treatment
Intramuscular/oral therapy can be considered for women with mild-to-moderately severe acute PID, because the clinical outcomes among women treated with these regimens are similar to those treated with intravenous therapy (729). Women who do not respond to IM/oral therapy within 72 hours should be reevaluated to confirm the diagnosis and should be administered intravenous therapy.
| Recommended Intramuscular/Oral Regimens |
|
|
| Ceftriaxone 250 mg IM in a single dose |
| PLUS |
| Doxycycline 100 mg orally twice a day for 14 days |
| WITH* or WITHOUT |
| Metronidazole 500 mg orally twice a day for 14 days |
| OR |
| Cefoxitin 2 g IM in a single dose and Probenecid, 1 g orally administered concurrently in a single dose |
| PLUS |
| Doxycycline 100 mg orally twice a day for 14 days |
| WITH or WITHOUT |
| Metronidazole 500 mg orally twice a day for 14 days |
| OR |
| Other parenteral third-generation cephalosporin (e.g., ceftizoxime or cefotaxime) |
| PLUS |
| Doxycycline 100 mg orally twice a day for 14 days |
| WITH* or WITHOUT |
| Metronidazole 500 mg orally twice a day for 14 days |
|
|
| *The recommended third-generation cephalsporins are limited in the coverage of anaerobes. Therefore, until it is known that extended anaerobic coverage is not important for treatment of acute PID, the addition of metronidazole to treatment regimens with third-generation cephalosporins should be considered (Source: Walker CK, Wiesenfeld HC. Antibiotic therapy for acute pelvic inflammatory disease: the 2006 CDC Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis 2007;28[Supp 1]:S29–36). |
These regimens provide coverage against frequent etiologic agents of PID, but the optimal choice of a cephalosporin is unclear. Cefoxitin, a second-generation cephalosporin, has better anaerobic coverage than ceftriaxone, and in combination with probenecid and doxycycline has been effective in short-term clinical response in women with PID. Ceftriaxone has better coverage against N. gonorrhoeae. The addition of metronidazole will also effectively treat BV, which is frequently associated with PID.
Alternative IM/Oral Regimens
Although information regarding other IM and oral regimens is limited, a few have undergone at least one clinical trial and have demonstrated broad-spectrum coverage. Azithromycin has demonstrated short-term clinical effectiveness in one randomized trial when used as monotherapy (500 mg IV daily for 1–2 doses, followed by 250 mg orally daily for 12–14 days) or in combination with metronidazole (745), and in another study, it was effective when used 1 g orally once a week for 2 weeks in combination with ceftriaxone 250 mg IM single dose (746). When considering these alternative regimens, the addition of metronidazole should be considered to provide anaerobic coverage. No data have been published regarding the use of oral cephalosporins for the treatment of PID. As a result of the emergence of quinolone-resistant N. gonorrhoeae, regimens that include a quinolone agent are no longer routinely recommended for the treatment of PID. If allergy precludes the use of cephalosporin therapy, if the community prevalence and individual risk for gonorrhea are low, and if follow-up is likely, use of fluoroquinolones for 14 days (levofloxacin 500 mg orally once daily, ofloxacin 400 mg twice daily, or moxifloxacin 400 mg orally once daily) with metronidazole for 14 days (500 mg orally twice daily) can be considered (747–749). Diagnostic tests for gonorrhea must be obtained before instituting therapy, and persons should be managed as follows.
If the culture for gonorrhea is positive, treatment should be based on results of antimicrobial susceptibility testing.
If the isolate is determined to be quinolone-resistant N. gonorrhoeae (QRNG) or if antimicrobial susceptibility cannot be assessed (e.g., if only NAAT testing is available), consultation with an infectious-disease specialist is recommended.
Other Management Considerations
To minimize disease transmission, women should be instructed to abstain from sexual intercourse until therapy is completed, symptoms have resolved, and sex partners have been adequately treated (See chlamydia and gonorrhea sections). All women who received a diagnosis of acute PID should be tested for HIV, as well as GC and chlamydia, using NAAT.
Follow-Up
Women should demonstrate clinical improvement (e.g., defervescence; reduction in direct or rebound abdominal tenderness; and reduction in uterine, adnexal, and cervical motion tenderness) within 3 days after initiation of therapy. If no clinical improvement has occurred within 72 hours after outpatient IM/oral therapy, hospitalization, assessment of the antimicrobial regimen, and additional diagnostics (including consideration of diagnostic laparoscopy for alternative diagnoses) are recommended. All women who have received a diagnosis of chlamydial or gonococcal PID should be retested 3 months after treatment, regardless of whether their sex partners were treated (480). If retesting at 3 months is not possible, these women should be retested whenever they next present for medical care in the 12 months following treatment.
Management of Sex Partners
Men who have had sexual contact with a woman with PID during the 60 days preceding her onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea, regardless of the etiology of PID or pathogens isolated from the woman. If a woman’s last sexual intercourse was >60 days before onset of symptoms or diagnosis, the most recent sex partner should be treated. Male partners of women who have PID caused by C. trachomatis and/or N. gonorrhoeae frequently are asymptomatic. Arrangements should be made to link male partners to care. If linkage is delayed or unlikely, EPT and enhanced referral are alternative approaches to treating male partners of women who have chlamydia or gonococcal infections (see Partner Services) (93,94). Partners should be instructed to abstain from sexual intercourse until they and their sex partners have been adequately treated (i.e., until therapy is completed and symptoms have resolved, if originally present).
Special Considerations
Allergy, Intolerance, and Adverse Reactions
The cross reactivity between penicillins and cephalosporins is <2.5% in persons with a history of penicillin allergy (428–431,464). The risk for penicillin cross-reactivity is highest with first-generation cephalosporins, but is negligible between most second-generation (cefoxitin) and all third-generation (ceftriaxone) cephalosporins (428–431) (see Management of Persons who Have a History of Penicillin Allergy).
Pregnancy
Pregnant women suspected to have PID are at high risk for maternal morbidity and preterm delivery. These women should be hospitalized and treated with intravenous antibiotics.
HIV Infection
Differences in the clinical manifestations of PID between women with HIV infection and women without HIV infection have not been well delineated. In early observational studies, women with HIV infection and PID were more likely to require surgical intervention. More comprehensive observational and controlled studies have demonstrated that women with HIV infection and PID have similar symptoms when compared with HIV-negative women with PID (266,750,751), except they are more likely to have a tubo-ovarian abscess; women with HIV infection responded equally well to recommended parenteral and IM/oral antibiotic regimens as women without HIV infection. The microbiologic findings for women with HIV infection and women without HIV infection were similar, except women with HIV infection had higher rates of concomitant M. hominis and streptococcal infections. These data are insufficient for determining whether women with HIV infection and PID require more aggressive management (e.g., hospitalization or intravenous antimicrobial regimens).
Intrauterine Contraceptive Devices
IUDs are one of the most effective contraceptive methods. Copper-containing and levonorgestrel-releasing IUDs are available in the United States. The risk for PID associated with IUD use is primarily confined to the first 3 weeks after insertion (752,753). If an IUD user receives a diagnosis of PID, the IUD does not need to be removed (63). However, the woman should receive treatment according to these recommendations and should have close clinical follow-up. If no clinical improvement occurs within 48–72 hours of initiating treatment, providers should consider removing the IUD. A systematic review of evidence found that treatment outcomes did not generally differ between women with PID who retained the IUD and those who had the IUD removed (754). These studies primarily included women using copper or other nonhormonal IUDs. No studies are available regarding treatment outcomes in women using levonorgestrel-releasing IUDs.
Epididymitis
Acute epididymitis is a clinical syndrome consisting of pain, swelling, and inflammation of the epididymis that lasts <6 weeks (755). Sometimes the testis is also involved— a condition referred to as epididymo-orchitis. A high index of suspicion for spermatic cord (testicular) torsion must be maintained in men who present with a sudden onset of symptoms associated with epididymitis, as this condition is a surgical emergency.
Among sexually active men aged <35 years, acute epididymitis is most frequently caused by C. trachomatis or N. gonorrhoeae. Acute epididymitis caused by sexually transmitted enteric organisms (e.g., Escherichia coli) also occurs among men who are the insertive partner during anal intercourse. Sexually transmitted acute epididymitis usually is accompanied by urethritis, which frequently is asymptomatic. Other nonsexually transmitted infectious causes of acute epididymitis (e.g., Fournier’s gangrene) are uncommon and should be managed in consultation with a urologist.
In men aged ≥35 years who do not report insertive anal intercourse, sexually transmitted acute epididymitis is less common. In this group, the epididymis usually becomes infected in the setting of bacteriuria secondary to bladder outlet obstruction (e.g., benign prostatic hyperplasia) (756). In older men, nonsexually transmitted acute epididymitis is also associated with prostate biopsy, urinary tract instrumentation or surgery, systemic disease, and/or immunosuppression.
Chronic epididymitis is characterized by a ≥6 week history of symptoms of discomfort and/or pain in the scrotum, testicle, or epididymis. Chronic infectious epididymitis is most frequently seen in conditions associated with a granulomatous reaction; Mycobacterium tuberculosis (TB) is the most common granulomatous disease affecting the epididymis and should be suspected, especially in men with a known history of or recent exposure to TB. The differential diagnosis of chronic non-infectious epididymitis, sometimes termed “orchalgia/epididymalgia” is broad (i.e., trauma, cancer, autoimmune, and idiopathic conditions); men with this diagnosis should be referred to a urologist for clinical management (755,757).
Diagnostic Considerations
Men who have acute epididymitis typically have unilateral testicular pain and tenderness, hydrocele, and palpable swelling of the epididymis. Although inflammation and swelling usually begins in the tail of the epididymis, it can spread to involve the rest of the epididymis and testicle. The spermatic cord is usually tender and swollen. Spermatic cord (testicular) torsion, a surgical emergency, should be considered in all cases, but it occurs more frequently among adolescents and in men without evidence of inflammation or infection. In men with severe, unilateral pain with sudden onset, those whose test results do not support a diagnosis of urethritis or urinary-tract infection, or men in whom diagnosis of acute epididymitis is questionable, immediate referral to a urologist for evaluation of testicular torsion is important because testicular viability might be compromised.
Bilateral symptoms should raise suspicion of other causes of testicular pain. Radionuclide scanning of the scrotum is the most accurate method to diagnose epididymitis, but it is not routinely available. Ultrasound should be primarily used for ruling out torsion of the spermatic cord in cases of acute, unilateral, painful scrotum swelling. However, because partial spermatic cord torsion can mimic epididymitis on scrotal ultrasound, when torsion is not ruled out by ultrasound, differentiation between spermatic cord torsion and epididymitis must be made on the basis of clinical evaluation. Although ultrasound can demonstrate epididymal hyperemia and swelling associated with epididymitis, it provides minimal utility for men with a clinical presentation consistent with epididymitis, because a negative ultrasound does not alter clinical management. Ultrasound should be reserved for men with scrotal pain who cannot receive an accurate diagnosis by history, physical examination, and objective laboratory findings or if torsion of the spermatic cord is suspected.
All suspected cases of acute epididymitis should be evaluated for objective evidence of inflammation by one of the following point-of-care tests.
Gram or methylene blue or gentian violet (MB/GV) stain of urethral secretions demonstrating ≥2 WBC per oil immersion field (478). These stains are preferred point-of-care diagnostic tests for evaluating urethritis because they are highly sensitive and specific for documenting both urethral inflammation and the presence or absence of gonococcal infection. Gonococcal infection is established by documenting the presence of WBC-containing intracellular Gram-negative or purple diplococci on urethral Gram stain or MB/GV smear, respectively.
Positive leukocyte esterase test on first-void urine.
Microscopic examination of sediment from a spun first-void urine demonstrating ≥10 WBC per high power field.
All suspected cases of acute epididymitis should be tested for C. trachomatis and for N. gonorrhoeae by NAAT. Urine is the preferred specimen for NAAT testing in men (394). Urine cultures for chlamydia and gonococcal epididymitis are insensitive and are not recommended. Urine bacterial culture might have a higher yield in men with sexually transmitted enteric infections and in older men with acute epididymitis caused by genitourinary bacteriuria.
Treatment
To prevent complications and transmission of sexually transmitted infections, presumptive therapy is indicated at the time of the visit before all laboratory test results are available. Selection of presumptive therapy is based on risk for chlamydia and gonorrhea and/or enteric organisms. The goals of treatment of acute epididymitis are 1) microbiologic cure of infection, 2) improvement of signs and symptoms, 3) prevention of transmission of chlamydia and gonorrhea to others, and 4) a decrease in potential chlamydia/gonorrhea epididymitis complications (e.g., infertility and chronic pain). Although most men with acute epididymitis can be treated on an outpatient basis, referral to a specialist and hospitalization should be considered when severe pain or fever suggests other diagnoses (e.g., torsion, testicular infarction, abscess, and necrotizing fasciitis) or when men are unable to comply with an antimicrobial regimen. Because high fever is uncommon and indicates a complicated infection, hospitalization for further evaluation is recommended.
| Recommended Regimens |
|
|
| For acute epididymitis most likely caused by sexually transmitted chlamydia and gonorrhea |
| Ceftriaxone 250 mg IM in a single dose |
| PLUS |
| Doxycycline 100 mg orally twice a day for 10 days |
| For acute epididymitis most likely caused by sexually-transmitted chlamydia and gonorrhea and enteric organisms (men who practice insertive anal sex) |
| Ceftriaxone 250 mg IM in a single dose |
| PLUS |
| Levofloxacin 500 mg orally once a day for 10 days |
| OR |
| Ofloxacin 300 mg orally twice a day for 10 days |
| For acute epididymitis most likely caused by enteric organisms |
| Levofloxacin 500 mg orally once daily for 10 days |
| OR |
| Ofloxacin 300 mg orally twice a day for 10 days |
|
|
Therapy including levofloxacin or ofloxacin should be considered if the infection is most likely caused by enteric organisms and gonorrhea has been ruled out by gram, MB, or GV stain. This includes men who have undergone prostate biopsy, vasectomy, and other urinary-tract instrumentation procedures. As an adjunct to therapy, bed rest, scrotal elevation, and nonsteroidal anti-inflammatory drugs are recommended until fever and local inflammation have subsided. Complete resolution of discomfort might not occur until a few weeks after completion of the antibiotic regimen.
Other Management Considerations
Men who have acute epididymitis confirmed or suspected to be caused by N. gonorrhoeae or C. trachomatis should be advised to abstain from sexual intercourse until they and their partners have been adequately treated and symptoms have resolved. All men with acute epididymitis should be tested for other STDs, including HIV.
Follow-Up
Men should be instructed to return to their health-care providers if their symptoms fail to improve within 72 hours of the initiation of treatment. Signs and symptoms of epididymitis that do not subside within 3 days require re-evaluation of the diagnosis and therapy. Men who experience swelling and tenderness that persist after completion of antimicrobial therapy should be evaluated for alternative diagnoses, including tumor, abscess, infarction, testicular cancer, tuberculosis, and fungal epididymitis.
Management of Sex Partners
Men who have acute sexually transmitted epididymitis confirmed or suspected to be caused by N. gonorrhoeae or C. trachomatis should be instructed to refer for evaluation, testing, and presumptive treatment all sex partners with whom they have had sexual contact within the 60 days preceding onset of symptoms (see Chlamydial Infections and Gonorrheal Infections). If the last sexual intercourse was >60 days before onset of symptoms or diagnosis, the most recent sex partner should be treated. Arrangements should be made to link female partners to care. EPT and enhanced referral (see Partner Services) are effective strategies for treating female sex partners of men who have chlamydia or gonorrhea for whom linkage to care is anticipated to be delayed (93,94). Partners should be instructed to abstain from sexual intercourse until they and their sex partners are adequately treated and symptoms have resolved.
Special Considerations
Allergy, Intolerance, and Adverse Reactions
The cross reactivity between penicillins and cephalosporins is <2.5% in persons with a history of penicillin allergy (428–431,464). The risk for penicillin cross-reactivity is highest with first-generation cephalosporins, but is negligible between most second-generation (cefoxitin) and all third-generation (ceftriaxone) cephalosporins (428–431) (see Management of Persons with a History of Penicillin Allergy). Alternative regimens have not been studied; therefore, clinicians should consult infectious-disease specialists if such regimens are required.
HIV Infection
Men with HIV infection who have uncomplicated acute epididymitis should receive the same treatment regimen as those who are HIV negative. Other etiologic agents have been implicated in acute epididymitis in men with HIV infection, including CMV, salmonella, toxoplasmosis, Ureaplasma urealyticum, Corynebacterium sp., Mycoplasma sp., and Mima polymorpha. Fungi and mycobacteria also are more likely to cause acute epididymitis in men with HIV infection than in those who are immunocompetent.
Human Papillomavirus Infection
Approximately 100 types of human papillomavirus infection (HPV) have been identified, at least 40 of which can infect the genital area (758). Most HPV infections are self-limited and are asymptomatic or unrecognized. Most sexually active persons become infected with HPV at least once in their lifetime (533,759). Oncogenic, high-risk HPV infection (e.g., HPV types 16 and 18) causes most cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancers and precancers (760), whereas nononcogenic, low-risk HPV infection (e.g., HPV types 6 and 11) causes genital warts and recurrent respiratory papillomatosis. Persistent oncogenic HPV infection is the strongest risk factor for development of HPV-associated precancers and cancers. A substantial burden of cancers and anogenital warts are attributable to HPV in the United States: in 2009, an estimated 34,788 new HPV-associated cancers (761,762) and approximately 355,000 new cases of anogenital warts were associated with HPV infection (763).
Prevention
HPV Vaccines
There are several HPV vaccines licensed in the United States: a bivalent vaccine (Cervarix) that prevents infection with HPV types 16 and 18, a quadrivalent vaccine (Gardasil) that prevents infection with HPV types 6, 11, 16, and 18, and a 9-valent vaccine that prevents infection with HPV types 6, 11, 16, and 18, 31, 33, 45, 52, and 58. The bivalent and quadrivalent vaccines offer protection against HPV types 16 and 18, which account for 66% of all cervical cancers, and the 9-valent vaccine protects against five additional types accounting for 15% of cervical cancers. The quadrivalent HPV vaccine also protects against types 6 and 11, which cause 90% of genital warts.
All HPV vaccines are administered as a 3-dose series of IM injections over a 6-month period, with the second and third doses given 1–2 and 6 months after the first dose, respectively. The same vaccine product should be used for the entire 3-dose series. For girls, either vaccine is recommended routinely at ages 11–12 years and can be administered beginning at 9 years of age (16); girls and women aged 13–26 years who have not started or completed the vaccine series should receive the vaccine. The quadrivalent or 9-valent HPV vaccine is recommended routinely for boys aged 11–12 years; boys can be vaccinated beginning at 9 years of age (http://www.cdc.gov/vaccines/hcp/acip-recs/index.html). Boys and men aged 13–21 years who have not started or completed the vaccine series should receive the vaccine (16) (http://www.cdc.gov/vaccines/hcp/acip-recs/index.html). For previously unvaccinated, immunocompromised persons (including persons with HIV infection) and MSM, vaccination is recommended through age 26 years (16). In the United States, the vaccines are not licensed or recommended for use in men or women aged >26 years (16). HPV vaccines are not recommended for use in pregnant women. HPV vaccines can be administered regardless of history of anogenital warts, abnormal Pap/HPV tests, or anogenital precancer. Women who have received HPV vaccine should continue routine cervical cancer screening if they are aged ≥21 years. HPV vaccine is available for eligible children and adolescents aged <19 years through the Vaccines for Children (VFC) program (information available by calling CDC INFO [800-232-4636]). For uninsured persons aged 19–26 years, patient assistance programs are available from the vaccine manufacturers. Prelicensure and postlicensure safety evaluations have found the vaccine to be well tolerated (764) (http://www.cdc.gov/vaccinesafety/Vaccines/HPV/index.html). Impact-monitoring studies in the United States have demonstrated reductions of genital warts, as well as the HPV types contained within the quadrivalent vaccine (765,766). The current recommendations for HPV vaccination are available at http://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Settings that provide STD services should either administer the vaccine to eligible clients who have not started or completed the vaccine series or refer these persons to another facility equipped to provide the vaccine. Clinicians providing services to children, adolescents, and young adults should be knowledgeable about HPV and HPV vaccine (http://www.cdc.gov/vaccines/who/teens/for-hcp/hpv-resources.html). HPV vaccination has not been associated with initiation of sexual activity or sexual risk behaviors or perceptions about sexually transmitted infections (128).
Abstaining from sexual activity is the most reliable method for preventing genital HPV infection. Persons can decrease their chances of infection by practicing consistent and correct condom use and limiting their number of sex partners. Although these interventions might not fully protect against HPV, they can decrease the chances of HPV acquisition and transmission.
Diagnostic Considerations
HPV tests are available to detect oncogenic types of HPV infection and are used in the context of cervical cancer screening and management or follow-up of abnormal cervical cytology or histology (see Cervical Cancer, Screening Recommendations). These tests should not be used for male partners of women with HPV or women aged <25 years, for diagnosis of genital warts, or as a general STD test.
The application of 3%–5% acetic acid, which might cause affected areas to turn white, has been used by some providers to detect genital mucosa infected with HPV. The routine use of this procedure to detect mucosal changes attributed to HPV infection is not recommended because the results do not influence clinical management.
Treatment
Treatment is directed to the macroscopic (e.g., genital warts) or pathologic precancerous lesions caused by HPV. Subclinical genital HPV infection typically clears spontaneously; therefore, specific antiviral therapy is not recommended to eradicate HPV infection. Precancerous lesions are detected through cervical cancer screening (see Cervical Cancer, Screening Recommendations); HPV-related precancer should be managed based on existing guidance.
Counseling
Key Messages for Persons with HPV Infection
General
Anogenital HPV infection is very common. It usually infects the anogenital area but can infect other areas including the mouth and throat. Most sexually active people get HPV at some time in their lives, although most never know it.
Partners who have been together tend to share HPV, and it is not possible to determine which partner transmitted the original infection. Having HPV does not mean that a person or his/her partner is having sex outside the relationship.
Most persons who acquire HPV clear the infection spontaneously and have no associated health problems. When the HPV infection does not clear, genital warts, precancers, and cancers of the cervix, anus, penis, vulva, vagina, head, and neck might develop.
The types of HPV that cause genital warts are different from the types that can cause cancer.
Many types of HPV are sexually transmitted through anogenital contact, mainly during vaginal and anal sex. HPV also might be transmitted during genital-to-genital contact without penetration and oral sex. In rare cases, a pregnant woman can transmit HPV to an infant during delivery.
Having HPV does not make it harder for a woman to get pregnant or carry a pregnancy to term. However, some of the precancers or cancers that HPV can cause, and the treatments needed to treat them, might lower a woman’s ability to get pregnant or have an uncomplicated delivery. Treatments are available for the conditions caused by HPV, but not for the virus itself.
No HPV test can determine which HPV infection will clear and which will progress. However, in certain circumstances, HPV tests can determine whether a woman is at increased risk for cervical cancer. These tests are not for detecting other HPV-related problems, nor are they useful in women aged<25 years or men of any age.
Prevention of HPV
Two HPV vaccines can prevent diseases and cancers caused by HPV. The Cervarix and Gardasil vaccines protect against most cases of cervical cancer; Gardasil also protects against most genital warts. HPV vaccines are recommended routinely for boys and girls aged 11–12 years; either vaccine is recommended for girls/women, whereas only one vaccine (Gardasil) is recommended for boys/men (http://www.cdc.gov/vaccines/vpd-vac/hpv). These vaccines are safe and effective.
Condoms used consistently and correctly can lower the chances of acquiring and transmitting HPV and developing HPV-related diseases (e.g., genital warts and cervical cancer). However, because HPV can infect areas not covered by a condom, condoms might not fully protect against HPV.
Limiting number of sex partners can reduce the risk for HPV. However, even persons with only one lifetime sex partner can get HPV.
Abstaining from sexual activity is the most reliable method for preventing genital HPV infection.
Anogenital Warts
Of anogenital warts, 90% are caused by nononcogenic HPV types 6 or 11; these types can be commonly identified before or at the same time anogenital warts are detected (767). HPV types 16, 18, 31, 33, and 35 are also occasionally found in anogenital warts (usually as co-infections with HPV 6 or 11) and can be associated with foci of high-grade squamous intraepithelial lesions (HSIL), particularly in persons who have HIV infection. In addition to anogenital warts, HPV types 6 and 11 have been associated with conjunctival, nasal, oral, and laryngeal warts.
Anogenital warts are usually asymptomatic, but depending on the size and anatomic location, they can be painful or pruritic. They are usually flat, papular, or pedunculated growths on the genital mucosa. Anogenital warts occur commonly at certain anatomic sites, including around the vaginal introitus, under the foreskin of the uncircumcised penis, and on the shaft of the circumcised penis. Warts can also occur at multiple sites in the anogenital epithelium or within the anogenital tract (e.g., cervix, vagina, urethra, perineum, perianal skin, anus, and scrotum). Intra-anal warts are observed predominantly in persons who have had receptive anal intercourse, but they also can occur in men and women who have not had a history of anal sexual contact.
Diagnostic Considerations
Diagnosis of anogenital warts is usually made by visual inspection. The diagnosis of anogenital warts can be confirmed by biopsy, which is indicated if lesions are atypical (e.g., pigmented, indurated, affixed to underlying tissue, bleeding, or ulcerated lesions). Biopsy might also be indicated in the following circumstances, particularly if the patient is immunocompromised (including those infected with HIV): 1) the diagnosis is uncertain; 2) the lesions do not respond to standard therapy; or 3) the disease worsens during therapy. HPV testing is not recommended for anogenital wart diagnosis, because test results are not confirmatory and do not guide genital wart management.
Treatment
The aim of treatment is removal of the wart and amelioration of symptoms, if present. The appearance of warts also can result in significant psychosocial distress, and removal can relieve cosmetic concerns. In most patients, treatment results in resolution of the wart(s). If left untreated, anogenital warts can resolve spontaneously, remain unchanged, or increase in size or number. Because warts might spontaneously resolve within 1 year, an acceptable alternative for some persons is to forego treatment and wait for spontaneous resolution. Available therapies for anogenital warts might reduce, but probably do not eradicate, HPV infectivity. Whether the reduction in HPV viral DNA resulting from treatment reduces future transmission remains unknown.
Recommended Regimens
Treatment of anogenital warts should be guided by wart size, number, and anatomic site; patient preference; cost of treatment; convenience; adverse effects; and provider experience. No definitive evidence suggests that any one recommended treatment is superior to another, and no single treatment is ideal for all patients or all warts. The use of locally developed and monitored treatment algorithms has been associated with improved clinical outcomes and should be encouraged. Because all available treatments have shortcomings, some clinicians employ combination therapy (e.g., provider-administered cryotherapy with patient-applied topical therapy between visits to the provider). However, limited data exist regarding the efficacy or risk for complications associated with combination therapy. Treatment regimens are classified as either patient-applied or provider-administered modalities. Patient-applied modalities are preferred by some persons because they can be administered in the privacy of their home. To ensure that patient-applied modalities are effective, instructions should be provided to patients while in the clinic, and all anogenital warts should be accessible and identified during the clinic visit. Follow-up visits after several weeks of therapy enable providers to answer any questions about the use of the medication and address any side effects experienced; follow-up visits also facilitate the assessment of the response to treatment.
| Recommended Regimens for External Anogenital Warts (i.e., penis, groin, scrotum, vulva, perineum, external anus, and perianus*) |
|
|
| Patient-Applied: |
| Imiquimod 3.75% or 5% cream† |
| OR |
| Podofilox 0.5% solution or gel |
| OR |
| Sinecatechins 15% ointment† |
| Provider–Administered: |
| Cryotherapy with liquid nitrogen or cryoprobe |
| OR |
| Surgical removal either by tangential scissor excision, tangential shave excision, curettage, laser, or electrosurgery |
| OR |
| Trichloroacetic acid (TCA) or bichloroacetic acid (BCA) 80%–90% solution |
|
|
| *Many persons with external anal warts also have intra-anal warts. Thus, persons with external anal warts might benefit from an inspection of the anal canal by digital examination, standard anoscopy, or high-resolution anoscopy. |
| †Might weaken condoms and vaginal diaphragms. |
Imiquimod is a patient-applied, topically active immune enhancer that stimulates production of interferon and other cytokines. Imiquimod 5% cream should be applied once at bedtime, three times a week for up to 16 weeks (768). Similarly, imiquimod 3.75% cream should be applied once at bedtime, but is applied every night (769). With either formulation, the treatment area should be washed with soap and water 6–10 hours after the application. Local inflammatory reactions, including redness, irritation, induration, ulceration/erosions, and vesicles might occur with the use of imiquimod, and hypopigmentation has also been described (770). A small number of case reports demonstrate an association between treatment with imiquimod cream and worsened inflammatory or autoimmune skin diseases (e.g., psoriasis, vitiligo, and lichenoid dermatoses) (771–773). Data from studies of human subjects are limited regarding use of imiquimod in pregnancy, but animal data suggest that this therapy poses low risk (317).
Podofilox (podophyllotoxin) is a patient-applied antimitotic drug that causes wart necrosis. Podofilox solution (using a cotton swab) or podofilox gel (using a finger) should be applied to anogenital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle can be repeated, as necessary, for up to four cycles. The total wart area treated should not exceed 10 cm2, and the total volume of podofilox should be limited to 0.5 mL per day. If possible, the health-care provider should apply the initial treatment to demonstrate proper application technique and identify which warts should be treated. Mild to moderate pain or local irritation might develop after treatment. Podofilox is contraindicated in pregnancy (317).
Sinecatechins is a patient-applied, green-tea extract with an active product (catechins). Sinecatechins 15% ointment should be applied three times daily (0.5 cm strand of ointment to each wart) using a finger to ensure coverage with a thin layer of ointment until complete clearance of warts is achieved. This product should not be continued for longer than 16 weeks (774–776). The medication should not be washed off after use. Genital, anal, and oral sexual contact should be avoided while the ointment is on the skin. The most common side effects of sinecatechins are erythema, pruritus/burning, pain, ulceration, edema, induration, and vesicular rash. The medication is not recommended for persons with HIV infection, other immunocompromised conditions, or with genital herpes because the safety and efficacy of therapy has not been evaluated. The safety of sinecatechins during pregnancy is unknown.
Cryotherapy is a provider-applied therapy that destroys warts by thermal-induced cytolysis. Health-care providers must be trained on the proper use of this therapy because over- and under-treatment can result in complications or low efficacy. Pain during and after application of the liquid nitrogen, followed by necrosis and sometimes blistering, is common. Local anesthesia (topical or injected) might facilitate therapy if warts are present in many areas or if the area of warts is large.
Surgical therapy has the advantage of eliminating most warts at a single visit, although recurrence can occur. Surgical removal requires substantial clinical training, additional equipment, and sometimes a longer office visit. After local anesthesia is applied, anogenital warts can be physically destroyed by electrocautery, in which case no additional hemostasis is required. Care must be taken to control the depth of electrocautery to prevent scarring. Alternatively, the warts can be removed either by tangential excision with a pair of fine scissors or a scalpel, by carbon dioxide (CO2) laser, or by curettage. Because most warts are exophytic, this procedure can be accomplished with a resulting wound that only extends into the upper dermis. Hemostasis can be achieved with an electrocautery unit or, in cases of very minor bleeding, a chemical styptic (e.g., an aluminum chloride solution). Suturing is neither required nor indicated in most cases. In patients with large or extensive warts, surgical therapy, including CO2 laser, might be most beneficial; such therapy might also be useful for intraurethral warts, particularly for those persons who have not responded to other treatments. Treatment of anogenital and oral warts should be performed in an appropriately ventilated room using standard precautions (http://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf#page=2) and local exhaust ventilation (e.g., a smoke evacuator) (777) (http://www.cdc.gov/niosh/docs/hazardcontrol/hc11.html).
Trichloroacetic acid (TCA) and bichloroacetic acid (BCA) are provider-applied caustic agents that destroy warts by chemical coagulation of proteins. Although these preparations are widely used, they have not been investigated thoroughly. TCA solution has a low viscosity comparable with that of water and can spread rapidly and damage adjacent tissues if applied excessively. A small amount should be applied only to the warts and allowed to dry (i.e., develop white frost on tissue) before the patient sits or stands. If pain is intense or an excess amount of acid is applied, the area can be covered with sodium bicarbonate (i.e., baking soda), washed with liquid soap preparations, or be powdered with talc to neutralize the acid or remove unreacted acid. TCA/BCA treatment can be repeated weekly if necessary.
Alternative Regimens for External Genital Warts
Less data are available regarding the efficacy of alternative regimens for treating anogenital warts, which include podophyllin resin, intralesional interferon, photodynamic therapy, and topical cidofovir. Further, alternative regimens might be associated with more side effects. Podopyllin resin is no longer a recommended regimen because of the number of safer regimens available, and severe systemic toxicity has been reported when podophyllin resin was applied to large areas of friable tissue and was not washed off within 4 hours (778–780). Podophyllin resin 10%–25% in a compound tincture of benzoin might be considered for provider-administered treatment under conditions of strict adherence to recommendations. Podophyllin should be applied to each wart and then allowed to air-dry before the treated area comes into contact with clothing. Over-application or failure to air-dry can result in local irritation caused by spread of the compound to adjacent areas and possible systemic toxicity. The treatment can be repeated weekly, if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, 1) application should be limited to <0.5 mL of podophyllin or an area of <10 cm2 of warts per session; 2) the area to which treatment is administered should not contain any open lesions, wounds, or friable tissue; and 3) the preparation should be thoroughly washed off 1–4 hours after application. Podophyllin resin preparations differ in the concentration of active components and contaminants. Shelf-life and stability of podophyllin preparations are unknown. The safety of podophyllin during pregnancy has not been established.
| Recommended Regimens for Urethral Meatus Warts |
|
|
| Cryotherapy with liquid nitrogen |
| OR |
| Surgical removal |
|
|
| Recommended Regimens for Vaginal Warts |
|
|
| Cryotherapy with liquid nitrogen. The use of a cryoprobe in the vagina is not recommended because of the risk for vaginal perforation and fistula formation. |
| OR |
| Surgical removal |
| OR |
| TCA or BCA 80%–90% solution |
|
|
| Recommended Regimens for Cervical Warts |
|
|
| Cryotherapy with liquid nitrogen |
| OR |
| Surgical removal |
| OR |
| TCA or BCA 80%–90% solution |
| Management of cervical warts should include consultation with a specialist. |
| For women who have exophytic cervical warts, a biopsy evaluation to exclude high-grade SIL must be performed before treatment is initiated. |
|
|
| Recommended Regimens for Intra-anal Warts |
|
|
| Cryotherapy with liquid nitrogen |
| OR |
| Surgical removal |
| OR |
| TCA or BCA 80%–90% solution |
| Management of intra-anal warts should include consultation with a specialist. |
|
|
Follow-Up
Most anogenital warts respond within 3 months of therapy. Factors that might affect response to therapy include immunosuppression and treatment compliance. In general, warts located on moist surfaces or in intertriginous areas respond best to topical treatment. A new treatment modality should be selected when no substantial improvement is observed after a complete course of treatment or in the event of severe side effects; treatment response and therapy-associated side effects should be evaluated throughout the course of therapy. Complications occur rarely when treatment is administered properly. Persistent hypopigmentation or hyperpigmentation can occur with ablative modalities (e.g., cryotherapy and electrocautery) and have been described with immune modulating therapies (e.g., imiquimod cream). Depressed or hypertrophic scars are uncommon but can occur, especially if patients have insufficient time to heal between treatments. Rarely, treatment can result in chronic pain syndromes (e.g., vulvodynia and hyperesthesia of the treatment site) or, in the case of anal warts, painful defecation or fistulas.
Counseling
Key Messages for Persons with Anogenital Warts
If left untreated, genital warts may go away, stay the same, or increase in size or number. The types of HPV that cause genital warts are different from the types that can cause cancer.
Women with genital warts do not need Pap tests more often than other women.
Time of HPV acquisition cannot be definitively determined. Genital warts can develop months or years after getting HPV. HPV types that cause genital warts can be passed on to another person even in the absence of visible signs of warts. Sex partners tend to share HPV, even though signs of HPV (e.g., warts) might occur in only one partner or in neither partner.
Although genital warts are common and benign, some persons might experience considerable psychosocial impact after receiving this diagnosis.
Although genital warts can be treated, such treatment does not cure the virus itself. For this reason, it is common for genital warts to recur after treatment, especially in the first 3 months.
Because genital warts can be sexually transmitted, patients with genital warts benefit from testing for other STDs. Sexual activity should be avoided with new partners until the warts are gone or removed. HPV might remain present and can still be transmitted to partners even after the warts are gone.
Condoms might lower the chances of transmitting genital warts if used consistently and correctly; however, HPV can infect areas that are not covered by a condom and might not fully protect against HPV.
A vaccine is available for males and females to prevent genital warts (Gardasil), but it will not treat existing HPV or genital warts. This vaccine can prevent most cases of genital warts in persons who have not yet been exposed to wart-causing types of HPV.
Management of Sex Partners
Persons should inform current partner(s) about having genital warts because the types of HPV that cause warts can be passed on to partners. Partners should receive counseling messages that partners might already have HPV despite no visible signs of warts, so HPV testing of sex partners of persons with genital warts is not recommended. Partner(s) might benefit from a physical examination to detect genital warts and tests for other STDs. No recommendations can be made regarding informing future sex partners about a diagnosis of genital warts because the duration of viral persistence after warts have resolved is unknown.
Special Considerations
Pregnancy
Podofilox (podophyllotoxin), podophyllin, and sinecatechins should not be used during pregnancy. Imiquimod appears to pose low risk but should be avoided until more data are available. Anogenital warts can proliferate and become friable during pregnancy. Although removal of warts during pregnancy can be considered, resolution might be incomplete or poor until pregnancy is complete. Rarely, HPV types 6 and 11 can cause respiratory papillomatosis in infants and children, although the route of transmission (i.e., transplacental, perinatal, or postnatal) is not completely understood. Whether cesarean section prevents respiratory papillomatosis in infants and children also is unclear (781); therefore, cesarean delivery should not be performed solely to prevent transmission of HPV infection to the newborn. Cesarean delivery is indicated for women with anogenital warts if the pelvic outlet is obstructed or if vaginal delivery would result in excessive bleeding. Pregnant women with anogenital warts should be counseled concerning the low risk for warts on the larynx of their infants or children (recurrent respiratory papillomatosis).
HIV Infection and Other Causes of Immunosuppression
Persons with HIV infection or who are otherwise immunosuppressed are more likely to develop anogenital warts than those who do not have HIV infection (782). Moreover, such persons can have larger or more numerous lesions, might not respond to therapy as well as those who are immunocompetent, and might have more frequent recurrences after treatment (782–785). Despite these factors, data do not support altered approaches to treatment for persons with HIV infection. Squamous cell carcinomas arising in or resembling anogenital warts might occur more frequently among immunosuppressed persons, therefore requiring biopsy for confirmation of diagnosis for suspicious cases (786–788).
High-grade Squamous Intraepithelial Lesions (HSIL)
Biopsy of an atypical wart might reveal HSIL or cancer of the anogenital tract. In this instance, referral to a specialist for treatment is recommended.
HPV-Associated Cancers and Precancers
Persistent infection with oncogenic types of HPV has a causal role in nearly all cervical cancers and in many vulvar, vaginal, penile, anal, and oropharyngeal cancers (789). However, the only HPV-associated cancer for which routine screening is recommended is cervical cancer.
Cervical Cancer
Screening Recommendations
Recommendations for cervical cancer screening in the United States are based on systematic evidence reviews and are largely consistent across the major medical organizations, including ACS, ACOG, and USPSTF (124–126) (http://www.cdc.gov/cancer/cervical/index.htm). Routine cervical screening should be performed starting at age 21 years and continue through age 65 years to prevent invasive cervical cancer. Testing can be performed using either conventional or liquid-based cytologic tests (i.e., Pap tests). For women aged ≥30 years, screening can include several FDA-approved oncogenic or high risk HPV (HR-HPV) tests. For cytopathologic and HR-HPV testing, clinics should use CLIA-certified laboratories using acceptable terminology (Bethesda 2001 or LAST terminology) (790,791). Annual cervical cancer screening is no longer recommended for all women. Instead, Pap testing is recommended every 3 years from ages 21–29 years. During age 30–65 years, women should either receive a Pap test every 3 years or a Pap test plus HPV test (co-test) every 5 years; co-testing can be done by either collecting one swab for the Pap test and another for the HPV test or by using the remaining liquid cytology material for the HPV test. Because of the high negative predictive value of two tests, women who test negative for both HPV and Pap test should not be screened again for 5 years. Cervical screening programs should screen women who have received HPV vaccination in the same manner as unvaccinated women. All major medical organizations concur that no Pap testing is recommended before age 21 years.
Women should be given a copy of their test results (Pap and HPV, if applicable); those with normal results should also be provided with general recommendations regarding when to schedule follow-up visits and the importance of cervical cancer screening. Women with abnormal screening tests should be referred to providers who are experienced in managing these cases (see Follow-Up). Women should be reassured and counseled about abnormal cervical cancer screening test results and informed about any implications for sex partner(s). (See counseling messages for HPV infection and for women receiving cervical cancer screening.)
The following additional management considerations are associated with performing Pap tests:
The Pap test should not be considered a screening test for STDs.
All women should receive cervical cancer screening, regardless of sexual orientation (i.e., women who identify as lesbian, bisexual, or heterosexual).
Ideally, women should be advised to have a Pap test 10–20 days after the first day of menses. However, this test can be performed during menstruation depending on menstrual flow and type of cytology used (liquid-based cytology can differentiate cells from blood and mucus; conventional Pap test might not).
If specific infections other than HPV (e.g., chlamydia or gonorrhea) are identified at the visit, the woman might need to have a repeat Pap test after appropriate treatment for those infections. However, in most instances (even in the presence of some severe infections), Pap tests will be reported as satisfactory for evaluation, and reliable final reports can be produced without the need to repeat the Pap test after treatment is received.
The presence of a mucopurulent discharge should not postpone Pap testing. The test can be performed after removal of the discharge with a saline-soaked cotton swab.
In the presence of cervical friability (see Cervicitis), liquid-based cytology should be used; conventional pap testing might need to be deferred in the presence of heavy bleeding until cervicitis is treated.
In the absence of other indications, women who have external genital warts do not need Pap tests more frequently than women who do not have warts.
The sequence of Pap testing in relation to collection of other endocervical specimens does not influence Pap test results or their interpretation (792). In general, vaginal specimens are preferred for chlamydia and gonorrhea screening in women, but in the setting of a pelvic exam, endocervical specimens for STD testing can be collected first.
Women who have had a total hysterectomy do not require a routine Pap test unless the hysterectomy was performed because of cervical cancer or its precursor lesions. In women whose cervix remains intact after a hysterectomy, regularly scheduled Pap tests should be performed as indicated (793–795).
Health-care facilities that train providers on Pap test collection and employ simple quality assurance measures are more likely to obtain satisfactory test results (as determined by the laboratory).
The use of instruments designed to sample to the cervical transformation zone (e.g., cytobrushes) improves the accuracy of Pap tests (796).
Liquid-based cytology is an acceptable alternative to conventional Pap tests, as it has similar test-performance characteristics.
At an initial visit, providers should ask women about their most recent Pap test and results and history of evaluation and treatment (e.g., loop electrosurgical excision procedure and colposcopy) to assist with management; every effort should be made to obtain copies of recent results. The importance and frequency of Pap testing or co-testing (Pap and HPV testing) should be reinforced.
HPV Tests for Cervical Cancer Screening
Clinical tests for oncogenic types of HPV are used for 1) cervical cancer screening in conjunction with a Pap test, 2) triage of abnormal cervical cytology results, and 3) follow-up after treatment of cervical precancers. These tests are only approved for use with cervical specimens, not oral or anal specimens. The role of testing for non-oncogenic HPV types (e.g., 6 and 11) is unclear and is not recommended.
Current FDA-cleared HPV tests detect viral nucleic acid (DNA) or messenger RNA (mRNA). Several FDA-cleared tests for HPV are available for use in the United States, but use of non-oncogenic (e.g., types 6 and 11) tests is not recommended (110). The Hybrid Capture 2 High-Risk HPV DNA test (Qiagen, Gaithersburg, Maryland) and the Cervista HPV High-Risk DNA test (Hologics, Beford, Massachusetts) detect presence of 13–14 oncogenic HPV types, whereas the Cervista HPV 16/18 DNA test only detects oncogenic HPV types 16 and 18. The Digene HC2 HPV DNA test (Qiagen, Gaithersburg, Maryland) detects 13 oncogenic or five non-oncogenic HPV types. The Cobas 4500 (Roche, Pleasanton California) test detects 14 oncogenic HPV DNA types and can detect individual types HPV 16 and 18, while the APTIMA HR HPV (Gen Probe, San Diego CA) test detects 14 oncogenic HPV types of HPV mRNA. Aptima HPV 16/18/45 test is also FDA-cleared to triage its pooled Aptima HR HPV test further, although there are no algorithms for HPV 16/18/45 testing in any clinical guidelines. HPV assays should be FDA-cleared and used only for the appropriate indications (110).
In the United States, HPV tests to detect oncogenic types of HPV infection are most commonly used to triage Pap test results indicating atypical squamous cells of undetermined significance (ASC-US) in women aged ≥25 years (110). HPV testing for oncogenic types are now being incorporated into cervical cancer screening recommendations with Pap tests (i.e., co-testing) to reduce follow-up visits in women aged ≥30 years (see Screening Recommendations). HPV testing can be performed on the same swab as used for the Pap test or a separate supplied swab; reflex testing of residual material of a liquid-based cytology specimen is another option. HPV testing for 16 and 18 is also used to triage discordant test results (i.e., in the case of a negative Pap test and positive HPV test). In the future, oncogenic (high-risk) HPV tests might be considered for primary cervical cancer screening, but no such recommendation has been made by any medical organization.
HPV testing (including oncogenic HPV and HPV 16/18 tests) should not be performed in the following situations:
Deciding whether to vaccinate against HPV;
Conducting STD screening in women or men at risk for STDs;
Providing care to persons with genital warts or their partners;
Conducting screening for cervical cancer as a stand-alone test (i.e., without a concurrent Pap test);
Testing women aged <30 years as part of routine cervical cancer screening; or
Testing oral or anal specimens.
Follow-Up
If the results of the Pap test are abnormal, follow-up care should be provided according to ASCCP 2012 Consensus Guidelines for Management of Abnormal Cervical Cytology (110). If clinic resources do not allow for follow-up of women with abnormal results, protocols for linkage to follow-up care and management should be in place. The following are highlights of the ASCCP guidelines.
Women aged 21–24 years are managed more conservatively than other women because of potential harms of overtreatment and low risk for cancer. For women in this age group who have ASC-US or LSIL, cytology should be repeated in 12 months.
For women with ASC-US cytology, either cytology can be repeated in 12 months (for women of all ages) or reflex HPV testing can be performed (for women aged ≥25 years).
For women with ASC-US who are HPV negative, a repeat HPV and Pap test in 3 years is recommended.
For women who have normal cytology but lack endocervical cells, a repeat Pap is not required. For women who have unsatisfactory cytology, regardless of negative HPV result, a repeat cytology is required in 2–4 months.
HPV 16/18 testing is one follow-up option for women who have discordant results (normal Pap test accompanied by a positive HPV test). If the HPV 16/18 test is positive, women should immediately receive colposcopy. If negative, these women should repeat the HPV co-test in 1 year.
For women with LSIL or HSIL, management should be provided by a specialist according to existing guidelines (http://www.asccp.org).
Clinics in settings serving women who might not adhere to follow-up recommendations for whom linkage to care is unlikely should consider offering in-house colposcopy and biopsy services. ASCCP has an app available for purchase and download for management of abnormal cytologic and histologic results. Although this app takes current results into consideration, clinicians are required to have knowledge of past abnormal Pap or cervical procedures to provide management guidance (http://www.asccp.org/Bookstore/ASCCP-Algorithms-Mobile-App).
Counseling
Women might believe the Pap test screens for conditions other than cervical cancer or might be confused by abnormal results (797–799). Health-care providers, a trusted source of information about HPV and abnormal Pap test results, are critical in educating women about high-risk HPV and can moderate the psychosocial impact of abnormal results (1,735,800,801). Women should be counseled on the risks, uncertainties, and benefits of screening (126,802). Education, counseling, and follow-up reminders by phone, text, or email might increase screening and adherence to follow-up (803). Multiple forms of communication (e.g., in-person counseling and printed or online information) might be more effective than one form alone (804). Print materials and online resources are available at http://www.cdc.gov/cancer/cervical/basic_info/screening.htm; http://www.cdc.gov/std/hpv/common/; http://www.ashasexualhealth.org/stdsstis/hpv/hpv-cervical-cancer.
Abnormal Pap test and/or HPV test results can cause short-term anxiety, stress, fear, and confusion, decreasing women’s ability to absorb and retain information and possibly acting as a barrier to follow-up care (798,805–807). A positive HPV test might exacerbate these feelings and elicit concerns about partner(s); worry about disclosure; and feelings of guilt, anger, and stigmatization (800,806). Providers should frame oncogenic HPV positivity in a neutral, nonstigmatizing context and emphasize its common, asymptomatic, and transient nature. Providers also should emphasize that HPV is often shared between partners. Therefore, having HPV does not imply infidelity, nor should it raise concerns about a partner’s health (800).
Key Messages for Women Regarding Cervical Cancer Screening
Cervical cancer can be prevented with regular screening tests, like the Pap test and the HPV DNA test (HPV test). All women should start getting regular Pap tests at age 21 years.
The Pap test can find abnormal cells on a woman’s cervix, which could lead to cervical cancer over time, and an HPV test detects HPV infection of the cervix. The HPV test can be used at the same time as the Pap test (known as “co-testing”) for women aged ≥30 years. The HPV test also can be used after an inconclusive Pap test among women aged ≥25 years; testing for this purpose is known as “reflex HPV testing.”
Positive Pap and HPV tests are markers of early signs of cervical cancer, which often does not cause symptoms until it is advanced. Appropriate follow-up is essential to ensure that cervical cancer does not develop. All women, even those who feel healthy, should receive screening for cervical cancer.
HPV is a common infection and is often cleared from the body without any medical interventions. A positive HPV test does not mean that a person has cancer.
HPV is often shared between partners and can lie dormant for many years; having HPV does not imply infidelity, nor should it necessarily raise concerns about a partner’s health (http://www.cdc.gov/cancer/hpv/basic_info/screening).
Management of Sex Partners
The benefit of disclosing a positive oncogenic HPV test to current and future sex partners is unclear. The following counseling messages can be communicated to sex partners:
Sex partners do not need to be tested for HPV.
Sex partners tend to share HPV, even though signs of HPV such as an abnormal Pap-test result might occur in only one partner. Sex partners of persons with HPV infection also likely have HPV.
When used correctly and consistently, condoms might lower the risk for HPV infection and might decrease the time to clear in women with HPV infection. However, HPV can infect areas not covered by the condom and might not fully protect against HPV.
Additional messages for partners include the messages for persons with HPV (see Counseling Messages for Persons with HPV).
Special Considerations
Pregnancy
Pregnant women should be screened at the same intervals as nonpregnant women. However, pregnant women with abnormal screening tests should be referred to a specialist (808–810), because treatment recommendations differ for this population. A swab, Ayre’s spatula, or cytobrush can be used for obtaining Pap tests in pregnant women (811–813).
HIV Infection
Several studies have documented an increased risk for cervical precancers and cancers in women with HIV infection (814,815). Women with HIV infection should be screened within 1 year of sexual activity or initial HIV diagnosis using conventional or liquid-based cytology (Pap test); testing should be repeated 6 months later. Management recommendations for women with HIV infection are detailed elsewhere (http://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/0) (247).
Adolescents
Prevalence of oncogenic HPV types are high among adolescents aged <21 years (816), and oncogenic HPV and squamous intraepithelial lesions caused by HPV in adolescent girls are more likely to regress than those in older women. For these reasons, cervical cancer screening and HPV testing are not recommended in adolescents. However, for adolescents with HIV infection, providers should screen 1 year after onset of sexual activity, regardless of age or mode of HIV infection (e.g., perinatally acquired or sexually acquired) (247); such screening is warranted because of the reported high rate of progression of abnormal cytology in adolescents with HIV infection.
Anal Cancer
Data are insufficient to recommend routine anal cancer screening with anal cytology in persons with HIV infection, MSM without HIV infection, and the general population. However, anal cytology might be useful for detecting anal cancer in persons who have masses on palpation, particularly persons with HIV infection and HIV-negative MSM with a history of receptive anal intercourse (247). More evidence is needed concerning the natural history of anal intraepithelial neoplasia, the best screening methods and target populations, the safety and response to treatments, and other programmatic considerations before screening can be routinely recommended. However, some clinical centers perform anal cytology to screen for anal cancer among high-risk populations (e.g., persons with HIV infection, MSM, and history of receptive anal intercourse), followed by high-resolution anoscopy (HRA) for those with abnormal cytologic results (e.g., ASC-US or worse). Oncogenic HPV tests are not clinically useful for anal cancer screening among MSM because of a high prevalence of anal HPV infection (817,818).
Viral Hepatitis
Hepatitis A
Hepatitis A, caused by infection with the hepatitis A virus (HAV), has an incubation period of approximately 28 days (range: 15–50 days) (819). HAV replicates in the liver and is shed in high concentrations in feces from 2–3 weeks before to 1 week after the onset of clinical illness. HAV infection produces a self-limited disease that does not result in chronic infection or CLD. However, up to 10% of patients experience a relapse of symptoms during the 6 months after acute illness. Acute liver failure from hepatitis A is rare (overall case-fatality rate: 0.5%). The risk for symptomatic infection is directly related to age, with >70% of adults having symptoms compatible with acute viral hepatitis and most children having either asymptomatic or unrecognized infection. Antibody produced in response to HAV infection persists for life and confers protection against reinfection (820).
HAV infection is primarily transmitted by the fecal-oral route, by either person-to-person contact or through consumption of contaminated food or water (821). Transmission of HAV during sexual activity probably results from fecal-oral contact; however, efforts to promote good personal hygiene have not been successful in interrupting outbreaks of hepatitis A. Although viremia occurs early in infection and can persist for several weeks after onset of symptoms, bloodborne transmission of HAV is uncommon (822). Transmission by saliva has not been demonstrated.
In the United States, of the hepatitis A cases accompanied by risk information reported during 2010, a particular risk was identified in only 25% (823). Among adults with identified risk factors, most cases occurred among sexual and household contacts; those with children attending a nursery, daycare, or preschool and persons working in such settings; MSM; IDUs (823); international travelers; and persons exposed to a common-source food or water outbreak.
Diagnostic Considerations
The diagnosis of hepatitis A cannot be made on a clinical basis alone, but rather requires serologic testing. The presence of IgM antibody to HAV is diagnostic of acute HAV infection. A positive test for total anti-HAV indicates immunity to HAV infection but does not differentiate current from previous HAV infection. Although usually not sensitive enough to detect the low level of protective antibody after vaccination, anti-HAV tests also might be positive after hepatitis A vaccination.
Treatment
Patients with acute hepatitis A usually require only supportive care, with no restrictions in diet or activity. Hospitalization might be necessary for patients who become dehydrated because of nausea and vomiting and is critical for patients with signs or symptoms of acute liver failure. Medications that might cause liver damage or are metabolized by the liver should be used with caution among persons with hepatitis A.
Prevention
Vaccination is the most effective means of preventing HAV transmission among persons at risk for infection (e.g., MSM, drug users, and persons with CLD). Hepatitis A vaccines are prepared from formalin-inactivated, cell-culture–derived HAV. Two monovalent vaccines (HAVRIX, GlaxoSmithKline; VAQTA, Merck and Co., Inc.) are cleared by FDA for persons aged ≥12 months (Table 2), and these vaccines are available to eligible children and adolescents aged <19 years through the VFC program (telephone: 800-232-4636).
TABLE 2.
Recommended regimens: dose and schedule for hepatitis A vaccines
| Vaccine | Age (yrs) | Dose | Volume (mL) | Two-dose schedule (months)* |
|---|---|---|---|---|
| HAVRIX† | 1–18 | 720 (EL.U.) | 0.5 | 0 (6–12) |
| >18 | 1,440 (EL.U.) | 1.0 | 0 (6–12) | |
| VAQTA§ | 1–18 | 25 (U) | 0.5 | 0 (6–18) |
| >18 | 50 (U) | 1.0 | 0 (6–18) |
Source: CDC. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(No. RR-7).
Abbreviations: EL.U = Enzyme-linked immunosorbent assay (ELISA) units; U = units.
0 months represents timing of the initial dose; subsequent numbers represent months after the initial dose.
Hepatitis A vaccine, inactivated, GlaxoSmithKline Biologicals; this vaccine is also licensed for a 3-dose series in children aged 2–18 years, with 360 EL.U, 0.5 mL doses at 0, 1, and 6–12 months.
Hepatitis A vaccine, inactivated, Merck and Co., Inc.
Administered IM in a 2-dose series at 0 and 6–18 months, hepatitis A vaccines induce protective antibody levels in virtually all adults: by 1 month after the first dose, 94%–100% of adults have protective antibody levels and after a second dose, 100% achieve protective levels (2). Kinetic models of antibody decline indicate that protective levels of antibody persist for at least 20 years. A study in persons who are Alaska Natives demonstrated that seropositivity for hepatitis A persists for at least 10 years after completing 2-dose vaccination at age 12–21 months (824). Sustained protection and the need for booster dosing will continue to be assessed (825,826). A combined hepatitis A and hepatitis B vaccine (Twinrix) has been developed and licensed for use as a 3-dose series in adults aged ≥18 years at risk for hepatitis A and hepatitis B infections. When administered IM on a 0-, 1-, and 6-month schedule, the vaccine has equivalent immunogenicity to that of the monovalent vaccines.
Immune globulin (IG) administered IM can provide postexposure prophylaxis against HAV. IG is a sterile solution of concentrated immunoglobulins prepared from pooled human plasma processed by cold ethanol fractionation. In the United States, IG is produced only from plasma that has tested negative for hepatitis B surface antigen, antibodies to HIV and HCV, and HIV and HCV RNA. In addition, the process used to manufacture IG inactivates viruses (e.g., HBV, HCV, and HIV). When administered IM within 2 weeks after exposure to HAV, IG is >85% effective in preventing HAV infections (827).
Pre-exposure Vaccination
The following persons seeking STD services should be offered hepatitis A vaccine: 1) all MSM; 2) drug users (injection and noninjection illicit drugs); and 3) persons with CLD, including persons with chronic HBV and HCV infection who have evidence of CLD. If persons are at risk for both hepatitis A and hepatitis B, the combined vaccine can be considered.
Prevaccination Serologic Testing
Approximately one third of the U.S. population has serologic evidence of previous HAV infection, the prevalence of which increases with age (828). The potential cost-savings of prevaccination testing for susceptibility should be weighed against cost and the likelihood that testing will interfere with initiating vaccination; serologic testing should not be a barrier to vaccination of at-risk populations. In these cases, the first vaccine dose should be administered immediately after collection of the blood sample for serologic testing. Vaccination of a person who is already immune is not harmful. Persons who have a documented history of ≥2-dose hepatitis A vaccination do not need further vaccination or serologic testing.
Postvaccination Serologic Testing
Postvaccination serologic testing for immunity is not indicated because most persons respond to the vaccine. In addition, the commercially available serologic test is not sensitive enough to detect the low but protective levels of antibody produced by vaccination.
Postexposure Prophylaxis
Persons who recently have been exposed to HAV and who previously have not received hepatitis A vaccine should be administered a single dose of monovalent hepatitis A vaccine or IG (0.02 mL/kg) as soon as possible, ideally within 2 weeks of exposure because the efficacy of vaccine or IG or vaccine when administered >2 weeks after exposure has not been established (820). Information about the relative efficacy of vaccine compared with IG postexposure is limited, and no data are available for persons aged >40 years or those with underlying medical conditions. Therefore, decisions to use vaccine versus IG should be informed by patient characteristics associated with more severe manifestations of hepatitis A (including older age and CLD) and the magnitude of the risk for HAV transmission resulting from the exposure.
IG should be used for children aged <12 months, immunocompromised persons, persons who have had diagnosed CLD, and persons for whom vaccine is contraindicated. For persons aged >40 years, IG is preferred because of the absence of information regarding vaccine performance and the more severe manifestations of hepatitis A in this age group; vaccine can be used if IG cannot be obtained. For healthy persons aged 12 months to 40 years, monovalent hepatitis A vaccine at the age-appropriate dose is preferred over IG because of the advantages associated with vaccination, including long-term protection and ease of administration.
If IG is administered to persons for whom hepatitis A vaccine also is recommended, a dose of vaccine should be provided simultaneously with IG, and the second vaccine dose should be administered according to the licensed schedule to complete the series. The combined vaccine can be considered in persons for whom both hepatitis A and hepatitis B vaccine is recommended.
Special Considerations
Limited data indicate that hepatitis A vaccination of persons with CLD and of persons with advanced HIV infection results in lower efficacy and antibody concentrations (247). In persons with HIV infection, antibody response can be directly related to CD4+ levels.
Hepatitis B
Hepatitis B is caused by infection with the hepatitis B virus (HBV). The incubation period from time of exposure to onset of symptoms is 6 weeks to 6 months. The highest concentrations of HBV are found in blood, with lower concentrations found in other body fluids including wound exudates, semen, vaginal secretions, and saliva (829,830). HBV is more infectious and more stable in the environment than other bloodborne pathogens (e.g., HCV and HIV).
HBV infection can be self-limited or chronic. In adults, approximately half of newly acquired HBV infections are symptomatic, and approximately 1% of reported cases result in acute liver failure and death (831). Risk for chronic infection is inversely related to age at acquisition; approximately 90% of infected infants and 30% of infected children aged <5 years become chronically infected compared with 2%–6% of persons who become infected as adults (832). Among persons with chronic HBV infection, the risk for premature death from cirrhosis or hepatocellular carcinoma (HCC) is 15%–25% (833).
HBV is efficiently transmitted by percutaneous or mucous membrane exposure to HBV-infected blood or body fluids that contain HBV. The primary risk factors associated with infection among adolescents and adults are unprotected sex with an infected partner, multiple partners, MSM, history of other STDs, and injection-drug use. In addition, several studies have demonstrated other modes of HBV transmission, including premastication and lapses in health-care infection-control procedures, as less common sources of transmission (244,834–836).
CDC’s national strategy to eliminate transmission of HBV infection includes 1) prevention of perinatal infection through routine screening of all pregnant women for HBsAg and immunoprophylaxis of infants born to mothers with HBsAg or mothers whose HBsAg status is unknown, 2) routine infant vaccination, 3) vaccination of previously unvaccinated children and adolescents through age 18 years, and 4) vaccination of previously unvaccinated adults at increased risk for infection (3,4). High vaccination coverage rates with subsequent declines in acute hepatitis B incidence have been achieved among infants and adolescents (4,823,837). The aging of persons vaccinated as children and adolescents likely has led to improved vaccination coverage in adults aged <30 years (838) and corresponding lower rates of acute HBV infection in this group. In contrast, vaccination coverage among most high-risk adult populations aged ≥30 years (e.g., persons with multiple sex partners, MSM, and IDUs) has remained low; these groups account for the highest rates of preventable acute infections (3,169,838–840). STD clinics and other settings providing STD services to high-risk adults should administer hepatitis B vaccine to those who are unvaccinated, as adults seeking STD services are at risk for this infection.
Diagnosis
Diagnosis of acute or chronic HBV infection requires serologic testing (Table 3). Because HBsAg is present in both acute and chronic infection, the presence of IgM antibody to hepatitis B core antigen (IgM anti-HBc) is diagnostic of acute or recently acquired HBV infection. Antibody to HBsAg (anti-HBs) is produced after a resolved infection and is the only HBV antibody marker present after vaccination. The presence of HBsAg and total anti-HBc, with a negative test for IgM anti-HBc, indicates chronic HBV infection. The presence of anti-HBc alone might indicate acute, resolved, or chronic infection or a false-positive result.
TABLE 3.
Interpretation of serologic test results* for HBV infection
| Serologic marker
|
Interpretation | |||
|---|---|---|---|---|
| HBsAg | Total anti-HBc | IgM anti-HBc | Anti-HBs | |
| − | − | − | − | Never infected |
| +† | − | − | − | Early acute infection; transient (up to 18 days) after vaccination |
| + | + | + | − | Acute infection |
| − | + | + | − | Acute resolving infection |
| − | + | − | + | Recovered from past infection and immune |
| + | + | − | − | Chronic infection |
| − | + | − | − | False positive (i.e., susceptible); past infection; “low-level” chronic infection§; passive transfer to infant born to HBsAg-positive mother |
| − | − | − | + | Immune if concentration is >10 mIU/mL, passive transfer after HBIG administration |
Abbreviations: anti-HBc = antibody to hepatitis B core antigen; anti-HBs = antibody to hepatitis B surface antigen; HBsAg = hepatitis B surface antigen; IgM = immunoglobulin M; mIU/mL = Milli-international units per milliliter.
Symbol for negative test result, “−“; symbol for positive test result, “+.”
To ensure that an HBsAg-positive test result is not false-positive, samples with repeatedly reactive HBsAg results should be tested with an FDA-cleared neutralizing confirmatory test.
Persons positive for only anti-HBc are unlikely to be infectious except under unusual circumstances involving direct percutaneous exposure to large quantities of blood (e.g., blood transfusion and organ transplantation).
Treatment
No specific therapy is available for persons with acute hepatitis B; treatment is supportive. Persons with chronic HBV infection should be referred for evaluation to a provider experienced in the management of chronic HBV infection. Therapeutic agents cleared by FDA for treatment of chronic hepatitis B can achieve sustained suppression of HBV replication and remission of liver disease (841).
Prevention
Two products have been approved for hepatitis B prevention: hepatitis B immune globulin (HBIG) for postexposure prophylaxis and hepatitis B vaccine (3,4). HBIG provides temporary (i.e., 3–6 months) protection from HBV infection and is typically used as PEP as an adjunct to hepatitis B vaccination (in previously unvaccinated persons) or in persons who have not responded to vaccination. HBIG is prepared from plasma known to contain high concentrations of anti-HBs. The recommended dose of HBIG is 0.06 mL/kg.
Hepatitis B vaccine contains HBsAg produced in yeast by recombinant DNA technology and provides protection from HBV infection when used for both pre-exposure vaccination and PEP. The two available monovalent hepatitis B vaccines for use in the United States are Recombivax HB (Merck and Co., Inc., Whitehouse Station, New Jersey) and Engerix-B (GlaxoSmithKline Biologicals, Pittsburgh, Pennsylvania). A combination hepatitis A and hepatitis B vaccine for use in persons ≥18 years, Twinrix (GlaxoSmithKline Biologicals, Pittsburgh, Pennsylvania), also is available.
When selecting a hepatitis B vaccination schedule, health-care providers should consider the need to achieve completion of the vaccine series. The recommended HBV dose and schedule varies by product and age of recipient (Table 4). Three different 3-dose schedules for adolescents and adults have been approved for both monovalent hepatitis B vaccines (i.e., Engerix-B and Recombivax HB); these vaccines can be administered at 0, 1, and 6 months; 0, 1, and 4 months; and 0, 2, and 4 months. A 4-dose schedule of Engerix-B at 0, 1, 2, and 12 months is licensed for all age groups. A 2-dose schedule of Recombivax HB adult formulation (10 μg) is licensed for adolescents aged 11–15 years, with a 4 month minimal interval between doses. When scheduled to receive the second dose, adolescents aged 16–19 years should be switched to a 3-dose series, with doses two and three consisting of the pediatric formulation (5 μg) administered on an appropriate schedule. Twinrix is a 3-dose schedule administered at 0, 1, and 6 months to persons aged ≥18 years at risk for both HAV and HBV infections.
TABLE 4.
Recommended doses of currently licensed formulations of adolescent and adult hepatitis B vaccines
| Group | Single-antigen vaccine
|
Combination vaccine
|
||||
|---|---|---|---|---|---|---|
| Recombivax HB
|
Engerix-B
|
Twinrix*
|
||||
| Dose (μg)† | Volume (mL) | Dose (μg)† | Volume (mL) | Dose (μg)† | Volume (mL) | |
| Adolescents aged 11–19 years§ | 5 | 0.5 | 10 | 0.5 | NA | NA |
| Adolescents aged 11–15 years¶ | 10 | 1.0 | NA | NA | NA | NA |
| Adults (aged ≥20 years) | 10 | 1.0 | 20 | 1.0 | 20 | 1.0 |
| Hemodialysis and other immunocompromised persons aged <20 years§ | 5 | 0.5 | 10 | 0.5 | NA | NA |
| Hemodialysis and other immunocompromised persons aged ≥20 years | 40** | 1.0 | 40†† | 2.0 | NA | NA |
Sources: CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(No. RR-16). CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006;55(No. RR-16).
Combined hepatitis A and hepatitis B vaccine. This vaccine is recommended for persons aged ≥18 years at increased risk for both hepatitis B and hepatitis A virus infections.
Recombinant hepatitis B surface antigen protein dose, in micrograms.
Pediatric formulation administered on a 3-dose schedule; higher doses might be more immunogenic, but no specific recommendations have been made.
Adult formulation administered on a 2-dose schedule.
Dialysis formulation administered on a 3-dose schedule at 0, 1, and 6 months.
Two 1.0-mL doses of the adult formulation administered at one site on a 4-dose schedule at 0, 1, 2, and 6 months.
Hepatitis B vaccine should be administered IM in the deltoid muscle and can be administered simultaneously with other vaccines. If the vaccine series is interrupted after the first or second dose of vaccine, the missed dose should be administered as soon as possible. The series does not need to be restarted after a missed dose. HBV vaccination is available for eligible children and adolescents aged <19 years through the VFC program (telephone: 800-232-4636).
In adolescents and healthy adults aged <40 years, approximately 30%–55% achieve a protective antibody response (i.e., anti-HBs ≥10 mIU/mL) after the first vaccine dose, 75% after the second, and >90% after the third. Vaccine-induced immune memory has been demonstrated to persist for at least 20 years (837,842,843). Periodic testing to determine antibody levels after routine vaccination in immunocompetent persons is not necessary, and booster doses of vaccine are not currently recommended.
Hepatitis B vaccination is generally well tolerated by most recipients. Pain at the injection site and low-grade fever are reported by a minority of recipients. For children and adolescents, a causal association exists between receipt of hepatitis B vaccination and anaphylaxis: for each 1.1 million doses of vaccine administered, approximately one vaccinee will experience this type of reaction. No deaths have been reported in these patients (3,4,839). Vaccine is contraindicated in persons with a history of anaphylaxis after a previous dose of hepatitis B vaccine and in persons with a known anaphylactic reaction to any vaccine component. No other adverse events after administration of hepatitis B vaccine have been demonstrated.
Pre-exposure Vaccination
Hepatitis B vaccination is recommended for all unvaccinated children and adolescents, all unvaccinated adults at risk for HBV infection (especially IDU, MSM, and adults with multiple sex partners), and all adults seeking protection from HBV infection (3). For adults, acknowledgment of a specific risk factor is not a requirement for vaccination.
Hepatitis B vaccine should be routinely offered to all unvaccinated persons attending STD clinics and to all unvaccinated persons seeking evaluation or treatment for STDs in other settings, especially correctional facilities, facilities providing drug-abuse treatment and prevention services, federally qualified health centers, and settings serving MSM (e.g., HIV care and prevention settings). If hepatitis B vaccine is unavailable at a particular facility, persons should be linked to a setting where they can receive vaccine. Persons with a reliable vaccination history (i.e., a written, dated record of each dose of a complete series) or reliable history of hepatitis B infection (i.e., a written record of infection and serologic results showing evidence of past infection) do not require vaccination. In all settings, vaccination should be initiated at the initial visit, even if concerns about completion of the vaccine series exist.
Prevaccination Serologic Testing
Conducting prevaccination serologic testing for susceptibility just before the initial vaccine dose is administered might be considered to reduce the cost of completing the vaccination series in adult populations that have an expected high prevalence (20%–30%) of HBV infection (e.g., IDUs and MSM, especially those in older age groups). In addition, prevaccination testing for susceptibility is recommended for unvaccinated household, sexual, and needle-sharing contacts of HBsAg-positive persons (169). Serologic testing should not be a barrier to vaccination. The first vaccine dose should be administered immediately after collection of the blood sample for serologic testing. Vaccination of persons who are immune to HBV infection because of current or previous infection or vaccination is not harmful and does not increase the risk for adverse events.
Anti-HBc is the test of choice for prevaccination testing. Persons who are anti-HBc–positive should be tested for HBsAg. If persons are determined to be HBsAg negative, no further action is required. Persons with HBsAg should be referred to a specialist in the management of hepatitis B infection and receive further serologic evaluation, prevention counseling, and evaluation for antiviral treatment (see Management of HBsAg-Positive Persons).
Postvaccination Serologic Testing for Response
Postvaccination serologic testing for immunity is not necessary after routine vaccination of adolescents or adults. However, such testing is recommended for persons whose subsequent clinical management depends on knowledge of their immune status (e.g., health-care workers or public safety workers at high risk for continued percutaneous or mucosal exposure to blood or body fluids). In addition, postvaccination testing is recommended for 1) persons with HIV infection and other immunocompromised persons to determine the need for revaccination and 2) sex and needle-sharing partners of HBsAg-positive persons to determine the need for revaccination and other methods to protect themselves from HBV infection.
If indicated, anti-HBs testing should be performed 1–2 months after administration of the last dose of the vaccine series. Persons determined to have anti-HBs levels of <10 mIU/mL after the primary vaccine series should be revaccinated with a 3-dose series and tested again for anti-HBs 1–2 months after the third dose. Persons who do not respond to revaccination should be tested for HBsAg. If HBsAg positive, the person should receive appropriate management (see Management of HBsAg-Positive Persons); if HBsAg negative, the person should be considered susceptible to HBV infection and counseled concerning precautions to prevent HBV infection and the need for HBIG PEP for any known exposure (see Postexposure Prophylaxis).
Postexposure Prophylaxis
Both passive-active PEP (the simultaneous administration of HBIG [i.e., 0.06 mL/kg] and hepatitis B vaccine at separate sites) and active PEP (the administration of hepatitis B vaccination alone) have been demonstrated to be highly effective in preventing transmission after exposure to HBV (4). HBIG alone also has been demonstrated to be effective in preventing HBV transmission, but with the availability of hepatitis B vaccine, HBIG typically is used as an adjunct to vaccination.
Exposure to an HBsAg-Positive Source
Unvaccinated persons or persons known not to have responded to a complete hepatitis B vaccine series should receive both HBIG and hepatitis vaccine as soon as possible (preferably ≤24 hours) after a discrete, identifiable exposure to blood or body fluids that contain blood from a person with HBsAg (Table 5). Hepatitis B vaccine should be administered simultaneously with HBIG at a separate injection site, and the vaccine series should be completed by using the age-appropriate vaccine dose and schedule (Table 4). Exposed persons who are in the process of being vaccinated but who have not completed the vaccine series should receive HBIG (i.e., 0.06 mL/kg) and complete the vaccine series. Exposed persons who are known to have responded to vaccination are considered protected; therefore, they need no additional doses of vaccine or HBIG. Persons who have written documentation of a complete hepatitis B vaccine series who did not receive postvaccination testing should receive a single vaccine booster dose. These persons should be managed according to guidelines for management of persons with occupational exposure to blood or body fluids that contain HBV (844).
TABLE 5.
Guidelines for postexposure prophylaxis* of persons with nonoccupational exposure† to blood or body fluids that contain blood, by exposure type and vaccination status
| Source of exposure | Treatment
|
|
|---|---|---|
| Unvaccinated person§ | Previously vaccinated person¶ | |
| HBsAg-positive source | ||
| Percutaneous (e.g., bite or needlestick) or mucosal exposure to HBsAg-positive blood or body fluids | Administer hepatitis B vaccine series and HBIG | Administer hepatitis B vaccine booster dose |
| Sex or needle-sharing contact of an HBsAg-positive person | Administer hepatitis B vaccine series and HBIG | Administer hepatitis B vaccine booster dose |
| Victim of sexual assault/abuse by a perpetrator who is HBsAg positive | Administer hepatitis B vaccine series and HBIG | Administer hepatitis B vaccine booster dose |
| Source with unknown HBsAg status | ||
| Victim of sexual assault/abuse by a perpetrator with unknown HBsAg status | Administer hepatitis B vaccine series | No treatment |
| Percutaneous (e.g., bite or needlestick) or mucosal exposure to potentially infectious blood or body fluids from a source with unknown HBsAg status | Administer hepatitis B vaccine series | No treatment |
| Sex or needle-sharing contact of person with unknown HBsAg status | Administer hepatitis B vaccine series | No treatment |
Source: CDC. Postexposure prophylaxis to prevent hepatitis B virus infection. MMWR Recomm Rep 2006;55(No. RR-16).
Abbreviations: HBIG = hepatitis B immune globulin. HBsAg = hepatitis B surface antigen.
When indicated, immunoprophylaxis should be initiated as soon as possible, preferably within 24 hours. Studies are limited on the maximum interval after exposure during which postexposure prophylaxis is effective, but the interval is unlikely to exceed 7 days for percutaneous exposures or 14 days for sexual exposures. The hepatitis B vaccine series should be completed.
These guidelines apply to nonoccupational exposures. Guidelines for management of occupational exposures have been published separately and also can be used for management of nonoccupational exposures, if feasible. Source: CDC. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013;62(No. RR-10).
A person who is in the process of being vaccinated but who has not completed the vaccine series should complete the series and receive treatment as indicated.
A person who has written documentation of a complete hepatitis B vaccine series and who did not receive postvaccination testing.
Exposure to a Source with Unknown HBsAg Status
Unvaccinated persons and persons with previous nonresponse to hepatitis B vaccination who have a discrete, identifiable exposure to blood or body fluids containing blood from a person with unknown HBsAg status should receive the hepatitis B vaccine series, with the first dose initiated as soon as possible after exposure (preferably <24 hours) and the series completed using the age-appropriate dose and schedule. Exposed persons who are not fully vaccinated should complete the vaccine series. Generally, exposed persons with written documentation of a complete hepatitis B vaccine series who did not receive postvaccination testing require no further treatment.
Special Considerations
Pregnancy
Regardless of whether they have been previously tested or vaccinated, all pregnant women should be tested for HBsAg at the first prenatal visit and at delivery if at high risk for HBV infection (see Special Populations Pregnant Women). Pregnant women at risk for HBV infection should receive hepatitis B vaccination. All pregnant women with HBsAg should be reported to state and local perinatal hepatitis B prevention programs and referred to a specialist. Information on the management of pregnant women with HBsAg and their infants is available at http://www.cdc.gov/mmwr/PDF/rr/rr5416.pdf.
HIV Infection
HIV infection can impair the response to hepatitis B vaccination. Persons with HIV infection should be tested for anti-HBs 1–2 months after the third vaccine dose (see Postvaccination Serologic Testing). Modified dosing regimens, including a doubling of the standard antigen dose and administration of additional doses, might increase the response rate (247). Additional recommendations for management of persons with HBsAg and HIV infection are available (247).
Management of HBsAg-Positive Persons
Recommendations for management of all persons with HBsAg-include the following:
All persons with HBsAg documented on laboratory results should be reported to the state or local health department.
To verify the presence of chronic HBV infection, persons with HBsAg should be retested. The absence of IgM anti-HBc or the persistence of HBsAg for 6 months indicates chronic HBV infection.
Persons with chronic HBV infection should be referred for evaluation to a specialist experienced in the management of chronic hepatitis B infection.
Household, sexual, and needle-sharing contacts of chronically infected persons should be evaluated. Unvaccinated sex partners and household and needle-sharing contacts should be tested for susceptibility to HBV infection (see Prevaccination Antibody Screening) and receive the first dose of hepatitis B vaccine immediately after collection of the blood sample for serologic testing. Susceptible persons should complete the vaccine series by using an age-appropriate vaccine dose and schedule.
Sex partners of persons with HBsAg should be counseled to use latex condoms (32) to protect themselves from sexual exposure to infectious body fluids (e.g., semen and vaginal secretions), unless they have been demonstrated to be immune after vaccination (anti-HBs ≥10 mIU/mL) or previously infected (anti-HBc positive).
- To prevent or reduce the risk for transmission to others in addition to vaccination, persons with HBsAg also should be advised to:
-
–use methods (e.g., condoms) to protect nonimmune sex partners from acquiring HBV infection from sexual activity until the partner can be vaccinated and immunity documented;
-
–cover cuts and skin lesions to prevent spread by infectious secretions or blood;
-
–refrain from donating blood, plasma, body organs, other tissue, or semen; and
-
–refrain from sharing household articles (e.g., toothbrushes, razors, or personal injection equipment) that could become contaminated with blood and refrain from premastication of food.
-
–
- To protect the liver from further harm, persons with HBsAg should be advised to:
-
–avoid or limit alcohol consumption because of the effects of alcohol on the liver;
-
–refrain from starting any new medicines, including OTC and herbal medicines, without checking with their health-care provider; and
-
–obtain vaccination against hepatitis A.
-
–
When seeking medical or dental care, HBsAg-positive persons should be advised to inform their health-care providers of their HBsAg status so that they can be appropriately evaluated and managed. The following are key counseling messages for persons with HBsAg:
HBV is not usually spread by hugging, coughing, food or water, sharing eating utensils or drinking glasses, or casual contact.
Persons should not be excluded from work, school, play, child care, or other settings because they are infected with HBV.
Involvement with a support group might help patients cope with chronic HBV infection.
Proctitis, Proctocolitis, and Enteritis
Sexually transmitted gastrointestinal syndromes include proctitis, proctocolitis, and enteritis. Evaluation for these syndromes should include appropriate diagnostic procedures (e.g., anoscopy or sigmoidoscopy, stool examination, and culture).
Proctitis is inflammation of the rectum (i.e., the distal 10–12 cm) that can be associated with anorectal pain, tenesmus, or rectal discharge. N. gonorrhoeae, C. trachomatis (including LGV serovars), T. pallidum, and HSV are the most common sexually transmitted pathogens involved. In persons with HIV infection, herpes proctitis can be especially severe. Proctitis occurs predominantly among persons who participate in receptive anal intercourse.
Proctocolitis is associated with symptoms of proctitis, diarrhea or abdominal cramps, and inflammation of the colonic mucosa extending to 12 cm above the anus. Fecal leukocytes might be detected on stool examination, depending on the pathogen. Pathogenic organisms include Campylobacter sp., Shigella sp., Entamoeba histolytica, and LGV serovars of C. trachomatis. CMV or other opportunistic agents can be involved in immunosuppressed HIV-infected patients. Proctocolitis can be acquired through receptive anal intercourse or by oral-anal contact, depending on the pathogen.
Enteritis usually results in diarrhea and abdominal cramping without signs of proctitis or proctocolitis; it occurs among persons whose sexual practices include oral-anal contact. In otherwise healthy persons, Giardia lamblia is most frequently implicated. When outbreaks of gastrointestinal illness occur among social or sexual networks of MSM, clinicians should consider sexual transmission as a mode of spread and provide counseling accordingly. Among persons with HIV infection, enteritis can be caused by pathogens that may not be sexually transmitted, including CMV, Mycobacterium avium–intracellulare, Salmonella sp., Campylobacter sp., Shigella sp., Cryptosporidium, Microsporidium, and Isospora. Multiple stool examinations might be necessary to detect Giardia, and special stool preparations are required to diagnose cryptosporidiosis and microsporidiosis. In addition, enteritis can be directly caused by HIV infection. Diagnostic and treatment recommendations for all enteric infections are beyond the scope of these guidelines.
Diagnostic Considerations for Acute Proctitis
Persons who present with symptoms of acute proctitis should be examined by anoscopy. A Gram-stained smear of any anorectal exudate from anoscopic or anal examination should be examined for polymorphonuclear leukocytes. All persons should be evaluated for HSV (by PCR or culture), N. gonorrhoeae (NAAT or culture), C. trachomatis (NAAT), and T. pallidum (Darkfield if available and serologic testing) (see pathogen-specific sections). If the C. trachomatis test is positive on a rectal swab, a molecular test PCR for LGV should be performed, if available, to confirm an LGV diagnosis (see LGV) (394).
Treatment for Acute Proctitis
Acute proctitis of recent onset among persons who have recently practiced receptive anal intercourse is usually sexually acquired (845,846). Presumptive therapy should be initiated while awaiting results of laboratory tests for persons with anorectal exudate detected on examination or polymorphonuclear leukocytes detected on a Gram-stained smear of anorectal exudate or secretions; such therapy also should be initiated when anoscopy or Gram stain is unavailable and the clinical presentation is consistent with acute proctitis in persons reporting receptive anal intercourse.
| Recommended Regimen |
|
|
| Ceftriaxone 250 mg IM in a single dose |
| PLUS |
| Doxycycline 100 mg orally twice a day for 7 days |
|
|
Bloody discharge, perianal ulcers, or mucosal ulcers among MSM with acute proctitis and either a positive rectal chlamydia NAAT or HIV infection should be offered presumptive treatment for LGV with doxycycline 100 mg twice daily orally for a total of 3 weeks (847,848) (see LGV). If painful perianal ulcers are present or mucosal ulcers are detected on anoscopy, presumptive therapy should also include a regimen for genital herpes (see Genital HSV Infections).
Other Management Considerations
To minimize transmission and reinfection, men treated for acute proctitis should be instructed to abstain from sexual intercourse until they and their partner(s) have been adequately treated (i.e., until completion of a 7-day regimen and symptoms resolved). All persons with acute proctitis should be tested for HIV and syphilis.
Follow-Up
Follow-up should be based on specific etiology and severity of clinical symptoms. For proctitis associated with gonorrhea or chlamydia, retesting for the respective pathogen should be performed 3 months after treatment.
Management of Sex Partners
Partners who have had sexual contact with persons treated for GC, CT, or LGV within the 60 days before the onset of the persons symptoms should be evaluated, tested, and presumptively treated for the respective pathogen. Partners of persons with sexually transmitted enteric infections should be evaluated for any diseases diagnosed in the person with acute proctitis. Sex partners should abstain from sexual intercourse until they and their partner with acute proctitis are adequately treated.
Allergy, Intolerance, and Adverse Reactions
Allergic reactions with third-generation cephalosporins (e.g., ceftriaxone) are uncommon in persons with a history of penicillin allergy (428,430,464). In those persons with a history of an IgE mediated penicillin allergy (e.g., those who have had anaphylaxis, Stevens Johnson syndrome, or toxic epidermal necrolysis), the use of ceftriaxone is contraindicated (428,431).
HIV Infection
Persons with HIV infection and acute proctitis may present with bloody discharge, painful perianal ulcers, or mucosal ulcers. Presumptive treatment should include a regimen for genital herpes and LGV.
Ectoparasitic Infections
Pediculosis Pubis
Persons who have pediculosis pubis (i.e., pubic lice) usually seek medical attention because of pruritus or because they notice lice or nits on their pubic hair. Pediculosis pubis is usually transmitted by sexual contact (849).
Treatment
| Recommended Regimens |
|
|
| Permethrin 1% cream rinse applied to affected areas and washed off after 10 minutes |
| OR |
| Pyrethrins with piperonyl butoxide applied to the affected area and washed off after 10 minutes |
|
|
| Alternative Regimens |
|
|
| Malathion 0.5% lotion applied to affected areas and washed off after 8–12 hours |
| OR |
| Ivermectin 250 µg/kg repeated in 2 weeks |
|
|
Reported resistance to pediculcides (permethrin and pyrethrins) has been increasing and is widespread (850,851). Malathion can be used when treatment failure is believed to have occurred as a result of resistance. The odor and long duration of application associated with malathion therapy make it a less attractive alternative compared with the recommended pediculcides. Ivermectin has limited ovicidal activity (852). Ivermectin might not prevent recurrences from eggs at the time of treatment, and therefore treatment should be repeated in 14 days (853,854). Ivermectin should be taken with food because bioavailability is increased, in turn increasing penetration of the drug into the epidermis. Adjustment of ivermectin dosage is not required for persons with renal impairment, but the safety of multiple doses in persons with severe liver disease is not known.
Lindane is recommended as an alternative therapy because it can cause toxicity, as indicated by seizure and aplastic anemia (855); it should only be used when other therapies cannot be tolerated or have failed. Lindane toxicity has not been reported when treatment was limited to the recommended 4-minute period. Lindane should not be used immediately after a bath or shower, and it should not be used by persons who have extensive dermatitis, women who are breastfeeding, or children aged <10 years (855).
Other Management Considerations
The recommended regimens should not be applied to the eyes. Pediculosis of the eyelashes should be treated by applying occlusive ophthalmic ointment or petroleum jelly to the eyelid margins twice a day for 10 days. Bedding and clothing should be decontaminated (i.e., machine-washed and dried using the heat cycle or dry cleaned) or removed from body contact for at least 72 hours. Fumigation of living areas is not necessary. Persons with pediculosis pubis should be evaluated for other STDs, including HIV.
Follow-Up
Evaluation should be performed after 1 week if symptoms persist. Re-treatment might be necessary if lice are found or if eggs are observed at the hair-skin junction. If no clinical response is achieved to one of the recommended regimens, retreatment with an alternative regimen is recommended.
Management of Sex Partners
Sex partners within the previous month should be treated. Sexual contact should be avoided until patients and partners have been treated, bedding and clothing decontaminated, and reevaluation performed to rule out persistent infection.
Special Considerations
Pregnancy
Existing data from human subjects suggest that pregnant and lactating women should be treated with either permethrin or pyrethrins with piperonyl butoxide. Because no teratogenicity or toxicity attributable to ivermectin has been observed in human pregnancy experience, ivermectin is classified as “human data suggest low risk” in pregnancy and probably compatible with breastfeeding (317). Use of lindane during pregnancy has been associated with neural tube defects and mental retardation, and it can accumulate in the placenta and in breast milk (855).
HIV Infection
Persons who have pediculosis pubis and also HIV infection should receive the same treatment regimen as those who are HIV negative. For more information, see Pediculosis pubis.
Scabies
The predominant symptom of scabies is pruritus. Sensitization to Sarcoptes scabiei occurs before pruritus begins. The first time a person is infested with S. scabiei, sensitization takes up to several weeks to develop. However, pruritus might occur within 24 hours after a subsequent reinfestation. Scabies in adults frequently is sexually acquired, although scabies in children usually is not (856,857).
Treatment
| Recommended Regimens |
|
|
| Permethrin 5% cream applied to all areas of the body from the neck down and washed off after 8–14 hours* |
| OR |
| Ivermectin 200ug/kg orally, repeated in 2 weeks† |
|
|
| *Infants and young children should be treated with permethrin. |
| †Infants and young children aged <10 years should not be treated with lindane. |
| Alternative Regimens |
|
|
| Lindane (1%) 1 oz of lotion or 30 g of cream applied in a thin layer to all areas of the body from the neck down and thoroughly washed off after 8 hours |
|
|
Permethrin is effective, safe, and less expensive than ivermectin (858). One study demonstrated increased mortality among elderly, debilitated persons who received ivermectin, but this observation has not been confirmed in subsequent reports (859). Ivermectin has limited ovicidal activity and may not prevent recurrences of eggs at the time of treatment; therefore, a second dose of ivermectin should be administered 14 days after the first dose. Ivermectin should be taken with food because bioavailability is increased, thereby increasing penetration of the drug into the epidermis. Adjustments to ivermectin dosing are not required in patients with renal impairment, but the safety of multiple doses in patients with severe liver disease is not known.
Lindane is an alternative regimen because it can cause toxicity (855); it should only be used if the patient cannot tolerate the recommended therapies or if these therapies have failed (860–862). Lindane should not be used immediately after a bath or shower, and it should not be used by persons who have extensive dermatitis or children aged <10 years. Seizures have occurred when lindane was applied after a bath or used by patients who had extensive dermatitis. Aplastic anemia after lindane use also has been reported. Lindane resistance has been reported in some areas of the world, including parts of the United States.
Other Management Considerations
Bedding and clothing should be decontaminated (i.e., either machine-washed, machine-dried using the hot cycle, or dry cleaned) or removed from body contact for at least 72 hours. Fumigation of living areas is unnecessary. Persons with scabies should be advised to keep fingernails closely trimmed to reduce injury from excessive scratching.
Crusted Scabies
Crusted scabies (i.e., Norwegian scabies) is an aggressive infestation that usually occurs in immunodeficient, debilitated, or malnourished persons, including persons receiving systemic or potent topical glucocorticoids, organ transplant recipients, persons with HIV infection or human T-lymphotrophic virus-1-infection, and persons with hematologic malignancies. Crusted scabies is transmitted more easily than scabies (863). No controlled therapeutic studies for crusted scabies have been conducted, and the appropriate treatment remains unclear. Substantial treatment failure might occur with a single-dose topical scabicide or with oral ivermectin treatment. Combination treatment is recommended with a topical scabicide, either 5% topical benzyl benzoate or 5% topical permethrin cream (full-body application to be repeated daily for 7 days then 2x weekly until discharge or cure), and treatment with oral ivermectin 200 ug/kg on days 1,2,8,9, and 15. Additional ivermectin treatment on days 22 and 29 might be required for severe cases (864). Lindane should be avoided because of the risks for neurotoxicity with heavy applications or denuded skin.
Follow-Up
The rash and pruritus of scabies might persist for up to 2 weeks after treatment. Symptoms or signs persisting for >2 weeks can be attributed to several factors. Treatment failure can occur as a result of resistance to medication or faulty application of topical scabicides. These medications do not easily penetrate into thick, scaly skin of persons with crusted scabies, perpetuating the harboring of mites in these difficult-to-penetrate layers. In the absence of appropriate contact treatment and decontamination of bedding and clothing, persisting symptoms can be attributed to reinfection by family members or fomites. Finally, other household mites can cause symptoms to persist as a result of cross reactivity between antigens. Even when treatment is successful, reinfection is avoided, and cross reactivity does not occur, symptoms can persist or worsen as a result of allergic dermatitis.
Retreatment 2 weeks after the initial treatment regimen can be considered for those persons who are still symptomatic or when live mites are observed. Use of an alternative regimen is recommended for those persons who do not respond initially to the recommended treatment.
Management of Sex Partners and Household Contacts
Persons who have had sexual, close personal, or household contact with the patient within the month preceding scabies infestation should be examined. Those found to be infested should be provided treatment.
Management of Outbreaks in Communities, Nursing Homes, and Other Institutional Settings
Scabies epidemics frequently occur in nursing homes, hospitals, residential facilities, and other communities (865). Control of an epidemic can only be achieved by treating the entire population at risk. Ivermectin can be considered in these settings, especially if treatment with topical scabicides fails. Epidemics should be managed in consultation with a specialist.
Special Considerations
Infants, Young Children, and Pregnant or Lactating Women
Infants and young children should be treated with permethrin; the safety of ivermectin in children who weigh <15 kg has not been determined. Infants and young children aged<10 years should not be treated with lindane. Ivermectin likely poses a low risk to pregnant women and is likely compatible with breastfeeding (See Pediculosis pubis); however, because of limited data regarding its use in pregnant and lactating women, permethrin is the preferred treatment (317).
HIV Infection
Persons with HIV infection who have uncomplicated scabies should receive the same treatment regimens as those who are HIV negative. Persons with HIV infection and others who are immunosuppressed are at increased risk for crusted scabies. Such persons should be managed in consultation with a specialist.
Sexual Assault and Abuse and STDs
Adolescents and Adults
These guidelines are primarily limited to the identification, prophylaxis, and treatment of STDs and conditions among adolescent and adult female sexual assault survivors. However, some of the following guidelines might still apply to male sexual assault survivors. The documentation of findings, collection of nonmicrobiologic specimens for forensic purposes, and the management of potential pregnancy or physical and psychological trauma are beyond the scope of these guidelines.
Examinations of survivors of sexual assault should be conducted by an experienced clinician in a way that minimizes further trauma to the survivor. The decision to obtain genital or other specimens for STD diagnosis should be made on an individual basis. Care systems for survivors should be designed to ensure continuity (including timely review of test results), support adherence, and monitor adverse reactions to any prescribed therapeutic or prophylactic regimens. Laws in all 50 states strictly limit the evidentiary use of a survivor’s previous sexual history, including evidence of previously acquired STDs, as part of an effort to undermine the credibility of the survivor’s testimony. Evidentiary privilege against revealing any aspect of the examination or treatment also is enforced in most states. Although it rarely occurs, STD diagnoses might later be accessed, and the survivor and clinician might opt to defer testing for this reason. While collection of specimens at initial examination for laboratory STD diagnosis gives the survivor and clinician the option to defer empiric prophylactic antimicrobial treatment, compliance with follow-up visits is typically poor (866,867). Among sexually active adults, the identification of an STD might represent an infection acquired before the assault, and therefore might be more important for the medical management of the patient than for legal purposes.
Trichomoniasis, BV, gonorrhea, and chlamydial infection are the most frequently diagnosed infections among women who have been sexually assaulted. Such conditions are prevalent in the population, and detection of these infections after an assault does not necessarily imply acquisition during the assault. However, a post-assault examination presents an important opportunity to identify or prevent STDs. Chlamydial and gonococcal infections in women are of particular concern because of the possibility of ascending infection. In addition, HBV infection can be prevented through postexposure vaccination (see Hepatitis B) (Table 5). Because female survivors also are at risk for acquiring HPV infection and the efficacy of the HPV vaccine is high (868,869), HPV vaccination is also recommended for females through age 26 years (16). Reproductive-aged female survivors should be evaluated for pregnancy.
Evaluating Adolescents and Adults for STDs
Initial Examination
Decisions to perform these tests should be made on an individual basis. An initial examination might include the following procedures:
NAATs for C. trachomatis and N. gonorrhoeae at the sites of penetration or attempted penetration (394). These tests are preferred for the diagnostic evaluation of adolescent or adult sexual assault survivors.
NAATs from a urine or vaginal specimen or point-of-care testing (i.e., DNA probes) from a vaginal specimen for T. vaginalis. Point-of-care testing and/or wet mount with measurement of vaginal pH and KOH application for the whiff test from vaginal secretions should be done for evidence of BV and candidiasis, especially if vaginal discharge, malodor, or itching is present.
A serum sample for evaluation of HIV, hepatitis B, and syphilis infections.
Treatment
Compliance with follow-up visits is poor among survivors of sexual assault (866,867). As a result, the following routine presumptive treatment after a sexual assault is recommended:
An empiric antimicrobial regimen for chlamydia, gonorrhea, and trichomonas.
Emergency contraception. This measure should be considered when the assault could result in pregnancy in the survivor.
Postexposure hepatitis B vaccination (without HBIG) if the hepatitis status of the assailant is unknown and the survivor has not been previously vaccinated. If the assailant is known to be HBsAg-positive, unvaccinated survivors should receive both hepatitis B vaccine and HBIG. The vaccine and HBIG, if indicated, should be administered to sexual assault survivors at the time of the initial examination, and follow-up doses of vaccine should be administered 1–2 and 4–6 months after the first dose. Survivors who were previously vaccinated but did not receive postvaccination testing should receive a single vaccine booster dose (see hepatitis B).
HPV vaccination is recommended for female survivors aged 9–26 years and male survivors aged 9–21 years. For MSM with who have not received HPV vaccine or who have been incompletely vaccinated, vaccine can be administered through age 26 years. The vaccine should be administered to sexual assault survivors at the time of the initial examination, and follow-up dose administered at 1–2 months and 6 months after the first dose.
Recommendations for HIV PEP are individualized according to risk (see Risk for Acquiring HIV Infection and Postexposure HIV Risk Assessment for PEP).
| Recommended Regimens |
|
|
| Ceftriaxone 250 mg IM in a single dose |
| PLUS |
| Azithromycin 1 g orally in a single dose |
| PLUS |
| Metronidazole 2 g orally in a single dose |
| OR |
| Tinidazole 2 g orally in a single dose |
|
|
If alcohol has been recently ingested or emergency contraception is provided, metronidazole or tinidazole can be taken by the sexual assault survivor at home rather than as directly observed therapy to minimize potential side effects and drug interactions. Clinicians should counsel persons regarding the possible benefits and toxicities associated with these treatment regimens; gastrointestinal side effects can occur with this combination. The efficacy of these regimens in preventing infections after sexual assault has not been evaluated. For those requiring alternative treatments, refer to the specific sections in this report relevant to the specific organism.
Other Management Considerations
At the initial examination and, if indicated, at follow-up examinations, patients should be counseled regarding symptoms of STDs and the need for immediate examination if symptoms occur. Further, they should be instructed to abstain from sexual intercourse until STD prophylactic treatment is completed.
Follow-up
After the initial postassault examination, follow-up examinations provide an opportunity to 1) detect new infections acquired during or after the assault; 2) complete hepatitis B and HPV vaccinations, if indicated; 3) complete counseling and treatment for other STDs; and 4) monitor side effects and adherence to postexposure prophylactic medication, if prescribed.
If initial testing was done, follow-up evaluation should be conducted within 1 week to ensure that results of positive tests can be discussed promptly with the survivor, treatment is provided if not given at the initial visit, and any follow-up for the infection(s) can be arranged. If initial tests are negative and treatment was not provided, examination for STDs can be repeated within 1–2 weeks of the assault; repeat testing detects infectious organisms that might not have reached sufficient concentrations to produce positive test results at the time of initial examination. For survivors who are treated during the initial visit, regardless of whether testing was performed, post-treatment testing should be conducted only if the survivor reports having symptoms. A follow-up examination at 1–2 months should also be considered to reevaluate for development of anogenital warts, especially among sexual assault survivors who received a diagnosis of other STDs. If initial test results were negative and infection in the assailant cannot be ruled out, serologic tests for syphilis can be repeated at 4–6 weeks and 3 months; HIV testing can be repeated at 6 weeks and at 3 and 6 months using methods to identify acute HIV infection (see Sexual Assault and STDs, Risk for Acquiring HIV Infection).
Risk for Acquiring HIV Infection
HIV seroconversion has occurred in persons whose only known risk factor was sexual assault or sexual abuse, but the frequency of this occurrence likely is low (870,871). In consensual sex, the per-act risk for HIV transmission from vaginal intercourse is 0.1%–0.2%, and for receptive rectal intercourse, 0.5%–3% (872). The per-act risk for HIV transmission from oral sex is substantially lower. Specific circumstances of an assault (e.g., bleeding, which often accompanies trauma) might increase risk for HIV transmission in cases involving vaginal, anal, or oral penetration. Site of exposure to ejaculate, viral load in ejaculate, and the presence of an STD or genital lesions in the assailant or survivor also might increase risk for HIV.
Postexposure prophylaxis with a 28-day course of zidovudine was associated with an 81% reduction in risk for acquiring HIV in a study of health-care workers who had percutaneous exposures to HIV-infected blood (873). On the basis of these results and results from animal studies, PEP has been recommended for health-care workers who have occupational exposures to HIV (874). These findings have been extrapolated to nonoccupational injection and sexual HIV exposures, including sexual assault. The possibility of HIV exposure from the assault should be assessed at the initial examination; survivors determined to be at risk for HIV should be informed about the possible benefit of nonoccupational postexposure prophylaxis (nPEP) in preventing HIV infection. Initiation of nPEP as soon as possible after the exposure increases the likelihood of prophylactic benefit.
Several factors impact the medical recommendation for nPEP and affect the assault survivor’s acceptance of that recommendation, including 1) the likelihood of the assailant having HIV; 2) any exposure characteristics that might increase the risk for HIV transmission; 3) the time elapsed after the event; and 4) the potential benefits and risks associated with the nPEP (312). Determination of the assailant’s HIV status at the time of the assault examination is usually not possible. Therefore, health-care providers should assess any available information concerning the 1) characteristics and HIV risk behaviors of the assailant(s) (e.g., being an MSM or using injection drugs), 2) local epidemiology of HIV/AIDS, and 3) exposure characteristics of the assault. When an assailant’s HIV status is unknown, determinations regarding risk for HIV transmission to the survivor should be based on 1) whether vaginal or anal penetration occurred; 2) whether ejaculation occurred on mucous membranes; 3) whether multiple assailants were involved; 4) whether mucosal lesions are present in the assailant or survivor; and 5) any other characteristics of the assault, survivor, or assailant that might increase risk for HIV transmission.
If nPEP is offered, the following information should be discussed with the survivor: 1) the necessity of early initiation of nPEP to optimize potential benefits (i.e., as soon as possible after and up to 72 hours after the assault; 2) the importance of close follow-up; 3) the benefit of adherence to recommended dosing; and 4) potential adverse effects of antiretrovirals. Providers should emphasize that severe adverse effects are rare from nPEP (875–877). Clinical management of the survivor should be implemented according to the HIV nPEP guidelines and in collaboration with specialists (312). However, distress after an assault also might prevent the survivor from accurately weighing exposure risks and benefits of nPEP and from making an informed decision regarding initiating therapy, even when such therapy is considered warranted by the health-care provider. In this instance, the survivor can be provided a 3–5-day supply of nPEP and scheduled for follow-up at a time that allows for provision of the remaining 23 days of medication (if nPEP has been initiated by the survivor) without interruption in dosing. A follow-up visit also creates opportunity for additional counseling as needed.
Recommendations for Postexposure HIV Risk Assessment of Adolescent and Adult Survivors Within 72 Hours of Sexual Assault
Assess risk for HIV infection in the assailant, and test that person for HIV whenever possible.
Use the algorithm to evaluate the survivor for the need for HIV nPEP (Figure) (312).
Consult with a specialist in HIV treatment if nPEP is being considered.
If the survivor appears to be at risk for acquiring HIV from the assault, discuss nPEP, including benefits and risks.
If the survivor chooses to start nPEP (312), provide enough medication to last until the follow-up visit at 3–7 days after initial assessment and assess tolerance to medications.
If nPEP is started, perform CBC and serum chemistry at baseline.
Perform an HIV antibody test at original assessment; repeat at 6 weeks, 3 months, and 6 months.
FIGURE. Algorithm for evaluation and treatment of possible nonoccupational HIV exposures.
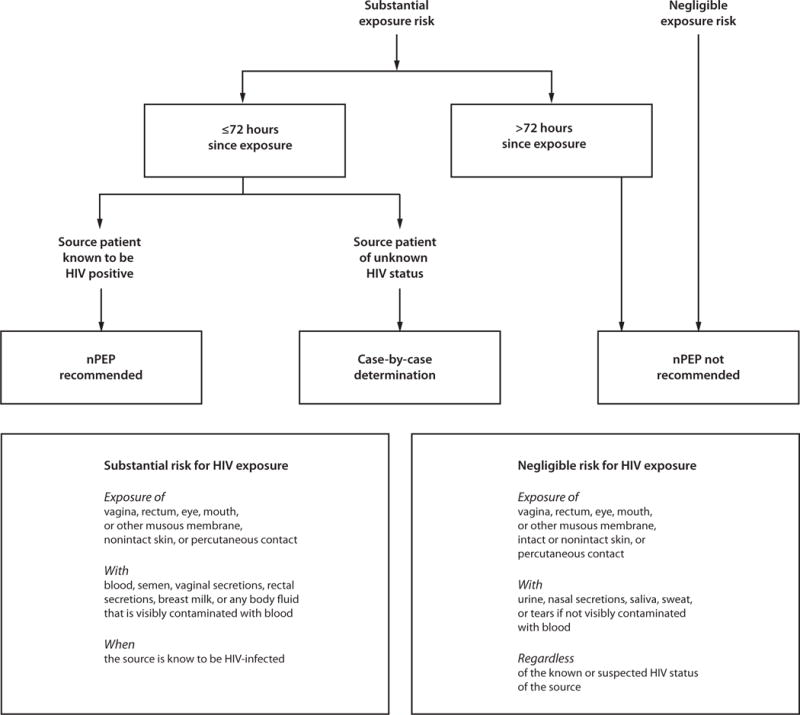
Source: CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States. MMWR Recomm Rep 2005;54(No. RR-2).
Assistance with nPEP-related decisions can be obtained by calling the National Clinician’s Post Exposure Prophylaxis Hotline (PEP Line) (telephone: 888-448-4911).
Sexual Assault or Abuse of Children
These guidelines are limited to the identification and treatment of STDs in pre-pubertal children. Management of the psychosocial or legal aspects of the sexual assault or abuse of children is beyond the scope of these guidelines.
The identification of sexually transmissible agents in children beyond the neonatal period strongly suggests sexual abuse (878). The significance of the identification of a sexually transmitted organism in such children as evidence of possible child sexual abuse varies by pathogen. Postnatally acquired gonorrhea and syphilis; chlamydia infection; and nontransfusion, nonperinatally acquired HIV are indicative of sexual abuse. Chlamydia infection might be indicative of sexual abuse in children ≥3 years of age and among those aged <3 years when infection is not likely perinatally acquired. Sexual abuse should be suspected when genital herpes, T. vaginalis, or anogenital warts are diagnosed. The investigation of sexual abuse among children who have an infection that could have been transmitted sexually should be conducted in compliance with recommendations by clinicians who have experience and training in all elements of the evaluation of child abuse, neglect, and assault. The social significance of an infection that might have been acquired sexually varies by the specific organism, as does the threshold for reporting suspected child sexual abuse (Table 6). In cases in which any STD has been diagnosed in a child, efforts should be made in consultation with a specialist to evaluate the possibility of sexual abuse, including conducting a history and physical examination for evidence of abuse and diagnostic testing for other commonly occurring STDs (879,880).
TABLE 6.
Implications of commonly encountered sexually transmitted or sexually associated infections for diagnosis and reporting of sexual abuse among infants and prepubertal children
| ST/SA confirmed | Evidence for sexual abuse | Suggested action |
|---|---|---|
| Gonorrhea* | Diagnostic | Report† |
| Syphilis* | Diagnostic | Report† |
| HIV§ | Diagnostic | Report† |
| Chlamydia trachomatis* | Diagnostic | Report† |
| Trichomonas vaginalis* | Highly suspicious | Report† |
| Genital herpes | Highly suspicious (HSV-2 especially) | Report†,¶ |
| Condylomata acuminata (anogenital warts)* | Suspicious | Consider report†,¶,** |
| Bacterial vaginosis | Inconclusive | Medical follow-up |
Source: Adapted from Kellogg N, American Academy of Pediatrics Committee on Child Abuse and Neglect. The evaluation of child abuse in children. Pediatrics 2005;116:506–12.
Abbreviations: HIV = human immunodeficiency virus; SA = sexually associated; ST = sexually transmitted.
If not likely to be perinatally acquired and rare vertical transmission is excluded.
Reports should be made to the agency in the community mandated to receive reports of suspected child abuse or neglect.
If not likely to be acquired perinatally or through transfusion.
Unless a clear history of autoinoculation exists.
Report if evidence exists to suspect abuse, including history, physical examination, or other identified infections.
The general rule that sexually transmissible infections beyond the neonatal period are evidence of sexual abuse has exceptions. For example, genital infection with T. vaginalis (881) or rectal or genital infection with C. trachomatis among young children might be the result of perinatally acquired infection and has, in some cases of chlamydia infection, persisted for as long as 2–3 years (882,883), though perinatal CT infection is now uncommon because of prenatal screening and treatment of pregnant women. Genital warts have been diagnosed in children who have been sexually abused (868), but also in children who have no other evidence of sexual abuse (884,885). BV has been diagnosed in children who have been abused, but its presence alone does not prove sexual abuse. Most HBV infections in children result from household exposure to persons who have chronic HBV infection rather than sexual abuse.
Reporting
All U.S. states and territories have laws that require the reporting of child abuse. Although the exact requirements differ by state, if a health-care provider has reasonable cause to suspect child abuse, a report must be made. Health-care providers should contact their state or local child-protection service agency regarding child-abuse reporting requirements in their states.
Evaluating Children for STDs
Evaluations of children for sexual assault or abuse should be conducted in a manner designed to minimize pain and trauma to the child. Examinations and collection of vaginal specimens in prepubertal children can be very uncomfortable and should be performed by an experienced clinician to avoid psychological and physical trauma to the child. The decision to obtain genital or other specimens from a child to evaluate for STDs must be made on an individual basis; however, children who received a diagnosis of one STD should be screened for all STDs. Because STDs are not common in prepubertal children or infants evaluated for abuse, testing all sites for all organisms is not routinely recommended. Factors that should lead the physician to consider screening for STD include (878):
Child has experienced penetration or has evidence of recent or healed penetrative injury to the genitals, anus, or oropharynx.
Child has been abused by a stranger.
Child has been abused by a perpetrator known to be infected with an STD or at high risk for STDs (e.g., intravenous drug abusers, MSM, persons with multiple sexual partners, and those with a history of STDs).
Child has a sibling, other relative, or another person in the household with an STD.
Child lives in an area with a high rate of STD in the community.
Child has signs or symptoms of STDs (e.g., vaginal discharge or pain, genital itching or odor, urinary symptoms, and genital lesions or ulcers).
Child or parent requests STD testing.
If a child has symptoms, signs, or evidence of an infection that might be sexually transmitted, the child should be tested for common STDs before the initiation of any treatment that could interfere with the diagnosis of those other STDs. Because of the legal and psychosocial consequences of a false-positive diagnosis, only tests with high specificities should be used. The potential benefit to the child of a reliable STD diagnosis justifies deferring presumptive treatment until specimens for highly specific tests are obtained by providers with experience in the evaluation of sexually abused and assaulted children.
Evaluations should be scheduled on a case-by-case basis according to history of assault or abuse and in a manner that minimizes the possibility for psychological trauma and social stigma. If the initial exposure was recent, the infectious organisms acquired through the exposure might not have produced sufficient concentrations of organisms to result in positive test results or examination findings (886). Alternatively, positive test results following a recent exposure might represent the assailant’s secretions (but would nonetheless be an indication for treatment of the child). A second visit approximately 2 weeks after the most recent sexual exposure should be scheduled to include a repeat physical examination and collection of additional specimens to identify any infection that might not have been detected at the time of initial evaluation. A single evaluation might be sufficient if the child was abused for an extended period of time and if a substantial amount of time elapsed between the last suspected episode of abuse and the medical evaluation. Compliance with follow-up appointments might be improved when law enforcement personnel or child protective services are involved.
Initial Examination
The following should be performed during the initial examination.
Visual inspection of the genital, perianal, and oral areas for genital discharge, odor, bleeding, irritation, warts, and ulcerative lesions. The clinical manifestations of some STDs are different in children than in adults. For example, typical vesicular lesions might be absent even in the presence of HSV infection. Because HSV can be indicative of sexual abuse, specimens should be obtained from all vesicular or ulcerative genital or perianal lesions and then sent for viral culture or PCR.
Culture for N. gonorrhoeae from specimens collected from the pharynx and anus in boys and girls, the vagina in girls, and the urethra in boys. Cervical specimens are not recommended for prepubertal girls. For boys with a urethral discharge, a meatal specimen discharge is an adequate substitute for an intraurethral swab specimen. Because of the legal implications of a diagnosis of N. gonorrhoeae infection in a child, if culture for the isolation of N. gonorrhoeae is done, only standard culture procedures should be performed. Gram stains are inadequate to evaluate prepubertal children for gonorrhea and should not be used to diagnose or exclude gonorrhea. Specimens from the vagina, urethra, pharynx, or rectum should be streaked onto selective media for isolation of N. gonorrhoeae, and all presumptive isolates of N. gonorrhoeae should be identified definitively by at least two tests that involve different approaches (e.g., biochemical, enzyme substrate, or serologic). Isolates should be preserved to enable additional or repeated testing. Data on use of NAAT for detection of N. gonorrhoeae in children are limited, and performance is test dependent (394). Consultation with an expert is necessary before using NAAT in this context, both to minimize the possibility of cross-reaction with nongonococcal Neisseria species and other commensals (e.g., N. meningitidis, N. sicca, N. lactamica, N. cinerea, and Moraxella catarrhalis) and to ensure appropriate interpretation of positive results. When testing vaginal secretions or urine from girls, NAAT can be used as an alternative to culture; however, culture remains the preferred method for testing urethral specimens or urine from boys and extragenital specimens (pharynx and rectum) from all children (394). All positive specimens should be retained for additional testing.
Culture for C. trachomatis from specimens collected from the anus in both boys and girls and from the vagina in girls. The likelihood of recovering C. trachomatis from the urethra of prepubertal boys is too low to justify the trauma involved in obtaining an intraurethral specimen. However, a meatal specimen should be obtained if urethral discharge is present. Pharyngeal specimens for C. trachomatis are not recommended for children of either sex because the likelihood of recovering chlamydia is low, perinatally acquired infection might persist beyond infancy, and culture systems in some laboratories do not distinguish between C. trachomatis and C. pneumoniae. Only standard culture systems for the isolation of C. trachomatis should be used. The isolation of C. trachomatis should be confirmed by microscopic identification of inclusions by staining with fluorescein-conjugated monoclonal antibody specific for C. trachomatis. Isolates should be preserved for additional testing. Nonculture tests for chlamydia (e.g., DFA) are not specific enough for use in cases of possible child abuse or assault. NAATs can be used for detection of C. trachomatis in vaginal specimens or urine from girls (394). No data are available regarding the use of NAAT from urine in boys or for extragenital specimens (e.g., those obtained from the rectum) in boys and girls. Culture remains the preferred method for extragenital sites. All specimens should be retained for additional testing.
Culture for T. vaginalis infection and wet mount of a vaginal swab specimen for T. vaginalis infection. Testing for T. vaginalis should not be limited to girls with vaginal discharge if other indications for vaginal testing exist, as there is some evidence to indicate that asymptomatic sexually abused children might be infected with T. vaginalis and might benefit from treatment (887,888). Data on use of NAAT for detection of T. vaginalis in children are too limited to inform recommendations, but no evidence suggests that performance of NAAT for detection of T. vaginalis in children would differ from that in adults.
Wet mount of a vaginal swab specimen for BV.
Collection of serum samples to be evaluated, preserved for subsequent analysis, and used as a baseline for comparison with follow-up serologic tests. Sera can be tested for antibodies to T. pallidum, HIV, and HBV. Decisions regarding the infectious agents for which to perform serologic tests should be made on a case-by-case basis.
Treatment
The risk of a child acquiring an STD as a result of sexual abuse or assault has not been well studied. Presumptive treatment for children who have been sexually assaulted or abused is not recommended because 1) the incidence of most STDs in children is low after abuse/assault, 2) prepubertal girls appear to be at lower risk for ascending infection than adolescent or adult women, and 3) regular follow-up of children usually can be ensured. However, some children or their parent(s) or guardian(s) might be concerned about the possibility of infection with an STD, even if the risk is perceived to be low by the health-care provider. Such concerns might be an appropriate indication for presumptive treatment in some settings and might be considered after all relevant specimens for diagnostic tests have been collected.
Other Management Considerations
Because child sexual-assault survivors are at increased risk for future unsafe sexual practices that have been linked to higher risk of HPV acquisition (868,889) and are more likely to engage in these behaviors at an earlier age, ACIP recommends vaccination of children who are victims of sexual abuse or assault at age ≥9 years who have not initiated or completed immunization (see HPV prevention section) (16). Although HPV vaccine will not protect against progression of infection already acquired or promote clearance of the infection, the vaccine protects against vaccine types not yet acquired.
Follow-Up
If no infections were identified at the initial examination after the last suspected sexual exposure and if this exposure was recent, a follow-up evaluation approximately 2 weeks after the last exposure can be considered. Likewise, if no physical examination or diagnostic testing was done at the initial visit, then a complete examination can be scheduled approximately 2 weeks after the last exposure to identify any evidence of STDs.
In circumstances in which transmission of syphilis, HIV, hepatitis B, or HPV is a concern but baseline tests for syphilis, HIV, and HBV are negative and examinations for genital warts are negative, follow-up serologic testing and an examination approximately 6 weeks and 3 months after the last suspected sexual exposure is recommended to allow time for antibodies to develop and signs of infection to appear. In addition, results of HBsAg testing must be interpreted carefully, because HBV can be transmitted nonsexually. Decisions regarding which tests should be performed must be made on an individual basis.
Risk for Acquiring HIV Infection
HIV infection has been reported in children for whom sexual abuse was the only known risk factor. Children might be at higher risk for HIV acquisition than adolescent and adult sexual assault or sexual abuse survivors because the sexual abuse of children is frequently associated with multiple episodes of assault and mucosal trauma might be more likely. Serologic testing for HIV infection should be considered for sexually abused children. The decision to test for HIV infection should involve the family, if possible, and be made on a case-by-case basis depending on the likelihood of infection among assailant(s) (890). Although data are insufficient concerning the efficacy of nPEP among children, treatment is well tolerated by infants and children with and without HIV infection, and children have a minimal risk for serious adverse reactions because of the short period recommended for prophylaxis (312,891). In considering whether to offer nPEP, health-care providers should consider whether the child can be treated soon after the sexual exposure (i.e., within 72 hours), the likelihood that the assailant is infected with HIV, and the likelihood of high compliance with the prophylactic regimen. The potential benefit of treating a sexually abused child should be weighed against the risk for adverse reactions. If nPEP is being considered, a provider specializing in evaluating or treating children with HIV infection should be consulted.
Recommendations for Postexposure HIV Risk Assessment of Children within 72 Hours of Sexual Assault
Review HIV/AIDS local epidemiology, assess risk for HIV infection in the assailant, and test for HIV infection.
Evaluate circumstances of assault that might affect risk for HIV transmission.
Consult with a specialist in treating children with HIV infection to select age-appropriate dosing and regimens if nPEP is considered.
For children determined to be at risk for HIV transmission from the assault, discuss nPEP with the caregiver(s), including its toxicity, unknown efficacy, and possible benefits.
If nPEP is begun, adequate doses of medication should be provided to last until the follow-up visit at 3–7 days after the initial assessment, at which time the child should be reevaluated and tolerance of medication assessed (105,312,892).
If nPEP is started, perform CBC and serum chemistry at baseline.
Perform HIV antibody testing during the original assessment and again at 6 weeks, 3 months, and 6 months after the assault.
Terms and Abbreviations Used in This Report
- AIDS
Acquired immunodeficiency syndrome
- ALT
Alanine aminotransferase
- anti-HBc
Antibody to hepatitis B core antigen
- anti-HCV
Hepatitis C antibodies
- ASC-US
Atypical squamous cells of undetermined significance
- BCA
Bichloroacetic acid
- BV
Bacterial vaginosis
- CBC
Complete blood count
- CI
Confidence interval
- CIN
Cervical intraepithelial neoplasia
- CLD
Chronic liver disease
- CLIA
Clinical Laboratory Improvement Amendments
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- DFA
Direct fluorescent antibody
- DGI
Disseminated gonococcal infection
- dL
Deciliter
- DNA
Deoxyribonucleic acid
- EC
Emergency contraception
- EIA
Enzyme immunoassay
- ELISA
Enzyme-linked immunosorbent assay
- EPT
Expedited partner therapy
- FDA
Food and Drug Administration
- FTA-ABS
Fluorescent treponemal antibody absorbed
- gG
Glycoprotein G
- GNID
Gram-negative intracellular diplococcic
- HAART
Highly active antiretroviral therapy
- HAV
Hepatitis A virus
- HBIG
Hepatitis B immune globulin
- HbsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- IFA
Immunofluorescence assay
- IgE
Immunoglobulin E
- Ig
Immune globulin
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- IM
Intramuscularly
- IUD
Intrauterine device
- IV
Intravenous or intravenously
- KOH
Potassium hydroxide
- LGV
Lymphogranuloma venereum
- MAC
Mycobacterium avium complex
- MIC
Minimum inhibitory concentration
- MSM
Men who have sex with men
- N-9
Nonoxynol-9
- NAAT
Nucleic acid amplification test
- NGU
Nongonococcal urethritis
- nPEP
Nonoccupational postexposure prophylaxis
- Pap
Papanicolaou
- PCR
Polymerase chain reaction
- PEP
Postexposure prophylaxis
- PID
Pelvic inflammatory disease
- PO
By mouth
- PPV
Positive predictive value
- QRNG
Quinolone-resistant Neisseria gonorrhoeae
- RNA
Ribonucleic acid
- RPR
Rapid plasma regain
- RT-PCR
Reverse transcriptase polymerase chain reaction
- RVVC
Recurrent vulvovaginal candidiasis
- SIL
Squamous intraepithelial lesion
- STD
Sexually transmitted disease
- TCA
Trichloroacetic acid
- TE
Toxoplasmic encephalitis
- TP-PA
Treponema pallidum particle agglutation
- VDRL
Venereal Disease Research Laboratory
- VVC
Vulvovaginal candidiasis
- WB
Western blot
- WBC
White blood count
- WSW
Women who have sex with women
Sexually Transmitted Diseases Treatment Guidelines, 2015 Workgroup Members
Chairperson: Kimberly A. Workowski, MD, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), CDC and Emory University, Atlanta, Georgia.
Presenters: D’Jahna Akinyemi, MD, MSCE, Johns Hopkins School of Medicine, Baltimore, Maryland; Laura Bachmann, MD, MPH, Wake Forest University Health Sciences, Winston-Salem, North Carolina; Gale Burstein, MD, Erie County Department of Health, Buffalo, New York; Stephanie Cohen, MD, San Francisco Department of Public Health, San Francisco, California; Carolyn Gardella, MD, University of Washington, Seattle, Washington; William M. Geisler, MD, University of Alabama, Birmingham, Alabama; Khalil Ghanem, MD, Johns Hopkins University, Baltimore, Maryland; Matt Golden, MD, University of Washington, Seattle, Washington; Linda Gorgos, MD, El Rio Community Health Center, Tucson, Arizona; Margaret Hammerschlag, MD, State University of New York, Downstate Medical Center, Brooklyn, New York; Lisa Hollier, MD, Baylor University, Houston, Texas; Katherine Hsu, MD, MPH, Massachusetts Department of Public Health, Jamaica Plains, Massachusetts; Peter Leone, MD, University of North Carolina School of Medicine, Chapel Hill, North Carolina; Lisa Manhart, PhD, University of Washington, Seattle, Washington; Jeanne Marrazzo, MD, University of Washington, Seattle, Washington; Kenneth Hugh Mayer, MD, Harvard Medical School, Boston, Massachusetts; Leandro Mena, MD, University of Mississippi School of Medicine, Jackson, Mississippi; Paul Nyirjesy, MD, Drexel University College of Medicine, Philadelphia, Pennsylvania; Ina Park, MD, STD Control Branch, California Department of Public Health, Department of Public Health; Susan Philip, MD, San Francisco Department of Public Health, San Francisco, California; Cornelius “Kees” Rietmeijer, MD, PhD, Rietmeijer Consulting, LLC, Denver, Colorado; Anne Rompalo, MD, Johns Hopkins School of Medicine, Baltimore, Maryland; Pablo Sanchez, MD, University of Texas Southwestern Medical Center, Dallas, Texas; Bradley Stoner, MD, PhD, Washington University, St. Louis, Missouri; Stephanie N. Taylor, MD, Louisiana State University Health Sciences Center, New Orleans, Louisiana; Anna Wald, MD, University of Washington, Seattle, Washington; George Wendel, MD, American Board of Ob-Gyn, Dallas, Texas; Harold C. Wiesenfeld, MD, University of Pittsburgh, Pittsburgh, Pennsylvania.
Moderators: Willard Cates, Jr., MD, Family Health International, Durham, North Carolina; King K. Holmes, MD, PhD, University of Washington, Seattle, Washington; Edward W. Hook III, MD, University of Alabama at Birmingham, Alabama; David Martin, MD, Louisiana State University Health Sciences Center, New Orleans, Louisiana.
Rapporteurs: William M. Geisler, MD, University of Alabama, Birmingham, Alabama; Jeanne Marrazzo, MD, University of Washington, Seattle, Washington; Anne Rompalo, MD, Johns Hopkins School of Medicine, Baltimore, Maryland.
Workgroup Members: Virginia A. Caine, MD, Marion County Public Health Department; Susan Cu-Uvin, MD, Brown University, Providence, Rhode Island; Carolyn Deal, PhD, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland ; Jordan Dimitrakoff, MD, PhD, Beth Israel Deaconess Medical Center, Boston, Massachusetts; J. Dennis Fortenberry, MD, Indiana University School of Medicine, Indianapolis, Indiana; Sarah Guerry, MD, Los Angeles County Department of Public Health, Los Angeles, California; Edward W. Hook III, MD, University of Alabama at Birmingham, Alabama; H. Hunter Handsfield, MD, University of Washington, Seattle, Washington; Patricia Kissinger, PhD, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana; Jill E. (Brown) Long, MD, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; Harvey J. Makadon, MD, The Fenway Institute, Boston, Massachusetts; Jane R. Schwebke, MD, University of Alabama, Birmingham, Alabama; Arlene Sena, MD, University of North Carolina, Chapel Hill, North Carolina; Jack D. Sobel, MD, Wayne State University School of Medicine, Detroit, Michigan; David Soper, MD, Medical University of South Carolina, Charleston, South Carolina; Richard Sweet, MD, University of California Davis School of Medicine, Sacramento, California; Jonathan M. Zenilman, MD, Johns Hopkins Bayview Medical Center, Baltimore, Maryland.
Liaison Participants: Lynn Barclay, MD, American Social Health Association; Anton Best, MBBS, Barbados Ministry of Health, HIV/AIDS; Gale Burstein, MD, American Academy of Pediatrics; Society of Adolescent Health and Medicine; National Association of City and County Health Officials; Carolyn Deal, PhD, National Institute of Allergy and Infectious Diseases, National Institutes of Health; Carlos Del Rio, MD, HIV Medical Association and Infectious Disease Society of America; Rupali Kotwal Doshi, MD, Health Resources and Services Administration, HIV/AIDS Bureau; Paul Etkind, DrPH, National Association of City and County Health Officials; Carolyn Gardella, MD, Infectious Disease Society of Obstetrics and Gynecology; Eric Garges, MD, U. S. Department of Defense; Tina Groat, MD, United Healthcare; Seiji Hayashi, MD, Health Resources and Services Administration – Bureau of Primary Health Care; Katherine Hsu, MD, MPH, National Network of STD/HIV Prevention Training Centers; Jeanne Marrazzo, MD, CDC/HRSA Advisory Committee on HIV and STD Prevention and Treatment; Beth Jordan Mynett, MD, Association of Reproductive Health Professionals; Deborah Nucatola, MD/Karen Shea, MSN, Planned Parenthood Federation of America; Pilar Ramón-Pardo, MD, Pan American Health Organization; Keith Radcliffe, MD, British Association of Sexual Health and International Union against STIs Europe, Birmingham, United Kingdom; Jennifer Rooke, MD, American College of Preventative Medicine; Bisan Salhi, MD, American College of Emergency Physicians; Daniel A. Shoskes, MD, American Urological Association; Frances Smith, MD, Barbados Ministry of Health, STIs; Nathaniel Smith, MD, Association of State and Territorial Health Officials; William (Bill) Smith, MD, National Coalition of STD Directors; David Soper, MD, American College of Obstetrics and Gynecology; Anne Spaulding, MD, National Commission on Correctional Health; Bradley Stoner, MD, PhD, American Sexually Transmitted Disease Association; Teodora Elvira Wi, MD, World Health Organization, Geneva, Switzerland; Tom Wong, MD, Public Health Agency of Canada, Ottawa, Ontario, Canada; Scott J. Zimmerman, DrPH, Association of Public Health Laboratories.
CDC, Division of Sexually Transmitted Disease Prevention Treatment Guidelines 2015 Project Coordinator: Kimberly A. Workowski, MD, NCHHSTP, CDC and Emory University, Atlanta, Georgia.
Project Manager: Melinda Flock, NCHHSTP, CDC, Atlanta, Georgia.
NCHHSTP/CDC Presenters: John T. Brooks, MD; Eileen Dunne, MD; Scott Holmberg, MD; Camille Introcaso, MD; Sarah Kidd, MD; Elissa Meites, MD; Mona Saraiya, MD.
CDC Consultants: Faruque Ahmed, MD; Sevgi O. Aral, PhD; John Brooks, MD; John Douglas, MD; Eileen Dunne, MD; Camille Introcaso, MD; Jill S. Huppert, MD; Sarah Kidd, MD; Elissa Meites, MD; and Mona Saraiya, MD.
Support Staff: Sheila McKenzie, Michele Thomas, Deborah McElroy and Angela Jones, NCHHSTP, CDC, Atlanta, Georgia.
Conflicts of Interest
Laura Bachmann, CDC funding; Virginia Caine, CDC funding; Ward Cates, Contraceptive Technology Communications, grant funding from FHI 360, Gates Foundation; William Geisler, research funding Warner Chilcott; Matt Golden, research funding from Genprobe, Biorad, Cempra; Sara Guerry, speaker for Merck; Ned Hook, research funding from Cempra, Glaxo Smith Kline, Becton Dickenson, Roche, Cepheid, GenProbe/Hologic, CDC, NIH; Peter Leone, speaker for Abbott Diagnostics; Lisa Manhart, previous conference support and travel funds from BioRad, previous reagents from Pfizer and GenProbe; Jeanne Marrazzo, research funding from Cepheid, Hologic/GenProbe, Medicis, and MethylGene, and consultant for Starpharma, Symbiomix, and Toltec; Leandro Mena, research funding from Becton Dickinson; Paul Nyirjesy, consultant at Hologic, receives research funding from Genesis Biotech Group; Susan Philip, research funding from Roche Diagnostics, Abbott Diagnostics, SeraCare Inc, Cepheid Inc;Jane Schwebke, consultant and receives research funding from Hologic/Genprobe, BD Diagnostics, LabCorp, Medicis, Methylgene, Embal; Jack Sobel, consultant with Medical Diagnostic Laboratories; Stephanie Taylor, consultant with Hologic/Genprobe, receives research funding from Roche, Becton Dickinson, Cepheid; Harold Wiesenfeld, research funding from CDC, Methylgene Inc., Genprobe Inc.
Footnotes
STI is the term used by USPSTF to describe the syndromes caused by various pathogens that can be acquired and transmitted through sexual activity.
Regardless of condom use during exposure.
Commerically available NAATs have not been cleared by FDA for these indications, but they can be used by laboratories that have met all regulatory requirements for an off-label procedure. Source: CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae — 2014. MMWR Recomm Rep 2014;63(No RR-2):1–19.
The absence of a fourfold or greater titer for a neonate does not exclude congenital syphilis.
CSF test results obtained during the neonatal period can be difficult to interpret; normal values differ by gestational age and are higher in preterm infants. Values as high as 25 white blood cells (WBCs)/mm3 and/or protein of 150 mg/dL might occur among normal neonates; lower values (i.e., 5 WBCs/mm3 and protein of 40 mg/dL) might be considered the upper limits of normal. Other causes of elevated values should be considered when an infant is being evaluated for congenital syphilis.
A women treated with a regimen other than recommended in these guidelines should be considered untreated.
References
- 1.CDC. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep. 2010:59. (No. RR-12) [PubMed] [Google Scholar]
- 2.CDC. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006:55. (No. RR-7) [PubMed] [Google Scholar]
- 3.CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part II: immunization of adults. MMWR Recomm Rep. 2006:55. (No. RR-16) [PubMed] [Google Scholar]
- 4.CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005:54. (No. RR-16) [PubMed] [Google Scholar]
- 5.CDC. A guide to taking a sexual history. Atlanta, GA: US Department of Health and Human Services, CDC; Available at http://www.cdc.gov/std/treatment/SexualHistory.pdf. [Google Scholar]
- 6.O’Connor EA, Lin JS, Burda BU, et al. USPSTF: behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections. Ann Intern Med. 2014;161:874–83. doi: 10.7326/M14-0475. [DOI] [PubMed] [Google Scholar]
- 7.LeFevre ML. USPSTF: behavioral counseling interventions to prevent sexually transmitted infections. Ann Intern Med. 2014;161:894–901. doi: 10.7326/M14-1965. [DOI] [PubMed] [Google Scholar]
- 8.Kamb ML, Fishbein M, Douglas JM, Jr, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280:1161–7. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 9.Metsch LR, Feaster DJ, Gooden L, et al. Effect of risk-reduction counseling with rapid HIV testing on risk of acquiring sexually transmitted infections: the AWARE randomized clinical trial. JAMA. 2013;310:1701–10. doi: 10.1001/jama.2013.280034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel P, Bush T, Mayer K, et al. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis. 2012;39:470–4. doi: 10.1097/OLQ.0b013e31824b3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warner L, Klausner JD, Rietmeijer CA, et al. Effect of a brief video intervention on incident infection among patients attending sexually transmitted disease clinics. PLoS Med. 2008;5:919–27. doi: 10.1371/journal.pmed.0050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC, Health Resources and Services Administration, National Institutes of Health, American Academy of HIV Medicine, Association of Nurses in AIDS Care, International Association of Providers of AIDS Care, the National Minority AIDS Council, and Urban Coalition for HIV/AIDS Prevention Services. Recommendations for HIV Prevention with Adults and Adolescents with HIV in the United States, 2014. Available at http://stacks.cdc.gov/view/cdc/26062.
- 13.Gilbert P, Ciccarone D, Gansky SA, et al. Interactive “video doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3:e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson JL, Milam J, McCutchan A, et al. Effect of brief safer-sex counseling by medical providers to HIV-1 seropositive patients: a multi-clinic assessment. AIDS. 2004;18:1179–86. doi: 10.1097/00002030-200405210-00011. [DOI] [PubMed] [Google Scholar]
- 15.Fisher JD, Cornman DH, Osborn CY, et al. Clinician-initiated HIV risk reduction intervention for HIV-positive persons: formative research, acceptability, and fidelity of the Options Project. J Acquir Immune Defic Syndr. 2004;37(Suppl 2):S78–S87. doi: 10.1097/01.qai.0000140605.51640.5c. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR RecommRep. 2014:63. (No. RR-05) [PubMed] [Google Scholar]
- 17.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 18.Anderson C, Gallo MF, Hylton-Kong T, et al. Randomized controlled trial on the effectiveness of counseling messages for avoiding unprotected sexual intercourse during sexually transmitted infection and reproductive tract infection treatment among female sexually transmitted infection clinic patients. Sex Transm Dis. 2013;40:105–10. doi: 10.1097/OLQ.0b013e31827938a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatcher RA, Trussel J, Nelson AL, et al. Contraceptive Technology. 20th. New York: Ardent Media; 2012. [Google Scholar]
- 20.Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev. 2002;1:CD003255. doi: 10.1002/14651858.CD003255. [DOI] [PubMed] [Google Scholar]
- 21.Smith DK, Herbst JH, Zhang X, et al. Condom efficacy by consistency of use among MSM; Paper presented at: US 20th Conference on Retroviruses and Opportunistic Infections; Atlanta. 2013. http://www.aidsmap.com/Consistent-condom-use-in-anal-sex-stops-70-of-HIV-infections-study-finds-but-intermittent-use-has-no-effect/page/2586976/ [Google Scholar]
- 22.Crosby RA, Charnigo RA, Weathers C, et al. Condom effectiveness against non-viral sexually transmitted infections: a prospective study using electronic daily diaries. Sex Transm Infect. 2012;88:484–9. doi: 10.1136/sextrans-2012-050618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ. 2004;82:454–61. [PMC free article] [PubMed] [Google Scholar]
- 24.Warner L, Stone KM, Macaluso M, et al. Condom use and risk of gonorrhea and Chlamydia: a systematic review of design and measurement factors assessed in epidemiologic studies. Sex Transm Dis. 2006;33:36–51. doi: 10.1097/01.olq.0000187908.42622.fd. [DOI] [PubMed] [Google Scholar]
- 25.Ness RB, Randall H, Richter HE, et al. Condom use and the risk of recurrent pelvic inflammatory disease, chronic pelvic pain, or infertility following an episode of pelvic inflammatory disease. Am J Public Health. 2004;94:1327–9. doi: 10.2105/ajph.94.8.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koss CA, Dunne EF, Warner L. A systematic review of epidemiologic studies assessing condom use and risk of syphilis. Sex Transm Dis. 2009;36:401–5. doi: 10.1097/OLQ.0b013e3181a396eb. [DOI] [PubMed] [Google Scholar]
- 27.Martin ET, Krantz E, Gottlieb SL, et al. A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Arch Intern Med. 2009;169:1233–40. doi: 10.1001/archinternmed.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winer RL, Hughes JP, Feng QH, et al. Condom use and the risk of genital human papillomavirus infection in young women. New England Journal of Medicine. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 29.Bleeker MCG, Hogewoning CJA, Voorhorst FJ, et al. Condom use promotes regression of human papillomavirus-associated penile lesions in male sexual partners of women with cervical intraepithelial neoplasia. International Journal of Cancer. 2003;107:804–10. doi: 10.1002/ijc.11473. [DOI] [PubMed] [Google Scholar]
- 30.Hogewoning CJA, Bleeker MCG, van den Brule AJC, et al. Condom use promotes regression of cervical intraepithelial neoplasia and clearance of human papillomavirus: a randomized clinical trial. International Journal of Cancer. 2003;107:811–6. doi: 10.1002/ijc.11474. [DOI] [PubMed] [Google Scholar]
- 31.Bernabe-Ortiz A, Carcamo CP, Scott JD, et al. HBV infection in relation to consistent condom use: a population-based study in Peru. PLoS One. 2011;6:e24721. doi: 10.1371/journal.pone.0024721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minuk GY, Bohme CE, Bowen TJ, et al. Efficacy of commercial condoms in the prevention of hepatitis B virus infection. Gastroenterology. 1987;93:710–4. doi: 10.1016/0016-5085(87)90431-8. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez-Romieu AC, Siegler AJ, Sullivan PS, et al. How often do condoms fail? A cross-sectional study exploring incomplete use of condoms, condom failures and other condom problems among black and white MSM in southern USA. Sex Transm Infect. 2014 doi: 10.1136/sextrans-2014-051581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Anna LH, Margolis AD, Warner L, et al. Condom use problems during anal sex among men who have sex with men (MSM): findings from the Safe in the City study. AIDS Care. 2012;24:1028–38. doi: 10.1080/09540121.2012.668285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiner MJ, Cates W, Jr, Warner L. The real problem with male condoms is nonuse. Sex Transm Dis. 1999;26:459–62. doi: 10.1097/00007435-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Gallo MF, Kilbourne-Brook M, Coffey PS. A review of the effectiveness and acceptability of the female condom for dual protection. Sex Health. 2012;9:18–26. doi: 10.1071/SH11037. [DOI] [PubMed] [Google Scholar]
- 37.Mantell JE, Kelvin EA, Exner TM, et al. Anal use of the female condom: does uncertainty justify provider inaction? AIDS Care. 2009;21:1185–94. doi: 10.1080/09540120902730005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg MJ, Davidson AJ, Chen JH, et al. Barrier contraceptives and sexually transmitted diseases in women: a comparison of female-dependent methods and condoms. Am J Public Health. 1992;82:669–74. doi: 10.2105/ajph.82.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Bruyn G, Shiboski S, van der Straten A, et al. The effect of the vaginal diaphragm and lubricant gel on acquisition of HSV-2. Sex Transm Infect. 2011;87:301–5. doi: 10.1136/sti.2010.047142. [DOI] [PubMed] [Google Scholar]
- 40.Ramjee G, van der Straten A, Chipato T, et al. The diaphragm and lubricant gel for prevention of cervical sexually transmitted infections: results of a randomized controlled trial. PLoS One. 2008;3:e3488. doi: 10.1371/journal.pone.0003488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson D, Tholandi M, Ramjee G, et al. Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: systematic review and meta-analysis of randomised controlled trials including more than 5000 women. Lancet Infect Dis. 2002;2:613–7. doi: 10.1016/s1473-3099(02)00396-1. [DOI] [PubMed] [Google Scholar]
- 42.McCormack S, Ramjee G, Kamali A, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376:1329–37. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–87. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 44.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 45.Feldblum PJ, Adeiga A, Bakare R, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One. 2008;3:e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hooton TM, Roberts PL, Stamm WE. Effects of recent sexual activity and use of a diaphragm on the vaginal microflora. Clin Infect Dis. 1994;19:274–8. doi: 10.1093/clinids/19.2.274. [DOI] [PubMed] [Google Scholar]
- 47.Fihn SD, Boyko EJ, Normand EH, et al. Association between use of spermicide-coated condoms and Escherichia coli urinary tract infection in young women. Am J Epidemiol. 1996;144:512–20. doi: 10.1093/oxfordjournals.aje.a008958. [DOI] [PubMed] [Google Scholar]
- 48.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13:797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 49.WHO. Hormonal contraception and HIV. World Health Organization; Geneva: 2012. [PubMed] [Google Scholar]
- 50.CDC. Update to CDC’s US medical eligibility criteria for contraceptive use, 2010: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection or infected with HIV. MMWR Morb Mortal Wkly Rep. 2012;61:449–52. [PubMed] [Google Scholar]
- 51.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 52.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 53.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auvert B, Sobngwi-Tambekou J, Cutler E, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in Orange Farm, South Africa. J Infect Dis. 2009;199:14–9. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sobngwi-Tambekou J, Taljaard D, Nieuwoudt M, et al. Male circumcision and Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis: observations after a randomised controlled trial for HIV prevention. Sex Transm Infect. 2009;85:116–20. doi: 10.1136/sti.2008.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS. 2012;26:609–15. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehta SD, Moses S, Parker CB, et al. Circumcision status and incident herpes simplex virus type 2 infection, genital ulcer disease, and HIV infection. AIDS. 2012;26:1141–9. doi: 10.1097/QAD.0b013e328352d116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WHO/UNAIDS. WHO/UNAIDS Technical Consultation on Male Circumcision and HIV Prevention: Research Implications for Policy and Programming. Montreux: Mar 6–8, 2007. New data on male circumcision and HIV prevention: policy and programme implications. 2007. [Google Scholar]
- 60.American Academy of Pediatrics Task Force on Circumcision. Circumcision policy statement. Pediatrics. 2012;130:585–6. [Google Scholar]
- 61.American Urological Association. Circumcision policy statement. 2007 Available at http://www.auanet.org/about/policy-statements.cfm.
- 62.Wiysonge CS, Kongnyuy EJ, Shey M, et al. Male circumcision for prevention of homosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2011;6:CD007496. doi: 10.1002/14651858.CD007496.pub2. [DOI] [PubMed] [Google Scholar]
- 63.CDC. U.S. Selected practice recommendations for contraceptive use, 2013: Adapted from the World Health Organization selected practice recommendations for contraceptive use. MMWR Recomm Rep. (2nd) 2013:62. (No. RR-05) [PubMed] [Google Scholar]
- 64.Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115:257–63. doi: 10.1097/AOG.0b013e3181c8e2aa. [DOI] [PubMed] [Google Scholar]
- 65.Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555–62. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- 66.Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Human reproduction. 2012;27:1994–2000. doi: 10.1093/humrep/des140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng L, Che Y, Gulmezoglu AM. Interventions for emergency contraception. Cochrane Database Syst Rev. 2012;8:CD001324. doi: 10.1002/14651858.CD001324.pub4. [DOI] [PubMed] [Google Scholar]
- 68.Myer L, Kuhn L, Stein ZA, et al. Intravaginal practices, bacterial vaginosis, and women’s susceptibility to HIV infection: epidemiological evidence and biological mechanisms. Lancet Infect Dis. 2005;5:786–94. doi: 10.1016/S1473-3099(05)70298-X. [DOI] [PubMed] [Google Scholar]
- 69.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2013 Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 71.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grohskopf LA, Chillag KL, Gvetadze R, et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64:79–86. doi: 10.1097/QAI.0b013e31828ece33. [DOI] [PubMed] [Google Scholar]
- 74.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 77.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 78.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112–4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Straten A, Van Damme L, Haberer JE, et al. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–9. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 80.Golden MR, Dombrowski JC, Kerani RP, et al. Failure of serosorting to protect African American men who have sex with men from HIV infection. Sex Transm Dis. 2012;39:659–64. doi: 10.1097/OLQ.0b013e31825727cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Philip SS, Yu X, Donnell D, et al. Serosorting is associated with a decreased risk of HIV seroconversion in the EXPLORE Study Cohort. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vallabhaneni S, Li X, Vittinghoff E, et al. Seroadaptive practices: association with HIV acquisition among HIV-negative men who have sex with men. PLoS One. 2012;7:e45718. doi: 10.1371/journal.pone.0045718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jin F, Prestage GP, Templeton DJ, et al. The impact of HIV seroadaptive behaviors on sexually transmissible infections in HIV-negative homosexual men in Sydney, Australia. Sex Transm Dis. 2012;39:191–4. doi: 10.1097/OLQ.0b013e3182401a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hotton AL, Gratzer B, Mehta SD. Association between serosorting and bacterial sexually transmitted infection among HIV-negative men who have sex with men at an urban lesbian, gay, bisexual, and transgender health center. Sex Transm Dis. 2012;39:959–64. doi: 10.1097/OLQ.0b013e31826e870d. [DOI] [PubMed] [Google Scholar]
- 85.Turner AN, Feldblum PJ, Hoke TH. Baseline infection with a sexually transmitted disease is highly predictive of reinfection during follow-up in Malagasy sex workers. Sex Transm Dis. 2010;37:559–62. doi: 10.1097/OLQ.0b013e3181d70a03. [DOI] [PubMed] [Google Scholar]
- 86.Peterman TA, Tian LH, Metcalf CA, et al. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med. 2006;145:564–72. doi: 10.7326/0003-4819-145-8-200610170-00005. [DOI] [PubMed] [Google Scholar]
- 87.Trelle S, Shang A, Nartey L, et al. Improved effectiveness of partner notification for patients with sexually transmitted infections: systematic review. BMJ. 2007;334:354. doi: 10.1136/bmj.39079.460741.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson TE, Hogben M, Malka ES, et al. A randomized controlled trial for reducing risks for sexually transmitted infections through enhanced patient-based partner notification. Am J Public Health. 2009;99(Supp1):S104–10. doi: 10.2105/AJPH.2007.112128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu YY, Frasure-Williams JA, Dunne EF, et al. Chlamydia partner services for females in California family planning clinics. Sex Transm Dis. 2011;38:913–8. doi: 10.1097/OLQ.0b013e3182240366. [DOI] [PubMed] [Google Scholar]
- 90.Mickiewicz T, Al-Tayyib A, Thrun M, et al. Implementation and effectiveness of an expedited partner therapy program in an urban clinic. Sex Transm Dis. 2012;39:923–9. doi: 10.1097/OLQ.0b013e3182756f20. [DOI] [PubMed] [Google Scholar]
- 91.Golden MR, Hogben M, Handsfield HH, et al. Partner notification for HIV and STD in the United States: low coverage for gonorrhea, chlamydial infection, and HIV. Sex Transm Dis. 2003;30:490–6. doi: 10.1097/00007435-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Vest JR, Valadez AM, Hanner A, et al. Using e-mail to notify pseudonymous e-mail sexual partners. Sex Transm Dis. 2007;34:840–5. doi: 10.1097/OLQ.0b013e318073bd5d. [DOI] [PubMed] [Google Scholar]
- 93.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676–85. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]
- 94.Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women – a randomized, controlled trial. Sex Transm Dis. 2003;30:49–56. doi: 10.1097/00007435-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Kissinger P, Mohammed H, Richardson-Alston G, et al. Patient-delivered partner treatment for male urethritis: a randomized, controlled trial. Clin Infect Dis. 2005;41:623–9. doi: 10.1086/432476. [DOI] [PubMed] [Google Scholar]
- 96.Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Human reproduction. 2009;24:888–95. doi: 10.1093/humrep/den475. [DOI] [PubMed] [Google Scholar]
- 97.Kissinger P, Schmidt N, Mohammed H, et al. Patient-delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sex Transm Dis. 2006;33:445–50. doi: 10.1097/01.olq.0000204511.84485.4c. [DOI] [PubMed] [Google Scholar]
- 98.Schwebke JR, Desmond RA. A randomized controlled trial of partner notification methods for prevention of trichomoniasis in women. Sex Transm Dis. 2010;37:392–6. doi: 10.1097/OLQ.0b013e3181dd1691. [DOI] [PubMed] [Google Scholar]
- 99.Stephens SC, Bernstein KT, Katz MH, et al. The effectiveness of patient-delivered partner therapy and chlamydial and gonococcal reinfection in San Francisco. Sex Transm Dis. 2010;37:525–9. doi: 10.1097/OLQ.0b013e3181d8920f. [DOI] [PubMed] [Google Scholar]
- 100.Kerani RP, Fleming M, DeYoung B, et al. A randomized, controlled trial of inSPOT and patient-delivered partner therapy for gonorrhea and chlamydial infection among men who have sex with men. Sex Transm Dis. 2011;38:941–6. doi: 10.1097/OLQ.0b013e318223fcbc. [DOI] [PubMed] [Google Scholar]
- 101.Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787–93. doi: 10.1086/428043. [DOI] [PubMed] [Google Scholar]
- 102.McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection–what would be missed? Sex Transm Dis. 2008;35:834–6. doi: 10.1097/OLQ.0b013e3181761993. [DOI] [PubMed] [Google Scholar]
- 103.Chou R, Cantor AG, Zakher B, et al. Screening for HIV in pregnant women: systematic review to update the 2005 US Preventive Services Task Force recommendation. Ann Intern Med. 2012;157:719–28. doi: 10.7326/0003-4819-157-10-201211200-00009. [DOI] [PubMed] [Google Scholar]
- 104.Moyer VA, US Preventive Services Task Force Screening for HIV: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;159:51–60. doi: 10.7326/0003-4819-159-1-201307020-00645. [DOI] [PubMed] [Google Scholar]
- 105.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/perinatalgl.pdf.
- 106.US Preventive Services Task Force. Screening for syphilis infection in pregnancy: reaffirmation recommendation statement. Ann Intern Med. 2009;150:705–9. doi: 10.7326/0003-4819-150-10-200905190-00008. [DOI] [PubMed] [Google Scholar]
- 107.US Prevention ServicesTask Force. Screening for hepatitis B virus infection in pregnancy: reaffirmation recommendation statement. Ann Intern Med. 2009;150:869–73. doi: 10.7326/0003-4819-150-12-200906160-00011. [DOI] [PubMed] [Google Scholar]
- 108.LeFevre ML. USPSTF: screening for chlamydia and gonorrhea. Ann Intern Med. 2014;161:902–10. doi: 10.7326/M14-1981. [DOI] [PubMed] [Google Scholar]
- 109.Moyer VA. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–57. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 110.Massad LS, Einstein MH, Huh WK, et al. 2012 ASCCP Consensus Guidelines Conference 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(Suppl 1):S1–27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 111.US Preventive Services Task Force. Screening for bacterial vaginosis in pregnancy to prevent preterm delivery: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:214–9. doi: 10.7326/0003-4819-148-3-200802050-00007. [DOI] [PubMed] [Google Scholar]
- 112.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 6th. Washington, DC: American College of Obstetricians and Gynecologists; 2007. [Google Scholar]
- 113.CDC. Rapid HIV antibody testing during labor and delivery for women of unknown HIV status: a practical guide and model protocol, 2004. Atlanta, GA: CDC; 2004. [Google Scholar]
- 114.American College of Obstetricians and Gynecologists. Viral hepatitis in pregnancy. ACOG Practice Bulletin No. 86. Obstet Gynecol. 2007;110:941–56. doi: 10.1097/01.AOG.0000263930.28382.2a. [DOI] [PubMed] [Google Scholar]
- 115.Public Health Agency of Canada. Canadian guidelines on sexually transmitted infections. 2006. Centre for Communicable Diseases and Infection Control; 2010. Available at: http://www.phac-aspc.gc.ca/std-mts/sti-its/ [Google Scholar]
- 116.Meyers D, Wolff T, Gregory K, et al. USPSTF Recommendations for STI screening. Am Fam Physician. 2008;77:819–24. [PubMed] [Google Scholar]
- 117.Forhan SE, Gottlieb SL, Sternberg MR, et al. Prevalence of sexually transmitted infections smong female adolescents aged 14 to 19 in the United States. Pediatrics. 2009;124:1505–12. doi: 10.1542/peds.2009-0674. [DOI] [PubMed] [Google Scholar]
- 118.CDC. Sexually transmitted disease surveillance 2013. Atlanta: US Department of Health and Human Services; 2014. [Google Scholar]
- 119.English A, Benson GR, Nash E, et al. Confidentiality for individuals insured as dependents: a review of state laws and policies. New York: Guttmacher institute and public health solutions; 2012. Available at: http://www.guttmacher.org/pubs/confidentiality-review.pdf. 2012. [Google Scholar]
- 120.US Preventive Services Task Force. Screening for chlamydial infection: recommendation statement. Ann Intern Med. 2007;147:128–34. doi: 10.7326/0003-4819-147-2-200707170-00172. [DOI] [PubMed] [Google Scholar]
- 121.CDC. Male Chlamydia Consultation, March 28–29, 2006. Atlanta, GA: May 22, 2007. Meeting report. [Google Scholar]
- 122.CDC. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006:55. (No. RR-14) [PubMed] [Google Scholar]
- 123.US Preventive Services Task Force. USPSTF: Screening for HIV: final recommendation statement. AHRQ Publication No 12-05173-EF-3. Available at http://www.uspreventiveservicestaskforce.org/uspstf13/hiv/hivfinalrs.htm.
- 124.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.American College of Obstetricians and Gynecologists (ACOG) Screening for cervical cancer. ACOG Practice Bulletin Number 131. Obstet Gynecol. 2012;120:1222–38. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 126.Moyer VA. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–91. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 127.Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ. 2009;339:b2968. doi: 10.1136/bmj.b2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mayhew A, Mullins TL, Ding L, et al. Risk perceptions and subsequent sexual behaviors after HPV vaccination in adolescents. Pediatrics. 2014;133:404–11. doi: 10.1542/peds.2013-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.CDC. Evaluation of large jail STD screening programs, 2008–2009. Atlanta, GA: CDC, NCHHSTP; 2011. Available at http://www.cdc.gov/std/publications/JailScreening2011.pdf. 2011. [Google Scholar]
- 130.Pathela P, Hennessy RR, Blank S, et al. The contribution of a urine-based jail screening program to citywide male chlamydia and gonorrhea case rates in New York City. Sex Transm Dis. 2009;36(2 Suppl):S58–61. doi: 10.1097/OLQ.0b013e31815615bb. [DOI] [PubMed] [Google Scholar]
- 131.Joesoef MR, Weinstock HS, Kent CK, et al. Corrections STD Prevalence Monitoring Group Sex and age correlates of Chlamydia prevalence in adolescents and adults entering correctional facilities, 2005: implications for screening policy. Sex Transm Dis. 2009;36(Suppl):S67–71. doi: 10.1097/OLQ.0b013e31815d6de8. [DOI] [PubMed] [Google Scholar]
- 132.Kahn RH, Voigt RF, Swint E, et al. Early syphilis in the United States identified in corrections facilities, 1999–2002. Sex Transm Dis. 2004;31:360–4. doi: 10.1097/00007435-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 133.Kahn RH, Peterman TA, Arno J, et al. Identifying likely syphilis transmitters: implications for control and evaluation. Sex Transm Dis. 2006;33:630–5. doi: 10.1097/01.olq.0000216063.75575.f8. [DOI] [PubMed] [Google Scholar]
- 134.Owusu-Edusei K, Jr, Gift TL, Chesson HW, et al. Investigating the potential public health benefit of jail-based screening and treatment programs for chlamydia. Am J Epidemiol. 2013;177:463–73. doi: 10.1093/aje/kws240. [DOI] [PubMed] [Google Scholar]
- 135.Sutcliffe S, Newman SB, Hardick A, et al. Prevalence and correlates of Trichomonas vaginalis infection among female US federal prison inmates. Sex Transm Dis. 2010;37:585–90. doi: 10.1097/olq.0b013e3181de4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Freeman AH, Katz KA, Pandori MW, et al. Prevalence and correlates of Trichomonas vaginalis among incarcerated persons assessed using a highly sensitive molecular assay. Sex Transm Dis. 2010;37:165–8. doi: 10.1097/OLQ.0b013e3181bcd3fc. [DOI] [PubMed] [Google Scholar]
- 137.Sosman J, Macgowan R, Margolis A, et al. Project START Biologics Study Group Sexually transmitted infections and hepatitis in men with a history of incarceration. Sex Transm Dis. 2011;38:634–9. doi: 10.1097/OLQ.0b013e31820bc86c. [DOI] [PubMed] [Google Scholar]
- 138.Mayer KH, Bekker LG, Stall R, et al. Comprehensive clinical care for men who have sex with men: an integrated approach. Lancet. 2012;380:378–87. doi: 10.1016/S0140-6736(12)60835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chesson HW, Sternberg M, Leichliter JS, et al. Changes in the state-level distribution of primary and secondary syphilis in the USA, 1985–2007. Sex Transm Infect. 2010;86(Suppl 3):iii, 58–62. doi: 10.1136/sti.2009.040865. [DOI] [PubMed] [Google Scholar]
- 140.Patton ME, Su JR, Nelson R, et al. Primary and secondary syphilis—United States, 2005–2013. MMWR Morb Mortal Wkly Rep. 2014;63:1402–6. [PMC free article] [PubMed] [Google Scholar]
- 141.Kerani RP, Handsfield HH, Stenger MS, et al. Rising rates of syphilis in the era of syphilis elimination. Sex Transm Dis. 2007;34:154–61. doi: 10.1097/01.olq.0000233709.93891.e5. [DOI] [PubMed] [Google Scholar]
- 142.Zetola NM, Bernstein KT, Wong E, et al. Exploring the relationship between sexually transmitted diseases and HIV acquisition by using different study designs. J Acquir Immune Defic Syndr. 2009;50:546–51. doi: 10.1097/qai.0b013e318195bd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Solomon MM, Mayer KH, Glidden DV, et al. Syphilis Predicts HIV Incidence Among Men and Transgender Women Who Have Sex With Men in a Preexposure Prophylaxis Trial. Clin Infect Dis. 2014;59:1020–6. doi: 10.1093/cid/ciu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Paz-Bailey G, Meyers A, Blank S, et al. A case-control study of syphilis among men who have sex with men in New York City: association With HIV infection. Sex Transm Dis. 2004;31:581–7. doi: 10.1097/01.olq.0000140009.28121.0f. [DOI] [PubMed] [Google Scholar]
- 145.Pathela P, Braunstein SL, Schillinger JA, et al. Men who have sex with men have a 140-fold higher risk for newly diagnosed HIV and syphilis compared with heterosexual men in New York City. J Acquir Immune Defic Syndr. 2011;58:408–16. doi: 10.1097/QAI.0b013e318230e1ca. [DOI] [PubMed] [Google Scholar]
- 146.Chew Ng RA, Samuel MC, Lo T, et al. Sex, drugs (methamphetamines), and the Internet: increasing syphilis among men who have sex with men in California, 2004–2008. Am J Public Health. 2013;103:1450–6. doi: 10.2105/AJPH.2012.300808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bernstein KT, Stephens SC, Strona FV, et al. Epidemiologic characteristics of an ongoing syphilis epidemic among men who have sex with men, San Francisco. Sex Transm Dis. 2013;40:11–7. doi: 10.1097/OLQ.0b013e31827763ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cohen SE, Chew Ng RA, Katz KA, et al. Repeat syphilis among men who have sex with men in California, 2002–2006: implications for syphilis elimination efforts. Am J Public Health. 2012;102:e1–8. doi: 10.2105/AJPH.2011.300383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Newman LM, Dowell D, Bernstein K, et al. A tale of two gonorrhea epidemics: results from the STD Surveillance Network (SSuN) 2012 doi: 10.1177/003335491212700308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim AA, Kent CK, Klausner JD. Risk factors for rectal gonococcal infection amidst resurgence in HIV transmission. Sex Transm Dis. 2003;30:813–7. doi: 10.1097/01.OLQ.0000086603.55760.54. [DOI] [PubMed] [Google Scholar]
- 151.Lafferty WE, Hughes JP, Handsfield HH. Sexually transmitted diseases in men who have sex with men – acquisition of gonorrhea and nongonococcal urethritis by fellatio and implications for STD/HIV prevention. Sex Transm Dis. 1997;24:272–8. doi: 10.1097/00007435-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 152.Bernstein KT, Stephens SC, Barry PM, et al. Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the oropharynx to the urethra among men who have sex with men. Clin Infect Dis. 2009;49:1793–7. doi: 10.1086/648427. [DOI] [PubMed] [Google Scholar]
- 153.Park J, Marcus JL, Pandori M, et al. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis. 2012;39:482–4. doi: 10.1097/OLQ.0b013e3182495e2f. [DOI] [PubMed] [Google Scholar]
- 154.CDC. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:716–19. [PubMed] [Google Scholar]
- 155.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537–43. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 156.Pathela P, Braunstein SL, Blank S, et al. HIV incidence among men with and those without sexually transmitted rectal infections: estimates from matching against an HIV case registry. Clin Infect Dis. 2013;57:1203–9. doi: 10.1093/cid/cit437. [DOI] [PubMed] [Google Scholar]
- 157.Scott KC, Philip S, Ahrens K, et al. High prevalence of gonococcal and chlamydial infection in men who have sex with men with newly diagnosed HIV infection – an opportunity for same-day presumptive treatment. J Acq Imm Def. 2008;48:109–12. doi: 10.1097/QAI.0b013e318165dc0b. [DOI] [PubMed] [Google Scholar]
- 158.Chesson HW, Bernstein KT, Gift TL, et al. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis. 2013;40:366–71. doi: 10.1097/OLQ.0b013e318284e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ackers ML, Greenberg AE, Lin CY, et al. High and persistent HIV seroincidence in men who have sex with men across 47 U.S. cities. PLoS One. 2012;7:e34972. doi: 10.1371/journal.pone.0034972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.CDC. HIV infection among young black men who have sex with men—Jackson, Mississippi, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58 [PubMed] [Google Scholar]
- 161.Wall KM, Khosropour CM, Sullivan PS. Offering of HIV screening to men who have sex with men by their health care providers and associated factors. Journal of the International Association of Physicians in AIDS Care. 2010;9:284–8. doi: 10.1177/1545109710379051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2010;37:771–6. doi: 10.1097/OLQ.0b013e3181e50058. [DOI] [PubMed] [Google Scholar]
- 163.Carter JW, Jr, Hart-Cooper GD, Butler MO, et al. Provider barriers prevent recommended sexually transmitted disease screening of HIV-infected men who have sex with men. Sex Transm Dis. 2014;41:137–42. doi: 10.1097/OLQ.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 164.Makadon HJ. The Fenway guide to lesbian, gay, bisexual and transgender health. 2009 [Google Scholar]
- 165.Moskowitz DA, Melton D, Owczarzak J. PowerON: the use of instant message counseling and the Internet to facilitate HIV/STD education and prevention. Patient Educ Couns. 2009;77:20–6. doi: 10.1016/j.pec.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mimiaga MJ, Reisner SL, Tetu AM, et al. Partner notification after STD and HIV exposures and infections: knowledge, attitudes, and experiences of Massachusetts men who have sex with men. Public Health Rep. 2009;124:111–9. doi: 10.1177/003335490912400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.CDC. Recommendations on the use of quadrivalent human papillomavirus vaccine in males: Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. [PubMed] [Google Scholar]
- 168.Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–85. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 169.CDC. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008:57. (No. RR-8) [PubMed] [Google Scholar]
- 170.Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52:1497–505. doi: 10.1002/hep.23808. [DOI] [PubMed] [Google Scholar]
- 171.Frederick T, Burian P, Terrault N, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women’s Interagency HIV Study (WIHS) AIDS Patient Care STDS. 2009;23:915–23. doi: 10.1089/apc.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hershow RC, Kalish LA, Sha B, et al. Hepatitis C virus infection in Chicago women with or at risk for HIV infection – Evidence for sexual transmission. Sex Transm Dis. 1998;25:527–32. doi: 10.1097/00007435-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 173.Wandeler G, Gsponer T, Bregenzer A, et al. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis. 2012;55:1408–16. doi: 10.1093/cid/cis694. [DOI] [PubMed] [Google Scholar]
- 174.Palacios R, Mata R, Aguilar I, et al. High seroprevalence but low incidence of HCV infection in a cohort of patients with sexually transmitted HIV in Andalusia, Spain. Journal of the International Association of Physicians in AIDS Care. 2009;8:100–5. doi: 10.1177/1545109709331474. [DOI] [PubMed] [Google Scholar]
- 175.Garg S, Taylor LE, Grasso C, et al. Prevalent and incident hepatitis C virus infection among HIV-infected men who have sex with men engaged in primary care in a Boston community health center. Clin Infect Dis. 2013;56:1480–7. doi: 10.1093/cid/cit054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Linas BP, Wong AY, Schackman BR, et al. Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men. Clin Infect Dis. 2012;55:279–90. doi: 10.1093/cid/cis382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taylor LE, DeLong AK, Maynard MA, et al. Acute hepatitis C virus in an HIV clinic: a screening strategy, risk factors, and perception of risk. AIDS Patient Care STDS. 2011;25:571–7. doi: 10.1089/apc.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013:62. [PMC free article] [PubMed] [Google Scholar]
- 179.Muzny CA, Sunesara IR, Martin DH, et al. Sexually transmitted infections and risk behaviors among African American women who have sex with women: does sex with men make a difference? Sex Transm Dis. 2011;38:1118–25. doi: 10.1097/OLQ.0b013e31822e6179. [DOI] [PubMed] [Google Scholar]
- 180.Eisenberg M. Differences in sexual risk behaviors between college students with same-sex and opposite-sex experience: results from a national survey. Archives of Sexual Behavior. 2001;30:575–89. doi: 10.1023/a:1011958816438. [DOI] [PubMed] [Google Scholar]
- 181.Koh AS, Gomez CA, Shade S, et al. Sexual risk factors among self-identified lesbians, bisexual women, and heterosexual women accessing primary care settings. Sex Transm Dis. 2005;32:563–9. doi: 10.1097/01.olq.0000175417.17078.21. [DOI] [PubMed] [Google Scholar]
- 182.Lindley L, Burcin M. STD diagnoses among sexually active female college students: does sexual orientation or gender of sex partner(s) make a difference?. National STD Prevention Conference; 2008; Chicago, IL. [Google Scholar]
- 183.Goodenow C, Szalacha LA, Robin LE, et al. Dimensions of sexual orientation and HIV-related risk among adolescent females: evidence from a statewide survey. Am J Public Health. 2008;98:1051–8. doi: 10.2105/AJPH.2005.080531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schick V, Rosenberger JG, Herbenick D, et al. Sexual behaviour and risk reduction strategies among a multinational sample of women who have sex with women. Sex Transm Infect. 2012;88:407–12. doi: 10.1136/sextrans-2011-050404. [DOI] [PubMed] [Google Scholar]
- 185.Richters J, Prestage G, Schneider K, et al. Do women use dental dams? Safer sex practices of lesbians and other women who have sex with women. Sex Health. 2010;7:165–9. doi: 10.1071/SH09072. [DOI] [PubMed] [Google Scholar]
- 186.Rowen TS, Breyer BN, Lin TC, et al. Use of barrier protection for sexual activity among women who have sex with women. Int J Gynaecol Obstet. 2013;120:42–5. doi: 10.1016/j.ijgo.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lindley LL, Friedman DB, Struble C. Becoming visible: assessing the availability of online sexual health information for lesbians. Health Promot Pract. 2012;13:472–80. doi: 10.1177/1524839910390314. [DOI] [PubMed] [Google Scholar]
- 188.Fethers K, Marks C, Mindel A, et al. Sexually transmitted infections and risk behaviours in women who have sex with women. Sex Transm Infect. 2000;76:345–9. doi: 10.1136/sti.76.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marrazzo JM, Koutsky LA, Eschenbach DA, et al. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–13. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 190.Kellock D, O’Mahony CP. Sexually acquired metronidazole-resistant trichomoniasis in a lesbian couple. Genitourin Med. 1996;72:60–1. doi: 10.1136/sti.72.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muzny CA, Rivers CA, Mena LA, et al. Genotypic characterization of Trichomonas vaginalis isolates among women who have sex with women in sexual partnerships. Sex Transm Dis. 2012;39:556–8. doi: 10.1097/OLQ.0b013e31824f1c49. [DOI] [PubMed] [Google Scholar]
- 192.Chan SK, Thornton LR, Chronister KJ, et al. Likely female-to-female sexual transmission of HIV—Texas, 2012. MMWR Morb Mortal Wkly Rep. 2014;63:209–12. [PMC free article] [PubMed] [Google Scholar]
- 193.Kwakwa HA, Ghobrial MW. Female-to-female transmission of human immunodeficiency virus. Clin Infect Dis. 2003;36:e40–1. doi: 10.1086/345462. [DOI] [PubMed] [Google Scholar]
- 194.Diamant AL, Schuster MA, McGuigan K, et al. Lesbians’ sexual history with men: implications for taking a sexual history. Arch Intern Med. 1999;159:2730–6. doi: 10.1001/archinte.159.22.2730. [DOI] [PubMed] [Google Scholar]
- 195.Xu F, Sternberg MR, Markowitz LE. Women who have sex with women in the United States: prevalence, sexual behavior and prevalence of herpes simplex virus type 2 infection-results from national health and nutrition examination survey 2001–2006. Sex Transm Dis. 2010;37:407–13. doi: 10.1097/OLQ.0b013e3181db2e18. [DOI] [PubMed] [Google Scholar]
- 196.Marrazzo JM, Stine K, Wald A. Prevalence and risk factors for infection with herpes simplex virus type-1 and-2 among lesbians. Sex Transm Dis. 2003;30:890–5. doi: 10.1097/01.OLQ.0000091151.52656.E5. [DOI] [PubMed] [Google Scholar]
- 197.Marrazzo JM, Koutsky LA, Stine KL, et al. Genital human papillomavirus infection in women who have sex with women. J Infect Dis. 1998;178:1604–9. doi: 10.1086/314494. [DOI] [PubMed] [Google Scholar]
- 198.Marrazzo JM, Koutsky LA, Kiviat NB, et al. Papanicolaou test screening and prevalence of genital human papillomavirus among women who have sex with women. Am J Public Health. 2001;91:947–52. doi: 10.2105/ajph.91.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bailey JV, Kavanagh J, Owen C, et al. Lesbians and cervical screening. Br J Gen Pract. 2000;50:481–2. [PMC free article] [PubMed] [Google Scholar]
- 200.Ferris DG, Batish S, Wright TC, et al. A neglected lesbian health concern: cervical neoplasia. J Fam Pract. 1996;43:581–4. [PubMed] [Google Scholar]
- 201.O’Hanlan KA, Crum CP. Human papillomavirus-associated cervical intraepithelial neoplasia following lesbian sex. Obstet Gynecol. 1996;88(4 Pt 2):702–3. doi: 10.1016/0029-7844(96)00206-2. [DOI] [PubMed] [Google Scholar]
- 202.Singh D, Fine DN, Marrazzo JM. Chlamydia trachomatis infection among women reporting sexual activity with women screened in family planning clinics in the Pacific Northwest, 1997 to 2005. Am J Public Health. 2011;101:1284–90. doi: 10.2105/AJPH.2009.169631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–9. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 204.Evans AL, Scally AJ, Wellard SJ, et al. Prevalence of bacterial vaginosis in lesbians and heterosexual women in a community setting. Sex Transm Infect. 2007;83:470–5. doi: 10.1136/sti.2006.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Marrazzo JM, Antonio M, Agnew K, et al. Distribution of genital Lactobacillus strains shared by female sex partners. J Infect Dis. 2009;199:680–3. doi: 10.1086/596632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Marrazzo JM, Fiedler TL, Srinivasan S, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis. 2012;205:1580–8. doi: 10.1093/infdis/jis242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mitchell C, Manhart LE, Thomas K, et al. Behavioral predictors of colonization with Lactobacillus crispatus or Lactobacillus jensenii after treatment for bacterial vaginosis: a cohort study. Infect Dis Obstet Gynecol. 2012;2012:706540. doi: 10.1155/2012/706540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mitchell C, Manhart LE, Thomas KK, et al. Effect of sexual activity on vaginal colonization with hydrogen peroxide-producing lactobacilli and Gardnerella vaginalis. Sex Transm Dis. 2011;38:1137–44. doi: 10.1097/OLQ.0b013e31822e6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fethers K, Twin J, Fairley CK, et al. Bacterial vaginosis (BV) candidate bacteria: associations with BV and behavioural practices in sexually-experienced and inexperienced women. PLoS One. 2012;7:e30633. doi: 10.1371/journal.pone.0030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56:777–86. doi: 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- 211.Marrazzo JM, Thomas KK, Fiedler TL, et al. Risks for acquisition of bacterial vaginosis among women who report sex with women: a cohort study. PLoS One. 2010;5:e11139. doi: 10.1371/journal.pone.0011139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vodstrcil LA, Walker SM, Hocking JS, et al. Incident Bacterial Vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu1130. pii: ciu1130. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 213.Marrazzo JM, Thomas KK, Ringwood K. A behavioural intervention to reduce persistence of bacterial vaginosis among women who report sex with women: results of a randomised trial. Sex Transm Infect. 2011;87:399–405. doi: 10.1136/sti.2011.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1–17. doi: 10.1007/s10461-007-9299-3. [DOI] [PubMed] [Google Scholar]
- 215.Operario D, Soma T, Underhill K. Sex work and HIV status among transgender women: systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2008;48:97–103. doi: 10.1097/QAI.0b013e31816e3971. [DOI] [PubMed] [Google Scholar]
- 216.Baral SD, Poteat T, Stromdahl S, et al. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:214–22. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 217.Clements-Nolle K, Marx R, Guzman R, et al. HIV prevalence, risk behaviors, health care use, and mental health status of transgender persons: implications for public health intervention. Am J Public Health. 2001;91:915–21. doi: 10.2105/ajph.91.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nuttbrock L, Hwahng S, Bockting W, et al. Lifetime risk factors for HIV/sexually transmitted infections among male-to-female transgender persons. J Acquir Immune Defic Syndr. 2009;52:417–21. doi: 10.1097/QAI.0b013e3181ab6ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2010. 2012 Jun; Published. [Google Scholar]
- 220.Sevelius J. “There’s no pamphlet for the kind of sex I have”: HIV-related risk factors and protective behaviors among transgender men who have sex with nontransgender men. J Assoc Nurses AIDS Care. 2009;20:398–410. doi: 10.1016/j.jana.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Reisner SL, Perkovich B, Mimiaga MJ. A mixed methods study of the sexual health needs of New England transmen who have sex with nontransgender men. AIDS Patient Care STDS. 2010;24:501–13. doi: 10.1089/apc.2010.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881–9. doi: 10.1002/hep.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tohme RA, Holmberg SD. Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clin Infect Dis. 2012;54:1167–78. doi: 10.1093/cid/cir991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brettler DB, Mannucci PM, Gringeri A, et al. The low risk of hepatitis C virus transmission among sexual partners of hepatitis C-infected hemophilic males: an international, multicenter study. Blood. 1992;80:540–3. [PubMed] [Google Scholar]
- 226.Kao JH, Hwang YT, Chen PJ, et al. Transmission of hepatitis C virus between spouses: The important role of exposure duration. American Journal of Gastroenterology. 1996;91:2087–90. [PubMed] [Google Scholar]
- 227.Marincovich B, Castilla J, del Romero J, et al. Absence of hepatitis C virus transmission in a prospective cohort of heterosexual serodiscordant couples. Sex Transm Infect. 2003;79:160–2. doi: 10.1136/sti.79.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tahan V, Karaca C, Yildirim B, et al. Sexual transmission of HCV between spouses. Am J Gastroenterol. 2005;100:821–4. doi: 10.1111/j.1572-0241.2005.40879.x. [DOI] [PubMed] [Google Scholar]
- 229.Vandelli C, Renzo F, Romano L, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: Results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99:855–9. doi: 10.1111/j.1572-0241.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 230.Feldman JG, Minkoff H, Landesman S, et al. Heterosexual transmission of hepatitis C, hepatitis B, and HIV-1 in a sample of inner city women. Sex Transm Dis. 2000;27:338–42. doi: 10.1097/00007435-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 231.Fierer DS, Uriel AJ, Carriero DC, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–6. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fierer DS, Mullen MP, Dieterich DT, et al. Early-onset liver fibrosis due to primary hepatitis C virus infection is higher over time in HIV-infected men. Clin Infect Dis. 2012;55:887–9. doi: 10.1093/cid/cis538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dionne-Odom J, Osborn MK, Radziewicz H, et al. Acute hepatitis C and HIV coinfection. Lancet Infect Dis. 2009;9:775–83. doi: 10.1016/S1473-3099(09)70264-6. [DOI] [PubMed] [Google Scholar]
- 234.van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Urbanus AT, van de Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS. 2009;23:F1–7. doi: 10.1097/QAD.0b013e32832e5631. [DOI] [PubMed] [Google Scholar]
- 236.Hammer GP, Kellogg TA, McFarland WC, et al. Low incidence and prevalence of hepatitis C virus infection among sexually active non-intravenous drug-using adults, San Francisco, 1997–2000. Sex Transm Dis. 2003;30:919–24. doi: 10.1097/01.OLQ.0000091152.31366.E6. [DOI] [PubMed] [Google Scholar]
- 237.Roy KM, Goldberg DJ, Hutchinson S, et al. Hepatitis C virus among self declared non-injecting sexual partners of injecting drug users. J Med Virol. 2004;74:62–6. doi: 10.1002/jmv.20146. [DOI] [PubMed] [Google Scholar]
- 238.Mele A, Stroffolini T, Tosti ME, et al. Heterosexual transmission of hepatitis C in Italy. J Med Virol. 1999;57:111–3. [PubMed] [Google Scholar]
- 239.Rauch A, Rickenbach M, Weber R, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV cohort study. Clin Infect Dis. 2005;41:395–402. doi: 10.1086/431486. [DOI] [PubMed] [Google Scholar]
- 240.van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230–8. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- 241.Browne R, Asboe D, Gilleece Y, et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex Transm Infect. 2004;80:326–7. doi: 10.1136/sti.2003.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–91. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 243.Ghosn J, Pierre-Francois S, Thibault V, et al. Acute hepatitis C in HIV-infected men who have sex with men. HIV Med. 2004;5:303–6. doi: 10.1111/j.1468-1293.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 244.Thompson ND, Perz JF, Moorman AC, et al. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med. 2009;150:33–9. doi: 10.7326/0003-4819-150-1-200901060-00007. [DOI] [PubMed] [Google Scholar]
- 245.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012:61. (No. RR-4) [PubMed] [Google Scholar]
- 246.Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158:109–13. doi: 10.7326/0003-4819-158-2-201301150-00575. [DOI] [PubMed] [Google Scholar]
- 247.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- 248.Briat A, Dulioust E, Galimand J, et al. Hepatitis C virus in the semen of men coinfected with HIV-1: prevalence and origin. AIDS. 2005;19:1827–35. doi: 10.1097/01.aids.0000189847.98569.2d. [DOI] [PubMed] [Google Scholar]
- 249.Tully JG, Taylor-Robinson D, Cole RM, et al. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1:1288–91. doi: 10.1016/s0140-6736(81)92461-2. [DOI] [PubMed] [Google Scholar]
- 250.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huppert JS, Mortensen JE, Reed JL, et al. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis. 2008;35:250–4. doi: 10.1097/OLQ.0b013e31815abac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mena L, Wang X, Mroczkowski TF, et al. Mycoplasma genitalium infections in asymptomatic men and men with urethritis attending a sexually transmitted diseases clinic in New Orleans. Clin Infect Dis. 2002;35:1167–73. doi: 10.1086/343829. [DOI] [PubMed] [Google Scholar]
- 253.Falk L. The overall agreement of proposed definitions of mucopurulent cervicitis in women at high risk of chlamydia infection. Acta Derm Venereol. 2010;90:506–11. doi: 10.2340/00015555-0924. [DOI] [PubMed] [Google Scholar]
- 254.Anagrius C, Lore B, Jensen JS. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect. 2005;81:458–62. doi: 10.1136/sti.2004.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Falk L, Fredlund H, Jensen JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005;81:73–8. doi: 10.1136/sti.2004.010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis. 2003;187:650–7. doi: 10.1086/367992. [DOI] [PubMed] [Google Scholar]
- 257.Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36:598–606. doi: 10.1097/OLQ.0b013e3181b01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mobley VL, Hobbs MM, Lau K, et al. Mycoplasma genitalium infection in women attending a sexually transmitted infection clinic: diagnostic specimen type, coinfections, and predictors. Sex Transm Dis. 2012;39:706–9. doi: 10.1097/OLQ.0b013e318255de03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lusk MJ, Konecny P, Naing ZW, et al. Mycoplasma genitalium is associated with cervicitis and HIV infection in an urban Australian STI clinic population. Sex Transm Infect. 2011;87:107–9. doi: 10.1136/sti.2010.045138. [DOI] [PubMed] [Google Scholar]
- 260.Casin I, Vexiau-Robert D, De La Salmoniere P, et al. High prevalence of Mycoplasma genitalium in the lower genitourinary tract of women attending a sexually transmitted disease clinic in Paris, France. Sex Transm Dis. 2002;29:353–9. doi: 10.1097/00007435-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 261.Korte JE, Baseman JB, Cagle MP, et al. Cervicitis and genitourinary symptoms in women culture positive for Mycoplasma genitalium. Am J Reprod Immunol. 2006;55:265–75. doi: 10.1111/j.1600-0897.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 262.Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. Brit J Obstet Gynecol. 2010;117:361–4. doi: 10.1111/j.1471-0528.2009.02455.x. [DOI] [PubMed] [Google Scholar]
- 263.Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet. 2002;359:765–6. doi: 10.1016/S0140-6736(02)07848-0. [DOI] [PubMed] [Google Scholar]
- 264.Cohen CR, Mugo NR, Astete SG, et al. Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect. 2005;81:463–6. doi: 10.1136/sti.2005.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Haggerty CL, Totten PA, Astete SG, et al. Mycoplasma genitalium among women with nongonococcal, nonchlamydial pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2006:30184. doi: 10.1155/IDOG/2006/30184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Irwin KL, Moorman AC, O’Sullivan MJ, et al. Influence of human immunodeficiency virus infection on pelvic inflammatory disease. Obstet Gynecol. 2000;95:525–34. doi: 10.1016/s0029-7844(99)00621-3. [DOI] [PubMed] [Google Scholar]
- 267.Short VL, Totten PA, Ness RB, et al. Clinical presentation of Mycoplasma genitalium infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin Infect Dis. 2009;48:41–7. doi: 10.1086/594123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Simms I, Eastick K, Mallinson H, et al. Associations between Mycoplasma genitalium, Chlamydia trachomatis, and pelvic inflammatory disease. Sex Transm Infect. 2003;79:154–6. doi: 10.1136/sti.79.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Taylor-Robinson D, Jensen JS, Svenstrup H, et al. Difficulties experienced in defining the microbial cause of pelvic inflammatory disease. International journal of STD and AIDS. 2012;23:18–24. doi: 10.1258/ijsa.2011.011066. [DOI] [PubMed] [Google Scholar]
- 270.Wiesenfeld HC, Hillier SL, Meyn L, et al. Mycoplasma genitalium – is it a pathogen in acute pelvic inflammatory disease (PID)?. STI & AIDS World Congress 2013 (Joint Meeting of the 20th ISSTDR and 14th IUSTI Meeting); July 14–27, 2013; Vienna, Austria. [Google Scholar]
- 271.Bjartling C, Osser S, Persson K. Mycoplasma genitalium and Chlamydia trachomatis in laparoscopically diagnosed pelvic inflammatory disease. STI & AIDS World Congress 2013 (Joint Meeting of the 20th ISSTDR and 14th IUSTI Meeting); July 14–17, 2013; Vienna, Austria. [Google Scholar]
- 272.Vandepitte J, Bukenya J, Hughes P, et al. Clinical characteristics associated with Mycoplasma genitalium infection among women at high risk of HIV and other STI in Uganda. Sex Transm Dis. 2012;39:487–91. doi: 10.1097/OLQ.0b013e31824b1cf3. [DOI] [PubMed] [Google Scholar]
- 273.Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ. 2010;340:c1642. doi: 10.1136/bmj.c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Edwards RK, Ferguson RJ, Reyes L, et al. Assessing the relationship between preterm delivery and various microorganisms recovered from the lower genital tract. J Matern Fetal Neonatal Med. 2006;19:357–63. doi: 10.1080/00207170600712071. [DOI] [PubMed] [Google Scholar]
- 275.Hitti J, Garcia P, Totten P, et al. Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex Transm Dis. 2010;37:81–5. doi: 10.1097/OLQ.0b013e3181bf5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mena LA, Mroczkowski TF, Nsuami M, et al. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649–54. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 277.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens—a randomized clinical trial. Clin Infect Dis. 2011;52:163–70. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Manhart LE, Gillespie CW, Lowens MS, et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis. 2013;56:934–42. doi: 10.1093/cid/cis1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: a controlled clinical trial. Sex Transm Infect. 2008;84:72–6. doi: 10.1136/sti.2007.027375. [DOI] [PubMed] [Google Scholar]
- 280.Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: an open study. International journal of STD and AIDS. 2008;19:676–9. doi: 10.1258/ijsa.2008.008038. [DOI] [PubMed] [Google Scholar]
- 281.Anagrius C, Lore B, Jensen JS. Treatment of Mycoplasma genitalium: observations from a Swedish STD clinic. PLoS One. 2013;8:e61481. doi: 10.1371/journal.pone.0061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Totten PA, Jensen NL, Khosropour CM, et al. Azithromycin and doxycycline resistance profiles of recent clinical isolates of Mycoplasma genitalium. STI & AIDS World Congress Joint Meeting of the 20th International Society of Sexually Transmitted Disease Research; July 14–17, 2013; Vienna, Austria. [Google Scholar]
- 283.Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One. 2008;3:e3618. doi: 10.1371/journal.pone.0003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Terada M, Izumi K, Ohki E, et al. Antimicrobial efficacies of several antibiotics against uterine cervicitis caused by Mycoplasma genitalium. J Infect Chemother. 2012;18:313–7. doi: 10.1007/s10156-011-0329-8. [DOI] [PubMed] [Google Scholar]
- 285.Manhart LE, Khosropour CM, Gillespie CW, et al. Treatment outcomes for persistent Mycoplasma genitalium-associated NGU: evidence of moxifloxacin treatment failures. STI & AIDS World Congress Joint Meeting of the 20th International Society for Sexually Transmitted Disease Research; July 14–17, 2013; Vienna, Austria. [Google Scholar]
- 286.Couldwell DL, Tagg KA, Jeoffreys NJ, et al. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. International journal of STD and AIDS. 2013;24:822–8. doi: 10.1177/0956462413502008. [DOI] [PubMed] [Google Scholar]
- 287.Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245–9. doi: 10.1128/JCM.00495-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ross JD, Cronje HS, Paszkowski T, et al. Moxifloxacin versus ofloxacin plus metronidazole in uncomplicated pelvic inflammatory disease: results of a multicentre, double blind, randomised trial. Sex Transm Infect. 2006;82:446–51. doi: 10.1136/sti.2005.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–9. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 290.Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177:116–25. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis. 2014;58:1312–21. doi: 10.1093/cid/ciu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 294.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 295.Miller WC, Rosenberg NE, Rutstein SE, et al. Role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:277–82. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pilcher CD, Tien HC, Eron JJ, Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–92. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 297.Weintrob AC, Giner J, Menezes P, et al. Infrequent diagnosis of primary human immunodeficiency virus infection: missed opportunities in acute care settings. Arch Intern Med. 2003;163:2097–100. doi: 10.1001/archinte.163.17.2097. [DOI] [PubMed] [Google Scholar]
- 298.Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 299.CDC. HIV/AIDS Surveillance report. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. [Google Scholar]
- 300.CDC. Vital signs: HIV testing and diagnosis among adults— United States, 2001–2009. MMWR Morbid Mortal Wkly Rep. 2010;59:1550–5. [PubMed] [Google Scholar]
- 301.CDC. Previous HIV testing among adults and adolescents newly diagnosed with HIV infection—National HIV Surveillance System, 18 jurisdictions, United States, 2006–2009. MMWR Morbid Mortal Wkly Rep. 2012;61:441–5. [PubMed] [Google Scholar]
- 302.CDC. Persons tested for HIV—United States, 2006. MMWR Morbid Mortal Wkly Rep. 2008;57:845–9. [PubMed] [Google Scholar]
- 303.Masciotra S, McDougal JS, Feldman J, et al. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. J Clin Virol. 2011;52(Suppl 1):S17–22. doi: 10.1016/j.jcv.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 304.CDC and Association of Public Health Laboratories. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Available at http://stacks.cdc.gov/view/cdc/23447.
- 305.CDC. HIV-2 Infection surveillance—United States, 1987–2009. MMWR Morbid Mortal Wkly Rep. 2011;60:985–8. [PubMed] [Google Scholar]
- 306.Pilcher CD, Eron JJ, Jr, Vemazza PL, et al. Sexual transmission during the incubation period of primary HIV infection. JAMA. 2001;286:1713–4. doi: 10.1001/jama.286.14.1713. [DOI] [PubMed] [Google Scholar]
- 307.Wingood GM, DiClemente RJ, Mikhail I, et al. A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: The WILLOW Program. J Acquir Immune Defic Syndr. 2004;37(Suppl 2):S58–67. doi: 10.1097/01.qai.0000140603.57478.a9. [DOI] [PubMed] [Google Scholar]
- 308.Richardson JL, Milam J, Stoyanoff S, et al. Using patient risk indicators to plan prevention strategies in the clinical care setting. J Acquir Immune Defic Syndr. 2004;37(Supp1):S88–94. doi: 10.1097/01.qai.0000140606.59263.16. [DOI] [PubMed] [Google Scholar]
- 309.Myers JJ, Shade SB, Rose CD, et al. Interventions delivered in clinical settings are effective in reducing risk of HIV transmission among people living with HIV: results from the Health Resources and Services Administration (HRSA)’s Special Projects of National Significance Initiative. AIDS Behav. 2010;14:483–92. doi: 10.1007/s10461-010-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Marks G, Crepaz N, Senterfitt JW, et al. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 311.CDC. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008:57. (No. RR-9) [PubMed] [Google Scholar]
- 312.CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005:54. (No. RR-2) [PubMed] [Google Scholar]
- 313.DiCarlo RP, Martin DH. The clinical diagnosis of genital ulcer disease in men. Clin Infect Dis. 1997;25:292–8. doi: 10.1086/514548. [DOI] [PubMed] [Google Scholar]
- 314.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lockett AE, Dance DA, Mabey DC, et al. Serum-free media for isolation of Haemophilus ducreyi. Lancet. 1991;338:326. doi: 10.1016/0140-6736(91)90473-3. [DOI] [PubMed] [Google Scholar]
- 316.Lewis DA. Chancroid: clinical manifestations, diagnosis, and management. Sex Transm Infect. 2003;79:68–71. doi: 10.1136/sti.79.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Briggs GC, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 9th. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 318.Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis. 2014;209:325–33. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 319.Ryder N, Jin F, McNulty AM, et al. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992–2006. Sex Transm Infect. 2009;85:416–9. doi: 10.1136/sti.2008.033902. [DOI] [PubMed] [Google Scholar]
- 320.Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30:797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- 321.Bernstein DI, Bellamy AR, Hook EW, 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56:344–51. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847–54. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 323.Engelberg R, Carrell D, Krantz E, et al. Natural history of genital herpes simplex virus type 1 infection. Sex Transm Dis. 2003;30:174–7. doi: 10.1097/00007435-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 324.Scoular A. Using the evidence base on genital herpes: optimising the use of diagnostic tests and information provision. Sex Transm Infect. 2002;78:160–5. doi: 10.1136/sti.78.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Scoular A, Gillespie G, Carman WF. Polymerase chain reaction for diagnosis of genital herpes in a genitourinary medicine clinic. Sex Transm Infect. 2002;78:21–5. doi: 10.1136/sti.78.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wald A, Huang ML, Carrell D, et al. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345–51. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 327.Van Der Pol B, Warren T, Taylor SN, et al. Type-specific identification of anogenital herpes simplex virus infections by use of a commercially available nucleic acid amplification test. J Clin Microbiol. 2012;50:3466–71. doi: 10.1128/JCM.01685-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Caviness AC, Oelze LL, Saz UE, et al. Direct immunofluorescence assay compared to cell culture for the diagnosis of mucocutaneous herpes simplex virus infections in children. J Clin Virol. 2010;49:58–60. doi: 10.1016/j.jcv.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 329.Song B, Dwyer DE, Mindel A. HSV type specific serology in sexual health clinics: use, benefits, and who gets tested. Sex Transm Infect. 2004;80:113–7. doi: 10.1136/sti.2003.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Whittington WL, Celum CL, Cent A, et al. Use of a glycoprotein G-based type-specific assay to detect antibodies to herpes simplex virus type 2 among persons attending sexually transmitted disease clinics. Sex Transm Dis. 2001;28:99–104. doi: 10.1097/00007435-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 331.Zimet GD, Rosenthal SL, Fortenberry JD, et al. Factors predicting the acceptance of herpes simplex virus type 2 antibody testing among adolescents and young adults. Sex Transm Dis. 2004;31:665–9. doi: 10.1097/01.olq.0000143089.77493.c2. [DOI] [PubMed] [Google Scholar]
- 332.Turner KR, Wong EH, Kent CK, et al. Serologic herpes testing in the real world: validation of new type-specific serologic herpes simplex virus tests in a public health laboratory. Sex Transm Dis. 2002;29:422–5. doi: 10.1097/00007435-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 333.Eing BR, Lippelt L, Lorentzen EU, et al. Evaluation of confirmatory strategies for detection of type-specific antibodies against herpes simplex virus type 2. J Clin Microbiol. 2002;40:407–13. doi: 10.1128/JCM.40.2.407-413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Golden MR, Ashley-Morrow R, Swenson P, et al. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex Transm Dis. 2005;32:771–7. doi: 10.1097/01.olq.0000175377.88358.f3. [DOI] [PubMed] [Google Scholar]
- 335.Morrow RA, Friedrich D, Meier A, et al. Use of “biokit HSV-2 Rapid Assay” to improve the positive predictive value of Focus HerpeSelect HSV-2 ELISA. BMC Infect Dis. 2005;5:84. doi: 10.1186/1471-2334-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ngo TD, Laeyendecker O, La H, et al. Use of commercial enzyme immunoassays to detect antibodies to the herpes simplex virus type 2 glycoprotein G in a low-risk population in Hanoi, Vietnam. Clinical and Vaccine Immunology. 2008;15:382–4. doi: 10.1128/CVI.00437-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clinical Microbiology and Infection. 2006;12:463–9. doi: 10.1111/j.1469-0691.2006.01370.x. [DOI] [PubMed] [Google Scholar]
- 338.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 339.Leone PA, Trottier S, Miller JM. Valacyclovir for episodic treatment of genital herpes: a shorter 3-day treatment course compared with 5-day treatment. Clin Infect Dis. 2002;34:958–62. doi: 10.1086/339326. [DOI] [PubMed] [Google Scholar]
- 340.Wald A, Carrell D, Remington M, et al. Two-day regimen of acyclovir for treatment of recurrent genital herpes simplex virus type 2 infection. Clin Infect Dis. 2002;34:944–8. doi: 10.1086/339325. [DOI] [PubMed] [Google Scholar]
- 341.Aoki FY, Tyring S, Diaz-Mitoma F, et al. Single-day, patient-initiated famciclovir therapy for recurrent genital herpes: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2006;42:8–13. doi: 10.1086/498521. [DOI] [PubMed] [Google Scholar]
- 342.Chosidow O, Drouault Y, Leconte-Veyriac F, et al. Famciclovir vs. aciclovir in immunocompetent patients with recurrent genital herpes infections: a parallel-groups, randomized, double-blind clinical trial. Br J Dermatol. 2001;144:818–24. doi: 10.1046/j.1365-2133.2001.04139.x. [DOI] [PubMed] [Google Scholar]
- 343.Bodsworth NJ, Crooks RJ, Borelli S, et al. International Valaciclovir HSV Study Group Valaciclovir versus aciclovir in patient initiated treatment of recurrent genital herpes: a randomised, double blind clinical trial. Genitourin Med. 1997;73:110–6. doi: 10.1136/sti.73.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Fife KH, Barbarash RA, Rudolph T, et al. The Valaciclovir International Herpes Simplex Virus Study Group Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection: results of an international, multicenter, double-blind, randomized clinical trial. Sex Transm Dis. 1997;24:481–6. doi: 10.1097/00007435-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 345.Diaz-Mitoma F, Sibbald RG, Shafran SD, et al. Collaborative Famciclovir Genital Herpes Research Group Oral famciclovir for the suppression of recurrent genital herpes: a randomized controlled trial. JAMA. 1998;280:887–92. doi: 10.1001/jama.280.10.887. [DOI] [PubMed] [Google Scholar]
- 346.Mertz GJ, Loveless MO, Levin MJ, et al. Collaborative Famciclovir Genital Herpes Research Group Oral famciclovir for suppression of recurrent genital herpes simplex virus infection in women: a multicenter, double-blind, placebo-controlled trial. Arch Intern Med. 1997;157:343–9. [PubMed] [Google Scholar]
- 347.Reitano M, Tyring S, Lang W, et al. International Valaciclovir HSV Study Group Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. J Infect Dis. 1998;178:603–10. doi: 10.1086/515385. [DOI] [PubMed] [Google Scholar]
- 348.Romanowski B, Marina RB, Roberts JN, et al. Patients’ preference of valacyclovir once-daily suppressive therapy versus twice-daily episodic therapy for recurrent genital herpes: a randomized study. Sex Transm Dis. 2003;30:226–31. doi: 10.1097/00007435-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 349.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 350.Goldberg LH, Kaufman R, Kurtz TO, et al. Acyclovir Study Group Long-term suppression of recurrent genital herpes with acyclovir: a 5-year benchmark. Arch Dermatol. 1993;129:582–7. [PubMed] [Google Scholar]
- 351.Fife KH, Crumpacker CS, Mertz GJ, et al. Acyclovir Study Group Recurrence and resistance patterns of herpes simplex virus following cessation of ≥6 years of chronic suppression with acyclovir. J Infect Dis. 1994;169:1338–41. doi: 10.1093/infdis/169.6.1338. [DOI] [PubMed] [Google Scholar]
- 352.Bartlett BL, Tyring SK, Fife K, et al. Famciclovir treatment options for patients with frequent outbreaks of recurrent genital herpes: the RELIEF trial. J Clin Virol. 2008;43:190–5. doi: 10.1016/j.jcv.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 353.Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis. 2006;33:529–33. doi: 10.1097/01.olq.0000204723.15765.91. [DOI] [PubMed] [Google Scholar]
- 354.Gilbert LK, Wyand F. Genital herpes education and counselling: testing a one-page ‘FAQ’ intervention. Herpes. 2009;15:51–6. [PubMed] [Google Scholar]
- 355.Rosenthal SL, Zimet GD, Leichliter JS, et al. The psychosocial impact of serological diagnosis of asymptomatic herpes simplex virus type 2 infection. Sex Transm Infect. 2006;82:154–8. doi: 10.1136/sti.2005.016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Miyai T, Turner KR, Kent CK, et al. The psychosocial impact of testing individuals with no history of genital herpes for herpes simplex virus type 2. Sex Transm Dis. 2004;31:517–21. doi: 10.1097/01.olq.0000137901.71284.6b. [DOI] [PubMed] [Google Scholar]
- 357.Ross K, Johnston C, Wald A. Herpes simplex virus type 2 serological testing and psychosocial harm: a systematic review. Sex Transm Infect. 2011;87:594–600. doi: 10.1136/sextrans-2011-050099. [DOI] [PubMed] [Google Scholar]
- 358.Wald A, Langenberg AGM, Krantz E, et al. The relationship between condom use and herpes simplex virus acquisition. Ann Intern Med. 2005;143:707–13. doi: 10.7326/0003-4819-143-10-200511150-00007. [DOI] [PubMed] [Google Scholar]
- 359.Wald A, Langenberg AG, Link K, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA. 2001;285:3100–6. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- 360.Henry RE, Wegmann JA, Hartle JE, et al. Successful oral acyclovir desensitization. Ann Allergy. 1993;70:386–8. [PubMed] [Google Scholar]
- 361.Posavad CM, Wald A, Kuntz S, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis. 2004;190:693–6. doi: 10.1086/422755. [DOI] [PubMed] [Google Scholar]
- 362.Tobian AA, Grabowski MK, Serwadda D, et al. Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis. 2013;208:839–46. doi: 10.1093/infdis/jit252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Conant MA, Schacker TW, Murphy RL, et al. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. International journal of STD and AIDS. 2002;13:12–21. doi: 10.1258/0956462021924550. [DOI] [PubMed] [Google Scholar]
- 364.Romanowski B, Aoki FY, Martel AY, et al. Collaborative Famciclovir HIV Study Group Efficacy and safety of famciclovir for treating mucocutaneous herpes simplex infection in HIV-infected individuals. AIDS. 2000;14:1211–7. doi: 10.1097/00002030-200006160-00019. [DOI] [PubMed] [Google Scholar]
- 365.DeJesus E, Wald A, Warren T, et al. Valacyclovir for the suppression of recurrent genital herpes in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:1009–16. doi: 10.1086/378416. [DOI] [PubMed] [Google Scholar]
- 366.Mujugira A, Magaret AS, Celum C, et al. Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: a randomized controlled trial. J Infect Dis. 2013;208:1366–74. doi: 10.1093/infdis/jit333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Reyes M, Shaik NS, Graber JM, et al. Acyclovir-resistant genital herpes among persons attending sexually transmitted disease and human immunodeficiency virus clinics. Arch Intern Med. 2003;163:76–80. doi: 10.1001/archinte.163.1.76. [DOI] [PubMed] [Google Scholar]
- 368.Safrin S, Crumpacker C, Chatis P, et al. The AIDS Clinical Trials Group A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. N Engl J Med. 1991;325:551–5. doi: 10.1056/NEJM199108223250805. [DOI] [PubMed] [Google Scholar]
- 369.Levin MJ, Bacon TH, Leary JJ. Resistance of herpes simplex virus infections to nucleoside analogues in HIV-infected patients. Clin Infect Dis. 2004;39(Suppl 5):S248–57. doi: 10.1086/422364. [DOI] [PubMed] [Google Scholar]
- 370.Perkins N, Nisbet M, Thomas M. Topical imiquimod treatment of aciclovir-resistant herpes simplex disease: case series and literature review. Sex Transm Infect. 2011;87:292–5. doi: 10.1136/sti.2010.047431. [DOI] [PubMed] [Google Scholar]
- 371.McElhiney LF. Topical cidofovir for treatment of resistant viral infections. Int J Pharm Compd. 2006;10:324–8. [PubMed] [Google Scholar]
- 372.Erard V, Wald A, Corey L, et al. Use of long-term suppressive acyclovir after hematopoietic stem-cell transplantation: impact on herpes simplex virus (HSV) disease and drug-resistant HSV disease. J Infect Dis. 2007;196:266–70. doi: 10.1086/518938. [DOI] [PubMed] [Google Scholar]
- 373.Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337:509–15. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 374.Pinninti SG, Kimberlin DW. Maternal and neonatal herpes simplex virus infections. Am J Perinatol. 2013;30:113–9. doi: 10.1055/s-0032-1332802. [DOI] [PubMed] [Google Scholar]
- 375.Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203–9. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 376.Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247–52. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- 377.Stone KM, Reiff-Eldridge R, White AD, et al. Pregnancy outcomes following systemic prenatal acyclovir exposure: Conclusions from the international acyclovir pregnancy registry, 1984–1999. Birth Defects Res A Clin Mol Teratol. 2004;70:201–7. doi: 10.1002/bdra.20013. [DOI] [PubMed] [Google Scholar]
- 378.Sheffield JS, Hollie LM, Hill JB, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396–403. doi: 10.1016/j.obstetgynecol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 379.Watts DH, Brown ZA, Money D, et al. A double-blind, randomized, placebo-controlled trial of acyclovir in late pregnancy for the reduction of herpes simplex virus shedding and cesarean delivery. Am J Obstet Gynecol. 2003;188:836–43. doi: 10.1067/mob.2003.185. [DOI] [PubMed] [Google Scholar]
- 380.Scott LL, Hollier LM, McIntire D, et al. Acyclovir suppression to prevent recurrent genital herpes at delivery. Infect Dis Obstet Gynecol. 2002;10:71–7. doi: 10.1155/S1064744902000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr. 2012;161:134–8. doi: 10.1016/j.jpeds.2011.12.053. [DOI] [PubMed] [Google Scholar]
- 382.Kimberlin DW, Baley J, Committee on Infectious Diseases et al. Guidance on management of asymptomatic neonates born to women with active genital herpes lesions. Pediatrics. 2013;131:383–6. doi: 10.1542/peds.2012-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.O’Farrell N. Donovanosis. Sex Transm Infect. 2002;78:452–7. doi: 10.1136/sti.78.6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Bowden FJ, National Donovanosis Eradication Advisory Committee Donovanosis in Australia: going, going. Sex Transm Infect. 2005;81:365–6. doi: 10.1136/sti.2004.013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Velho PE, Souza EM, Belda Junior W. Donovanosis. The Brazilian Journal of Infectious Diseases. 2008;12:521–5. doi: 10.1590/s1413-86702008000600015. [DOI] [PubMed] [Google Scholar]
- 386.Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect. 2002;78:90–2. doi: 10.1136/sti.78.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.White JA. Manifestations and management of lymphogranuloma venereum. Curr Opin Infect Dis. 2009;22:57–66. doi: 10.1097/QCO.0b013e328320a8ae. [DOI] [PubMed] [Google Scholar]
- 388.Ward H, Martin I, Macdonald N, et al. Lymphogranuloma venereum in the United Kingdom. Clin Infect Dis. 2007;44:26–32. doi: 10.1086/509922. [DOI] [PubMed] [Google Scholar]
- 389.Martin-Iguacel R, Llibre JM, Nielsen H, et al. Lymphogranuloma venereum proctocolitis: a silent endemic disease in men who have sex with men in industrialised countries. Eur J Clin Microbiol Infect Dis. 2010;29:917–25. doi: 10.1007/s10096-010-0959-2. [DOI] [PubMed] [Google Scholar]
- 390.Pallawela SN, Sullivan AK, Macdonald N, et al. Clinical predictors of rectal lymphogranuloma venereum infection: results from a multicentre case-control study in the U.K. Sex Transm Infect. 2014;90:269–74. doi: 10.1136/sextrans-2013-051401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.de Vrieze NH, de Vries HJ. Lymphogranuloma venereum among men who have sex with men. An epidemiological and clinical review. Expert Rev Anti Infect Ther. 2014;12:697–704. doi: 10.1586/14787210.2014.901169. [DOI] [PubMed] [Google Scholar]
- 392.Koper NE, van der Sande MA, Gotz HM, et al. Lymphogranuloma venereum among men who have sex with men in the Netherlands: regional differences in testing rates lead to underestimation of the incidence, 2006–2012. Euro Surveill. 2013;18:20561. doi: 10.2807/1560-7917.es2013.18.34.20561. [DOI] [PubMed] [Google Scholar]
- 393.Haar K, Dudareva-Vizule S, Wisplinghoff H, et al. Lymphogranuloma venereum in men screened for pharyngeal and rectal infection, Germany. Emerg Infect Dis. 2013;19:488–92. doi: 10.3201/eid1903.121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Papp JR, Schachter J, Gaydos C, et al. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014:63. (No. RR-02) [PMC free article] [PubMed] [Google Scholar]
- 395.CDC Association of Public Health Laboratories. Laboratory diagnostic testing for Treponema pallidum, Expert Consultation Meeting Summary Report. Atlanta, GA: Jan 13–15, 2009. Available at: http://www.aphl.org/aphlprograms/infectious/std/Documents/ID_2009Jan_Laboratory-Guidelines-Treponema-pallidum-Meeting-Report.pdf. 2009. [Google Scholar]
- 396.Nandwani R, Evans DT. Are you sure it’s syphilis? A review of false positive serology. International journal of STD and AIDS. 1995;6:241–8. doi: 10.1177/095646249500600404. [DOI] [PubMed] [Google Scholar]
- 397.Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114:1005–9. doi: 10.7326/0003-4819-114-12-1005. [DOI] [PubMed] [Google Scholar]
- 398.CDC. Syphilis testing algorithms using treponemal tests for initial screening–four laboratories, New York City, 2005–2006. MMWR Morbid Mortal Wkly Rep. 2008;57:872–5. [PubMed] [Google Scholar]
- 399.CDC. Discordant results from reverse sequence syphilis screening—five laboratories, United States, 2006–2010. MMWR Morbid Mortal Wkly Rep. 2011;60:133–7. [PubMed] [Google Scholar]
- 400.Park IU, Chow JM, Bolan G, et al. Screening for syphilis with the treponemal immunoassay: analysis of discordant serology results and implications for clinical management. J Infect Dis. 2011;204:1297–304. doi: 10.1093/infdis/jir524. [DOI] [PubMed] [Google Scholar]
- 401.Wong EH, Klausner JD, Caguin-Grygiel G, et al. Evaluation of an IgM/IgG sensitive enzyme immunoassay and the utility of index values for the screening of syphilis infection in a high-risk population. Sex Transm Dis. 2011;38:528–32. doi: 10.1097/OLQ.0b013e318205491a. [DOI] [PubMed] [Google Scholar]
- 402.Lukehart SA, Hook EW, III, Baker-Zander SA, et al. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988;109:855–62. doi: 10.7326/0003-4819-109-11-855. [DOI] [PubMed] [Google Scholar]
- 403.Jaffe HW, Larsen SA, Peters M, et al. Tests for treponemal antibody in CSF. Arch Intern Med. 1978;138:252–5. [PubMed] [Google Scholar]
- 404.Marra CM, Maxwell CL, Smith SL, et al. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis. 2004;189:369–76. doi: 10.1086/381227. [DOI] [PubMed] [Google Scholar]
- 405.CDC. Inadvertent use of Bicillin® C-R to treat syphilis infection—Los Angeles, California, 1999–2004. MMWR Morbid Mortal Wkly Rep. 2005;54:217–9. [PMC free article] [PubMed] [Google Scholar]
- 406.Rolfs RT, Joesoef MR, Hendershot EF, et al. The Syphilis and HIV Study Group A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. N Engl J Med. 1997;337:307–14. doi: 10.1056/NEJM199707313370504. [DOI] [PubMed] [Google Scholar]
- 407.Taiwan HIV and Syphilis Study Group. Comparison of effectiveness of 1 dose versus 3 doses of benzathine penicillin in treatment of early syphilis in HIV-infected patients: multicenter, prospective observational study in Taiwan [Abstract# S-119]. Presented at: the 20th Conference on Retroviruses and Opportunistic Infections (CROI 2013); March 3–6, 2013; Atlanta, GA. [Google Scholar]
- 408.Ghanem KG, Erbelding EJ, Wiener ZS, et al. Serological response to syphilis treatment in HIV-positive and HIV-negative patients attending sexually transmitted diseases clinics. Sex Transm Infect. 2007;83:97–101. doi: 10.1136/sti.2006.021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Sena AC, Wolff M, Martin DH, et al. Predictors of serological cure and Serofast State after treatment in HIV-negative persons with early syphilis. Clin Infect Dis. 2011;53:1092–9. doi: 10.1093/cid/cir671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Sena AC, Wolff M, Behets F, et al. Response to therapy following retreatment of serofast early syphilis patients with benzathine penicillin. Clin Infect Dis. 2013;56:420–2. doi: 10.1093/cid/cis918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Ghanem KG, Erbelding EJ, Cheng WW, et al. Doxycycline compared with benzathine penicillin for the treatment of early syphilis. Clin Infect Dis. 2006;42:e45–e9. doi: 10.1086/500406. [DOI] [PubMed] [Google Scholar]
- 412.Wong T, Singh AE, De P. Primary syphilis: serological treatment response to doxycycline/tetracycline versus benzathine penicillin. Am J Med. 2008;121:903–8. doi: 10.1016/j.amjmed.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 413.Hook EW, 3rd, Roddy RE, Handsfield HH. Ceftriaxone therapy for incubating and early syphilis. J Infect Dis. 1988;158:881–4. doi: 10.1093/infdis/158.4.881. [DOI] [PubMed] [Google Scholar]
- 414.Riedner G, Rusizoka M, Todd J, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353:1236–44. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 415.Hook EW, 3rd, Martin DH, Stephens J, et al. A randomized, comparative pilot study of azithromycin versus benzathine penicillin G for treatment of early syphilis. Sex Transm Dis. 2002;29:486–90. doi: 10.1097/00007435-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 416.Hook EW, 3rd, Behets F, Van Damme K, et al. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. J Infect Dis. 2010;201:1729–35. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 417.Lukehart SA, Godornes C, Molini BJ, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154–8. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 418.Mitchell SJ, Engelman J, Kent CK, et al. Azithromycin-resistant syphilis infection: San Francisco, California, 2000–2004. Clin Infect Dis. 2006;42:337–45. doi: 10.1086/498899. [DOI] [PubMed] [Google Scholar]
- 419.A2058G Prevalence Workgroup. Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex Transm Dis. 2012;39:794–8. doi: 10.1097/OLQ.0b013e31826f36de. [DOI] [PubMed] [Google Scholar]
- 420.Collart P, Poitevin M, Milovanovic A, et al. Kinetic study of serum penicillin concentrations after single doses of benzathine and benethamine penicillins in young and old people. Br J Vener Dis. 1980;56:355–62. doi: 10.1136/sti.56.6.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Hagdrup HK, Lange Wantzin G, Secher L, et al. Penicillin concentrations in serum following weekly injections of benzathine penicillin G. Chemotherapy. 1986;32:99–101. doi: 10.1159/000238397. [DOI] [PubMed] [Google Scholar]
- 422.Frentz G, Nielsen PB, Espersen F, et al. Penicillin concentrations in blood and spinal fluid after a single intramuscular injection of penicillin G benzathine. Eur J Clin Microbiol. 1984;3:147–9. doi: 10.1007/BF02014334. [DOI] [PubMed] [Google Scholar]
- 423.Nathan L, Bawdon RE, Sidawi JE, et al. Penicillin levels following the administration of benzathine penicillin G in pregnancy. Obstet Gynecol. 1993;82:338–42. [PubMed] [Google Scholar]
- 424.Marra CM, Maxwell CL, Tantalo L, et al. Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter? Clin Infect Dis. 2004;38:1001–6. doi: 10.1086/382532. [DOI] [PubMed] [Google Scholar]
- 425.Marra CM, Maxwell CL, Tantalo LC, et al. Normalization of serum rapid plasma reagin titer predicts normalization of cerebrospinal fluid and clinical abnormalities after treatment of neurosyphilis. Clin Infect Dis. 2008;47:893–9. doi: 10.1086/591534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Hook EW, 3rd, Baker-Zander SA, Moskovitz BL, et al. Ceftriaxone therapy for asymptomatic neurosyphilis: case report and Western blot analysis of serum and cerebrospinal fluid IgG response to therapy. Sex Transm Dis. 1986;133(3 Suppl):185–8. [PubMed] [Google Scholar]
- 427.Shann S, Wilson J. Treatment of neurosyphilis with ceftriaxone. Sex Transm Infect. 2003;79:415–6. doi: 10.1136/sti.79.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Ahmed KA, Fox SJ, Frigas E, et al. Clinical outcome in the use of cephalosporins in pediatric patients with a history of penicillin allergy. International Arch Allergy Immunol. 2012;158:405–10. doi: 10.1159/000333553. [DOI] [PubMed] [Google Scholar]
- 429.Park MA, Koch CA, Klemawesch P, et al. Increased adverse drug reactions to cephalosporins in penicillin allergy patients with positive penicillin skin test. International Arch Allergy Immunol. 2010;153:268–73. doi: 10.1159/000314367. [DOI] [PubMed] [Google Scholar]
- 430.Novalbos A, Sastre J, Cuesta J, et al. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy. 2001;31:438–43. doi: 10.1046/j.1365-2222.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- 431.Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: a meta-analysis. Otolaryngol Head Neck Surg. 2007;136:340–7. doi: 10.1016/j.otohns.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 432.Kingston AA, Vujevich J, Shapiro M, et al. Seronegative secondary syphilis in 2 patients coinfected with human immunodeficiency virus. Arch Dermatol. 2005;141:431–3. doi: 10.1001/archderm.141.4.431. [DOI] [PubMed] [Google Scholar]
- 433.CDC. Symptomatic early neurosyphilis among HIV-positive men who have sex with men—four cities, United States, January 2002–June 2004. MMWR Morb Mortal Wkly Rep. 2007;56:625–8. [PMC free article] [PubMed] [Google Scholar]
- 434.Ghanem KG, Moore RD, Rompalo AM, et al. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS. 2008;22:1145–51. doi: 10.1097/QAD.0b013e32830184df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Ghanem KG, Moore RD, Rompalo AM, et al. Antiretroviral therapy is associated with reduced serologic failure rates for syphilis among HIV-infected patients. Clin Infect Dis. 2008;47:258–65. doi: 10.1086/589295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Libois A, De Wit S, Poll B, et al. HIV and syphilis: When to perform a lumbar puncture. Sex Transm Dis. 2007;34:141–4. doi: 10.1097/01.olq.0000230481.28936.e5. [DOI] [PubMed] [Google Scholar]
- 437.Ghanem KG. Sensitivity and specificity of lumbar puncture in HIV-infected patients with syphilis and no neurologic symptoms reply. Clin Infect Dis. 2009;49:162–3. doi: 10.1086/599616. [DOI] [PubMed] [Google Scholar]
- 438.Marra CM, Boutin P, McArthur JC, et al. A pilot study evaluating ceftriaxone and penicillin G as treatment agents for neurosyphilis in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2000;30:540–4. doi: 10.1086/313725. [DOI] [PubMed] [Google Scholar]
- 439.Dowell ME, Ross PG, Musher DM, et al. Response of latent syphilis or neurosyphilis to ceftriaxone therapy in persons infected with human immunodeficiency virus. Am J Med. 1992;93:481–8. doi: 10.1016/0002-9343(92)90574-u. [DOI] [PubMed] [Google Scholar]
- 440.Smith NH, Musher DM, Huang DB, et al. Response of HIV-infected patients with asymptomatic syphilis to intensive intramuscular therapy with ceftriaxone or procaine penicillin. Int J STD AIDS. 2004;15:328–32. doi: 10.1177/095646240401500511. [DOI] [PubMed] [Google Scholar]
- 441.Hollier LM, Hill J, Sheffield JS, et al. State laws regarding prenatal syphilis screening in the United States. Am J Obstet Gynecol. 2003;189:1178–83. doi: 10.1067/s0002-9378(03)00547-7. [DOI] [PubMed] [Google Scholar]
- 442.Newman L, Kamb M, Hawkes S, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med. 2013;10:e1001396. doi: 10.1371/journal.pmed.1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Alexander JM, Sheffield JS, Sanchez PJ, et al. Efficacy of treatment for syphilis in pregnancy. Obstet Gynecol. 1999;93:5–8. doi: 10.1016/s0029-7844(98)00338-x. [DOI] [PubMed] [Google Scholar]
- 444.Walker GJ. Antibiotics for syphilis diagnosed during pregnancy. Cochrane Database Syst Rev. 2001:CD001143. doi: 10.1002/14651858.CD001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Wendel GD, Jr, Sheffield JS, Hollier LM, et al. Treatment of syphilis in pregnancy and prevention of congenital syphilis. Clin Infect Dis. 2002;35(Suppl 2):S200–9. doi: 10.1086/342108. [DOI] [PubMed] [Google Scholar]
- 446.Zhu L, Qin M, Du L, et al. Maternal and congenital syphilis in Shanghai, China, 2002 to 2006. Int J Infect Dis. 2010;14(Suppl 3):e45–8. doi: 10.1016/j.ijid.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 447.Hawkes S, Matin N, Broutet N, et al. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11:684–91. doi: 10.1016/S1473-3099(11)70104-9. [DOI] [PubMed] [Google Scholar]
- 448.Hollier LM, Harstad TW, Sanchez PJ, et al. Fetal syphilis: clinical and laboratory characteristics. Obstet Gynecol. 2001;97:947–53. doi: 10.1016/s0029-7844(01)01367-9. [DOI] [PubMed] [Google Scholar]
- 449.Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herxheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75(3 Pt 1):375–80. [PubMed] [Google Scholar]
- 450.Kruger C, Malleyeck I. Congenital syphilis: still a serious, under-diagnosed threat for children in resource-poor countries. World Journal of Pediatrics. 2010;6:125–31. doi: 10.1007/s12519-010-0028-z. [DOI] [PubMed] [Google Scholar]
- 451.Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122:778. doi: 10.1016/j.amjmed.2009.01.034. (e1–7) [DOI] [PubMed] [Google Scholar]
- 452.Albin S, Agarwal S. Prevalence and characteristics of reported penicillin allergy in an urban outpatient adult population. Allergy Asthma Proc. 2014;35:489–94. doi: 10.2500/aap.2014.35.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Gadde J, Spence M, Wheeler B, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:2456–63. [PubMed] [Google Scholar]
- 454.Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med. 2000;160:2819–22. doi: 10.1001/archinte.160.18.2819. [DOI] [PubMed] [Google Scholar]
- 455.Hsu MH, Yen JC, Chiu WT, et al. Using health smart cards to check drug allergy history: the perspective from Taiwan’s experiences. J Med Syst. 2011;35:555–8. doi: 10.1007/s10916-009-9391-5. [DOI] [PubMed] [Google Scholar]
- 456.Jares EJ, Sanchez-Borges M, Cardona-Villa R, et al. Multinational experience with hypersensitivity drug reactions in Latin America. Ann Allergy Asthma Immunol. 2014;113:282–9. doi: 10.1016/j.anai.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 457.del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol. 2007;98:355–9. doi: 10.1016/S1081-1206(10)60882-4. [DOI] [PubMed] [Google Scholar]
- 458.Wong BB, Keith PK, Waserman S. Clinical history as a predictor of penicillin skin test outcome. Annals Asthma Allergy Immunol. 2006;97:169–74. doi: 10.1016/S1081-1206(10)60008-7. [DOI] [PubMed] [Google Scholar]
- 459.Sogn DD, Evans R, 3rd, Shepherd GM, et al. Results of the National Institute of Allergy and Infectious Diseases Collaborative Clinical Trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch Intern Med. 1992;152:1025–32. [PubMed] [Google Scholar]
- 460.Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1:258–63. doi: 10.1016/j.jaip.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 461.Forrest DM, Schellenberg RR, Thien VV, et al. Introduction of a practice guideline for penicillin skin testing improves the appropriateness of antibiotic therapy. Clin Infect Dis. 2001;32:1685–90. doi: 10.1086/320752. [DOI] [PubMed] [Google Scholar]
- 462.Park M, Markus P, Matesic D, et al. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol. 2006;97:681–7. doi: 10.1016/S1081-1206(10)61100-3. [DOI] [PubMed] [Google Scholar]
- 463.Raja AS, Lindsell CJ, Bernstein JA, et al. The use of penicillin skin testing to assess the prevalence of penicillin allergy in an emergency department setting. Annals Emerg Med. 2009;54:72–7. doi: 10.1016/j.annemergmed.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Romano A, Gaeta F, Valluzzi RL, et al. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010;126:994–9. doi: 10.1016/j.jaci.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 465.Blanca M, Perez E, Garcia J, et al. Anaphylaxis to amoxycillin but good tolerance for benzyl penicillin: in vivo and in vitro studies of specific IgE antibodies. Allergy. 1988;43:508–10. doi: 10.1111/j.1398-9995.1988.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 466.Vega JM, Blanca M, Garcia JJ, et al. Immediate allergic reactions to amoxicillin. Allergy. 1994;49:317–22. doi: 10.1111/j.1398-9995.1994.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 467.Wendel GD, Jr, Stark BJ, Jamison RB, et al. Penicillin allergy and desensitization in serious infections during pregnancy. N Engl J Med. 1985;312:1229–32. doi: 10.1056/NEJM198505093121905. [DOI] [PubMed] [Google Scholar]
- 468.Borish L, Tamir R, Rosenwasser LJ. Intravenous desensitization to beta-lactam antibiotics. J Allergy Clin Immunol. 1987;80(3 Pt 1):314–9. doi: 10.1016/0091-6749(87)90037-6. [DOI] [PubMed] [Google Scholar]
- 469.Legere HJ, 3rd, Palis RI, Rodriguez Bouza T, et al. A safe protocol for rapid desensitization in patients with cystic fibrosis and antibiotic hypersensitivity. Journal of Cystic Fibrosis. 2009;8:418–24. doi: 10.1016/j.jcf.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Manhart LE, Holmes KK, Hughes JP, et al. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health. 2007;97:1118–25. doi: 10.2105/AJPH.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Ross JDC, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect. 2006;82:269–71. doi: 10.1136/sti.2005.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Taylor-Robinson D, Gilroy CB, Thomas BJ, et al. Mycoplasma genitalium in chronic non-gonococcal urethritis. International journal of STD and AIDS. 2004;15:21–5. doi: 10.1258/095646204322637209. [DOI] [PubMed] [Google Scholar]
- 473.Dupin N, Bijaoui G, Schwarzinger M, et al. Detection and quantification of Mycoplasma genitalium in male patients with urethritis. Clin Infect Dis. 2003;37:602–5. doi: 10.1086/376990. [DOI] [PubMed] [Google Scholar]
- 474.Krieger JN, Riley DE, Roberts MC, et al. Prokaryotic DNA sequences in patients with chronic idiopathic prostatitis. J Clin Microbiol. 1996;34:3120–8. doi: 10.1128/jcm.34.12.3120-3128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol. 2009;200:e181–7. doi: 10.1016/j.ajog.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 476.Bradshaw CS, Tabrizi SN, Read TR, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis. 2006;193:336–45. doi: 10.1086/499434. [DOI] [PubMed] [Google Scholar]
- 477.Reitmeijer CA, Mettenbrink CJ. Recalibrating the gram stain diagnosis of male urethritis in the era of nucleic acid amplification testing. Sex Transm Dis. 2012;39:18–20. doi: 10.1097/OLQ.0b013e3182354da3. [DOI] [PubMed] [Google Scholar]
- 478.Taylor SN, DiCarlo RP, Martin DH. Comparison of methylene blue/gentian violet stain to Gram’s stain for the rapid diagnosis of gonococcal urethritis in men. Sex Transm Dis. 2011;38:995–6. doi: 10.1097/OLQ.0b013e318225f7c2. [DOI] [PubMed] [Google Scholar]
- 479.Geisler WM, Yu SY, Hook EW., 3rd Chlamydial and gonococcal infection in men without polymorphonuclear leukocytes on gram stain: implications for diagnostic approach and management. Sex Transm Dis. 2005;32:630–4. doi: 10.1097/01.olq.0000175390.45315.a1. [DOI] [PubMed] [Google Scholar]
- 480.Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478–89. doi: 10.1097/OLQ.0b013e3181a2a933. [DOI] [PubMed] [Google Scholar]
- 481.Fung M, Scott KC, Kent CK, et al. Chlamydial and gonococcal reinfection among men: a systematic review of data to evaluate the need for retesting. Sex Transm Infect. 2007;83:304–9. doi: 10.1136/sti.2006.024059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Lusk MJ, Konecny P. Cervicitis: a review. Curr Opin Infect Dis. 2008;21:49–55. doi: 10.1097/QCO.0b013e3282f3d988. [DOI] [PubMed] [Google Scholar]
- 483.Marrazzo JM, Martin DH. Management of women with cervicitis. Clin Infect Dis. 2007;44(Suppl 3):S102–10. doi: 10.1086/511423. [DOI] [PubMed] [Google Scholar]
- 484.Taylor SN, Lensing S, Schwebke J, et al. Prevalence and treatment outcome of cervicitis of unknown etiology. Sex Transm Dis. 2013;40:379–85. doi: 10.1097/OLQ.0b013e31828bfcb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Marrazzo JM, Wiesenfeld HC, Murray PJ, et al. Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis. 2006;193:617–24. doi: 10.1086/500149. [DOI] [PubMed] [Google Scholar]
- 486.Moi H, Reinton N, Moghaddam A. Mycoplasma genitalium in women with lower genital tract inflammation. Sex Transm Infect. 2009;85:10–4. doi: 10.1136/sti.2008.032748. [DOI] [PubMed] [Google Scholar]
- 487.Bjartling C, Osser S, Persson K. Mycoplasma genitalium in cervicitis and pelvic inflammatory disease among women at a gynecologic outpatient service. Am J Obstet Gynecol. 2012;206:e471–8. doi: 10.1016/j.ajog.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 488.Geisler WM, Yu S, Venglarik M, et al. Vaginal leucocyte counts in women with bacterial vaginosis: relation to vaginal and cervical infections. Sex Transm Infect. 2004;80:401–5. doi: 10.1136/sti.2003.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Steinhandler L, Peipert JF, Heber W, et al. Combination of bacterial vaginosis and leukorrhea as a predictor of cervical chlamydial or gonococcal infection. Obstet Gynecol. 2002;99:603–7. doi: 10.1016/s0029-7844(01)01788-4. [DOI] [PubMed] [Google Scholar]
- 490.Manhart LE. Has the time come to systematically test for Mycoplasma genitalium? Sex Transm Dis. 2009;36:607–8. doi: 10.1097/OLQ.0b013e3181b9d825. [DOI] [PubMed] [Google Scholar]
- 491.Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(Suppl 3):S129–42. doi: 10.1093/cid/cir702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Coleman JS, Hitti J, Bukusi EA, et al. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS. 2007;21:755–9. doi: 10.1097/QAD.0b013e328012b838. [DOI] [PubMed] [Google Scholar]
- 493.Johnson LR, Starkey CR, Palmer J, et al. A comparison of two methods to determine the presence of high-risk HPV cervical infections. Am J Clin Pathol. 2008;130:401–8. doi: 10.1309/4DXEAFG2JXYF34N3. [DOI] [PubMed] [Google Scholar]
- 494.McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–10. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 495.Gatski M, Martin DH, Theall K, et al. Mycoplasma genitalium infection among HIV-positive women: prevalence, risk factors and association with vaginal shedding. Int J STD AIDS. 2011;22:155–9. doi: 10.1258/ijsa.2010.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Gitau RW, Graham SM, Masese LN, et al. Effect of acquisition and treatment of cervical infections on HIV-1 shedding in women on antiretroviral therapy. AIDS. 2010;24:2733–7. doi: 10.1097/QAD.0b013e32833f9f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Scholes D, Stergachis A, Heidrich FE, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med. 1996;334:1362–6. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 498.Kamwendo F, Forslin L, Bodin L, et al. Decreasing incidences of gonorrhea- and chlamydia-associated acute pelvic inflammatory disease: a 25-year study from an urban area of central Sweden. Sex Transm Dis. 1996;23:384–91. doi: 10.1097/00007435-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 499.Gift TL, Blake DR, Gaydos CA, et al. The cost-effectiveness of screening men for Chlamydia trachomatis: a review of the literature. Sex Transm Dis. 2008;35(Supp1):S51–60. doi: 10.1097/OLQ.0b013e3181723dba. [DOI] [PubMed] [Google Scholar]
- 500.Gift TL, Gaydos CA, Kent CK, et al. The program cost and cost-effectiveness of screening men for Chlamydia to prevent pelvic inflammatory disease in women. Sex Transm Dis. 2008;35(Supp1):S66–75. doi: 10.1097/OLQ.0b013e31818b64ac. [DOI] [PubMed] [Google Scholar]
- 501.Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an Internet-based screening program. J Clin Microbiol. 2009;47:1663–7. doi: 10.1128/JCM.02387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Knox J, Tabrizi SN, Miller P, et al. Evaluation of self-collected samples in contrast to practitioner-collected samples for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis by polymerase chain reaction among women living in remote areas. Sex Transm Dis. 2002;29:647–54. doi: 10.1097/00007435-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 503.Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005;32:725–8. doi: 10.1097/01.olq.0000190092.59482.96. [DOI] [PubMed] [Google Scholar]
- 504.Doshi JS, Power J, Allen E. Acceptability of chlamydia screening using self-taken vaginal swabs. Int J STD AIDS. 2008;19:507–9. doi: 10.1258/ijsa.2008.008056. [DOI] [PubMed] [Google Scholar]
- 505.Schachter J, Moncada J, Liska S, et al. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis. 2008;35:637–42. doi: 10.1097/OLQ.0b013e31817bdd7e. [DOI] [PubMed] [Google Scholar]
- 506.Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis. 2008;35:495–8. doi: 10.1097/OLQ.0b013e31816471ae. [DOI] [PubMed] [Google Scholar]
- 507.Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol. 2010;48:1827–32. doi: 10.1128/JCM.02398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae oropharyngeal infections. J Clin Microbiol. 2009;47:902–7. doi: 10.1128/JCM.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sexton ME, Baker JJ, Nakagawa K, et al. How reliable is self-testing for gonorrhea and chlamydia among men who have sex with men? J Fam Pract. 2013;62:70–8. [PMC free article] [PubMed] [Google Scholar]
- 510.van der Helm JJ, Hoebe CJ, van Rooijen MS, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis. 2009;36:493–7. doi: 10.1097/OLQ.0b013e3181a44b8c. [DOI] [PubMed] [Google Scholar]
- 511.Dodge B, Van Der Pol B, Reece M, et al. Rectal self-sampling in nonclinical venues for detection of sexually transmissible infections among behaviourally bisexual men. Sex Health. 2012;9:190–1. doi: 10.1071/SH11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Chernesky M, Freund GG, Hook E, 3rd, et al. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae infections in North American women by testing SurePath liquid-based Pap specimens in APTIMA assays. J Clin Microbiol. 2007;45:2434–8. doi: 10.1128/JCM.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Geisler WM, Wang C, Morrison SG, et al. The natural history of untreated Chlamydia trachomatis infection in the interval between screening and returning for treatment. Sex Transm Dis. 2008;35:119–23. doi: 10.1097/OLQ.0b013e318151497d. [DOI] [PubMed] [Google Scholar]
- 514.Lau CY, Qureshi AK. Azithromycin versus doxycycline for genital chlamydial infections: a meta-analysis of randomized clinical trials. Sex Transm Dis. 2002;29:497–502. doi: 10.1097/00007435-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 515.Hathorn E, Opie C, Goold P. What is the appropriate treatment for the management of rectal Chlamydia trachomatis in men and women? Sex Transm Infect. 2012;88:352–4. doi: 10.1136/sextrans-2011-050466. [DOI] [PubMed] [Google Scholar]
- 516.Steedman NM, McMillan A. Treatment of asymptomatic rectal Chlamydia trachomatis: is single-dose azithromycin effective? International journal of STD and AIDS. 2009;20:16–8. doi: 10.1258/ijsa.2008.008211. [DOI] [PubMed] [Google Scholar]
- 517.Marcus JL, Kohn RP, Barry PM, et al. Chlamydia trachomatis and Neisseria gonorrhoeae transmission from the female oropharynx to the male urethra. Sex Transm Dis. 2011;38:372–3. doi: 10.1097/OLQ.0b013e3182029008. [DOI] [PubMed] [Google Scholar]
- 518.Geisler WM, Koltun WD, Abdelsayed N, et al. Safety and efficacy of WC2031 versus vibramycin for the treatment of uncomplicated urogenital Chlamydia trachomatis infection: a randomized, double-blind, double-dummy, active-controlled, multicenter trial. Clin Infect Dis. 2012;55:82–8. doi: 10.1093/cid/cis291. [DOI] [PubMed] [Google Scholar]
- 519.Renault CA, Israelski DM, Levy V, et al. Time to clearance of Chlamydia trachomatis ribosomal RNA in women treated for chlamydial infection. Sex Health. 2011;8:69–73. doi: 10.1071/SH10030. [DOI] [PubMed] [Google Scholar]
- 520.Dunne EF, Chapin JB, Rietmeijer CA, et al. Rate and predictors of repeat Chlamydia trachomatis infection among men. Sex Transm Dis. 2008;35(11 Supp1):S40–4. doi: 10.1097/OLQ.0b013e31817247b2. [DOI] [PubMed] [Google Scholar]
- 521.Kjaer HO, Dimcevski G, Hoff G, et al. Recurrence of urogenital Chlamydia trachomatis infection evaluated by mailed samples obtained at home: 24 weeks’ prospective follow up study. Sex Transm Infect. 2000;76:169–72. doi: 10.1136/sti.76.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Whittington WL, Kent C, Kissinger P, et al. Determinants of persistent and recurrent Chlamydia trachomatis infection in young women: results of a multicenter cohort study. Sex Transm Dis. 2001;28:117–23. doi: 10.1097/00007435-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 523.Jacobson GF, Autry AM, Kirby RS, et al. A randomized controlled trial comparing amoxicillin and azithromycin for the treatment of Chlamydia trachomatis in pregnancy. Am J Obstet Gynecol. 2001;184:1352–4. doi: 10.1067/mob.2001.115050. [DOI] [PubMed] [Google Scholar]
- 524.Kacmar J, Cheh E, Montagno A, et al. A randomized trial of azithromycin versus amoxicillin for the treatment of Chlamydia trachomatis in pregnancy. Infect Dis Obstet Gynecol. 2001;9:197–202. doi: 10.1155/S1064744901000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Rahangdale L, Guerry S, Bauer HM, et al. An observational cohort study of Chlamydia trachomatis treatment in pregnancy. Sex Transm Dis. 2006;33:106–10. doi: 10.1097/01.olq.0000187226.32145.ea. [DOI] [PubMed] [Google Scholar]
- 526.Aggarwal A, Spitzer RF, Caccia N, et al. Repeat screening for sexually transmitted infection in adolescent obstetric patients. J Obstet Gynaecol Can. 2010;32:956–61. doi: 10.1016/s1701-2163(16)34683-7. [DOI] [PubMed] [Google Scholar]
- 527.Hood EE, Nerhood RC. The utility of screening for chlamydia at 34–36 weeks gestation. W V Med J. 2010;106:10–1. [PubMed] [Google Scholar]
- 528.Phillips Campbell R, Kintner J, Whittimore J, et al. Chlamydia muridarum enters a viable but non-infectious state in amoxicillin-treated BALB/c mice. Microbes and Infection. 2012;14:1177–85. doi: 10.1016/j.micinf.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Wyrick PB. Chlamydia trachomatis persistence in vitro: an overview. J Infect Dis. 2010;201(Suppl 2):S88–95. doi: 10.1086/652394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Hammerschlag MR, Gelling M, Roblin PM, et al. Treatment of neonatal chlamydial conjunctivitis with azithromycin. Ped Infect Dis J. 1998;17:1049–50. doi: 10.1097/00006454-199811000-00020. [DOI] [PubMed] [Google Scholar]
- 531.Hammerschlag MR, Chandler JW, Alexander ER, et al. Longitudinal studies on chlamydial infections in the first year of life. Ped Infect Dis. 1982;1:395–401. doi: 10.1097/00006454-198211000-00007. [DOI] [PubMed] [Google Scholar]
- 532.Beem MO, Saxon E, Tipple MA. Treatment of chlamydial pneumonia of infancy. Pediatrics. 1979;63:198–203. [PubMed] [Google Scholar]
- 533.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–93. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 534.Drake C, Barenfanger J, Lawhorn J, et al. Comparison of easy-flow copan liquid Stuart’s and starplex swab transport systems for recovery of fastidious aerobic bacteria. J Clin Microbiol. 2005;43:1301–3. doi: 10.1128/JCM.43.3.1301-1303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Wade JJ, Graver MA. Survival of six auxotypes of Neisseria gonorrhoeae in transport media. J Clin Microbiol. 2003;41:1720–1. doi: 10.1128/JCM.41.4.1720-1721.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163–8. doi: 10.1016/s0732-8893(99)00134-0. [DOI] [PubMed] [Google Scholar]
- 537.Workowski KA, Berman SM, Douglas JM., Jr Emerging antimicrobial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Ann Intern Med. 2008;148:606–13. doi: 10.7326/0003-4819-148-8-200804150-00005. [DOI] [PubMed] [Google Scholar]
- 538.Schwarcz SK, Zenilman JM, Schnell D, et al. National surveillance of antimicrobial resistance in Neisseria gonorrhoeae. JAMA. 1990;264:1413–7. [PubMed] [Google Scholar]
- 539.CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morbid Mortal Wkly Rep. 2007;56:332–6. [PubMed] [Google Scholar]
- 540.CDC. Update to CDC’s Sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morbid Mortal Wkly Rep. 2012;61:590–4. [PubMed] [Google Scholar]
- 541.Muratani T, Akasaka S, Kobayashi T, et al. Outbreak of cefozopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimicrob Agents Chemother. 2001;45:3603–6. doi: 10.1128/AAC.45.12.3603-3606.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Deguchi T, Yasuda M, Yokoi S, et al. Treatment of uncomplicated gonococcal urethritis by double-dosing of 200 mg cefixime at a 6-h interval. J Infect Chemother. 2003;9:35–9. doi: 10.1007/s10156-002-0204-8. [DOI] [PubMed] [Google Scholar]
- 543.Yokoi S, Deguchi T, Ozawa T, et al. Threat to cefixime treatment for gonorrhea. Emerg Infect Dis. 2007;13:1275–7. doi: 10.3201/eid1308.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Lo JY, Ho KM, Leung AO, et al. Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection. Antimicrob Agents Chemother. 2008;52:3564–7. doi: 10.1128/AAC.00198-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Unemo M, Golparian D, Syversen G, et al. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveillance. 2010;15:19721. doi: 10.2807/ese.15.47.19721-en. [DOI] [PubMed] [Google Scholar]
- 546.Ison CA, Hussey J, Sankar KN, et al. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveillance. 2011;16:19833. [PubMed] [Google Scholar]
- 547.Forsyth S, Penney P, Rooney G. Cefixime-resistant Neisseria gonorrhoeae in the UK: a time to reflect on practice and recommendations. International journal of STD and AIDS. 2011;22:296–7. doi: 10.1258/ijsa.2009.009191. [DOI] [PubMed] [Google Scholar]
- 548.Unemo M, Golparian D, Stary A, et al. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveillance. 2011;16:19998. [PubMed] [Google Scholar]
- 549.Unemo M, Golparian D, Nicholas R, et al. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56:1273–80. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Lewis DA, Sriruttan C, Muller EE, et al. Phenotypic and genetic characterization of the first two cases of extended-spectrum-cephalosporin-resistant Neisseria gonorrhoeae infection in South Africa and association with cefixime treatment failure. J Antimicrob Chemother. 2013;68:1267–70. doi: 10.1093/jac/dkt034. [DOI] [PubMed] [Google Scholar]
- 551.Ota KV, Fisman DN, Tamari IE, et al. Incidence and treatment outcomes of pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections in men who have sex with men: a 13-year retrospective cohort study. Clin Infect Dis. 2009;48:1237–43. doi: 10.1086/597586. [DOI] [PubMed] [Google Scholar]
- 552.Allen VG, Mitterni L, Seah C, et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA. 2013;309:163–70. doi: 10.1001/jama.2012.176575. [DOI] [PubMed] [Google Scholar]
- 553.Chen MY, Stevens K, Tideman R, et al. Failure of 500 mg of ceftriaxone to eradicate pharyngeal gonorrhoea, Australia. J Antimicrob Chemother. 2013;68:1445–7. doi: 10.1093/jac/dkt017. [DOI] [PubMed] [Google Scholar]
- 554.Tapsall J, Read P, Carmody C, et al. Two cases of failed ceftriaxone treatment in pharyngeal gonorrhoea verified by molecular microbiological methods. J Med Microbiol. 2009;58(Pt 5):683–7. doi: 10.1099/jmm.0.007641-0. [DOI] [PubMed] [Google Scholar]
- 555.Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148–9. doi: 10.3201/eid1701.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Unemo M, Golparian D, Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 557.Unemo M, Golparian D, Potocnik M, et al. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveillance. 2012;17:20200. [PubMed] [Google Scholar]
- 558.National Committee for Clinical Laboratory Standards. Approved Standard M100-S20 performance standards for antimicrobial susceptibility testing: twentieth informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 559.Carnicer-Pont D, Smithson A, Fina-Homar E, et al. First cases of Neisseria gonorrhoeae resistant to ceftriaxone in Catalonia, Spain, May 2011. Enferm Infecc Microbiol Clin. 2012;30:218–9. doi: 10.1016/j.eimc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 560.Camara J, Serra J, Ayats J, et al. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother. 2012;67:1858–60. doi: 10.1093/jac/dks162. [DOI] [PubMed] [Google Scholar]
- 561.Steingrimsson O, Olafsson JH, Thorarinsson H, et al. Azithromycin in the treatment of sexually transmitted disease. J Antimicrob Chemother. 1990;25(Suppl A):109–14. doi: 10.1093/jac/25.suppl_a.109. [DOI] [PubMed] [Google Scholar]
- 562.Waugh MA. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women. J Antimicrob Chemother. 1993;31(Suppl E):193–8. doi: 10.1093/jac/31.suppl_e.193. [DOI] [PubMed] [Google Scholar]
- 563.Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea—is dual therapy the way forward? International journal of STD and AIDS. 2007;18:647–8. doi: 10.1258/095646207781568556. [DOI] [PubMed] [Google Scholar]
- 564.Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539–45. doi: 10.1093/cid/cit084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Lyss SB, Kamb ML, Peterman TA, et al. Chlamydia trachomatis among patients infected with and treated for Neisseria gonorrhoeae in sexually transmitted disease clinics in the United States. Ann Intern Med. 2003;139:178–85. doi: 10.7326/0003-4819-139-3-200308050-00007. [DOI] [PubMed] [Google Scholar]
- 566.Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis. 1995;20(Suppl 1):S47–65. doi: 10.1093/clinids/20.supplement_1.s47. [DOI] [PubMed] [Google Scholar]
- 567.Newman LM, Moran JS, Workowski KA. Update on the management of gonorrhea in adults in the United States. Clin Infect Dis. 2007;44(Suppl 3):S84–101. doi: 10.1086/511422. [DOI] [PubMed] [Google Scholar]
- 568.Yu RX, Yin Y, Wang GQ, et al. Worldwide susceptibility rates of Neisseria gonorrhoeae isolates to cefixime and cefpodoxime: a systematic review and meta-analysis. PLoS One. 2014;9:e87849. doi: 10.1371/journal.pone.0087849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083–91. doi: 10.1093/cid/ciu521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.CDC. Discontinuation of Spectinomycin. MMWR Morb Mortal Wkly Rep. 2006;55:370. [Google Scholar]
- 571.Soge OO, Harger D, Schafer S, et al. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011) Sex Transm Dis. 2012;39:877–9. doi: 10.1097/OLQ.0b013e3182685d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Waters LJ, Boag FC, Betournay R. Efficacy of azithromycin 1 g single dose in the management of uncomplicated gonorrhoea. Int J STD AIDS. 2005;16:84. doi: 10.1258/0956462052932746. [DOI] [PubMed] [Google Scholar]
- 573.McLean CA, Wang SA, Hoff GL, et al. The emergence of Neisseria gonorrhoeae with decreased susceptibility to azithromycin in Kansas City, Missouri, 1999 to 2000. Sex Transm Dis. 2004;31:73–8. doi: 10.1097/01.OLQ.0000109514.91508.FC. [DOI] [PubMed] [Google Scholar]
- 574.Chisholm SA, Neal TJ, Alawattegama AB, et al. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother. 2009;64:353–8. doi: 10.1093/jac/dkp188. [DOI] [PubMed] [Google Scholar]
- 575.Mayer KH, Klausner JD, Handsfield HH. Intersecting epidemics and educable moments—Sexually transmitted disease risk assessment and screening in men who have sex with men. Sex Transm Dis. 2001;28:464–7. doi: 10.1097/00007435-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 576.Linhart Y, Shohat T, Amitai Z, et al. Sexually transmitted infections among brothel-based sex workers in Tel-Aviv area, Israel: high prevalence of pharyngeal gonorrhoea. Int J STD AIDS. 2008;19:656–9. doi: 10.1258/ijsa.2008.008127. [DOI] [PubMed] [Google Scholar]
- 577.Kissinger PJ, Reilly K, Taylor SN, et al. Early repeat Chlamydia trachomatis and Neisseria gonorrhoeae infections among heterosexual men. Sex Transm Dis. 2009;36:498–500. doi: 10.1097/OLQ.0b013e3181a4d147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Lo JY, Ho KM, Lo AC. Surveillance of gonococcal antimicrobial susceptibility resulting in early detection of emerging resistance. J Antimicrob Chemother. 2012;67:1422–6. doi: 10.1093/jac/dks036. [DOI] [PubMed] [Google Scholar]
- 579.CDC. Cephalosporin-resistant Neisseria gonorrhoeae public health response plan. Available at http://www.cdc.gov/std/treatment/Ceph-R-ResponsePlanJuly30-2012.pdf.
- 580.Haimovici R, Roussel TJ. Treatment of gonococcal conjunctivitis with single-dose intramuscular ceftriaxone. Am J Ophthalmol. 1989;107:511–4. doi: 10.1016/0002-9394(89)90495-9. [DOI] [PubMed] [Google Scholar]
- 581.Belkacem A, Caumes E, Ouanich J, et al. Changing patterns of disseminated gonococcal infection in France: cross-sectional data 2009–2011. Sex Transm Infect. 2013;89:613–5. doi: 10.1136/sextrans-2013-051119. [DOI] [PubMed] [Google Scholar]
- 582.Scott WJ, Eck CD. Povidone-iodine and ophthalmia neonatorum. Ophthalmology. 2012;119:653–4. doi: 10.1016/j.ophtha.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 583.David M, Rumelt S, Weintraub Z. Efficacy comparison between povidone iodine 2.5% and tetracycline 1% in prevention of ophthalmia neonatorum. Ophthalmology. 2011;118:1454–8. doi: 10.1016/j.ophtha.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 584.Binenbaum G, Bruno CJ, Forbes BJ, et al. Periocular ulcerative dermatitis associated with gentamicin ointment prophylaxis in newborns. J Pediatr. 2010;156:320–1. doi: 10.1016/j.jpeds.2009.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Nathawad R, Mendez H, Ahmad A, et al. Severe ocular reactions after neonatal ocular prophylaxis with gentamicin ophthalmic ointment. Pediatr Infect Dis J. 2011;30:175–6. doi: 10.1097/INF.0b013e3181f6c2e5. [DOI] [PubMed] [Google Scholar]
- 586.CDC. CDC Guidance on shortage of erythromycin (0.5%) ophthalmic ointment-September 2009. Atlanta, GA: 2010. [Google Scholar]
- 587.MacDonald N, Mailman T, Desai S. Gonococcal infections in newborns and in adolescents. Adv Exp Med Biol. 2008;609:108–30. doi: 10.1007/978-0-387-73960-1_9. [DOI] [PubMed] [Google Scholar]
- 588.Laga M, Meheus A, Piot P. Epidemiology and control of gonococcal ophthalmia neonatorum. Bull World Health Organ. 1989;67:471–7. [PMC free article] [PubMed] [Google Scholar]
- 589.Fethers KA, Fairley CK, Morton A, et al. Early sexual experiences and risk factors for bacterial vaginosis. J Infect Dis. 2009;200:1662–70. doi: 10.1086/648092. [DOI] [PubMed] [Google Scholar]
- 590.Laxmi U, Agrawal S, Raghunandan C, et al. Association of bacterial vaginosis with adverse fetomaternal outcome in women with spontaneous preterm labor: a prospective cohort study. J Matern Fetal Neonatal Med. 2012;25:64–7. doi: 10.3109/14767058.2011.565390. [DOI] [PubMed] [Google Scholar]
- 591.Nelson DB, Hanlon A, Hassan S, et al. Preterm labor and bacterial vaginosis-associated bacteria among urban women. J Perinat Med. 2009;37:130–4. doi: 10.1515/JPM.2009.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202:1907–15. doi: 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33:747–52. doi: 10.1097/01.olq.0000218869.52753.c7. [DOI] [PubMed] [Google Scholar]
- 594.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacteria vaginosis outcomes in women. Sex Transm Dis. 2012;39:822–30. doi: 10.1097/OLQ.0b013e3182631d89. [DOI] [PubMed] [Google Scholar]
- 596.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 597.Schwebke JR, Hillier SL, Sobel JD, et al. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstet Gynecol. 1996;88(4 Pt 1):573–6. doi: 10.1016/0029-7844(96)00233-5. [DOI] [PubMed] [Google Scholar]
- 598.Fredricks DN, Fiedler TL, Thomas KK, et al. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007;45:3270–6. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Cartwright CP, Lembke BD, Ramachandran K, et al. Development and validation of a semiquantitative, multitarget PCR assay for diagnosis of bacterial vaginosis. J Clin Microbiol. 2012;50:2321–9. doi: 10.1128/JCM.00506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol. 2007;196:517.e1–e6. doi: 10.1016/j.ajog.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Livengood CH, 3rd, Ferris DG, Wiesenfeld HC, et al. Effectiveness of two tinidazole regimens in treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2007;110(2 Pt 1):302–9. doi: 10.1097/01.AOG.0000275282.60506.3d. [DOI] [PubMed] [Google Scholar]
- 602.Sobel J, Peipert JF, McGregor JA, et al. Efficacy of clindamycin vaginal ovule (3-day treatment) vs. clindamycin vaginal cream (7-day treatment) in bacterial vaginosis. Infect Dis Obstet Gynecol. 2001;9:9–15. doi: 10.1155/S1064744901000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Antonio MA, Meyn LA, Murray PJ, et al. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous Lactobacilli. J Infect Dis. 2009;199:1506–13. doi: 10.1086/598686. [DOI] [PubMed] [Google Scholar]
- 604.Abad CL, Safdar N. The role of lactobacillus probiotics in the treatment or prevention of urogenital infections—a systematic review. J Chemother. 2009;21:243–52. doi: 10.1179/joc.2009.21.3.243. [DOI] [PubMed] [Google Scholar]
- 605.Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009:CD006289. doi: 10.1002/14651858.CD006289.pub2. [DOI] [PubMed] [Google Scholar]
- 606.Mastromarino P, Macchia S, Meggiorini L, et al. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clinical Microbiology and Infection. 2009;15:67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 607.Hemmerling A, Harrison W, Schroeder A, et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex Transm Dis. 2010;37:745–50. doi: 10.1097/OLQ.0b013e3181e50026. [DOI] [PubMed] [Google Scholar]
- 608.Ferris MJ, Masztal A, Aldridge KE, et al. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis. 2004;4:5. doi: 10.1186/1471-2334-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Bradshaw CS, Tabrizi SN, Fairley CK, et al. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J Infect Dis. 2006;194:828–36. doi: 10.1086/506621. [DOI] [PubMed] [Google Scholar]
- 610.Marrazzo JM, Thomas KK, Fiedler TL, et al. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Ann Intern Med. 2008;149:20–8. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Meltzer MC, Desmond RA, Schwebke JR. Association of Mobiluncus curtisii with recurrence of bacterial vaginosis. Sex Transm Dis. 2008;35:611–3. doi: 10.1097/OLQ.0b013e318167b105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Beigi RH, Austin MN, Meyn LA, et al. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am J Obstet Gynecol. 2004;191:1124–9. doi: 10.1016/j.ajog.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 613.Nyirjesy P, McIntosh MJ, Steinmetz JI, et al. The effects of intravaginal clindamycin and metronidazole therapy on vaginal mobiluncus morphotypes in patients with bacterial vaginosis. Sex Transm Dis. 2007;34:197–202. doi: 10.1097/01.olq.0000235152.98601.d7. [DOI] [PubMed] [Google Scholar]
- 614.Bunge KE, Beigi RH, Meyn LA, et al. The efficacy of retreatment with the same medication for early treatment failure of bacterial vaginosis. Sex Transm Dis. 2009;36:711–3. doi: 10.1097/OLQ.0b013e3181af6cfd. [DOI] [PubMed] [Google Scholar]
- 615.Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0. 75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis Am J Obstet Gynecol. 2006;194:1283–9. doi: 10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 616.Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732–4. doi: 10.1097/OLQ.0b013e3181b08456. [DOI] [PubMed] [Google Scholar]
- 617.McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect Dis. 2008;197:1361–8. doi: 10.1086/587490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Hauth JC, Goldenberg RL, Andrews WW, et al. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med. 1995;333:1732–6. doi: 10.1056/NEJM199512283332603. [DOI] [PubMed] [Google Scholar]
- 619.Morales WJ, Schorr S, Albritton J. Effect of metronidazole in patients with preterm birth in preceding pregnancy and bacterial vaginosis: a placebo-controlled, double-blind study. Am J Obstet Gynecol. 1994;171:345–9. doi: 10.1016/s0002-9378(94)70033-8. [DOI] [PubMed] [Google Scholar]
- 620.Yudin MH, Landers DV, Meyn L, et al. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstet Gynecol. 2003;102:527–34. doi: 10.1016/s0029-7844(03)00566-0. [DOI] [PubMed] [Google Scholar]
- 621.Ugwumadu A, Reid F, Hay P, et al. Natural history of bacterial vaginosis and intermediate flora in pregnancy and effect of oral clindamycin. Obstet Gynecol. 2004;104:114–9. doi: 10.1097/01.AOG.0000130068.21566.4e. [DOI] [PubMed] [Google Scholar]
- 622.Burtin P, Taddio A, Ariburnu O, et al. Safety of metronidazole in pregnancy: a meta-analysis. Am J Obstet Gynecol. 1995;172(2 Pt 1):525–9. doi: 10.1016/0002-9378(95)90567-7. [DOI] [PubMed] [Google Scholar]
- 623.Piper JM, Mitchel EF, Ray WA. Prenatal use of metronidazole and birth-defects – no association. Obstet Gynecol. 1993;82:348–52. [PubMed] [Google Scholar]
- 624.Lamont RF, Nhan-Chang CL, Sobel JD, et al. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205:177–90. doi: 10.1016/j.ajog.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Brocklehurst P, Gordon A, Heatley E, et al. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2013;1:CD000262. doi: 10.1002/14651858.CD000262.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Odendaal HJ, Popov I, Schoeman J, et al. Preterm labour—is bacterial vaginosis involved? South African Medical Journal. 2002;92:231–4. [PubMed] [Google Scholar]
- 627.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342:534–40. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 628.Vermeulen GM, Bruinse HW. Prophylactic administration of clindamycin 2% vaginal cream to reduce the incidence of spontaneous preterm birth in women with an increased recurrence risk: a randomised placebo-controlled double-blind trial. Br J Obstet Gynaecol. 1999;106:652–7. doi: 10.1111/j.1471-0528.1999.tb08363.x. [DOI] [PubMed] [Google Scholar]
- 629.McDonald HM, O’Loughlin JA, Vigneswaran R, et al. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): a randomised, placebo controlled trial. Br J Obstet Gynaecol. 1997;104:1391–7. doi: 10.1111/j.1471-0528.1997.tb11009.x. [DOI] [PubMed] [Google Scholar]
- 630.Ugwumadu A, Manyonda I, Reid F, et al. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet. 2003;361:983–8. doi: 10.1016/S0140-6736(03)12823-1. [DOI] [PubMed] [Google Scholar]
- 631.Lamont RF, Duncan SL, Mandal D, et al. Intravaginal clindamycin to reduce preterm birth in women with abnormal genital tract flora. Obstet Gynecol. 2003;101:516–22. doi: 10.1016/s0029-7844(02)03054-5. [DOI] [PubMed] [Google Scholar]
- 632.McGregor JA, French JI, Jones W, et al. Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase – results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol. 1994;170:1048–60. doi: 10.1016/s0002-9378(94)70098-2. [DOI] [PubMed] [Google Scholar]
- 633.Joesoef MR, Hillier SL, Wiknjosastro G, et al. Intravaginal clindamycin treatment for bacterial vaginosis: effects on preterm delivery and low birth weight. Am J Obstet Gynecol. 1995;173:1527–31. doi: 10.1016/0002-9378(95)90644-4. [DOI] [PubMed] [Google Scholar]
- 634.Koss CA, Baras DC, Lane SD, et al. Investigation of metronidazole use during pregnancy and adverse birth outcomes. Antimicrob Agents Chemother. 2012;56:4800–5. doi: 10.1128/AAC.06477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Erickson SH, Oppenheim GL, Smith GH. Metronidazole in breast milk. Obstet Gynecol. 1981;57:48–50. [PubMed] [Google Scholar]
- 636.Passmore CM, McElnay JC, Rainey EA, et al. Metronidazole excretion in human milk and its effect on the suckling neonate. Br J Clin Pharmacol. 1988;26:45–51. doi: 10.1111/j.1365-2125.1988.tb03362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Golightly P, Kearney L. Metronidazole— is it safe to use with breastfeeding? United Kingdom National Health Service. UKMI. 2012 [Google Scholar]
- 638.Jamieson DJ, Duerr A, Klein RS, et al. Longitudinal analysis of bacterial vaginosis: findings from the HIV epidemiology research study. Obstet Gynecol. 2001;98:656–63. doi: 10.1016/s0029-7844(01)01525-3. [DOI] [PubMed] [Google Scholar]
- 639.Sutton M, Sternberg M, Koumans EH, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis. 2007;45:1319–26. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 640.Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601–8. doi: 10.1128/JCM.00748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Meites E, Llata E, Braxton J, et al. Trichomonas vaginalis in selected U.S. sexually transmitted disease clinics: testing, screening, and prevalence. Sex Transm Dis. 2013;40:865–9. doi: 10.1097/OLQ.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Willers DM, Peipert JF, Allsworth JE, et al. Prevalence and predictors of sexually transmitted infection among newly incarcerated females. Sex Transm Dis. 2008;35:68–72. doi: 10.1097/OLQ.0b013e318154bdb2. [DOI] [PubMed] [Google Scholar]
- 643.Nijhawan AE, DeLong AK, Celentano DD, et al. The association between Trichomonas infection and incarceration in HIV-seropositive and at-risk HIV-seronegative women. Sex Transm Dis. 2011;38:1094–100. doi: 10.1097/OLQ.0b013e31822ea147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Shuter J, Bell D, Graham D, et al. Rates of and risk factors for trichomoniasis among pregnant inmates in New York City. Sex Transm Dis. 1998;25:303–7. doi: 10.1097/00007435-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 645.Sosman JM, MacGowan RJ, Margolis AD, et al. Screening for sexually transmitted diseases and hepatitis in 18–29-year-old men recently released from prison: feasibility and acceptability. Int J STD AIDS. 2005;16:117–22. doi: 10.1258/0956462053057594. [DOI] [PubMed] [Google Scholar]
- 646.Mayer KH, Bush T, Henry K, et al. Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: implications for prevention interventions. Sex Transm Dis. 2012;39:1–7. doi: 10.1097/OLQ.0b013e31823b1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Kelley CF, Rosenberg ES, O’Hara BM, et al. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis. 2012;39:739. doi: 10.1097/OLQ.0b013e318264248b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Peterman TA, Tian LH, Metcalf CA, et al. Persistent, undetected Trichomonas vaginalis infections? Clin Infect Dis. 2009;48:259–60. doi: 10.1086/595706. [DOI] [PubMed] [Google Scholar]
- 649.Gatski M, Kissinger P. Observation of probable persistent, undetected Trichomonas vaginalis infection among HIV-positive women. Clin Infect Dis. 2010;51:114–5. doi: 10.1086/653443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Sena AC, Miller WC, Hobbs MM, et al. Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment, and prevention. Clin Infect Dis. 2007;44:13–22. doi: 10.1086/511144. [DOI] [PubMed] [Google Scholar]
- 651.Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200:e41–7. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Tsai CS, Shepherd BE, Vermund SH. Does douching increase risk for sexually transmitted infections? A prospective study in high-risk adolescents. Am J Obstet Gynecol. 2009;200:e31–8. doi: 10.1016/j.ajog.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 654.Van Der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–54. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 655.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–65. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426–33. doi: 10.1136/sextrans-2012-051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Minkoff H, Grunebaum AN, Schwarz RH, et al. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol. 1984;150:965–72. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- 658.Cotch MF, Pastorek JG, 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Transm Dis. 1997;24:353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 659.Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis. 2002;34:519–22. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]
- 660.Cosentino LA, Campbell T, Jett A, et al. Use of nucleic acid amplification testing for diagnosis of anorectal sexually transmitted infections. J Clin Microbiol. 2012;50:2005–8. doi: 10.1128/JCM.00185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Francis SC, Kent CK, Klausner JD, et al. Prevalence of rectal Trichomonas vaginalis and Mycoplasma genitalium in male patients at the San Francisco STD clinic, 2005–2006. Sex Transm Dis. 2008;35:797–800. doi: 10.1097/OLQ.0b013e318177ec39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Hoots BE, Peterman TA, Torrone EA, et al. A Trich-y question: should Trichomonas vaginalis infection be reportable? Sex Transm Dis. 2013;40:113–6. doi: 10.1097/OLQ.0b013e31827c08c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Hollman D, Coupey SM, Fox AS, et al. Screening for Trichomonas vaginalis in high-risk adolescent females with a new transcription-mediated nucleic acid amplification test (NAAT): associations with ethnicity, symptoms, and prior and current STIs. J Pediatr Adolesc Gynecol. 2010;23:312–6. doi: 10.1016/j.jpag.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 664.Roth AM, Williams JA, Ly R, et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis. 2011;38:398–400. doi: 10.1097/OLQ.0b013e318203e3ce. [DOI] [PubMed] [Google Scholar]
- 665.Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol. 2011;49:4106–11. doi: 10.1128/JCM.01291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Huppert JS, Mortensen JE, Reed JL, et al. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin Infect Dis. 2007;45:194–8. doi: 10.1086/518851. [DOI] [PubMed] [Google Scholar]
- 667.Dize L, Agreda P, Quinn N, et al. Comparison of self-obtained penile-meatal swabs to urine for the detection of C. trachomatis, N. gonorrhoeae and T. vaginalis. Sex Transm Infect. 2013;89:305–7. doi: 10.1136/sextrans-2012-050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Campbell L, Woods V, Lloyd T, et al. Evaluation of the OSOM Trichomonas rapid test versus wet preparation examination for detection of Trichomonas vaginalis vaginitis in specimens from women with a low prevalence of infection. J Clin Microbiol. 2008;46:3467–9. doi: 10.1128/JCM.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Huppert JS, Hesse E, Kim G, et al. Adolescent women can perform a point-of-care test for trichomoniasis as accurately as clinicians. Sex Transm Infect. 2010;86:514–9. doi: 10.1136/sti.2009.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol. 2011;49:866–9. doi: 10.1128/JCM.02367-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Brown HL, Fuller DD, Jasper LT, et al. Clinical evaluation of affirm VPIII in the detection and identification of Trichomonas vaginalis, Gardnerella vaginalis, and Candida species in vaginitis/vaginosis. Infect Dis Obstet Gynecol. 2004;12:17–21. doi: 10.1080/1064744042000210375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Mohamed OA, Cohen CR, Kungu D, et al. Urine proves a poor specimen for culture of Trichomonas vaginalis in women. Sex Transm Infect. 2001;77:78–9. doi: 10.1136/sti.77.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Lawing LF, Hedges SR, Schwebke JR. Detection of trichomonosis in vaginal and urine specimens from women by culture and PCR. J Clin Microbiol. 2000;38:3585–8. doi: 10.1128/jcm.38.10.3585-3588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Stoner KA, Rabe LK, Meyn LA, et al. Survival of Trichomonas vaginalis in wet preparation and on wet mount. Sex Transm Infect. 2013;89:485–8. doi: 10.1136/sextrans-2012-051001. [DOI] [PubMed] [Google Scholar]
- 675.Kingston MA, Bansal D, Carlin EM. ‘Shelf life’ of Trichomonas vaginalis. Int J STD AIDS. 2003;14:28–9. doi: 10.1258/095646203321043228. [DOI] [PubMed] [Google Scholar]
- 676.Wood BA, Monro AM. Pharmacokinetics of tinidazole and metronidazole in women after single large oral doses. Br J Vener Dis. 1975;51:51–3. doi: 10.1136/sti.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Viitanen J, Haataja H, Mannisto PT. Concentrations of metronidazole and tinidazole in male genital tissues. Antimicrob Agents Chemother. 1985;28:812–4. doi: 10.1128/aac.28.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Mannisto P, Karhunen M, Mattila J, et al. Concentrations of metronidazole and tinidazole in female reproductive organs after a single intravenous infusion and after repeated oral administration. Infection. 1984;12:197–201. doi: 10.1007/BF01640899. [DOI] [PubMed] [Google Scholar]
- 679.Spence MR, Harwell TS, Davies MC, et al. The minimum single oral metronidazole dose for treating trichomoniasis: a randomized, blinded study. Obstet Gynecol. 1997;89(5 Pt 1):699–703. doi: 10.1016/s0029-7844(97)81437-8. [DOI] [PubMed] [Google Scholar]
- 680.Gabriel G, Robertson E, Thin RN. Single dose treatment of trichomoniasis. J Int Med Res. 1982;10:129–30. doi: 10.1177/030006058201000212. [DOI] [PubMed] [Google Scholar]
- 681.Thin RN, Symonds MA, Booker R, et al. Double-blind comparison of a single dose and a five-day course of metronidazole in the treatment of trichomoniasis. Br J Vener Dis. 1979;55:354–6. doi: 10.1136/sti.55.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Prasertsawat PO, Jetsawangsri T. Split-dose metronidazole or single-dose tinidazole for the treatment of vaginal trichomoniasis. Sex Transm Dis. 1992;19:295–7. doi: 10.1097/00007435-199209000-00011. [DOI] [PubMed] [Google Scholar]
- 683.Apte VV, Packard RS. Tinidazole in the treatment of trichomoniasis, giardiasis and amoebiasis: report of a multicentre study. Drugs. 1978;15(Suppl 1):43–8. doi: 10.2165/00003495-197800151-00009. [DOI] [PubMed] [Google Scholar]
- 684.Anjaeyulu R, Gupte SA, Desai DB. Single-dose treatment of trichomonal vaginitis: a comparison of tinidazole and metronidazole. J Int Med Res. 1977;5:438–41. doi: 10.1177/030006057300100210. [DOI] [PubMed] [Google Scholar]
- 685.Mati JK, Wallace RJ. The treatment of trichomonal vaginitis using a single dose of tinidazole by mouth. East Afr Med J. 1974;51:883–8. [PubMed] [Google Scholar]
- 686.Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev. 2003;2:CD000218. doi: 10.1002/14651858.CD000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Van Der Pol B, Williams JA, Orr DP, et al. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis. 2005;192:2039–44. doi: 10.1086/498217. [DOI] [PubMed] [Google Scholar]
- 688.Williams JA, Van Der Pol B, Ofner S, et al. Time from treatment to negative PCR results for C. trachomatis, N. gonorrhoeae and T. vaginalis. National STD Prevention Conference; March 10–13, 2008; Chicago, IL. 2008. [Google Scholar]
- 689.Mohammed H, Leichliter JS, Schmidt N, et al. Does patient-delivered partner treatment improve disclosure for treatable sexually transmitted diseases? AIDS Patient Care STDS. 2010;24:183–8. doi: 10.1089/apc.2009.0237. [DOI] [PubMed] [Google Scholar]
- 690.Kirkcaldy RD, Augostini P, Asbel LE, et al. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009–2010. Emerg Infect Dis. 2012;18:939–43. doi: 10.3201/eid1806.111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Schwebke JR, Barrientes FJ. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother. 2006;50:4209–10. doi: 10.1128/AAC.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Crowell AL, Sanders-Lewis KA, Secor WE. In vitro metronidazole and tinidazole activities against metronidazole-resistant strains of Trichomonas vaginalis. Antimicrob Agents Chemother. 2003;47:1407–9. doi: 10.1128/AAC.47.4.1407-1409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Bosserman EA, Helms DJ, Mosure DJ, et al. Utility of antimicrobial susceptibility testing in Trichomonas vaginalis-infected women with clinical treatment failure. Sex Transm Dis. 2011;38:983–7. doi: 10.1097/OLQ.0b013e318224db39. [DOI] [PubMed] [Google Scholar]
- 694.Nyirjesy P, Gilbert J, Mulcahy LJ. Resistant trichomoniasis: successful treatment with combination therapy. Sex Transm Dis. 2011;38:962–3. doi: 10.1097/OLQ.0b013e31822037e4. [DOI] [PubMed] [Google Scholar]
- 695.Tayal SC, Ochogwu SA, Bunce H. Paromomycin treatment of recalcitrant Trichomonas vaginalis. Int J STD AIDS. 2010;21:217–8. doi: 10.1258/ijsa.2009.009085. [DOI] [PubMed] [Google Scholar]
- 696.Sobel JD, Nyirjesy P, Brown W. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin Infect Dis. 2001;33:1341–6. doi: 10.1086/323034. [DOI] [PubMed] [Google Scholar]
- 697.Muzny C, Barnes A, Mena L. Symptomatic Trichomonas vaginalis infection in the setting of severe nitroimidazole allergy: successful treatment with boric acid. Sex Health. 2012;9:389–91. doi: 10.1071/SH11114. [DOI] [PubMed] [Google Scholar]
- 698.Aggarwal A, Shier RM. Recalcitrant Trichomonas vaginalis infections successfully treated with vaginal acidification. Journal of obstetrics and gynaecology Canada. 2008;30:55–8. doi: 10.1016/S1701-2163(16)32714-1. [DOI] [PubMed] [Google Scholar]
- 699.Dan M, Sobel JD. Failure of nitazoxanide to cure trichomoniasis in three women. Sex Transm Dis. 2007;34:813–4. doi: 10.1097/NMD.0b013e31802f5d9a. [DOI] [PubMed] [Google Scholar]
- 700.Sena AC, Bachmann LH, Hobbs MM. Persistent and recurrent Trichomonas vaginalis infections: epidemiology, treatment and management considerations. Expert Rev Anti Infect Ther. 2014;12:673–85. doi: 10.1586/14787210.2014.887440. [DOI] [PubMed] [Google Scholar]
- 701.Obiero J, Mwethera PG, Wiysonge CS. Topical microbicides for prevention of sexually transmitted infections. Cochrane Database Syst Rev. 2012;(6):CD007961. doi: 10.1002/14651858.CD007961.pub2. [DOI] [PubMed] [Google Scholar]
- 702.Helms DJ, Mosure DJ, Secor WE, et al. Management of Trichomonas vaginalis in women with suspected metronidazole hypersensitivity. Am J Obstet Gynecol. 2008;198:e371–7. doi: 10.1016/j.ajog.2007.10.795. [DOI] [PubMed] [Google Scholar]
- 703.Mann JR, McDermott S, Gill T. Sexually transmitted infection is associated with increased risk of preterm birth in South Carolina women insured by Medicaid. J Matern Fetal Neonatal Med. 2010;23:563–8. doi: 10.3109/14767050903214574. [DOI] [PubMed] [Google Scholar]
- 704.Mann JR, McDermott S, Barnes TL, et al. Trichomoniasis in pregnancy and mental retardation in children. Ann Epidemiol. 2009;19:891–9. doi: 10.1016/j.annepidem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 705.Mann JR, McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord. 2011;15:667–73. doi: 10.1177/1087054710370566. [DOI] [PubMed] [Google Scholar]
- 706.Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. New Engl J Med. 2001;345:487–93. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 707.Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2003;189:1398–400. doi: 10.1067/s0002-9378(03)00777-4. [DOI] [PubMed] [Google Scholar]
- 708.Mann JR, McDermott S, Zhou L, et al. Treatment of trichomoniasis in pregnancy and preterm birth: an observational study. J Womens Health. 2009;18:493–7. doi: 10.1089/jwh.2008.0964. [DOI] [PubMed] [Google Scholar]
- 709.Gulmezoglu AM, Azhar M. Interventions for trichomoniasis in pregnancy. Cochrane Database Syst Rev. 2011:CD000220. doi: 10.1002/14651858.CD000220.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Stringer E, Read JS, Hoffman I, et al. Treatment of trichomoniasis in pregnancy in sub-Saharan Africa does not appear to be associated with low birth weight or preterm birth. S Afr Med J. 2010;100:58–64. [PMC free article] [PubMed] [Google Scholar]
- 711.Trintis J, Epie N, Boss R, et al. Neonatal Trichomonas vaginalis infection: a case report and review of literature. Int J STD AIDS. 2010;21:606–7. doi: 10.1258/ijsa.2010.010174. [DOI] [PubMed] [Google Scholar]
- 712.Carter JE, Whithaus KC. Neonatal respiratory tract involvement by Trichomonas vaginalis: a case report and review of the literature. Am J Trop Med Hygiene. 2008;78:17–9. [PubMed] [Google Scholar]
- 713.Gumbo FZ, Duri K, Kandawasvika GQ, et al. Risk factors of HIV vertical transmission in a cohort of women under a PMTCT program at three peri-urban clinics in a resource-poor setting. J Perinatol. 2010;30:717–23. doi: 10.1038/jp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Goldenberg RL, Mwatha A, Read JS, et al. The HPTN 024 Study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. Am J Obstet Gynecol. 2006;194:650–61. doi: 10.1016/j.ajog.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 715.Cu-Uvin S, Ko H, Jamieson DJ, et al. Prevalence, incidence, and persistence or recurrence of trichomoniasis among human immunodeficiency virus (HIV)-positive women and among HIV-negative women at high risk for HIV infection. Clin Infect Dis. 2002;34:1406–11. doi: 10.1086/340264. [DOI] [PubMed] [Google Scholar]
- 716.Miller M, Liao Y, Wagner M, et al. HIV, the clustering of sexually transmitted infections, and sex risk among African American women who use drugs. Sex Transm Dis. 2008;35:696–702. doi: 10.1097/OLQ.0b013e31816b1fb8. [DOI] [PubMed] [Google Scholar]
- 717.Anderson BL, Firnhaber C, Liu T, et al. Effect of trichomoniasis therapy on genital HIV viral burden among African women. Sex Transm Dis. 2012;39:638–42. doi: 10.1097/OLQ.0b013e31825725ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis. 2009;36:11–6. doi: 10.1097/OLQ.0b013e318186decf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Kissinger P, Mena L, Levison J, et al. A randomized treatment trial: single versus 7-day dose of metronidazole for the treatment of Trichomonas vaginalis among HIV-infected women. J Acquir Immune Defic Syndr. 2010;55:565–71. doi: 10.1097/QAI.0b013e3181eda955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Balkus JE, Richardson BA, Mochache V, et al. A prospective cohort study comparing the effect of single-dose 2 g metronidazole on Trichomonas vaginalis infection in HIV-seropositive versus HIV-seronegative women. Sex Transm Dis. 2013;40:499–505. doi: 10.1097/OLQ.0b013e31828fce34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Kissinger P, Adamski A, Clark RA, et al. Does antiretroviral therapy interfere with the treatment of Trichomonas vaginalis among HIV+ women? Sex Transm Dis. 2013;40:506–7. doi: 10.1097/OLQ.0b013e31829335fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Bruggemann RJ, Alffenaar JW, Blijlevens NM, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441–58. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 723.Shahid Z, Sobel JD. Reduced fluconazole susceptibility of Candida albicans isolates in women with recurrent vulvovaginal candidiasis: effects of long-term fluconazole therapy. Diagn Microbiol Infect Dis. 2009;64:354–6. doi: 10.1016/j.diagmicrobio.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 724.Marchaim D, Lemanek L, Bheemreddy S, et al. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol. 2012;120:1407–14. doi: 10.1097/aog.0b013e31827307b2. [DOI] [PubMed] [Google Scholar]
- 725.Kennedy MA, Sobel JD. Vulvovaginal candidiasis caused by non-albicans Candida species: new insights. Curr Infect Dis Rep. 2010;12:465–70. doi: 10.1007/s11908-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 726.Sobel JD, Chaim W, Nagappan V, et al. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol. 2003;189:1297–300. doi: 10.1067/s0002-9378(03)00726-9. [DOI] [PubMed] [Google Scholar]
- 727.Vazquez JA, Peng G, Sobel JD, et al. Evolution of antifungal susceptibility among Candida species isolates recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis. Clin Infect Dis. 2001;33:1069–75. doi: 10.1086/322641. [DOI] [PubMed] [Google Scholar]
- 728.Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32:400–5. doi: 10.1097/01.olq.0000154508.26532.6a. [DOI] [PubMed] [Google Scholar]
- 729.Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929–37. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 730.Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30:1114–7. doi: 10.1016/j.ajem.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 731.Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162:585–90. doi: 10.1093/aje/kwi243. [DOI] [PubMed] [Google Scholar]
- 732.Jurstrand M, Jensen JS, Magnuson A, et al. A serological study of the role of Mycoplasma genitalium in pelvic inflammatory disease and ectopic pregnancy. Sex Transm Infect. 2007;83:319–23. doi: 10.1136/sti.2007.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Oakeshott P, Aghaizu A, Hay P, et al. Is Mycoplasma genitalium in women the “New Chlamydia?” A community-based prospective cohort study. Clin Infect Dis. 2010;51:1160–6. doi: 10.1086/656739. [DOI] [PubMed] [Google Scholar]
- 734.Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104:761–9. doi: 10.1097/01.AOG.0000139512.37582.17. [DOI] [PubMed] [Google Scholar]
- 735.Peipert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol. 2001;184:856–64. doi: 10.1067/mob.2001.113847. [DOI] [PubMed] [Google Scholar]
- 736.Gaitan H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002;10:171–80. doi: 10.1155/S1064744902000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Jacobson L, Westrom L. Objectivized diagnosis of acute pelvic inflammatory disease. Diagnostic and prognostic value of routine laparoscopy. Am J Obstet Gynecol. 1969;105:1088–98. doi: 10.1016/0002-9378(69)90132-x. [DOI] [PubMed] [Google Scholar]
- 738.Sellors J, Mahony J, Goldsmith C, et al. The accuracy of clinical findings and laparoscopy in pelvic inflammatory disease. Am J Obstet Gynecol. 1991;164(1 Pt 1):113–20. doi: 10.1016/0002-9378(91)90639-9. [DOI] [PubMed] [Google Scholar]
- 739.Bevan CD, Johal BJ, Mumtaz G, et al. Clinical, laparoscopic and microbiological findings in acute salpingitis: report on a United Kingdom cohort. Br J Obstet Gynaecol. 1995;102:407–14. doi: 10.1111/j.1471-0528.1995.tb11294.x. [DOI] [PubMed] [Google Scholar]
- 740.Wiesenfeld HC, Hillier SL, Meyn LA, et al. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol. 2012;120:37–43. doi: 10.1097/AOG.0b013e31825a6bc9. [DOI] [PubMed] [Google Scholar]
- 741.Sweet RL. Treatment of acute pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2011;2011:561909. doi: 10.1155/2011/561909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Smith KJ, Ness RB, Wiesenfeld HC, et al. Cost-effectiveness of alternative outpatient pelvic inflammatory disease treatment strategies. Sex Transm Dis. 2007;34:960–6. [PubMed] [Google Scholar]
- 743.Haggerty CL, Ness RB, Amortegui A, et al. Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Am J Obstet Gynecol. 2003;188:141–8. doi: 10.1067/mob.2003.87. [DOI] [PubMed] [Google Scholar]
- 744.McGregor JA, Crombleholme WR, Newton E, et al. Randomized comparison of ampicillin-sulbactam to cefoxitin and doxycycline or clindamycin and gentamicin in the treatment of pelvic inflammatory disease or endometritis. Obstet Gynecol. 1994;83:998–1004. doi: 10.1097/00006250-199406000-00020. [DOI] [PubMed] [Google Scholar]
- 745.Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. J Int Med Res. 2003;31:45–54. doi: 10.1177/147323000303100108. [DOI] [PubMed] [Google Scholar]
- 746.Savaris RF, Teixeira LM, Torres TG, et al. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol. 2007;110:53–60. doi: 10.1097/01.AOG.0000268801.90261.27. [DOI] [PubMed] [Google Scholar]
- 747.Heystek M, Ross JD. A randomized double-blind comparison of moxifloxacin and doxycycline/metronidazole/ciprofloxacin in the treatment of acute, uncomplicated pelvic inflammatory disease. Int J STD AIDS. 2009;20:690–5. doi: 10.1258/ijsa.2008.008495. [DOI] [PubMed] [Google Scholar]
- 748.Boothby M, Page J, Pryor R, et al. A comparison of treatment outcomes for moxifloxacin versus ofloxacin/metronidazole for first-line treatment of uncomplicated non-gonococcal pelvic inflammatory disease. Int J STD AIDS. 2010;21:195–7. doi: 10.1258/ijsa.2009.009374. [DOI] [PubMed] [Google Scholar]
- 749.Judlin P, Liao Q, Liu Z, et al. Efficacy and safety of moxifloxacin in uncomplicated pelvic inflammatory disease: the MONALISA study. Br J Obstet Gynaecol. 2010;117:1475–84. doi: 10.1111/j.1471-0528.2010.02687.x. [DOI] [PubMed] [Google Scholar]
- 750.Bukusi EA, Cohen CR, Stevens CE, et al. Effects of human immunodeficiency virus 1 infection on microbial origins of pelvic inflammatory disease and on efficacy of ambulatory oral therapy. Am J Obstet Gynecol. 1999;181:1374–81. doi: 10.1016/s0002-9378(99)70378-9. [DOI] [PubMed] [Google Scholar]
- 751.Mugo NR, Kiehlbauch JA, Nguti R, et al. Effect of human immunodeficiency virus-1 infection on treatment outcome of acute salpingitis. Obstet Gynecol. 2006;107:807–12. doi: 10.1097/01.AOG.0000207597.70524.e8. [DOI] [PubMed] [Google Scholar]
- 752.Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet. 2000;356:1013–9. doi: 10.1016/S0140-6736(00)02699-4. [DOI] [PubMed] [Google Scholar]
- 753.Viberga I, Odlind V, Lazdane G, et al. Microbiology profile in women with pelvic inflammatory disease in relation to IUD use. Infect Dis Obstet Gynecol. 2005;13:183–90. doi: 10.1080/10647440500097601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Tepper NK, Steenland MW, Gaffield ME, et al. Retention of intrauterine devices in women who acquire pelvic inflammatory disease: a systematic review. Contraception. 2013;87:655–60. doi: 10.1016/j.contraception.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 755.Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35:101–8. doi: 10.1016/j.ucl.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 756.Tracy CR, Costabile RA. The evaluation and treatment of acute epididymitis in a large university based population: are CDC guidelines being followed? World J Urol. 2009;27:259–63. doi: 10.1007/s00345-008-0338-0. [DOI] [PubMed] [Google Scholar]
- 757.Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79:583–7. [PubMed] [Google Scholar]
- 758.de Villiers EM, Fauquet C, Broker TR, et al. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 759.Myers ER, McCrory DC, Nanda K, et al. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151:1158–71. doi: 10.1093/oxfordjournals.aje.a010166. [DOI] [PubMed] [Google Scholar]
- 760.Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses. Lancet Oncology. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 761.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Patel H, Wagner M, Singhal P, et al. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13:39. doi: 10.1186/1471-2334-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Chesson HW, Ekwueme DU, Saraiya M, et al. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30:6016–9. doi: 10.1016/j.vaccine.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.CDC. Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013—United States. MMWR Morbid Mortal Wkly Rep. 2013;62:591–5. [PMC free article] [PubMed] [Google Scholar]
- 765.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–93. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 766.Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health. 2013;103:1428–35. doi: 10.2105/AJPH.2012.301182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 768.Gotovtseva EP, Kapadia AS, Smolensky MH, et al. Optimal frequency of imiquimod (aldara) 5% cream for the treatment of external genital warts in immunocompetent adults: a meta-analysis. Sex Transm Dis. 2008;35:346–51. doi: 10.1097/OLQ.0b013e31815ea8d1. [DOI] [PubMed] [Google Scholar]
- 769.Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies. Infect Dis Obstet Gynecol. 2011;2011:806105. doi: 10.1155/2011/806105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2008;33:74–6. doi: 10.1111/j.1365-2230.2007.02520.x. [DOI] [PubMed] [Google Scholar]
- 771.Domingues E, Chaney KC, Scharf MJ, et al. Imiquimod reactivation of lichen planus. Cutis. 2012;89:276–7. , 83. [PubMed] [Google Scholar]
- 772.Patel U, Mark NM, Machler BC, et al. Imiquimod 5% cream induced psoriasis: a case report, summary of the literature and mechanism. Br J Dermatol. 2011;164:670–2. doi: 10.1111/j.1365-2133.2010.10124.x. [DOI] [PubMed] [Google Scholar]
- 773.Kumar B, Narang T. Local and systemic adverse effects to topical imiquimod due to systemic immune stimulation. Sex Transm Infect. 2011;87:432. doi: 10.1136/sextrans-2011-050025. [DOI] [PubMed] [Google Scholar]
- 774.Tatti S, Swinehart JM, Thielert C, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet Gynecol. 2008;111:1371–9. doi: 10.1097/AOG.0b013e3181719b60. [DOI] [PubMed] [Google Scholar]
- 775.Stockfleth E, Beti H, Orasan R, et al. Topical Polyphenon E in the treatment of external genital and perianal warts: a randomized controlled trial. Br J Dermatol. 2008;158:1329–38. doi: 10.1111/j.1365-2133.2008.08520.x. [DOI] [PubMed] [Google Scholar]
- 776.Gross G, Meyer KG, Pres H, et al. A randomized, double-blind, four-arm parallel-group, placebo-controlled Phase II/III study to investigate the clinical efficacy of two galenic formulations of Polyphenon E in the treatment of external genital warts. J Eur Acad Dermatol Venereol. 2007;21:1404–12. doi: 10.1111/j.1468-3083.2007.02441.x. [DOI] [PubMed] [Google Scholar]
- 777.CDC. DHHS, editor. (NIOSH Publication Number 96–128).Control of smoke from laser/electric surgical procedures. 2013 Available at http://www.cdc.gov/niosh/docs/hazardcontrol/hc11.html.
- 778.Filley CM, Graff-Richard NR, Lacy JR, et al. Neurologic manifestations of podophyllin toxicity. Neurology. 1982;32:308–11. doi: 10.1212/wnl.32.3.308. [DOI] [PubMed] [Google Scholar]
- 779.Conard PF, Hanna N, Rosenblum M, et al. Delayed recognition of podophyllum toxicity in a patient receiving epidural morphine. Anesth Analg. 1990;71:191–3. doi: 10.1213/00000539-199008000-00013. [DOI] [PubMed] [Google Scholar]
- 780.Karol MD, Conner CS, Watanabe AS, et al. Podophyllum: suspected teratogenicity from topical application. Clin Toxicol. 1980;16:283–6. doi: 10.3109/15563658008989950. [DOI] [PubMed] [Google Scholar]
- 781.Silverberg MJ, Thorsen P, Lindeberg H, et al. Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet Gynecol. 2003;101:645–52. doi: 10.1016/s0029-7844(02)03081-8. [DOI] [PubMed] [Google Scholar]
- 782.Dolev JC, Maurer T, Springer G, et al. Incidence and risk factors for verrucae in women. AIDS. 2008;22:1213–9. doi: 10.1097/QAD.0b013e3283021aa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Silverberg MJ, Ahdieh L, Munoz A, et al. The impact of HIV infection and immunodeficiency on human papillomavirus type 6 or 11 infection and on genital warts. Sex Transm Dis. 2002;29:427–35. doi: 10.1097/00007435-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 784.De Panfilis G, Melzani G, Mori G, et al. Relapses after treatment of external genital warts are more frequent in HIV-positive patients than in HIV-negative controls. Sex Transm Dis. 2002;29:121–5. doi: 10.1097/00007435-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 785.Conley LJ, Ellerbrock TV, Bush TJ, et al. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminata and intraepithelial neoplasia: a prospective cohort study. Lancet. 2002;359:108–13. doi: 10.1016/S0140-6736(02)07368-3. [DOI] [PubMed] [Google Scholar]
- 786.Schlecht HP, Fugelso DK, Murphy RK, et al. Frequency of occult high-grade squamous intraepithelial neoplasia and invasive cancer within anal condylomata in men who have sex with men. Clin Infect Dis. 2010;51:107–10. doi: 10.1086/653426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Maniar KP, Ronnett BM, Vang R, et al. Coexisting high-grade vulvar intraepithelial neoplasia (VIN) and condyloma acuminatum: independent lesions due to different HPV types occurring in immunocompromised patients. Am J Surg Pathol. 2013;37:53–60. doi: 10.1097/PAS.0b013e318263cda6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Massad LS, Xie X, Darragh T, et al. Genital warts and vulvar intraepithelial neoplasia: natural history and effects of treatment and human immunodeficiency virus infection. Obstet Gynecol. 2011;118:831–9. doi: 10.1097/AOG.0b013e31821a0f4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 790.Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266–97. doi: 10.5858/arpa.LGT200570. [DOI] [PubMed] [Google Scholar]
- 791.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 792.Ghanem KG, Koumans EH, Johnson RE, et al. Effect of specimen order on Chlamydia trachomatis and Neisseria gonorrhoeae test performance and adequacy of Papanicolaou smear. J Pediatr Adolesc Gynecol. 2006;19:23–30. doi: 10.1016/j.jpag.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 793.Saraiya M, Lee NC, Blackman D, et al. Self-reported Papanicolaou smears and hysterectomies among women in the United States. Obstet Gynecol. 2001;98:269–78. doi: 10.1016/s0029-7844(01)01447-8. [DOI] [PubMed] [Google Scholar]
- 794.Sirovich BE, Welch HG. Cervical cancer screening among women without a cervix. JAMA. 2004;291:2990–3. doi: 10.1001/jama.291.24.2990. [DOI] [PubMed] [Google Scholar]
- 795.Stokes-Lampard H, Wilson S, Waddell C, et al. Vaginal vault smears after hysterectomy for reasons other than malignancy: a systematic review of the literature. Br J Obstet Gynaecol. 2006;113:1354–65. doi: 10.1111/j.1471-0528.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 796.Arbyn M, Herbert A, Schenck U, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for collecting samples for conventional and liquid-based cytology. Cytopathology. 2007;18:133–9. doi: 10.1111/j.1365-2303.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 797.Daley E, Perrin K, Vamos C, et al. Confusion about Pap smears: lack of knowledge among high-risk women. J Womens Health. 2013;22:67–74. doi: 10.1089/jwh.2012.3667. [DOI] [PubMed] [Google Scholar]
- 798.Drolet M, Brisson M, Maunsell E, et al. The psychosocial impact of an abnormal cervical smear result. Psychooncology. 2012;21:1071–81. doi: 10.1002/pon.2003. [DOI] [PubMed] [Google Scholar]
- 799.Ogbechie OA, Hacker MR, Dodge LE, et al. Confusion regarding cervical cancer screening and chlamydia screening among sexually active young women. Sex Transm Infect. 2012;88:35–7. doi: 10.1136/sextrans-2011-050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Dunne EF, Friedman A, Datta SD, et al. Updates on human papillomavirus and genital warts and counseling messages from the 2010 Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2011;53(Suppl 3):S143–52. doi: 10.1093/cid/cir703. [DOI] [PubMed] [Google Scholar]
- 801.Fry AM, Ferries-Rowe EA, Learman LA, et al. Pap smear versus speculum examination: can we teach providers to educate patients? J Womens Health. 2010;19:1715–9. doi: 10.1089/jwh.2009.1862. [DOI] [PubMed] [Google Scholar]
- 802.Adab P, Marshall T, Rouse A, et al. Randomised controlled trial of the effect of evidence based information on women’s willingness to participate in cervical cancer screening. J Epidemiol Community Health. 2003;57:589–93. doi: 10.1136/jech.57.8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Han HR, Kim J, Lee JE, et al. Interventions that increase use of Pap tests among ethnic minority women: a meta-analysis. Psychooncology. 2011;20:341–51. doi: 10.1002/pon.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 804.Dyson S, Pitts M, Lyons A, et al. Providing high quality information about human papillomavirus for women after treatment for high-grade cervical dysplasia. Sex Health. 2010;7:49–54. doi: 10.1071/SH09059. [DOI] [PubMed] [Google Scholar]
- 805.McCaffery KJ, Irwig L, Turner R, et al. Psychosocial outcomes of three triage methods for the management of borderline abnormal cervical smears: an open randomised trial. BMJ. 2010;340:b4491. doi: 10.1136/bmj.b4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Daley EM, Perrin KM, McDermott RJ, et al. The psychosocial burden of HPV: a mixed-method study of knowledge, attitudes and behaviors among HPV+ women. J Health Psychol. 2010;15:279–90. doi: 10.1177/1359105309351249. [DOI] [PubMed] [Google Scholar]
- 807.Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect. 2009;85:508–13. doi: 10.1136/sti.2009.037028. [DOI] [PubMed] [Google Scholar]
- 808.Sadler L, Saftlas A, Wang W, et al. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100–6. doi: 10.1001/jama.291.17.2100. [DOI] [PubMed] [Google Scholar]
- 809.Serati M, Uccella S, Laterza RM, et al. Natural history of cervical intraepithelial neoplasia during pregnancy. Acta Obstet Gynecol Scand. 2008;87:1296–300. doi: 10.1080/00016340802482986. [DOI] [PubMed] [Google Scholar]
- 810.Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 811.Stillson T, Knight AL, Elswick RK., Jr The effectiveness and safety of two cervical cytologic techniques during pregnancy. J Fam Pract. 1997;45:159–63. [PubMed] [Google Scholar]
- 812.Foster JC, Smith HL. Use of the cytobrush for Papanicolaou smear screens in pregnant women. J Nurse Midwifery. 1996;41:211–7. doi: 10.1016/0091-2182(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 813.Paraiso MF, Brady K, Helmchen R, et al. Evaluation of the endocervical cytobrush and cervex-brush in pregnant women. Obstet Gynecol. 1994;84:539–43. [PubMed] [Google Scholar]
- 814.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–10. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 815.Chaturvedi AK, Madeleine MM, Biggar RJ, et al. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Widdice LE, Moscicki AB. Updated guidelines for papanicolaou tests, colposcopy, and human papillomavirus testing in adolescents. J Adolesc Health. 2008;43(4 Suppl):S41–51. doi: 10.1016/j.jadohealth.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 818.Salit IE, Lytwyn A, Raboud J, et al. The role of cytology (Pap tests) and human papillomavirus testing in anal cancer screening. AIDS. 2010;24:1307–13. doi: 10.1097/QAD.0b013e328339e592. [DOI] [PubMed] [Google Scholar]
- 819.Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985;313:1059–67. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- 820.CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morbid Mortal Wkly Rep. 2007;56 [PubMed] [Google Scholar]
- 821.Bell BP, Shapiro CN, Alter MJ, et al. The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies. J Infect Dis. 1998;178:1579–84. doi: 10.1086/314518. [DOI] [PubMed] [Google Scholar]
- 822.Bower WA, Nainan OV, Han X, et al. Duration of viremia in hepatitis A virus infection. J Infect Dis. 2000;182:12–7. doi: 10.1086/315701. [DOI] [PubMed] [Google Scholar]
- 823.CDC. Update to: CDC viral hepatitis surveillance, United States 2010. Available at http://www.cdc.gov/hepatitis/Statistics/2010Surveillance/PDFs/2010HepSurveillanceRpt.pdf.
- 824.Sharapov UM, Bulkow LR, Negus SE, et al. Persistence of hepatitis A vaccine induced seropositivity in infants and young children by maternal antibody status: 10-year follow-up. Hepatology. 2012;56:516–22. doi: 10.1002/hep.25687. [DOI] [PubMed] [Google Scholar]
- 825.Ott JJ, Irving G, Wiersma ST. Long-term protective effects of hepatitis A vaccines: a systematic review. Vaccine. 2012;31:3–11. doi: 10.1016/j.vaccine.2012.04.104. [DOI] [PubMed] [Google Scholar]
- 826.Raczniak GA, Bulkow LR, Bruce MG, et al. Long-term immunogenicity of hepatitis A virus vaccine in Alaska 17 years after initial childhood series. J Infect Dis. 2013;207:493–6. doi: 10.1093/infdis/jis710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Winokur PL, Stapleton JT. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis. 1992;14:580–6. doi: 10.1093/clinids/14.2.580. [DOI] [PubMed] [Google Scholar]
- 828.Klevens RM, Kruszon-Moran D, Wasley A, et al. Seroprevalence of hepatitis A virus antibodies in the U.S.: results from the National Health and Nutrition Examination Survey. Public Health Rep. 2011;126:522–32. doi: 10.1177/003335491112600408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.Alter HJ, Purcell RH, Gerin JL, et al. Transmission of hepatitis B to chimpanzees by hepatitis B surface antigen-positive saliva and semen. Infect Immun. 1977;16:928–33. doi: 10.1128/iai.16.3.928-933.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Villarejos VM, Visona KA, Gutierrez A, et al. Role of saliva, urine and feces in the transmission of type B hepatitis. N Engl J Med. 1974;291:1375–8. doi: 10.1056/NEJM197412262912602. [DOI] [PubMed] [Google Scholar]
- 831.Busch K, Thimme R. Natural history of chronic hepatitis B virus infection. Med Microbiol Immunol. 2014 doi: 10.1007/s00430-014-0369-7. [DOI] [PubMed] [Google Scholar]
- 832.Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000. doi: 10.1093/clinids/20.4.992. [DOI] [PubMed] [Google Scholar]
- 833.Goldstein ST, Zhou F, Hadler SC, et al. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 834.Davis LG, Weber DJ, Lemon SM. Horizontal transmission of hepatitis B virus. Lancet. 1989;1:889–93. doi: 10.1016/s0140-6736(89)92876-6. [DOI] [PubMed] [Google Scholar]
- 835.Martinson FE, Weigle KA, Royce RA, et al. Risk factors for horizontal transmission of hepatitis B virus in a rural district in Ghana. Am J Epidemiol. 1998;147:478–87. doi: 10.1093/oxfordjournals.aje.a009474. [DOI] [PubMed] [Google Scholar]
- 836.CDC. Healthcare-associated hepatitis B and C outbreaks reported to the Centers for Disease Control and Prevention (CDC) in 2008–2013. CDC; c2010. Available at http://www.cdc.gov/hepatitis/Outbreaks/HealthcareHepOutbreakTable.htm. [Google Scholar]
- 837.Spradling PR, Xing J, Williams R, et al. Immunity to hepatitis B virus (HBV) infection two decades after implementation of universal infant HBV vaccination: association of detectable residual antibodies and response to a single HBV challenge dose. Clin Vaccine Immunol. 2013;20:559–61. doi: 10.1128/CVI.00694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Lu PJ, Byrd KK, Murphy TV, et al. Hepatitis B vaccination coverage among high-risk adults 18–49 years, U.S. 2009. Vaccine. 2011;29:7049–57. doi: 10.1016/j.vaccine.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 839.MacKellar DA, Valleroy LA, Secura GM, et al. Two decades after vaccine license: Hepatitis B immunization and infection among young men who have sex with men. Am J Public Health. 2001;91:965–71. doi: 10.2105/ajph.91.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.CDC. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50 (No. RR-11) [PubMed] [Google Scholar]
- 841.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 842.Mendy M, Peterson I, Hossin S, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One. 2013;8:e58029. doi: 10.1371/journal.pone.0058029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843.Poovorawan Y, Chongsrisawat V, Theamboonlers A, et al. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Human Vaccines and Immunotherapeutics. 2012;8:896–904. doi: 10.4161/hv.19989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.CDC. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep. 2013;62 (No. RR-10) [PubMed] [Google Scholar]
- 845.Klausner JD, Kohn R, Kent C. Etiology of clinical proctitis among men who have sex with men. Clin Infect Dis. 2004;38:300–2. doi: 10.1086/380838. [DOI] [PubMed] [Google Scholar]
- 846.Rompalo AM. Diagnosis and treatment of sexually acquired proctitis and proctocolitis: an update. Clin Infect Dis. 1999;28(Suppl 1):S84–90. doi: 10.1086/514721. [DOI] [PubMed] [Google Scholar]
- 847.McLean CA, Stoner BP, Workowski KA. Treatment of lymphogranuloma venereum. Clin Infect Dis. 2007;44(Suppl 3):S147–52. doi: 10.1086/511427. [DOI] [PubMed] [Google Scholar]
- 848.Van der Bij AK, Spaargaren J, Morre SA, et al. Diagnostic and clinical implications of anorectal lymphogranuloma venereum in men who have sex with men: a retrospective case-control study. Clin Infect Dis. 2006;42:186–94. doi: 10.1086/498904. [DOI] [PubMed] [Google Scholar]
- 849.Galiczynski EM, Jr, Elston DM. What’s eating you? Pubic lice (Pthirus pubis) Cutis. 2008;81:109–14. [PubMed] [Google Scholar]
- 850.Meinking TL, Serrano L, Hard B, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220–4. doi: 10.1001/archderm.138.2.220. [DOI] [PubMed] [Google Scholar]
- 851.Yoon KS, Gao JR, Lee SH, et al. Permethrin-resistant human head lice, Pediculus capitis, and their treatment. Arch Dermatol. 2003;139:994–1000. doi: 10.1001/archderm.139.8.994. [DOI] [PubMed] [Google Scholar]
- 852.Burkhart CG, Burkhart CN. Oral ivermectin for Phthirus pubis. J Am Acad Dermatol. 2004;51:1037–8. doi: 10.1016/j.jaad.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 853.Scott GR, Chosidow O. European guideline for the management of pediculosis pubis, 2010. Int J STD AIDS. 2011;22:304–5. doi: 10.1258/ijsa.2011.011114. [DOI] [PubMed] [Google Scholar]
- 854.Goldust M, Rezaee E, Raghifar R, et al. Comparing the efficacy of oral ivermectin vs malathion 0.5% lotion for the treatment of scabies. SkinMed. 2014;12:284–7. [PubMed] [Google Scholar]
- 855.Nolan K, Kamrath J, Levitt J. Lindane toxicity: a comprehensive review of the medical literature. Pediatr Dermatol. 2012;29:141–6. doi: 10.1111/j.1525-1470.2011.01519.x. [DOI] [PubMed] [Google Scholar]
- 856.Shimose L, Munoz-Price LS. Diagnosis, prevention, and treatment of scabies. Curr Infect Dis Rep. 2013;15:426–31. doi: 10.1007/s11908-013-0354-0. [DOI] [PubMed] [Google Scholar]
- 857.Monsel G, Chosidow O. Management of scabies. Skin Therapy Lett. 2012;17:1–4. [PubMed] [Google Scholar]
- 858.Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717–25. doi: 10.1056/NEJMct0910329. [DOI] [PubMed] [Google Scholar]
- 859.Barkwell R, Shields S. Deaths associated with ivermectin treatment of scabies. Lancet. 1997;349:1144–5. doi: 10.1016/S0140-6736(05)63020-6. [DOI] [PubMed] [Google Scholar]
- 860.Mounsey KE, Holt DC, McCarthy J, et al. Scabies: molecular perspectives and therapeutic implications in the face of emerging drug resistance. Future Microbiol. 2008;3:57–66. doi: 10.2217/17460913.3.1.57. [DOI] [PubMed] [Google Scholar]
- 861.Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840–1. doi: 10.1001/archdermatol.2009.125. [DOI] [PubMed] [Google Scholar]
- 862.Mounsey KE, McCarthy JS, Walton SF. Scratching the itch: new tools to advance understanding of scabies. Trends Parasitol. 2013;29:35–42. doi: 10.1016/j.pt.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 863.Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375–81. doi: 10.1016/j.jinf.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 864.Ortega-Loayza AG, McCall CO, Nunley JR. Crusted scabies and multiple dosages of ivermectin. Journal of Drugs in Dermatology. 2013;12:584–5. [PubMed] [Google Scholar]
- 865.Bouvresse S, Chosidow O. Scabies in healthcare settings. Curr Opin Infect Dis. 2010;23:111–8. doi: 10.1097/QCO.0b013e328336821b. [DOI] [PubMed] [Google Scholar]
- 866.Ackerman DR, Sugar NF, Fine DN, et al. Sexual assault victims: factors associated with follow-up care. Am J Obstet Gynecol. 2006;194:1653–9. doi: 10.1016/j.ajog.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 867.Parekh V, Brown CB. Follow up of patients who have been recently sexually assaulted. Sex Transm Infect. 2003;79:349. doi: 10.1136/sti.79.4.349-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Unger ER, Fajman NN, Maloney EM, et al. Anogenital human papillomavirus in sexually abused and nonabused children: a multicenter study. Pediatrics. 2011;128:e658–65. doi: 10.1542/peds.2010-2247. [DOI] [PubMed] [Google Scholar]
- 869.Kreimer AR, Rodriguez AC, Hildesheim A, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.Claydon E, Murphy S, Osborne EM, et al. Rape and HIV. Int J STD AIDS. 1991;2:200–1. doi: 10.1177/095646249100200310. [DOI] [PubMed] [Google Scholar]
- 871.Murphy S, Kitchen V, Harris JR, et al. Rape and subsequent seroconversion to HIV. BMJ. 1989;299:718. doi: 10.1136/bmj.299.6701.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.Varghese B, Maher JE, Peterman TA, et al. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29:38–43. doi: 10.1097/00007435-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 873.Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. N Engl J Med. 1997;337:1485–90. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 874.Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875–92. doi: 10.1086/672271. [DOI] [PubMed] [Google Scholar]
- 875.Du Mont J, Myhr TL, Husson H, et al. HIV postexposure prophylaxis use among Ontario female adolescent sexual assault victims: a prospective analysis. Sex Transm Dis. 2008;35:973–8. doi: 10.1097/OLQ.0b013e3181824f3c. [DOI] [PubMed] [Google Scholar]
- 876.Neu N, Heffernan-Vacca S, Millery M, et al. Postexposure prophylaxis for HIV in children and adolescents after sexual assault: a prospective observational study in an urban medical center. Sex Transm Dis. 2007;34:65–8. doi: 10.1097/01.olq.0000225329.07765.d8. [DOI] [PubMed] [Google Scholar]
- 877.Loutfy MR, Macdonald S, Myhr T, et al. Prospective cohort study of HIV post-exposure prophylaxis for sexual assault survivors. Antivir Ther. 2008;13:87–95. doi: 10.1177/135965350801300109. [DOI] [PubMed] [Google Scholar]
- 878.Jenny C, Crawford-Jakubiak JE, Committee on Child Abuse and Neglect et al. The evaluation of children in the primary care setting when sexual abuse is suspected. Pediatrics. 2013;132:e558–67. doi: 10.1542/peds.2013-1741. [DOI] [PubMed] [Google Scholar]
- 879.Girardet RG, Lahoti S, Howard LA, et al. Epidemiology of sexually transmitted infections in suspected child victims of sexual assault. Pediatrics. 2009;124:79–86. doi: 10.1542/peds.2008-2947. [DOI] [PubMed] [Google Scholar]
- 880.Black CM, Driebe EM, Howard LA, et al. Multicenter study of nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in children being evaluated for sexual abuse. Pediatr Infect Dis J. 2009;28:608–13. doi: 10.1097/INF.0b013e31819b592e. [DOI] [PubMed] [Google Scholar]
- 881.Schwandt A, Williams C, Beigi RH. Perinatal transmission of Trichomonas vaginalis: a case report. J Reprod Med. 2008;53:59–61. [PubMed] [Google Scholar]
- 882.Bell TA, Stamm WE, Wang SP, et al. Chronic Chlamydia trachomatis infections in infants. JAMA. 1992;267:400–2. [PubMed] [Google Scholar]
- 883.Schachter J, Grossman M, Sweet RL, et al. Prospective study of perinatal transmission of Chlamydia trachomatis. JAMA. 1986;255:3374–7. [PubMed] [Google Scholar]
- 884.Jones V, Smith SJ, Omar HA. Nonsexual transmission of anogenital warts in children: a retrospective analysis. The Scientific World Journal. 2007;7:1896–9. doi: 10.1100/tsw.2007.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Smith EM, Swarnavel S, Ritchie JM, et al. Prevalence of human papillomavirus in the oral cavity/oropharynx in a large population of children and adolescents. Pediatr Infect Dis J. 2007;26:836–40. doi: 10.1097/INF.0b013e318124a4ae. [DOI] [PubMed] [Google Scholar]
- 886.Gavril AR, Kellogg ND, Nair P. Value of follow-up examinations of children and adolescents evaluated for sexual abuse and assault. Pediatrics. 2012;129:282–9. doi: 10.1542/peds.2011-0804. [DOI] [PubMed] [Google Scholar]
- 887.Bandea CI, Joseph K, Secor EW, et al. Development of PCR assays for detection of Trichomonas vaginalis in urine specimens. J Clin Microbiol. 2013;51:1298–300. doi: 10.1128/JCM.03101-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Gallion HR, Dupree LJ, Scott TA, et al. Diagnosis of Trichomonas vaginalis in female children and adolescents evaluated for possible sexual abuse: a comparison of the InPouch TV culture method and wet mount microscopy. J Pediatr Adolesc Gynecol. 2009;22:300–5. doi: 10.1016/j.jpag.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 889.Lalor K, McElvaney R. Child sexual abuse, links to later sexual exploitation/high-risk sexual behavior, and prevention/treatment programs. Trauma Violence and Abuse. 2010;11:159–77. doi: 10.1177/1524838010378299. [DOI] [PubMed] [Google Scholar]
- 890.Girardet RG, Lemme S, Biason TA, et al. HIV post-exposure prophylaxis in children and adolescents presenting for reported sexual assault. Child Abuse Negl. 2009;33:173–8. doi: 10.1016/j.chiabu.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 891.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 892.Havens PL, American Academy of Pediatrics Committee on Pediatric AIDS Postexposure prophylaxis in children and adolescents for nonoccupational exposure to human immunodeficiency virus. Pediatrics. 2003;111(6 Pt 1):1475–89. doi: 10.1542/peds.111.6.1475. [DOI] [PubMed] [Google Scholar]


