Abstract
Elementary processes of photoperception by phytochrome A (PhyA) for the high-irradiance response (HIR) of hypocotyl elongation in Arabidopsis were examined using a newly designed irradiator with LED. The effect of continuous irradiation with far-red (FR) light could be replaced by intermittent irradiation with FR light pulses if given at intervals of 3 min or less for 24 h. In this response, the Bunsen-Roscoe law of reciprocity held in each FR light pulse. Therefore, we determined the action spectrum for the response by intermittent irradiation using phyB and phyAphyB double mutants. The resultant action spectrum correlated well with the absorption spectrum of PhyA in far-red-absorbing phytochrome (Pfr). Intermittent irradiation with 550 to 667 nm of light alone had no significant effect on the response. In contrast, intermittent irradiation with red light immediately after each FR light pulse completely reversed the effect of FR light in each cycle. The results indicate that neither red-absorbing phytochrome synthesized in darkness nor photoconverted Pfr are physiologically active, and that a short-lived signal is induced during photoconversion from Pfr to red-absorbing phytochrome. The mode of photoperception by PhyA for HIR is essentially different from that by PhyA for very-low-fluence responses and phytochrome B for low-fluence responses.
Light controls diverse processes of plant growth and development (Mohr and Shropshire, 1983). Early physiological and photochemical studies indicated that light responses in photomorphogenesis are classified as a low- or high-energy reactions according to their energy requirements (Mohr, 1962). Later, low-energy reactions induced by short pulses of irradiation with relatively small doses of light, were divided into low-fluence responses (LFRs, which early papers cited as low-irradiance responses) and a very-low-fluence responses (VLFRs, which early papers cited as very-low-irradiance responses) (Blaauw et al., 1968; Mandoli and Briggs, 1981). The high-energy reaction, which required a radiation with relatively high energy for a relatively long period of time, was renamed a high-irradiance response (HIR) (Briggs et al., 1984).
Phytochrome was first discovered as the photoreceptor for reversibility by red (R) and far-red (FR) light (Butler et al., 1959), which was only observed in LFRs. Recent studies using phytochrome-deficient mutants demonstrated that phytochromes are photoreceptors for VLFR (Shinomura et al., 1996) and HIR (Quail et al., 1995), although R/FR light reversibility was not observed in either response.
HIR has been examined extensively in photoinhibition of stem elongation and in anthocyanin production. Each of these responses requires prolonged light irradiation treatment and depends on fluence rate (Mohr, 1972; Smith et al., 1991). Action spectra for responses to the prolonged irradiation were determined as a way to define the spectral characteristics of putative photoreceptor(s), but they showed complicated features; action maxima were found in the UV-B, UV-A, blue light, R light, and FR light regions, and the action spectra varied depending on the plant species, developmental stage, and growth conditions before the spectral light treatment (Beggs et al., 1980; Goto et al., 1993; Mancinelli, 1994). HIRs showing a peak in the FR light region (Mohr, 1962) were called FR light-HIR, and the involvement of phytochrome in the mediation of the FR light-HIR was speculated based upon experimental results using prolonged dichromatic irradiation with continuous R and FR light (Hartmann, 1966).
The discovery of type I and II phytochromes showing different stability of far-red-absorbing phytochrome (Pfr) and molecular properties (Furuya, 1989) and of the phytochrome multigene family (Sharrock and Quail, 1989; Clack et al., 1994) opened new approaches to address which member of the phytochrome family is responsible for which phytochrome-mediated response(s) (Furuya, 1993). Among them, phytochrome A (PhyA) and phytochrome B (PhyB) were assigned to mediate HIR of photoinhibition of hypocotyl elongation in Arabidopsis (Quail et al., 1995). The response induced by continuous R light was mediated predominantly, if not exclusively, by PhyB (Nagatani et al., 1991; Reed et al., 1993). Conversely, the responses induced by continuous FR light were mediated predominantly by PhyA (Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993). Given that VLFRs of seed germination (Shinomura et al., 1994, 1996) and Cab gene expression (Hamazato et al., 1997) of Arabidopsis are induced by a brief light irradiation mediated by PhyA, the question arises whether the elementary process of photoperception by PhyA for induction of HIR is the same as that for the induction of PhyA-mediated VLFR.
For several HIRs, including fern spore germination (Sugai et al., 1977) and hypocotyl elongation in dicots (Mohr, 1957; Hillman and Purves, 1966), the physiological effects of continuous R light could be replaced by intermittent irradiation with R light pulses, and the effect was reversed by pulses of FR light after each R light pulse. The PhyB-mediated HIR of inhibition of hypocotyl elongation of Arabidopsis was also examined by 4-h intermittent instead of continuous R irradiation (McCormac et al., 1993). Using this light treatment, the R/FR-light-reversible regulation and the validity of the Bunsen-Roscoe law of reciprocity were shown at each pulse of R light (McCormac et al., 1993). In the response obeying the Bunsen-Roscoe law of reciprocity, the extent of the response is proportional to the photon fluence, irrespective of whether that photon fluence is produced by short irradiation with high photon fluence rate or longer irradiation with low photon fluence rate (Schäfer et al., 1983). The validity of the Bunsen-Roscoe law of reciprocity in PhyB-mediated HIR and LFR means that just one photoreceptor contributes to a primary reaction of each response as a rate limiting factor. Therefore, the elementary process of the photoperception by PhyB for HIR (McCormac et al., 1993) appears to be consistent with that by PhyB for LFR (Shinomura et al., 1996).
In contrast, the PhyA-mediated inhibition of hypocotyl elongation in Arabidopsis induced by continuous FR light was not induced by either FR light pulses at 4-h intervals (McCormac et al., 1993) or FR light pulses at 1-h intervals (Ahmad and Cashmore, 1997; Casal et al., 1998). Therefore, it was not known whether the PhyA action for this response is similar to PhyA-mediated VLFR, which is characterized by the required range of photon fluence, the effective range of wavelength, the validity of the Bunsen-Roscoe law of reciprocity, and the failure of R/FR light reversibility.
To solve this problem, we designed and built a custom-made irradiation system based on light-emitting diodes (LEDs) that allows us to control the intermittent irradiation with R and FR light pulses for durations of milliseconds to hours with any desired dark intervals. We describe here elementary processes of photoperception by PhyA for inhibition of hypocotyl elongation by cyclical pulse irradiation generated by LEDs, the action spectrum by intermittent irradiation generated by the Okazaki large spectrograph (Watanabe et al., 1982), and the opposite effects of R and FR light pulses on the response. We discuss why PhyA-mediated inhibition of hypocotyl elongation occurs under continuous (and intermittent) irradiation with FR light but not with R light (Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993), despite both PhyA (Butler et al., 1959) and PhyB (Abe et al., 1989) showing similar absorption spectra with peaks in the R light region of the spectrum.
MATERIALS AND METHODS
Plant Growth Measurement
Wild-type seedlings of Arabidopsis Heynh. were of ecotype Ler (Landsberg erecta); PhyA-deficient (phyA) mutant seedlings were phyA-201 (ecotype Ler); PhyB-deficient (phyB) mutant seedlings were phyB-1 (ecotype Ler); and PhyA- and PhyB-deficient (phyAphyB) double-mutant seedlings were produced from phyA-201 and phyB-1, unless otherwise indicated. Wild-type seedlings of ecotype Columbia and phyB-9 mutant seedlings (ecotype Columbia) were also examined when needed. For measurement of hypocotyl length, approximately 60 seeds were planted on agar plates (Murashige and Skoog medium diluted to one-tenth with 0.7% [w/v] agar) and kept at 4°C for 3 d. Duplicate or triplicate plates were used for the measurement of each data point. Plates were subsequently transferred to 23°C in the dark for 16 h, exposed to white light for 8 h to induce seed germination, and then transferred to the dark for an additional 40 h. These 2-d-old seedlings were irradiated with pulses of LEDs or monochromatic light for appropriate periods, then hypocotyls of the longest 30 seedlings were measured using a digimatic caliper (CD-15C, Mitsutoyo, Tokyo). Mean value and se were calculated with a processor (DP–1HS, Mitsutoyo).
Light Treatment
LEDs were used to develop a new irradiation system consisting of 600 R-LEDs (EBR5305S, Stanley Electronics, Tokyo; peak emission at 660 nm, halfwidth in 30 nm, maximum output of 2 mW) and 200 FR-LEDs (HE7601S, Hitachi, Tokyo; peak emission at 770 nm, halfwidth in 50 nm, maximum output of 30 mW). The LEDs were mounted on a clear polycarbonate board (20 × 26 cm) in rows (24 rows for R-LED arrays and six rows for FR-LED arrays). Each array contained 33 R-LEDs or FR-LEDs spaced 3.0 mm apart. Duration of pulses of R or FR light and their photon fluence rate were separately controlled by the electrical system. Photon fluence rate was measured using the optical power meter (1830–C, Newport, Irvine, CA). Maximum outputs generated from the LED irradiation system were 2.2 mW cm−2 for R light (corresponding to 120 μmol m−2 s−1 at 650 nm) and 12 mW cm−2 for FR light (corresponding to 1.2 mmol m−2 s−1 at 770 nm), respectively. General characteristics of the LED irradiation system for plants were reviewed elsewhere (Bula et al., 1991).
Determination of Action Spectra
Intermittent irradiation with monochromatic light for 1 min with intervals of 2 min of darkness was carried out using the Okazaki large spectrograph (Watanabe et al., 1982). Fluence-response curves in the phyB mutant and the phyAphyB mutant were determined at 20 different wavelengths from 320 to 780 nm at intervals of 10 to 67 nm. Action spectra for 20% inhibition of hypocotyl elongation at each wavelength were constructed from these curves. Photon effectiveness was calculated as the reciprocal of the fluence required for 20% inhibition of hypocotyl elongation, which is represented by 80% relative hypocotyl length to the dark level obtained from the fluence response curves.
RESULTS
Effects of Intermittent Irradiation with FR Light
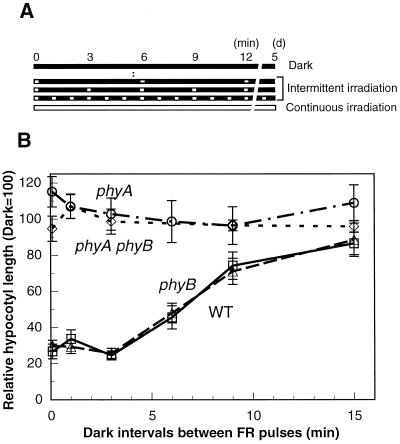
First we tested whether the effect of continuous FR irradiation on inhibition of hypocotyl elongation in the wild type and the phyB mutant of Arabidopsis could be replaced by intermittent irradiation with FR light pulses. Two-day-old etiolated seedlings were exposed intermittently to FR light pulses (180 μmol m−2 s−1 for 10 s) for 5 d (Fig. 1A), and the hypocotyl lengths were measured. The seedlings grown under FR light pulses at intervals of 3 min or shorter showed equivalent hypocotyl lengths as the seedlings grown in continuous FR irradiation (Fig. 1B). In contrast, when the seedlings were grown under FR light pulses at intervals of 15 min or longer, their hypocotyl lengths were indistinguishable from those of the dark-grown seedlings (Fig. 1B). The phyA mutant and the phyAphyB mutant did not show hypocotyl shortening under any of these conditions (Fig. 1B). These results indicated that intermittent irradiation with FR light at intervals of 3 min or less caused an equivalent effect on PhyA-dependent inhibition of hypocotyl elongation, as did continuous FR irradiation.
Figure 1.
Effect of intermittent irradiation with FR light on PhyA-mediated inhibition of hypocotyl elongation in Arabidopsis. A, Light regime. Two-day-old etiolated seedlings were transferred to intermittent FR light irradiation of various cycles, continuous FR light, or darkness and grown for 5 d, and their hypocotyl lengths were measured. Long white bar, Continuous irradiation with FR light (30 μmol m−2 s−1); long black bars, continuous darkness; small white boxes, intermittent irradiation, FR light pulses (180 μmol m−2 s−1 for 10 s each) with LEDs. B, Responses to intermittent irradiation with FR light in wild-type (WT, ▵), phyA (○), phyB (□), and phyAphyB (⋄) mutants. Values at a dark interval of 0 min correspond to continuous irradiation. Error bars represent se.
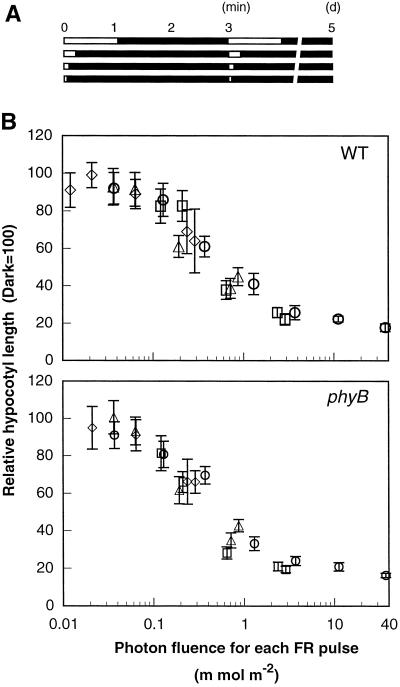
To determine whether the process of photoperception obeyed the Bunsen-Roscoe law of reciprocity, the response was measured after changing either the exposure time of cyclical FR light pulses or the total light energy using the 3-min cyclical irradiation regime (Fig. 2A). The wild-type seedlings showed an inhibition of hypocotyl elongation proportional to the total light energy per pulse across the range of photon fluences from 0.04 to 2 mmol m−2 (Fig. 2B, WT). The response did not show any dependency either on the exposure time within the range examined (1–60 s) or on the photon fluence rate (0.62–620 μmol m−2s−1) (Fig. 2B, WT). Essentially the same results were obtained with phyB mutant seedlings (Fig. 2B, phyB). Therefore, the Bunsen-Roscoe law of reciprocity held in this response, suggesting that photoperception by PhyA is a rate-limiting factor of this response.
Figure 2.
Relationship between the hypocotyl length and the photon fluence of FR light per pulse. A, Light regime. Two-day-old etiolated seedlings of the wild type were transferred and grown in the intermittent irradiation with different intensities of FR light for different durations of exposure time in 3-min cycles for 5 d, and their hypocotyl lengths were measured. White bars, FR light pulses with LEDs; black bars, darkness. B, Responses to intermittent irradiation with FR light pulses for 1 s (⋄), 3 s (▵), 10 s (□), and 60 s (○) in the wild type (top graph) and the phyB mutant (bottom graph). Error bars represent se.
Effects of Duration of Intermittent FR Light Treatment on Hypocotyl Growth
To find how long prolonged irradiation with FR light is needed for detection of this response, the phyB mutant seedlings were grown under continuous FR light (90 μmol m−2 s−1) for various durations from 5 min to 24 h and then kept in darkness for 5 d. Their hypocotyl lengths were measured and compared with those of the seedlings treated with continuous irradiation with FR light for 6 d as a control. In treatments 12 h or shorter, the hypocotyls were not as short as those of seedlings treated with continuous irradiation for 6 d (data not shown). When the seedlings were exposed to continuous FR light for 24 h, the hypocotyls were as long as those of seedlings treated with continuous irradiation for 6 d (data not shown).
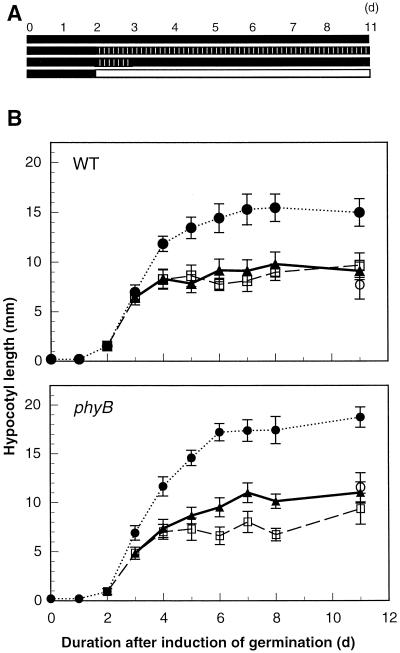
The time courses of hypocotyl elongation in seedlings grown under intermittent FR irradiation were examined. When 2-d-old wild-type seedlings were grown under intermittent irradiation with FR light pulses (100 μmol m−2 s−1 for 10 s) for 24 h, the time course of hypocotyl elongation was the same as the seedlings that were given intermittent FR irradiation throughout their hypocotyl growth (for several days) (Fig. 3B, WT). For both of these treatments, the hypocotyl growth rate increased during the 1st d from the beginning of the irradiation, and then decreased, reaching its maximum within 4 d after induction of germination (Fig. 3B, WT). In contrast, the hypocotyls of dark-grown seedlings continued to elongate until the 7th d after induction of germination (Fig. 3, WT). Essentially similar time courses of hypocotyl elongation were obtained in the phyB mutant seedlings, although the final hypocotyl length of the seedlings treated with FR light pulses for 24 h was slightly longer than in those treated with FR light pulses throughout their hypocotyl growth (Fig. 3B, phyB). These results show that, in either continuous or intermittent irradiation, FR irradiation of just 24 h can cause the equivalent effect on inhibition of hypocotyl elongation obtained by prolonged (more than 2 d) FR irradiation.
Figure 3.
Effect of intermittent irradiation with FR light pulses for 1 d on further growth of the hypocotyl in darkness. A, Light regime. Two-day-old etiolated seedlings were treated as follows: one group (□) was kept under intermittent irradiation with FR light pulses (100 μmol m−2 s−1 for 10 s each) in 3-min cycles throughout the experiment, the second group (▴) was exposed to the same intermittent FR light irradiation for 1 d, then grown in the dark, the third group (●) was kept in the dark, and the fourth group (○) was kept under continuous irradiation with FR light (5.6 μmol m−2 s−1) for 9 d. White lines in black bars, FR light pulses with LEDs; black bars, darkness; white bars, continuous irradiation with FR light. B, Time courses of the hypocotyl growth in the wild type (top graph) and the phyB mutant (bottom graph). Error bars represent se.
Action Spectrum for PhyA-Dependent HIR of Hypocotyl Elongation
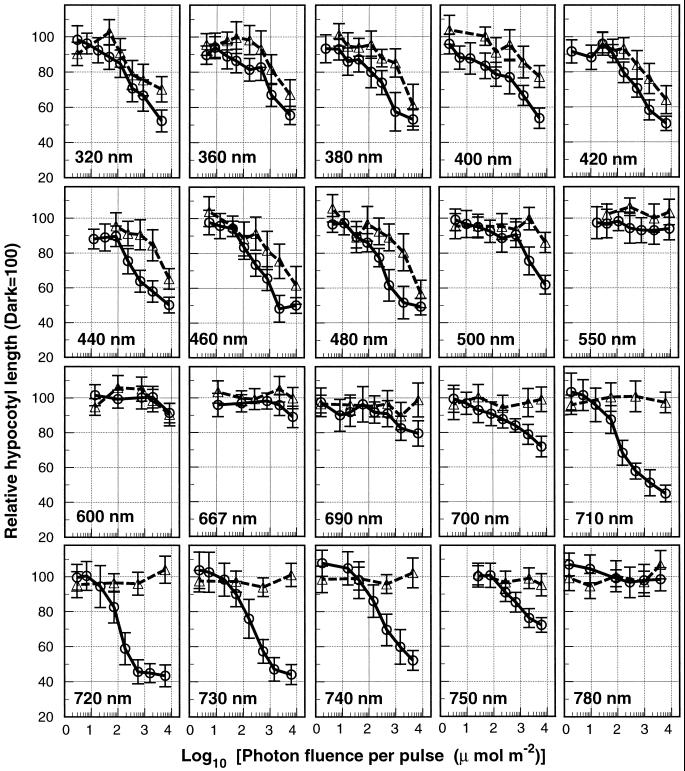
As the photoperception by PhyA under the intermittent irradiation conditions obeyed the Bunsen-Roscoe law of reciprocity (Fig. 2), we were able to analyze the relative efficacy of different wavelengths of light for inducing equivalent hypocotyl inhibition responses. The Okazaki large spectrograph and its threshold boxes were designed and built so that monochromatic lights are intermittently exposed in desired fluence rates at wavelengths of 250 to 1,000 nm (Watanabe et al., 1982). Etiolated seedlings with hypocotyls shorter than 2 mm were intermittently irradiated with monochromatic light in 3-min cycles for 24 h, then kept in darkness for 5 d, and their hypocotyl lengths were measured. Hypocotyl lengths were normalized to hypocotyl lengths of dark controls and plotted against the photon fluence per pulse to generate fluence response curves at each wavelength (Fig. 4). Based on the fluence-response curves, the photon fluences required for 20% inhibition of hypocotyl elongation were determined at each wavelength, and their reciprocals were plotted to construct the action spectra (Fig. 5).
Figure 4.
Effect of intermittent irradiation with 320 to 780 nm of light on inhibition of hypocotyl elongation in the phyB and the phyAphyB double mutants. Two-day-old etiolated seedlings of the phyB mutant (○) and the phyAphyB double mutant (▵) were exposed to intermittent irradiation with monochromatic light in 3-min cycles for 1 d, then grown in the dark for 5 d, and their hypocotyl lengths were measured. Error bars represent se.
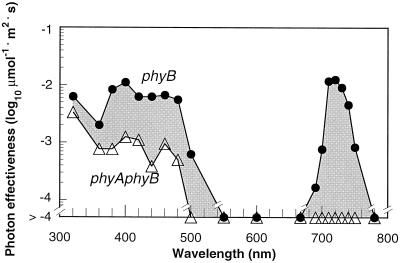
Figure 5.
Action spectra for inhibition of hypocotyl elongation in the phyB and the phyAphyB double mutants by intermittent light treatment. Photon effectiveness for the response was calculated as reciprocal of the fluence required for 20% inhibition of hypocotyl elongation based upon the data in Figure 4. The area shaded gray in the figure corresponds to the differences between the spectrum of the phyB mutant (●) and the phyAphyB double mutant (▵), and is due to the involvement of PhyA for the response.
We performed this analysis for the phyB and phyAphyB mutants. The difference between the action spectrum for the response in the phyB mutant and that in the phyAphyB mutant (Fig. 5, shaded region) was attributed to the deficiency of PhyA in the absence of PhyB. The action spectrum for 20% inhibition of hypocotyl elongation in the phyB mutant showed a range of effective wavelengths, with two regions occurring at 690 to 750 nm and 320 to 500 nm (Fig. 5). In contrast, the action spectrum in the phyAphyB mutant showed no effectiveness in the 500- to 750-nm region, but retained the peak in the UV-A to blue light region, although this was less effective than in the phyB mutant (Fig. 5). Neither of the mutants responded to 550 to 667 nm of light within the range of the photon fluences examined (Fig. 4).
The results indicated that PhyA perceived both FR light (690–750 nm) and UV-A to blue light (320–500 nm) and effectively mediated inhibition of hypocotyl elongation. In contrast, intermittently exposed R light (550–667 nm) induced no effect on PhyA-dependent hypocotyl elongation. The effective range of the resultant action spectra in vivo (Fig. 5) were within the effective range for the photoconversion from Pfr to red-absorbing phytochrome (Pr) in vitro (Butler et al., 1959; Furuya and Song, 1994; Mancinelli, 1994).
Photoreversible Effect of Intermittent R and FR Light Pulses
A question arose whether the effect of FR light pulses on hypocotyl elongation could be canceled by R light pulses given immediately after each FR light pulse in 3-min cycles. In the phyB mutant, irradiation with R light pulses given immediately after each FR light pulse caused hypocotyls to elongate as much as those of dark-grown seedlings (Table I, phyB-1). Consecutive exposure of FR light following FR and R light pulses resulted in hypocotyls as short as (or shorter than) those treated with FR light pulses alone (Table I, phyB-1). The phyAphyB double mutant showed no such photoreversible responses (Table I, phyAphyB). These results indicated that photoperception of each cycle is a photoreversible process mediated by PhyA.
Table I.
Effect of intermittent consecutive FR and R light pulses on hypocotyl elongation in wild-type, phyA, phyB, and phyAphyB mutants
| Cyclic Treatmenta | Hypocotyl Length
|
|||
|---|---|---|---|---|
| Wild Type | phyA-201 | phyB-1 | phyAphyB | |
| mm | ||||
| (R 60s/D 120s)n | 7.9 ± 1.1 | 6.4 ± 0.5 | 15.7 ± 1.2 | 15.7 ± 1.3 |
| (FR 60s/D 120s)n | 4.9 ± 0.8 | 11.8 ± 1.3 | 5.4 ± 0.6 | 15.4 ± 1.1 |
| (FR 20s/R 40s/D 120s)n | 6.4 ± 0.8 | 5.4 ± 0.5 | 15.3 ± 1.0 | 14.7 ± 1.4 |
| (FR 20s/R 20s/FR 20s/D 120s)n | 2.7 ± 0.5 | 11.7 ± 0.9 | 3.1 ± 0.3 | 15.0 ± 1.3 |
| D | 14.8 ± 0.8 | 11.9 ± 0.9 | 16.9 ± 1.0 | 15.5 ± 1.0 |
Two-day-old etiolated seedlings were transferred to intermittent irradiation with R light (2 mmol m−2) and/or FR light (5 mmol m−2) pulses in 3-min cycles using the LED irradiation system, grown for 5 d, and their hypocotyl lengths were measured. Values are means of 30 seedlings ± se.
D, Darkness; n = 2,400.
In contrast, the phyA mutant showed reversible responses in the opposite manner to the phyB mutant; namely, R light pulses inhibited hypocotyl elongation, and complete R/FR light reversibility was observed in the 3-min cyclical irradiation (Table I, phyA-201).
In wild-type seedlings, both FR and R light pulses in any combination resulted in shorter hypocotyls than those of dark-grown seedlings (Table I, WT). Interestingly, the hypocotyl lengths of the wild type caused by irradiation with FR light pulses or FR/R/FR light pulses were not significantly different from those of the phyB mutant (Table I), although the effects of R and FR light on the PhyA- and PhyB-dependent inhibition of hypocotyl elongation are opposite. Similarly, the hypocotyl length of the wild type caused by irradiation with R light pulses or FR/R light pulses were not significantly different from those of the phyA mutant (Table I). These results suggest that the PhyA-dependent inhibition of hypocotyl elongation by irradiation with FR light, and the PhyB-dependent inhibition of hypocotyl elongation by irradiation with R light do not interfere with each other in wild-type seedlings.
Essentially similar results were obtained with wild-type seedlings of ecotype Columbia and phyB-9 mutant seedlings (Columbia background) treated with intermittent FR and R light pulses (data not shown).
DISCUSSION
Photoconversion of PhyA from Pfr to Pr Induces FR Light-Mediated HIR
The results presented in this paper show that both PhyA- and PhyB-dependent HIR of hypocotyl inhibition responses are photoreversible, and that R and FR light act in opposite directions in responses mediated by the two photoreceptors. To our knowledge, this is the first description of a R/FR-light-reversible regulation of PhyA-mediated FR-HIR.
Our results lead us to a hypothesis that the PhyA-mediated HIR is induced by a signal that is transmitted during photoconversion from Pfr to Pr, and that neither dark-synthesized Pr nor photoconverted Pfr is effective. First, PhyA in Pr synthesized in seedlings in darkness showed no ability to induce HIR, because the presence or absence of PhyA did not affect the elongation of hypocotyl length in darkness (Table I), as was reported previously (Nagatani et al., 1993; Reed et al., 1994). Second, the evidence that R light (550–667 nm) pulses alone had no effect (Fig. 4) strongly suggested that the PhyA in Pfr does not induce this response. Third, the effective range of wavelength in the action spectrum for PhyA-dependent HIR of inhibition of hypocotyl elongation by intermittent light (Fig. 5) were within the effective range of wavelength in the absorption spectrum of Pfr (Butler et al., 1959, 1964; Furuya and Song, 1994). In contrast, the effective range of wavelengths in FR light region in the action spectrum were outside the range of the absorption spectrum of Pr. Fourth, the effect of FR light pulses on the response was repeatedly reversed by R light pulses (Table I). Fifth, intermittent irradiation with FR/R/FR light pulses led to significantly greater effect on inhibition of hypocotyl elongation than that with just FR light pulses in both the wild type and the phyB mutant (Table I). This finding is explained by the speculation that irradiation with FR/R/FR light pulses generates a relatively larger pool of photocycled Pr than irradiation with FR light pulses alone does. These results allow us to construct a simple model of PhyA action showing that this response correlates with the photoperception of PhyA in Pfr producing Pr, rather than the photoperception of Pr producing Pfr.
Over the last 4 decades, many scientists in this field have believed that Pfr is the only biologically active form of the molecule, although the reliability of this concept was questioned (Smith, 1983). Most of the action spectra for FR-HIR reported were constructed from data obtained under continuous irradiation before appreciation of the existence of a family of phytochromes (Mohr, 1962; Mancinelli, 1994). They showed effective ranges at 420 to 500 nm and 710 to 750 nm, and their spectrum peaks fluctuated depending on materials; peaks at 420, 450, and 728 nm (Mohr and Wehrung, 1960) or at 370, 440, and 716 nm (Hartmann, 1967) in inhibition of hypocotyl elongation in dark-grown lettuce seedlings, and at 450 and 730 nm in reopening of the plumular hook in lettuce seedlings (Mohr, 1962). They did not show a simple correlation with the photoconversion from Pr to Pfr or from Pfr to Pr (Mohr, 1962; Hartmann, 1967; Mancinelli, 1994). To explain those action spectra, many models for FR-HIR were proposed based on the molecular properties of phytochromes, including balances of biosynthesis, photoconversion, and degradation of phytochrome pools (Smith, 1970; Mohr, 1972; Schäfer, 1975; Johnson and Tasker, 1979). The application of intermittent irradiation for phytochrome-deficient mutants directed us to a model and interpretation based on the specific function of the phytochrome family for the action spectrum of FR-HIR (Fig. 5).
Recently, a positive function of Pr was suggested (Reed, 1999) by studies of phytochrome-dependent seed germination (Reed et al., 1994; Shinomura et al., 1994), morphogenesis in darkness (Kim et al., 1998), and autophosphorylation of phytochromes (Yeh et al., 1997; Yeh and Lagarias, 1998). Autophosphorylation of cyanobacterial phytochrome was promoted by continuous FR irradiation (when Pr should predominate) rather than by continuous R irradiation (when Pfr should predominate) (Yeh et al., 1997). In oat phytochrome, Pfr (Sommer et al., 1996) and also Pr (Yeh and Lagarias, 1998) were phosphorylated. The phytochrome action model (Yeh and Lagarias, 1998) based upon those molecular data is consistent with the idea from the present physiological study that photoconversion of PhyA into Pr by FR light perception promotes a biologically active signal.
PhyA Signal for HIR Is Short-Lived
The PhyA-dependent signal produced by photoconversion from Pfr to Pr appears to be short-lived because the effect of FR light pulses disappeared within 15 min (Fig. 1).In contrast, the PhyB-produced signal for HIR is stable because the effect of R light pulses persisted for 4 h (McCormac et al., 1993; Ahmad and Cashmore, 1997). Previously, before the existence of a family of phytochromes was known, it was found that induction of anthocyanin production by intermittent FR light pulses at intervals of a few minutes or shorter brought about the equivalent extent of the response as continuous FR light irradiation in milo seedlings (Downs and Siegelman, 1963) and in cabbage and mustard seedlings (Mancinelli and Rabino, 1975). The common requirement for frequent irradiation with FR light pulses for induction of these responses strongly suggests that the effects of intermittent FR light pulses in anthocyanin production resulted from PhyA action.
These results remind us of the existence of two pools of physiologically stable phytochrome and labile phytochrome in plant tissues (Furuya, 1989). Pfr of type II phytochrome, including PhyB and probably other phytochromes, is stable in the dark for long periods, for years in dry seeds, and then induces responses. In contrast, Pfr of Type I phytochrome, which corresponds to PhyA, is unstable and disappears rapidly after irradiation with R light in the subsequent dark period (Takimoto and Saji, 1984). However, the time course of disappearance of the effectiveness of each FR light pulse in the present study (Fig. 1) is faster than the time course of phytochrome degradation (Vierstra, 1994) or dark reversions (Briggs and Rice, 1972) previously reported. Therefore, it is still an open question whether the labile nature of PhyA contributes to the requirement of frequent FR light exposure in PhyA-dependent HIR.
Although rapid degradation is generally assumed to be specific to Pfr (Vierstra, 1994), rapid degradation of Pr (one-half of the pool degraded within 4 h) was observed under specific conditions in etiolated oat (Chorney and Gordon, 1965; Stone and Pratt, 1979). Degradation of Pr required that dark-synthesized Pr is converted to Pfr and then back to Pr for generating photocycled-Pr (Chorney and Gordon, 1965; Stone and Pratt, 1979). This requirement for Pr cycling is similar to that for PhyA-mediated HIR in Arabidopsis, but further analysis for physiological role of photocycled Pr degradation in PhyA is needed to conclude whether these two phenomena are related or not.
Another question arises whether the “photoconverted Pr” of PhyA is directly responsible for the induction of the response, or whether some “intermediate form(s)” of PhyA produced in photoconversion from Pfr to Pr (Bischoff et al., 1998) is responsible. Two possibilities may explain the short-lived PhyA signal: (a) Pr itself is responsible for the response but its activity disappears rapidly for an unknown reason, or (b) some unstable intermediate form produced by photoconversion from Pfr to Pr is responsible for the induction of the response. Various approaches have been applied to study the molecular and kinetic mechanisms that occur in the course of the Pr to Pfr phototransformation (Furuya, 1983; Rüdiger and Thümmler, 1994). In contrast, the back reaction from Pfr to Pr has not been as thoroughly investigated (Bischoff et al., 1998).
PhyA-Mediated HIR Is Fluence Dependent
It was well established that two of the defining characteristics of the FR-HIR are the requirement for prolonged irradiation and the dependency upon fluence rate (Briggs et al., 1984; Mancinelli, 1994). The present study revealed that for either intermittent or continuous irradiation, maximal inhibition of hypocotyl elongation mediated by PhyA is achieved during the first 24-h irradiation with FR light (Fig. 3). Although the reason why a minimum of 24 h of irradiation is still required to elicit the response is obscure, we speculate that a 24-h irradiation period leads to an irreversible change in hypocotyl growth rate that persists for several days in the absence of any further light stimulus.
The photon fluence dependency for PhyA-mediated FR-HIR by intermittent irradiation may also explain why the FR-HIR by continuous irradiation depends on the fluence rate. The present study revealed that the extent of inhibition of hypocotyl elongation mediated by PhyA shows a linear correlation with photon fluence per each FR light pulse they are given at intervals of 3 min or shorter (Figs. 1 and 2). If the duration of FR light pulse is extended to 3 min, which corresponds to “continuous irradiation,” an increase of fluence rate inevitably causes an increase of photon fluence. Under such continuous irradiation, the extent of the response appears to depend on fluence rate, but actually depends on photon fluence in each elementary process of photoperception by PhyA. In fact, in the wild type and the phyB mutant, similar inhibition of hypocotyl length was achieved by both continuous and intermittent irradiation if the same photon fluence (1 mmol m−2) was used during each 3 min (Fig. 3).
PhyA Cues Responses by Two Distinct Modes of Photoperception
The present study demonstrated conclusively, for the first time to our knowledge, that PhyA in Arabidopsis has at least two different modes of photoperception, one observed in “photoirreversible” VLFR and depending on the photoconversion from Pr to Pfr (Shinomura et al., 1996; Hamazato et al., 1997), and another, found here, observed in “FR/R light photoreversible” HIR depending on the photoconversion from Pfr to Pr. In PhyA-mediated VLFR of seed germination in Arabidopsis, either a pulse of FR light (1 μmol m−2 and higher of 726 nm of light) or a pulse of very low fluence of R light (1 nmol m−2 and higher of 667 nm of light) induced germination, and photoreversibility was not reported (Shinomura et al., 1996). The action spectrum for this response correlated well with the absorption spectrum of Pr (Shinomura et al., 1996), demonstrating that the concept of Pfr as the active form of phytochrome was consistent with that physiological response. On the other hand, in PhyA-mediated HIR of inhibition of hypocotyl elongation, only FR light pulses (66 μmol m−2 and higher of 726 nm of light each, Fig. 4) induced the response, but R light pulses given alone never induced this response.
The modes of photoperception by PhyA for HIR and VLFR are different from that for PhyB-mediated responses, although PhyA and PhyB have similar absorption spectra in vitro (Abe et al., 1989; Kunkel et al., 1993), amino acid sequences (Sharrock and Quail, 1989), and protein subdomain structures (Xu et al., 1995; Wagner et al., 1996). These results suggest that the PhyA-dependent inhibition of hypocotyl elongation may represent a specialized branch of the FR/R light sensing pathway, with unique transduction components not shared by other photoreceptors. This idea would help to explain many complicated physiological characteristics of the photoinhibition of stem elongation reported so far.
The ecological significance of phytochromes as spectral sensors for growth and photosynthesis has been well characterized and discussed in relation to the physiological function of phytochromes and their R/FR light reversible characteristics, but the significance of FR-HIR has been obscure (Smith, 1994). Interestingly, the present study showed that both R and FR light are effective for the inhibition of hypocotyl elongation in wild-type Arabidopsis (Table I). Given that FR irradiation causes simultaneous production of Pr in both PhyA and PhyB, it appears that the primary function of PhyA for the response was unaffected by PhyB in wild-type seedlings. Similarly, the primary function of PhyB by photoconversion into Pfr by irradiation with R light pulses was unaffected by PhyA of Pfr in the wild-type seedlings. These results reveal that plants use the overlapping functions of PhyA and PhyB separately, and have evolved different modes of action for the different phytochromes in order to occupy a greater variety of niches in the light landscape.
ACKNOWLEDGMENTS
We thank Pill-Soon Song and Jason W. Reed for insightful discussions; Norio Murata and Masakatsu Watanabe for hosting us at the National Institute for Basic Biology; Mamoru Kubota and Sho-ichi Higashi for help with operation of the Okazaki large spectrograph; Kenya Suzuki for the physiological experiment; Takao Yokota for discussion and support; Masashi Kiguchi for helpful advice on calculation of optical power; Ryoko Katayanagi for greenhouse plant care; Sigeo Kubota and Hiroshi Tohyama for building the LED irradiation system; and laboratory staff for discussion and support.
Footnotes
This work was carried out under Hitachi Advanced Research Laboratory project (no. B2023) and National Institute for Basic Biology Cooperative Research Program for the Okazaki large spectrograph (grant nos. 97–524 and 98–522). Research was partly supported by a grant from the Program for Promotion of Basic Research Activity for Innovative Biosciences to M.F.
This paper is dedicated to Hans Mohr who had made the greatest contribution to HIR.
LITERATURE CITED
- Abe H, Takio K, Titani K, Furuya M. Amino-terminal amino acid sequences of pea phytochrome II fragments obtained by limited proteolysis. Plant Cell Physiol. 1989;30:1089–1097. [Google Scholar]
- Ahmad M, Cashmore AR. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 1997;11:421–427. doi: 10.1046/j.1365-313x.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- Beggs CJ, Holmes MG, Jabben M, Schäfer E. Action spectra for the inhibition of hypocotyl growth by continuous irradiation in light and dark-grown Sinapis alba L. seedlings. Plant Physiol. 1980;66:615–618. doi: 10.1104/pp.66.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff M, Hermann G, Rentsch S, Strehlow D. Ultrashort processes of native phytochrome: femtosecond kinetics of the far-red-absorbing form Pfr. J Phys Chem A. 1998;102:4399–4404. [Google Scholar]
- Blaauw OH, Blaauw-Jansen G, van Leeuwen WJ. An irreversible red-light-induced growth response in Avena. Planta. 1968;82:87–104. doi: 10.1007/BF00384699. [DOI] [PubMed] [Google Scholar]
- Briggs WR, Mandoli DF, Shinkle JR, Kaufman LS, Watson JC, Thompson WF. Phytochrome regulation of plant development at the whole plant, physiological, and molecular levels. In: Colombetti G, Lenci F, Song P-S, editors. Sensory Perception and Transduction in Aneural Organisms. New York: Plenum; 1984. pp. 265–280. [Google Scholar]
- Briggs WR, Rice HV. Phytochrome: chemical and physical properties and mechanism of action. Annu Rev Plant Physiol. 1972;23:293–334. [Google Scholar]
- Bula RJ, Morrow RC, Tibbitts TW, Barta DJ, Ingatius RW, Martin TS. Light-emitting diodes as a radiation source for plants. HortScience. 1991;26:203–205. [PubMed] [Google Scholar]
- Butler WL, Hendricks SB, Siegelman HW. Action spectra of phytochrome in vitro. Photochem Phtotobiol. 1964;3:521–528. [Google Scholar]
- Butler WL, Norris KH, Siegelman HW, Hendricks SB. Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants. Proc Natl Acad Sci USA. 1959;45:1703–1708. doi: 10.1073/pnas.45.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Cerdan PD, Staneloni RJ, Cattaneo L. Different phototransduction kinetics of phytochrome A and phytochrome B in Arabidopsis thaliana. Plant Physiol. 1998;116:1533–1538. doi: 10.1104/pp.116.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorney W, Gordon SA. Action spectrum and characteristics of the light activated disappearance of phytochrome in oat seedlings. Plant Physiol. 1965;41:891–896. doi: 10.1104/pp.41.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Downs RJ, Siegelman HW. Photocontrol of anthocyanin synthesis in milo seedlings. Plant Physiol. 1963;38:25–30. doi: 10.1104/pp.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M. Molecular properties of phytochrome. Phil Trans R Soc Lond B. 1983;303:361–375. [Google Scholar]
- Furuya M. Molecular properties and biogenesis of phytochrome I and II. Adv Biophys. 1989;25:133–167. doi: 10.1016/0065-227x(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Furuya M. Phytochromes: their molecular species, gene families and functions. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:617–645. [Google Scholar]
- Furuya M, Song P-S. Assembly and properties of holophytochrome. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 105–140. [Google Scholar]
- Goto N, Yamamoto KT, Watanabe M. Action spectra for inhibition of hypocytyl growth of wild-type plants and of the hy2 long-hypocotyl mutant of Arabidopsis thaliana L. Photochem Photobiol. 1993;57:867–871. [Google Scholar]
- Hamazato F, Shinomura T, Hanzawa H, Chory J, Furuya M. Fluence and wavelength requirements for Arabidopsis CAB gene induction by different phytochromes. Plant Physiol. 1997;115:1533–1540. doi: 10.1104/pp.115.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann KM. A general hypothesis to interpret “high energy phenomena” of photomorphogenesis on the basis of phytochrome. Photochem Photobiol. 1966;5:349–366. [Google Scholar]
- Hartmann KM. Ein Wirkungsspektrum der Photomorphogenese unter Hochenergiebedingungen und seine Interpretation auf der Basis des Phytochroms (Hypokotylwachstumshemmung bei Lactuca sativa L.) Z Naturforsch. 1967;22b:1172–1175. [PubMed] [Google Scholar]
- Hillman WS, Purves WK. Light responses, growth factors and phytochrome transformations of Cucumis seedling tissues. Planta. 1966;70:275–284. doi: 10.1007/BF00396492. [DOI] [PubMed] [Google Scholar]
- Johnson CB, Tasker R. A scheme to account quantitatively for the reaction of phytochrome in etiolated and light-grown plants. Plant Cell Environ. 1979;2:259–265. [Google Scholar]
- Kim BC, Soh MS, Hong SH, Furuya M, Nam HG. Photomorphogenic development of the Arabidopsis shy2-1D mutation and its interaction with phytochromes in darkness. Plant J. 1998;15:61–68. doi: 10.1046/j.1365-313x.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T, Tomizawa K, Kern R, Furuya M, Chua NH, Schäfer E. In vitro formation of a photoreversible adduct of phycocyanobilin and tobacco apophytochrome B. Eur J Biochem. 1993;215:587–594. doi: 10.1111/j.1432-1033.1993.tb18069.x. [DOI] [PubMed] [Google Scholar]
- Mancinelli AL. The physiology of phytochrome action. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 211–269. [Google Scholar]
- Mancinelli AL, Rabino I. Photocontrol of anthocyanin synthesis. IV. Dose dependence and reciprocity relationships in anthocyanin synthesis. Plant Physiol. 1975;56:351–355. doi: 10.1104/pp.56.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli DF, Briggs WR. Phytochrome control of two low-irradiance responses in etiolated oat seedlings. Plant Physiol. 1981;67:733–739. doi: 10.1104/pp.67.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormac AC, Wagner D, Boylan MT, Quail PH, Smith H, Whitelam GC. Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome B-encoding cDNAs: evidence that phytochrome A and phytochrome B have distinct photoregulatory functions. Plant J. 1993;4:19–27. [Google Scholar]
- Mohr H. Der Einfluss monochromatischer Strahlung auf das Längenwachstum des Hypokotyls und auf die Anthocyanbildung bei Keimlingen von Sinapis alba L. (Brassica alba Boiss.) Planta. 1957;49:389–405. [Google Scholar]
- Mohr H. Primary effects of light on growth. Annu Rev Plant Physiol. 1962;13:465–488. [Google Scholar]
- Mohr H. Lectures on Photomorphogenesis. Berlin: Springer-Verlag; 1972. [Google Scholar]
- Mohr H, Shropshire W., Jr . An introduction to photomorphogenesis for the general reader. In: Shropshire W Jr, Mohr H, editors. Photomorphogenesis. Berlin: Springer-Verlag; 1983. pp. 24–38. [Google Scholar]
- Mohr H, Wehrung M. Die Sreuerung des Hypokotylwachstums bei den Keimlingen von Lactuca sativa L. durch sichtbare Strahlung. Planta. 1960;55:438–450. [Google Scholar]
- Nagatani A, Chory J, Furuya M. Phytochrome B is not detectable in the hy3 mutant of Arabidopsis, which is deficient in responding to end-of-day far-red light treatments. Plant Cell Physiol. 1991;32:1119–1122. [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH. hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Reed JW. Phytochromes are Pr-ipatetic kinases. Curr Opin Plant Biol. 1999;5:393–397. doi: 10.1016/s1369-5266(99)00011-4. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdiger W, Thümmler F. The phytochrome chromophore. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 51–69. [Google Scholar]
- Schäfer E. A new approach to explain the “high irradiance responses” of photomorphogenesis on the basis of phytochrome. J Math Biol. 1975;2:41–56. [Google Scholar]
- Schäfer E, Fukshansky L, Shropshire W., Jr . Action spectroscopy of photoreversible pigment systems. In: Shropshire W Jr, Mohr H, editors. Photomorphogenesis. Berlin: Springer-Verlag; 1983. pp. 39–68. [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Chory J, Furuya M. The induction of seed germination in Arabidopsis thaliana is regulated principally by phytochrome B and secondarily by phytochrome A. Plant Physiol. 1994;104:363–371. doi: 10.1104/pp.104.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Phytochrome and photomorphogenesis in plants. Nature. 1970;227:665–668. doi: 10.1038/227665a0. [DOI] [PubMed] [Google Scholar]
- Smith H. Is Pfr the active form of phytochrome? Phil Trans R Soc Lond B. 1983;303:443–452. [Google Scholar]
- Smith H. Sensing the light environment: the functions of the phytochrome family. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 377–416. [Google Scholar]
- Smith H, Whitelam GC, McCormac AC. Do the members of the phytochrome family have different roles? Physiological evidence from wild-type, mutant and transgenic plants. In: Thomas B, Johnson CB, editors. Phytochrome Properties and Biological Action. Berlin: Springer-Verlag; 1991. pp. 217–236. [Google Scholar]
- Sommer D, Wells TA, Song PS. A possible tyrosine phosphorylation of phytochrome. FEBS Lett. 1996;393:161–166. doi: 10.1016/0014-5793(96)00876-9. [DOI] [PubMed] [Google Scholar]
- Stone HJ, Pratt LH. Characterization of the destruction of phytochrome in the red-absorbing form. Plant Physiol. 1979;63:680–682. doi: 10.1104/pp.63.4.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai M, Takeno K, Furuya M. Diverse responses of spores in the light-dependent germination of Lygodium japonicum. Plant Sci Lett. 1977;8:333–338. [Google Scholar]
- Takimoto A, Saji H. A role of phytochrome in photoperiodic induction: two-phytochrome-pool theory. Physiol Plant. 1984;61:675–682. [Google Scholar]
- Vierstra RD. Phytochrome degradation. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 141–162. [Google Scholar]
- Wagner D, Fairchild CD, Kuhn RM, Quail PH. Chromophore-bearing NH2-terminal domains of phytochromes A and B determine their photosensory specificity and differential light lability. Proc Natl Acad Sci USA. 1996;93:4011–4015. doi: 10.1073/pnas.93.9.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Furuya M, Miyoshi Y, Inoue Y, Iwahashi I, Matsumoto K. Design and performance of the Okazaki large spectrograph for photobiological research. Photochem Photobiol. 1982;36:491–498. [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Parks BM, Short TW, Quail PH. Missense mutations define a restricted segment in the C-terminal domain of phytochrome A critical to its regulatory activity. Plant Cell. 1995;7:1433–1443. doi: 10.1105/tpc.7.9.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KC, Lagarias JC. Eukaryotic phytochromes: light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc Natl Acad Sci USA. 1998;95:13976–13981. doi: 10.1073/pnas.95.23.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KC, Wu SH, Murphy JT, Lagarias JC. A cyanobacterial phytochrome two-component light sensory system. Science. 1997;277:1505–1508. doi: 10.1126/science.277.5331.1505. [DOI] [PubMed] [Google Scholar]