Abstract
Objective:
The methanolic extracts of Nigella sativaL. seeds (MENS) and Berberis vulgaris L.(MEBV) were investigated for treatment of Haemoproteus columbae-infected pigeons (Columba livia domestica).
Materials and Methods:
One hundred twenty naturally-infected pigeons were randomly divided into four groups of thirty each. Two groups were treated separately with the extracts, while the positive and negative control groups were given buparvaquone (Butalex®) and distilled water, respectively. The parasitaemia rate was calculated in all groups before and after the experiment at four-day intervals for16 days.
Results:
The results showed a high therapeutic effect for MENS with a progressive decrease in average parasitaemia rate from 18.17% before treatment to 0.73% at the end of treatment (p<0.05), while Butalex® was able to suppress the parasitemia rate from 18.90% before treatment to 0.23% at the end of experiment (p<0.05). However, no significant changes in parasitemia rate were evident in groups treated with MEBV (p>0.05).
Conclusion:
Methanolic extracts of N. sativa showed therapeutic effects against H. columbae and may be regarded as a suitable choice for further studies to develop new drugs against blood parasites, in both animals and human beings.
Key Words: Nigella sativa, Berberis vulgaris, Haemoproteus columbae, Pigeon
Introduction
Infectious diseases threaten the health and survival of domesticated and wildlife populations around the world. Haemosporida of the genera Haemoproteus and Plasmodium (Phylum Apicomplexa, Order Haemosporida, class Sporozoa) are relatively well known. These parasites are common vector-borne blood parasites with a worldwidedistribution, which are transmitted to a wide variety of avian species. Plasmodium is genetically closely related to Haemoproteus, but there are differences in their life cycles and primary vectors (Martinsen et al., 2008 ▶). H.columbae, which is also known as pigeon malaria, is transmitted to pigeons by pigeon louse fly,Pseudolynchiacanariensis (OrderDiptera, FamilyHippoboscidae), which transmit the disease by inoculating the infective sporozoites. Schizogony occurs in lung endothelium and the merozoites are released; then, merozoites invade erythrocytes and develop into gametocytes. Gametocytes are visible in blood smears and partially surround the nucleus of RBC (Bishopp, 1929 ▶; Valkiunas, 2004 ▶). Some researchers have considered Haemoproteus spp. infection a mild or even nonpathogenic parasite in birds (Ashford, 1971 ▶; Garvin et al., 2003 ▶). Nowadays, it is well understood that Haemoproteus can affect avian body condition (Valkiūnas et al., 2006 ▶), immune and reproductive systems (Tomás et al., 2007 ▶), and community relationships (Ricklefs et al., 2004 ▶) and may lead to death or extinction of more susceptible bird species (Atkinson et al., 2000 ▶). An experiment done by Garvin et al. (2003) ▶ on pathologic effects of Haemoproteus-induced infection indicated that the erythrocytic form causes severe anemia (Cardona et al., 2002 ▶; O'roke, 1930 ▶), weakness and anorexia (Garvin et al., 2003 ▶). Also, Manwell and Loeffler (1961) ▶ revealed that the erythrocytic phase of H.columbae can consume glucose even 100 times more than that of uninfected RBC. Another investigation demonstrated that blood parasites such as Haemoproteus are common causes of death and reduce avian survival by increasing the predation risk under natural conditions (Møller and Nielsen, 2007 ▶). Plasmodium and Haemoproteus are commonly used as models for hypotheses evaluation in ecology (Knowles et al., 2010b ▶; Ricklefs et al., 2005 ▶) and also for investigation of diagnosis and control strategies for human malaria (Slater, 2005 ▶). Resistance of some blood parasites to standard drugs has motivated scientists to introduce more effective drugs with novel modes of action (Muregi et al., 2003 ▶). Therefore, studies on appropriate alternative compounds for development of new treatment strategies are needed. Herbal extracts (e.g. quinine and artemisinin) have been a valuable source of new drugs, especially anti-haemosporidial agents (Gessler et al., 1994 ▶). Considerable evidences have demonstrated that some plant products can be useful as anti-haemosporidial agents (Muregi et al., 2003 ▶; Okeola et al., 2011 ▶; Rodrigues and Gamboa, 2009 ▶). To the best of our knowledge, no documented research has studied the effects of plant extracts againstH.columbae. Nigella sativa L., commonly known as black seed, belongs to the Ranunculaceae family. This seed has been used in Middle and Far East communities as a natural drug for treatment of many diseases. Previously, N. sativa (“SiahDaneh” in Persian) was used as a drug for treatment of tumor (Ahmad et al., 2013 ▶), diabetes (El-Shabrawy and Nada, 1996), and cestode and nematode infections (Mahmoud et al., 2002 ▶). Furthermore, its methanolic extracts have shown antimalarial, antioxidant and anti-leishmanial activities, which were more effective than chloroquine in parasite clearance (Mahmoudvand et al., 2014a ▶; Okeola et al., 2011b ▶). Moreover, Berberisvulgaris L. called “zereshk” (a Persian name for the dried fruit of Berberis) is another desirable plant which showed high anti-leishmanial activity in BALB/c mice and culture models (Mahmoudvand et al., 2014 ▶; Salehabadi et al., 2014 ▶). Today, application of plants such as N. sativa and B. vulgaris are becoming very popular, some of them have been employed for decades to treat different infectious agents (Okeola et al., 2011 ▶; Salehabadi et al., 2014 ▶). The objective of the present study was to examine the therapeutic potential of N. sativa and B. vulgaris methanolic extracts in domesticated pigeons (Columba liviadomestica) naturally infected with H.columbae.
Materials and Methods
Plant materials
N. sativa seeds and dried B. vulgaris fruit were purchased from a local herbal market in Shiraz (Iran). The taxonomic identity of each plant was authenticated (N. sativa: NREF-96-201 and B. vulgaris: NREF-96-202) by F. Bahmanzadegan and M. Etemadi (Research Center of Agriculture, Natural Resources and Education, Fars Province, Iran). Methanolic extract of two plants were obtained according to the method previously described by Moazeni and Nazer (2010). Briefly, plants were dried under shade, and powdered mechanically using a commercial electrical blender. To obtain the methanolic extract of each plant, 500 g of powder was added to 1 liter of methanol and mixed gently for 1 hr using a magnetic stirrer. The obtained mixture was left at room temperature for 24 hr. The mixture was stirred again and filtered and then the solvent was removed by evaporation in a rotary evaporator. The obtained residue was placed into a sterile glass container and stored in the refrigerator at 4ºC for later use. Approximately 11 g of dried extracts from each 500 gr of dried powder of both plants were obtained.
Animals
The present study was carried out on a flock of domesticated pigeons (Columba liviadomestica) in Shiraz, Fars province, Iran. Blood smears were obtained from all the flock. One hundred twenty female pigeons, between nine months and one year old, weighing 450–500 g, and naturally infected with H. columbaewere chosen for the present experiment and randomly divided into different groups. The pigeons were not treated before experiment.
Parasitemia rate
Blood samples were obtained from the brachial vein punctured by a lancet and smears were prepared on clean microscopic slides, fixed by absolute methanol, and then stained with 10% aqueous Giemsa stain for 45 min. The number of parasitized red blood cells containing halter-shaped gametocyte in each smear was counted from at least 600 RBC and the parasitemia rate in each sample, was monitored at four-day intervals for 16 days (Ishtiaq et al., 2007 ▶; Salehabadi et al., 2014 ▶).
Field application and study design
The infected pigeons were randomly divided into four groups of 30animals each. The experimental groups were separately kept in cages with free access to food and water. Three naturally infected groups were treated with N.sativaseeds, dried B. vulgaris methanolic extract and Butalex® (positive control group) and the negative control group was treated with distilled water. Methanolic extracts of N. sativa seeds (MENS) was administered at a dose of 12.5% based on the previous studies (Al-Naggar et al., 2003 ▶; Okeola et al., 2011 ▶) and our preliminary study (data not shown). The recommended dose for B.vulgarismethanolic extract (MEBV) was 20% (Salehabadi et al., 2014 ▶). Both extracts were administered once a day by oral gavage for 16 days. Butalex® was administered intramuscularly (IM) as a single recommended dose (El-Metenawy, 1999 ▶). Parasitemia rate was calculated for all groups on days 4, 8, 12, 16.
Statistical analysis
Statistical analysis was performed using SPSS software Version 21.0. (IBM Corp. Released 2012. Armonk, NY: IBM Corp) and Graph Pad Prism version 7.00 (Graph Pad Software, La Jolla, California, USA). The results were presented as mean ± standard error of mean (SEM). One-way ANOVA and Tukey tests were used for comparison of mean of measured parameters among control and treatment groups. Kruskal-Wallis, a non-parametric test, was also used to compare the reduction in percentage of parasitemia rates among different groups. Values were considered statistically significant at p<0.05.
Results
To evaluate the therapeutic effects of MENS and MEBV, a field efficacy was carried out on 120 H. Columbae-infected pigeons. Dramatically, a progressive decrease in average parasitemia rate was observed in infected pigeons treated with MENS, from18.17% before treatment to 5.96% by day 4 and 0.73% at the end of treatment (p<0.05) (Figures 1), while MEBV was unable to show significant therapeutic effects, with a maximum decrease in average parasitemia from 19.89% on the first day to 13.70% on day 16 (Figure 2) (p>0.05).
Figure 1.
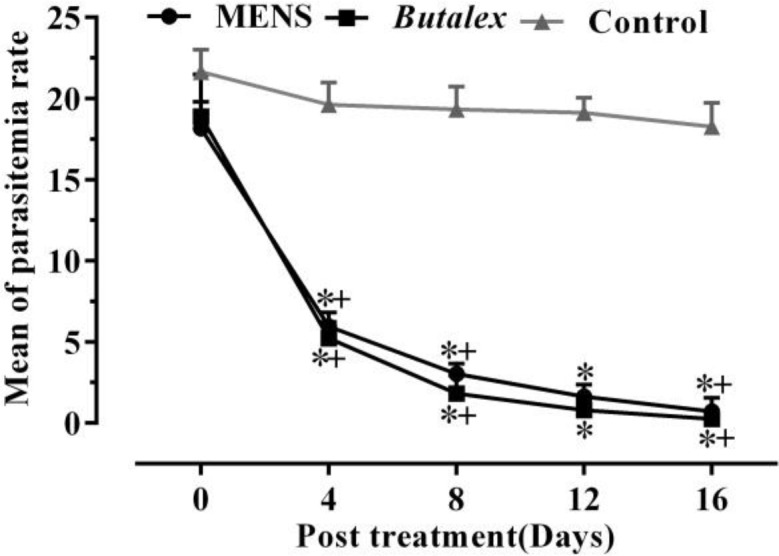
Changesinparasitemia rates following administration of N. sativa methanolic extract (MENS) and Butalex® compared to control. Data are presented as mean±SEM (n=30). *Significant differences (p<0.05) between the control group versus MENS and Butalex® groups. +Significant differences (p<0.05) between various days of each group. No significant difference was observed between MENS and Butalex groups.
Figure 2.
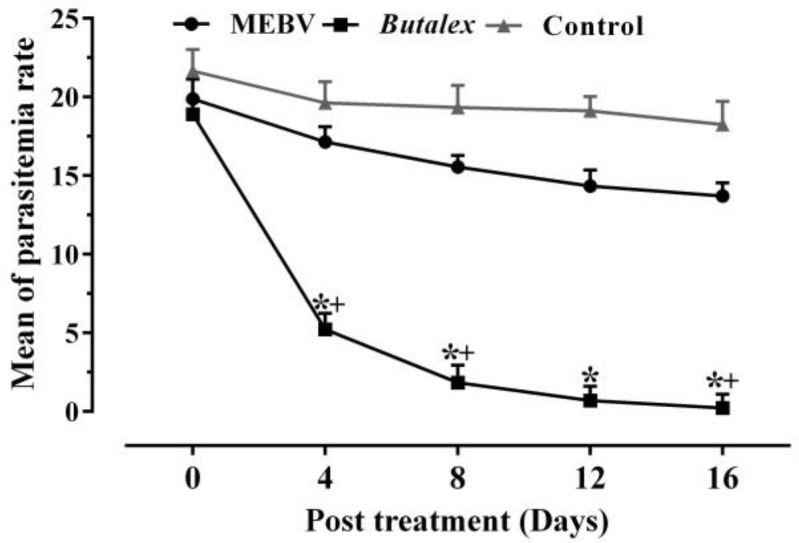
Changes in parasitemia rates following administration of B.vulgaris methanolic extract (MEBV) and Butalex® compared to control. Data are presented as mean±SEM (n=30).*Significant differences (p<0.05)between the Butalex® group versuscontrol and MEBV groups. +Significant differences (p<0.05)between various days of each group. No significant difference was observed between groups MEBV and Control.
The results also showed that Butalex®was able to suppress average parasitemia rate from 18.90% before treatment to 0.23% at the end of the experiment (day 16) (Figures1 and 2) (p<0.05). Figures 3 and 4 show reduction in percentage of parasitemia following treatment in all groups. Treatment of pigeons with Butalex®showed 96.19% and 98.74% reduction from day 12 to the end of experiment (day 16) (p<0.001). Compared to Butalex®, which is frequently used as a commercially available product, MENS-treated group with 68.02%, 84.49% and 96.14% reduction in percentage of parasitemia on days 4, 8 and 16, respectively (p<0.001) (Figures 1 and 3), showed highly effective therapeutic effectsin a time-dependent manner. Between group analysis showed a significant difference in reduction percentage of parasitemia in MENS and Butalex®-treated groups compared to MEBV group (p<0.001). However, compared to control, no significant changes in parasitemia rate were evident in groups treated with MEBV (p>0.05) (Figures2 and 4).
Figure 3.
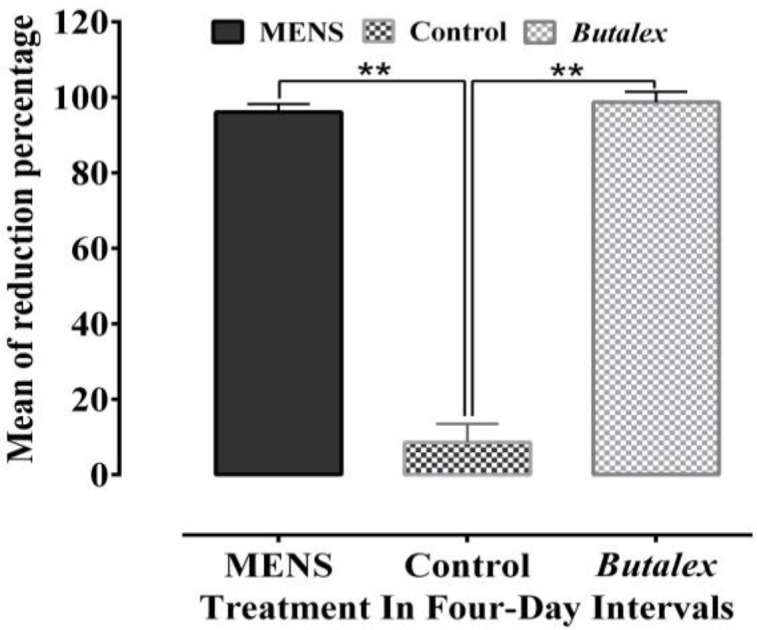
Mean of reduction percentage of parasitemia rates following treatment with N. sativa methanolic extract (MENS) and Butalex®. Significant differences have been shown at **p<0.001.Values are reported as Mean±SEM.
Figure 4.
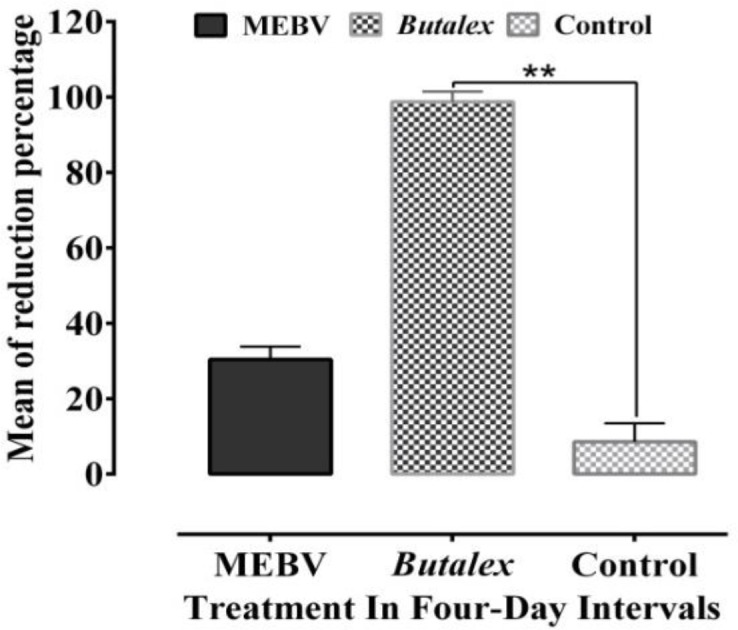
Mean of reduction percentage of parasitemia rates following treatment with B.vulgaris methanolic extract (MEBV) and Butalex®. Significant differences have been shown at **p<0.001.Values are reported as Mean±SEM.
Discussion
The phylum Apicomplexa forms a large and cosmopolitan assemblage of protozoan parasites. Genus Haemoproteus includes some species that parasitize a wide variety of birds including domestic and wild ones (Knowles et al., 2010a ▶). The application of Plant-Derived Products (PDPs) such as methanolic extracts and essential oils offers highly appropriate alternative agents compared to chemical products (El Zalabani et al., 2012 ▶). These substances are commonly used not only for keeping the social life of animals, but also for issues concerning food safety and economy, because PDPs are generally inexpensive and environmentally safe for both men and animals (Razavi et al., 2015 ▶). Some attempts have been made to find more effective anti-haemosporidial compounds and some herbal products were described as effective anti-malarial remedies (Okeola et al., 2011 ▶). In the present study, the possible therapeutic activities of MENS and MEBV against H. columbae in naturally-infected pigeons were investigated. Our results showed that treatment with MENS could significantly reduce the percentage of parasitemia rates by 96.14% (p<0.001) compared to 98.74% obtained by Butalex®at the end of treatments. Our data revealed a time-dependent pattern for the therapeutic effect of MENS. At the beginning of the study, mean of parasitemia rate in MENS-treated group was 18.17±4.33 which decreased to 0.73±1.01 on day 16 post treatment (p<0.05).This therapeutic activity of MENS was in accordance with that reported against P. yoelii infection (Okeola et al., 2011). Although B. vulgaris showed anti-leishmainal and anti-malarial activity in murine models (Fata et al., 2006 ▶; Salehabadi et al., 2014 ▶), in our study, MEBV showed a weak therapeutic activity against H. columbae which may be due to differences in final and intermediate host, transmission, and biology of these two parasites (Zhang et al., 2014 ▶). It is possible that higher doses of MEBV or even treatment of pigeons for a period longer than 16 days, may show better therapeutic effects. N. sativa has been studied as a natural medicine for its biological activity and therapeutic potential against various diseases (Ahmad et al., 2013 ▶). Based on the previous investigations, MENS has a high antioxidant activity and can protect rat hepatocytes against oxidative damages (Okeola et al., 201 ▶1). Nowadays, many synthetic drugs such as Butalex® are used to treat hemoparasites but they are expensive and possess narrow margins of safety and there is a risk of drug resistance (Cheesman, 2000 ▶).To reduce the side effects of conventional drugs, some works have been conducted to use natural drugs with minimal side effects (Ahmad et al., 2013; Rahman et al., 1999). Some previous studies which used MENS against different parasitic agents such as P. yoelii (Okeola et al., 2011a ▶), Schistosoma mansoni (Mahmoud et al., 2002 ▶) and Cryptosporidium parvum (Nasir et al., 2013 ▶) suggested that N. sativa has a broad-spectrum anti-parasitic activity and can be used as an alternative treatment with a different mode of action, and minimal sideeffects. Some investigations confirmed that applying N. sativa seed extract via either oral or intraperitoneal route, produces a low level of cytotoxicity in rat and mouse models (Ahmad et al., 2013 ▶; Zaoui et al., 2002 ▶).Therefore, it could be concluded that MENS is safer for mammalian cells, considering that even at high concentrations no significant cytotoxicity was observed in the host cells (Mahmoudvand et al., 2014 ▶; Salem and Hossain, 2000 ▶). Although the exact mechanism via which MENS affects the infectious agents is not completely understood, some studies were performed to elucidate its mechanisms of actions. Recent investigations suggested that antimicrobial effects of MENS are attributed to itsmost important bioactive ingredients, particularly thymoquinone and other important components such as thymohydroquinone (TQ), dithymoquinone, carvacrol and p-cymene (Ahmad et al., 2013 ▶; Mahmoudvand et al., 2014 ▶; Okeola et al., 2011 ▶). TQ has been shown to suppress the Fe-NTA- induced oxidative stress and many pathological changes in Wistar rat models (Khan and Sultana, 2005 ▶).
Majdalawieh et al. (2010) ▶ confirmed the potential immunomodulatory and anti-inflammatory effects of N. sativa in BALB/c mice. They reported that the aqueous extract of N. sativa induced Th2 versus Th1 cytokines secretion by splenocytes, while stimulation of pro-inflammatory mediators was suppressed significantly.
In conclusion, the present study confirmed that N. sativa has an effective anti-haematozoalproperty with unknown mode of action. Therefore, N. sativa may be a good candidate for developing new anti-protozoal drugs. Further studies are required to elucidate the exact mechanism(s), the mode(s) of action, and probable side effects of MENS, and investigate the application of its effective constituents.
Acknowledgment
We are thankful to F. Bahmanzadegan and M. Etemadi, Research Center of Agriculture and Natural Resources and Education for kindly helps in taxonomic identification of plants.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, Damanhouri ZA, Anwar F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Naggar T, Gomez-Serranillos M, Carretero M, Villar A. Neuropharmacological activity of Nigella sativa L extracts. J Ethnopharmacol. 2003;88:63–68. doi: 10.1016/s0378-8741(03)00157-0. [DOI] [PubMed] [Google Scholar]
- Ashford Rw. Blood parasites and migratory fat at Lake Chad.113. Ibis: John Wiley & Sons; 1971. pp. 100–101. [Google Scholar]
- Atkinson CT, Dusek RJ, Woods KL, Iko WM. Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J Wildl Dis. 2000;36:197–201. doi: 10.7589/0090-3558-36.2.197. [DOI] [PubMed] [Google Scholar]
- Bishopp F. The pigeon fly-an important pest of pigeons in the United States. J Econ Entomol. 1929;22:974–980. [Google Scholar]
- Cardona CJ, Ihejirika A, McClellan L. Haemoproteuslophortyx infection in bobwhite quail. Avian Dis. 2002;46:249–255. doi: 10.1637/0005-2086(2002)046[0249:HLIIBQ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cheesman S. The topoisomerases of protozoan parasites. Parasitol Today. 2000;16:277–281. doi: 10.1016/s0169-4758(00)01697-5. [DOI] [PubMed] [Google Scholar]
- El-Metenawy T. Therapeutic effects of some antihaematozoal drugs against Haemoproteuscolumbaein domestic pigeons. DtschTierarztlWochenschr. 1999;106:72–72. [PubMed] [Google Scholar]
- El-Shabrawy O, Nada S. Biological evaluation of multicomponent tea used as hypoglycemic in rats. Fitoterapia. 1996;67:99–102. [Google Scholar]
- El Zalabani SM, El-Askary HI, Mousa OM, Issa MY, Zaitoun AA, Abdel-Sattar E. Acaricidal activity of Swieteniamahogani and Swieteniamacrophyllaethanolic extracts against Varroa destructor in honeybee colonies. ExpParasitol. 2012;130:166–170. doi: 10.1016/j.exppara.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Fata A, Rakhshandeh H, Berenji F, Jalilianfard A. Treatment of Cutaneous Leishmaniasis in murine model by Alcoholic Extract of Berberis vulgaris. Iran J Parasitol. 2006;1:39–42. [Google Scholar]
- Garvin MC, Homer BL, Greiner EC. Pathogenicity of Haemoproteusdanilewskyi, Kruse, 1890, in blue jays (Cyanocittacristata) J Wildl Dis. 2003;39:161–169. doi: 10.7589/0090-3558-39.1.161. [DOI] [PubMed] [Google Scholar]
- Gessler M, Nkunya MH, Mwasumbi LB, Heinrich M, Tanner M. Screening Tanzanian medicinal plants for antimalarial activity. Acta Trop. 1994;56:65–77. doi: 10.1016/0001-706x(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Ishtiaq F, Gering E, Rappole JH, Rahmani AR, Jhala YV, Dove CJ, Milensky C, Olson SL, Peirce MA, Fleischer RC. Prevalence and diversity of avian hematozoan parasites in Asia: a regional survey. J Wildl Dis. 2007;43:382–398. doi: 10.7589/0090-3558-43.3.382. [DOI] [PubMed] [Google Scholar]
- Khan N, Sultana S. Inhibition of two stage renal carcinogenesis, oxidative damage and hyper proliferative response by Nigella sativa. Eur J Cancer Prev. 2005;14:159–168. doi: 10.1097/00008469-200504000-00012. [DOI] [PubMed] [Google Scholar]
- Knowles S, Palinauskas V, Sheldon B. Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J EvolBiol. 2010;23:557–569. doi: 10.1111/j.1420-9101.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud M, El-Abhar H, Saleh S. The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol. 2002;79:1–11. doi: 10.1016/s0378-8741(01)00310-5. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H, Sharififar F, Rahmat MS, Tavakoli R, Dezaki ES, Jahanbakhsh S, Sharifi I. Evaluation of anti-leishmanial activity and cytotoxicity of the extracts of Berberis vulgaris and Nigella sativa against Leishmaniatropica. J Vector Borne Dis. 2014;51:294. [PubMed] [Google Scholar]
- Majdalawieh AF, Hmaidan R, Carr RI. Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J Ethnopharmacol. 2010;131:268–275. doi: 10.1016/j.jep.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Manwell RD, Loeffler CA. Glucose consumption by Haemoproteuscolumbae. J Parasitol. 1961;47:285–290. [PubMed] [Google Scholar]
- Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. MolPhylogenetEvol. 2008;47:261–273. doi: 10.1016/j.ympev.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Moazeni M, Nazer A. In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. World J Surg. 2010;34:2677–2681. doi: 10.1007/s00268-010-0718-7. [DOI] [PubMed] [Google Scholar]
- Møller AP, Nielsen JT. Malaria and risk of predation: a comparative study of birds. Ecology. 2007;88:871–881. doi: 10.1890/06-0747. [DOI] [PubMed] [Google Scholar]
- Muregi F, Chhabra S, Njagi E, Lang'at-Thoruwa C, Njue W, Orago A, Omar S, Ndiege I. In vitro antiplasmodial activity of some plants used in Kisii, Kenya against malaria and their chloroquine potentiation effects. J Ethnopharmacol. 2003;84:235–239. doi: 10.1016/s0378-8741(02)00327-6. [DOI] [PubMed] [Google Scholar]
- Nasir A, Avais M, Khan M, Khan J, Hameed S, Reichel M. Treating Cryptosporidium parvum infection in calves. J Parasitol. 2013;99:715–717. doi: 10.1645/12-42.1. [DOI] [PubMed] [Google Scholar]
- O'roke EC. The Morphology, Transmission, and Life-history of HaemoproteuslophortyxO'Roke, a Blood Parasite of the California Valley Quail. Univ California Pub Zool. 1930;36:1–50. [Google Scholar]
- Okeola VO, Adaramoye OA, Nneji CM, Falade CO, Farombi EO, Ademowo OG. Antimalarial and antioxidant activities of methanolic extract of Nigella sativa seeds (black cumin) in mice infected with Plasmodium yoellinigeriensis. Parasitol Res. 2011;108:1507–1512. doi: 10.1007/s00436-010-2204-4. [DOI] [PubMed] [Google Scholar]
- Rahman NNNA, Furuta T, Takane K, Mohd MA. Antimalarial activity of extracts of Malaysian medicinal plants. J Ethnopharmacol. 1999;64:249–254. doi: 10.1016/s0378-8741(98)00135-4. [DOI] [PubMed] [Google Scholar]
- Razavi SM, Asadpour M, Jafari A, Malekpour SH. The field efficacy of Lepidiumlatifolium and Zataria multifloramethanolic extracts against Varroa destructor. Parasitol Res. 2015;114:4233–4238. doi: 10.1007/s00436-015-4661-2. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Fallon SM, Bermingham E. Evolutionary relationships, cospeciation, and host switching in avian malaria parasites. SystBiol. 2004;53:111–119. doi: 10.1080/10635150490264987. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Swanson BL, Fallon SM, MartÍnez-AbraÍn A, Scheuerlein A, Gray J, Latta SC. Community relationships of avian malaria parasites in southern Missouri. EcolMonogr. 2005;75:543–559. [Google Scholar]
- Rodrigues JR, Gamboa ND. Effect of dequalinium on the oxidative stress in Plasmodium berghei-infected erythrocytes. Parasitol Res. 2009;104:1491–1496. doi: 10.1007/s00436-009-1355-7. [DOI] [PubMed] [Google Scholar]
- Salehabadi A, Karamian M, Farzad MH, Namaei MH. Effect of root bark extract of Berberis vulgaris L on Leishmania major on BALB/c mice. Parasitol Res. 2014;113:953–957. doi: 10.1007/s00436-013-3727-2. [DOI] [PubMed] [Google Scholar]
- Salem ML, Hossain MS. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int J Immunopharmacol. 2000;22:729–740. doi: 10.1016/s0192-0561(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Slater LB. Malarial birds: Modeling infectious human disease in animals. Bull Hist Med. 2005;79:261–294. doi: 10.1353/bhm.2005.0092. [DOI] [PubMed] [Google Scholar]
- Tomás G, Merino S, Moreno J, Morales J, Martinez‐De La Puente J. Impact of blood parasites on immunoglobulin level and parental effort: a medication field experiment on a wild passerine. FunctEcol. 2007;21:125–133. [Google Scholar]
- Valkiunas G. Avian malaria parasites and other haemosporidia. CRC press, London: Taylor & Francis; 2004. pp. 12–16. [Google Scholar]
- Valkiunas G, Zickus T, Shapoval AP, Iezhova TA. Effect of Haemoproteusbelopolskyi (Haemosporida: Haemoproteidae) on body mass of the blackcap Sylvia atricapilla. J Parasitol. 2006;92:1123–1125. doi: 10.1645/GE-3564-RN.1. [DOI] [PubMed] [Google Scholar]
- Zaoui A, Cherrah Y, Mahassini N, Alaoui K, Amarouch H, Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine. 2002;9:69–74. doi: 10.1078/0944-7113-00084. [DOI] [PubMed] [Google Scholar]


