Abstract
Objective:
Ziziphus jujuba stimulates the release of nitric oxide (NO). Because NO is involved in cardiovascular regulations, in this study the effects of hydroalcoholic extract of Z. jujuba on cardiovascular responses in acute NG-nitro-L-arginine methyl ester (L-NAME) hypertensive rats were evaluated.
Materials and Methods:
Rats were divided into 6 group (n=6): 1) saline, 2) L-NAME received (10mg/kg) intravenously, 3) sodium nitroprusside (SNP) (50µg/kg)+L-NAME group received SNP before L-NAME and 4-6) three groups of Z. jujuba (100, 200 and 400mg/kg) that treated for four weeks and on the 28th day, L-NAME was injected. Femoral artery and vein were cannulated for recording cardiovascular responses and drug injection, respectively. Systolic blood pressure (SBP), Mean arterial pressure (MAP) and heart rate (HR) were recorded continuously. Maximal changes (∆) of SBP, MAP and HR were calculated and compared to control and L-NAME groups.
Results:
In L-NAME group, maximal ΔSBP (L-NAME: 44.15±4.0 mmHg vs control: 0.71±2.1 mmHg) and ΔMAP (L-NAME: 40.8±4.0 mmHg vs control: 0.57±1.6 mmHg) significantly increased (p<0.001 in both) but ∆HR was not significant as compared to control (p>0.05). All doses of Z. jujuba attenuated maximal ∆SBP and ∆MAP induced by L-NAME but only the lowest dose (100 mg/kg) had significant effects (ΔSBP: 20.36±5.6 mmHg vs L-NAME: 44.1±4.0 mmHg and ΔMAP: 20.8±4.5 mmHg vs L-NAME: 40.8±3.8 mmHg (p<0.05 to p<0.01)). The ∆HR at three doses was not significantly different from that of L-NAME group (p>0.05).
Conclusion:
Because long-term consumption of Z. jujuba extract, especially its lowest dose, attenuated cardiovascular responses induced by L-NAME, we suggest that Z. jujuba has potential beneficial effects in prevention of hypertension induced by NO deficiency.
Key Words: Ziziphus jujube, Nitro-L-arginine methyl ester, Blood pressure, Hypertension, Nitric oxide
Introduction
Nitric oxide (NO) is an active gaseous molecule that plays an important role in regulation of regional blood flow, blood pressure, platelet aggregation, vascular smooth muscle proliferation and mediator of nociception in acute and chronic pain conditions (Naseem, 2005 ▶; Sasser et al., 2011 ▶; Abbasnezhad et al., 2016 ▶). NO is synthetized from L-arginine by three isoforms (neuronal, inducible and endothelial) of nitric oxide synthases (NOS). From these isoforms, endothelial NOS (eNOS) is mostly involved in synthesis of NO in endothelium and has protective effect on cardiovascular system. Endothelium dysfunction results in decreased NO bioavailability and leads to several cardiovascular problem including development of hypertension (Naseem, 2005 ▶). In addition, agents that inhibit NOS activity increase cardiovascular responses. For example NG-nitro-L-arginine methyl ester (L-NAME), a NOS inhibitor induces hypertension by inhibition of NO synthesis, in animals (Khayyal et al., 2002 ▶). In addition, agents that increase NO bioavailability may potentially have therapeutic uses in hypertension treatment (Sasser et al., 2011 ▶).
Ziziphus jujuba (Z. jujuba) is a plant belonging to the Rhamnaceae family. Z. jujuba has a great history of usage both as a remedy and a fruit (Mahajan and Chopda, 2009 ▶). The main biologically active components of plant are vitamins C and E, flavonoids, phenols, triterpene acids, polysaccharides and saponins (Cheng et al., 2000 ▶; Gao et al., 2013 ▶). Recent pharmacological studies showed that Z. jujuba has many pharmacological effects such as anticancer (Huang et al., 2007 ▶), anti-inflammatory (Al-Reza et al., 2010 ▶), antioxidant (Zhang et al., 2010 ▶), hepatoprotective (Wang et al., 2012 ▶) and many other protective activities in organs and tissues. Hypotensive effect of Z. jujuba has been reported to be mediated via stimulation of the release of NO (Kim and Han, 1996 ▶). The hypotensive effect of Z. jujuba also has been reported previously (Mahajan and Chopda, 2009 ▶). Involvement of several compound of Z. jujuba such as jujuboside, saponins in cardiovascular regulation has also been shown (Steinkamp-Fenske et al., 2007 ▶; Zhao et al., 2016 ▶). However, the exact mechanism underlying the effect of Z. jujuba on cardiovascular system is yet to be determined. Because Z. jujuba stimulates the release of NO in vitro (in cultured endothelial cells) and in vivo (Kim and Han, 1996 ▶), it is possible that cardiovascular effect of Z. jujuba is mediated by NO. To determine if cardiovascular effect of Z. jujuba is mediated via NO system, the cardiovascular effects of Z. jujuba were assessed in acute L-NAME-treated hypertensive rats (Khayyal et al., 2002 ▶).
Materials and Methods
Extract preparation
Fruits of Z. jujuba were provided from herbs store, Birjand, Iran, and identified by botanists in Herbarium of Ferdowsi University of Mashhad. Then, 100 g of dried fruit without seed was powdered then macerated in 1000 ml ethanol 70% and shaked for 72 hr. After that, the mixture was filtered through different filter sizes. The solvent was evaporated by an oven at 40° C (Mohebbati et al., 2016 ▶). The yield percentage of Z. jujuba was 60%. Different concentrations of the Z. jujuba fruit extract were prepared by adding saline.
Animals and surgery
Forty-two male Wistar rats were used in this study. The animals were anesthetized using urethane (1.5 g/kg, i.p). Animal temperature was kept at 37 °C with a heating lamp. The left femoral artery was cannulated with a polyethylene catheter (PE-50) filled with heparinized saline then catheter was connected to a blood pressure transducer and blood pressure (BP) and heart rate (HR) were continuously recorded by a Power Lab system (ID instrument, Australia) (Shafei and Nasimi, 2011 ▶). The right femoral vein was also cannulated for drug injection.
Experimental protocol
The L-NAME group received L-NAME (10mg/kg) intravenously (i.v) (Hu et al. 1997 ▶), in sodium nitroprusside (SNP) group, SNP (50mg/kg, i.v) (Hirschl et al., 1997 ▶) was injected 5 min before L-NAME. In the Z. jujuba groups, rats were treated with three doses of hydroalcoholic extract of Z. jujuba (100, 200 and 400 mg/kg) (Goyal et al., 2011 ▶) by gavage for four week. On day 28, the L-NAME (10 mg/kg, i.v) was injected. In all groups, systolic blood pressure (SBP), mean atrial pressure (MAP) and heart rate (HR) were recorded throughout the trial period.
Drug and animal groups
The drugs including urethane, L-NAME and SNP were purchased from Sigma, USA. All drugs were dissolved in saline.
Rats were randomly divided into 6 groups as follow (n=7 in each group)
1. Control group received saline (i.v).
2. L-NAME group received L-NAME (10mg/kg, i.v).
3. SNP group received SNP (50µg/kg, i.v) before injection of L-NAME 10mg/kg (i.v).
4. Z. jujuba 100 group orally received 100 mg/kg of extract for four weeks and on day 28, they received L-NAME 10mg/kg (i.v).
5. Z. jujuba 200 group orally received 200 mg/kg of extract for four weeks and on day 28 received L-NAME 10mg/kg (i.v).
6. Z. jujuba 400 group orally received 400 mg/kg of extract for four weeks and on day 28 received L-NAME 10mg/kg (i.v).
Statistical analysis
Changes (∆) in MAP, SBP and HR values were calculated and expressed as mean±SEM. Statistical comparisons were done by one-way ANOVA followed by the Tukey’s post hoc test. A p<0.05 was considered statistically significant.
Results
Effects of saline on cardiovascular responses
After a stabilizing time of 10 min, saline was injected intravenously and cardiovascular responses were recorded. Injection of saline had no significant effects on SBP (before: 122±10 mmHg and after: 123±10 mmHg; p>0.05), MAP (before: 114±10 mmHg and after: 115±10 mmHg; p>0.05) or HR (before: 334±16 beats/min and after: 336±22 beats/min; p>0.05).
Effect of intravenous injection of L-NAME alone and after pre-treatment with SNP on cardiovascular responses
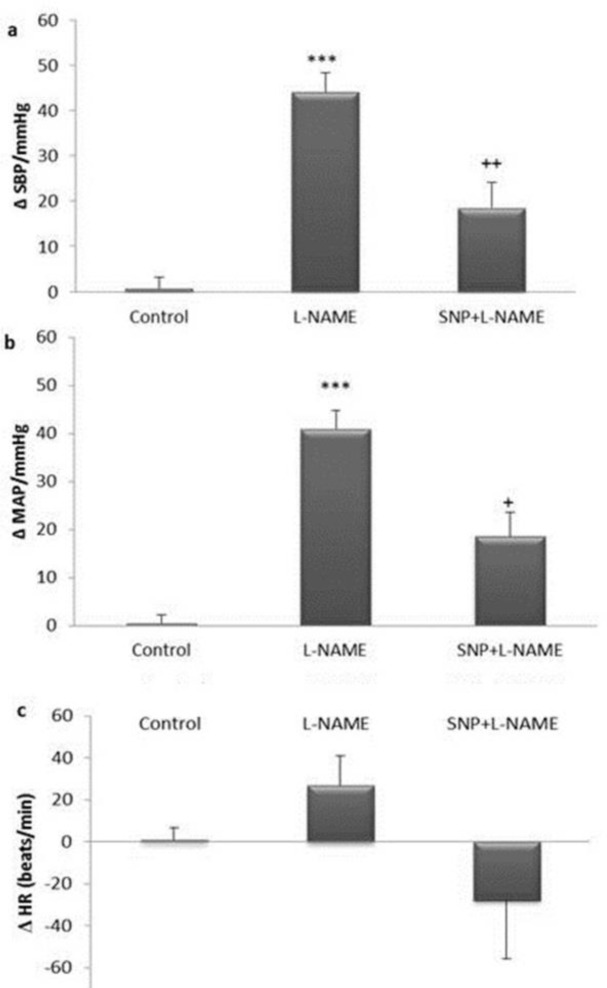
To evaluate the effects of L-NAME, L-NAME alone (10 mg/kg; i.v) was slowly injected and cardiovascular responses (SBP, MAP and HR) were recorded (Figure 1). Mean ∆SBP and ∆MAP after injections of L-NAME are shown in Figure 2a and b. It was observed that ∆SBP and ∆MAP significantly increased compared to control group (ΔSBP: L-NAME group 44.15±4.0 mmHg vs control group: 0.71±2.1 mmHg (p<0.001) and ΔMAP:L-NAME group (40.8±4.0 mmHg vs control group: 0.57±1.6 mmHg (p<0.001)). The HR also increased compared to control group but it was not significant (ΔHR: L-NAME group 26.6±14 vs control group 1±4.9 beats/min (p>0.05)) (Figure. 2 c).
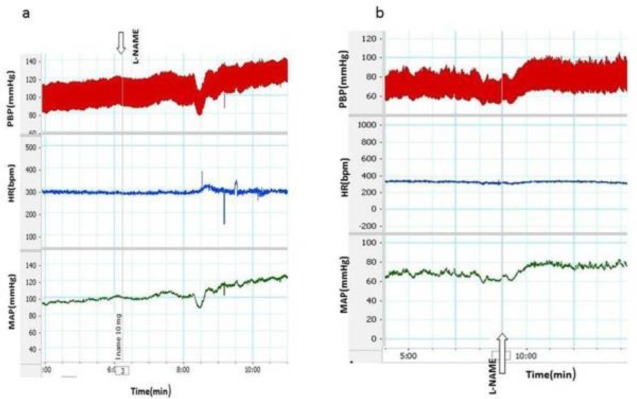
Figure 1.
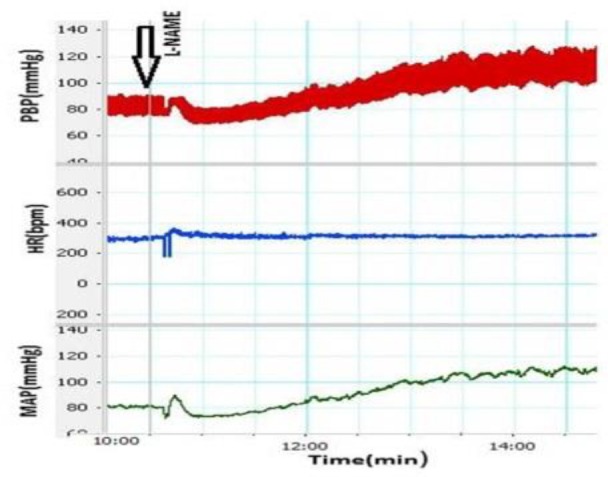
A sample of recording of cardiovascular parameter after i.v injection of L-NAME
Figure 2.
Effects of L-NAME (10mg/kg; i.v) and L-NAME+SNP (50 µg/kg) on ΔSBP (a), ΔMAP (b) and ΔHR (c) in anesthetized rats (n=6).
The data were compared with control group and expressed as mean ± SEM. One-way ANOVA was used for statistical analysis. *** p<0.001 compared to control.+ p<0.05 and ++ p<0.01 compared to L-NAME.
ΔSBP: Changes of systolic blood pressure, ΔMAP: Changes of mean arterial pressure and ΔHR: Changes of heart rate
In SNP group, SNP was injected (50 µg/kg, i.v) before L-NAME. SNP ameliorated increased cardiovascular responses induced by L-NAME. Figure 2 a and b show the effect of SNP on SBP and MAP. Based on our results, pre-treatment with SNP could significantly attenuate the effect of L-NAME on cardiovascular responses (ΔSBP in SNP+L-NAME group: 18.6±5.5 mmHg vs ΔSBP in L-NAME group: 44.1±4.0 mmHg (p<0.01) and ΔMAP in SNP+L-NAME group: 18.5±5.1 mmHg vs ΔMAP in L-NAME group: 40.8±3.8 mmHg (p<0.05). The changes in HR in SNP+L-NAME group were also lower compared to L-NAME group but the difference was not significant (ΔHR in SNP+L-NAME group: -27.9±27.7 vs ΔHR in L-NAME group: 26.6±14 beats/min (p>0.05) (Figure 1c). The HR changes in SNP+L-NAME group was also not significantly different from those of control group (p>0.05).
Effect of hydroalcoholic extract of Ziziphus jujuba fruits on cardiovascular responses in L-NAME hypertensive rats
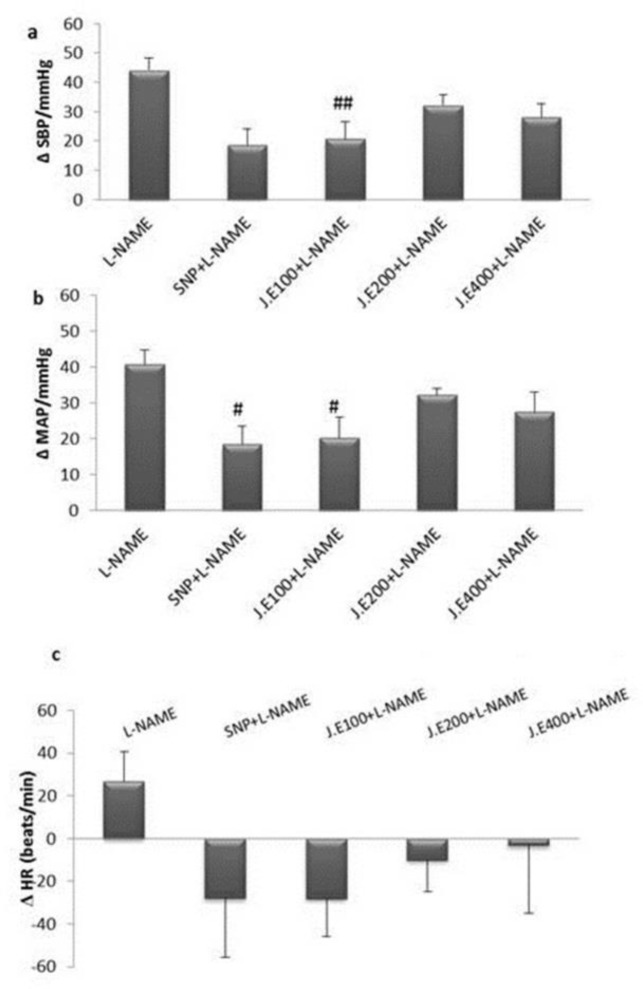
Rats treated with three doses of Z. jujuba (100, 200, and 400 mg/kg, orally), for 4 weeks, then received L-NAME (10 mg; i.v) slowly on day 28 and cardiovascular responses were recorded( Figures 3). In Z. jujuba 100mg/kg +L-NAME group, ΔSBP and ΔMAP significantly decreased compared to L-NAME group (ΔSBP for Z. jujuba 100mg/kg +L-NAME: 20.36±5.6 mmHg vs ΔSBP for L-NAME: 44.1±4.0 mmHg and ΔMAP for Z. jujuba 100mg/kg +L-NAME: 20.8±4.5 mmHg vs ΔMAP for L-NAME: 40.8±3.8 mmHg (p<0.05 to p<0.01) (Figure. 4 a and b). Changes in HR at this dose (100mg/kg) was not significantly different from those of L-NAME alone (ΔHR for Z. jujuba 100mg/kg +L-NAME: 28.1±17.8 vs ΔHR for L-NAME: 26.6±14.0, beats/min (p>0.05)) (Figure 4 c). Changes in all responses at this dose were not significantly different from those of SNP+ L-NAME group.
Figure 3.
Samples of recording of cardiovascular parameter induced by injection of L-NAME after pretreatment with two doses100 mg/kg (a) and 400 mg/kg(b) of Z. jujube
Figure 4.
Effects of hydroalcoholic extract of Z. jujuba on cardiovascular responses in L-NAME hypertensive rats (n= 6).
Rats were treated with three doses of Z. jujuba (100, 200 and 400mg/kg) for four weeks then, L-NAME was injected and cardiovascular responses were determined. The data from Z. jujuba-treated rats were compared with those of L-NAME group and expressed as mean±SEM. One-way ANOVA was used for statistical analysis.
# p<0.05 and ## p<0.01 compared to L-NAME
SBP: Systolic blood pressure, MAP: mean arterial pressure, and HR: heart rate
In rats treated with Z. jujuba 200 mg/kg+L-NAME, ΔSBP and ΔMAP did not significantly reduce compared to L-NAME group (ΔSBP in Z. jujuba 200 mg/kg+L-NAME group: 32.2±3.5 vs ΔSBP in L-NAME group: 44.1±4.0 mmHg (p>0.05) and ΔMAP in Z. jujuba 200 mg/kg+L-NAME group: 32.2±1.6 mmHg vs ΔMAP in L-NAME group: 40.8±3.8 mmHg (p>0.05)) (Figure 4a and b). Changes in HR at this dose (200 mg/kg) were not significantly different from those of L-NAME group (ΔHR in Z. jujuba 200 mg/kg+L-NAME group: -10±15.0 vs ΔHR in L-NAME group: 26.6±14.0, beats/min (p>0.05) (Figure 4c). Changes in all responsess at this dose were not significantly different from those of SNP + L-NAME group.
In rats treated with Z. jujuba 400 mg/kg+L-NAME, ΔSBP and ΔMAP were lower than L-NAME group but were not significantly different (ΔSBP in Z. jujuba 400 mg/kg+L-NAME 28.1±4.5 vs ΔSBP in L-NAME group: 44.1±4.0 mmHg (p>0.05) and ΔMAP in Z. jujuba 400 mg/kg+L-NAME: 27.5±5.4 mmHg vs ΔMAP in L-NAME group: 40.8±3.8 mmHg (p>0.05)) (Figure 4 a and b). Changes in HR at this dose (400 mg/kg) were also not significantly different from those of L-NAME group. (ΔHR in Z. jujuba 400 mg/kg+L-NAME: -3.1±31.7 vs ΔHR in L-NAME group: 26.6±14.0 beats/min (p>0.05)) (Figure 4c ). Changes in all responses at this dose (400 mg/kg) were not significantly different from those of SNP+L-NAME group.
Effect of hydroalcoholic extract of Ziziphus jujuba on body weight
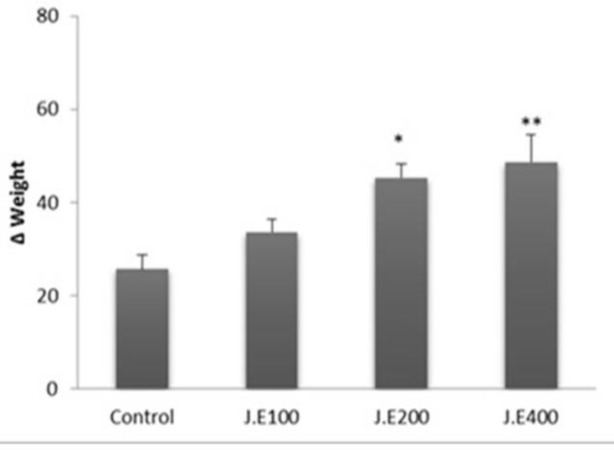
The body weight in all animals treated with the extract increased. However, Z. jujuba extract at 200 and 400 mg/kg significantly increased rats body weight compared to control group (p<0.05 to p<0.01; Figure 5).
Figure 5.
Effects of hydroalcoholic extract of Z. jujuba on body weight of rats after four week treatment (n=6). One-way ANOVA was used for statistical analysis.
* p<0. 05 and ** p<0.01 compared to control .
Discussion
In the present study, the effects of sub-chronic administration of Z. jujuba fruits hydroalcoholic extract to L-NAME hypertensive rats investigated.
Our results showed that four-week administration of three doses of Z. jujuba (100, 200 and 400 mg/kg) ameliorates L-NAME-induced hypertension.
NO, is a potent vasodilator that has an important role in cardiovascular regulation (Fadel, 2017 ▶). The vasodilatory effect of NO is mediated by increased production of guanosine 3′, 5′-cyclic monophosphate in vascular smooth muscle (Fadel, 2017 ▶). It is well known that inhibition of NO production is associated with increased blood pressure and induction of hypertension (Zicha et al., 2006 ▶). In the present study, we used L-NAME, a well-known NOS inhibitor for inhibition of NO production (Zicha et al., 2006 ▶). Our result indicated that L-NAME could increase MAP, SBP with a mild effect on HR that is consistent with previous studies (Khayyal et al., 2002 ▶). We observed that L-NAME at the dose of 10 mg/kg did not have a significant effect on HR. The pharmacological vasodilators, nitroglycerin and SNP both cause vasodilation by donation of exogenous NO or NO-like compounds (Mohebbati et al., 2016a). In this study, injection of SNP before L-NAME ameliorated L-NAME-induced hypertension that confirms involvement of NO in cardiovascular regulation (Jerkic et al., 2004 ▶). HR in SNP+L-NAME group, was also lower than baseline value which might be due to direct effect of NO released from SNP on nodes of heart or promotion of parasympathetic activity (Klimaschewski et al., 1992 ▶). The ameliorative effects of different doses of Z. jujuba on cardiovascular responsess in hypertension induced by L-NAME suggest NO involvement in cardiovascular effects of Z. jujuba fruits extract. Consistent with our results, a previous study reported that Z. jujuba stimulates the release of NO in cultured endothelial cells (Kim and Han, 1996 ▶). Therefore, it is conceivable that this effect of extract might be mediated via affecting endothelium of vessels and increasing NO production. In addition, there is evidence that sympathetic activity is increased in L-NAME hypertension (Biancardi et al., 2007 ▶) and it is conceivable that the effect of extract is mediated via inhibition of sympathetic nervous system. It has also been reported that NO under normal condition, inhibits the release of endothelin-1 from endothelium. Therefore, after blockade of NO in acute L-NAME hypertension, release of endothelin increased and caused vasoconstriction (Banting et al., 1992 ▶). It is possible that Z. jujuba antagonizes endothelin-1 receptors and decreases blood pressure. However, future studies are needed to clarify this hypothesis.
Our results also showed that the best effect of the extract was achieved at the lowest dose (100 mg/kg). The mechanism of this effect is unknown; but, it is possible that doses that we used in this study are high. Therefore, the lowest dose could induce the maximum vasorelaxant effect. In addition, endothelium beside production of vasorelaxant agents produces vasoconstrictor agents such as thromboxane A2 and prostaglandins (Kato et al., 1990 ▶). It is possible that at higher dose (400mg/kg), the extract activates vasoconstrictor agents and by amelioration of vasorelaxant effects of NO, it could increase cardiovascular responses.
The existence of active biological compounds such as flavonoids, phenols, alkaloids, terpenoids and vitamins has been shown in Z. jujuba (Cheng et al., 2000 ▶; Fisher et al., 2003 ▶). Many of these compounds affect NO production or show protective effects on cardiovascular system. For example in our previous study, Trigonella foenum plant could increase NO production in an endothelial cell line. This effect of T. foenum was mostly induced by diosgenin, which also named sapogenin (Mohebbati et al., 2016a ▶). As sapogenin has also been isolated from Z. jujuba, it is conceivable that this compound and its derivative are involved in cardiovascular effect of Z. jujuba by increasing NO production. Jujuboside is another active compound of Z. jujuba that can reduce the vascular tone by activation of NOS (Zhang et al., 2002 ▶; Zhao et al. 2016 ▶). Several studies showed that flavonoid (Freedman et al., 2001 ▶) compounds found in many herbs including Z. jujuba cause vasodilation via increment of endothelial NO production. Therefore, effect of Z. jujuba on blood pressure may be mediated by flavonoids (Achike and Kwan, 2003 ▶; Han et al., 2001 ▶; Mohebbati et al., 2016 ▶). Betulinic acid is a phenolic compound that has been isolated from Z. jujuba (Mahajan and Chopda, 2009 ▶). Betulinic acid also affects eNOS activity (Steinkamp-Fenske et al., 2007), increases bioavailability of NO and has protective effects on cardiovascular system.
There are several evidence showing that increased reactive oxygen species (ROS) production could alter several physiological functions of endothelium including NO production and are involved in pathogenesis of hypertension (Schulz et al., 2011 ▶). Antioxidant agents by improvement of endothelial function and increasing NO levels, have beneficial effect on cardiovascular system. Previous studies have shown antioxidant effect of Z. jujuba (Taati et al., 2011 ▶; Zhang et al., 2010 ▶). Therefore, cardiovascular effect of this plant maybe mediated by its antioxidant properties. Inflammation is another important factor involved in cardiovascular diseases and hypertension (Savoia and Schiffrin, 2006 ▶). Anti-inflammatory effect of Z. jujuba reported in previous studies (Al-Reza et al., 2010 ▶; Goyal et al., 2011 ▶). The jujubosides, flavonoids and terpenes are important compounds of Z. jujuba that have anti-inflammatory activities which may produce beneficial cardiovascular effect of Z. jujuba.
Also in rats treated with Z. jujuba, body weights increased dose dependently. This increase in weight may be due to appetite increment in rats (Stewart, 2004 ▶). It has been shown that NO is important in regulation of appetite (Morley et al., 2011 ▶). As body weight of animals increased dose-dependently, it is possible that Z. jujuba increases animal's appetite through a NO-dependent mechanism.
In summary, a few studies evaluated cardiovascular effect of Z. jujuba. Our results for the first time showed that hydroalcoholic extract of Z. jujuba attenuates the acute L-NAME hypertension. Therefore, it is conceivable that cardiovascular effect of this extract is mediated by NO production.
Because all doses of hydroalcoholic extract of Z. jujuba, especially the lowest does, could suppress cardiovascular responses induced by L-NAME, we suggest that long-term consumption of this plant has beneficial effects for prevention of hypertension induced by NO deficiency.
Acknowledgment
We would like to thank the Research Council of Mashhad University of Medical Sciences for their financial support.
Conflicts of interest
There is no conflict of interest to declare.
References
- Abbasnezhad A, Khazdair MR, Kianmehr M. The role of nitric oxide on the oxytocin induce analgesia in mice. Iran J Basic Med Sci. 2016;19:238–244. [PMC free article] [PubMed] [Google Scholar]
- Achike FI, Kwan CY. Nitric oxide, human diseases and the herbal products that affect the nitric oxide signalling pathway. Clin Exp Pharmacol Physiol. 2003;30:605–615. doi: 10.1046/j.1440-1681.2003.03885.x. [DOI] [PubMed] [Google Scholar]
- Al-Reza SM, Yoon JI, Kim HJ, Kim JS, Kang SC. Anti-inflammatory activity of seed essential oil from Zizyphus jujuba. Food Chem Toxicol. 2010;48:639–643. doi: 10.1016/j.fct.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Banting JD, Friberg P, Adams MA. acute hypertension after nitric oxide synthase inhibition is mediated primarily by increased endothelin vasoconstriction. J Hypertens. 1996;14:975–82. [PubMed] [Google Scholar]
- Biancardi VC, Bergamaschi CT, Lopes OU, Campos RR. Sympathetic activation in rats with L-NAME-induced hypertension. Braz J Med Biol Res. 2007;40:401–8. [PubMed] [Google Scholar]
- Cheng G, Bai Y, Zhao Y, Tao J, Liu Y, Tu G, Ma L, LiaO N, XU X. Flavonoids from Ziziphus jujuba Mill var spinosa. Tetrahedron. 2000;56:8915–8920. [Google Scholar]
- Fadel PJ. Nitric Oxide and Cardiovascular Regulation. Am Heart Assoc. 2017;2017:778–779. doi: 10.1161/HYPERTENSIONAHA.117.08999. [DOI] [PubMed] [Google Scholar]
- Fisher NDL, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Parker C, Li L, Perlman JA, Frei B, Ivanov V, Deak LR, Iafrati MD, Folts JD. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–2798. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- Gao Q-H, Wu C-S, Wang M. the jujube (Ziziphus jujuba Mill) fruit: a review of current knowledge of fruit composition and health benefits. J Agric Food Chem. 2013;61:3351–3363. doi: 10.1021/jf4007032. [DOI] [PubMed] [Google Scholar]
- Goyal R, Sharma PL, Singh M. Possible attenuation of nitric oxide expression in anti-inflammatory effect of Ziziphus jujuba in rat. J Nat Med. 2011;65:514–518. doi: 10.1007/s11418-011-0531-0. [DOI] [PubMed] [Google Scholar]
- Han YJ, Kwon YG, Chung HT, Lee SK, Simmons RL, Billiar TR, Kim YM. Antioxidant enzymes suppress nitric oxide production through the inhibition of NF-κB activation: role of H2O2 and nitric oxide in inducible nitric oxide synthase expression in macrophages. Nitric Oxide. 2001;5:504–513. doi: 10.1006/niox.2001.0367. [DOI] [PubMed] [Google Scholar]
- Hirschl MM, Binder M, Bur A, Herkner H, Müllner M, Woisetschläger C, Laggner AN. Safety and efficacy of urapidil and sodium nitroprusside in the treatment of hypertensive emergencies. Intensive Care Med. 1997;23:885–888. doi: 10.1007/s001340050426. [DOI] [PubMed] [Google Scholar]
- Hu CT, Chang KC, Wu CY, Chen HI. Acute effects of nitric oxide blockade with L‐NAME on arterial haemodynamics in the rat. Br J Pharmacol. 1997;122:1237–1243. doi: 10.1038/sj.bjp.0701496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Kojima-Yuasa A, Norikura T, Kennedy DO, Hasuma T, Matsui-Yuasa I. Mechanism of the anti-cancer activity of Zizyphus jujuba in HepG2 cells. Am J Chin Med. 2007;35:517–532. doi: 10.1142/S0192415X0700503X. [DOI] [PubMed] [Google Scholar]
- Jerkic M, Rivas-Elena JV, Prieto M, et al. Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J. 2004;18:609–611. doi: 10.1096/fj.03-0197fje. [DOI] [PubMed] [Google Scholar]
- Khayyal MT, El-Ghazaly MA, Abdallah DM, Nassar NN, Okpanyi SN, Kreuter M-H. Blood Pressure Lowering Effect of an Olive Leaf Extract {Olea europaed) in L-NAME Induced Hypertension in Rats. Arzneimittelforschung. 2002;52:797–802. doi: 10.1055/s-0031-1299970. [DOI] [PubMed] [Google Scholar]
- Kim H, Han S. Zizyphus jujuba and Codonopsis pilosula stimulate nitric oxide release in cultured endothelial cells and kidney tissues. Asia Pac J Pharmacol. 1996;11:121–128. [Google Scholar]
- Klimaschewski L, Kummer W, Mayer B, et al. Nitric oxide synthase in cardiac nerve fibers and neurons of rat and guinea pig heart. Circ Res. 1992;71:1533–1537. doi: 10.1161/01.res.71.6.1533. [DOI] [PubMed] [Google Scholar]
- Mahajan RT, Chopda M. Phyto-Pharmacology of Ziziphus jujuba Mill-A plant review. Pharmacogn Rev. 2009;3:320–329. [Google Scholar]
- Mohebbati R, Iranmanesh M, Beheshti F, et al. The Effect of Some Herbal Extracts on Nitric Oxide Production in Endothelial Cells 3T3 Cell Line. Iran J Pharm Sinc. 2016;12:1–10. [Google Scholar]
- Mohebbati R, Shafei MN, Soukhtanloo M, Roshan NM, Rad AK, Anaeigoudari A, Hosseinian S, Karimi S, Beheshti F. Adriamycin-induced oxidative stress is prevented by mixed hydro-alcoholic extract of Nigella sativa and Curcuma longa in rat kidney. Avicenna J Phytomed. 2016;6:86–94. [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Sell RL, Hileman SM, Banks WA. Nitric oxide is a central component in neuropeptide regulation of appetite. Peptides. 2011;32:776–780. doi: 10.1016/j.peptides.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Sasser JM, Molnar M, Baylis C. Relaxin ameliorates hypertension and increases nitric oxide metabolite excretion in angiotensin II but not Nω-nitro-L-arginine methyl ester hypertensive rats. Hypertension. 2011;58:197–204. doi: 10.1161/HYPERTENSIONAHA.110.164392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34:665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- Shafei MN, Nasimi A. Effect of glutamate stimulation of the cuneiform nucleus on cardiovascular regulation in anesthetized rats: Role of the pontine Kolliker–Fuse nucleus. Brain Res. 2011;1385:135–143. doi: 10.1016/j.brainres.2011.02.046. [DOI] [PubMed] [Google Scholar]
- Steinkamp-Fenske K, Bollinger L, Xu H, Yao Y, Horke S, Förstermann U, Li H. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J Pharmacol Exp Ther. 2007;322:836–842. doi: 10.1124/jpet.107.123356. [DOI] [PubMed] [Google Scholar]
- Stewart DE. Venlafaxine and sour date nut. A J Psychiatry. 2004;161:1129. doi: 10.1176/appi.ajp.161.6.1129-a. [DOI] [PubMed] [Google Scholar]
- Taati M, Alirezaei M, Meshkatalsadat M, Rasoulian B, Kheradmand A, Neamati S. Antioxidant effects of aqueous fruit extract of Ziziphus jujuba on ethanol-induced oxidative stress in the rat testes. Iran J Vet Res. 2011;12:39–45. [Google Scholar]
- Wang D, Zhao Y, Jiao Y, Yu L, Yang S, Yang X. Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohydr Polym. 2012;88:1453–1459. [Google Scholar]
- Xiaoling H, Zhiyun X, Yi N, Jinliang L, Xianqiong F. Outline of the Investigation on the leaf of F tataricum bythe Means of Traditional Chinese Medicine and Western Modem Medicine. 1992:470–476. [Google Scholar]
- Zhang D, Yuan B, Sun H. the effect of jujuboside on rats with spontaneous hypertension. J Xi'an Jiaotong Univers. 2002;24:59–60. [Google Scholar]
- Zhang H, Jiang L, Ye S, Ye Y, Ren F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill) from China. Food Chem Toxicol. 2010;48:1461–1465. doi: 10.1016/j.fct.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang X, Li J, Bian Y, Sheng M, Liu B, Fu Z, Zhang Y, Yang B. Jujuboside B Reduces Vascular Tension by Increasing Ca 2+ Influx and Activating Endothelial Nitric Oxide Synthase. PloS one. 2016;11:149386. doi: 10.1371/journal.pone.0149386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha J, Dobešová Z, Kuneš J. Antihypertensive mechanisms of chronic captopril or N-acetylcysteine treatment in L-NAME hypertensive rats. Hypertens Res. 2006;29:1021–1027. doi: 10.1291/hypres.29.1021. [DOI] [PubMed] [Google Scholar]