Abstract
Objective:
The aim of the present study was to evaluate the possible protective effect of Plantago major (P. major) extract against doxorubicin (DXR)-induced renal inflammation in rats.
Materials and Methods:
80 male albino rats were randomly divided into 8 groups as follows: control, DXR, Ext (extract) 600, Ext1200, dexamethasone+DXR, vitamin E+DXR, Ext600+DXR, and Ext1200+DXR. Duration of the study was 35 days and DXR was intravenously injected on the 7th day of the experiment. Tumor necrosis factor-alpha (TNF-α) production and monocyte chemoattractant protein-1 (MCP-1) expression levels were assessed in the left kidney. Serum creatinine concentration and osmolarity were determined on the 1st, 14th, 21st, 28th and 35th days of the experiment.
Results:
DXR caused a significant increase in renal expression of MCP-1 and TNF-α production compared to control animals. Administration of dexamethasone, vitamin E and P. major extract significantly improved the expression of these inflammatory mediators compared to DXR group. Compared to day 1 in DXR group, serum osmolarity showed a significant increase on days 21, 28 and 35. Also, on these days, serum osmolarity in DXR group was significantly higher than that on the same days in control group. In Vit E+DXR and Ext 1200+DXR groups, there was no significant changes in serum osmolarity among different days of the study. However, in these groups, serum osmolarity on days 21, 28 and 35 showed a significant decrease compared to the same days in DXR group.
Conclusion:
Present results suggest that hydroethanolic extract of P. major protected renal tissue against DXR–induced renal inflammation.
Key Words: Plantago major, Doxorubicin, Vitamin E, Dexamethasone, Inflammation
Introduction
Doxorubicin (DXR), also known as adriamycin, is a widely used antineoplastic chemotherapeutic that is used for the treatment of different neoplastic conditions including uterine sarcoma, acute lymphoblastic leukemia (ALL), and multiple myeloma, as well as breast, liver and lung cancers (Katzung et al., 2012 ▶). Some of molecular underllying mechanisms proposed for DXR are intercalation with DNA and inhibition of nucleic acid synthesis, as well as reactive oxygen species (ROS) generation (Cummings et al., 1991 ▶). In spite of DXR's high efficacy in the treatment of tumors, its clinical use is highly restricted due to severe side effects; one of the most important adverse effect of DXR is nephropathy. Nephropathy is an important cause of nephrotic syndrome, a combination of proteinuria, low blood albumin level, hyperlipidemia and edema (Orth and Ritz, 1998 ▶). DXR-induced nephropathy is a widely used experimental model in rodents to produce experimental proteinuric nephropathy (Okuda et al., 1986 ▶). Although the exact mechanisms underlying DXR-induced nephropathy are not fully understood, the role of oxidative stress and inflammation has been demonstrated in this model (Szalay et al., 2015 ▶). ROS generation is assumed to have a central role. It has been shown that DXR injection leads to a marked increase in renal lipid peroxidation and decreases in renal total antioxidant capacity and antioxidant enzyme activity in rats (Mohebbati et al., 2016 ▶). Also, DXR administration leads to prominent tubulointerstitial inflammation with marked lymphocytes and macrophages infiltration. This local inflammation may be due to production of cytokines and growth factors like tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β) and chemotactic factors (monocyte chemoattractant protein-1 (MCP-1)) in response to proinflammatory mediators and infiltrated cells (Szalay et al., 2015 ▶). Nuclear factor κB (NF-κB) is a transcription factor that controls cytokine production and many other inflammatory genes expression. It has been demonstrated that inhibition of NF-κB reduced tubulointerstitial injury in DXR-induced nephropathy (Yamashita et al., 2017 ▶). Increasing data suggest that modifying inflammatory and oxidative pathways may alleviate DXR-induced renal damage. Plantago major, a flowering plant belonging to the family Plantaginaceae, is one of the most commonly grown medicinal plants throughout the world (Samuelsen, 2000 ▶). P. major contains different biologically active compounds including flavonoids, terpenoids, alkaloids, lipids, and polysaccharides (Jamilah et al., 2012 ▶) that possess anticancer, anti-ulcerogenic, immunomodulatory, antimicrobial, anti-inflammatory and antioxidant properties (Miraj, 2016 ▶). The aim of the present study was to evaluate the possible protective effects of P. major against DXR-induced renal inflammation in rats.
Materials and Methods
Extract preparation
P. major whole plant was well-dried and then grounded to powder. The powder was extracted using a Soxhlet extractor using ethanol (70%). The extract was concentrated in a rotary evaporator. It was then kept at 4oC prior to use.
Chemicals
DXR was purchased from Ebewe Pharma Company (Austria). Vitamin E (Vit E) and dexamethasone (DEX) were obtained from Osve Pharmaceutical Company (Iran).
Animals
Eighty male Albino Wistar rats weighing 200-250 g were obtained from the Animal House of the School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. The animals were housed at room temperature (25±1 oC) on a regular 12 hr/12 hr light/dark cycle with free access to food and water ad libitum. All experiments were approved by ethics committee of Mashhad University of Medical Sciences, Mashhad, Iran.
Experimental design
In the present study, animals were randomly divided into 8 groups of 10 rats as follows: 1) Control: this group received an injection of normal saline via intravenous tail injection (i.v.) on the 7th day of the experiment, 2) DXR: this group received an injection of DXR (5 mg/kg, i.v.) on the 7th day of the experiment, 3) Ext- 600: this group received P. major extract (600 mg/kg, in drinking water) for 5 consecutive weeks and injected with normal saline on the 7th day of the experiment, 4) Ext-1200: this group received P. major extract (1200 mg/kg, in drinking water) for 5 consecutive weeks and injected with normal saline on the 7th day of the experiment, 5) DEX+DXR: this group received an injection of DEX (0.9 mg/kg i.p.) for 6 consecutive days before injection of DXR, and for 2 weeks after that, every other day, 6) Vit E+DXR: this group received vitamin E (100 mg/kg, in drinking water) for 5 consecutive weeks and injected with DXR on the 7th day of the experiment, 7) Ext 600+DXR: this group received P. major extract (600 mg/kg, in drinking water) for 5 consecutive weeks and injected with DXR on the 7th day of the experiment, and 8) Ext 1200+DXR: this group received P. major extract (1200 mg/kg, in drinking water) for 5 consecutive weeks and injected with DXR on the 7th day of the experiment.
Blood samples were collected from the orbital sinus on the 1st, 14th, 21st, 28th and 35th days of the experiment. Blood samples were centrifuged at 3000 rpm for 15 min, and serum was stored at -20 °C until used. Serum concentration of creatinine was measured by Convergys®100 Biochemistry Analyser using the commercial kit (Pars Azmoon Company, Iran). Serum osmolarity was determined by a cryoscopic osmometer (Osmomat 030, Germany). Four weeks after DXR injection, animals were killed and the left kidneys were removed and divided into two halves. One half was fixed in 10% formalin for the assessment of immunolocalization of MCP-1. The other half was kept at -80°C for determination of TNF-α concentration.
Immunohistochemical examinations
The fixed kidney tissues were embedded in paraffin and cut into 5-μm thick sections. Then, tissue sections were deparaffinized and incubated with MCP-1 antibody (rabbit polyclonal anti-MCP-1, Biorbyt, UK; dilution 1:100) at 4°C overnight; then; samples were incubated with HRP- conjugated secondary antibody (goat polyclonal secondary antibody to rabbit IgG, Biorbyt, UK; dilution 1:100). The sections were finally incubated with diaminobenzidine (DAB) as the chromogen. Then, the slides were counterstained with hematoxylin and finally examined by light microscopy. Three blind examiners evaluated the intensity of staining using the following scoring system: 0=no staining, 1=weak, 2=moderate, and 3=severe (Zhang et al, 2010 ▶).
Determination of TNF-α concentration
Briefly, kidney tissue was homogenized and centrifuged at 6000 rpm for 10 min. The supernatant was collected and TNF-α level was measured using anti-rat TNF-α ELISA kit (IBL international, US) according to the manufacturer's instructions.
Statistical analysis
All data were expressed as means±SEM. Comparison among groups was made using one-way ANOVA followed by LSD post hoc test. Intragroup comparisons were made using repeated measures. Differences were considered statistically significant when p<0.05.
Results
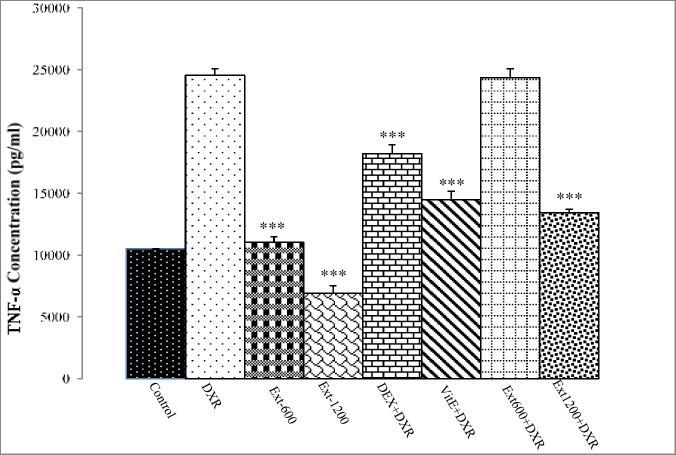
Renal TNF-α concentration significantly increased in DXR group compared to the control group (p<0.001). In Ext-600 and Ext-1200 groups, renal TNF-α production was significantly lower than that of the DXR group (p<0.001). Administration of DEX and Vit E significantly decreased renal TNF-α protein level compared to DXR-treated rats (p<0.001). Treatment of DXR-injected rats with P. major extract (1200 mg/kg) led to a significant reduction in renal TNF-α production compared to DXR group (Figure 1).
Figure 1.
Renal TNF-α concentration in different experimental groups. Values are expressed as mean±SEM. *** p<0.001 indicates significant differences compared to the DXR group. DXR: doxorubicin; DEX: dexamethasone; Vit E: vitamin E; and Ext: Plantago major extract).
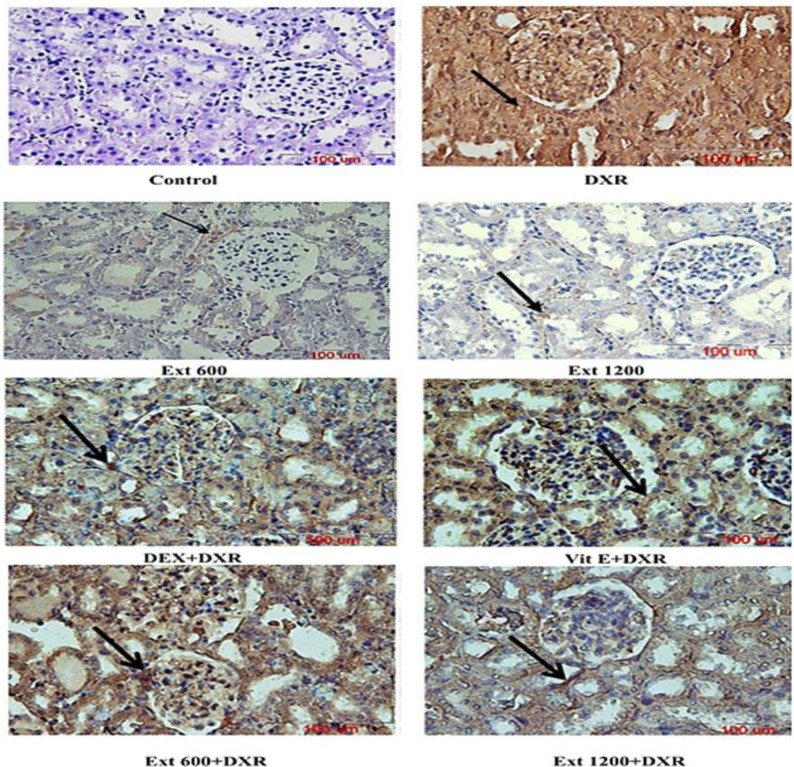
Figure 2 shows renal immunohistochemical localization of MCP-1 protein and Table 1 demonstrates the estimation of MCP-1 immunoreactivity in renal tissue in different experimental groups. The results of immunohistochemical staining showed very weak expression of MCP-1 in renal tissues of the control animals, as well as, the Ext-600 and Ext-1200 groups. However, immunoreactivity for MCP-1 was strong in DXR-treated rats.
Figure 2.
Photomicrographs showing immunolocalization of MCP-1 in the cortex (A) and medulla (B) of different experimental groups. Immunoreactivity is shown with arrows (magnification 400X; scale bar =100 µm). DXR: doxorubicin; DEX: dexamethasone; Vit E: vitamin E; and Ext: Plantago major extract.
Table 1.
The intensity scores of renal MCP-1 expression in different groups of animals.
| Control | DXR | Ext-600 | Ext-1200 | DEX+DXR | Vit E+DXR | Ext 600+DXR | Ext 1200+DXR | |
|---|---|---|---|---|---|---|---|---|
| MCP-1 | 1 | 4 | 1 | 1 | 2 | 2 | 2 | 1-2 |
Moderate MCP-1 immunoreactivity was observed in DEX+DXR and Vit E+DXR groups compared with DXR- treated rats. Also, the staining intensity of MCP-1 in kidney tissue decreased after administration of P. major extract at doses of 600 and 1200 mg/kg compared with DXR group (Figure 2 and Table 1).
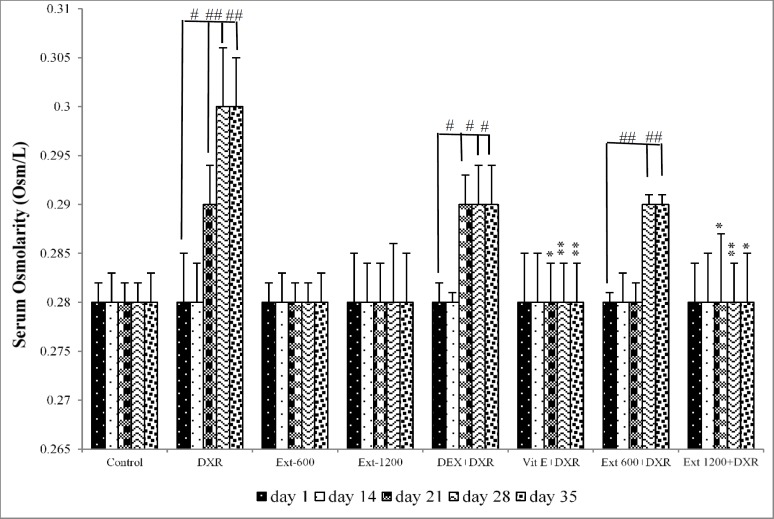
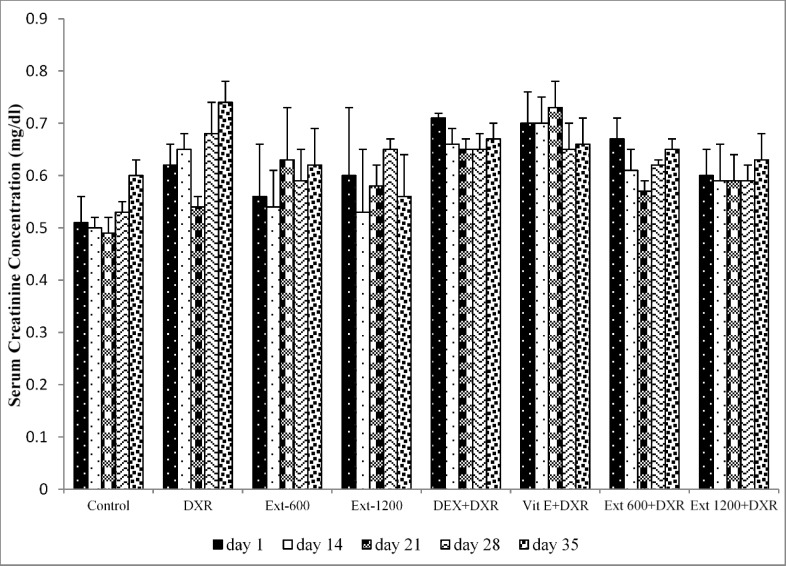
In the control, Ext-600 and Ext-1200 groups, there was no significant differences in serum osmolarity among different days of the experiment. However, compared to day 1 in DXR group, serum osmolarity showed a significant increase on days 21, 28 and 35 of the study (p<0.05, p<0.01 and p<0.01 respectively) (Figure 3). Additionally, on these days, serum osmolarity in DXR group was significantly higher than that of the control group on the same days (p<0.05, p<0.001 and p<0.01, respectively) (Figure 3). In the Vit E+DXR and Ext 1200+DXR groups, there was no significant differences in serum osmolarity among different days of the study. However, in the Vit E+DXR group, serum osmolarity on days 21, 28 and 35 showed a significant decrease compared to that of the DXR group on the same days (p<0.05, p<0.01 and p<0.05, respectively) (Figure 3). Furthermore, on these days, serum osmolarity in the Ext 1200+DXR group was also significantly lower than that of DXR-injected animals (p<0.05, p<0.01 and p<0.05, respectively) (Figure 3). However, serum creatinine concentration showed no significant difference among different days of the study (Figure 4).
Figure 3.
Serum osmolarity in all experimental groups. Values are expressed as mean±SEM.
*p<0.05 and **p<0.01 indicate significant differences compared to the results of the same days in DXR group.
#p<0.05 and ##p<0.01 indicate significant differences compared to day 1 in the same group.
(DXR: doxorubicin; DEX: dexamethasone; Vit E: vitamin E; and Ext: Plantago major extract).
Figure 4.
Serum creatinine concentration in all experimental groups. Values are presented as mean±SEM.
(DXR: doxorubicin; DEX: dexamethasone; Vit E: vitamin E; and Ext: Plantago major extract).
Discussion
The results of the current study indicated that P. major extract significantly improves the renal expression of MCP-1 and TNF-α in DXR-injected rats. Various mechanisms have been suggested to explain DXR-induced nephropathy (Lee and Harris, 2011 ▶). Although it is commonly accepted that proteinuria is caused by injury to glomerular endothelial cells, glomerular basement membrane and podocytes, the exact mechanism of the DXR-induced nephropathy is not fully understood. In many experimental models of renal injury including cisplatin-induced nephrotoxicity (Hosseinian et al., 2016 ▶; Parhizgar et al., 2016 ▶), renal ischemia-reperfusion (Havakhah et al., 2014), unilateral ureteral obstruction (Hosseinian et al., 2017 ▶), and DXR-induced nephropathy (Mohebbati et al., 2016 ▶; Mohebbati et al., 2017 ▶), the role of oxidative stress and inflammation has been demonstrated. It has been postulated that free radical generation, lipid peroxidation and antioxidant enzymes inhibition are the main mechanisms underlying DXR nephrotoxicity (Szalay et al., 2015 ▶). In addition, DXR leads to a severe tubulointerstitial inflammation by local generation of cytokines and chemotactic factors in response to cellular injury, plasma protein filtration and glomerular inflammatory mediators (Rangan et al., 2000 ▶). The exact mechanisms underlying inflammatory action of DXR are unclear, but in recent investigations, the role of nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs) signaling pathways and their interaction have been postulated (Kim et al., 2017 ▶). TNF-α, a pro-inflammatory cytokine, is produced after DXR administration by glomerular and tubular cells and extrinsic infiltrated inflammatory cells and acts through MAPKs and NF-κB signaling pathways (Neale et al., 1995 ▶). Activation of these pathways then, upregulates the expression of some inflammatory cytokines including TNF-α and MCP-1 (Hosseinian et al., 2017 ▶). In the present study, the nephropathy was created by a single dose intravenous injection of DXR (5 mg/kg). The results indicated a 2.33- fold increase in renal production of TNF-α, and a marked increase in renal expression of MCP-1 after DXR administration. These findings have also been reported by Rangan et al., (2000) ▶ and Benchetrit et al. (2001) ▶ who reported the upregulation of the above-mentioned inflammatory mediators following DXR administration (Rangan et al., 2000 ▶; Benchetrit et al., 2001 ▶). The present study revealed a significant decrease in TNF-α concentration in P. major extract-treated rats. Interestingly, the higher dose of the extract (1200 mg/kg) exerted more marked protection against TNF-α production compared to the lower dose (600 mg/kg). Also, a remarkable reduction was observed in MCP-1 expression in animals treated with P. major extract, showing that treatment with P. major extract attributes to improvement of inflammation. Our results also showed that these beneficial effects of P. major extract (1200 mg/kg) were comparable and even more pronounced than those of DEX and Vit E, which can confirm the anti-inflammatory and antioxidant effects of P. major extract. In the present work, serum osmolarity in DXR group showed a significant increase on the 21st, 28th and 35th days of the experiment. The exact mechanism of DXR effect on serum osmolarity has not been fully elucidated, but it seems that enhanced epithelial Na+/K+-ATPase activity, hypovolemia and sodium retention might be involved in this process (Deschenes and Doucet, 2000 ▶). Present results also showed that reducing effect of P. major extract on serum osmolarity in the Ext 1200+DXR group was more marked than those of the Ext 600+DXR group, which might indicate a dose-dependent improving effect for P. major extract on tubular transport processes. Meanwhile, based on our findings, serum creatinine concentration showed no significant differences among different experimental groups. This result possibly indicates that DXR administration with the dose used in this study, has no significant effect on renal processing of creatinine.
In conclusion, the present study showed that P. major extract in a dose-dependent manner could improve renal inflammation associated with DXR that might partly be due to its antioxidant and anti-inflammatory actions. However, the exact mechanisms underlying the effects of P. major needs to be further evaluated.
Acknowledgment
This study was part of an M.Sc. thesis and financially supported by Research Council of Mashhad University of Medical Sciences, Mashhad, Iran.
Conflicts of interest
Authors declare no conflicts of interest.
References
- Benchetrit S, Golan E, Podjarny E, Green J, Rashid G, Bernheim J. Low molecular weight heparin reduces proteinuria and modulates glomerular TNF-alpha production in the early phase of adriamycin nephropathy. Nephron. 2001;87:155–160. doi: 10.1159/000045905. [DOI] [PubMed] [Google Scholar]
- Cummings J, Anderson L, Willmott N, Smyth JF. The molecular pharmacology of doxorubicin in vivo. Eur J Cancer Clin Oncol. 1991;27:532–535. doi: 10.1016/0277-5379(91)90209-v. [DOI] [PubMed] [Google Scholar]
- Deschenes G, Doucet A. Collecting Duct Na+/K+-ATPase Activity Is Correlated with Urinary Sodium Excretion in Rat Nephrotic Syndromes. J Am Soc Nephrol. 2000;11:604–615. doi: 10.1681/ASN.V114604. [DOI] [PubMed] [Google Scholar]
- Havakhah S, Sadeghnia HR, Hadjzadeh MR, Mohammadian Roshan N, Hosseinzadeh H, Mohareri N, Khajavi Rad A. Effect of Nigella sativa on ischemia-reperfusion induced rat kidney damage. Iran J Basic Med Sci. 2014;17:986–992. [PMC free article] [PubMed] [Google Scholar]
- Hosseinian S, Khajavi Rad A, Ebrahimzadeh A, Soukhtanloo M, Sadeghnia HR, Shafei MN. Thymoquinone ameliorates renal damage in unilateral ureteral obstruction in rats. Pharmacol Rep. 2017;69:648–657. doi: 10.1016/j.pharep.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Hosseinian S, Khajavi Rad A, Hadjzadeh MR, Mohammadian Roshan N, Shafiee S. The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats. Avicenna J Phytomed. 2016;6:44–54. [PMC free article] [PubMed] [Google Scholar]
- Jamilah J, Sharifa A, Sharifah N. GC-MS analysis of various extracts from leaf of Plantago major used as traditional medicine. World Appl Sci J. 2012;17:67–70. [Google Scholar]
- Katzung B, Masters S, Trevor A. Basic and clinical pharmacology. NewYork: McGrow-Hill; 2012. p. 1108. [Google Scholar]
- Kim DR, Lee SY, Kim JS, Kim YG, Moon JY, Lee SH. Ameliorating Effect of Gemigliptin on Renal Injury in Murine Adriamycin-Induced Nephropathy. Biomed Res Int. 2017 doi: 10.1155/2017/7275109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrol. 2011;16:30–38. doi: 10.1111/j.1440-1797.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- Miraj S. A review study of pharmacological properties of plantago major l. Der Pharma Chemica. 2016;8:21–25. [Google Scholar]
- Mohebbati R, Shafei MN, Beheshti F, Soukhtanloo M, Mohammadian Roshan N, Anaeigoudari A, Parhizgar S, Hosseinian S, Khazdeir MR, Khajavi Rad A. Mixed Hydroalcoholic Extracts of Nigella sativa and Curcuma longa Improves Adriamycin-Induced Renal Injury in Rat. Saudi J Kidney Dis Transpl. 2017;28:1270–1281. doi: 10.4103/1319-2442.220880. [DOI] [PubMed] [Google Scholar]
- Mohebbati R, Shafei MN, Soukhtanloo M, Roshan NM, Khajavi Rad A, Anaeigoudari A, Hosseinian S, Karimi S, Beheshti F. Adriamycin-induced oxidative stress is prevented by mixed hydro-alcoholic extract of Nigella sativa and Curcuma longa in rat kidney. Avicenna J Phytomed. 2016;6:86–94. [PMC free article] [PubMed] [Google Scholar]
- Neale TJ, Rüger B, Macaulay H, Dunbar PR, Hasan Q, Bourke A. Tumor necrosis factor-alpha is expressed by glomerular visceral epithelial cells in human membranous nephropathy. Am J Pathol. 1995;146:1444–1454. [PMC free article] [PubMed] [Google Scholar]
- Orth SR, Ritz E. The nephrotic syndrome. New England J Med. 1998;338:1202–1211. doi: 10.1056/NEJM199804233381707. [DOI] [PubMed] [Google Scholar]
- Okuda S, Oh Y, Tsuruda H, Onoyama K, Fujimi S, Fujishima M. Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int. 1986;29:502–510. doi: 10.1038/ki.1986.28. [DOI] [PubMed] [Google Scholar]
- Parhizgar S, Hosseinian S, Hadjzadeh MR, Soukhtanloo M, Ebrahimzadeh Bideskan A, Mohebbati R, Naji Ebrahimi Yazd Z, Khajavi Rad A. Renoprotective Effect of Plantago Major Against Nephrotoxicity and Oxidative Stress Induced by Cisplatin. Iran J Kidney Dis. 2016;10:182–188. [PubMed] [Google Scholar]
- Rangan GK, Wang Y, Tay YC, Harris DC. Cytokine gene expression in Adriamycin nephropathy: Effects of antioxidant nuclear factor κB inhibitors in established disease. Nephron. 2000;86:482–490. doi: 10.1159/000045838. [DOI] [PubMed] [Google Scholar]
- Samuelsen AB. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J Ethnopharmacol. 2000;71:1–21. doi: 10.1016/S0378-8741(00)00212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay CI, Erdélyi K, Kökény G, Lajtár E, Godó M, Révész C. Oxidative/nitrative stress and inflammation drive progression of doxorubicin-induced renal fibrosis in rats as revealed by comparing a normal and a fibrosis-resistant rat strain. PloS One. 2015;10:e0127090. doi: 10.1371/journal.pone.0127090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Yoshida T, Suzuki S, Homma K, Hayashi M. Podocyte-specific NF-κB inhibition ameliorates proteinuria in adriamycin-induced nephropathy in mice. Clin Exp Nephrol. 2017;21:16–26. doi: 10.1007/s10157-016-1268-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol. 2010;21:966–973. doi: 10.1681/ASN.2009080872. [DOI] [PMC free article] [PubMed] [Google Scholar]