Abstract
In recent years, nerve growth factor (NGF) and the NGF receptor have become potential therapeutic targets in the treatment of acute and chronic pain states. NGF is a neurotrophin involved in regulating the function of sensory and sympathetic neurons during development. Numerous pain states have been linked to elevated levels of NGF and its role in increasing the perception of pain. Tanezumab, a recombinant humanized monoclonal antibody (IgG), was developed to target NGF, binding both circulating and local tissue NGF preventing interaction with the tropomyosin-related kinase-A and p75 receptors. Recent clinical studies with tanezumab in different patient populations to date, including osteoarthritis, low back pain, and diabetic peripheral neuropathy, demonstrate efficacy with few side effects, including transient arthralgias, paresthesias, hypoesthesia, and rarely, osteonecrosis. Anti-NGF antibodies are a novel therapy in pain management and have shown promise in the treatment of certain pain conditions, which at present are poorly treated. Tanezumab offers an exciting new class of analgesics that has the potential to change the treatment of pain.
Keywords: Monoclonal antibody, nerve growth factor, neuropathic pain, tanezumab, tropomyosin-related kinase
Introduction
Pain, both acute and chronic, remains a challenge for scientists and clinicians with regard to understanding its pathogenesis and efficacious treatments. In this regard, chronic pain is prevalent among Americans, affecting roughly 100 million people.[1] This condition has a significant impact on individual patients’ quality of life, as it is strongly associated with disability and poor self-rated health. In addition, patients suffering from chronic inflammatory and neuropathic pain have decreased productivity and increase health-care costs, which contribute significantly to the overall economic burden on society. In 2011, the Institute of Medicine estimated losses of up to $635 billion dollars each year for medical treatment of pain-related conditions and lost economic productivity.[1] Hence, treatments are being developed with the aim to improve functional status and to reduce suffering, thereby decreasing health care visits and costs. The current best practice involves multimodal analgesic therapy, including both pharmacologic and nonpharmacologic modalities, to optimize patient outcomes and to minimize adverse effects. Current therapies include, but are not limited to, nonsteroidal anti-inflammatory drugs (NSAIDs), neuropathic medications, antidepressants, opioids, and targeted spinal injections.[2] Each modality has a unique risk profile that necessitates thoughtful planning before instituting each specific therapy. For instance, the use of NSAIDs is limited in patients due to renal, cardiovascular and gastrointestinal side effects.[3,4] The high incidence of cognitive dysfunction, respiratory depression, and addiction in patients taking opioids mandates the development of new therapeutic targets.[5,6] Patient selection and predictors of success for spinal injections continue to be a subject of much debate with regard to efficacy and potential risks.[7]
In recent years, basic science and clinical research advancements have helped better understand the pathophysiology of pain. Some current areas of research have focused on compounds that attenuate glial activation (e.g., minocycline and methylxanthine derivatives); drugs that inhibit proinflammatory cytokine production (e.g., cytokine inhibitors and antagonists to toll-like receptor 4 activation); and anti-inflammatory agents that reduce inflammation.[8] One of the more promising specific targets that have evolved from this research is nerve growth factor (NGF) and its receptor.[5] Tanezumab is a recombinant humanized monoclonal antibody (IgG) that was developed by Pfizer to target NGF for the treatment of several pain conditions.[5,6] Figure 1 shows the molecular formula of tanezumab. The Fc mutation limits antibody dependent cell mediated toxicity and complement activation. The drug is an antibody that has high sensitivity and specificity for NGF.[6] Its favorable pharmacokinetic profile allows it to bind both circulating and local tissue NGF, thereby preventing interaction with the tropomyosin-related kinase-A (TrK-A) and p75 receptors. Tanezumab is a large protein and hence does not cross the blood–brain barrier. Its plasma half-life is 22–25 days.[7,8] Anti-NGF therapy appears to be antihyperalgesic (normalizing a decreased nociceptive threshold) as apposed to an analgesic (increasing normal and sensitized nociceptive thresholds).[8]
Figure 1.
Molecular formula of tanezumab
Pharmacological Properties
NGF is a neurotrophin involved in regulating the function of sensory and sympathetic neurons during development. In adults, it serves as a modulator of nociception and is found to be elevated in chronic pain conditions leading to increased perception of pain [11] [Figure 2]. These large tightly bound homodimer protein molecules bind to a family of receptors called tropomyosin-related kinase (TrK) with high affinity and to the p75 receptor with low affinity.[11,12] The most notable of the receptors is TrK-A, which regulates ion channels and molecules that are critical in the signaling of pain. NGF is elevated in inflammatory conditions due to its release by mast cells, macrophages, and lymphocytes. TrK-A is found in high quantities in nerve fibers in the dorsal root ganglion and has been shown in animal studies to propagate chronic pain.[12] On NGF binding TrK-A, the complex is taken up peripherally and transported to the cell soma where it activates transcription factors affecting gene expression. Both in rodents and humans, cutaneous administration of NGF had led to hyperalgesia within 1–3 h. The rapid nociceptor sensitization of cutaneous receptors shows NGF plays a pivotal role in both acute nociceptive responses and in chronic pain.[13,14]
Figure 2.
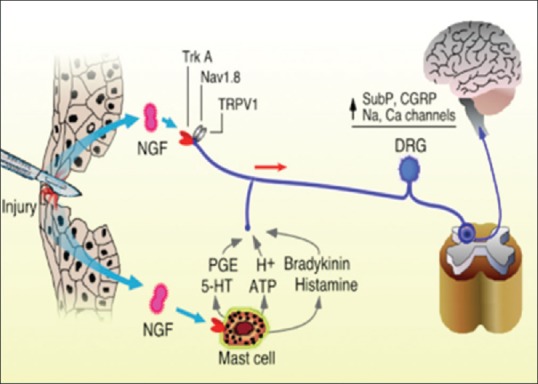
Role of nerve growth factor in modulating pain signals to the central nervous system
Clinical Indications
The Biological Investigational New Drug application for tanezumab was submitted to the Food and Drug Administration (FDA) in April 2004 for indications related to the treatment of moderate to severe chronic pain.[3] Clinical trials (Phase I and II) demonstrated the efficacy of tanezumab in treating the osteoarthritic pain of the hip and knee joint as well as chronic low back pain.[15,16,17,18] Due to reports of osteonecrosis and increased need for total joint replacements, the FDA placed the drug on clinical hold for on-going investigations related to osteoarthritis (OA) on June 22, 2010. On December 23, 2010, the drug was put on hold for all clinical investigations except for cancer. These events were investigated by an independent, expert adjudication committee, which did not find evidence indicating the aforementioned claims.[5,19] Increased risk for rapidly progressive OA was noted in patients who received concomitant therapy with anti-NGF agents and NSAIDs.[20] The Arthritis Advisory Committee meeting held on March 12, 2012 reviewed the results and endorsed the continued clinical development of tanezumab. On August 28, 2012, the partial clinical hold on tanezumab was lifted.[5]
Table 1 lists the current indications investigated in the literature. Tanezumab studies included placebo-controlled and active-controlled (comparing to naproxen and oxycodone) studies. Table demonstrates the different doses of tanezumab used for the treatment of patients with OA: 2.5 mg, 5 mg, 10 mg in Phase II trials and 10 μg/kg, 25 μg/kg, 50 μg/kg, 100 μg/kg, and 200 μg/kg in Phase III trials. The doses used for chronic low back pain ranged from 5 mg to 20 mg every 8 weeks or a single shot of intravenous 200 μg/kg.[18,21,22] Tanezumab demonstrated superiority in the efficacy of pain control compared to placebo in these patients.[17,18,21,22,23,24,25] Tanezumab compared to NSAIDs and opioids showed greater efficacy for Western Ontario and McMaster Universities osteoarthritis Index (WOMAC) pain, WOMAC function and Patient's Global Assessment in patients with hip or knee OA.[23,25] While comparing the average low back pain scores and Roland Morris Disability Questionnaire responses, patients receiving tanezumab reported significantly better efficacy in pain control and functional improvement.[21,22] Other investigations are exploring the use of tanezumab to provide relief for neuropathic pain. Bramson et al. showed effective pain reduction in patients with diabetic peripheral neuropathy when they received 20 mg subcutaneous injections every 8 weeks.[26] For doses ranging between 50 mcg/kg and 200 mcg/kg given intravenously to patients with post-herpetic neuralgia, there was no significant benefit demonstrated in the same study.[26] The differing NGF involvement in these two disease processes may be responsible for the difference in the effectiveness of the drug to treat each condition.
Table 1.
Clinical indications for tanezumab and the most important papers in the literature investigating its efficacy for different chronic pain conditions
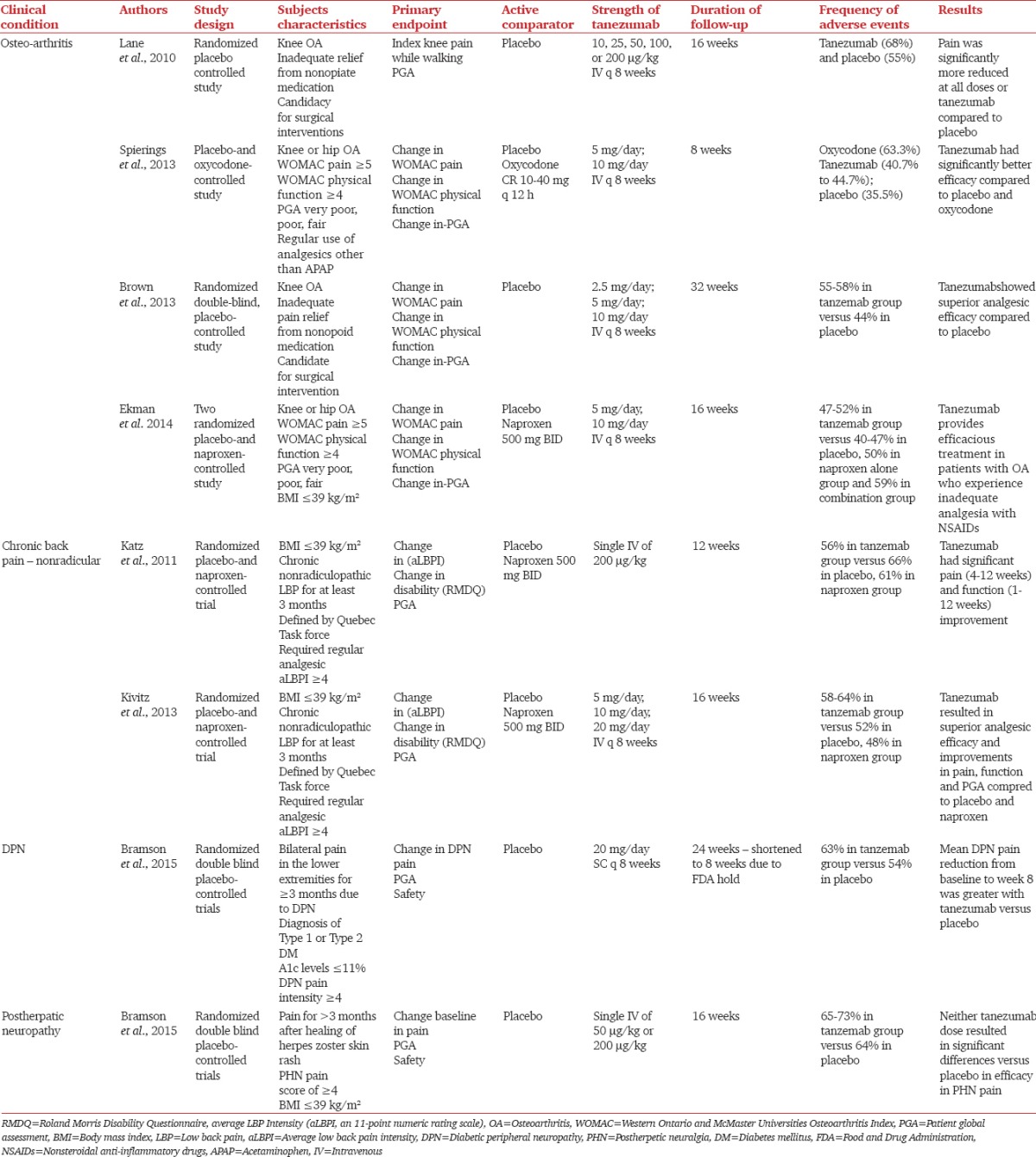
Patients suffering from OA and chronic low back pain reported greater pain reduction and improved functionality when compared to placebo and NSAIDs. The initial safety and tolerability profile compared favorably with current therapies. The adverse effects associated with cyclooxygenase-2 inhibitors and NSAIDs were not demonstrated in patients on tanezumab therapy. Neither does the novel drug appear to have any effects on the respiratory or central nervous system or raise concerns regarding abuse liability.[18] Adverse events (AEs) such as transient arthralgias, paresthesias, and hypoesthesia were noted in patients receiving tanezumab.[21,22] These AEs occurred more frequently in the tanezumab group when compared to placebo. However, the AEs were fewer compared to patients receiving opioids and mixed results were obtained when comparing to NSAIDs.[17,18,21,22,23,24,25] Safety, determined by odds ratios of withdrawals from studies due to AEs, was better at the lower doses than higher doses. These results reveal that antibodies to NGF provide efficacy in OA and that general safety at the lower doses appears similar to placebo. The severe adverse effects of osteonecrosis and earlier than expected joint replacement were investigated as noted above. Although a causal relationship was not seen, additional data on both efficacy and safety of these antibodies are needed to define the optimal dose to maximize benefit to risk. Based on the findings, the pharmaceutical companies, and the FDA negotiated restarting clinical trials, provided an avoidance of chronic concomitant NSAID and anti-NGF therapy, careful dose escalation in chronic pain patients, and discontinuing in patients who do not show improvement with initial doses. Patients with progressive OA are not to be included in further investigations.[5]
Conclusion
Acute and chronic pain states remain challenging to treat, yet there are several classes of drugs which are efficacious treatments with good safety profiles. Persistent chronic pain is a long-term condition which impacts the affected individuals by interfering with their quality of life both at home and at work and has significant societal consequences as well. Current therapies include NSAIDs, opioids, and neuropathic agents, but they may result in poorly tolerated side effects. Anti-NGF antibodies are a novel therapy in pain management and have shown promise in the treatment of conditions that are currently poorly treated.
To use anti-NGF antibodies for different pain conditions, further investigations are needed to determine the role NGF plays in pain maintenance. Based on the current findings, it has shown to have some benefit on chronic low back pain and osteoarthritic pain relief and functional improvement, but additional data are necessary to determine efficacy and safety. Further investigations are required as the data are lacking for the utility of the drug in postherpetic neuralgia. Other potential indications include treatment for interstitial cystitis, prostatitis, and pancreatitis. Continued attention to safety issues associated with tanezumab will also help determine its utility. Tanezumab offers an exciting new class of analgesics that has the potential to change the treatment of pain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Institute of Medicine, the National Institutes of Health. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. [Last accessed on 2015 Dec 12]. Available from: https://www.iom.nationalacademies.org/~/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf .
- 2.Bhangare KP, Kaye AD, Knezevic NN, Candido KD, Urman RD. An Analysis of New Approaches and Drug Formulations for Treatment of Chronic Low Back Pain. Anesthesiol Clin. 2017;;35:341–50. doi: 10.1016/j.anclin.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Vadivelu N, Gowda AM, Urman RD, Jolly S, Kodumudi V, Maria M, et al. Ketorolac tromethamine-routes and clinical implications. Pain Pract. 2015;15:175–93. doi: 10.1111/papr.12198. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Huffman LH. Medications for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration Center for Drug Evaluation and Research Background Materials. Meeting of the Arthritis Advisory Committee (AAC) [Last accessed on 2016 Mar 10]. Available from: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm301305.pdf .
- 6.Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27:85–91. doi: 10.1016/j.tips.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Friedly J, Standaert C, Chan L. Epidemiology of spine care: The back pain dilemma. Phys Med Rehabil Clin N Am. 2010;21:659–77. doi: 10.1016/j.pmr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–61. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdiche YN, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci. 2008;17:1326–35. doi: 10.1110/ps.035402.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115:128–41. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115:189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendell LM. Does NGF binding to p75 and trkA receptors activate independent signalling pathways to sensitize nociceptors? J Physiol. 2002;544(Pt 2):333. doi: 10.1113/jphysiol.2002.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45:252–61. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 15.Lane NE, Webster L, Shiao-Ping L, Gray M, Hefti F, Walicke P. RN624 (anti-NGF) improves pain and function in subjects with moderate knee osteoarthritis: A phase I study. Arthritis Rheumatol. 2005;52:S461. [Google Scholar]
- 16.Hefti F, Mokhtarani M, Gray M, Zhao C, Chan C. RN624 (anti-NGF) reduces pain and improves function in subjects with moderate to severe pain from osteoarthritis of the knee. Pain. 2006;7:S45. [Google Scholar]
- 17.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521–31. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152:2248–58. doi: 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC, Abramson SB, Hungerford DS, McCarthy E, Vignon EP, Smith MD, et al. Adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol. 2012;64:S113. doi: 10.1002/art.39492. [DOI] [PubMed] [Google Scholar]
- 20.Nickel JC, Atkinson G, Krieger JN, Mills IW, Pontari M, Shoskes DA, et al. Preliminary assessment of safety and efficacy in proof-of-concept, randomized clinical trial of tanezumab for chronic prostatitis/chronic pelvic pain syndrome. Urology. 2012;80:1105–10. doi: 10.1016/j.urology.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Kivitz AJ, Gimbel JS, Bramson C, Nemeth MA, Keller DS, Brown MT, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013;154:1009–21. doi: 10.1016/j.pain.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Gimbel JS, Kivitz AJ, Bramson C, Nemeth MA, Keller DS, Brown MT, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014;155:1793–801. doi: 10.1016/j.pain.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Spierings EL, Fidelholtz J, Wolfram G, Smith MD, Brown MT, West CR. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain. 2013;154:1603–12. doi: 10.1016/j.pain.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 24.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic hip pain: Results of a randomized, double-blind, placebo.controlled phase III trial. Arthritis Rheum. 2013;65:1795–803. doi: 10.1002/art.37950. [DOI] [PubMed] [Google Scholar]
- 25.Ekman EF, Gimbel JS, Bello AE, Smith MD, Keller DS, Annis KM, et al. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol. 2014;41:2249–59. doi: 10.3899/jrheum.131294. [DOI] [PubMed] [Google Scholar]
- 26.Bramson C, Herrmann DN, Carey W, Keller D, Brown MT, West CR, et al. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015;16:1163–76. doi: 10.1111/pme.12677. [DOI] [PubMed] [Google Scholar]


