Abstract
Medical gases are nowadays being used for a number of diverse clinical applications and its piped delivery is a landmark achievement in the field of patient care. Patient safety is of paramount importance in the design, installation, commissioning, and operation of medical gas pipeline systems (MGPS). The system has to be operational round the clock, with practically zero downtime and its failure can be fatal if not restored at the earliest. There is a lack of awareness among the clinicians regarding the medico-legal aspect involved with the MGPS. It is a highly technical field; hence, an in-depth knowledge is a must to ensure safety with the system.
Keywords: Central supply, hospital, nitrous oxide, oxygen
Introduction
Gas pipeline failures have been reported several times in the anesthesia literature. Petty reviewed pipeline-related deaths from 1972 to 1993 in the US and reported 26 deaths which were due to crossed pipelines, defective connectors, and error in the supply.[1] A survey by the Anesthesia Patient Safety Foundation showed a significant knowledge deficit among anesthesiologists regarding the medical gas pipeline systems (MGPS),[2] and adequacy of knowledge is a must to make the system safe.[3] The past decade witnessed many medico-legal cases compelling the clinicians to shift focus toward practical orientation of the system. Hence, maintaining safety standards is of prime importance starting from the main source at the manifold room to the final delivery point. The two main standards pertaining to MGPS are National Fire Protection Association 99 (US) and HTM 02-01 (UK). Others are British Standards EN 737 (BS EN 737), Compressed Gas Association, Canadian Standards Association, and the International Standards Organization. Cylinders should follow American Society for Testing and Materials standards, and pipes should have Lloyd's certification as per BS 2871.[4,5,6,7]
The core of the entire system is the manifold room which should be manned round the clock with trained personnel. It should have a suitable acoustic enclosure with 100% generator backup and fire security.[8] The location should be marked clearly for ease of identification in the event of an emergency.[9] It houses the control panel which allows a flow of 3000 L/min at 4.1 bar from the vacuum insulated evaporator (VIE) and relays alarm to secondary panels located throughout the hospital.[10]
Oxygen
Continuous supply of oxygen is the primary requisite of any medical unit. According to BS EN 737-3:2000, there should be three independent supply sources.[8] Primary, secondary, and a reserve source adequate to meet the demand in the event of primary and secondary supply failure. The manifold room should have 2 banks of D-type cylinders, each holding a minimum of 2 days consumption, attached to a fully automatic changeover control panel. Three-day consumption should be kept in reserve, as a contingency plan.[10] Aluminum or steel cylinders with Kevlar or Carbon fiber outer shell allow higher filling pressure,[11,12,13] enabling larger storage. Cylinders should be tested by manufacturers at intervals of 5 years.[12,13] Besides, oxygen concentrators or pressure swing adsorber system can supply a FiO2 ranging from 0.95 to 0.97, strictly monitored by a paramagnetic oxygen analyzer.[8] Liquid oxygen is an economical and convenient form of oxygen storage. The cryogenic liquid is stored in a VIE, usually of the capacity of 5 KL or 10 KL, although smaller Cryospeed™ vessels are also available.[14] VIE should be installed in a high security and fire safe zone after taking regulatory approval from the Fires and Explosives Act office in Nagpur.[8,15]
Compressed Air
It is used both as a driving force for equipment such as pneumatic drills (surgical air) or as an inhalational gas (medical air). The plant must ensure a flow of 3 KL/min at 8 bar, reduced thereafter as per requirement. Medical air needs a flow rate of 80 L/min at 4 bar and surgical airflow at the rate of 350 L/min at 7 bar. The medical air quality should meet the standards laid by the European Pharmacopoeia,[8] restricting the carbon monoxide level to 5 ml/m3. Integral dryers, filters, and dew point monitor control the humidity to its allowable limit of 67 ml/m3.
Vacuum and Anesthetic Gas Scavenging System
Vacuum pressure of −300 mmHg is required at the terminal unit with a flow of 40 L/min.
Anesthetic gases are considered to be substances hazardous to health as per the Control of Substances Hazardous to Health Regulations 2002 except where they are administered to a patient in the course of treatment. Exhaust of both the systems should be carefully positioned away from the windows and intake of air compressors and ventilators.[16] To control the greenhouse effect of the anesthetic gases, the anesthetic gas scavenging systems should incorporate a canister system which captures the unused gases, filters, and recycles them.[17]
Pipeline System
Copper seamless pipes with fluxless silver brazing are used which should be as per ASTM standard and Lloyd's certification.[8,13] They are intercepted by the area valve service units (AVSUs) and area alarm panels (AAPs)
[Figures 1 and 2]. AVSUs are placed in each clinical sector, to cutoff the gas delivery to the area beyond it during maintenance or to handle emergency. AAPs display the line pressures and have audiovisual alerts. All pipelines should be color coded with colored bands put at intervals of every 3 m[8] [Figure 3].
Figure 1.

Area valve service unit
Figure 2.

Area alarm panel
Figure 3.
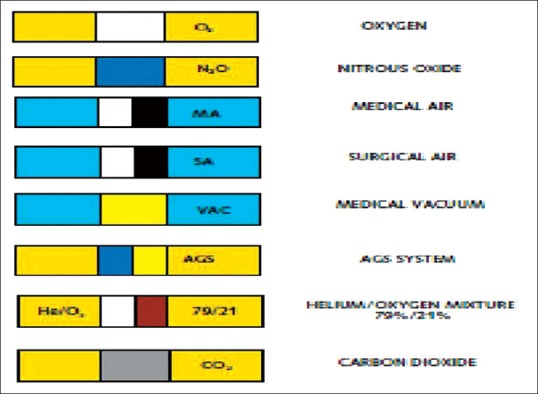
Pipeline color coding
Outlet Points
These are the final delivery points which are color coded, incorporating either the diameter index safety system or are of the quick connect type,[4] available in two varieties, the Diamond and Chemetron [Figures 4 and 5]. The Chemetron is a more sturdy variant and is resistant to leakage.
Figure 4.

Diamond-type oxygen outlet point
Figure 5.
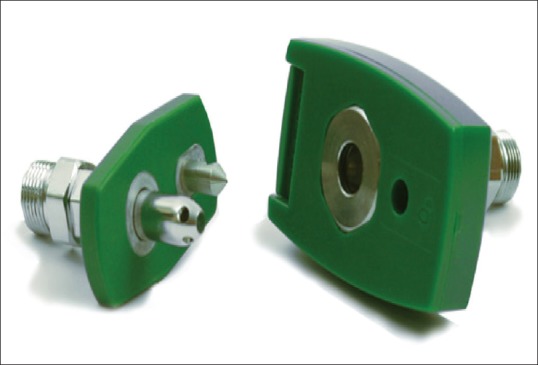
Chemetron-type oxygen outlet point
Every new installation needs to be tested and verified as per the laid guidelines before putting the system into use.[4,8,18]
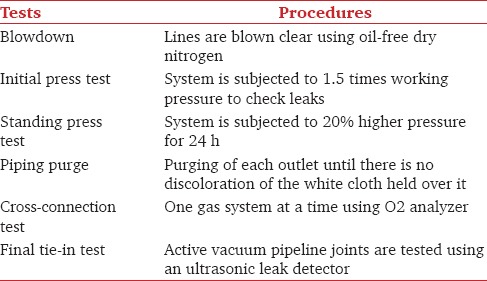
It is important to have a contingency plan to avoid crisis situations [Table 1].
Table 1.
Pipeline testing
Indigenous arrangement – all critical areas should have bulk oxygen cylinders, fitted with a double-stage regulator, tubing, and an adaptor. In case of manifold failure, the AVSU of the area is closed. The pipeline beyond it can now be fed with oxygen from this cylinder, by connecting the adaptor to any oxygen outlet point within the territory. Crisis due of vacuum pipeline failure can be tided over with portable electrical suction units.
The tender terms and conditions should specify that the supplier's bulk oxygen trailer should reach the hospital as soon as possible in the eventuality of pipeline failure.
However, besides quality control and knowledge, safety can be ensured only when there is a proper upkeep and maintenance of the system. This can be ensured by:
Formulating Standard Operating Procedures (SOP's)
Maintaining logbooks
Keeping all the equipment under Annual Maintenance Contract/ Comprehensive Maintenance Contract (AMC/CAMC), allotted to the original supplier of the Equipment or to vendors with minimum 3-year experience in the field
Preventive maintenance of equipment and leak test of pipeline should be ensured on quarterly basis
24 h manning by trained personnel
Periodic training of manifold personnel
Regular inspection of the pipelines, particularly after Public Works Department (PWD) work of every area
Daily checking of contingency plan
Mock drills of pipeline failure, fire, and explosion should be regularly conducted.
The vastness of the system has always remained a matter of concern whenever the issue of safety was raised. The anesthesiologists are an integral part of this system, but keeping in mind the medico-legal liabilities, the administrative control of the system should be vested upon the biomedical engineers, and it is imperative that all users should also be adequately trained.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Petty WC. AANA journal course: Update for nurse anesthetists – Medical gases, hospital pipelines, and medical gas cylinders: How safe are they? AANA J. 1995;63:307–24. [PubMed] [Google Scholar]
- 2.Peterson TG. Do you know your MGVS? Or what do anesthesiologists know about their MGVS? J Clin Monit. 1995;11:415–6. doi: 10.1007/BF01616753. [DOI] [PubMed] [Google Scholar]
- 3.Westwood M, Riley W. Medical gases, their storage and delivery. Anaesth Intensive Care Med. 2012;13:533–8. [Google Scholar]
- 4.Dorsch JA, Dorsch SE Medical gas pipeline system. Understanding Anesthesia Equipment. 5th ed. Ch. 2. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 25–50. [Google Scholar]
- 5.National Fire Protection Association. Quincy MA: National Fire Protection Association; 2005. Standard for Health Care Facilities (NFPA 99) [Google Scholar]
- 6.International Standards Organization. Geneva, Switzerland: International Standards Organization; 2002. Medical Gas Pipeline System. Part 1. [Google Scholar]
- 7.Canadian Standards Association. Toronto, Ontario: Canadian Standards Association; 1997. Medical Gas Terminal Units (CSA Z305.5.M86[R]) [Google Scholar]
- 8.Department of Health. Health Technical Memorandum 02-01. London: The Stationery Office; 2006. Medical Gas Pipeline Systems. Part A: Design, Installation, Validation and Verification; pp. 41–51. [Google Scholar]
- 9.Department of Health. Health Technical Memorandum 02.01. London: The Stationery Office; 2006. Medical Gas Pipeline Systems. Part B: Design, Installation, Validation and Verification. [Google Scholar]
- 10.Das S, Chattopadhyay S, Bose P. The anaesthesia gas supply system. Indian J Anaesth. 2013;57:489–99. doi: 10.4103/0019-5049.120145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.British Oxygen Company Group PLC. Cylinder Data Chart. [Last accessed on 2016 Dec 21]. Available from: http://www.bocmedical.co.uk .
- 12.Lovell T. Medical gases, their storage and delivery. Anaesth Intensive Care Med. 2004;5:10–4. [Google Scholar]
- 13.Spence AA, Fee JP, Nunn G, Ross J, Garrett M, Henrys P, et al. 2nd ed. Oxford:; 2005. Medical Gases: Their Properties and Uses; pp. 85–96. [Google Scholar]
- 14.British Oxygen Company Group PLC. Liquid Oxygen. [Last accessed on 2016 Dec 21]. Available from: http://www.bocmedical.co.uk .
- 15.Petroleum and Explosives safety Organization, Nagpur. Apex Manual ISO9001:2008. [Last accessed on 2016 Dec 26]. Available from: http://www.peso.gov.in/pdf .
- 16.HSC. General COSHH ACOP, Biological Agents, Control of Substances Hazardous to Health Regulations HSE Books. 2013 (Approved Codes of Practice and Guidance) [Google Scholar]
- 17.A Remedy for the Operating Room. Blue-Zone Technologies Ltd; [Last accessed 2016 Dec 14]. Available from: http://www.bluezone.ca/site05/Home/tabid/36/Default.aspx . [Google Scholar]
- 18.Bland H. The supply of anaesthetic and other medical gases. In: Davey A, Diba A, editors. Ward's Anaesthetic Equipment. 5th ed. Ch. 1. China: Elsevier Saunders; 2005. pp. 23–45. [Google Scholar]


