Abstract
Tuberculosis (TB) is one of the most fatal infectious diseases and a leading cause of mortality, with 95% of these deaths occurring in developing countries. The causative agent, Mycobacterium tuberculosis (Mtb), has a well-established ability to circumvent the host’s immune system for its intracellular survival. microRNAs (miRNAs) are small, non-coding RNAs having an important function at the post-transcriptional level and are involved in shaping immunity by regulating the repertoire of genes expressed in immune cells. It has been established in recent studies that the innate immune response against TB is significantly regulated by miRNAs. Moreover, differential expression of miRNA in Mtb infection can reflect the disease progression and may help distinguish between active and latent TB infection (LTBI). These findings encouraged the application of miRNAs as potential biomarkers. Similarly, active participation of miRNAs in modulation of autophagy and apoptosis responses against Mtb opens an exciting avenue for the exploitation of miRNAs as host directed therapy (HDT) against TB. Nanoparticles mediated delivery of miRNAs to treat various diseases has been reported and this technology has a great potential to be used in TB. In reality, this exploitation of miRNAs as biomarkers and in HDT is still in its infancy stage, and more studies using animal models mimicking human TB are advocated to assess the role of miRNAs as biomarkers and therapeutic targets. In this review, we attempt to summarize the recent advancements in the role of miRNAs in TB as immune modulator, miRNAs’ capability to distinguish between active and latent TB and, finally, usage of miRNAs as therapeutic targets against TB.
Keywords: Mycobacterium tuberculosis, miRNA expression, immune regulation, autophagy, apoptosis, biomarker, nanoparticles, host directed therapy
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) is one of the most fatal infectious diseases (Dye and Williams, 2010). Current estimations show that 1/3 of the world population is latently infected with Mtb. However, only 5–10% of infected people develop active TB in their lifetime (Bhatt and Salgame, 2007). It is the leading infectious cause of deaths, exceeding HIV/AID, resulting in approximately 1.3 million human deaths in 2016 (WHO, 2017). Mtb is an intracellular pathogen and its ability to survive inside host macrophages and tubercle granulomas constitutes the major attribute of its virulence. Macrophages are an important component of the host innate immune response and are able to generate a variety of antimicrobial molecular responses, such as antibacterial peptides, hydrolases and toxic reactive oxygen and nitrogen intermediates (Fenton and Vermeulen, 1996). Research on how Mtb is capable of surviving in this antibacterial environment remains an area of interest for many research groups. Accumulating evidence suggests that Mtb is capable of modulating cellular processes such as cytokine production, autophagy, apoptosis, MHC class II expression and phagolysosome maturation in macrophages and dendritic cells (Ahmad, 2011). Currently, many studies have reported that most of the above mentioned cellular mediated immune responses of eukaryotic cells are under the control of miRNAs (Bartel, 2004; Giraldez et al., 2005) and it is now established that modulation of miRNAs expression associated with these biological processes is one of the important strategies implemented by bacterial pathogens to survive inside host immune cells (Das et al., 2016).
miRNAs are small, non-coding RNAs having an important function at the post-transcriptional level to regulate gene expression (O’Connell et al., 2010; Watanabe and Kanai, 2011). Binding of miRNAs to their complementary sequences in the 3′ untranslated region (3′ UTR) of their respective protein coding mRNA targets results in transcript degradation or translational inhibition (Maute et al., 2014). The human genome may encode more than two thousands potentially functional miRNAs (Kozomara and Griffiths-Jones, 2014; Hammond, 2015) and it is estimated that one-half of all protein-coding transcripts are subjected to miRNA regulation (Bartel, 2009; Krol et al., 2010; Watanabe and Kanai, 2011; Vera et al., 2013). Each miRNA may suppress multiple genes and one mRNA can be targeted by multiple miRNAs. Therefore, disease-associated miRNAs represent a new class of diagnostic markers or therapeutic targets (Mayr et al., 2007; Pillai et al., 2007; Subramanian and Steer, 2010; Jackson and Levin, 2012; Mendell and Olson, 2012; Nana-Sinkam and Croce, 2013). Moreover, some bioinformatics studies hint at the existence of tens of thousands of short non-coding RNAs similar to miRNAs with the potential to regulate expression of most or all human genes, although it is unclear whether all of the small RNAs are functional or they are just fragments of larger RNAs (Brameier and Wiuf, 2007; Dieci et al., 2007; Bentwich, 2008). Previously, it was believed that transcription factors (TFs) are responsible for the regulation of gene expression for various cell phenotypes and responses of cells to environmental stimuli. But with the discovery of miRNAs, it is speculated that miRNAs, in combination with TFs, control the expression of thousands of mammalian genes, a process described as ‘tuning’ the transcriptional network (Zhou et al., 2007; Fabian et al., 2010). Some miRNAs, like miR-16, are widely expressed in body tissues while others are tissue or developmental stage specific (Poy et al., 2004). The tissue-specific expression of miRNAs is suggestive of their role in cell differentiation and function (Shi et al., 2010; Pauli et al., 2011).
It has been established that both adaptive and innate immune responses are regulated by miRNAs. For example, in the adaptive immune response, the differentiation of B cells, antibody generation, and T cell development and function are controlled by miRNAs (Singh et al., 2013). A number of recent studies described the regulation of mammalian miRNAs in response to bacterial infection (De Flora and Bonanni, 2011). Innate immune cell activation requires miRNAs, including miR-155, miR-146a, miR-21, and miR-9 (Belver et al., 2011). Components of inflammatory and immune pathways are regulated by miRNAs under the challenge of mycobacterial infections (Liu et al., 2011; Chatterjee et al., 2011). For example, TNF biosynthesis is inhibited by miR-125b in human alveolar macrophages during Mtb infection (Rajaram et al., 2011). Furthermore, many research groups have reported the differential expression of miRNAs in host cells challenged with Mtb, indicating the important role of these miRNAs in regulating the immune response in TB and proposing the miRNAs as potential biomarkers (Wang et al., 2011; Qi et al., 2012; Wu et al., 2012; Zhang et al., 2013). Many of these studies also provide an insight into the role of these differentially expressed miRNAs in TB, but a detailed understanding of the role of these miRNAs in the anti-mycobacterial response is a matter of highest interest for future investigations. These studies also provide a good foundation for the development of reliable biomarkers and therapeutic targets for TB.
Autophagy plays a pivotal role in controlling the bacterial load during TB (Deretic et al., 2013; Richetta and Faure, 2013). However, Mtb has evolved strategies to survive in macrophages by evading delivery to the lysosomes (Hmama et al., 2015) and like other intracellular pathogens Mtb also has the ability to exploit host degradative processes to breakdown cellular macromolecules into simple nutrients for its survival and multiplication (Steele et al., 2015). Similarly, cell apoptosis is also one of the important host defense mechanisms through which macrophages control Mtb infection (Weiss and Schaible, 2015). Mtb is able to inhibit phagolysosome biogenesis, inhibition of apoptosis as well as autophagy due to presence of lipoarabinomannan in its cell wall which is a major immunomodulatory lipoglycan (Vergne et al., 2015). The emerging roles of miRNAs in regulating Mtb–induced autophagy and apoptosis have attracted increased attention in recent years. Appreciating the potential to be exploited in host-directed therapies designed to control autophagy and apoptosis, many groups have focused their research on miRNAs (Ouimet et al., 2016; Zhang et al., 2016; Guo et al., 2017). These recent studies have opened a new avenue to exploit miRNAs in HDT. In the current review, we will summarize recent advances in the understanding of differential expression of miRNAs and their role in TB, and their potential to be used as biomarkers and therapeutic targets for diagnosis and treatment of this deadly disease.
Differential Expression of miRNAs in Different Cell Types Following Mtb Infection
Several studies have addressed the differential expression of miRNAs as reflecting disease prediction and progression in vivo and in vitro. For example, differentially expressed miRNAs have a close relationship with disease progression to hepatocellular carcinoma in both hepatitis B and C (Ura et al., 2009). Recently, attempts have been made to determine the effects of Mtb infection on the expression of miRNAs in the host (Ghorpade et al., 2012; Vegh et al., 2015; Zheng L. et al., 2015). In a recent study, mouse bone marrow derived macrophages (BMDMs) infected with Mtb showed up-regulation of miR-155 (Kumar et al., 2015) while infection of peripheral blood mononuclear cells (PBMCs) derived macrophages with the same pathogen led to down-regulation of miR-155 expression (Rajaram et al., 2011). This means different cell types may respond differently upon infection with Mtb. Sharbati et al. (2011) reported overexpression of let-7e, miR-29a and miR-886-5p in human monocyte derived macrophages (MDMs) in response to mycobacterial infection. Integrated analysis of microRNA and mRNA expression as well as target prediction revealed caspases 3 and 7 as potential targets of let-7e, and miR-29a, respectively. Liu et al. (2012) reported that differentially expressed hsa-mir-21 inhibits expression of two genes encoding vitamin D–dependent antimicrobial peptides, CAMP and DEFB4A, in human monocytes derived from leprosy patients. This inhibition is carried out by direct downregulation of Toll-like receptor (TLR) 2/1 heterodimer (TLR2/1)-induced CYP27B1 and IL1B expression as well as indirect upregulation of IL-10. Furci et al. (2013) studied Mtb-induced miRNA expression profile in primary human macrophages infected with virulent Mtb H37Rv and avirulent M. bovis BCG and showed that macrophages differentially expressed miRNAs, including miR-155, miR-146a, miR-145, miR-222∗, miR-27a, and miR-27b. In this study, miR-222∗, miR-27a, and miR-27b, which have been reported to control inflammatory response and lipid metabolism (McGregor and Choi, 2011; Graff et al., 2012) were significantly downregulated. miR-145, which has been reported to induce apoptosis (Spizzo et al., 2010), was also downregulated, in line with a reduced capacity of virulent Mtb strain to induce apoptosis (Keane et al., 2000; Arcila et al., 2007; Starczynowski et al., 2010; O’Neill et al., 2011). Downregulation of miR-145 results in overexpression of its targets and inhibition of apoptosis (Starczynowski et al., 2010).
Das et al. (2013) analyzed global changes in the miRNA expression profile using microarrays and found that nine miRNA genes (miR-30a, miR-30e, miR-155, miR-1275, miR-3665, miR-3178, miR-4484, miR-4668-5p, and miR-4497) were differentially expressed in THP-1 cells infected with Mtb H37Rv or Mtb H37Ra strains. These differentially expressed miRNAs perform various important functions. miR-30e is activated by β-catenin (Schepeler et al., 2012), while miR-30a inhibits the epithelial to mesenchymal transition (Kumarswamy et al., 2012). miR-1275 is associated with liver metastases of cancer (Kahlert et al., 2011) and miR-155 binds a negative regulator associated with TNF-α production (Rajaram et al., 2011). Spinelli et al. (2013) examined PBMCs and pleural fluid mononuclear cells (PFMCs), and reported that miRNAs expression is associated with IL-6 levels, a cytokine playing a substantial role in TB immunopathology. Lin et al. (2015) showed that the Beijing/W TB strains repressed a number of miRNAs in human macrophages as compared to the non-Beijing/W TB strains, which might reflect their virulence characteristics in altering the host response. Two other research groups identified a series of miRNA differentially expressed in PBMCs of Chinese patients with pulmonary TB by using miRNA expression profiling. Liu et al. (2011) showed that the expression of several miRNAs was significantly altered in patients with active TB, with miR-144∗ being mainly expressed in T cells. Functional analysis showed that miR-144∗ inhibits the secretion of two important cytokines, INF-γ and TNF-α, and also reduces T cell proliferation. Wang et al. (2011) demonstrated that miR-424 and miR-365 levels were significantly raised in patients with active TB compared to healthy controls. In recent years, many other studies have reported the differential expression of miRNAs in different cell types in response to mycobacterial infection (Ni et al., 2014; Zhang et al., 2015). The detailed role of these differentially expressed miRNAs in anti-mycobacterial response is a matter of high interest for many research groups. Furthermore, these studies also provide a good foundation for the development of reliable biomarkers for TB diagnosis.
miRNAs as Potential TB Biomarkers
An essential method to effectively control the spread of TB is to diagnose it at an early stage. Currently used test systems are insufficient and unable in practice to discriminate between active TB and latent TB infection (LTBI). Altered miRNA expression profiles may help differentiate between active TB and LTBI and can also act as reliable biomarkers for the diagnosis of the disease. This potential of miRNAs has received much attention in recent years, with several in vivo and in vitro studies proposing miRNAs as potential biomarkers (Miotto et al., 2013; Weiner et al., 2013) although suitable miRNA biomarkers have not been established yet (Walz et al., 2011) (Table 1). In a study using human PBMCs, the authors suggested that differentially expressed miRNAs combined with predicted differentially expressed mRNAs from the same whole genome transcriptional profiling may be used as a new way to differentiate among active TB, LTBI and healthy controls (Xu et al., 2013). Maertzdorf et al. (2012) identified a cluster of differentially expressed miRNAs in patients having active TB and sarcoidosis. Interestingly, strong correlations between miRNAs and gene expression were found. Similarly, lipomannan from virulent Mtb stimulated the expression of miR-125b in human macrophages while lipomannan from avirulent M. smegmatis -resulted in increased expression of miR-155 (Rajaram et al., 2011). These reports revealed that components from related bacterial species, with different virulence, lead to differential expression of miRNAs and consequently modulated the immune response.
Table 1.
Differential expression of miRNAs in tuberculosis and their potential as biomarkers.
| Species examined | Type of tissue/cells examined | Candidate biomarkers | Reference |
|---|---|---|---|
| Human | PBMCs | has-miR-21∗ and has-miR-26b | Xu et al., 2013 |
| Human | Macrophages | miR-125b and miR-155 | Rajaram et al., 2011 |
| Human | Macrophages | miR-29a and miR-361-5p | Draz et al., 2014 |
| Human | Macrophages | miR-31 | Wang et al., 2015 |
| Human | Serum | miR-4433b-5p, miR-424-5p, and miR-199b-5p | Wang et al., 2016 |
| Human | Whole blood | hsa-miR-21 hsa-miR-7f-1∗ | Latorre et al., 2015 |
| Human | Serum | miR-361-5p, miR-889, and miR-576-3p | Qi et al., 2012 |
| Human | Whole blood | miR-1, miR-155, miR-31, miR-146a, miR-10a, miR-125b, miR-150, and miR-29 | Zhou et al., 2016 |
| Human | Macrophages | miR-144 | Lv et al., 2016 |
| human | Serum | hsa-let-7b and hsa-miR-30b | Xin et al., 2016 |
| Mouse | Macrophages | let-7e, miR-29a, and miR-886-5p | Sharbati et al., 2011 |
| Human | Macrophages | miR-3179, miR-147, and miR-19b-2∗ | Yi et al., 2012 |
| Human | Macrophages | miR-155, miR-146a, miR-145, miR-222∗, miR-27a, and miR-27b | Furci et al., 2013 |
| Human | Serum | miR-424-5p, miR-493-5p, miR-296-5p, miR-27b-3p, miR-377-5p, miR-3680-5p, and miR-191-5p | Meng et al., 2014 |
| Human | T cells | miR-144∗ | Liu et al., 2011 |
| Human | Whole blood | miR-424 and miR-365 | Wang et al., 2011 |
In a very recent study, co-regulatory networks consisting of transcription factors and miRNAs as well as their target genes were analyzed from whole blood of TB patients. This TF-gene network showed that SPI1, CEBPB, STAT1, STAT2, STAT3, STAT4, and STAT5A directly regulate 22, 12, 11, 1, 10, 1, and 3 genes, respectively. The results suggested that TF-miRNA gene co-regulatory networks may help provide a way to discover future biomarker and therapeutic targets (Lin et al., 2017). Wu et al. (2014) uncovered several miRNA-gene interactions differentiating among active TB, LTBI and healthy subjects. Wang et al. (2015) discovered that expression of miRNA-31 in pediatric TB patients was significantly lower compared with that in normal children. Furthermore, miRNA-31 expression was negatively correlated with serum levels of IL-6, TNF-α, NF-κ0, and IFN-overed several m- analysis study reported that the current data do not support any association between miR-146a/499 polymorphisms and genetic susceptibility of humans to TB (Lu et al., 2016). Latorre et al. (2015) reported nine differentially expressed miRNAs in active TB patients with respect to those with LTBI and healthy controls. Qi et al. (2012) suggested that altered levels of serum miRNAs have great potential to serve as biomarkers for early detection of pulmonary TB. Similarly, Zhou et al. (2016) identified the expression profile of circulating miRNAs and demonstrated that miRNAs may act as effective biomarkers for the early diagnosis of childhood TB.
Barry et al. (2015) examined the appropriateness of 12 miRNAs and RNU6B to normalize circulating plasma miRNA levels in individuals with active TB. These data identify miR-93 as a suitable miRNA that can be used for normalizing miRNA levels in TB patients. Ren et al. (2015) acquired miRNA expression data from sensitive Mtb and MDR Mtb strains by using next generation sequencing (NGS) and revealed that 142 miRNAs were differentially expressed in the MDR Mtb strains but not in sensitive TB strains. This suggests that miRNAs may have a role in the development of drug-resistance in Mtb strains. Miotto et al. (2013) identified 15 serum miRNAs as signature in pulmonary TB with a diagnostic accuracy of 82%. These studies have contributed substantially to present the differentially expressed miRNAs as potential biomarker candidates for diagnosis of TB, but there is no established miRNA biomarker so far. Several factors may contribute to this slow discovery, including heterogeneous study designs having small study group sizes, noticeable inter-individual variability of miRNA expression and inadequate statistical evaluation for candidate selection (Ueberberg et al., 2014). Moreover, many other pathological conditions may also induce similar miRNAs expression profiles. Keeping in view these limitations, additional investigations are a prerequisite for the selection and use of miRNAs as biomarkers for diagnosis of TB.
miRNAs as Regulators of TB Immunity
Regulation of Inflammatory and Immuno-Modulatory Cytokines
Several miRNAs have been demonstrated to regulate the inflammatory and immune response signaling pathways (Table 2) in response to challenge with Mtb (Guo et al., 2010; Chatterjee et al., 2011; Fu et al., 2011; Huang et al., 2011; Li et al., 2011; Liu et al., 2011). Innate immune cell activation is regulated by miR-155, miR-146a, miR-21, and miR-9 (Belver et al., 2011). Similarly, miR-155 is a positive regulator of TLR signaling, and is induced upon stimulation of murine macrophages with interferon beta (IFN-β) or TLR ligands (Gantier, 2010; Liston et al., 2010). TNF biosynthesis is inhibited by miR-125b in Mtb-infected human alveolar macrophages (Rajaram et al., 2011). miR-29 helps control innate and adaptive immune responses against Mtb by targeting interferon-γ and it is suggested as a biomarker for pulmonary tuberculosis because it is related to the clinical manifestation of the disease (Ma et al., 2011).
Table 2.
miRNAs regulation of host immune response in tuberculosis.
| Functions | miRNA | Target | Species examined | Tissue/Cell examined | Reference |
|---|---|---|---|---|---|
| Apoptosis | miR-145 | TRAF6 | Mouse | Stem cells | Starczynowski et al., 2010 |
| let-7e | Caspase 3 | Human | Monocyte derived macrophages | Sharbati et al., 2011 | |
| miR-29a | Caspase 7 | Human | Monocyte derived macrophages | Sharbati et al., 2011 | |
| miR-155 | FOXO3 | Human | Monocytes | Huang et al., 2015 | |
| miR-20a-5p | JNK-2 | Human | Macrophages | Zhang et al., 2016 | |
| miR-21 | Bcl-2 | Mouse | RAW264.7 macrophages | Wang Q. et al., 2014 | |
| Cytokines | miR-144∗ | INF-γ and TNF-α | Human | Whole blood | Liu et al., 2011 |
| miR-146a | IRAK-1/TRAF-6 pathway | Mouse | RAW264.7 macrophages | Li et al., 2013 | |
| miR-146a | TNF-α | Human | Alveolar macrophages | Liu et al., 2014 | |
| miR-223 | CXCL2, CCL3 and IL-6 | Mouse | miR-223-/- mouse | Dorhoi et al., 2013 | |
| let-7f | A20, TNF, IL-1β | Mouse | RAW264.7 and BMDMs | Kumar et al., 2015 | |
| miR-125b | TNF | Human | Monocyte derived macrophages | Rajaram et al., 2011 | |
| miR-27a | IRAK4 | Human | THP-1 | Wang et al., 2017 | |
| miR-27a | IL-10 and TAB2 | Mouse | RAW264.7 and BMDMs | Hussain et al., 2018 | |
| miR-27a and miR-27b | IRF4 | Computational study | TargetScan database | Ahluwalia et al., 2017 | |
| miR-302c | IRF5 | Computational study | TargetScan database | Ahluwalia et al., 2017 | |
| miR-155, miR-132, and miR-455-5p | SOCS transcription factors | Computational study | TargetScan database | Ahluwalia et al., 2017 | |
| Nitric oxide suppression | miR-146a | NF-kB, MAPK | Mouse | RAW264.7 macrophages | Li et al., 2016 |
| miR-155 | C/EBPb | Mouse | RAW264.7 macrophages | Qin et al., 2016 | |
| Increased bacterial survival | miR-155 | SHIP1/protein kinase B (Akt) pathway | Mouse | Macrophages | Rothchild et al., 2016 |
| Inhibition of antimicrobial peptides | hsa-miR-21 | CYP27B1, IL1B | Human | Monocytes | Liu et al., 2012 |
| Autophagy | miR-155 | Rheb | Mouse | RAW264.7 and BMDMs | Wang et al., 2013 |
| miR-142-3p | N-Wasp | Mouse and human | J774A.1 and primary human macrophages | Bettencourt et al., 2013 | |
| miR-33 | ATG5, LAMP1 | Human | THP-1 and HEK293 cells | Ouimet et al., 2016 | |
| miR-125a-3p | UVRAG | Mouse | RAW264.7 and J774A.1 macrophages | Kim et al., 2015 | |
| miR-17-5p | ULK-1 | Mouse | RAW264.7 macrophages | Duan et al., 2015 | |
| miR-144-3p | ATG4a | Mouse | RAW264.7 macrophages | Guo et al., 2017 | |
| miR-20a | ATG7andATG16L1 | Mouse | RAW264.7 macrophages | Guo et al., 2016 | |
| miR-23a-5p | TLR2/MyD88/NF-κB | Mouse | RAW264.7 and BMDMs | Gu et al., 2017 | |
| miR-26a | KLF 4 | Mouse | RAW264.7 macrophages | Sahu et al., 2017 | |
| miR-17-5p | Mcl-1/STAT3 | Mouse | RAW264.7 macrophages | Kumar et al., 2016 | |
Role of miR-146a and miR-155 in Host Immune Response Regulation
With respect to TB, miR-146a and miR-155 are the most vastly studied miRNAs influencing the host–pathogen interaction. miR-146a expression is driven by the transcription factor NF-κB, predicted to base-pair with sequences in the 3′-UTRs of the TNF receptor-associated factor 6 and IL-1 receptor-associated kinase 1 genes (Taganov et al., 2006). Mice deficient in miR-146a showed fatal IFN-γ-dependent autoimmune disease, possibly involving the STAT1 signaling pathway (Lu et al., 2016). miR-146a also represses mycobacteria-mediated inflammatory response and facilitates bacterial proliferation in RAW264.7 macrophages via the IRAK-1/TRAF-6 pathway (Li et al., 2013). In addition, it is overexpressed in macrophages in response to M. bovis BCG while downregulated in alveolar macrophages of pulmonary TB patients and negatively regulating TNF-α (Liu et al., 2014). More recently, it has been described that miR-146a promotes mycobacterial survival in RAW264.7 macrophages through suppression of nitric oxide (NO) production (Li et al., 2016).
miR-155 also has been extensively studied and plays an important role in various physiological and pathological processes (Hacker and Karin, 2006; Faraoni et al., 2009; Kutty et al., 2010; Wang et al., 2010; Savan, 2014; Forster et al., 2015). It is induced upon infection of murine macrophages with mycobacteria and known to be a positive regulator of TLR signaling (Wang J. et al., 2014). Pharmacological inhibition of the Janus kinase (JNK) pathway blocked the induction of miR-155 in response to either polyriboinosinic: polyribocytidylic acid or TNF-α, suggesting that miR-155 signals through the JNK pathway (O’Connell et al., 2007). Iwai et al. (2015) unveiled that miRNA-155 knockout mice were susceptible to M. tuberculosis infection and died significantly earlier with significantly higher numbers of CFU in lungs, as compared to wild-type mice. These studies demonstrate the protective role of miRNA-155 against mycobacterial infection. On the other hand, miR-155 augments the survival of macrophages thereby providing a niche for the replication of Mtb, it promotes the survival of Mtb-specific T cells enabling an effective adaptive immune response. These effects of miR-155 on innate and adaptive immunity are associated with the observations that miR-155-deficient mice better control early Mtb infection, but are compromised in their ability to control late infection (Rothchild et al., 2016). Furthermore, miR-155 reportedly inhibits apoptosis in monocytes (Huang et al., 2015). Zhang et al. (2015) discovered that there was an inverse relationship between serum miR-155 abundance and NK cell cytotoxicity. Serum miR-155 levels were shown to be negatively associated with the TB-suppressing activity of NK cells. Similarly, Qin et al. (2016) reported that miR-155 expression is significantly increased in macrophages after M. marinum infection, resulting in decreased NO synthesis and increased mycobacterium load. These findings support the proposed theory that a single miRNA can target multiple genes at the same time and emphasizing the regulatory role of miRNAs (Mayr et al., 2007; Pillai et al., 2007).
Role of Other miRNAs in Host Immune Response Regulation
Besides these two extensively studied miRNAs, many other miRNAs have been reported to play a role in Mtb infection. miR-223 has a critical role in the control of TB and potentially other chronic inflammatory diseases. Chemoattractants like CXCL2, CCL3, and IL-6 have been identified as targets of miR-223 in myeloid cells (Dorhoi et al., 2013). Similarly, a transgenic mouse with endogenous blockage of miR-29 showed increased resistance against Mtb infection (Ma et al., 2011). A recent study has identified miR-27a as a restrainer of immune response in Mtb infection by targeting IRAK4. Expression levels of IFN-γ, IL-β, IL-6, and TNF-α were significantly decreased following transfection of miR-27a mimics (Wang et al., 2017). miRNAs expression and transcriptional regulation of target genes potentially affect the regulation of multiple immunological responses. These studies enhance our understanding of the role of miRNAs in host-pathogen interactions and offer a new way to improve our diagnostic tools and treatments regimes against TB.
miRNAs as Regulators of Autophagy
Autophagy is an intracellular process involving self-digestion or self-eating, whereby cytoplasmic constituents are transported to and degraded by lysosomes (Lamb et al., 2013). Besides being critical for other cell functions, autophagy plays a key role in immune responses against invading viral and bacterial pathogens (Huang and Brumell, 2014). Similarly, apoptosis is also one of the important host defense mechanisms through which the host cells limit the extent of damage caused by the infection. However, the capacity of Mtb to survive and replicate in host macrophages is central to Mtb pathogenesis and is often associated with its degree of virulence (Weiss and Schaible, 2015). It is important to explore how Mtb hijacks host immunomodulatory signaling pathways to survive and replicate in macrophages for appropriate control and treatment of the disease. It is well established that Mtb has developed several schemes to avoid the antimicrobial effects of macrophages to be able to survive intracellularly (Ahmad, 2011). One of these significant strategies is its ability to block phagosome maturation and deploy other countermeasures to impede autophagy, and evade the hostile environment of phagolysosomes (Vergne et al., 2004; Russell, 2011; Espert et al., 2015). Moreover, phagosome maturation and mycobacterial killing can be reinstated through exogenous induction of autophagy in infected macrophages (Deretic et al., 2013).
The pathways associated with autophagy are tightly regulated at the post-transcriptional level and have been well described, but the contribution of miRNAs in activation or inhibition of autophagy during Mtb infection had been largely unknown. However, in recent years many research groups have successfully unveiled the role of several miRNAs in autophagy regulation during Mtb infection. Ouimet et al. (2016) reported that miR-33 induction in THP-1 and HEK293 cells inhibits the integrated pathways involved in autophagy and also reprograms the host lipid metabolism for intracellular survival and persistence of Mtb. Guo et al. (2017) revealed that M. bovis BCG infection of macrophages leads to increased expression of miR-144-3p, which induces autophagy-related gene 4a (ATG4a) to inhibit autophagy. In another study, overexpression of miR-23a-5p dramatically prevented Mtb-induced activation of autophagy in macrophages by modulating TLR2/MyD88/NF-κB signaling (Gu et al., 2017). Similarly, miRNA-20a targets ATG7 and ATG16L1 and is able to inhibit autophagy (Guo et al., 2016).
In contrast to the inhibitory role of miRNAs in autophagy induction reported by the above studies, other studies have identified several miRNAs involved in enhancing autophagy during Mtb infection. Sahu et al. (2017) reported that miR-26a mimic attenuates Mtb survival in macrophages by targeting the transcription factor KLF4. This transcription factor is able to prevent trafficking of Mtb to lysosomes. miR-155 expression accelerates the autophagy-mediated anti-mycobacterial response by targeting Ras homolog enriched in brain (Rheb), a negative regulator of autophagy (Wang et al., 2013). Similarly, Kumar et al. (2016) demonstrated that a miR-17/PKCδ/STAT3 axis is involved in regulating autophagy during Mtb infection. Neural Wiskott-Aldrich syndrome protein (N-Wasp) is an actin-binding protein involved in phagocytosis during microbial challenge. Mtb induced expression of miR-142-3p targets N-Wasp leading to reduced phagocytosis (Bettencourt et al., 2013).
miRNAs as Regulators of Apoptosis
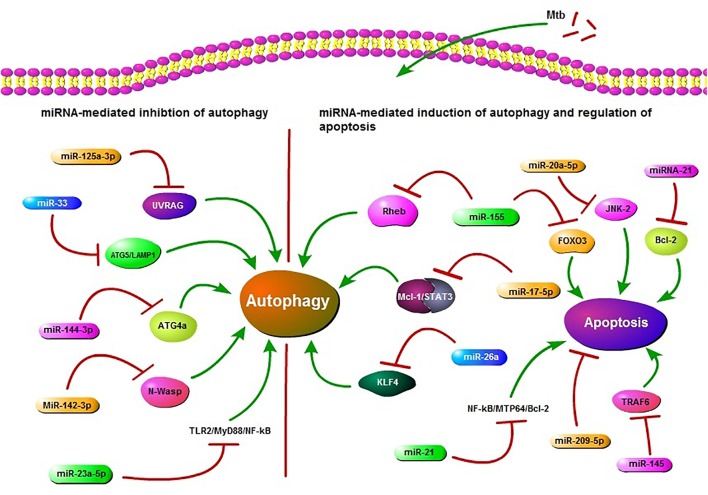
MiRNAs also play a role in regulation of apoptosis in Mtb infection. According to recent findings, miR-20a-5p functions as a negative regulator of mycobacterial-triggered apoptosis and inhibition of miR-20a-5p results in more efficient Mtb clearance (Zhang et al., 2016). MPT64 is one of the important secreted proteins by Mtb and it could inhibit apoptosis of RAW264.7 cells through the NF-kB-miRNA21-Bcl-2 pathway (Wang Q. et al., 2014). Huang et al. (2015) demonstrated that miR-155 targets FOXO3 and regulates apoptosis. Other studies have also reported the role of miRNAs in apoptosis in Mtb infection (Starczynowski et al., 2010; Sharbati et al., 2011). Collectively, these findings highlight the role of miRNAs in regulation of autophagy and apoptosis in TB (Figure 1). These findings also offer an opportunity for exogenous manipulation of miRNAs to restrain the multiplication and enhance the clearance of Mtb from the host cells.
FIGURE 1.
Schematic presentation of miRNA regulation of autophagy and apoptosis. miR-125a-3p, miR-33, miR-144-3p, miR-23a-5p, and miR-142-3p are potential inhibitors of autophagy in Mycobacterium tuberculosis (Mtb) infection. miR-125a-3p inhibits autophagy through targeting UV radiation resistance-associated gene (UVRAG), miR-33 targets ATG5/LAMP1, miR-144-3p targets autophagy-related gene 4a (ATG4a), miR-23a-5p inhibits the TLR2/MyD88/NF-κB leading to reduced autophagy and miR-33 also plays an inhibitory role via targeting some unknown factors. miR-142-3p targets Neural Wiskott-Aldrich syndrome protein (N-Wasp) leading to reduced phagocytosis. While miR-155, miR-17-5p, and miR-26a target Ras homolog enriched in brain (Rheb), Mcl-1/STAT3, KLF4, respectively, and play a positive role in autophagy regulation during Mtb infection. miR-155 also inhibits apoptosis by targeting FOXO3. miR-21 targets NF-kB-MTP64-Bcl-2 to down regulate the apoptosis. miR-20a-5p, miR-21, miR-209-5p, and miR-145 also regulate apoptosis. All of these miRNAs may act as potential targets in HDT.
miRNAs as Therapeutic Targets
Application of miRNAs as a novel class of drug targets for treatment of various diseases is an emerging area of research. For example, miRNAs have been used as therapeutic targets in hepatitis C virus infection, cancer and cardiovascular diseases (Lanford et al., 2010; Takeshita et al., 2010; Kotsinas et al., 2015). Exogenous manipulation in pathologically imbalanced miRNAs is able to transform the phenotype of cancer cells (Merhautova et al., 2016). Moreover, antitumor activity of miRNAs has been demonstrated and safety of use has been established in hepatic carcinoma (Callegari et al., 2015). The expression of miRNAs can be manipulated for therapeutic purposes either through positive or negative regulation. Novel intervention strategies exploit miRNA-mimics to restore optimal miRNA expression or anti-miRNAs to block abnormally produced miRNAs (Stenvang et al., 2012). For example, miRNAs can be targeted therapeutically to inhibit their maturation (Lee et al., 2006). Similarly, it is possible to increase the activity of downregulated anti-mycobacterial miRNAs by using synthetic oligos and reduce the effects of overexpressed pro-mycobacterial miRNAs through antisense oligonucleotides or anti-miRNA complementary to mature miRNA (Meister et al., 2004; Grimm et al., 2006; Baumann and Winkler, 2014). Another alternative strategy is to replace specific miRNA by gene therapy using viral vectors (Uprichard, 2005).
In Vivo Manipulation of miRNAs Expression
Besides these anti-miRNA techniques, substantial advances have been made concerning delivery of miRNAs into lungs and encouraging results have described modulation of TGF-β1 expression in a mouse model of TB (Rosas-Taraco et al., 2011). In vivo delivery of miRNAs has been performed by lentiviral vectors, lipid conjugates, or small exosome-like vesicles. Antigen-specific exosome-like nanovesicles have been transfected with mimics or anti-miRNA to restore miRNA expression in target cells (Bryniarski et al., 2013). In a similar study, primary B cells transfected with a specific anti-miRNA have been shown to deliver the molecule successfully to antigen-activated T cells (Almanza et al., 2013). Zheng D. et al. (2015) successfully silenced in vivo miR-195 expression by using anti-miR-195. Similarly, in vivo inhibition of miR-328 by intra-tracheal inoculation of anti-328 resulted in 4-fold enhanced non-typeable Haemophilus influenzae (NTHi) clearance from the lungs as compared to controls (Tay et al., 2015). Thus, miRNAs can be targeted in the lung to enhance host immunity against microbial infections and offer a potential new anti-mycobacterial approach for the treatment of TB. Further studies are required to define the most appropriate route of miRNA delivery and pharmaco-vigilance problems, providing a valid foundation for future miRNA-based HDTs of TB.
Nanoparticle-Mediated Delivery of miRNAs in TB
Technological developments at the micro and nanoscale can be used to modulate the immune response (Dacoba et al., 2017). Multiple materials and techniques have been developed to deliver drugs and a broad array of cargo to cells, including nanostructured materials (Vázquez-Hernández et al., 2017), synthetic nanoparticles (Ayer and Harm-Anton, 2017), proactive biomimetic delivery systems (Parodi et al., 2017), functional polymers (Jiang et al., 2017) or cell-penetrating peptides (Tashima, 2016), among others. Several materials have been proposed to help treat immune diseases with nanoparticles, including silica, iron oxide, gold, liposomes, or polylactic-co-glycolic acid (PLGA) (Prosperi et al., 2017). Of particular interest in infectious diseases are techniques that can encapsulate miRNAs and deliver them to the cells that are at the forefront of the immune response, i.e., macrophages or dendritic cells, taking advantage of the innate ability of these cells to internalize foreign bodies. The idea of miRNA delivery fits into the general concept of nanoparticle-based induction of immune responses by targeting phagocytes (i.e., macrophages), in contrast to the alternative method of targeting adaptive lymphocytes (i.e., T cells). MiRNA delivery has been explored for many applications related to inflammation (Zhou et al., 2013) and cancer (Fernandez-Piñeiro et al., 2017), and its potential use demonstrated in infectious diseases (Pegtel et al., 2010). MiRNAs can be incorporated into both natural –i.e., chitosan- and synthetic – i.e., (PLGA)- particle-forming materials. For example, nanoparticle- and liposome-loaded miRNAs have been delivered to macrophages (Duan et al., 2015; Moore et al., 2016). At the time of writing, however, very few reports are available on the implementation of this technique in TB. This implementation can be discussed by either describing current general uses of nanoparticles in immunology or broadly mentioning the utilization of nanoparticles with miRNAs. We will briefly combine these two views and describe nanoparticle methods used elsewhere that can be adopted in miRNA delivery in TB.
Direct Delivery of miRNAs to Modulate Host Immune Response
To help eliminate or substantially reduce mycobacterial activity by targeting macrophages and dendritic cells, several approaches can be implemented. An obvious approach is to directly deliver miRNAs that improve host immune responses against TB, or more generally that facilitate increased expression of anti-inflammatory molecules like IL-10. Among the candidates for this approach we can cite miR-155, miR-146a, miR-145, miR-99b, miR-19b-2∗, miR-27a, or miR-27b. Conversely, a second approach is to deliver siRNAs that help decrease expression of pro-inflammatory cytokines such as TNF-α that are induced by miRNAs. Candidates for this approach are those miRNAs that are overexpressed during TB infection, including let-7e, miR-29a, miR-886-5p, miR-147, or miR-3179. In addition to siRNAs, this approach could include the delivery of small molecules (drugs) that can selectively affect immune responses including dexamethasone or other small molecules which modify the host’s ability to regulate miRNA expression.
Direct miRNA delivery could knock-down, overexpress or downregulate appropriate host molecular targets against TB. An early example of this approach of direct delivery is the nanoparticle release of miR-223 in macrophages to facilitate phenotype transitions (Tran et al., 2016). To implement the direct delivery, the critical aspect is the selection of the miRNAs. When innate immune cell activation is desired, direct delivery of miR-155 may be an option, because of its role as a positive regulator of TLR signaling (Wang J. et al., 2014) and its promotion of autophagy and mycobacterial clearance (Wang et al., 2013). Similarly to miR-155, nanoparticle delivery of miR-125b during Mtb infection can be useful in inhibiting TNF biosynthesis. Delivery of these miRNAs have been demonstrated previously in pancreatic cancer cells (Su et al., 2016) or in lung cancer (Talekar et al., 2016), but not in infectious diseases. In general, this approach of direct delivery should be implemented for those miRNAs that are downregulated during TB infection, including miR-155, miR-146a, miR-145, miR-222∗, miR-27a, or miR-27b (Spizzo et al., 2010; Belver et al., 2011; McGregor and Choi, 2011; Graff et al., 2012). Only a few reports are available describing nanoparticle uses with mi-R155, miR-223, miR-146, or miR-125b, among the many miRNAs involved in TB cited in our manuscript.
Use of siRNAs to Downregulate the miRNAs
In the second approach mentioned –releasing siRNAs to downregulate miRNAs expression-, a clear candidate may be miR-146a, because it facilitates bacterial proliferation via the IRAK-1/TRAF-6 pathway, negatively regulates TNF-α and promotes mycobacterial survival in macrophages through suppression of NO production. Interestingly, nanoparticles loaded with miR-146 have been used to inhibit inflammation in keratinocytes (Urgard et al., 2016) and human dental pulp cells (Liu et al., 2016) and to inhibit kidney fibrosis (Morishita et al., 2015). This suggests that both methodologies –direct delivery and secondary molecular induction- can be complementary approaches in nanoparticle-mediated delivery of miRNAs. Delivery of molecular compounds that facilitate upregulation or downregulation of host’s expression of miRNAs is part of this second approach. For this to work, it would be necessary to know what class of molecules (or particular molecule) can directly regulate the host expression of the targeted miRNAs. In summary, there appears to be great potential for the broad implementation of nanoparticle-mediated release of miRNAs important during TB infection. Additional studies will be necessary to clarify the role of these differentially expressed miRNAs in mycobacterial infection and observe differential effects of direct miRNA delivery or indirect regulation to each particular set of cells.
Recent Studies Illustrating miRNAs as Potential Therapeutic Targets
In recent years, several research groups have pointed out many miRNAs that can be targeted to treat TB. For instance, miR-155 is negatively associated with the TB-suppressing activity of NK cells (Zhang et al., 2015). The same miRNA also leads to autophagy-mediated mycobacterial elimination by targeting Rheb (Wang et al., 2013). Ouimet et al. (2016) revealed that silencing of miR-33 and miR-33∗ by genetic or pharmacological means promotes autophagy flux through depression of key autophagy effectors and AMPK-dependent activation of the transcription factors FOXO3 and TFEB. In a similar study, miR-23a-5p modulated the host innate immune response by promoting Mtb survival and inhibiting autophagy induction through TLR2/MyD88/NF-κB pathway (Gu et al., 2017). Cytokines are important mediators of inflammatory and immune responses. miR-99b is able to inhibit the secretion of pro-inflammatory cytokines in Mtb infection, suggesting a new approach for designing miRNA-based therapies and control of TB (Singh et al., 2013). miR-206 has been suggested to function as an inflammatory regulator leading to the expression of MMP9 by targeting TIMP3 in Mtb infection (Fu et al., 2016). Similarly, Lou et al. (2017) demonstrated that miR-20b can alleviate the inflammatory response in TB mice by targeting the NLRP3/caspase-1/IL-1β pathway. Zhang et al. (2017) showed that the induction of miR-32-5p strongly increases the survival rate of Mtb by directly targeting Follistatin-like protein 1 (FSTL1) through the TLR-4/miRNA-32-5p/FSTL1 pathway. However, a possible limitation is that most of the miRNAs are not entirely gene-specific. One miRNA may target multiple mRNAs, suggesting that exogenous miRNA administration might exhibit off-target effects. In reality, the exploitation of miRNAs in HDT is still in its infancy stage but it has opened an exciting avenue for the control and treatment of TB.
Conclusion and Future Prospects
Tuberculosis (TB) is one of the world’s most deadly communicable diseases and Mtb is difficult to eradicate, due to its capability to persist within macrophages. Macrophages play a pivotal role in the host immune response against Mtb, a response tightly regulated by multiple factors, including miRNAs. The emerging role of miRNAs in regulating both adaptive and innate immune responses against Mtb has attracted increasing attention from many research groups in recent years. Many studies have revealed that differential expression of miRNA could reflect disease progression and may distinguish between active and LTBI. These findings provide insights into the potential utility of miRNAs as biomarkers for the diagnosis of TB. However, a possible limitation in the use of miRNAs as biomarkers is that most of the miRNAs are not completely gene-specific. Active participation of miRNAs in modulation of autophagy and apoptosis against Mtb, and the emerging role of nanotechnology in medicine opens an exciting avenue for the exploitation of nanoparticles-mediated delivery of miRNAs in host directed therapy (HDT) against TB.
These findings provide valuable information and a firm foundation for the development of HDT based on miRNAs. Moreover, these advancements in miRNA research have reduced our current limitations related to diagnostics, multidrug resistance and HDT for better control and treatment of TB. In this review, we present a comprehensive survey of the published literature on the differential expression and role of miRNAs, as well as recent advances in the development of miRNA-based biomarkers and therapeutics for TB. Further studies are required to elucidate the full potential of miRNAs as novel TB biomarkers, as well as their manipulation as adjunctive treatment for TB.
Author Contributions
NS collected the data and wrote the manuscript. TH and SS helped for figure and table compilation. AP assisted in writing the manuscript and provided the critical comments. XZ gave the idea behind the manuscript compilation. DZ reviewed the article before final submission. All authors read and approved the manuscript prior to submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by China Agriculture Research System (No. CARS-36), the National Key Research and Development Program (No. 2017YFD0500901), the National Natural Science Foundation of China (No. 31572487); the National Science and Technology Pillar Program during the twelfth 5-year plan period (No. 2015BAI09B01), and the MoSTRCUK International Cooperation Project (Project No. 2013DFG32500); and the High-End Foreign Experts Recruitment Program (Project No. GDW20161100071).
References
- Ahluwalia P. K., Pandey R. K., Sehajpal P. K., Prajapati V. K. (2017). Perturbed microRNA expression by Mycobacterium tuberculosis promotes macrophage polarization leading to pro-survival foam cell. Front. Immunol. 8:107. 10.3389/fimmu.2017.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S. (2011). Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2011:814943. 10.1155/2011/814943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanza G., Anufreichik V., Rodvold J. J., Chiu K. T., DeLaney A., Akerset J. C., et al. (2013). Synthesis and delivery of short, noncoding RNA by B lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 110 20182–20187. 10.1073/pnas.1311145110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcila M. L., Sanchez M. D., Ortiz B., Barrera L. F., Garcia L. F., Rojas M. (2007). Activation of apoptosis, but not necrosis, during Mycobacterium tuberculosis infection correlated with decreased bacterial growth: role of TNF-alpha, IL-10, caspases and phospholipase A2. Cell. Immunol. 249 80–93. 10.1016/j.cellimm.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Ayer M., Harm-Anton K. (2017). Cell-mediated delivery of synthetic nano- and microparticles. J. Control. Release 259 92–104. 10.1016/j.jconrel.2017.01.048 [DOI] [PubMed] [Google Scholar]
- Barry S. E., Chan B., Ellis M., Yang Y. R., Plit M. L., Guan G., et al. (2015). Identification of miR-93 as a suitable miR for normalizing miRNA in plasma of tuberculosis patients. J. Cell. Mol. Med. 19 1606–1613. 10.1111/jcmm.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116 281–297. 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Bartel D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136 215–233. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann V., Winkler J. (2014). MiRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med. Chem. 17 1967–1984. 10.4155/fmc.14.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belver L., Papavasiliou F. N., Ramiro A. R. (2011). MicroRNA control of lymphocyte differentiation and function. Curr. Opin. Immunol. 23 368–373. 10.1016/j.coi.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentwich I. (2008). Identifying human microRNAs. Curr. Top. Microbiol. Immunol. 320 257–269. 10.1007/978-3-540-75157-1_12 [DOI] [PubMed] [Google Scholar]
- Bettencourt P., Marion S., Pires D., Santos L. F., Lastrucci C., Carmo N., et al. (2013). Actin-binding protein regulation by microRNAs as a novel microbial strategy to modulate phagocytosis by host cells: the case of N-Wasp and miR-142-3p. Front. Cell. Infect. Microbiol. 3:19. 10.3389/fcimb.2013.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K., Salgame P. (2007). Host innate immune response to Mycobacterium tuberculosis. J. Clin. Immunol. 27 347–362. 10.1007/s10875-007-9084-0 [DOI] [PubMed] [Google Scholar]
- Brameier M., Wiuf C. (2007). Ab initio identification of human microRNAs based on structure motifs. BMC Bioinformatics 8:478. 10.1186/1471-2105-8-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryniarski K., Ptak W., Jayakumar A., Püllmann K., Caplan M. J., Chairoungdua A., et al. (2013). Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J. Allergy Clin. Immunol. 132 170–181. 10.1016/j.jaci.2013.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegari E., Gramantieri L., Domenicali M., D’Abundo L., Sabbioni S., Negrini M. (2015). MicroRNAs in liver cancer: a model for investigating pathogenesis and novel therapeutic approaches. Cell Death Differ. 22 46–57. 10.1038/cdd.2014.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Dwivedi V. P., Singh Y., Siddiqui I., Sharma P., Van Kaer L., et al. (2011). Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a Toll-Like receptor-2-dependent manner. PLoS Pathog. 7:e1002378. 10.1371/journal.ppat.1002378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacoba T. G., Olivera A., Torres D., Crecente-Campo J., Alonso M. J. (2017). Modulating the immune system through nanotechnology. Semin. Immunol. 34 78–102. 10.1016/j.smim.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K., Garnica O., Dhandayuthapani S. (2016). Modulation of host miRNAs by intracellular bacterial pathogens. Front. Cell. Infect. Microbiol. 6:79 10.3389/fcimb.2016.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K., Saikolappan S., Dhandayuthapani S. (2013). Differential expression of miRNAs by macrophages infected with virulent and avirulent Mycobacterium tuberculosis. Tuberculosis 93 S47–S50. 10.1016/S1472-9792(13)70010-6 [DOI] [PubMed] [Google Scholar]
- De Flora S., Bonanni P. (2011). The prevention of infection associated cancers. Carcinogenesis 32 787–795. 10.1093/carcin/bgr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Saitoh T., Akira S. (2013). Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13 722–737. 10.1038/nri3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieci G., Fiorino G., Castelnuovo M., Teichmann M., Pagano A. (2007). The expanding RNA polymerase III transcriptome. Trends Genet. 23 614–622. 10.1016/j.tig.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Dorhoi A., Iannaccone M., Farinacci M., Faé K. C., Schreiber J., Moura-Alves P., et al. (2013). MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J. Clin. Invest. 123 4836–4848. 10.1172/JCI67604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draz N. I., el Hady S. A., Elsayed M. S., Korraa E. E. A., el Magd N. M. (2014). Serum microRNA-29a and microRNA-361-5p as potential diagnostic biomarkers for active pulmonary tuberculosis. Egypt. J. Med. Microbiol. 23. [Google Scholar]
- Duan W., Yang T., Zhou S. F., Wang Z. L., Zhou Z. W., He Z. X. (2015). Novel targeting of PEGylated liposomes for codelivery of TGF-beta1 siRNA and four antitubercular drugs to human macrophages for the treatment of mycobacterial infection: a quantitative proteomic study. Drug Des. Dev. Ther. 9 4441–4470. 10.2147/DDDT.S79369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C., Williams B. G. (2010). The population dynamics and control of tuberculosis. Science 328 856–861. 10.1126/science.1185449 [DOI] [PubMed] [Google Scholar]
- Espert L., Beaumelle B., Vergne I. (2015). Autophagy in Mycobacterium tuberculosis and HIV infections. Front. Cell. Infect. Microbiol. 5:49. 10.3389/fcimb.2015.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian M. R., Sonenberg N., Filipowicz W. (2010). Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79 351–379. 10.1146/annurev-biochem-060308-103103 [DOI] [PubMed] [Google Scholar]
- Faraoni I., Antonetti F. R., Cardone J., Bonmassar E. (2009). MiR-155 gene: a typical multifunctional microRNA. Biochim. Biophys. Acta 1792 497–505. 10.1016/j.bbadis.2009.02.013 [DOI] [PubMed] [Google Scholar]
- Fenton M. J., Vermeulen M. W. (1996). Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect. Immun. 64 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Piñeiro I., Badiola I., Sanchez A. (2017). Nanocarriers for microRNA delivery in cancer medicine. Biotechnol. Adv. 35 350–360. 10.1016/j.biotechadv.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Forster S. C., Michelle D. T., Hertzog P. J. (2015). MicroRNA as type i interferon-regulated transcripts and modulators of the innate immune response. Front. Immunol. 6:334. 10.3389/fimmu.2015.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D., Zeng L., Liu Z., Ke X., Lei L., Li G. (2016). MicroRNA-206 regulates the secretion of inflammatory cytokines and MMP9 expression by targeting TIMP3 in Mycobacterium tuberculosis–infected THP-1 human macrophages. Biochem. Biophys. Res. Commun. 477 167–173. 10.1016/j.bbrc.2016.06.038 [DOI] [PubMed] [Google Scholar]
- Fu Y., Yi Z., Wu X., Wu X., Li J., Xu F., et al. (2011). Circulating microRNAs in patients with active pulmonary tuberculosis. J. Clin. Microbiol. 49 4246–4251. 10.1128/JCM.05459-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furci L., Schena E., Miotto P., Daniela M. C. (2013). Alteration of human macrophages microRNA expression profile upon infection with Mycobacterium tuberculosis. Int. J. Mycobacteriol. 2 128–134. 10.1016/j.ijmyco.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Gantier M. P. (2010). New perspectives in MicroRNA regulation of innate immunity. J. Interferon Cytokine Res. 30 283–289. 10.1089/jir.2010.0037 [DOI] [PubMed] [Google Scholar]
- Ghorpade D. S., Leyland R., Kurowska-Stolarska M., Patil S. A., Balajia K. N. (2012). MicroRNA-155 is required for Mycobacterium bovis BCG-mediated apoptosis of macrophages. Mol. Cell. Biol. 12 2239–2253. 10.1128/MCB.06597-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez A. J., Cinalli R. M., Glasner M. E., Enright A. J., Thomson J. M., Baskerville S., et al. (2005). MicroRNAs regulate brain morphogenesis in zebrafish. Science 308 833–838. 10.1126/science.1109020 [DOI] [PubMed] [Google Scholar]
- Graff J. W., Dickson A. M., Clay G., McCaffrey A. P., Wilson M. E. (2012). Identifying functional microRNAs in macrophages with polarized phenotypes. J. Biol. Chem. 287 21816–21825. 10.1074/jbc.M111.327031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D., Streetz K. L., Jopling C. L., Storm T. A., Pandey K., Davis C. R., et al. (2006). Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 7092 537–541. 10.1038/nature04791 [DOI] [PubMed] [Google Scholar]
- Gu X., Gao Y., Mu D. G., Fu E. Q. (2017). MiR-23a-5p modulates mycobacterial survival and autophagy during Mycobacterium tuberculosis infection through TLR2/MyD88/NF-κB pathway by targeting TLR2. Exp. Cell Res. 354 71–77. 10.1016/j.yexcr.2017.03.039 [DOI] [PubMed] [Google Scholar]
- Guo L., Zhao J., Qu Y., Yin R., Gao Q., Ding S., et al. (2016). MicroRNA-20a inhibits autophagic process by targeting ATG7 and ATG16L1 and favors mycobacterial survival in macrophage cells. Front. Cell. Infect. Microbiol. 6:134. 10.3389/fcimb.2016.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Zhou L., Gao Q., Zhang A., Wei J., Hong D., et al. (2017). MicroRNA-144-3p inhibits autophagy activation and enhances Bacillus Calmette-Guerin infection by targeting ATG4a in RAW264.7 macrophage cells. PLoS One 12:e0179772. 10.1371/journal.pone.0179772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Li J. T., Pan X., Wei L., Wu J. Y. (2010). Candidate Mycobacterium tuberculosis genes targeted by human microRNAs. Protein Cell 1 419–421. 10.1007/s13238-010-0056-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H., Karin M. (2006). Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006:re13. 10.1126/stke.3572006re13 [DOI] [PubMed] [Google Scholar]
- Hammond S. M. (2015). An overview of microRNAs. Adv. Drug Deliv. Rev. 87 3–14. 10.1016/j.addr.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmama Z., Pena-Diaz S., Joseph S., Av-Gay Y. (2015). Immuno-evasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol. Rev. 264 220–232. 10.1111/imr.12268 [DOI] [PubMed] [Google Scholar]
- Huang J., Brumell J. H. (2014). Bacteria-autophagy interplay: a battle for survival. Nat. Rev. Microbiol. 12 101–114. 10.1038/nrmicro3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Junhua J., Xu W., Zhao H., Zhang C., Shi Y., et al. (2015). MiR-155 is up-regulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO3. Mol. Med. Rep. 12 7102–7108. 10.3892/mmr.2015.4250 [DOI] [PubMed] [Google Scholar]
- Huang Y., Shen X. J., Zou Q., Shen X. J., Zou Q., Wang S. P., et al. (2011). Biological functions of microRNAs: a review. J. Physiol. Biochem. 67 129–139. 10.1007/s13105-010-0050-6 [DOI] [PubMed] [Google Scholar]
- Hussain T., Zhao D., Shah S. Z. A., Wang J., Yue R., Liao Y., et al. (2018). MicroRNA 27a-3p regulates antimicrobial responses of murine macrophages infected by Mycobacterium avium subspecies paratuberculosis by targeting interleukin-10 and TGF-β-activated protein kinase 1 binding protein 2. Front. Immunol. 8:1915. 10.3389/fimmu.2017.01915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H., Funatogawa K., Matsumura K., Kato-Miyazawa M., Kirikae F., Kiga K., et al. (2015). MicroRNA-155 knockout mice are susceptible to Mycobacterium tuberculosis infection. Tuberculosis 95 246–250. 10.1016/j.tube.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Jackson A. L., Levin A. A. (2012). Developing microRNA therapeutics: approaching the unique complexities. Nucleic Acid Ther. 22 213–225. 10.1089/nat.2012.0356 [DOI] [PubMed] [Google Scholar]
- Jiang B., Yang J., Rahoui N., Taloub N., Huang Y. D. (2017). Functional polymer materials affecting cell attachment. Adv. Colloid Interface Sci. 250 185–194. 10.1016/j.cis.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Kahlert C., Klupp F., Brand K., Lasitschka F., Diederichs S., Kirchberg J., et al. (2011). Invasion front specific expression and prognostic significance of microRNA in colorectal liver metastases. Cancer Sci. 102 1799–1807. 10.1111/j.1349-7006.2011.02023.x [DOI] [PubMed] [Google Scholar]
- Keane J., Remold H. G., Kornfeld H. (2000). Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 164 2016–2020. 10.4049/jimmunol.164.4.2016 [DOI] [PubMed] [Google Scholar]
- Kim J. K., Yuk J. M., Kim S. Y., Kim T. S., Jin H. S., Yang C. S. (2015). MicroRNA-125a inhibits autophagy activation and antimicrobial responses during mycobacterial infection. J. Immunol. 194 5355–5365. 10.4049/jimmunol.1402557 [DOI] [PubMed] [Google Scholar]
- Kotsinas A., Sigala F., Garbis S. D., Galyfos G., Filis K., Vougas K., et al. (2015). MicroRNAs determining inflammation as novel biomarkers and potential therapeutic targets. Curr. Med. Chem. 22 2666–2679. 10.2174/0929867322666150716113304 [DOI] [PubMed] [Google Scholar]
- Kozomara A., Griffiths-Jones S. (2014). miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42 D68–D73. 10.1093/nar/gkt1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J., Loedige I., Filipowicz W. (2010). The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11 597–610. 10.1038/nrg2843 [DOI] [PubMed] [Google Scholar]
- Kumar M., Sahu S. K., Kumar R., Subuddhi A., Maji R. K., Jana K., et al. (2015). MicroRNA let-7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-kB pathway. Cell Host Microbe 11 345–356. 10.1016/j.chom.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Kumar R., Sahu S. K., Kumar M., Jana K., Gupta P., Gupta U. D., et al. (2016). MicroRNA 17-5p regulates autophagy in Mycobacterium tuberculosis-infected macrophages by targeting Mcl-1 and STAT3. Cell. Microbiol. 18 679–691. 10.1111/cmi.12540 [DOI] [PubMed] [Google Scholar]
- Kumarswamy R., Mudduluru G., Ceppi P., Muppala S., Kozlowski M., Niklinski J., et al. (2012). MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int. J. Cancer 130 2044–2053. 10.1002/ijc.26218 [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Nagineni C. N., Samuel W., Vijayasarathy C., Hooks J. J., Redmond T. M. (2010). Inflammatory cytokines regulate microRNA-155 expression in human retinal pigment epithelial cells by activating JAK/STAT pathway. Biochem. Biophys. Res. Commun. 402 390–395. 10.1016/j.bbrc.2010.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. A., Yoshimori T., Tooze S. A. (2013). The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 14 759–774. 10.1038/nrm3696 [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Hildebrandt-Eriksen E. S., Petri A., Persson R., Lindow M., Munk M. E., et al. (2010). Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327 198–201. 10.1126/science.1178178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre I., Leidinger P., Backes C., Domínguez J., de Souza-Galvão M. L., Maldonado J., et al. (2015). A novel whole-blood miRNA signature for a rapid diagnosis of pulmonary tuberculosis. Eur. Respir. J. 45 1173–1176. 10.1183/09031936.00221514 [DOI] [PubMed] [Google Scholar]
- Lee Y., Han J., Yeom K. H., Jin H., Kim V. N. (2006). Drosha in primary microRNA processing. Cold Spring Harb. Symp. Quant. Biol. 71 51–57. 10.1101/sqb.2006.71.041 [DOI] [PubMed] [Google Scholar]
- Li D., Wang T., Song X., Qucuo M., Yang B., Zhang J., et al. (2011). Genetic study of two single nucleotide polymorphisms within corresponding microRNAs and susceptibility to tuberculosis in a Chinese Tibetan and Han population. Hum. Immunol. 72 598–602. 10.1016/j.humimm.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Li M., Wang J., Fang Y., Gong S., Li M., Wu M., et al. (2016). MicroRNA-146a promotes mycobacterial survival in macrophages through suppressing nitric oxide production. Sci. Rep. 6:23351. 10.1038/srep23351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Yue Y., Xu W., Xiong S. (2013). MicroRNA-146a represses mycobacteria-induced inflammatory response and facilitates bacterial replication via targeting IRAK-1 and TRAF-6. PLoS One 12:e81438. 10.1371/journal.pone.0081438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Duan Z., Xu F., Zhang J., Shulgina M. V., Li F. (2017). Construction and analysis of the transcription factor-microRNA co-regulatory network response to Mycobacterium tuberculosis: a view from the blood. Am. J. Transl. Res. 9 1962–1976. [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Leung E., Lee N., Lui G., To K., Chan R. C. Y. (2015). Differential MicroRNA expression in human macrophages with Mycobacterium tuberculosis infection of Beijing/W and non-Beijing/W strain types. PLoS One 10:e0126018. 10.1371/journal.pone.0126018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A., Linterman M., Lu L. F. (2010). MicroRNA in the adaptive immune system, in sickness and in health. J. Clin. Immunol. 30 339–346. 10.1007/s10875-010-9378-5 [DOI] [PubMed] [Google Scholar]
- Liu L., Shu S., Cheung G. S., Wei X. (2016). Effect of miR-146a/bFGF/PEG-PEI nanoparticles on inflammation response and tissue regeneration of human dental pulp cells. Biomed Res. Int. 2016:3892685. 10.1155/2016/3892685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. T., Wheelwright M., Teles R., Komisopoulou E., Edfeldt K., Ferguson B., et al. (2012). MicroRNA-21 targets the vitamin D-dependent antimicrobial pathway in leprosy. Nat. Med. 18 267–273. 10.1038/nm.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. H., Wang X. J., Jiang J., Cao Z. H., Yang B. F., Cheng X. (2011). Modulation of T cell cytokine production by miR-144∗ with elevated expression in patients with pulmonary tuberculosis. Mol. Immunol. 48 1084–1090. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhou G., Deng X., Yuc Q., Hu Y., Sun H., et al. (2014). Analysis of miRNA expression profiling in human macrophages responding to Mycobacterium infection: induction of the immune regulator miR-146a. J. Infect. 68 553–561. 10.1016/j.jinf.2013.12.017 [DOI] [PubMed] [Google Scholar]
- Lou J., Wang Y., Zhang Z., Qiu W. (2017). MiR-20b inhibits Mycobacterium tuberculosis induced inflammation in the lung of mice through targeting NLRP3. Exp. Cell Res. 358 120–128. 10.1016/j.yexcr.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Lu Y. J., Shen N., Wang X. (2016). Genetic associations between miR-146a/499 polymorphisms and tuberculosis: a meta-analysis. Int. J. Clin. Exp. Med. 9 6445–6452. [Google Scholar]
- Lv Y., Guo S., Li X., Chi J., Qu Y., Zhong H. (2016). Sputum and serum microRNA-144 levels in patients with tuberculosis before and after treatment. Int. J. Infect. Dis. 43 68–73. 10.1016/j.ijid.2015.12.014 [DOI] [PubMed] [Google Scholar]
- Ma F., Xu S., Liu X., Zhang Q., Xu X., Liu M., et al. (2011). The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat. Immunol. 12 861–869. 10.1038/ni.2073 [DOI] [PubMed] [Google Scholar]
- Maertzdorf J., Weiner J., Mollenkopf H. J., Bornot T. B., Bauer T., Prasse A., et al. (2012). Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc. Natl. Acad. Sci. U.S.A. 109 7853–7858. 10.1073/pnas.1121072109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maute R. L., Dalla-Favera R., Basso K. (2014). RNAs with multiple personalities. Wiley Interdiscip. Rev. RNA 5 1–13. 10.1002/wrna.1193 [DOI] [PubMed] [Google Scholar]
- Mayr C., Hemann M. T., Bartel D. P. (2007). Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315 1576–1579. 10.1126/science.1137999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor R. A., Choi M. S. (2011). MicroRNAs in the regulation of adipogenesis and obesity. Curr. Mol. Med. 11 304–316. 10.2174/156652411795677990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G., Landthaler M., Dorsett Y., Tuschl T. (2004). Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA 10 544–550. 10.1261/rna.5235104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J. T., Olson E. N. (2012). MicroRNAs in stress signaling and human disease. Cell 148 1172–1187. 10.1016/j.cell.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q. L., Liu F., Yang X., Liu X., Zhang X., Zhang C., et al. (2014). Identification of latent tuberculosis infection related microRNAs in human U937 macrophages expressing Mycobacterium tuberculosis Hsp16.3. BMC Microbiol. 14:37. 10.1186/1471-2180-14-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhautova J., Demlova R., Slaby O. (2016). MicroRNA-based therapy in animal models of selected gastrointestinal cancers. Front. Pharmacol. 7:329. 10.3389/fphar.2016.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto P., Mwangoka G., Valente I. C., Norbis L., Sotgiu G., Bosu R., et al. (2013). MiRNA signatures in sera of patients with active pulmonary tuberculosis. PLoS One 8:e80149. 10.1371/journal.pone.0080149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. B., Sawyer A. J., Saucier-Sawyer J., Saltzman W. M., Kyriakides T. R. (2016). Nanoparticle delivery of miR-223 to attenuate macrophage fusion. Biomaterials 89 127–135. 10.1016/j.biomaterials.2016.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita Y., Imai T., Yoshizawa H., Watanabe M., Ishibashi K., Muto S., et al. (2015). Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int. J. Nanomedicine 11 3475–3488. 10.2147/IJN.S82587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nana-Sinkam S. P., Croce C. M. (2013). Clinical applications for microRNAs in cancer. Clin. Pharmacol. Ther. 93 98–104. 10.1038/clpt.2012.192 [DOI] [PubMed] [Google Scholar]
- Ni B., Rajaram M. V. S., Lafuse W. P., Landes M. B., Schlesinger L. S. (2014). Mycobacterium tuberculosis decreases human macrophage IFN-g responsiveness through miR-132 and miR-26a. J. Immunol. 193 4537–4547. 10.4049/jimmunol.1400124 [DOI] [PubMed] [Google Scholar]
- O’Connell R. M., Rao D. S., Chaudhuri A. A., Baltimore D. (2010). Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10 111–122. 10.1038/nri2708 [DOI] [PubMed] [Google Scholar]
- O’Connell R. M., Taganov K. D., Boldin M. P., Cheng G., Baltimore D. (2007). MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 5 1604–1609. 10.1073/pnas.0610731104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill L. A., Sheedy F. J., McCoy C. E. (2011). MicroRNAs: the fine tuners of Toll-like receptor signaling. Nat. Rev. Immunol. 11 163–175. 10.1038/nri2957 [DOI] [PubMed] [Google Scholar]
- Ouimet M., Koster S., Sakowski E., Ramkhelawon B., van Solingen C., Oldebeken S., et al. (2016). Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 17 677–686. 10.1038/ni.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A., Molinaro R., Sushnitha M., Evangelopoulos M., Martinez J. O., Arrighetti N., et al. (2017). Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials 147 155–168. 10.1016/j.biomaterials.2017.09.020 [DOI] [PubMed] [Google Scholar]
- Pauli A., Rinn J. L., Schier A. F. (2011). Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 12 136–149. 10.1038/nrg2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel D. M., Cosmopoulos K., Thorley-Lawson D. A., van Eijndhoven M. A., Hopmans E. S., Lindenberg J. L., et al. (2010). Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. U.S.A. 107 6328–6333. 10.1073/pnas.0914843107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R. S., Bhattacharyya S. N., Filipowicz W. (2007). Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 17 118–126. 10.1016/j.tcb.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Poy M. N., Eliasson L., Krutzfeldt J., Kuwajima S., Ma X., Macdonald P. E., et al. (2004). A pancreatic islet specific microRNA regulates insulin secretion. Nature 432 226–230. 10.1038/nature03076 [DOI] [PubMed] [Google Scholar]
- Prosperi D., Colombo M., Zanonia I., Granucci F. (2017). Drug nanocarriers to treat autoimmunity and chronic inflammatory diseases. Semin. Immunol. 34 61–67. 10.1016/j.smim.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Cui L., Ge Y., Shi Z., Zhao K., Guo X., et al. (2012). Altered serum microRNAs as biomarkers for the early diagnosis of pulmonary tuberculosis infection. BMC Infect. Dis. 12:384. 10.1186/1471-2334-12-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Wang Q., Zhou Y., Duan Y., Gao Q. (2016). Inhibition of IFN-γ-induced nitric oxide dependent antimycobacterial activity by miR-155 and C/EBPβ. Int. J. Mol. Sci. 17:535. 10.3390/ijms17040535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram M. V., Ni B., Morris J. D., Brooks M. N., Carlson T. K., Bak-thavachalu B., et al. (2011). Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2(MK2) and microRNA miR-125b. Proc. Natl. Acad. Sci. U.S.A. 108 17408–17413. 10.1073/pnas.1112660108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren N., Gao G., Sun Y., Zhang L., Wang H., Hua W., et al. (2015). MicroRNA signatures from multidrug-resistant Mycobacterium tuberculosis. Mol. Med. Rep. 12 6561–6567. 10.3892/mmr.2015.4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetta C., Faure M. (2013). Autophagy in antiviral innate immunity. Cell. Microbiol. 15 368–376. 10.1111/cmi.12043 [DOI] [PubMed] [Google Scholar]
- Rosas-Taraco A. G., Higgins D. M., Sanchez-Campillo J., Lee E. J., Orme I. M., González-Juarrero M. (2011). Local pulmonary immunotherapy with siRNA targeting TGFbeta1 enhances antimicrobial capacity in Mycobacterium tuberculosis infected mice. Tuberculosis 91 98–106. 10.1016/j.tube.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothchild A. C., Sissonsa J. R., Shafiani S., Plaisier C., Mina D., Maia D., et al. (2016). MiR-155–regulated molecular network orchestrates cell fate in the innate and adaptive immune response to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 113 E6172–E6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G. (2011). Mycobacterium tuberculosis and the intimated is course of a chronic infection. Immunol. Rev. 240 252–268. 10.1111/j.1600-065X.2010.00984.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu S. K., Kumar M., Chakraborty S., Banerjee S. K., Kumar R., Gupta P., et al. (2017). MicroRNA 26a (miR-26a)/KLF4 and CREB-C/EBPβ regulate innate immune signaling, the polarization of macrophages and the trafficking of Mycobacterium tuberculosis to lysosomes during infection. PLoS Pathog. 13:e1006410. 10.1371/journal.ppat.1006410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savan R. (2014). Post-transcriptional regulation of interferons and their signaling pathways. J. Interferon Cytokine Res. 5 318–329. 10.1089/jir.2013.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepeler T., Holm A., Halvey P., Nordentoft I., Lamy P., Riising E. M., et al. (2012). Attenuation of the beta-catenin/TCF4 complex in colorectal cancer cells induces several growth-suppressive microRNAs that target cancer promoting genes. Oncogene 31 50–60. 10.1038/onc.2011.453 [DOI] [PubMed] [Google Scholar]
- Sharbati J., Lewin A., Kutz-Lohroff B., Kamal E., Einspanier R., Sharbati S. (2011). Integrated microRNA-mRNA-analysis of human monocyte derived macrophages upon Mycobacterium avium subsp. hominissuis infection. PLoS One 6:e20258. 10.1371/journal.pone.0020258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhao X., Hsieh J., Wichterle H., Impey S., Banerjee S., et al. (2010). MicroRNA regulation of neural stem cells and neurogenesis. J. Neurosci. 30 14931–14936. 10.1523/JNEUROSCI.4280-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y., Kau V., Mehra A., Chatterjee S., Tousif S., Dwivedi V. P., et al. (2013). Mycobacterium tuberculosis controls MicroRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J. Biol. Chem. 288 5056–5061. 10.1074/jbc.C112.439778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S. V., Diaza A., D’Attilio L., Marchesinib M. M., Bogueb C., Baya M. L., et al. (2013). Altered microRNA expression levels in mononuclear cells of patients with pulmonary and pleural tuberculosis and their relation with components of the immune response. Mol. Immunol. 53 265–269. 10.1016/j.molimm.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Spizzo R., Nicoloso M. S., Lupini L., Lu Y., Fogarty J., Rossi S., et al. (2010). MiR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-alpha in human breast cancer cells. Cell Death Differ. 17 246–254. 10.1038/cdd.2009.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starczynowski D. T., Kuchenbauer F., Argiropoulos B., Sung S., Morin R., Muranyi A., et al. (2010). Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat. Med. 16 49–58. 10.1038/nm.2054 [DOI] [PubMed] [Google Scholar]
- Steele S., Brunton J., Kawula T. (2015). The role of autophagy in intracellular pathogen nutrient acquisition. Front. Cell. Infect. Microbiol. 5:51 10.3389/fcimb.2015.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvang J., Petri A., Lindow M., Obad S., Kauppinen S. (2012). Inhibition of microRNA function by antimiR oligonucleotides. Silence 3:1. 10.1186/1758-907X-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M. J., Aldawsari H., Amiji M. (2016). Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of MicroRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci. Rep. 22:30110. 10.1038/srep30110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S., Steer C. J. (2010). MicroRNAs as gatekeepers of apoptosis. J. Cell. Physiol. 223 289–298. 10.1002/jcp.22066 [DOI] [PubMed] [Google Scholar]
- Taganov D., Boldin M. P., Chang K., Baltimore D. (2006). NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses Konstantin. Proc. Natl. Acad. Sci. U.S.A. 33 12481–12486. 10.1073/pnas.0605298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita F., Patrawala L., Osaki M., Takahashi R. U., Yamamoto Y., Kosaka N., et al. (2010). Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via down regulation of multiple cell-cycle genes. Mol. Ther. 18 181–187. 10.1038/mt.2009.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talekar M., Trivedi M., Shah P., Ouyang Q., Oka A., Gandham S., et al. (2016). Combination wt-p53 and MicroRNA-125b transfection in a genetically engineered lung cancer model using dual CD44/EGFR-targeting nanoparticles. Mol. Ther. 24 759–769. 10.1038/mt.2015.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashima T. (2016). Intelligent substance delivery into cells using cell-penetrating peptides. Bioorg. Med. Chem. Lett. 27 121–130. 10.1016/j.bmcl.2016.11.083 [DOI] [PubMed] [Google Scholar]
- Tay H. L., Kaiko G. E., Plank M., Li J., Maltby S., Essilfie A. T., et al. (2015). Antagonism of miR-328 increases the antimicrobial function of macrophages and neutrophils and rapid clearance of nontypeable Haemophilus influenzae (NTHi) from infected lung. PLoS Pathog. 11:e1004549. 10.1371/journal.ppat.1004549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T., Krishnan S., Amiji M. M. (2016). MicroRNA-223 induced repolarization of peritoneal macrophages using CD44 targeting hyaluronic acid nanoparticles for anti-inflammatory effects. PLoS One 11:e152024. 10.1371/journal.pone.0152024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueberberg B., Kohns M., Mayatepek E., Jacobsen M. (2014). Are microRNAs suitable biomarkers of immunity to tuberculosis? Mol. Cell. Pediatr. 1:8. 10.1186/s40348-014-0008-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uprichard S. L. (2005). The therapeutic potential of RNA interference. FEBS Lett. 579 5996–6007. 10.1016/j.febslet.2005.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura S., Honda M., Yamashita T., Ueda T., Takatori H., Nishino R., et al. (2009). Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology 49 1098–1112. 10.1002/hep.22749 [DOI] [PubMed] [Google Scholar]
- Urgard E., Lorents A., Klaas M., Padari K., Viil J., Runnel T., et al. (2016). Pre-administration of PepFect6-microRNA-146a nanocomplexes inhibits inflammatory responses in keratinocytes and in a mouse model of irritant contact dermatitis. J. Control. Release 235 195–204. 10.1016/j.jconrel.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Vázquez-Hernández F., Granada-Ramírez D. A., Arias-Cerón J. S., Rodriguez-Fragoso P., Mendoza-Álvarez J. G. E., Ramón-Gallegos A., et al. (2017). “Use of nanostructured materials in drug delivery,” Nanobiomaterials, ed. Narayan R. (Sawston: Woodhead Publishing; ), 503–549. [Google Scholar]
- Vegh P., Magee D. A., Nalpas N. C., Bryan K., McCabe M. S., John A., et al. (2015). MicroRNA profiling of the bovine alveolar macrophage response to Mycobacterium bovis infection suggests pathogen survival is enhanced by microRNA regulation of endocytosis and lysosome trafficking. Tuberculosis 95 60–67. 10.1016/j.tube.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Vera J., Lai X., Schmitz U., Wolkenhauer O. (2013). MicroRNA-regulated networks: the perfect storm for classical molecular biology, the ideal scenario for systems biology. Adv. Exp. Med. Biol. 774 55–76. 10.1007/978-94-007-5590-1_4 [DOI] [PubMed] [Google Scholar]
- Vergne I., Chua J., Singh S. B., Deretic V. (2004). Cell biology of Mycobacterium tuberculosis phagosome. Annu. Rev. Cell Dev. Biol. 20 367–394. 10.1146/annurev.cellbio.20.010403.114015 [DOI] [PubMed] [Google Scholar]
- Vergne I., Gilleron M., Nigou J. (2015). Manipulation of the endocytic pathway and phagocyte functions by Mycobacterium tuberculosis lipoarabinomannan. Front. Cell. Infect. Microbiol. 4:187. 10.3389/fcimb.2014.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Ronacher K., Hanekom W., Scriba T. J., Zumla A. (2011). Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 11 343–354. 10.1038/nri2960 [DOI] [PubMed] [Google Scholar]
- Wang C., Liu C., Wei L., Shi L., Pan Z., Mao L., et al. (2016). A group of novel serum diagnostic biomarkers for multidrug-resistant tuberculosis by iTRAQ-2D LC-MS/MS and Solexa sequencing. Int. J. Biol. Sci. 12 246–256. 10.7150/ijbs.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Yang S., Sun G., Tang X., Lu S., Neyrolles O., et al. (2011). Comparative miRNA expression profiles in individuals with latent and active tuberculosis. PLoS One 6:e25832. 10.1371/journal.pone.0025832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wu M., Wen J., Yang K., Li M., Zhan X., et al. (2014). MicroRNA-155 induction by Mycobacterium bovis BCG enhances ROS production through targeting SHIP. Mol. Immunol. 62 29–36. 10.1016/j.molimm.2014.05.012 [DOI] [PubMed] [Google Scholar]
- Wang J., Yang K., Zhou L., Minhaowu Wu Y., Zhu M., et al. (2013). MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 9:e1003697. 10.1371/journal.ppat.1003697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. X., Xu J., Han Y. F., Zhu Y. B., Zhang W. J. (2015). Diagnostic values of microRNA-31 in peripheral blood mononuclear cells for pediatric pulmonary tuberculosis in Chinese patients. Genet. Mol. Res. 14 17235–17243. 10.4238/2015.December.16.23 [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Jia Z., Wei B., Zhou Y., Niu C., Bai S., et al. (2017). MicroRNA-27a restrains the immune response to Mycobacterium tuberculosis infection by targeting IRAK4, a promoter of the NF-κB pathway. Int. J. Clin. Exp. Pathol. 10 9894–9901. [PMC free article] [PubMed] [Google Scholar]
- Wang P., Hou J., Lin L., Wang C., Liu X., Li D., et al. (2010). Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J. Immunol. 185 6226–6233. 10.4049/jimmunol.1000491 [DOI] [PubMed] [Google Scholar]
- Wang Q., Liu S., Tang Y., Liu Q., Yao Y. (2014). MPT64 protein from Mycobacterium tuberculosis inhibits apoptosis of macrophages through NF-kB-miRNA21-Bcl-2 pathway. PLoS One 9:e100949. 10.1371/journal.pone.0100949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Kanai A. (2011). Systems biology reveals microRNA-mediated gene regulation. Front. Genet. 2:29 10.3389/fgene.2011.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J., Maertzdorf J., Kaufmann S. H. (2013). The dual role of biomarkers for understanding basic principles and devising novel intervention strategies in tuberculosis. Ann. N. Y. Acad. Sci. 1283 22–29. 10.1111/j.1749-6632.2012.06802.x [DOI] [PubMed] [Google Scholar]
- Weiss G., Schaible U. E. (2015). Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 264 182–203. 10.1111/imr.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2017). Global Tuberculosis Report. Available at: http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1 [Google Scholar]
- Wu J., Lu C., Diao N., Zhang S., Wang S., Wang F., et al. (2012). Analysis of microRNA expression profiling identifies miR-155 and miR-155∗ as potential diagnostic markers for active tuberculosis: a preliminary study. Hum. Immunol. 73 31–37. 10.1016/j.humimm.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Wu S., Lee S., Huang K., Lee T., Hsu P. W., Weng J. T. (2014). Systematic expression profiling analysis identifies specific MicroRNA-gene interactions that may differentiate between active and latent tuberculosis infection. Biomed Res. Int. 2014:895179. 10.1155/2014/895179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H., Yang Y., Liu J., Li X., Li M., Feng B., et al. (2016). Association between tuberculosis and circulating microRNA hsa-let-7b and hsa-miR-30b: a pilot study in a Chinese population. Tuberculosis 99 63–69. 10.1016/j.tube.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Xu Y., Ren W., Liu Y., Zhang X., Li C., Sun Z. (2013). Tuberculosis-related miRNAs have potential as disease biomarkers. J. Tuberc. Res. 1 17–27. 10.4236/jtr.2013.12005 [DOI] [Google Scholar]
- Yi Z., Fu Y., Ji R., Li R., Guan Z. (2012). Altered microRNA signatures in sputum of patients with active pulmonary tuberculosis. PLoS One 7:e43184. 10.1371/journal.pone.0043184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Xi X., Wang Q., Jiao J., Zhang L., Zhao H. (2015). The association between serum miR-155 and natural killer cells from tuberculosis patients. Int. J. Clin. Exp. Med. 8 9168–9172. [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Liu X., Wang W., Cai Y., Li S., Chen Q. (2016). Down-regulation of miR-20a-5p triggers cell apoptosis to facilitate mycobacterial clearance through targeting JNK2 in human macrophages. Cell Cycle 15 2527–2538. 10.1080/15384101.2016.1215386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Guo J., Fan S., Li Y., Wei L., Yang X., et al. (2013). Screening and identification of six serum microRNAs as novel potential combination biomarkers for pulmonary tuberculosis diagnosis. PLoS One 12:e81076. 10.1371/journal.pone.0081076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. M., Zhang A. R., Xu M., Lou J., Qiu W. Q. (2017). TLR-4/miRNA-32-5p/FSTL1 signaling regulates mycobacterial survival and inflammatory responses in Mycobacterium tuberculosis-infected macrophages. Exp. Cell Res. 352 313–321. 10.1016/j.yexcr.2017.02.025 [DOI] [PubMed] [Google Scholar]
- Zheng D., Yu Y., Li M., Wang G., Chen R., Fan G., et al. (2015). Inhibition of microRNA 195 prevents apoptosis and multiple-organ injury in mouse models of sepsis. J. Infect. Dis. 213 1661–1670. 10.1093/infdis/jiv760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Leung E., Lee N., Lui G., To K., Chan R. C. Y., et al. (2015). Differential MicroRNA expression in human macrophages with Mycobacterium tuberculosis infection of Beijing/W and non-Beijing/W strain types. PLoS One 10:e0126018. 10.1371/journal.pone.0126018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Ferguson J., Chang J. T. (2007). Inter- and intra-combinatorial regulation by transcription factors and microRNAs. BMC Genomics 8:396. 10.1186/1471-2164-8-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Yu G., Yang X., Zhu C., Zhang Z., Zhan X. (2016). Circulating microRNAs as biomarkers for the early diagnosis of childhood tuberculosis infection. Mol. Med. Rep. 13 4620–4626. 10.3892/mmr.2016.5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang L., Zhao W., Wu Y., Zhu C., Yang Y. (2013). Nanoparticle-mediated delivery of TGF-β1 miRNA plasmid for preventing flexor tendon adhesion formation. Biomaterials 34 8269–8278. 10.1016/j.biomaterials.2013.07.072 [DOI] [PubMed] [Google Scholar]