Abstract
Background:
This study was planned to compare and evaluate the staining efficacy of Leishman–Giemsa cocktail (LG), Papanicolaou, and Giemsa stain (G) in potentially malignant disorders and malignant lesions.
Aims:
To evaluate the quality of nuclear and cytoplasmic staining of LG with G, and rapid Papanicolaou stain (Pap) and to compare the total staining efficiency of LG against G and P.
Materials and Methods:
One hundred and eighty participants were studied under three groups – 60 as healthy controls, 60 with potentially malignant disorders, and 60 with malignant lesions; smears were taken thrice from the buccal mucosa. One smear was fixed with Bio-Fix spray and other two smears were allowed to air dry for 2–3 minutes. Then, the ethyl alcohol-fixed smear was stained with Pap and the two other air-dried smears were stained with G and LG stains. Analysis was done using Friedman test and Wilcoxon Signed Rank Test with SPSS Version 15.0.
Results:
In the normal group, staining of LG was highly significant (P < 0.001). Among potentially malignant lesions, LG was observed to be highly significant (P < 0.001) when compared with G and was not significant when compared with Pap (P = 0.186). In the malignant group, LG was highly significant (P < 0.001). LG was superior with the highest average staining score of (2.018) than Pap and G.
Conclusion:
LG cocktail is a better stain with excellent cytoplasmic and nuclear staining intensity compared to Pap and G stains.
Keywords: Exfoliative cytology, Giemsa stain, Leishman–Giemsa cocktail stain, rapid Papanicolaou method, staining efficiency
INTRODUCTION
Oral cancer is the most common malignancy worldwide. It is a substantial healthcare burden on the economy, and with the increasing incidence of oral cancer in developing countries like India and other southeast-Asian countries, the role of screening is becoming more vital.[1]
Exfoliative cytology is an economical, easy, noninvasive, and feasible method for detection of malignancies. It is the microscopic examination of shed cells from an epithelial surface. It is a rapid procedure which may be equally valuable in screening for oral squamous cell carcinoma (OSCC). However, population screening for OSCC has not yet received sufficient support to establish it as a public health measure.[2]
The most commonly followed procedure for staining exfoliative cytology smears is the Papanicolaou (PAP) technique. It is beneficial in differential staining of cells from various layers. However, the procedure is expensive and time consuming with multiple steps.[1] Routine PAP staining is a commonly employed chair-side cytological procedure in the diagnosis of suspicious oral smears as it yields a polychromatic, transparent staining reaction with crisp nuclear/cytological features.[3] Various modifications of PAP staining such as Ultra-Fast method (90 seconds) and Rapid PAP method (4–5 minutes) are also available. These two techniques overcome the time limitation of routine PAP but the issues of ethanol fixation and color preservation still remain.[3]
Romanowsky stains are universally employed for staining blood films. Giemsa staining method is used for the cytological diagnosis of specimens in addition to PAP. However, some of the drawbacks include the tendency to precipitate, high background staining, the need to prepare fresh solution every day, and technique sensitivity. Many new modifications to the original scrape smear with a wooden spatula are available for good diagnosis. It includes oral rinse, imprint cytology, and use of sponge. The new analytical techniques which have been gaining popularity in this decade include Oral CDx and computer-based deposition of a thin layer of cells.[4]
Leishman–Giemsa cocktail (LG) being a new staining technique has not been used widely in exfoliative cytology. This easy and one-step procedure warrants further investigation because of its potential application in oral cancer screening.[1]
This study was conducted to evaluate and compare routinely used stains such as PAP and Giemsa stain (G) with the relatively new LG stain in oral exfoliated cells of healthy controls, potentially malignant disorders, and malignant oral lesions such as oral cancer. We compared the efficiency of the three staining procedures. We also review the current indications for oral exfoliative cytology and add more valuable data to the existing literature.
MATERIALS AND METHODS
Sixty healthy controls, 60 cases of potentially malignant disorders in the oral cavity, and 60 cases of malignant oral lesions such as oral cancer were selected from the outpatient department of a dental college and group of hospitals in Rajasthan. The study was conducted from August 2014 to May 2016. A written informed consent was obtained from all the patients.
Patients with potentially malignant disorders such as leukoplakia, lichen planus, and oral submucous fibrosis and patients with malignant disorders such as oral squamous cell carcinoma were included in the study. Patients with systemic disorders and uncooperative patients were excluded.
Participants were asked to rinse their mouth with tapwater to rinse off the cellular debris. The exfoliated cell samples were obtained by gently scraping the buccal mucosa in a clockwise direction using a wooden tongue blade. Three smears each were taken from the buccal mucosa in case of healthy controls. Similarly, three individual smears were made from the area of lesion in case of potentially malignant and malignant disorders. One of the smears was fixed with BIOFIX spray and the other two smears were allowed to air-dry for 2–3 minutes. Thus, a total of 540 smears were prepared for this study.
One fixed smear was stained with Rapid PAP stain with accordance to Asthana et al.[3] It was stained with Rapid PAP nuclear stain by dipping it for 60 seconds. Then 3 drops of Scott's tapwater buffer was added and washed after 10 seconds. The excess water was blotted out from the slide. It was dipped with two changes of Rapid PAP dehydrant for 30 seconds each. Then, it was dipped for 45 seconds in Rapid PAP cytoplasmic stain, and was washed in tapwater, dipped in saline, and kept for drying. Thus, the staining time for Rapid PAP method was approximately 3 minutes.
One of the air-dried smears was stained with Giemsa stain. Giemsa working solution was prepared by mixing 5% Giemsa stain with 95% distilled water. The slide was immersed in this working solution for 20–30 minutes and kept for air-drying. Thus, the staining time for Giemsa method was 30 minutes.
The other air-dried smear was stained with LG stain. LG was prepared by filtering a unit volume of Giemsa and mixing it with an equal volume of distilled water to prepare Giemsa working solution. Equal volume of Leishman's stain was filtered and mixed with an equal volume of Giemsa working solution (1:1) to prepare the LG stain. The cocktail was used and stored just like Leishman's stain. The air-dried smears were flooded with LG stain and left for 1 minute. An equal volume of buffer (pH, 6.8) was added and left for 5 minutes with gentle blowing. The slides were washed in tapwater and left for drying. Thus, the staining time for LG cocktail method was 6 minutes.
The slides were evaluated for staining characteristics such as nuclear and cytoplasmic detail. Each stained slide was evaluated for 10 consecutive cells and recorded to the scoring criteria as per Sujathan et al.[5] To determine the consistency, intraobserver variation was checked, wherein the same method was used and the counting was repeated after a gap of 5 days. The identical scores of both assessments were only included in final analysis.
The stained slides were evaluated for staining characteristics such as nuclear and cytoplasmic detail. An area of sufficient cellularity was assessed.
Nuclear detail was assessed based on the nature of the chromatin, pyknosis, vesicularity, and membrane integrity and scored as: 0 poor preservation, 1+ smudgy, 2+ fair preservation but chromatin granularity not appreciable, 3+ excellent preservation with crisp chromatin.
Cytoplasmic details were evaluated based on the transparency and nature of cell membrane and scored as 0 not preserved, 1+ non-transparent with intact cell membrane, 2+ non-transparent masking nuclear details, and 3+ transparent, intact cell membrane without masking nuclear details. Intraobserver variation was checked by repeating counting after a gap of 5 days. The identical scores of both assessments were only included in the final analysis.
The results were tabulated and statistically evaluated using Friedman test and Wilcoxon Matched-pairs Signed Rank test using SPSS 15 (SPSS INC, Chicago, IL) software.
RESULTS
The present study was undertaken to compare and evaluate the efficacy of LG stain, Giemsa stain, and PAP in cytological diagnosis of normal cases, potentially malignant disorders, and malignant oral lesions.
In the normal group, cytoplasmic stain of LG was compared with PAP vs. Giemsa. Mean value calculated for each stain were as follows: Rapid PAP was 2.01, Giemsa was 1.65, and for LG stain was 2.32 [Table 1]. LG was observed to be highly significant with a P value of <0.001. Pap was also highly significant in comparison with Giemsa with a P value of <0.001. Thus, in our study, LG was superior to PAP and Giemsa in the cytoplasmic staining of the normal group.
Table 1.
Mean score of nuclear and cytoplasmic details in the normal group, malignant group, and premalignant group with PAP, Giemsa, and LG cocktail stains
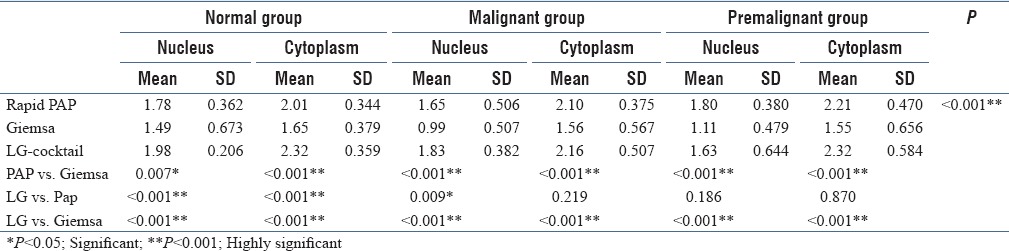
For normal group, we calculated total scoring for both nuclear and cytoplasmic staining with all the three stains – LG, PAP, and Giemsa to calculate the total efficiency. The total score for PAP stain was 2272, Giemsa stain was 1886, and LG stain was 2532. The average of the total nuclear and cytoplasmic staining scores was 3.7 for PAP stain, 3.1 for Giemsa, and 4.2 for LG stain. Thus, LG stain showed superior staining in normal cases.
The cytoplasmic staining of the three stains was compared among potentially malignant lesions. The staining of LG was compared with PAP vs. Giemsa, and the mean value calculated for each stain was 2.32, 2.01, and 1.65, respectively. LG was observed to be highly significant with a P value of <0.001when compared with Giemsa and was not significant when compared with PAP, with a P value 0.870 [Table 1]. Pap was highly significant in comparison with Giemsa with a P value of < 0.001. Hence, LG and PAP were found to be more superior to Giemsa in the cytoplasmic staining of potentially malignant group. In potentially malignant cases, the total score was calculated for PAP was 2406, Giemsa was 1590, and LG was 2290. There was only a small difference between PAP and LG stain having an average of 4.0, 2.6, and 3.8 for the three stains. Thus, PAP and LG showed superior staining results in comparison with Giemsa.
LG in comparison with PAP and G for nuclear staining in malignant group had a mean value of 1.83, 1.65, and 0.99, respectively, for each stain. LG was highly significant with a P value of <0.001. PAP was found to be more significant in comparison with Giemsa showing a P value of 0.007 [Table 1]. The total score calculated for both nuclear and cytoplasmic staining in case of malignant group with PAP was 2224, Giemsa was 1534, and LG had a score of 2390. The average was calculated as 3.7, 2.5, and 3.9. Thus, LG cocktail was the superior stain among the three in the staining of malignant group.
When compared for nuclear staining of LG, Giemsa and PAP, the mean value calculated for each stain was 1.80, 1.11, and 1.63. LG was observed to be highly significant with a P value of <0.001 in comparison with G and was not significant when compared with PAP with a P value 0.186. PAP was also highly significant in comparison with Giemsa with a P value of <0.001 [Table 1]. Hence, LG and PAP were found to be more superior to Giemsa in the nuclear staining of potentially malignant group. LG was also found to be superior to PAP and Giemsa in the cytoplasmic staining of malignant group.
The mean quality index was also calculated for PAP, Giemsa, and LG by dividing the total score obtained per stain with the maximum possible score (3600). It also showed that LG cocktail was superior to PAP and Giemsa stain.
The average scores were also calculated for the three stains on the whole in normal, potentially malignant, and malignant cases, which showed LG cocktail superior among PAP and Giemsa with the highest average score of 2.018.
As per the Wilcoxon signed ranks test–ranks table, it is clear that LG was a better cytoplasmic stain than PAP and Giemsa in normal cases. In comparison with potentially malignant cases, both LG and PAP stains had a similar score and could not achieve statistical significance. However, both the stains were superior to Giemsa in nuclear and cytoplasmic staining. In comparison of malignant cases, LG had higher mean scores than PAP and Giemsa stains.
DISCUSSION
Oral mucosa is an index to the general health of the body. Everything which passes through the oral cavity in various forms like food and drink comes first in contact with it.[6] Exfoliated cells, when collected and appropriately stained, gives information on the living epithelium from which they are derived. Thus, by studying the morphological alterations of the exfoliated cells and their pattern, various pathological conditions could be diagnosed.[7]
Exfoliative cytology relies on the presence of cells that are shed spontaneously and collection of cells is considered only a minimally invasive procedure with little risk of complication. Cells can be collected from the epithelial surfaces by lightly scraping the surface, swabbing, aspirating, or washing the surfaces.[7,8] It can serve an important role by identifying oral mucosal lesions that need to be biopsied despite a “benign” appearance. Hence, it can be a powerful tool for the early detection of malignant and premalignant lesions as well as for some viral and fungal infections. It is a simple, painless, bloodless technique that can be repeated frequently with minimal discomfort to the patient.[6,9,10]
The present study was carried out in normal cases, potentially malignant, and malignant lesions excluding patients with systemic diseases and uncooperative behavior. The study participants comprised 540 smears taken from 180 participants, including 60 normal patients as controls, 60 potentially malignant disorders in oral region, and 60 patients of malignant lesions such as oral cancer to compare and evaluated the efficacy of the three stains. Belgaumi and Shetty performed a study on 100 healthy controls and 100 patients diagnosed with squamous cell carcinoma.[1] Garbyal et al. analyzed 720 cases in their study.[11] Mitra et al. performed as similar study with 25 cases of oral lesions.[12] Asthana et al. compared 100 smears from 50 patients.[7]
PAP staining is the most widely used staining technique for cytological diagnosis comprising nuclear and cytoplasmic staining. PAP staining is a very reliable technique and imparts blue color to the nuclei due to hematoxylin, and OG-6 imparts an orange/pink color to the keratinized cells. Superficial cells are stained orange/pink in color, green/blue in intermediate and parabasal cells, and metaplastic cells stain green and pink. Alcohol is used as a coagulative fixative that causes cell shrinkage by removing intracellular water and gives a sharp nuclear detail. The cytoplasmic features become less well defined. Delay in fixation also interferes with good interpretation. Leishman stain is a good nuclear stain with intense staining of extracellular ground substance. Nuclei are stained deep blue to blue-violet and cytoplasm is stained light blue.[13] Giemsa stain is a good cytoplasmic stain which gives blue color to the nuclei and cytoplasm is stained pink. When Giemsa stain is mixed with Leishman stain at a ratio of 1:1, the resultant LG cocktail gives a moderate metachromasia to the ground substance and brilliantly stained cellular components with a deep blue color to the nuclei and light blue color to the cytoplasm. Hence, the chromatin granularity and vesicularity is better appreciated in air-dried LG-stained smears. The staining is also completed in 6–7 minutes with fewer steps and minimal expenditure.[1,13]
Our study comprised the use of three stains such as PAP, Giemsa, and LG stain. Belgaumi and Shetty in their study used PAP, Giemsa, and LG stains to calculate the efficiency of each stain.[1] Garbyal et al. in a study on air-dried cytologic smears compared the use of LG and Giemsa.[11] Mitra et al. used LG and PAP stain in their study.[11]
It was observed in our study that LG was better appreciated than PAP and G (P < 0.001) in the nuclear staining for both normal and malignant group, whereas in the potentially malignant group, there was no statistical difference between LG and PAP (P value of 0.186). Our results for PAP and Giemsa were almost similar to a study by Idris and Hussain,[14] but are in contrast to the study on fine needle aspiration by Garbyal et al.,[11] in which the cytoplasmic staining gave good results with LG and excellent results with Giemsa.
The current study showed that the overall mean quality index of LG cocktail, PAP, and Giemsa as 3.9, 2.8, and 3.8, respectively, which highlighted that LG had better staining quality compared to other stains. LG was superior with good differential cytoplasmic details, decent cytoplasmic transparency, along with superior staining of nuclear chromatin.[14]
In our results, according to Friedman test, there was a statistical significant difference among the stains with χ2(2) = 67.821, 28.417, 54.478; P = 0.0005 for cytoplasmic staining and χ2(2) = 26.327, 44.947, 55.483; P = 0.0005 for nuclear staining. The results of the Wilcoxon signed rank test for cytoplasmic staining showed that LG had statistically significant difference than Giemsa and PAP in normal cases (Z = −5.380, −4.538, −5.873; P = 0.000) in malignant cases (Z = −6.418, −4.865, −4.444, P = 0.000) and in potentially malignant cases (Z = −4.968; P = 0.000). Our results were in accordance to study by Belgaumi and Shetty who also observed statistically significant difference when the cytoplasmic staining was compared for PAP vs. Giemsa (P = 0.0001) and Giemsa vs. LG (P = 0.0001).[1]
Hence, from the above data, we found that LG stain was more efficient in cytoplasmic and nuclear staining in comparison to PAP and Giemsa stain, but the difference was negligible in comparison to PAP stain in potentially malignant cases. The overall observations of the present study were that the LG stain is comparable and slightly superior to PAP stain in cytoplasmic and nuclear details, which is in accordance with the study by Garbyal et al. and Mitra et al. LG is also superior to Giemsa stain both in staining characteristics.[11,12] The comparison of few reported studies is tabulated [Table 2].
Table 2.
Comparison of previous studies on staining with PAP, Giemsa, LG cocktail stains

A stain to be used in a community screening setup should have good staining characteristics and the technique should also be easy, rapid, and inexpensive. Rapid PAP stain took around 3 minutes, but it involved multiple steps and required alcohol fixation. The fixing and staining time with Giemsa was about 30 minutes in our study. However, the LG cocktail staining procedure did not require additional fixation with alcohol and could be completed in 6–7 minutes. Hence, it was more rapid and efficient in nature. Hence, LG shows better feasibility to be used in mass-screening programs. Thus, the added advantages of the staining procedure include single-step procedure, speed of the technique, reduced staining time, saving critical manpower, and good staining results.[1] Our study supports the idea of utilizing LG cocktail stain even in situ ations with heavy outpatient load, screening intraoperative smears, and community screening programs.
Smear interpretation becomes difficult due to the blind technique and inadequate sampling. Hence, staining quality is critical. LG cocktail provides comparable cytoplasmic staining and better nuclear staining to PAP and has potential application in the screening of oral cancer.[10,15,16] It is always better to confirm the dysplastic cells with conventional PAP staining and subsequent tissue biopsy.
We have attempted to perform the study within our limitations and are planning for a large-scale studies to further ascertain our findings by comparing conventional PAP with these newer rapid staining methods. The future prospects include the use of specialized techniques such as liquid-based cytology and molecular analyses to enhance the specificity and sensitivity of LG cocktail stain and also to compare its efficacy with such modern techniques. Liquid-based cytology with cytomorphometric assessment along with molecular and DNA analysis [10,17] could be included in our further studies.
CONCLUSION
We conclude that LG stain could be used as a vital procedure in oral exfoliative cytology along with Rapid PAP stain and can be indicated for the early detection as well as follow-up of the patients with either a potentially malignant disorders or oral malignancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Belgaumi UI, Shetty P. Leishman Giemsa cocktail as a new, potentially useful cytological technique comparable to Papanicolaou staining for oral cancer diagnosis. J Cytol. 2013;30:18–22. doi: 10.4103/0970-9371.107507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugerman PB, Savage NW. Exfoliative cytology in clinical oral pathology. Aust Dent J. 1996;41:71–4. doi: 10.1111/j.1834-7819.1996.tb05915.x. [DOI] [PubMed] [Google Scholar]
- 3.Asthana A, Singh AK. Comparison of the routine Papanicolaou staining technique with the rapid, economic, acetic acid, Papanicolaou (REAP) technique. Int J Med Dent Sci. 2014;3:484–9. [Google Scholar]
- 4.Dhanapal R, Saraswathi TR, Sreenivas B, Kumar G, Ramachandran CR. Concentrated full smear technique a comparison with Oral scrape - “A liquid based cytology”. J Clin Dent. 2012;6:57–62. [Google Scholar]
- 5.Sujathan K, Pillai RK, Kannan S, Chandralekha B, Mathew A, Nair KM. Cytodiagnosis of serous effusions: A combined approach to morphological features in Papanicolaou and May Grunewald Giemsa stain and a modified cell block preparation. J Cytol. 2000;17:89–95. [Google Scholar]
- 6.Kumar A, Kumar A, Ritu, Parveen S, Akhtar MJ. Clinico-pathological and cytological changes in oral mucosa of patients having tobacco smoking habit. J Evolution Med Dent Sci. 2014;3:14250–6. [Google Scholar]
- 7.Agarwal SP. Role of diagnostic cytology. Manual for cytology in National Cancer Control Programme, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. 2005:9–44. [Google Scholar]
- 8.Ibrahim ZA. Comparison between Papanicolaou and May-Grunewald Giemsa stains in thyroid fine needle aspiration. Faculty of medical laboratory sciences, University of Khartoum. 2004:1–35. [Google Scholar]
- 9.Kaur M, Saxena S, Samantha YP, Chawla G, Yadav G. Usefulness of oral exfoliative cytology in dental practise. J Oral Health Comm Dent. 2013;7:161. [Google Scholar]
- 10.Rajput DV, Tupkari JV. Early detection of oral cancer: Pap and AgNOR staining in brush biopsies. J Oral Maxillofac Pathol. 2010;14:52–8. doi: 10.4103/0973-029X.72501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garbyal RS, Agarwal N, Kumar P. Leishman-Giemsa Cocktail: An effective Romanowsky stain for air-dried cytologic smears. Acta Cytol. 2006;50:403–6. doi: 10.1159/000325981. [DOI] [PubMed] [Google Scholar]
- 12.Mitra S, Bose S, Mukherjee G. Comparative studies on the Leishman-Giemsa stains and Papanicolaou stains for cytological diagnosis of oral lesion. Sci Cult. 2011;77:139–40. [Google Scholar]
- 13.Joshi PS, Kaljkar MS. Cytomorphometric analysis of oral premalignant and malignant lesions using feulgen stain and exfoliative brush cytology. J Interdiscipl Histopathol. 2013;1:204–11. [Google Scholar]
- 14.Idris A, Hussain M. Comparison of the efficacy of three stains used for the detection of cytological changes in Sudanese females with breast lumps. Sudanese J Public Health. 2009;4:275–7. [Google Scholar]
- 15.Singh A. Role of exfoliative cytology in oral lesions. J Coll Med Sci Nepal. 2010;6:29–37. [Google Scholar]
- 16.Suryalakshmi S, Thangamani P, Ravi S, Niveditha T, Kumar IVS, Mohan KR, et al. Comparison of Leishman staining with H and E staining technique in the study of FNAC smears. IOSR J Med Dent Sci. 2016;15:34–42. [Google Scholar]
- 17.Navone R. Cytology of the oral cavity: A re-evaluation. Pathologica. 2009;101:6–8. [PubMed] [Google Scholar]


