Abstract
Field soil atmospheres have higher CO2 and lower O2 concentrations compared with ambient atmosphere, but little is known about the impact of such conditions on root exudation patterns. We used altered levels of CO2 and O2 relative to ambient conditions to examine the influence of the atmosphere on the production of root border cells by pea (Pisum sativum) root tips. During germination, atmospheres with high CO2 and low O2 inhibited root development and border cell separation in pea seedlings. Later in development, the same atmospheric composition stimulated border cell separation without significantly influencing root growth. Increased CO2, not low O2, was responsible for the observed stimulation of border cell number. High CO2 apparently can override endogenous signals that regulate the number of border cells released from pea roots into the rhizosphere. The same conditions that stimulated border cell production in pea had no such effect in alfalfa (Medicago sativa).
CO2 and oxygen O2 are crucial components of the rhizosphere, “the narrow zone subject to the influence of living roots, as manifested by the leakage or exudation of substances that affect microbial activity” (Curl and Truelove, 1986). In field soil, CO2 and O2 concentrations vary depending on soil type, soil moisture, organic matter content, temperature, type of crops, and soil microflora (Abrosimova and Revut, 1964; Yamaguchi et al., 1967; Buyanovsky and Wagner, 1983; Wood et al., 1993). Compared with 0.03% (v/v) CO2 and 21% (v/v) O2 levels that exist under ambient atmospheric conditions, the CO2 concentration at some soil depths may reach 10% or higher and the O2 concentration can decline to lower than 10% (for review, see Stolzy, 1974; Buyanovsky and Wagner, 1983). The different CO2:O2 levels in field soil compared with ambient atmosphere are thought to be due mainly to the respiration of soil microorganisms and plant roots (Wood et al., 1993) and to the slow gas exchange rates that occur in the soil (Stolzy and Zentmyer, 1975). The increase in CO2 levels in the soil corresponds to a decline in O2 levels so that the sum of these two gases remains at about 21%, while the N2 concentration in the soil remains at 79% (Griffin, 1972).
Colonization of plant roots by microorganisms is sensitive to fluctuations in atmospheric concentrations of CO2 and O2. Root colonization by certain bacteria can be stimulated by up to 138% under atmospheres containing high CO2/low O2 concentrations (Kim et al., 1996). One explanation for such results is that high CO2/low O2 atmospheres influence the quantity and composition of root exudates needed to support microbial growth in the rhizosphere. For example, Gal and dihydroxyacetone concentrations of peanut root exudate are sensitive to O2 and CO2 levels (Rittenhouse and Hale, 1971) and hypoxia can cause increases in organic acids, sugars, and amino acids in exudates of young corn and sunflower (Grineva, 1962).
Root border cells are major contributors of root exudates in most agronomically important crop species (Hawes et al., 1998). Border cells, formerly termed “sloughed root cap” cells, are defined as those cells separated from each other and loosely associated with the root so that they disperse into suspension upon immersion of the root tip into water (Hawes, 1990; Hawes and Lin, 1990). In young roots of legumes, up to 98% of the exudates released from hydroponically grown seedlings derive from the process of border cell separation (Griffin et al., 1976; Hawes and Pueppke, 1986). In addition to the thousands of detached cells, these exudates include a high Mr mucilage that encases border cells, the cell wall breakdown products solubilized during the separation of the cells, and an array of extracellular chemicals that are exported by border cells after they separate from the root (Brigham et al., 1995; Zhu et al., 1997).
In the past, border cell production was incorrectly thought to be a continuous by-product of constitutive turnover of the root cap (Clowes, 1994). However, more recent studies have revealed that border cell separation is not necessarily continuous but can be turned on and off by the plant and can be induced experimentally (Hawes and Lin, 1990; Brigham et al., 1998). Under laboratory conditions (24°C, dark), the process of border cell separation in pea (Pisum sativum cv Little Marvel), our primary model system, can be divided into two stages. Stage I is radicle emergence. Border cells can be isolated when the root is 5 mm long, and from this point on, border cell numbers increase with increasing root length until the root is 25 mm long. At this point in development, if the cells are not removed from the tip, the production of border cells ceases so that cell number per root tip remains constant regardless of root length. Border cells apparently secrete a factor that accumulates extracellularly until it reaches a concentration that inhibits further turnover of the root cap (Brigham et al., 1998).
Stage II is renewed border cell separation. When the existing border cells are removed together with their associated exudates by agitation of the root tip in water, renewed production of border cells by the root cap is induced. New border cells can be isolated from such induced root tips within 1 h, and 24 h later a full set of approximately 4,000 border cells is present on each root tip, at which time border cell separation again ceases (Hawes and Lin, 1990; Stephenson and Hawes, 1994). Similar border cell separation processes exist on other legumes, although the number of cells produced daily may vary. For example, alfalfa (Medicago sativa cv Lew) root tips yield about 2,000 cells per day, and the maximum cell number is reached when root length is 20 mm.
Factors that regulate border cell production under natural conditions are unknown. If this process is responsive to environmental conditions that normally develop underground, then border cell number would be predicted to vary accordingly within specific microenvironments. Such variation would be predicted to exert a large effect on the properties of the rhizosphere, particularly with respect to colonization and infection by microorganisms that specifically respond to border cells and their exudates (Hawes et al., 1998). In this study, the effect of atmospheres with altered CO2:O2 concentrations on the production of border cells was examined.
MATERIALS AND METHODS
Plant Material and Border Cell Isolation
Plant species included pea (Pisum sativum cv Little Marvel, Royal Seeds, Kansas City, MO) and alfalfa (Medicago sativa cv Lew, a gift from Dr. Steve Smith, Department of Plant Sciences, University of Arizona, Tucson).
Pea seeds were surface-sterilized by immersion in 95% (v/v) ethanol for 10 min and then in full-strength commercial bleach (5.25% [v/v] NaOCl) for 30 min. Alfalfa seeds were sterilized in ethanol and bleach for 10 min each. Seeds then were rinsed in sterilized distilled water six times, followed by immersion in sterilized distilled water for 6 h for pea and 2 h for alfalfa. The imbibed seeds were germinated at 24°C on 1.0% (w/v) water agar (Sigma-Aldrich, St. Louis) plates overlaid with sterilized germination paper (Hawes and Lin, 1990).
Border cells were isolated as described previously (Hawes and Lin, 1990). Tips of intact roots were immersed in 1 mL of sterilized distilled water for 1 to 2 min and border cells were removed by gently agitation. Border cell numbers were determined by direct counts using a light microscope.
Preparation of Atmospheres with Altered CO2:O2 Concentrations
A portable gas-mixing device (Misaghi and Stowell, 1991) was used to provide atmospheres with defined N2, O2, and CO2 concentrations and constant flow rates. The device uses adjustable flow control valves and a mass flowmeter to mix and deliver precise, predetermined quantities of three gases in different combinations at a constant flow rate. The O2 and CO2 concentrations were varied by adjusting the flow rate from the source O2, CO2, and N2 cylinders. At the early stage of experiments, the test atmospheres included CO2:O2 concentrations (v/v) of 0.03%:21% (ambient atmospheric conditions) and 3%:18%, 6%:15%, 9%:12%, and 12%:9%, N2 concentration was kept at 79%.
Atmospheres prepared by increasing the CO2 level or decreasing the O2 level only were used in some experiments. The atmospheric components of these gas mixtures were CO2:O2 concentrations (v/v) of 3%:21%, 6%:21%, 0.03%:18%, and 0.03%:15%, and the N2 concentrations were changed proportionally to 76%, 73%, 82%, and 85%. In preliminary tests, significant effects were observed in the absence of any changes in N2 level, so subsequent experiments focused on the effects of CO2:O2 concentrations, and no further studies were carried on the effects of N2. The actual concentration of each gas in the mixture was determined using an analytical gas chromatograph (Series 100, Hach Carle Chromatography Company, Loveland, CO) by analysis of 1 mL of the mixed air sample.
Glass jars with two ports (4 mm in diameter) on the lid serving as an inlet and an outlet were used for exposing seeds or whole seedlings to the test atmospheres (Kim and Misaghi, 1992). Glass jars (3.8 L) were flushed with 100% (v/v) N2 for about 30 min to eliminate the existing O2 and CO2 before running the test atmospheres. Seeds or seedlings were placed in water agar plates overlaid with germination paper. To promote gas exchange, a 10-mm-long, 3-mm-wide opening was made on the wall of each plate. A maximum of 10 plates, each with 25 mL of water agar containing seeds or seedlings, were placed inside each glass jar. When assembled, the volume in each jar left for air phases was about 3.5 L. When plates containing seeds or seedlings in each jar were fewer than 10, empty water agar plates were used to make up the number so as to keep the desired volume. To eliminate fluctuations in CO2 and O2 concentrations caused by seed respiration, the jar was flushed once every 30 min; the flow rate of mixed gases was set at 7.0 L/h. One jar was used for each treatment. To eliminate the effects of other environmental factors, all of the experiments were carried out in a controlled-temperature chamber at 24°C in darkness.
Effect of Altered CO2:O2 Concentrations on Germination, Root Growth, and Border Cell Number
Stage I: During Radicle Emergence
Pea seeds were germinated under the test atmospheres (CO2:O2 concentrations [v/v] of 0.03%:21%, 3%:18%, 6%:15%, 9%:12%, and 12%:9%).
Percentage germination and root lengths were measured 2 d later. For germination tests there were three replicate plates each containing 10 seeds. Root lengths for these seedlings were measured individually. Ungerminated seeds were considered as having a root length of “0 mm.” The whole test was carried out twice.
Once the radicle emerged, seedlings with specific root lengths (5, 10, 15, 20, or 25 mm) were selected at 5-h intervals from each treatment. For each specific root length, 12 seedlings were sampled and border cells were harvested and counted individually. The experiment was performed twice.
Stage II: Renewed Border Cell Separation
Pea or alfalfa seeds were germinated and grown under ambient atmosphere in an incubator. Pea seedlings with 25-mm-long roots or alfalfa seedlings with 20-mm-long roots were selected for use in further tests in which they were exposed to CO2:O2 concentrations (v/v) of 0.03%:21%, 3%:18%, and 6%:15%.
Root growth was monitored by measuring root length before transferring (0) and 1, 2, or 3 d after treatment. Fifteen seedlings were measured for root length in each treatment each day. The test was performed twice.
The effect of altered CO2:O2 levels on renewed border cell separation was evaluated by exposing 15 pea seedlings (25 mm) to test atmospheres. Border cells were removed and counted daily before exposing (0) and 1, 2, and 3 d after treatment, and the pea seedlings with washed root tips were placed back into the glass jars under the test atmospheres. There were three replicates (plates) for each treatment and each sample was the average of five seedlings in the same plates. The whole test was performed twice.
Pea and alfalfa seedlings containing a full set of border cells were transferred to indicated test atmospheres. Fifteen seedlings were collected from each treatment everyday at d 0 (before transferring) and 1, 2, or 3 d after treatment and border cell numbers were counted. There were three replicate plates for each treatment and each sample was the average of five seedlings in the same plate. The whole test was performed twice.
Effects of Seed Density on CO2 Concentration and Border Cell Number
Effect of Seed Density on CO2 Concentration
Standard Petri plates containing water agar (1.0% [w/v], Sigma) and either 10 or 30 seeds per plate, were used. A sterilized needle was used to make a hole in the center of the Petri plate lid, then the hole was sealed with clear plastic tape. Seeds were germinated under ambient atmosphere at 24°C in an incubator. CO2 and O2 concentrations within Petri plates were determined after 2 d when root lengths of most of the seedlings had reached about 20 to 25 mm. One-milliliter air samples were taken from each plate with a syringe inserted through the hole on the Petri plate cover. Samples were analyzed with an analytical gas chromatograph. Each treatment included three replicates (plates) and two independent experiments were conducted.
Effect of Seed Density on Border Cell Number
The whole test was carried out as described above under “Effects of Seed Density on CO2 Concentration.” Five seedlings were removed from each plate after air samples were taken and border cells were isolated and counted. Border cell numbers were the average of five seedlings and each treatment included three replicates (plates). The whole test was carried out twice.
Statistical Analysis
The whole experiment was a complete random design and the data from all experiments were subjected to analysis of variance and Duncan's test for multiple range test using Costat Statistical Software (Cohort Software, Berkeley, CA).
RESULTS
High CO2/Low O2 Concentrations Inhibit Root Development and Border Cell Separation during the Period of Radicle Emergence
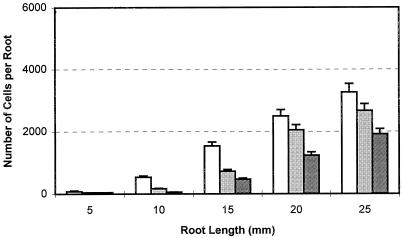
Under ambient atmospheric conditions, border cells can be collected from the root tip when the root is 5 mm in length. Subsequently, border cell number increases with increasing root length and reaches the maximum number when root length is 25 mm, and then remains stable (Hawes and Lin, 1990). Under the tested CO2:O2 concentrations, the number of border cells separated at each root length during this early period of development was significantly decreased (Fig. 1) and the percentage germination and average root length of each treatment also declined (Table I). The higher the CO2 and lower the O2 level, the greater was the reduction in germination, root growth, and border cell numbers. Since the 9% and 12% (v/v) CO2 greatly inhibited the germination and root growth of pea (Table I), subsequent experiments employed CO2 concentrations of 3% and 6%. These levels are in the concentration range known to occur in cultivated field soil (Stolzy, 1974; Buyanovsky and Wagner, 1983).
Figure 1.
Effects of high CO2/low O2 atmospheres on border cell numbers during germination. Pea seeds were germinated under the indicated test atmospheres. In each treatment, 12 seedlings were collected and border cells were harvested and counted individually for each specific root length. The experiment was performed twice. Values are the means of 24 replicates and bars represent se. White bars, 0.03%CO2:21%O2; light gray bars, 3% CO2:18%O2; dark gray bars, 6%CO2:15%O2.
Table I.
Effects of high CO2/low O2 atmospheres on pea seed germination and radicle emergence
| (%CO2:%O2) | 0.03%:21% | 3%:18% | 6%:15% | 9%:12% | 12%:9% |
|---|---|---|---|---|---|
| Germination (%) | 90.6 ± 3.6a | 86.8 ± 3.4a | 83.4 ± 2.2ab | 66.3 ± 4.7b | 53.3 ± 6.1bc |
| Root length (mm) | 21.2 ± 2.4a | 15.6 ± 1.2ab | 12.5 ± 0.9b | 8.2 ± 0.7c | 5.6 ± 1.2cd |
Pea seeds were germinated on water agar plates overlaid with germination paper under the test atmospheres as indicated. The percentage of germination and root length were measured 2 d later. For each treatment there were three replicate plates, each containing 10 seedlings. Root lengths for these seedlings were measured individually. The whole test was carried out twice. Values for germination rates are means of six replicates and values for root length are means of 60 seedlings ± se.
Atmospheres with High CO2/Low O2 Have No Significant Effect on Growth of Established Roots of Pea and Alfalfa Seedlings
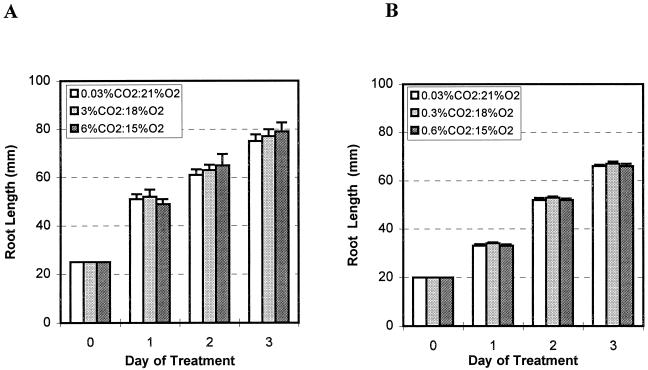
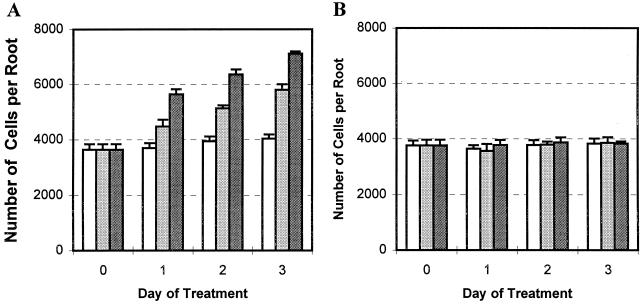
When pea and alfalfa seedlings are grown under ambient atmosphere conditions until roots are 25 and 20 mm long, respectively, the species-specific maximum number of border cells has been reached, and the roots are referred to herein as “established” roots. Seedlings then were transferred into gas jars for exposure to the test atmospheres. During the 3-d treatment, root growth was monitored by measuring length daily (Fig. 2). The differences in root growth under each test atmospheric treatment were not statistically significant.
Figure 2.
Effects of high CO2/low O2 atmospheres on root growth of pea (A) and alfalfa (B) seedlings with established roots. Pea and alfalfa seeds were germinated and grown under ambient atmospheres in an incubator. Seedlings with established roots (25 mm for pea and 20 mm for alfalfa) were transferred to the indicated test atmospheres. Root lengths of 15 seedlings were measured every day for 3 d. The experiment was performed twice. Values are the means of 30 replicates and bars represent se.
Atmospheres with High CO2/Low O2 Have No Effect on the Number of Border Cells Produced by Established Roots during a 24-h Period
When border cells are removed from established roots, renewed border cell separation is initiated immediately, and by 24 h a new set of approximately 4,000 border cells accumulates at the tip of each root (Hawes and Lin, 1990). Experiments were carried out to determine whether high CO2/low O2 atmospheres influence the number of border cells that can be produced by a root tip daily. Established roots with a full set of border cells were washed to remove border cells and then placed under test atmospheres. After 24 h, newly synthesized border cells were harvested and counted, and the process was repeated over 3 consecutive d. Each day, roots produced 4,000 ± 500 new border cells whether the root was maintained at high CO2/low O2 atmospheres or in ambient atmosphere. No significant differences were observed in the daily production of border cells under high CO2/low O2 or ambient atmosphere.
Atmospheres with High CO2/Low O2 Concentrations Override the Endogenous Regulation of Border Cell Production in Pea Roots
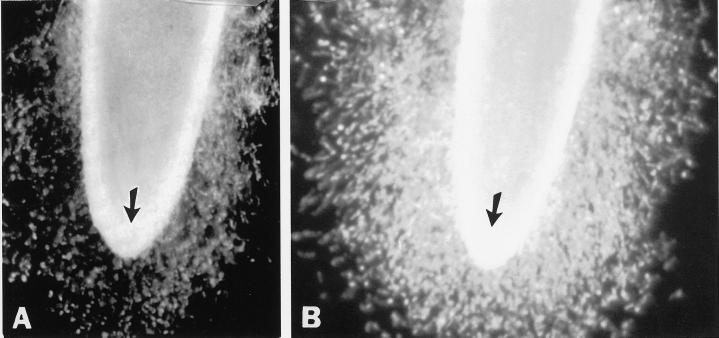
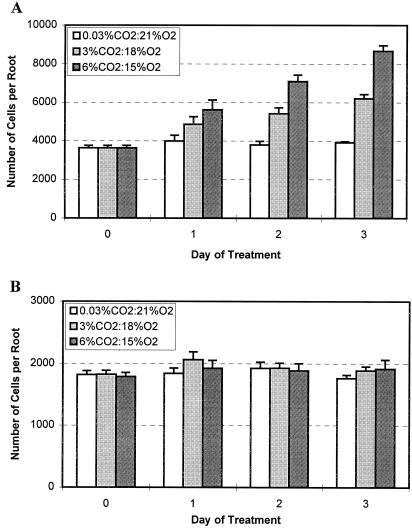
Established pea roots with a full set of border cells, in which border cell number normally remains unchanged as roots grow (Hawes and Lin, 1990), were exposed to the test atmospheres. After 3 d in 6% (v/v) CO2, the appearance of the root cap was indistinguishable from that of roots that had been maintained in ambient atmospheric conditions (Fig. 3, arrows). However, the number of border cells present at the root tip of seedlings maintained in ambient atmosphere (Fig. 3A) appeared to be much smaller than at the root tips of seedlings maintained in high CO2/low O2 (Fig. 3B). The halo of border cells surrounding the root tip was much denser, and its diameter was nearly doubled. To confirm the changes in cell number, the experiment was repeated, with sample seedlings removed daily to count border cell number. During the 3-d experimental period, border cell numbers from roots maintained under ambient atmospheric conditions had not increased significantly, as reported previously (Hawes and Lin, 1990). In contrast, pea seedlings under high CO2/low O2 atmospheres responded by a progressive, dosage-dependent increase in border cell number throughout the test period (Fig. 4A). At the end of the 3 d, border cell numbers in 3% and 6% (v/v) CO2 were 1.5- and 2-fold higher, respectively, than those maintained in ambient conditions.
Figure 3.
Effects of high CO2/low O2 atmospheres on border cell separation from pea. Pea seeds were germinated and grown under ambient atmospheric conditions in a controlled-temperature incubator, and seedlings with established roots (25 mm) with border cells were transferred into ambient (0.03% CO2:21% O2) and high CO2/low O2 (6% CO2:15% O2) atmospheric conditions. Seedlings from both treatments were collected 3 d later, root tips were immersed in 1 mL of distilled water for 1 min, and the separation of border cells was observed under a dissecting microscope.
Figure 4.
Different effects of high CO2/low O2 atmospheres on border cell separation from pea (A) and alfalfa (B). Pea and alfalfa seeds were germinated and grown under ambient atmospheric conditions in a controlled-temperature incubator and seedlings with established roots (25 mm for pea and 20 mm for alfalfa) and a whole set of border cells were transferred into indicated test atmospheres. Seedlings were collected and border cell numbers were counted from each treatment before (0) and 1, 2, and 3 d after the treatment. There were three replicates in each treatment and each sample was the average of five seedlings. The whole test was performed twice. Values are the means of six replicates and bars represent se.
Border Cell Production in Alfalfa Is Not Stimulated by High CO2/Low O2 Atmospheres
Alfalfa seedlings with established roots were transferred into the test atmospheres, and border cell number was monitored over the next 3 d. In contrast to results obtained with pea, border cell number on alfalfa roots did not change significantly over time under any CO2:O2 regime (Fig. 4B).
CO2:O2 Changes Generated by Plant Respiration Stimulate Border Cell Production as Effectively as Laboratory Atmospheres with Altered CO2:O2 Concentrations
The results from the above experiments were consistent with the hypothesis that altered CO2:O2 concentrations can affect border cell production. If correct, then any conditions that result in similarly altered CO2:O2 concentrations would also result in altered border cell production. The possibility that such effects occuring in response to plant respiration affect border cell numbers was tested. The density of seeds germinated in Petri plates, which results in significantly altered CO2:O2 concentrations, was determined. Gas chromatographic analysis was used to demonstrate that in plates containing 30 seeds each, the CO2 concentration (v/v) reached 1.6% and the O2 level decreased to 17%, while at 10 seedlings per plate the CO2 level (v/v) was 0.3% and the O2 level was 19% (Table II). Border cell number on established roots maintained at higher seed density was nearly 1.5-fold higher than on roots under low density (Table II).
Table II.
Relationship of seed density, pea border cell number, and CO2:O2 concentration
| No. of Seeds per Plate | Border Cells/Root | CO2 | O2 |
|---|---|---|---|
| % | |||
| 10 | 4,180 ± 105b | 0.3 ± 0.04b | 19 ± 0.61a |
| 30 | 6,510 ± 164a | 1.6 ± 0.12a | 17 ± 0.42ab |
Pea seeds were germinated on water agar plates with different seed densities. Three days later, 1-mL air samples were collected from each plate and analyzed with a gas chromatograph. Border cell numbers were the average from five seedlings from each plates and there were three replicates (plates) for each treatment. The whole test was carried out twice. Values for gas contents and border cell numbers are mean of six replicates ± se.
Increased CO2 Concentration Is Responsible for Increased Border Cell Number
The experimental results indicated that high CO2/low O2 conditions can affect border cell production in pea, under certain conditions. To distinguish which factor, increased CO2 or decreased O2, caused the observed results, a set of tests was designed using increased CO2 and normal O2 or decreased O2 and normal CO2 levels. The experiment was carried out using pea seedlings with established roots (25 mm) with a full set of border cells, and the experimental process was as described above. Responses to high CO2/normal O2 treatment were indistinguishable from responses to high CO2/low O2 treatments (Fig. 5A). In contrast, no significant changes were found in response to decreased O2 and normal CO2 treatments (Fig. 5B), indicating that increased CO2, rather than reduced O2, was the factor responsible for the increase in border cell number.
Figure 5.
Effects of increased CO2 (A) or decreased O2 (B) on border cell numbers of pea. Pea seeds were germinated and grown under ambient atmosphere in an incubator. Pea seedlings with established roots (25 mm) were exposed to the indicated increased CO2 or decreased O2 treatments. A certain number of seedlings were collected and border cell numbers were counted from each treatment before (0) and 1, 2, and 3 d after treatment. There were three replicates in each treatment and each sample was the average of five seedlings. The whole test was performed twice. Values are the means of six replicates and bars represent se. White bars, 0.03%CO2:21%O2; light gray bars, 3% CO2:21%O2; dark gray bars, 6%CO2:21%O2.
DISCUSSION
The results from this study demonstrate that whole seedlings exposed to atmospheres with high CO2/low O2 can respond with altered production of border cells at their root tips, but that this effect varies according to plant developmental stage and plant species. During the period of radicle emergence, high CO2/low O2 concentrations were correlated with reduced production of border cells as well as reduced seed germination and root growth. During the post-germination phase, however, no significant reduction in root growth or the daily production of border cells occurred in pea or alfalfa seedlings in response to atmospheres with high CO2/low O2. Surprisingly, in fact, the effect of high CO2/low O2 on established pea roots was to increase the total number of border cells that accumulated over time. A change in atmosphere can therefore exert opposite effects on root formation within a narrow window of development.
To our knowledge, this study is the first to demonstrate that a specific environmental signal can create large changes in the process of border cell production. As the connections between cells dissolve during the process of border cell separation, border cells are encased within a high-Mr mucilage surrounding the root tip (Hawes and Brigham, 1992; Hawes et al., 1998). This mucilage remains rather dry in the absence of free water, which has the effect of holding the cells together at the tip. Only when free water is introduced do the cells disperse away into the rhizosphere. In the absence of free water, border cells remain tightly appressed to the root surface. Once the number of accumulated cells within the mucilage has reached its species-dependent maximum, mitosis within the root cap meristem ceases and border cell number stops increasing. Even after many days of growth, this number remains constant (Hawes and Lin, 1990; Brigham et al., 1998). In response to increased CO2 and reduced O2, however, the numbers of border cells do not level off as in ambient atmosphere, but instead increase steadily over time. The response is dosage dependent. The higher the CO2 levels, the more border cells accumulate, such that a given root in 6% CO2:15% O2 (v/v) has more than twice as many cells (8,300 versus 4,000) after 3 d as those grown in ambient conditions. In previous studies, moderate changes in relative humidity, temperature, and water availability did not cause significant changes in border cell number during development (Hawes and Lin, 1990; Hawes and Brigham, 1992).
The mechanism by which controlled atmospheres override the normal regulation of border cell number in pea is not known. Alterations of plant physiology and biochemistry corresponding to either O2 deficiency or CO2 excess have been reported (for review, see Stolzy, 1974; Mistrik et al., 1992; Rouhier et al., 1996). In the current study, low O2 had no effect on border cell number, while high CO2 caused effects identical to those observed with high CO2/low O2 in combination, suggesting that increased border cell number is entirely due to high levels of CO2, not to reduced O2.
One possible mechanism by which CO2 could influence the process is by altered pH. CO2 can cause a slight decrease in pH (Umbreit, 1964), since it can form carbonic acid and dicarbonate once dissolved in water. Extracellular and intracellular pH changes caused by CO2 treatment have been reported. For example, by bubbling air samples containing 5% (v/v) CO2 into Acer pseudoplatanus cell suspensions, the extracellular pH in the suspension decreased from 7.5 to 6.4 and the intracellular pH of plant cells decreased from 7.0 to 6.4 (Bown, 1985); pH decreases were also observed in the cytoplasm and vacuoles of lettuce leaf tissue treated with 15% CO2 (Sirphanich and Kader, 1986). Similar pH changes could influence border cell production. During border cell development, a molecule produced by border cells accumulates to a threshold that inhibits mitosis in the root cap meristem, thereby autoregulating the number of cells produced by a given root (Brigham et al., 1998). A change in the solubility or biological activity of this molecule as a result of a change in extracellular pH could alter its ability to modulate mitotic activity leading to border cell production.
In contrast to pea, alfalfa border cell production by established roots was completely insensitive to changes on atmospheric CO2 levels. The basis for this differential sensitivity is not known but such distinctions could have significant consequences at the rhizosphere. Since its discovery in 1904, research on the rhizosphere has yielded one uncontroverted principle: populations of microorganisms are much higher in the region surrounding roots than in bulk soil, as a result of the nutrient-rich exudates released from plants. On a daily basis, from 15% to more than 50% of plant-fixed carbon can be released in root exudates (for review, see Lynch and Whipps, 1991). Recognition of this principle has spawned countless efforts to exploit it to improve plant health, with mixed results. Successes have been achieved in the areas such as nitrogen fixation (Carroll, 1991), biocontrol of plant diseases (Handelsman and Stabb, 1996), and applications of plant growth promoting rhizobacteria (Loper et al., 1997), yet a large portion of attempted rhizosphere manipulations result in inconsistency or failure (for review, see Cook and Baker, 1983; Weller, 1988; Handelsman and Stabb, 1996). As Rovira (1991) put it, “this frustration spreads across biological control of root diseases, promotion of plant growth, maintenance of high populations of effective rhizobia and is not surprising considering the complexity of the rhizosphere environment.”
A lack of attention to the impact of the plant-regulated production of border cells into the rhizosphere has prevailed for many years (Hawes et al., 1998), and may in part account for continued difficulty in understanding and manipulating the biology of the rhizosphere. The results of this study reveal that changes in soil atmospheric conditions within a physiologically relevant range can result in thousands more or less border cells released from the root tip, depending on developmental stage as well as plant species or genotype. Root border cells can attract fungal zoospores in seconds (Goldberg et al., 1989), synthesize defense structures (Sherwood, 1987), and induce expression of microbial genes required for pathogenesis and symbiosis (Zhu et al., 1997). The delivery of more than 8,000 border cells in response to changes in CO2 levels would be expected to stimulate qualitative and quantitative variations in associated microbial populations compared with a rhizosphere with 4,000 cells. An awareness of these variations is likely to facilitate efforts to manage the ecology of the rhizosphere.
Footnotes
This work was supported by grants from the U.S. Department of Agriculture and the Department of Energy, Division of Energy Biosciences.
LITERATURE CITED
- Abrosimova LN, Revut IB. Biological activity and the composition of soil air in the plow layer. Sov Soil Sci. 1964;7:682–691. [Google Scholar]
- Bown AW. CO2 and intracellular pH. Plant Cell Environ. 1985;8:459–465. [Google Scholar]
- Brigham LA, Woo HH, Hawes MC. Differential expression of proteins and mRNAs from border cells and root tips of pea. Plant Physiol. 1995;109:457–463. doi: 10.1104/pp.109.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham LA, Woo HH, Wen F, Hawes MC. Meristem-specific suppression of mitosis and a global switch in gene expression in the root cap of pea (Pisum sativam) by endogenous signals. Plant Physiol. 1998;118:1223–1231. doi: 10.1104/pp.118.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyanovsky GA, Wagner GH. Annual cycles of carbon dioxide level in soil air. Soil Sci Soc Am J. 1983;47:1139–1145. [Google Scholar]
- Carroll PV. Root-bacteria interactions: symbiotic nitrogen fixation. In: Keister DL, Cregan PB, editors. The Rhizosphere and Plant Growth. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 723–756. [Google Scholar]
- Clowes FAL. Origin of the epidermis in root meristems. New Phytol. 1994;127:335–347. doi: 10.1111/j.1469-8137.1994.tb04284.x. [DOI] [PubMed] [Google Scholar]
- Cook RJ, Baker KF. The Nature and Practice of Biological Control of Plant Pathogens. St. Paul: The American Phytopathological Society; 1983. [Google Scholar]
- Curl EA, Truelove B. The Rhizosphere. Berlin: Springer-Verlag; 1986. [Google Scholar]
- Goldberg NP, Hawes MC, Stanghellini ME. Specific attraction to and infection of isolated cotton root cap cells by zoospores of Pythium dissotocum. Can J Bot. 1989;67:1760–1767. [Google Scholar]
- Griffin DM. Ecology of Soil Fungi. Syracuse, NY: Syracuse University Press; 1972. [Google Scholar]
- Griffin DM, Hale MG, Shay F. Nature and quantity of sloughed organic matter produced by roots of axenic peanut plants. Soil Biol Biochem. 1976;8:29–32. [Google Scholar]
- Grineva GM. Excretion of plant roots during brief periods of anaerobiosis. Sov Plant Physiol. 1962;8:549–552. [Google Scholar]
- Handelsman J, Stabb EV. Biocontrol of soilborne plant pathogens. Plant Cell. 1996;8:1855–1869. doi: 10.1105/tpc.8.10.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes MC. Sloughed root cap cells: a regulator of microbial populations in the rhizosphere? Plant Soil. 1990;129:19–27. [Google Scholar]
- Hawes MC, Brigham LA. Impact of root border cells on microbial populations in the rhizosphere. Adv Plant Pathol. 1992;8:119–148. [Google Scholar]
- Hawes MC, Brigham LA, Wen F, Woo H-H, Zhu Y. Function of root border cells in plant health: pioneers in the rhizosphere. Annu Rev Phytopathol. 1998;36:311–327. doi: 10.1146/annurev.phyto.36.1.311. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Lin HJ. Correlation of pectolytic enzyme activity with the programmed release of cells from root caps of pea (Pisum sativum) Plant Physiol. 1990;94:1855–1859. doi: 10.1104/pp.94.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes MC, Pueppke SG. Isolated peripheral root cap cells: yield from different plants, and callus formation from single cells. Am J Bot. 1986;73:1466–1473. [Google Scholar]
- Kim DH, Misaghi IJ. Siderophore production by fluorescent pseudomonads is sensitive to changes in O2 and CO2 levels. Soil Biol Biochem. 1992;24:821–824. [Google Scholar]
- Kim DH, Misaghi IJ, Pierson EA. Fluorescent pseudomonad populations in modified rhizosphere atmosphere. Soil Biol Biochem. 1996;28:497–501. [Google Scholar]
- Loper JE, Nowak-Thompson B, Whistler CA, Hagen MJ, Corbell NA, Henkels MD, Stockwell VO. Biological control mediated by antifungal metabolite production and resource competition: an overview. In: Ogoshi A, Kobayashi K, Homma Y, Kodama F, Kondo N, Akino S, editors. Plant Growth-Promoting Rhizobacteria: Present Status and Future Prospects. The 4th Plant Growth Promoting Rhizobacteria International Workshop Organizing Committee 1997. Sapporo, Japan: Nakanishi Printing; 1997. pp. 73–79. [Google Scholar]
- Lynch JM, Whipps JM. Substrate role in the rhizosphere. In: Keister DL, Cregan PB, editors. The Rhizosphere and Plant Growth. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 15–24. [Google Scholar]
- Misaghi IJ, Stowell LJ. Portable device for preparation delivery of gas mixture. Appl Environ Microbiol. 1991;57:843–846. doi: 10.1128/aem.57.3.843-846.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistrik I, Holobrada M, Ciamporova M. The root in unfavorable conditions. In: Kolek J, Kozinka V, editors. Physiology of the Plant Root System. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 286–312. [Google Scholar]
- Rittenhouse RL, Hale MG. Loss of organic compounds from roots: II. Effect of O2 and CO2 tension on release of sugars from peanut roots under axenic conditions. Plant Soil. 1971;35:311–321. [Google Scholar]
- Rouhier H, Billès G, Billès L, Bottner P. Carbon fluxes in the rhizosphere of sweet chestnut seedlings (Castanea sativa) grown under two atmospheric CO2 concentrations: 14C partitioning after pulse labeling. Plant Soil. 1996;180:101–111. [Google Scholar]
- Rovira AD. Rhizosphere research: 85 years of progress and frustration. In: Keister DL, Cregan PB, editors. The Rhizosphere and Plant Growth. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 3–13. [Google Scholar]
- Sherwood R. Papilla formation in corn root cap cells and leaves inoculated with Colletotrichum graminicola. Phytopathology. 1987;77:930–934. [Google Scholar]
- Sirphanich J, Kader AA. Changes in cytoplasmic and vacuolar pH in harvested lettuce tissue as influenced by CO2. J Am Soc Hort Sci. 1986;111:73–77. [Google Scholar]
- Stephenson MB, Hawes MC. Correlation of pectinmethylesterase activity in root caps of pea with root border cell separation. Plant Physiol. 1994;111:959–964. doi: 10.1104/pp.106.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzy LH. Soil atmosphere. In: Carson EW, editor. The Plant Root and Its Environment. University Press of Virginia, Charlottesville. 1974. pp. 335–362. [Google Scholar]
- Stolzy LH, Zentmyer GA. Dynamic and measurement of oxygen diffusion and concentration in the root zone and other microsites. In: Bruehl GW, editor. Biology and Control of Soilborne Plant Pathogens. American Phytopathological Society, St. Paul. 1975. pp. 50–54. [Google Scholar]
- Umbreit WW. Carbon dioxide and bicarbonate. In: Umbreit WW, Burris RH, Stauffer JF, editors. Manometric Techniques and Related Methods for the Study of Tissue Metabolism. Manometric Techniques. Minneapolis: Burgess Publishing Co.; 1964. pp. 18–27. [Google Scholar]
- Weller DM. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol. 1988;26:379–407. [Google Scholar]
- Wood RD, Keller CK, Johnstone DL. In situ measurement of microbial activity and controls on microbial CO2 production in the unsaturated zone. Water Resour Res. 1993;29:647–659. [Google Scholar]
- Yamaguchi M, Flocker WJ, Howard FD. Soil atmosphere as influenced by temperature and moisture. Soil Sci Soc Am Proc. 1967;31:164–167. [Google Scholar]
- Zhu Y, Pierson LS, III, Hawes MC. Induction of microbial genes for pathogenesis and symbiosis by chemicals from root border cells. Plant Physiol. 1997;115:1691–1698. doi: 10.1104/pp.115.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]