Abstract
Background:
Cytologic examination of body fluids commonly involves the use of direct or sediment smears, cytocentrifuge preparations, membrane filter preparations, or cell block sections. Cytospin and cell block techniques are extremely useful in improving cell yield of thin serous effusions and urine samples, and ensure high diagnostic efficacy.
Materials and Methods:
We studied cytospin preparations and cell block sections prepared from 180 samples of body fluids and urine samples to compare the relative efficiency of cell retrieval, preservation of cell morphology, ease of application of special stains, and diagnostic efficacy. Samples were collected and processed to prepare cytospin smears and cell block sections.
Results:
We observed that overall, cell yield and preservation of individual cell morphology were better in cytospin preparations as compared to cell blocks, while preservation of architectural pattern was better in cell block sections. The number of suspicious cases also decreased on cell block sections, with increased detection of malignancy. It was difficult to prepare cell blocks from urine samples due to low cellularity.
Conclusions:
Cytospin technology is a quick, efficient, and cost-effective method of increasing cell yield in hypocellular samples, with better preservation of cell morphology. Cell blocks are better prepared from high cellularity fluids; however, tissue architecture is better studied, with improved rate of diagnosis and decrease in ambiguous results. Numerous sections can be prepared from a small amount of material. Special stains and immunochemical stains can be easily applied to cell blocks. It also provides a source of archival material.
Keywords: Body fluids, cell block, cytospin, urine sample
INTRODUCTION
Diagnostic cytology is the scientific art of interpretation of cells from human body that exfoliate or are removed from their physiologic milieu. During the last two decades, cytology laboratories have experienced a dramatic increase in the number and types of specimens received for cytological evaluation.
Accurate cytological interpretation of cellular material is dependent on (1) methods of specimen collection, (2) fixation and fixatives, (3) preservation of fluids specimens prior to processing, (4) preparation of material for microscopic examination and staining, and (5) mounting of the cell sample.
The most commonly used procedures for cytological sample preparation are: (1) direct smear on glass slides, (2) cytocentrifuge preparation, (3) simple centrifugation, and (4) preparation of cell blocks. With the ordinary centrifuge, the problem of insufficient material has been frequently observed and better results can be obtained using cytocentrifuge.[1] The cell block method should be used for processing all residual material remaining after cytological preparations.[2]
The present study has been undertaken to evaluate and compare the relative efficiency of cell retrieval, preservation of cell morphology, and diagnostic accuracy of cytocentrifuge and cell block preparations in processing body fluids and urine specimens.
MATERIALS AND METHODS
The present study analyzed 180 fluid samples, including aspirations from pleural cavity, peritoneal cavity, pelvis and female genital tract as well as urine specimens. These specimens were processed by cytospin and cell block techniques and the slides were studied and compared for cell retrieval, preservation of cellular morphology, and diagnostic accuracy. A detailed patient history and records of relevant investigations were also obtained in each case.
Processing of fluid samples by cytospin method
Fresh samples were collected in anticoagulant vials. For nonhemorrhagic fluids the anticoagulant used was ethylenediaminetetraacetic acid (EDTA), whereas heparin was preferred for hemorrhagic fluids. The samples were loaded into an automated cytospin machine (Shandon cytospin 4, Thermo Electron Corporation, Pittsburgh, USA) following the manufacturer's instructions and centrifuged at 500–700 revolutions per minute (rpm) for 5 min. Slides prepared by cytospin technique were fixed by immersing in 95% ethyl alcohol for 20–30 min.
Processing of fluid samples by cell block method
The cell blocks were prepared by the fixed sediment technique.[2] The fluid samples were centrifuged at 3000 rpm for 10 min using a Remi centrifuge to obtain sediment; the sediment was allowed to stand undisturbed for a time period ranging from 2 h to overnight. The supernatant was decanted and the tube was allowed to drain on a filter paper. The residual sediment or fibrin clot was then dislodged using a spatula and wrapped in filter paper, placed in a cassette, and fixed in 10% formalin. This was followed by paraffin embedding and blocks preparation. Then, 4 to 6 μm thick sections were then cut and mounted on albuminized glass slides.
Following routine laboratory protocol, the cytospin smears were stained with hematoxylin and eosin (H and E) and Papanicolaou (Pap) stains, while the cell block sections were stained with H and E. Periodic acid Schiff stain (PAS) was applied wherever required.
The difference between the two techniques—cytospin and cell block method—was statistically tested for significance by “Z” test for proportion.
RESULTS
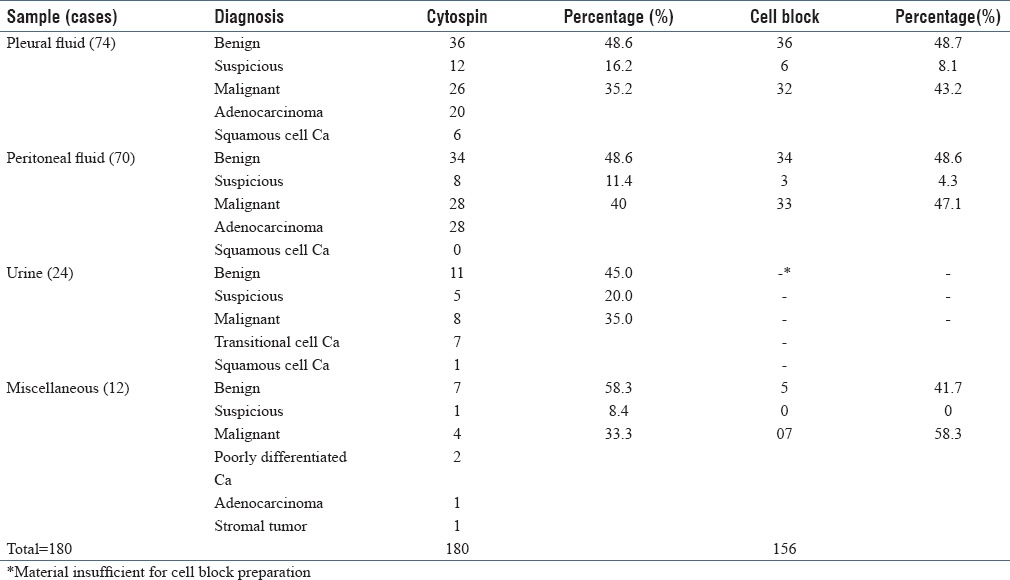
A total of 180 cases were analyzed in the present study. Majority of the patients were females (109 cases) and the age ranged from 15 to 70 years. These included 74 samples of pleural fluid, 70 samples of peritoneal fluid, 24 urine specimens, and 12 samples of miscellaneous fluids. All the 180 samples were processed by cytospin method and cell block technique, and diagnosis was rendered on the slides thus prepared [Table 1].
Table 1.
Summary of diagnosis
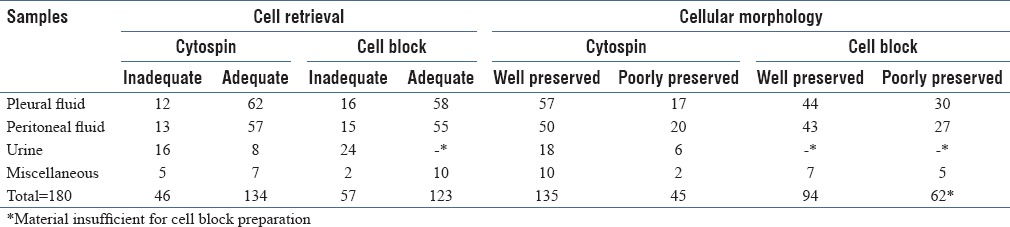
A comparative assessment of results obtained by both cytospin and cell block technique was evaluated [Table 2]. It was observed that among 74 pleural fluid samples, both cellularity and preservation of cell morphology were better in cytospin preparations as compared to cell blocks. However, the number of suspicious cases diagnosed on cytospin smears was seen to reduce from 12 to 6 cases when they were reassessed by cell block sections, as a diagnosis of adenocarcinoma was possible. In contrast to cytospin smears, there was a higher rate of detection of malignancy on cell block sections. Similar observations were made with respect to samples of peritoneal fluid.
Table 2.
Comparative evaluation of results obtained on cytospin and cell block examination
Arrangement of cells in sheets and clusters was the commonest pattern, in pleural fluid, while dispersed cells were most commonly encountered in peritoneal fluids. Glandular arrangement and acinar formation were observed in some cases [Figures 1 and 2]. Further by using PAS stain on cell block sections, active mesothelial cells could be distinguished from mucus producing tumor cells [Figure 2d].
Figure 1.
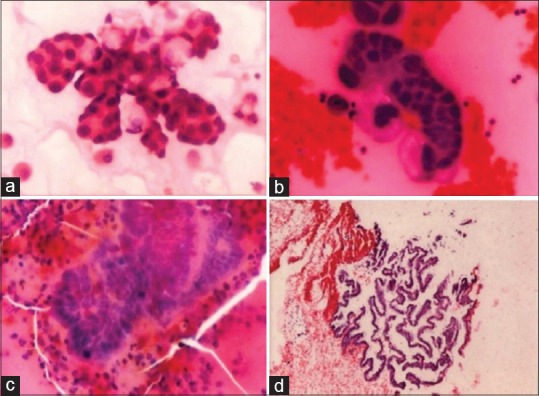
(a and b) Cytospin smears showing metastatic adenocarcinoma with papillary sheet of malignant cells and hemorrhagic background (H and E stain, ×40). (c) Cell block showing papillae of malignant cells in fluid from Pouch of Douglas (H and E stain, ×40). (d) Cell block of peritoneal fluid with metastatic papillary cystadenocarcinoma ovary (H and E stain, ×10)
Figure 2.
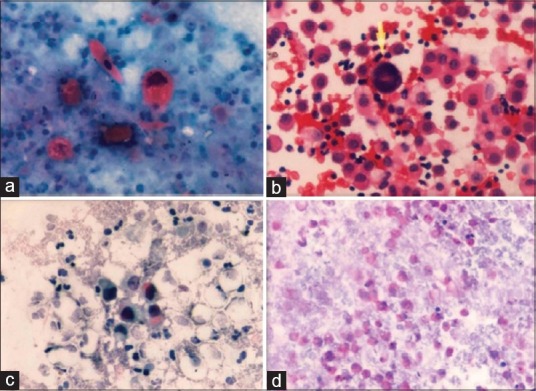
(a)Cytospin smear with malignant keratinized squamous cells in pleural fluid (Pap stain, ×40). (b) Cell block with isolated malignant cells and mesothelial cells in pleural fluid (adenocarcinoma) (H and E stain, ×40). (c) Cytospin smear of urine sample–transitional cell carcinoma (Pap stain, ×40). (d) Cell block of pleural fluid showing PAS stain positivity in tumor cells (PAS stain, x 40)
Out of 12 samples of the miscellaneous category, adequate cellularity was reported in 7 cases on cytospin smears, as compared to 10 cases on cell blocks. Maintenance of tissue architecture and diagnosis of malignancy was, once again, better on cell block sections. However, the nuclear and cytoplasmic details were better preserved by cytospin technique. No major artifacts or distortions were noted. Out of 7 benign cases, 2 cases with cells having atypical changes associated with inflammation and 1 case belonging to suspicious category were placed in the malignant category (adenocarcinoma of ovary) on cell block sections.
Seven cases of transitional cell carcinoma and one of squamous cell carcinoma were detected on cytospin smears of urine samples Figure 2a and c]. An attempt was made to prepare cell blocks from all the urine samples processed by cytospin. However, it was seen that because of inadequate sediment yield after centrifugation due to poor cellularity, the tissue contained in the blocks was grossly insufficient for a histological diagnosis.
The differences between cytospin and cell block method were tested for significance by using “Z” test for proportion. The value of Z-test on comparing cytospin and cell block techniques for cell retrieval and morphology [Table 2] showed that the results obtained by the two techniques were significant only for cell morphology of malignant pleural fluid samples (Z = 2.1), indicating better preservation of cellular morphology by cytospin method. No significant difference was seen between the two techniques with regard to adequacy of cell retrieval.
DISCUSSION
Cytological examination of serous effusions has increasingly gained acceptance in clinical medicine to such an extent that a positive diagnosis is often considered the definitive diagnosis and obviates exploratory surgery. However, cytological diagnosis of effusions has a sensitivity of 40–70%.[3] This can be attributed to low cellular yield and spreading of cells over a large area, which reduces the rate of detection. Moreover, overcrowding, overlapping, cell loss, and cellular changes due to processing are important factors.[4] It is therefore important for the cytologist to strive to attain the best possible results. Cytopreparation of fluids is as important as cytodiagnosis in providing good diagnostic service. In this study, the specimens were processed by both cytospin and cell block methods prior to diagnosis.
On comparing results obtained by both cytospin and cell block technique, it was observed that cytospin technique is better for concentrations of cells from fluid sample especially in hypocellular fluids. Cytospin technique also allows better preservation of cell morphology as compared to cell block method. This may be attributed to the various changes that occur in the tissue at different stages of fixation and tissue processing by the latter method. Additionally, cytospin technique is less time consuming, relatively inexpensive, easy, and involves less technical manpower. The drawback is that all cells including red and white cells and debris are concentrated in a small area, which often tends to obscure any epithelial cells. Aggregation of mesothelial cells into clusters, rosettes, or acinar structures can confound the picture in cytospin smears. Therefore, use of cytospin as the initial method of investigating fluid samples is augmented by cell blocks, which in selected cases may help in resolving diagnostic dilemmas.
Several studies have analyzed the utility of cell block preparations. Tissue architecture is better delineated and special stains can be easily applied. Serial sections can also be obtained for immunostaining. The slides can be better preserved for long durations.[5,6,7,8,9,10,11] The procedure has also been applied to fluid-based (Thin Prep) gynecologic specimens with good results.[12]
However, cell block preparation is time-consuming, and microscopic picture is often dependent on the effect of fixative used. During processing, a lower fixative concentration (7.5% buffered formalin) and shorter period of immersion in xylene are considered to give optimal results.[13] Another study has recommended immediate fixation of aspirates in 4% paraformaldehyde to shorten the processing time.[14] The procedure also requires ample amount of sediment for adequate sections, as observed in this study with regard to urine samples. Therefore, low cellularity samples may not yield adequate cell block sections.
Scrape cell block technique, miniblock technique, cotton block method, and recently, a capsule-based method have been described as an alternative to conventional cell block preparation.[9,11,15,16,17] These modifications help in increasing cell yield and enable shorter processing time.
In conclusion, we observed that both methods have their own merits and demerits. Most importantly, the cell block serves as an adjunct to the cytospin method in enhancing diagnostic yield of fluid samples.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mishra RK, Juna RP, Sharma SP, Kapoor N. A critical evaluation of cytological preparations by different techniques: A semiquantitative and statistical analysis. J Cytol. 2002;19:41–7. [Google Scholar]
- 2.Bales CE. Laboratory techniques. In: Koss LG, Melamed MR, editors. Koss' diagnostic cytology and its histopathologic bases. 5th edition. II. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 1569. [Google Scholar]
- 3.Mezger J, Stotzer O, Schilli G, Bauer S, Wilmanns W. Identification of carcinoma cells in ascitic and pleural fluid. Comparison of four panepithelial antigens with carcinoembryonic antigen. Acta Cytol. 1992;36:75–81. [PubMed] [Google Scholar]
- 4.Sujathan K, Kannan S, Mathew A, Pillai KR, Chandralekha B, Nair MK. Cytodiagnosis of serous effusions: A combined approach to morphological features in Papanicolaou and May-Grunwald Giemsa stained smears and a modified cellblock technique. J Cytol. 2000;17:75–89. [Google Scholar]
- 5.Shivakumarswamy U, Arakeri SU, Karigowdar MH, Yelikar BR. Diagnostic utility of the cell block method versus the conventional smear study in pleural fluid cytology. J Cytol. 2012;29:1–5. doi: 10.4103/0970-9371.93210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thapar M, Mishra RK, Sharma A, Goyal V, Goyal V. Critical analysis of cell block versus smear examination in effusions. J Cytol. 2009;26:60–4. doi: 10.4103/0970-9371.55223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni MB, Desai SB, Ajit D, Chinoy RF. Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagn Cytopathol. 2009;37:86–90. doi: 10.1002/dc.20963. [DOI] [PubMed] [Google Scholar]
- 8.Mayall F, Chang B, Darlington A. A review of 50 consecutive cytology cell block preparations in a large general hospital. J Clin Pathol. 1997;50:985–90. doi: 10.1136/jcp.50.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung SW, Bedard YC. Simple miniblock technique for cytology. Mod Pathol. 1993;6:630–2. [PubMed] [Google Scholar]
- 10.Fetsch PA, Simsir A, Brosky K, Abati A. A comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol. 2002;26:6166. doi: 10.1002/dc.10039. [DOI] [PubMed] [Google Scholar]
- 11.Nga ME, Lim G-L, Barbo N, Chan NH. Successful retrieval of fine-needle aspiration biopsy material from previously stained smears for immunocytochemistry: A novel technique applied to three soft tissue tumors. Mod Pathol. 2005;18:728–32. doi: 10.1038/modpathol.3800356. [DOI] [PubMed] [Google Scholar]
- 12.Rofagha SK, Shecket MV. Diagnostic value, feasibility, and validity of preparing cell blocks from fluid-based gynecologic cytology specimens. Cancer Cytopath. 2002;96:204–9. doi: 10.1002/cncr.10716. [DOI] [PubMed] [Google Scholar]
- 13.Kung ITM, Yuen RWS, Chan JKC. Optimal formalin fixation and processing schedule of cell blocks from fine needle aspirates. Pathology. 1989;21:143–5. doi: 10.3109/00313028909059552. [DOI] [PubMed] [Google Scholar]
- 14.Zito FA, Gadaleta CD, Salvatore C, Filotico R, Labriola A, Marzullo A, et al. A modified cell block technique for fine needle aspiration cytology. Acta Cytol. 1995;39:93–9. [PubMed] [Google Scholar]
- 15.Bhatia P, Dey P, Uppal R, Shifa R, Srinivasan R, Nijhawan R. Cell blocks from scraping of cytology smear: Comparison with conventional cell block. Acta Cytol. 2008;52:329–33. doi: 10.1159/000325516. [DOI] [PubMed] [Google Scholar]
- 16.Musso C, Silva-Santos MC, Pereira FE. Cotton block method: One-step method of cell block preparation after fine needle aspiration. Acta Cytol. 2005;49:22–6. doi: 10.1159/000326090. [DOI] [PubMed] [Google Scholar]
- 17.Wen CH, Tsao SC, Su YC, Wu CC, Chai CY. Utility of the capsule-based technique for cell block preparation-in body fluids and Liqui-PREP™ specimens. Acta Cytol. 2011;55:460–6. doi: 10.1159/000330674. [DOI] [PubMed] [Google Scholar]


