Abstract
Proton pump inhibitors (PPIs) potently inhibit gastric acid secretion and are widely used for treatment of acid-related diseases including gastroesophageal reflux disease and secondary prevention of aspirin/NSAID-induced ulcers. Although clinically important adverse effects of PPIs can occur, just as with other drugs, those are not frequently observed during or after administration. Thus, PPIs are regarded as relatively safe and considered to be clinically beneficial. Recently, PPIs have become frequently administered to patients with functional gastrointestinal diseases or primary prevention of drug-related gastroduodenal damage, even though their beneficial effects for those conditions have not been fully confirmed. PPIs tend to be given for conditions in which the necessity of the drug has not been clarified, thus otherwise rare adverse effects are presented as clinically relevant. Although several PPI-related adverse effects have been reported, their clinical relevance is not yet clear, since the evidence reported in those studies is not at a high enough level, as the majority are based on retrospective observational studies and the reported hazard ratios are low. It is important to administer PPIs only for patients who will gain a substantial clinical benefit and to continue to investigate their adverse effects with high quality prospective studies.
Keywords: Adverse effect, Allergic disease, Gastric acid, Gastroesophageal reflux, Ulcer
Introduction
The stomach is the only organ that secretes acidic fluid as low as pH 2. Such gastric secretion is important not only for sterilization of bacteria contained in ingested foods, but also for digestion and absorption of various nutritional factors, such as protein, iron, calcium, and vitamin B12. However, since secreted acid may damage the gastrointestinal tract, various types of protective mechanisms, including mucosal mucous/bicarbonate secretion and sphincter contraction of the gastroesophageal junction, are present to prevent gastric secretion-induced gastroesophageal damage. When those protective mechanisms are overcome by acid secretion, gastrointestinal mucosa can become damaged and irritated, resulting in unpleasant symptoms or even organic disease. Such pathological conditions are referred to as acid-related diseases and include gastroduodenal ulcers, gastroesophageal reflux disease (GERD), Barrett’s esophagus, and functional dyspepsia.
For treatment of acid-related diseases, various inhibitors of gastric acid secretion and neutralizing agents have been developed. Initially, neutralizing drugs containing aluminum or magnesium, and anti-cholinergic agents were employed. However, the effects of those for acid inhibition are limited and their use frequently has adverse effects, including cardio-vascular events, diarrhea, and constipation. In addition, acid-neutralizing drugs are prone to interact with other drugs in the gastrointestinal tract and change the bioavailability of co-administered medication. Therefore, those originally used for acid-related diseases have been nearly completely replaced by histamine H2 receptor antagonists (H2RAs), which were developed in the late 1970s. An H2RA cancels the acid secretory effects of histamine. Because of the stimulating effects of gastrin and acetylcholine, especially during the post-prandial period, an H2RA most effectively inhibits gastric acid secretion during the nocturnal period.1 Accordingly, H2RAs are not potent enough for treatment of GERD with post-prandial reflux, though they are effective for gastroduodenal ulcers. Thereafter, along with the increase in prevalence of GERD from 1980 to 1990, proton pump inhibitors (PPIs) were developed for more potent acid inhibition during the daytime period and used as first-line treatment for acid-related diseases.2
Employment of PPIs steadily and remarkably increased after starting clinical use for treatment of acid-related diseases, and they are now some of the most frequently prescribed drugs throughout the world,3 with large numbers of affected patients provided ongoing treatment with PPI administration for several years. Along with their popularity, adverse events possibly related to long-term administration of PPIs have been reported, though the level of risk is not high. In this review, we describe the pharmacological characteristics of PPIs and compare them with those of H2RAs. Furthermore, we present a balanced interpretation of reports concerning the advantages and disadvantages of PPI use.
Pharmacological Characteristics of Proton Pump Inhibitors
H2RAs competitively bind to histamine H2 receptors on the basolateral plasma membrane of parietal cells and inhibit binding of histamine to these receptors, resulting in inhibition of gastric acid secretion mainly during the nocturnal period, since histamine-stimulated acid secretion is important at that time.1,4 H2RAs do not effectively inhibit gastrin- or acetylcholine-induced stimulation of gastric acid secretion, which is important in regard to post-prandial acid secretion. The acid suppressing effect of an H2RA quickly appears when its concentration in plasma increases after the first dose.5 However, as with many types of receptor antagonists, H2RAs show gradually weakened acid suppression, a tolerance phenomenon, following repetitive administration of only approximately 2 weeks.1,4 Thus, H2RAs are considered to be short-distance track sprinters and not long-distance marathon runners.
Potassium competitive acid blockers (P-CABs) inhibit acid secretion by binding to the potassium binding site of alpha-subunit of proton pumps with iron bonds.6,7 Although P-CABs show a very quick acid suppressing effect with oral administration, their superior clinical benefits as compared to conventional PPIs are not confirmed, except for Helicobacter pylori eradication therapy and for PPI-resistant GERD.8–11 Various P-CABs have indeed been found to have therapeutic effects similar to those of standard PPIs when used for treatment of uncomplicated GERD.12,13 In fact, revaprazan and vonoprazan, P-CABs available throughout the world, are now used only in several countries including Korea and Japan, different from standard PPIs.
PPIs are the most widely used medication for gastric acid inhibition in the world. All the PPIs available in Japan, including omeprazole, esomeprazole, lansoprazole, and rabeprazole, have a benzimidazole nucleus in their molecules along with various types of branch structures. These drugs covalently bind to SH residues of cysteine molecules in the alpha-subunit of proton pumps on the secretary canalicular membranes of gastric parietal cells and inhibit the acid secretory function of those pumps, resulting in inhibition of gastric acid secretion. Since all currently available PPIs share the same molecular structure, they also have similar pharmacological characteristics. A PPI is unstable in an acidic condition. Therefore, an enteric coating or co-administration with an acid-neutralizing agent such as sodium bicarbonate is necessary to obtain adequate per-oral bioavailability. Following absorption in the small intestine, a significant percentage of first-generation PPIs (omeprazole and lansoprazole) are degraded by hepatic enzymes including CYP2C19. In contrast, second-generation PPIs (esomeprazole and rabeprazole) are more stable and their plasma concentration is not strongly influenced by different CYP2C19 hepatic enzyme activities.14,15 Although their plasma half-life is only 2–3 hours, these drugs remain bind to proton pumps for an extended period and inhibit pump activity, until new pumps are finally synthesized and replace the old ones in parietal cells. According to a previous study, 25% of proton pumps in a parietal cell will be replaced by newly synthesized pumps within 1 day.16,17
PPIs must be activated by highly concentrated hydrogen irons before binding to proton pumps. For that activation, the parietal cells must actively secrete hydrogen irons into the secretory canaliculi when the PPI reaches that network. When gastric acid secretion has been inhibited by a pathological condition or medication, even partially, complete activation of the PPI may be prevented and its acid suppressing effect weakened. Only after acid-induced activation has occurred, PPIs bind to SH residues of proton pump cysteines.17 Since only a part of the proton pump is in an active acid secreting state when a PPI is administered, repeated administrations of the drug are necessary for adequate and complete inhibition of proton pumps. Even during the period of stable acid inhibition following several initial oral doses, acid inhibition during the nocturnal period is weaker with a once daily morning dose, since approximately 25% of proton pumps are replaced by newly synthesized ones within 24 hours and the newly synthesized pumps after the morning PPI administration will begin to secrete acid during the nocturnal period.18 PPIs are almost exclusively metabolized by the liver and not by the kidneys, thus their potency is not influenced by impaired renal function. Furthermore, their acid inhibitory effect does not decrease even after long-term continuous administration, which is different from H2RAs. Therefore, PPIs are effective for long-term acid inhibition, especially during the daytime period, because of their lack of tolerance phenomenon. PPIs are considered to be long-range marathon runners and not short-range track sprinters.
Advantages of Long-term Proton Pump Inhibitor Use
PPIs potently inhibit gastric acid secretion, especially during the daytime period following a daily single morning dose. Acid inhibition provided by per-oral administration gradually increases during the first 3–5 days after the start of administration. PPIs do not show tolerance phenomenon, even after long-term treatment. Since nocturnal acid inhibition is not so strong and intra-gastric pH during the nocturnal period remains at around 2.0 in the majority of administered cases, the pre-breakfast plasma gastrin concentration measured in the early morning does not show a remarkable elevation. These characteristics of PPIs may be considered to be advantageous for long-term control of gastric acid secretion.
Long-term inhibition of gastric acid secretion is necessary for GERD maintenance therapy and prevention of occurrence of gastroduodenal ulcers during administration of aspirin or NSAIDs.19–23 For control of dyspepsia, which affects patients with functional dyspepsia (FD), acid inhibitors are intermittently administered or given on an on-demand basis, but not continuously. Therefore, GERD maintenance therapy and prevention of drug-related ulcer recurrence are 2 important conditions that necessitate long-term PPI administration. Patients with GERD mainly complain of reflux symptoms after meals, since gastroesophageal refluxes frequently occur during the postprandial period. Since the acid suppressing effect of a single morning dose of a PPI remains potent during the daytime period, PPIs are reported to be effective to prevent recurrence of reflux symptoms and esophageal erosions/ulcers.19–21 With their continuous administration, recurrence of GERD during maintenance therapy for 1 year is reported to be less than 15%, while recurrence within 1 year without maintenance therapy is estimated to be greater than 50%.24,25 When the preventive effect of PPIs for GERD recurrence was compared with that of H2RAs, PPIs were found to be much more effective. Their long-term administration may also be effective for prevention of neoplastic transition of Barrett’s esophagus to dysplastic Barrett’s esophagus, such as an adenocarcinoma. It has not been completely established whether PPIs effectively prevent dysplastic changes of Barrett’s esophagus. However, no report has indicated that these drugs aggravate dysplastic changes, though several have suggested that they effectively prevent dysplastic changes.26 Thus, long-term maintenance therapy with a PPI is effective for preventing recurrence of GERD and may also prevent neoplastic progression of Barrett’s esophagus.
Long-term PPI administration is also helpful for preventing recurrence of aspirin-induced gastroduodenal ulcers and is more effective than H2RAs, with a decrease in recurrence to approximately one-tenth of that seen in placebo-treated groups.23,27–29 Similarly, in cases administered with an NSAID, PPIs were reported to decrease the recurrence rate to one-tenth of that of a placebo group over a 6- to 12-month observation period.22 Since many patients with cerebrovascular or cardiovascular diseases are treated with aspirin as an anti-thrombotic drug, prevention of aspirin-induced ulcers is critically important for prevention of NSAID-induced ulcers. Thus, PPIs are first-line drugs used for the prevention of aspirin/NSAID-related ulcer recurrence, and their continuous administration is effective and potent for the prevention of recurrence as well as maintenance therapy of GERD.
Disadvantages of Long-term Proton Pump Inhibitors Use
All clinical drugs have both therapeutic and adverse effects, including PPIs. Since the basic chemical structure of available PPIs is similar, the adverse effects of the drugs are also similar and can be divided into 2 types, those related and unrelated to acid inhibition. The majority of acid inhibition-related adverse effects are observed during long-term treatment with a PPI, while those unrelated to acid inhibition are observed in patients with long-term as well as those with short-term treatment (Table).
Table.
Adverse Events Reported in Patients Treated With Proton Pump Inhibitors
| Adverse events unrelated to acid inhibition | Adverse events related to acid inhibition |
|---|---|
| Allergic reaction to drug chemicals | Pneumonia |
| Collagenous colitis | Gastrointestinal infection |
| Acute interstitial nephritis | Gastric carcinoid tumor |
| Chronic kidney disease | Gastric fundic mucosal hypertrophy |
| Drug interaction | Changes in gut microbiome |
| Dementia | Small intestinal bacterial overgrowth |
| Cerebral ischemic diseases | Iron deficiency |
| Ischemic cardiac diseases | Bone fracture |
| Vitamin B12 deficiency | |
| Hypomagnesemia | |
| Gastric fundic gland polyps | |
| Gastric cancer | |
| Colon cancer | |
| Spontaneous bacterial peritonitis | |
| Hepatic encephalopathy | |
| Drug interaction |
Adverse Events Unrelated to Acid Inhibition
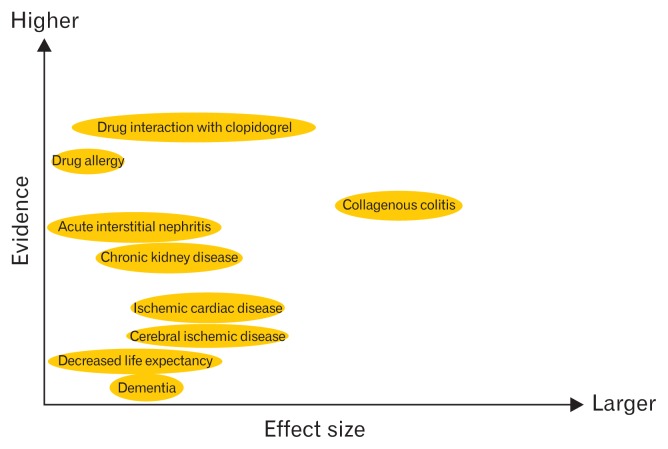
The relative importance of acid inhibition unrelated adverse events is shown in Figure 1.
Figure 1.
Acid-inhibition unrelated adverse events reported during proton pump inhibitor administration. Possible evidence level and effect size of each adverse effect is shown.
Possible Allergic Reaction to Drug Chemicals
Anaphylaxis, pancytopenia, agranulocytosis, thrombocytopenia, hemolytic anemia, acute liver damage, Lyell syndrome, Stevens-Johnson syndrome, interstitial nephritis, and rhabdomyolysis may occur in a small number of cases treated with a PPI, as well many other types of drugs.30–34 Since prediction of occurrence of these possibly life-threatening effects is difficult, it is important to correctly consider indications and administer PPIs only to patients who truly need potent acid suppression. In addition, patients should be instructed to report unexplained skin eruptions, fever, or general malaise appearing after starting a PPI administration to their attending physician without delay. Doctors also should check for possible adverse effects within the first few weeks after starting an administration by scheduling follow-up consultation sessions.
Collagenous Colitis
Diarrhea is frequently experienced by patients being treated with PPIs.35 Some cases of PPI-related diarrhea may be complicated with collagenous colitis, which is characterized by diarrhea and histo-pathological identification of thick collagen bands beneath the colonic epithelium. According to the case-control study, PPI use is associated with an increased risk for collagenous colitis (hazard ratio 4.5).36 Although a variety of drugs, including all available PPIs, are reported to be possible pathogenetic factors of collagenous colitis, the PPI lansoprazole is most frequently reported as responsible.37 Although the pathogenetic mechanism of PPI-related as well as that of non-drug-related collagenous colitis has yet to be clarified, PPI-induced augmentation of collagen gene expression in colonocytes may partially be responsible for it.38 PPIs-related collagenous colitis usually resolves spontaneously after cessation of the drug.39 Therefore, when patients complain of diarrhea after starting a PPI, possible occurrence of collagenous colitis must be considered.
Acute Interstitial Nephritis and Chronic Kidney Disease
It has been reported that interstitial nephritis may occur in patients treated with a PPI, possibly because of an allergic reaction to the drug, though the precise mechanism is not clear.40,41 In biopsy-proven cases, 70% of acute interstitial nephritis was reported to be caused by the drugs and as high as 14% of them is caused by PPIs.42 In addition to an acute renal injury, it is reported by many investigators that long-term PPIs administration is associated with chronic kidney disease when the renal functions were evaluated by the serum creatinine levels or estimated glomerular filtration rate, though the hazard ratio is very modest (1.1–1.5) and the results are based only on observational studies.43–47 Thus, patients must be regularly checked while under prescription for the possible occurrence of renal dysfunction, although it is not clear whether acute interstitial nephritis and chronic kidney disease are causally related or not.48
Drug Interaction During Activation and/or Degradation Phase in Liver
Many drugs including PPIs, diazepam, phenytoin, and warfarin are at least partially degraded by hepatic drug metabolizing enzyme CYP2C19. However, the capacity of that enzyme is not large sufficient, thus PPI administration may decrease the level of degradation of other drugs, augmenting their pharmacological effects. On the other hand, for activation of clopidogrel, CYP2C19 enzyme activity is necessary. Therefore, PPIs administration in patients treated with clopidogrel may decrease its anti-thrombotic activity and increase the risk of cardiovascular events.
Based on the pharmacodynamic studies, PPIs attenuate clopidogrel-induced inhibition of platelet aggregation. In these studies, ADP-induced platelet aggregation inhibition was measured by thromboelastography with and without PPIs administration in cases treated with clopidogrel. The results of the study and a systematic review clearly showed that clopidogrel-induced inhibition of platelet aggregation is weakened by the co-administration of PPIs, although some reports showed that rabeprazole, which is not mainly metabolized by CYP2C19, did not statistically significantly attenuate the anti-thrombotic effect of clopidogrel.49–52
This consistent pharmacodynamic study result, however, was not confirmed in clinical studies. Firstly performed epidemiological study supported this concept and suggested that PPIs interfered with clopidogrel activation and elevated cardiovascular risk.53 However, some retrospective studies thereafter did not support this.54,55 A meta-analysis of retrospective studies suggested the possible risk of increased major cardiac events in association with PPI administration in cases chronically treated with clopidogrel, though not all such studies reached the same conclusion.56,57 The level of evidence presented by those retrospective studies was not adequate to make clinically relevant conclusions. Additionally, 3 prospective randomized controlled studies, including the famous (the Clopidogrel and the Optimization of Gastrointestinal Events Trial) COGENT study, suggested a lack of PPI-induced increase of major cardiac events, even when administered to patients treated with clopidogrel.58–60 Although further studies may be necessary to conclude whether PPIs and clopidogrel have clinically meaningful interactions, the available data in the literature considers to not prevent the use of PPIs in patients treated with clopidogrel, if they need PPI administration.
Dementia
Recently, a group of investigators reported 2 publications and suggested the increased risk of dementia in elderly persons treated with PPIs based on retrospective studies on a German database.61,62 Hazard ratios of these 2 studies were modest and were 1.38 and 1.44 respectively, though they claimed potentially enhanced amyloid beta peptide levels in the brain by direct inhibition of the enzymes beta- and gamma-secretase with PPIs as a possible causative mechanism.63 Three following retrospective studies done in USA and in Europe, on the other hands, did not detect the statistically significant risk of dementia in patients treated with PPIs.64–66 In addition, recently reported prospective population-based study clearly indicated that PPIs use was not associated with the dementia risk, even for people with high cumulative exposure and suggested that PPI administration should not be avoided out of concern about dementia risk.67
Others
Increased risks of cerebral ischemic diseases, ischemic cardiac diseases un-related to clopidogrel administration and even decreased life expectancy were recently reported in cases treated with long-term PPIs administration.68–71 However, studies that presented findings suggesting those as risks were retrospectively performed using a database constructed for other purposes, and no prospective investigation has been done to determine the true risk of these pathological conditions developing from PPI use. The reliability of those retrospective studies is not high. Only a large risk over odds ratio (OR) greater than 2–3 is considered to be clinically relevant. Since the reported OR values of these diseases are 1–2, they may not be clinically relevant. Furthermore, these studies did not fill the Hill criteria for Causation.72,73 Additional studies, possibly even prospective investigations, are needed in the future to establish the true risk of PPI administration in regard to these diseases.
Acid Inhibition Related Adverse Events
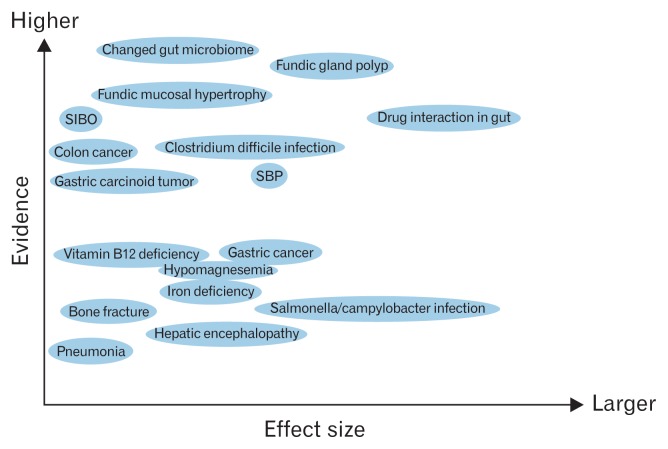
The relative importance of acid inhibition related adverse events is shown in Figure 2.
Figure 2.
Acid-inhibition related adverse events reported during proton pump inhibitor administration. Possible evidence level and effect size of each adverse effect is shown. SIBO, small intestinal bacterial overgrowth; SBP, spontaneous bacterial peritonitis.
Pneumonia
Administration of PPIs decreases the bactericidal effect of gastric juice by increasing intra-gastric and lower esophageal intra-luminal pH. In addition, PPIs may also decrease anti-bacterial immunity by decreasing lysosomal enzyme activity.74 The increased risk of pneumonia in cases treated with PPIs was originally reported based on retrospective studies of a primary care based database.75–77 The majority of PPI-administered cases in the database were patients with GERD. Only during the period less than 30 days after starting administration was the risk of pneumonia shown to be elevated. On the other hand, long-term administration over 30 days was not associated with a risk of pneumonia.78 The mechanism of this observed risk is difficult to understand. In addition, a study of cases administered PPIs for prevention of recurrent NSAIDs related ulcers did not show an increased risk of pneumonia after that administration.79,80 These results bring into question the reliability of the primary care diagnosis of GERD. Cough or laryngo-pharyngeal symptoms caused by a respiratory infection can be misdiagnosed as laryngo-pharyngeal symptoms related to GERD, with a PPI thus administered. The small risk of pneumonia observed only in the short term after starting PPI administration may not actually be a clinically relevant risk for long-term treatment of GERD. Additionally, different from retrospective studies, a meta-analysis of prospective randomized controlled studies did not show increased risk of pneumonia during the administration of various PPIs.81
Increased Gastrointestinal Infection
Bacteria that may cause a gastrointestinal infection can be divided into acid-resistant and acid-labile. Acid-labile bacteria, including Salmonella, Campylobacter, and the vegetative form of Clostridium difficile, may have an increased chance to infect and grow in the gastrointestinal tract when gastric acid secretion is suppressed by a PPI.82,83 As for infection with Salmonella and Campylobacter, many retrospective studies have suggested increased infection rates during administration of PPIs, though some cohort studies have failed to show such increased infection in PPI-treated cases.84,85 Because of diverse study results, it is difficult to conclude whether PPIs increase the risk of Salmonella and/or Campylobacter infection.
C. difficile enteritis is an increasing problem especially in western countries, because of the growing population of strains resistant to antibiotics. Several studies have examined the risk of C. difficile enteritis in cases treated with PPIs, which found that long-term administration may be responsible for an increased risk, whereas short-term treatment may not increase that risk.84,86 The study results investigating whether PPI administration increases risk of recurrent C. difficile enterocolitis and/or severe-complicated diseases are not consistent.87–90 Thus, long-term administration of PPIs should be limited to patients who truly need medication, as that may increase the risk of C. difficile enteritis to an OR of 1.5–2.0.
Gastric Neuroendocrine Tumor
PPI administration increases plasma gastrin concentration by increasing intra-gastric pH. Since gastrin stimulates proliferation of gastric enterochromaffin like (ECL) cells with abundant gastrin receptors, PPIs have been consistently reported to increase the number of ECL cells in the gastric fundic mucosa.91 Patients with high grade chronic atrophic gastritis have an increased concentration of gastrin in plasma and occasionally show development of type 1 gastric neuroendocrine and carcinoid tumors, though hypergastrinemia is considered not to be a sufficient condition for the progression from hyperplasia to dysplasia of ECL cells.92,93 Gastric carcinoid tumors are divided into 3 types, type 1 with hypergastrinemia, type 2 with multiple endocrine adenomatosis, and type 3 without hypergastrinemia.94 Type 1 gastric carcinoid tumors, which are most frequently found in the stomach in association with hypergastrinemia, are benign in their biological characteristics and rarely invade deeper into the gastric wall or metastasize to distant organs, while type 3 gastric carcinoid tumors without hypergastrinemia are malignant.95,96
Based on a study done on experimental animals, long-term PPI-administration was found to develop gastric neuroendocrine tumors with accompanying hypergastrinemia at least in rodents. The result of the animal study temporally halted the clinical development of PPIs in the past.97 After the start of worldwide clinical use of PPIs, only several case reports have noted cases of PPI administration-related gastric neuroendocrine tumors, though the reliability of those reports is not adequate. Some are too limited to be convincing of a pathogenic role of administered PPI and some cases were reported to have developed a carcinoid tumor soon after start of administration.98,99 In addition, the prognosis of patients with type 1 carcinoid tumors has recently been reported to be quite good even without treatment, though endoscopic resection is standard.96 These reports suggest that the risk of gastric carcinoid tumor development during long-term PPI administration is not clinically relevant, though periodic endoscopic screening may be recommended during the period of administration.
Gastric Fundic Mucosal Hypertrophy
Hypergastrinemia increases proliferation of gastric mucosal stem cells located in the neck area of gastric fundic glands in addition to gastric ECL cells.100,101 Therefore, hypergastrinemia caused by PPIs increases gastric fundic mucosal thickness and gastric acid secretion when the administration is stopped.102 Such thickening of gastric fundic mucosa is reported to be especially prominent when these drugs are administered for a long period to patients not infected with H. pylori. Furthermore, similar thickening has been observed in cases of Zollinger-Ellison syndrome. An abrupt stop of PPI administration in patients with Zollinger-Ellison syndrome is accompanied by an abrupt rebound of acid hypersecretion, resulting in acid-induced complications such as higher grade reflux esophagitis.103 Therefore, long-term PPI administration will lead to proliferation of gastric fundic mucosa, resulting in rebound acid hypersecretion once the administration is stopped. This phenomenon may make intermittent PPI use difficult, thus affected patients will likely be treated with the widely used long-term administration for maintenance of GERD.
Changes in Gut Microbiome and Small Intestinal Bacterial Overgrowth
PPIs have been reported to change the gut microbiome and increase the number of Streptococcus organisms, which densely colonize the oral cavity.104–106 In addition, PPI administration increases bacterial density in the duodenum and jejunum. The presence of 100 000 bacterial colonies/mL in small intestinal contents is called small intestinal bacterial overgrowth (SIBO). PPI administration is considered to be a risk factor of SIBO.107 These results suggest that PPIs decrease gastric acid secretion and the bactericidal effect of the gastric juice, with a resulting increase in microbial density in the small intestine and in Streptococcus even in the gut microbiome. In addition, the possible lability of PPIs administered to patients with trans-oral infection of other pathogenetic bacteria has been suggested, though the clinical relevance of that has not been clarified. At present, the clinical importance of a changed microbiome in PPI-treated patients is not clear.
Hypomagnesemia
Magnesium, important for regulation of neuromuscular and various enzyme activities, is absorbed from the small intestine and excreted in urine. Therefore, chronic diarrhea is a risk factor for hypomagnesemia and affected patients may experience convulsions, muscle cramps, seizures, or anorexia. Chronic PPI administration has been reported to cause hypomagnesemia, possibly through decreased absorption of magnesium in the small intestine.108,109 Other reports, on the other hand, did not confirm the association between PPI administration and hypomagnesemia.110 A reported systematic review and meta-analysis of observational studies suggested the statistically significant association between PPI administration and hypomagnesemia, although the hazard ratio is modest (1.43) and the causation relationship is not clear.111 One of the reported possible mechanism is that the PPIs-induced alteration in the gut microenvironment especially intra-luminal pH, may change the activity of gut magnesium transporters including transient receptor potential melastin 6 and 7.108,112 Based on this hypothesis, hypomagnesemia may be classified as an acid inhibition related adverse event. Although the number of reported cases with clinically meaningful hypomagnesemia that developed during chronic PPI administration is not high, periodic measurement of serum magnesium concentrations may be necessary to detect the possible subclinical hypomagnesemia.
Decreased Absorption of Other Nutrients
Some nutrients require gastric acid for effective absorption, including iron, calcium, and vitamin B12. Long-term PPI administration may decrease gastric acid secretion, especially during the daytime postprandial period, thus decreasing levels of iron, calcium, and vitamin B12 absorption, and possibly causing a pathological condition associated with lack of those nutrients.
Iron usually requires acid-induced solubilization from foods before being absorbed. However, the duodenal mucosal iron absorption system has a potent regulatory power for iron absorption and up-regulates that even without gastric acid when storage of iron in the body is decreased. Because of this regulating power of the iron absorption system, iron shortage rarely occurs, even in patients treated long-term with a PPI.113,114 However, in cases with a deficient regulatory mechanism including hereditary hemochromatosis, decreased iron absorption may occur during long-term administration.115,116
For absorption of calcium, gastric acid may have important roles. Decreased absorption of insoluble calcium such as calcium carbonate during potent acid suppression has been reported, though that caused by diet during acid suppression has not been clearly shown.117 Such decreased absorption from the diet, if caused by PPI administration, may cause calcium deficiency, leading to osteoporosis and increased risk of bone fracture, although a study did not support the concept that PPI administration decreases calcium absorption.118 Several observational studies have suggested elevated femoral neck fracture risk in patients treated with PPIs.119–121 Surprisingly, no positive correlation between the length of PPI administration and increased risk of fracture has yet been shown.119 Some prospective observational studies failed to show decreased bone mineral density during long-term administration of PPIs, and other studies have shown that their administration did not increase fracture risk in cases without additional clear risk factors related to osteoporosis and bone fracture.122–125 Thus, the results of many studies suggest that PPI use does not increase the risk of osteoporosis or bone fracture.
Vitamin B12 contained in food binds to dietary protein. For its absorption, vitamin B12 must be released from protein by digestion with acid and pepsin, and then binds with an intrinsic factor produced by parietal cells in the stomach. Only intrinsic factor-bound vitamin B12 can be absorbed from the terminal ileum. PPIs may decrease vitamin B12 absorption by decreasing protein digestion in the stomach. However, the results of several studies that measured changes of serum vitamin B12 during long-term PPI administration were diverse and suggested little clinically relevant adverse effect of the drugs on absorption of vitamin B12.126–129
Gastric Fundic Gland Polyp
Gastric fundic gland polyps are small and benign growths found in the gastric fundic gland area in cases without H. pylori infection, which consist of hyperplastic and often cystic fundic glands. When PPIs are continuously administered to H. pylori-negative patients, multiple fundic gland polyps are reported to develop in fundic gland mucosa.130–132 Though beta-catenin mutations have been reported to be found in some fundic gland polyps found during PPI administration, these polyps show regression and finally disappear when PPI administration is stopped.133,134 Therefore, those polyps formed during chronic PPI administration are considered to be not clinically important, although follow-up endoscopy is still necessary for detection of possible neoplastic changes.
Gastric Cancers
In patients infected with H. pylori, the administration of PPIs has been repeatedly reported to augment mucosal inflammation and accelerate mucosal atrophy, which may increase the risk of gastric cancer, although some diversities in study results are found.91,135,136 Furthermore, some observational studies have suggested that PPIs increase gastric cancer risk in patients infected with H. pylori, whereas others have presented contrasting findings.132,137–139 A recent study reported that PPI administration even after successful eradication of H. pylori augmented the risk of gastric cancer up to a hazard ratio value of 4.29.140 The level of risk reported by different investigators is not homogenous, thus it is difficult to reach a conclusion based on current findings. Additional studies will be necessary to determine whether PPIs increase gastric cancer risk in patients with H. pylori infection, as well as those who have successfully undergone eradication.
Colon Cancers
Some colon cancer cells have been reported to have gastrin receptors, which were found to be linked to cell proliferation.141,142 Therefore, hypergastrinemia caused by PPI administration may stimulate neoplastic colonic cells and increase the risk of colon cancer. Based on an observational study, the long-term use of PPI did not influence the frequency, growth or histology of colonic adenomatous polyps.143 Two large independent observational studies investigated the risk of colon cancer during chronic administration of PPIs, and both found no evidence of increased risk.144,145 Therefore, PPIs are considered not to increase the risk of colon cancer despite their association with elevated plasma gastrin levels.
Spontaneous Bacterial Peritonitis and Hepatic Encephalopathy
Spontaneous bacterial peritonitis is a bacterial infection of the abdominal cavity observed in cases with ascites caused by liver cirrhosis. Because of the increased permeability of the intestinal mucosa in cases with cirrhosis, gut bacteria may penetrate the intestinal wall and proliferate in the ascites fluid without macroscopic intestinal damage. PPIs administration has been repeatedly reported to increase the risk of spontaneous bacterial peritonitis from a hazard ratio of 1.4 to 5.0 by many investigators, although there are some inconsistencies in the study results.146–150
Recently, in addition, PPI administration is also reported to be associated with hepatic encephalopathy in patients with cirrhosis.70,148 The PPI-induced gut microbial changes or PPI-induced hypomagnesemia and vitamin B12 deficiency are considered to be possible links between hepatic encephalopathy and PPI administration, though the precise mechanism is not yet clarified.
The risk of PPI use in cases with cirrhosis, thus, need to be considered, though these study results are just an association and do not show the causal relationship. PPI administration is necessary in some cases with cirrhosis, since PPI decreases the risk of esophageal varix rupture and the occurrence of peptic ulcers.151,152 Balancing the benefit and risk of PPI administration should always be considered when doctors prescribe PPIs to cases with liver cirrhosis.
Drug Interactions in the Gastrointestinal Tract
Absorption of several different drugs is under the strong influence of gastric acid secretion, such as digitalis, which is degraded by gastric acid in the stomach. Therefore, the pharmacological effect of digitalis and others may be augmented during PPI administration. On the other hand, effective absorption of several drugs including itraconazole and atazanavir becomes difficult when PPIs are administered, since their solubility is low at neutral pH. Drugs that are not absorbed and remain functioning in the gastrointestinal tract, such as polycarbophil calcium, may partially lose their potency with PPIs, since acid-induced activation of those drugs is incomplete in an acid neutral environment. Although all drugs that inhibit gastric acid secretion may cause drug interactions in the gastrointestinal tract, the interaction with PPIs may be strong because of their potent acid suppression.
Balancing Advantages and Disadvantages of Proton Pump Inhibitors
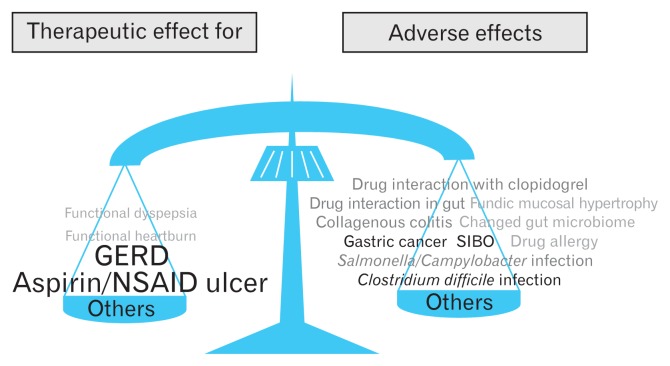
All PPIs currently available show both beneficial and adverse effects. Therefore, of course, the appropriate indications of PPIs therapy should be confirmed as a first step when we decide the potential administration of PPIs.153,154 When deciding when to administer any medicine, it is important to re-confirm that the beneficial effects will outweigh any potential adverse events. The beneficial therapeutic effects of PPIs for the treatment of GERD, gastroduodenal ulcers, and H. pylori eradication are quite clear, while their effects for symptomatic improvement in patients with functional dyspepsia and for patients with esophageal hypersensitivity are limited.155 PPIs are not effective for symptom improvement in functional heartburn cases. Therefore, when administered to patients with functional dyspepsia, esophageal hypersensitivity, or functional heartburn, attention must be given to potential adverse effects, even if the risk is reported to be low. To gain only a small therapeutic benefit, a low risk of adverse effects should be avoided. On the other hand, when the expected therapeutic effect is large enough, a low risk of adverse effects can be accepted. Thus, expected therapeutic effects must always be balanced with possible adverse events (Fig. 3).
Figure 3.
Beneficial effects and possible adverse effects need to be balanced, when proton pump inhibitors (PPIs) are administered as many other drugs. Beneficial effects of PPIs for the long-term treatment of gastroesophageal reflux disease (GERD) and prevention of aspirin/non-steroidal anti-inflammatory drug (NSAID)-induced ulcers are strong enough when PPIs are administered only to appropriate patients. PPIs also have several acid-inhibition related and unrelated adverse effects although the clinical impacts of these adverse events are not so serious. However, balancing beneficial and adverse effects as well as selecting appropriate patients who will get larger benefits by the PPIs administration are critically important. SIBO, small intestinal bacterial overgrowth.
The majority of adverse effects related to PPI administration are reported to occur after long-term administration has been given. For symptom control in patients with low grade reflux esophagitis, intermittent or on-demand administration may be effective enough. Low grade reflux esophagitis, such as Los Angeles grade A or B, has repeatedly been reported not to progress to high grade reflux esophagitis or develop clinically relevant complications, including bleeding and esophageal stricture, even without intensive treatment. Therefore, long-term PPI administration may be necessary for maintenance treatment mainly in patients with high grade reflux esophagitis, Los Angeles grade C or D. Patients with high grade reflux esophagitis are reported to comprise only 5–10% of all cases of reflux esophagitis. For treatment of low grade reflux esophagitis and non-erosive GERD, long-term PPI administration should be avoided, if possible.
Long-term PPIs are also frequently given for prevention of NSAID- or aspirin-related ulcers. High dose NSAID/aspirin administration, elderly age, past history of ulcers, and bleeding ulcers are well known to increase the risk for ulcer recurrence. Therefore, for risky cases, PPI administration for prevention of recurrence is considered to be a reasonable option. However, for patients without such risks, long-term PPI use should be avoided.
Majority of reported adverse effects of PPIs are conflicting and their clinical impacts are not large enough. Since most of the studies concerning the adverse events of PPIs are retrospective and observational, the inclusion risks of potential bias are not negligible. In these conditions, only the study results showing the large clinical impact can be reliable and clinically important. Therefore, the presence of clinically meaningful risk of long-term PPIs administration is considered to be not fully established. However, even under these conditions, specialists in gastroenterology must always be careful to balance the merits and demerits of long-term PPI administration in daily medical practice.
In conclusion, known risks of long-term PPI administration must be considered in clinical practice, though the majority of evidence presented in regard to such risks is not consistent or adequate to make firm conclusions.
Footnotes
Financial support: None.
Conflicts of interest: Outside of this article, Yoshikazu Kinoshita received honoraria from Eisai pharmaceutical Co, EA pharma, Zeria pharmaceutical Co, Astellas pharma, AstraZeneca KK, Takeda pharma, Daiichi-Sankyo pharma, Abbott Japan, Otsuka pharma, Sucampo pharma, Mylan, Sato pharma, Ono pharma, Taisho-Toyama pharma, Sumitomo-Dainippon pharma, and Mochida pharma.
Author contributions: Yoshikazu Kinoshita wrote this article; and Norihisa Ishimura and Shunji Ishihara gave important information and advices for the construction of this article. All of them read the final version of the article and agreed the contents of the article.
References
- 1.Komazawa Y, Adachi K, Mihara T, et al. Tolerance to famotidine and ranitidine treatment after 14 days of administration in healthy subjects without Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:678–682. doi: 10.1046/j.1440-1746.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 2.Iwakiri K, Kinoshita Y, Habu Y, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51:751–767. doi: 10.1007/s00535-016-1227-8. [DOI] [PubMed] [Google Scholar]
- 3.Lanas A. We are using too many PPIs, and we need to stop: a European perspective. Am J Gastroenterol. 2016;111:1085–1086. doi: 10.1038/ajg.2016.166. [DOI] [PubMed] [Google Scholar]
- 4.Fujisawa T, Adachi K, Komazawa Y, et al. Helicobacter pylori infection prevents the occurrence of the tolerance phenomenon of histamine H2 receptor antagonists. Aliment Pharmacol Ther. 2004;20:559–565. doi: 10.1111/j.1365-2036.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- 5.Adachi K, Komazawa Y, Mihara T, et al. Comparative study of the speed of acid-suppressing effects of oral administration of cimetidine and famotidine. J Gastroenterol Hepatol. 2005;20:1012–1015. doi: 10.1111/j.1440-1746.2005.03917.x. [DOI] [PubMed] [Google Scholar]
- 6.Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438) J Pharmacol Exp Ther. 2011;339:412–420. doi: 10.1124/jpet.111.185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott DR, Munson KB, Marcus EA, Lambrecht NW, Sachs G. The binding selectivity of vonoprazan (TAK-438) to the gastric H+, K+-ATPase. Aliment Pharmacol Ther. 2015;42:1315–1326. doi: 10.1111/apt.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurai Y, Nishimura A, Kennedy G, et al. Safety, Tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol. 2015;6:e94. doi: 10.1038/ctg.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects--a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42:719–730. doi: 10.1111/apt.13325. [DOI] [PubMed] [Google Scholar]
- 10.Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43:240–251. doi: 10.1111/apt.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–1446. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahrilas PJ, Dent J, Lauritsen K, et al. A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol. 2007;5:1385–1391. doi: 10.1016/j.cgh.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Dent J, Kahrilas PJ, Hatlebakk J, et al. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol. 2008;103:20–26. doi: 10.1111/j.1572-0241.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 14.Shirai N, Furuta T, Moriyama Y, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929–1937. doi: 10.1046/j.1365-2036.2001.01108.x. [DOI] [PubMed] [Google Scholar]
- 15.Adachi K, Katsube T, Kawamura A, et al. CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther. 2000;14:1259–1266. doi: 10.1046/j.1365-2036.2000.00840.x. [DOI] [PubMed] [Google Scholar]
- 16.Gedda K, Scott D, Besancon M, Lorentzon P, Sachs G. Turnover of the gastric H+, K+-adenosine triphosphatase alpha subunit and its effect on inhibition of rat gastric acid secretion. Gastroenterology. 1995;109:1134–1141. doi: 10.1016/0016-5085(95)90571-5. [DOI] [PubMed] [Google Scholar]
- 17.Huber R, Kohl B, Sachs G, Senn-Bilfinger J, Simon WA, Sturm E. Review article: the continuing development of proton pump inhibitors with particular reference to pantoprazole. Aliment Pharmacol Ther. 1995;9:363–378. doi: 10.1111/j.1365-2036.1995.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 18.Katsube T, Adachi K, Kawamura A, et al. Helicobacter pylori infection influences nocturnal gastric acid breakthrough. Aliment Pharmacol Ther. 2000;14:1049–1056. doi: 10.1046/j.1365-2036.2000.00799.x. [DOI] [PubMed] [Google Scholar]
- 19.Hongo M, Fujimoto K Gastric Polyps Study Group. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol. 2010;45:618–624. doi: 10.1007/s00535-010-0207-7. [DOI] [PubMed] [Google Scholar]
- 20.Labenz J, Armstrong D, Lauritsen K, et al. Esomeprazole 20 mg vs. pantoprazole 20 mg for maintenance therapy of healed erosive oesophagitis: results from the EXPO study. Aliment Pharmacol Ther. 2005;22:803–811. doi: 10.1111/j.1365-2036.2005.02643.x. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita Y, Kato M, Fujishiro M, et al. Efficacy and safety of twice-daily rabeprazole maintenance therapy for patients with reflux esophagitis refractory to standard once-daily proton pump inhibitor: the Japan-based EXTEND study. J Gastroenterol. 2017;28:1–11. doi: 10.1007/s00535-017-1417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugano K, Choi MG, Lin JT, et al. Multinational, double-blind, randomised, placebo-controlled, prospective study of esomeprazole in the prevention of recurrent peptic ulcer in low-dose acetylsalicylic acid users: the LAVENDER study. Gut. 2014;63:1061–1068. doi: 10.1136/gutjnl-2013-304722. [DOI] [PubMed] [Google Scholar]
- 23.Sugano K, Kinoshita Y, Miwa H, Takeuchi T Esomeprazole NSAID Preventive Study Group. Randomised clinical trial: esomeprazole for the prevention of nonsteroidal anti-inflammatory drug-related peptic ulcers in Japanese patients. Aliment Pharmacol Ther. 2012;36:115–125. doi: 10.1111/j.1365-2036.2012.05133.x. [DOI] [PubMed] [Google Scholar]
- 24.Pace F, Annese V, Prada A, et al. Rabeprazole is equivalent to omeprazole in the treatment of erosive gastro-oesophageal reflux disease. A randomised, double-blind, comparative study of rabeprazole and omeprazole 20 mg in acute treatment of reflux oesophagitis, followed by a maintenance open-label, low-dose therapy with rabeprazole. Dig Liver Dis. 2005;37:741–750. doi: 10.1016/j.dld.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229–1237. doi: 10.1136/gutjnl-2013-305997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkey CJ, Karrasch JA, Szczepañski L, et al. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus misoprostol for NSAID-induced ulcer management (OMNIUM) study group. N Engl J Med. 1998;338:727–734. doi: 10.1056/NEJM199803123381105. [DOI] [PubMed] [Google Scholar]
- 28.Yeomans ND, Tulassay Z, Juhász L, et al. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid suppression trial: ranitidine versus omeprazole for NSAID-associated ulcer treatment (ASTRONAUT) study group. N Engl J Med. 1998;338:719–726. doi: 10.1056/NEJM199803123381104. [DOI] [PubMed] [Google Scholar]
- 29.Sugano K, Kontani T, Katsuo S, et al. Lansoprazole for secondary prevention of gastric or duodenal ulcers associated with long-term non-steroidal anti-inflammatory drug (NSAID) therapy: results of a prospective, multicenter, double-blind, randomized, double-dummy, active-controlled trial. J Gastroenterol. 2012;47:540–552. doi: 10.1007/s00535-012-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi SW, Han JM, Bae YJ, et al. Lessons from two cases of anaphylaxis to proton pump inhibitors. J Clin Pharm Ther. 2012;37:614–616. doi: 10.1111/j.1365-2710.2012.01348.x. [DOI] [PubMed] [Google Scholar]
- 31.Dury S, Nardi J, Gozalo C, Lebargy F, Deslee G. Agranulocytosis induced by proton pump inhibitors. J Clin Gastroenterol. 2012;46:859. doi: 10.1097/MCG.0b013e318236f18a. [DOI] [PubMed] [Google Scholar]
- 32.Zaccardi F, Pitocco D, Martini F, et al. A case of esomeprazole-induced transient diabetes and hepatitis: the role of liver inflammation in the pathogenesis of insulin resistance. Acta Diabetol. 2014;51:151–153. doi: 10.1007/s00592-012-0382-5. [DOI] [PubMed] [Google Scholar]
- 33.Lin CY, Wang CW, Hui CR, et al. Delayed-type hypersensitivity reactions induced by proton pump inhibitors: a clinical and in vitro T-cell reactivity study. Allergy. 2017;73:221–229. doi: 10.1111/all.13235. [DOI] [PubMed] [Google Scholar]
- 34.Tröger U, Reiche I, Jepsen MS, Huth C, Bode-Böger SM. Esomeprazole-induced rhabdomyolysis in a patient with heart failure. Intensive Care Med. 2010;36:1278–1279. doi: 10.1007/s00134-010-1854-0. [DOI] [PubMed] [Google Scholar]
- 35.Shimura S, Hamamoto N, Yoshino N, et al. Diarrhea caused by proton pump inhibitor administration: comparisons among lansoprazole, rabeprazole, and omeprazole. Curr Ther Res Clin Exp. 2012;73:112–120. doi: 10.1016/j.curtheres.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keszthelyi D, Jansen SV, Schouten GA, et al. Proton pump inhibitor use is associated with an increased risk for microscopic colitis: a case-control study. Aliment Pharmacol Ther. 2010;32:1124–1128. doi: 10.1111/j.1365-2036.2010.04453.x. [DOI] [PubMed] [Google Scholar]
- 37.Umeno J, Esaki M, Nuki Y, Kim H, Kitazono T, Matsumoto T. Letter: lansoprazole consumption is more common in Japanese patients with collagenous colitis. Aliment Pharmacol Ther. 2013;38:208–209. doi: 10.1111/apt.12356. [DOI] [PubMed] [Google Scholar]
- 38.Mori S, Kadochi Y, Luo Y, et al. Proton pump inhibitor induced collagen expression in colonocytes is associated with collagenous colitis. World J Gastroenterol. 2017;23:1586–1593. doi: 10.3748/wjg.v23.i9.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capurso G, Marignani M, Attilia F, et al. Lansoprazole-induced microscopic colitis: an increasing problem? Results of a prospective case-series and systematic review of the literature. Dig Liver Dis. 2011;43:380–385. doi: 10.1016/j.dld.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Torpey N, Barker T, Ross C. Drug-induced tubulo-interstitial nephritis secondary to proton pump inhibitors: experience from a single UK renal unit. Nephrol Dial Transplant. 2004;19:1441–1446. doi: 10.1093/ndt/gfh137. [DOI] [PubMed] [Google Scholar]
- 41.Berney-Meyer L, Hung N, Slatter T, Schollum JB, Kitching AR, Walker RJ. Omeprazole-induced acute interstitial nephritis: a possible Th1–Th17-mediated injury? Nephrology (Carlton) 2014;19:359–365. doi: 10.1111/nep.12226. [DOI] [PubMed] [Google Scholar]
- 42.Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993–2011: a case series. Am J Kidney Dis. 2014;64:558–566. doi: 10.1053/j.ajkd.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238–246. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27:3153–3163. doi: 10.1681/ASN.2015121377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arora P, Gupta A, Golzy M, Patel N, Carter RL, Jalal K, Lohr JW. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016;17:112. doi: 10.1186/s12882-016-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klatte DCF, Gasparini A, Xu H, et al. Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology. 2017;153:702–710. doi: 10.1053/j.gastro.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 47.Nochaiwong S, Ruengorn C, Awiphan R, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;33:331–342. doi: 10.1093/ndt/gfw470. [DOI] [PubMed] [Google Scholar]
- 48.Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29:611–616. doi: 10.1007/s40620-016-0309-2. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Chen S, Lian J, Zeng X, Luo T. Pharmacodynamic impacts of proton pump inhibitors on the efficacy of clopidogrel in vivo-a systematic review. Clin Cardiol. 2013;36:184–189. doi: 10.1002/clc.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Funck-Brentano C, Szymezak J, Steichen O, et al. Effects of rabeprazole on the antiplatelet effects and pharmacokinetics of clopidogrel in healthy volunteers. Arch Cardiovasc Dis. 2013;106:661–671. doi: 10.1016/j.acvd.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Hokimoto S, Mizobe M, Akasaka T, et al. Impact of CYP2C19 polymorphism and proton pump inhibitors on platelet reactivity to clopidogrel and clinical outcomes following stent implantation. Thrombosis Research. 2014;133:599–605. doi: 10.1016/j.thromres.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhu P, Gao Z, Tang XF, et al. Impact of proton-pump inhibitors on the pharmacodynamic effect and clinical outcomes in patients receiving dual antiplatelet therapy after percutaneous coronary intervention: a propensity score analysis. Chin Med J. 2017;130:2899–2905. doi: 10.4103/0366-6999.220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 54.O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989–997. doi: 10.1016/S0140-6736(09)61525-7. [DOI] [PubMed] [Google Scholar]
- 55.Simon T, Steg PG, Gilard M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French registry of acute ST-elevation and non-ST-elevation myocardial infarction (FAST-MI) registry. Circulation. 2011;123:474–482. doi: 10.1161/CIRCULATIONAHA.110.965640. [DOI] [PubMed] [Google Scholar]
- 56.Serbin MA, Guzauskas GF, Veenstra DL. Clopidogrel-proton pump inhibitor drug-drug interaction and risk of adverse clinical outcomes among PCI-treated ACS patients: a meta-analysis. J Manag Care Spec Pharm. 2016;22:939–947. doi: 10.18553/jmcp.2016.22.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melloni C, Washam JB, Jones WS, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes. 2015;8:47–55. doi: 10.1161/CIRCOUTCOMES.114.001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 59.Gao QP, Sun Y, Sun YX, Wang LF, Fu L. Early use of omeprazole benefits patients with acute myocardial infarction. J Thromb Thrombolysis. 2009;28:282–287. doi: 10.1007/s11239-008-0282-2. [DOI] [PubMed] [Google Scholar]
- 60.Ren YH, Zhao M, Chen YD, et al. Omeprazole affects clopidogrel efficacy but not ischemic events in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Chin Med J. 2011;124:856–861. [PubMed] [Google Scholar]
- 61.Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265:419–428. doi: 10.1007/s00406-014-0554-0. [DOI] [PubMed] [Google Scholar]
- 62.Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410–416. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 63.Badiola N, Alcalde V, Pujol A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One. 2013;8:e58837. doi: 10.1371/journal.pone.0058837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lochhead P, Hagan K, Joshi AD, et al. Association between proton pump inhibitor use and cognitive function in women. Gastroenterology. 2017;153:971–979. e4. doi: 10.1053/j.gastro.2017.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldstein FC, Steenland K, Zhao L, Wharton W, Levey AI, Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J Am Geriatr Soc. 2017;65:1969–1974. doi: 10.1111/jgs.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taipale H, Tolppanen AM, Tiihonen M, Tanskanen A, Tiihonen J, Hartikainen S. No association between proton pump inhibitor use and risk of Alzheimer’s disease. Am J Gastroenterol. 2017;112:1802–1808. doi: 10.1038/ajg.2017.196. [DOI] [PubMed] [Google Scholar]
- 67.Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc. 2017;66:247–253. doi: 10.1111/jgs.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang YF, Chen YT, Luo JC, Chen TJ, Wu JC, Wang SJ. Proton-pump inhibitor use and the risk of first-time ischemic stroke in the general population: a nationwide population-based study. Am J Gastroenterol. 2017;112:1084–1093. doi: 10.1038/ajg.2017.101. [DOI] [PubMed] [Google Scholar]
- 69.Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsai CF, Chen MH, Wang YP, et al. Proton pump inhibitors increase risk for hepatic encephalopathy in patients with cirrhosis in a population study. Gastroenterology. 2017;152:134–141. doi: 10.1053/j.gastro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open. 2017;7:e015735. doi: 10.1136/bmjopen-2016-015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153:35–48. doi: 10.1053/j.gastro.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 73.Bjorkman DJ. Proton pump inhibitors and chronic kidney disease: causation or another false alarm? Gastroenterology. 2017;153:638–640. doi: 10.1053/j.gastro.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 74.Liu W, Baker SS, Trinidad J, et al. Inhibition of lysosomal enzyme activities by proton pump inhibitors. J Gastroenterol. 2013;48:1343–1352. doi: 10.1007/s00535-013-0774-5. [DOI] [PubMed] [Google Scholar]
- 75.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 76.Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med. 2007;167:950–955. doi: 10.1001/archinte.167.9.950. [DOI] [PubMed] [Google Scholar]
- 77.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149:391–398. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 78.Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther. 2010;31:1165–1177. doi: 10.1111/j.1365-2036.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- 79.Estborn L, Joelson S. Occurrence of community-acquired respiratory tract infection in patients receiving esomeprazole: retrospective analysis of adverse events in 31 clinical trials. Drug Saf. 2008;31:627–636. doi: 10.2165/00002018-200831070-00008. [DOI] [PubMed] [Google Scholar]
- 80.Filion KB, Chateau D, Targownik LE, et al. Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: replicated cohort studies with meta-analysis. Gut. 2014;63:552–558. doi: 10.1136/gutjnl-2013-304738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sultan N, Nazareno J, Gregor J. Association between proton pump inhibitors and respiratory infections: a systematic review and meta-analysis of clinical trials. Can J Gastroenterol. 2008;22:761–766. doi: 10.1155/2008/821385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23:3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 83.Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651–668. doi: 10.1046/j.1365-2036.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 84.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 85.Brophy S, Jones KH, Rahman MA, et al. Incidence of Campylobacter and Salmonella infections following first prescription for PPI: a cohort study using routine data. Am J Gastroenterol. 2013;108:1094–1100. doi: 10.1038/ajg.2013.30. [DOI] [PubMed] [Google Scholar]
- 86.Faleck DM, Salmasian H, Furuya EY, Larson EL, Abrams JA, Freedberg DE. Proton Pump Inhibitors Do Not Increase Risk for Clostridium difficile Infection in the Intensive Care Unit. Am J Gastroenterol. 2016;111:1641–1648. doi: 10.1038/ajg.2016.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khanna S, Aronson SL, Kammer PP, Baddour LM, Pardi DS. Gastric acid suppression and outcomes in Clostridium difficile infection: a population-based study. Mayo Clin Proc. 2012;87:636–642. doi: 10.1016/j.mayocp.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shivashankar R, Khanna S, Kammer PP, et al. Clinical factors associated with development of severe-complicated Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1466–1471. doi: 10.1016/j.cgh.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170:772–778. doi: 10.1001/archinternmed.2010.73. [DOI] [PubMed] [Google Scholar]
- 90.Freedberg DE, Salmasian H, Friedman C, Abrams JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. Am J Gastroenterol. 2013;108:1794–1801. doi: 10.1038/ajg.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas PJ. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42:649–663. doi: 10.1111/apt.13324. [DOI] [PubMed] [Google Scholar]
- 92.Campana D, Ravizza D, Ferolla P, et al. Risk factors of type 1 gastric neuroendocrine neoplasia in patients with chronic atrophic gastritis. A retrospective, multicentre study. Endocrine. 2017;56:633–638. doi: 10.1007/s12020-016-1099-y. [DOI] [PubMed] [Google Scholar]
- 93.Lamberts R. Morphological changes of the human gastric mucosa under long-term proton pump inhibitor therapy and their clinical relevance. Microsc Res Tech. 2000;48:357–366. doi: 10.1002/(SICI)1097-0029(20000315)48:6<357::AID-JEMT6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 94.Delle Fave G, Capurso G, Annibale B, Panzuto F. Gastric neuroendocrine tumors. Neuroendocrinology. 2004;80(suppl 1):16–19. doi: 10.1159/000080734. [DOI] [PubMed] [Google Scholar]
- 95.Yu JY, Wang LP, Meng YH, Hu M, Wang JL, Bordi C. Classification of gastric neuroendocrine tumors and its clinicopathologic significance. World J Gastroenterol. 1998;4:158–161. doi: 10.3748/wjg.v4.i2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sato Y, Imamura H, Kaizaki Y, et al. Management and clinical outcomes of type I gastric carcinoid patients: retrospective, multicenter study in Japan. Dig Endosc. 2014;26:377–384. doi: 10.1111/den.12197. [DOI] [PubMed] [Google Scholar]
- 97.Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov. 2003;2:132–139. doi: 10.1038/nrd1010. [DOI] [PubMed] [Google Scholar]
- 98.Dawson R, Manson JM. Omeprazole in oesophageal reflux disease. Lancet. 2000;356:1770–1771. doi: 10.1016/S0140-6736(05)71964-4. [DOI] [PubMed] [Google Scholar]
- 99.Haga Y, Nakatsura T, Shibata Y, et al. Human gastric carcinoid detected during long-term antiulcer therapy of H2 receptor antagonist and proton pump inhibitor. Dig Dis Sci. 1998;43:253–257. doi: 10.1023/A:1018881617038. [DOI] [PubMed] [Google Scholar]
- 100.Chiba T, Fukui H, Kinoshita Y. Reg protein: a possible mediator of gastrin-induced mucosal cell growth. J Gastroenterol. 2000;35(suppl 12):52–56. [PubMed] [Google Scholar]
- 101.Kinoshita Y, Ishihara S, Kadowaki Y, Fukui H, Chiba T. Reg protein is a unique growth factor of gastric mucosal cells. J Gastroenterol. 2004;39:507–513. doi: 10.1007/s00535-004-1354-5. [DOI] [PubMed] [Google Scholar]
- 102.Gillen D, Wirz AA, Ardill JE, McColl KE. Rebound hypersecretion after omeprazole and its relation to on-treatment acid suppression and Helicobacter pylori status. Gastroenterology. 1999;116:239–247. doi: 10.1016/S0016-5085(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 103.Poitras P, Gingras MH, Rehfeld JF. The Zollinger-Ellison syndrome: dangers and consequences of interrupting antisecretory treatment. Clin Gastroenterol Hepatol. 2012;10:199–202. doi: 10.1016/j.cgh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 104.Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mishiro T, Oka K, Kuroki Y, et al. Proton pump inhibitor alters oral microbiome in gastrointestinal tract of healthy volunteers. J Gastroenterol Hepatol. doi: 10.1111/jgh.14040. Published Online First: 4 Nov 2017. [DOI] [Google Scholar]
- 107.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483–490. doi: 10.1016/j.cgh.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 108.William JH, Danziger J. Proton-pump inhibitor-induced hypomagnesemia: current research and proposed mechanisms. World J Nephrol. 2016;5:152–157. doi: 10.5527/wjn.v5.i2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoorn EJ, van der Hoek J, de Man RA, Kuipers EJ, Bolwerk C, Zietse R. A case series of proton pump inhibitor-induced hypomagnesemia. Am J Kidney Dis. 2010;56:112–116. doi: 10.1053/j.ajkd.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 110.Biyik M, Solak Y, Ucar R, et al. Hypomagnesemia among outpatient long-term proton pump inhibitor users. Am J Ther. 2017;24:e52–e55. doi: 10.1097/MJT.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 111.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37:1237–1241. doi: 10.3109/0886022X.2015.1057800. [DOI] [PubMed] [Google Scholar]
- 112.Bai JPF, Hausman E, Lionberger R, Zhang X. Modeling and simulation of the effect of proton pump inhibitors on magnesium homeostasis. 1.Oral absorption of magnesium. Mol Pharm. 2012;9:3495–3505. doi: 10.1021/mp300323q. [DOI] [PubMed] [Google Scholar]
- 113.Koop H, Bachem MG. Serum iron, ferritin, and vitamin B12 during prolonged omeprazole therapy. J Clin Gastroenterol. 1992;14:288–292. doi: 10.1097/00004836-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 114.Stewart CA, Termanini B, Sutliff VE, et al. Iron absorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment Pharmacol Ther. 1998;12:83–98. doi: 10.1046/j.1365-2036.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- 115.Hutchinson C, Geissler CA, Powell JJ, Bomford A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut. 2007;56:1291–1295. doi: 10.1136/gut.2006.108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanclooster A, van Deursen C, Jaspers R, Cassiman D, Koek G. Proton pump inhibitors decrease phlebotomy need in HFE hemochromatosis: double-blind randomized placebo-controlled trial. Gastroenterology. 2017;153:678–680. e2. doi: 10.1053/j.gastro.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 117.Wood RJ, Serfaty-Lacrosniere C. Gastric acidity, atrophic gastritis, and calcium absorption. Nutr Rev. 1992;50:33–40. doi: 10.1111/j.1753-4887.1992.tb02510.x. [DOI] [PubMed] [Google Scholar]
- 118.Hansen KE, Jones AN, Lindstrom MJ, et al. Do proton pump inhibitors decrease calcium absorption? J Bone Miner Res. 2010;25:2786–2795. doi: 10.1002/jbmr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209–1218. doi: 10.1038/ajg.2011.113. [DOI] [PubMed] [Google Scholar]
- 120.Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170:765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896–904. doi: 10.1053/j.gastro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 123.Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos) Am J Gastroenterol. 2012;107:1361–1369. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy. 2008;28:951–959. doi: 10.1592/phco.28.8.951. [DOI] [PubMed] [Google Scholar]
- 125.Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hirschowitz BI, Worthington J, Mohnen J. Vitamin B12 deficiency in hypersecretors during long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2008;27:1110–1121. doi: 10.1111/j.1365-2036.2008.03658.x. [DOI] [PubMed] [Google Scholar]
- 127.Attwood SE, Ell C, Galmiche JP, et al. Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: data from the SOPRAN and LOTUS studies. Aliment Pharmacol Ther. 2015;41:1162–1174. doi: 10.1111/apt.13194. [DOI] [PubMed] [Google Scholar]
- 128.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 129.Hartman B, Donnelly-VanderLoo M, Watson T, O’Connor C, Madill J. Proton-pump inhibitor therapy and vitamin B12 status in an inpatient hospital setting. Appl Physiol Nutr Metab. 2016;41:1071–1076. doi: 10.1139/apnm-2016-0020. [DOI] [PubMed] [Google Scholar]
- 130.Jalving M, Koornstra JJ, Wesseling J, Boezen HM, DE Jong S, Kleibeuker JH. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1341–1348. doi: 10.1111/j.1365-2036.2006.03127.x. [DOI] [PubMed] [Google Scholar]
- 131.Ally MR, Veerappan GR, Maydonovitch CL, et al. Chronic proton pump inhibitor therapy associated with increased development of fundic gland polyps. Dig Dis Sci. 2009;54:2617–2622. doi: 10.1007/s10620-009-0993-z. [DOI] [PubMed] [Google Scholar]
- 132.Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706–1719. e5. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 133.Torbenson M, Lee JH, Cruz-Correa M, et al. Sporadic fundic gland polyposis: a clinical, histological, and molecular analysis. Mod Pathol. 2002;15:718–723. doi: 10.1097/01.MP.0000018976.15044.9B. [DOI] [PubMed] [Google Scholar]
- 134.Choudhry U, Boyce HW, Jr, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol. 1998;110:615–621. doi: 10.1093/ajcp/110.5.615. [DOI] [PubMed] [Google Scholar]
- 135.Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018–1022. doi: 10.1056/NEJM199604183341603. [DOI] [PubMed] [Google Scholar]
- 136.Brunner G, Athmann C, Schneider A. Long-term, open-label trial: safety and efficacy of continuous maintenance treatment with pantoprazole for up to 15 years in severe acid-peptic disease. Aliment Pharmacol Ther. 2012;36:37–47. doi: 10.1111/j.1365-2036.2012.05106.x. [DOI] [PubMed] [Google Scholar]
- 137.Poulsen AH, Christensen S, McLaughlin JK, et al. Proton pump inhibitors and risk of gastric cancer: a population-based cohort study. Br J Cancer. 2009;100:1503–1507. doi: 10.1038/sj.bjc.6605024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brusselaers N, Wahlin K, Engstrand L, Lagergren J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population-based cohort study in Sweden. BMJ Open. 2017;7:e017739. doi: 10.1136/bmjopen-2017-017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wennerström ECM, Simonsen J, Camargo MC, Rabkin CS. Acid-suppressing therapies and subsite-specific risk of stomach cancer. Br J Cancer. 2017;116:1234–1238. doi: 10.1038/bjc.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018;67:28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 141.Colucci R, Blandizzi C, Tanini M, Vassalle C, Breschi MC, Del Tacca M. Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br J Pharmacol. 2005;144:338–348. doi: 10.1038/sj.bjp.0706053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Takhar AS, Eremin O, Watson SA. The role of gastrin in colorectal carcinogenesis. Surgeon. 2004;2:251–257. doi: 10.1016/S1479-666X(04)80093-3. [DOI] [PubMed] [Google Scholar]
- 143.Singh M, Dhindsa G, Friedland S, Triadafilopoulos G. Long-term use of proton pump inhibitors does not affect the frequency, growth, or histologic characteristics of colon adenomas. Aliment Pharmacol Ther. 2007;26:1051–1061. doi: 10.1111/j.1365-2036.2007.03450.x. [DOI] [PubMed] [Google Scholar]
- 144.Yang YX, Hennessy S, Propert K, Hwang WT, Sedarat A, Lewis JD. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology. 2007;133:748–754. doi: 10.1053/j.gastro.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 145.Robertson DJ, Larsson H, Friis S, Pedersen L, Baron JA, Sørensen HT. Proton pump inhibitor use and risk of colorectal cancer: a population-based, case-control study. Gastroenterology. 2007;133:755–760. doi: 10.1053/j.gastro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 146.Yu T, Tang Y, Jiang L, Zheng Y, Xiong W, Lin L. Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: a meta-analysis. Dig Liver Dis. 2016;48:353–359. doi: 10.1016/j.dld.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 147.Khan MA, Kamal S, Khan S, Lee WM, Howden CW. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2015;27:1327–1336. doi: 10.1097/MEG.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 148.Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–1272. doi: 10.1002/hep.28737. [DOI] [PubMed] [Google Scholar]
- 149.Kim JH, Lim KS, Min YW, et al. Proton pump inhibitors do not increase the risk for recurrent spontaneous bacterial peritonitis in patients with cirrhosis. J Gastroenterol Hepatol. 2017;32:1064–1070. doi: 10.1111/jgh.13637. [DOI] [PubMed] [Google Scholar]
- 150.Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. 2015;62:1056–1060. doi: 10.1016/j.jhep.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 151.Okamoto E, Amano Y, Fukuhara H, et al. Does gastroesophageal reflux have an influence on bleeding from esophageal varices? J Gastroenterol. 2008;43:803–808. doi: 10.1007/s00535-008-2232-3. [DOI] [PubMed] [Google Scholar]
- 152.Hidaka H, Nakazawa T, Wang G, et al. Long-term administration of PPI reduces treatment failures after esophageal variceal band ligation: a randomized, controlled trial. J Gastroenterol. 2012;47:118–126. doi: 10.1007/s00535-011-0472-0. [DOI] [PubMed] [Google Scholar]
- 153.Goh KL, Choi MG, Hsu PI, et al. Pharmacological and safety profile of dexlansoprazole: a new proton pump inhibitor-implications for treatment of gastroesophageal reflux disease in the Asia Pacific region. J Neurogastroenterol Motil. 2016;22:355–366. doi: 10.5056/jnm15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xie C, Sifrim D, Li Y, Chen M, Xiao Y. Esophageal baseline impedance reflects mucosal integrity and predicts symptomatic outcome with proton pump inhibitor treatment. J Neurogastroenterol Motil. 2018;24:43–50. doi: 10.5056/jnm17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]