Abstract
Background/Aims
The novel prokinetic drug acotiamide is used for treatment of functional dyspepsia. It is still unclear how acotiamide has effects on esophageal motor function. Esophageal peristalsis and esophagogastric junction (EGJ) compliance has an important role for prevention of esophageal mucosal damage caused by gastroesophageal reflux, however, few studies have analyzed the effects of acotiamide on those former activities and none have investigated its effects on EGJ compliance. The aim of our research was to examine the effects of acotiamide on esophageal motility and EGJ compliance.
Methods
We enrolled 3 gastroesophageal reflux disease (GERD) patients as well as 9 healthy volunteers. Using high-resolution manometry, we examined esophageal motor activity parameters, including esophageal body contractions and lower esophageal sphincter (LES) pressure. While, EGJ compliance was evaluated using a functional lumen imaging probe. Following determination of baseline values for esophageal motor activities and EGJ compliance, acotiamide at a standard dose of 300 mg/day was administered for 3 days. All measurements were performed again 2 hours after the last acotiamide administration.
Results
In the healthy volunteers, as compared with the baseline values, acotiamide administration did not significantly change esophageal body contractions and LES pressure. And EGJ distensibility was not significantly changed (distensibility index in 40-mL distension: 3.5 ± 0.4 vs 3.3 ± 0.5 mm2/mmHg). Similarly in the GERD patients, there were no differences in either esophageal motility or EGJ compliance between before and after acotiamide administration (distensibility index in 40-mL distension: 6.2 ± 0.5 vs 6.5 ± 1.1 mm2/mmHg).
Conclusion
In both healthy individuals and GERD patients, standard dose acotiamide dose does not have significant effects on esophageal motor activities or EGJ compliance.
Keywords: Acotiamide, Esophageal motility, Esophagogastric junction, Gastroesophageal reflux disease, Manometry
Introduction
Patients with gastroesophageal reflux disease (GERD), a gastrointestinal disorder induced by gastric acidic contents reflux, generally suffer from heartburn and acid regurgitation, leading to significant quality of life impairment.1 Presently, the most common initial treatment is administration of a proton pump inhibitor (PPI), because of reported excellent suppression of gastric acid secretion.2 On the other hand, approximately 30% of GERD patients show PPI resistance.3,4
Prokinetic drugs are anticipated to be effective for GERD, because they improve esophageal motor activity and facilitate gastric emptying. In addition, previous reports have suggested that addition of a prokinetic drug to PPI therapy may improve GERD symptoms.5,6 However, the beneficial effects of administration of a prokinetic drug on esophageal motility remain controversial.7
The prokinetic agent acotiamide is a novel gastric motility modulator used for treating functional dyspepsia (FD), and known to enhance both gastric emptying and gastric accommodation in experimental animals as well as patients. Acotiamide inhibits acetylcholine-esterase in peripheral nerve endings and suppresses degradation of acetylcholine, resulting in gastric motility augmentation and enhancement of gastric emptying.8–10 Nevertheless, the effects of acotiamide on esophageal motility have not been fully elucidated. We previously reported that acotiamide at a standard dose had no significant effects on esophageal motor activity or gastroesophageal reflux in healthy adults.11 On the other hand, Yamashita et al12 found that when given to healthy adults, acotiamide reduced transient lower esophageal sphincter relaxation (TLESR) and enhanced esophageal bolus clearance. Presently, it is unclear whether acotiamide affects esophageal motility and no previous study has evaluated the effect of acotiamide on esophagogastric junction (EGJ) compliance.
The concept of EGJ compliance has recently attracted the attention of researchers investigating GERD, as it was reported to have important role for prevention of mucosal damage caused by gastroesophageal reflux, with higher EGJ compliance considered to be related to more gastric contents back to the esophagus.13–15 An endo-luminal functional lumen imaging probe (EndoFLIP; Crospon Ltd, Galway, Ireland) is a newly developed device used to obtain a distensibility at the EGJ. With use of that probe and esophageal manometry, we previously showed that high-dose mosapride (prokinetic drug with activities toward 5-hydroxytryptamine 4-receptors [5-HT4]) augmented esophageal body contractions and lower esophageal sphincter (LES) pressure, and also significantly reduced distensibility at the EGJ.16 In another study, we found that metoclopramide (prokinetic agent that inhibits presynaptic dopamine D2 receptors) augmented peristaltic contractions and LES pressure without reducing EGJ compliance, which were different from the effects of mosapride.17 Accordingly, among drugs currently available, the effects on EGJ compliance by different prokinetic agents may be diverse.
Since the effects of acotiamide on the distensibility at the EGJ have not been evaluated, in this study we utilized the EndoFLIP system and high-resolution manometry to investigate the effects of acotiamide on esophageal motility and EGJ compliance in GERD patients as well as healthy volunteers.
Materials and Methods
Subjects and Study Protocol
Nine healthy individuals (6 males, 3 females; mean age 51.2 years, range 43–63 years) who did not have chest or abdominal symptoms were enrolled. None of the subjects had a history of cardiac, respiratory, neurologic, or gastrointestinal disorders, nor were any taking medications that influence esophageal motility. Additionally, 3 GERD patients (1 male, 2 females; mean age 66.7 years, range 47–78 years) were enrolled in this study. All patients were under treatment with a PPI and the study was done under the administration with a PPI.
Esophageal motor activity function was evaluated using high-resolution manometry (Starlet, Star Medical, Inc. Tokyo, Japan), while EGJ compliance was evaluated with the EndoFLIP system. After baseline measurements of esophageal motor activities and EGJ compliance, acotiamide was administered with 100 mL of water 3 times a day for 3 days, which is the standard dose for adult patients with FD. On the last day of acotiamide administration, the measurements were repeated in the same manner. This administration period was decided based on the drug information from the manufacturer. Blood concentration of acotiamide stabilizes after the 3-day-long per-oral administration.
The study protocol was approved by the ethical committee of Shimane University Faculty of Medicine prior to subject enrollment (IRB No. 1785). Written informed consent was obtained from each subject before enrollment in the study, which was carried out in accordance with the Declaration of Helsinki. This study was registered with the University Hospital Medical Information Network (UMIN) clinical trials registry, number UMIN (000021344).
Determination of Esophageal Motor Activity
Esophageal motor activity was evaluated using the Starlet system, which includes a control unit and catheter equipped with 36 solid-state pressure sensors spaced at 1-cm intervals. Manometry examinations were performed 2 hours after administration of acotiamide, because its Tmax value is reported to be 2.42 ± 0.97 in the drug information usage manual provided by the manufacturer. Based on the manufacturer’s recommendations, the catheter was inserted transnasally after local anesthesia. Measurements were performed in a supine position at 5 minutes after insertion. Resting LES pressure was determined for at least 5 minutes. Next, esophageal body peristaltic contractions were evaluated when the subjects swallowed 5 mL of water and repeated at 1-minute intervals until 10 complete contraction records were obtained. Measurement data obtained with the Starlet system were analyzed using specialized software (Star Medical, Inc), which is able to calculate parameters defined by the Chicago criteria.18 Distal latency was defined as the interval between upper esophageal sphincter relaxation and the contractile deceleration point, which is the inflection point where propagation velocity slows. Contractile front velocity was defined as the slope of the tangent approximating the 30-mmHg isobaric contour between the proximal trough and contractile deceleration point. The distal contractile integral value was calculated by multiplying the length of the smooth muscle esophagus by the duration of propagation of the contractile wave front, with the mean pressure in the entire box determined after excluding measurements below 20 mmHg. Integrated relaxation pressure was measured at the time of the lowest 4-second cumulative pressure value that occurred during the 10-second post-deglutition time window in the electronically generated e-sleeve signal through the anatomic zone defining the EGJ.19
Determination of Esophagogastric Compliance
EGJ compliance was evaluated by use of an EndoFLIP system. This system is equipped with a probe that includes a 240-cm long catheter with a 3-mm outer diameter and a 14-cm long bag at the distal end. The bag has 16 paired impedance electrodes along with a pressure sensor and is distended to a maximal diameter of 25 mm. As the bag is filled with a specially formulated conductive solution, impedance across each segment becomes inversely proportional to the CSA of the bag at that locus, which enables determinations of bag diameter and CSA, along with intra-bag pressure. Data obtained from the sensor probe are sampled with a recording unit and displayed in real time on a monitor. After calibrating the catheter according to the manufacturer’s directions, the probe was inserted in a transnasal manner until the distal bag straddled the EGJ. Esophageal peristaltic contraction waves were often displayed on the monitor, though ignored. The bag was distended and we recorded parameters of EGJ compliance for 30 seconds at distension volumes of 20, 30, and 40 mL. The parameters used for EGJ compliance were smallest CSA values, intra-bag pressures, and distensibility indexes (DI, mm/mmHg) at each distension volume, as previously reported. EGJ DI was defined as the smallest CSA value in relation to the corresponding intra-bag pressure value and calculated using the following equation: smallest CSA/intra-bag pressure + intragastric pressure offset.13,16,17
Statistical Methods
Data are expressed as the mean ± SE. Statistical comparisons between acotiamide and baseline values were performed used a Wilcoxon signed rank test. P < 0.05 was considered to indicate a statistically significant difference. All analyses were performed using SPSS version 19.0 (IBM SPSS Japan Inc, Tokyo, Japan).
Results
All 12 subjects completed the study protocol, and there was no adverse events, with Starlet manometry data for esophageal motor activity successfully recorded for each. Because of technical problems, EndoFLIP data for 1 of the healthy subjects were not recorded, thus data obtained from 8 of those subjects were analyzed with the EndoFLIP system.
Consistent with our previous reports, mean resting LES pressure was not significantly augmented in the healthy subjects after acotiamide administration (19.2 ± 2.5 vs 19.9 ± 2.8 mmHg, P = 0.575). Also, that administration did not change parameters defined by the Chicago criteria used to judge esophageal motor activity (integrated relaxation pressure: 15.4 ± 1.2 vs. 14.2 ± 1.1 mmHg, P = 0.314; distal contractile integral: 3921.8 ± 535.3 vs 4030.0 ± 722.8 mmHg·sec·cm, P = 0.953; contractile front velocity: 5.1 ± 0.9 vs 4.5 ± 0.5 cm/sec, P = 0.374; distal latency: 7.1 ± 0.2 vs 7.5 ± 0.3 seconds, P = 0.374) (Table 1). As for the GERD patients, even though they had slightly weaker esophageal body peristaltic activity and lower esophageal sphincter resting pressure in basal measurements, there was no change in esophageal motility following acotiamide administration (Table 1).
Table 1.
Esophageal Motor Activity
| Healthy subjects (n = 9) | GERD patients (n = 3) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Baseline | After acotiamide | P-value | Baseline | After acotiamide | P-value | |
| Resting LES pressure | 19.2 ± 2.5 | 19.9 ± 2.8 | 0.575 | 14.1 ± 2.3 | 14.5 ± 3.3 | 0.958 |
| 4s-IRP (mmHg) | 15.4 ± 1.2 | 14.2 ± 1.1 | 0.314 | 9.2 ± 2.9 | 11.0 ± 4.0 | 0.285 |
| DCI (mmHg·sec·cm) | 3921.8 ± 535.3 | 4030.0 ± 722.8 | 0.953 | 1219.3 ± 617.3 | 1445.0 ± 848.0 | 0.593 |
| CFV (cm/sec) | 5.1 ± 0.9 | 4.5 ± 0.5 | 0.374 | 3.1 ± 0.1 | 3.6 ± 0.5 | 0.285 |
| DL (sec) | 7.1 ± 0.2 | 7.5 ± 0.3 | 0.374 | 7.1 ± 0.4 | 7.7 ± 0.6 | 0.109 |
GERD, gastroesophageal reflux disease; LES, lower esophageal sphincter; 4s-IRP, 4-second integrated relaxation pressure; DCI, distal contractile integral; CFV, contractile front velocity; DL, distal latency.
Values are expressed as the mean ± SE.
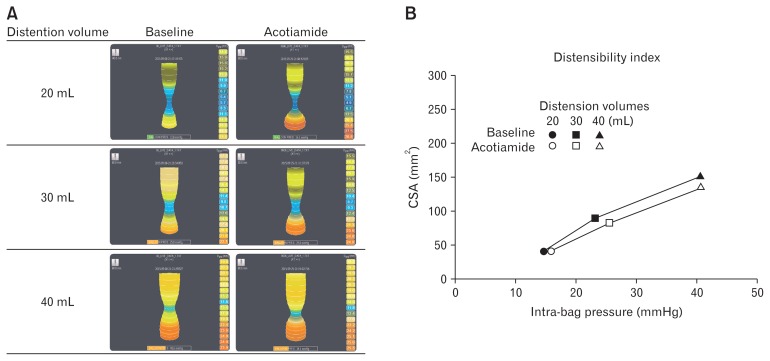
As for our analysis of EGJ compliance investigation, when the bag used with the EndoFILP system was distended straddling the EGJ, it was deformed the shape of an hourglass by contraction at the EGJ during both acotiamide administration and at the baseline (Figure). In healthy subjects, there were no statistically significant differences in regard to CSA (150.3 ± 12.2 vs 133.7 ± 15.5 mm2, distention volume 40 mL, P = 0.237) or DI (3.5 ± 0.4 vs 3.3 ± 0.5 mm2/mmHg, distention volume 40 mL, P = 0.674) values before and after administration of acotiamide (Table 2). In contrast, the EGJ DI value in the GERD patients was higher as compared to that for the normal volunteers (DI in 40-mL distension: 3.5 ± 0.4 vs 6.2 ± 0.5 mm2/mmHg). However, following administration of acotiamide, there was no significant change in EGJ compliance (DI in 40-mL distension: 6.2 ± 0.5 vs 6.5 ± 1.1 mm2/mmHg) (Table 2).
Figure.
Esophagogastric junction (EGJ) compliance with and without acotiamide. (A) An endo-luminal functional lumen imaging probe (EndoFLIP) system was used to monitor EGJ compliance at baseline and following acotiamide administration. Using the special solution provided, the bag included with the system was distended by 20, 30, and 40 mL by which its shape changed to an hourglass form corresponding to distention volume with both acotiamide administration and at the baseline. There were no differences in bag shape following acotiamide administration. (B) Baseline and acotiamide administration values presented as distensibility index graph. To evaluate distensibility index, intra-bag pressure (x-axis) and hiatal cross-sectional area (CSA) (y-axis) values were determined with the EndoFLIP bag distended to 20 mL (circles), 30 mL (squares), and 40 mL (triangles). There were no significant differences between results obtained at baseline and those with acotiamide administration.
Table 2.
Esophagogastric Junction Compliance
| Healthy subjects (n = 8) | GERD patients (n = 3) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Baseline | After acotiamide | P-value | Baseline | After acotiamide | P-value | |
| Distention volume: 20 mL | ||||||
| Hiatal CSA (mm2) | 40.4 ± 9.0 | 41.1 ± 7.1 | 0.866 | 47.2 ± 14.5 | 35.7 ± 9.8 | 0.108 |
| Intra-bag pressure (mmHg) | 14.7 ± 0.8 | 15.7 ± 1.6 | 0.575 | 13.3 ± 1.2 | 12.6 ± 2.7 | 0.583 |
| DI (mm2/mmHg) | 2.2 ± 0.5 | 2.0 ± 0.2 | 0.674 | 2.8 ± 1.1 | 2.5 ± 1.1 | 0.108 |
| Distention volume: 30 mL | ||||||
| Hiatal CSA (mm2) | 89.4 ± 10.4 | 81.5 ± 9.9 | 0.263 | 88.0 ± 5.2 | 111.8 ± 14.4 | 0.108 |
| Intra-bag pressure (mmHg) | 23.4 ± 2.1 | 25.6 ± 2.6 | 0.161 | 18.7 ± 1.3 | 17.7 ± 2.2 | 0.285 |
| DI (mm2/mmHg) | 3.4 ± 0.5 | 2.8 ± 0.4 | 0.093 | 3.9 ± 0.4 | 5.4 ± 1.34 | 0.108 |
| Distention volume: 40 mL | ||||||
| Hiatal CSA (mm2) | 150.3 ± 12.2 | 133.7 ± 155 | 0.237 | 161.7 ± 12.5 | 187.3 ± 19.9 | 0.285 |
| Intra-bag pressure (mmHg) | 40.8 ± 2.0 | 40.8 ± 3.9 | 0.889 | 24.6 ± 0.9 | 24.4 ± 3.2 | 0.972 |
| DI (mm2/mmHg) | 3.5 ± 0.4 | 3.3 ± 0.5 | 0.674 | 6.2 ± 0.5 | 6.5 ± 1.1 | 0.593 |
GERD, gastroesophageal reflux disease; CSA, cross-sectional area; DI, distensibility index.
Values are expressed as the mean ± SE.
Discussion
This is the first clinical study to use the EndoFLIP system to investigate the effects of acotiamide on EGJ compliance in GERD patients as well as healthy subjects. In addition, effects of acotiamide on esophageal motor activities, which have not been previously reported, were examined using high resolution manometry. Our findings showed no effects on esophageal motor activities or EGJ compliance by acotiamide administration in both GERD patients and healthy subjects. We previously reported that acotiamide did not have any effects on esophageal motor activities based on findings obtained with high-resolution manometry.11 However, the present study employed some methods differently as compared to our previous investigation. First, the esophageal manometry examinations were performed at 2 hours after acotiamide administration, when a stronger pharmacological effect can be expected. Also, the manometry device differed from that used in our other study. Finally, we utilized parameters based on the Chicago classification, version 3.0, to evaluate esophageal motor function. Nevertheless, the present results were consistent with those of our previous study despite these differences, and confirmed no effects on esophageal motor function following acotiamide administration.
Esophageal motor function disorder is an important factor related to GERD occurrence. In patients with lower grade GERD, gastroesophageal reflux occurs only during TLESR, with no remarkable esophageal motor abnormality apparent. On the other hand, in patients with higher grade GERD low LES pressure and impaired esophageal peristalsis have been reported, while they are also known to frequently experience both free and stress-induced gastroesophageal refluxes.20–23 To prevent gastroesophageal reflux as well as reflux of clear gastric contents into the esophagus, effective control of esophageal peristalsis and LES pressure is important.24–26
EGJ compliance as an additional factor of GER has recently received interest from researchers. Increased compliance in GERD patients results in greater reflux volume per event, resulting in a more proximal spreading of reflux contents into the esophagus. On the other hand, decreased EGJ compliance reduces the amount of fluid reflux from the stomach, restricts gastric gas regurgitation by belching, and decreases the probability of reflux events becoming symptomatic.14,27–31 Although both basal LES pressure and EGJ compliance are important in regard to the physiological function of GERD, their relationship is still unclear. Pandolfino et al30 reported finding only a weak negative relationship between LES pressure and EGJ compliance, and concluded that LES pressure considers to play a small role in defining the EGJ distensibility. The EGJ is a complex structure composed of the LES, and crural diaphragm, as well as clasp and sling fibers at the angle of His. The influence of individual prokinetic agents on each of those factors is difficult to evaluate with the EndoFLIP system alone, however, it seems that acotiamide does not have significant effects on EGJ compliance as a whole.32
Prokinetic drugs may be beneficial, because they are able to accelerate gastric emptying, increase LES pressure, and improve clearance of reflux from the stomach. However, few findings have been presented regarding their efficacy on esophageal motor function and GERD, thus those effects remain to be established.7,33 Furthermore, only a few studies have investigated the effects of prokinetic drugs on EGJ compliance. We previously found that high-dose (40 mg) mosapride augmented esophageal body peristaltic contractions and basal LES pressure, in addition to reducing EGJ compliance, while other studies have reported that a standard dose had no effects on esophageal motor activity.16,34 Additionally, we previously found that metoclopramide had pharmacological actions similar to mosapride and augmented esophageal motor activities without changing EGJ distensibility.17 These differences may be derived from the pharmacological mechanisms and dosage of the specific administered drugs. Furthermore, other studies have found that the effects of standard dose oral mosapride and metoclopramide on esophageal motor activity are reduced as compared to high dose and intravenous administrations.34,35
The novel prokinetic drug acotiamide developed in Japan, functions as a selective acetylcholine-esterase inhibitor in the peripheral autonomic motor nervous system of the stomach, which consequently elevates the concentration of acetylcholine in the neuromuscular junction along with augmented gastric motility.8–10 It is prescribed as a prokinetic agent, and shows pharmacological effects similar to those of mosapride and metoclopramide. In our previous study, mosapride and metoclopramide were shown to augment resting LES pressure and esophageal body contractions, whereas acotiamide did not augment either of those in the present examinations. In addition, acotiamide did not have an effect on EGJ compliance, which was different from our previous finding for mosapride. These results are important for understanding the individual pharmacological characteristics of mosapride, metoclopramide, and acotiamide. Although each of these drugs are administrated similarly for patients impaired gastrointestinal motility, mosapride mainly stimulates 5-HT4 serotonin receptors, while metoclopramide mainly inhibits dopamine D2 receptors in the peripheral nervous plexus of the autonomic motor nervous system. We consider that the pharmacological differences among these 3 drugs are likely related to their different effects on esophageal motor activity and EGJ compliance.
This study has some limitations. First, the subjects were not randomized and we did not use a placebo control protocol. Also, even though acotiamide was administered to both healthy volunteers and GERD patients, the total number of subjects was small, thus a larger more controlled study is needed to firmly establish the effects of acotiamide on esophageal motility. Furthermore, esophageal motility examinations were performed during the pre-prandial period, because insertion of the EndoFLIP catheter may cause unintended vomiting or even aspiration pneumonia. Examination of the pre-prandial period may be one of the limitations of this study, because the majority of gastroesophageal refluxes occur during the post-prandial period. Finally, no positive effect of acotiamide was found after a 3-day administration. However, with a longer administration, more favorable chronic effects of acotiamide may be observed. Therefore, a study with a longer drug administration period is needed in the future.
In conclusion, administration of acotiamide at 300 mg/day did not augment esophageal motor activity or change EGJ compliance in both healthy individuals and GERD patients. Additional studies are needed to investigate whether the drug has beneficial effects for patients with impaired esophageal motor activity as well as on EGJ compliance.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Yoshikazu Kinoshita: study concept and design; Hironobu Mikami, Mayumi Okada, Eiko Okimoto, and Daisuke Izumi: acquisition, analysis, and interpretation of data; Hironobu Mikami and Norihisa Ishimura: drafting of the manuscript; Norihisa Ishimura, Shunji Ishihara, and Yoshikazu Kinoshita: critical revision of the manuscript for important intellectual content; Hironobu Mikami: statistical analysis; and Yoshikazu Kinoshita: study supervision.
References
- 1.Suzuki H, Matsuzaki J, Masaoka T, Inadomi JM. Greater loss of productivity among Japanese workers with gastro-esophageal reflux disease (GERD) symptoms that persist vs resolve on medical therapy. Neurogastroenterol Motil. 2014;26:764–771. doi: 10.1111/nmo.12319. [DOI] [PubMed] [Google Scholar]
- 2.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 3.Goh KL, Choi MG, Hsu WP, et al. Unmet treatment needs of gastroesophageal reflux disease in Asia: gastroesophageal reflux disease in Asia Pacific Survey. J Gastroenterol Hepatol. 2014;29:1969–1975. doi: 10.1111/jgh.12655. [DOI] [PubMed] [Google Scholar]
- 4.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1340–1354. doi: 10.1136/gutjnl-2011-301897. [DOI] [PubMed] [Google Scholar]
- 5.Cho YK, Choi MG, Park EY, et al. Effect of mosapride combined with esomeprazole improves esophageal peristaltic function in patients with gastroesophageal reflux disease: a study using high resolution manometry. Dig Dis Sci. 2013;58:1035–1041. doi: 10.1007/s10620-012-2430-y. [DOI] [PubMed] [Google Scholar]
- 6.Miwa H, Inoue K, Ashida K, et al. Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non-erosive reflux disease – a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:323–332. doi: 10.1111/j.1365-2036.2010.04517.x. [DOI] [PubMed] [Google Scholar]
- 7.Boeckxstaens G, El-Serag HB, Smout AJ, Kahrilas PJ. Symptomatic reflux disease: the present, the past and the future. Gut. 2014;63:1185–1193. doi: 10.1136/gutjnl-2013-306393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsueda K, Hongo M, Tack J, Saito Y, Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut. 2012;61:821–828. doi: 10.1136/gutjnl-2011-301454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsueda K, Hongo M, Ushijima S, Akiho H. A long-term study of acotiamide in patients with functional dyspepsia: results from an open-label phase III trial in Japan on efficacy, safety and pattern of administration. Digestion. 2011;84:261–268. doi: 10.1159/000332404. [DOI] [PubMed] [Google Scholar]
- 10.Kusunoki H, Haruma K, Manabe N, et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil. 2012;24:540–545. e250–e251. doi: 10.1111/j.1365-2982.2012.01897.x. [DOI] [PubMed] [Google Scholar]
- 11.Ishimura N, Mori M, Mikami H, et al. Effects of acotiamide on esophageal motor function and gastroesophageal reflux in healthy volunteers. BMC Gastroenterol. 2015;15:117. doi: 10.1186/s12876-015-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita H, Kanamori A, Fukuchi T, et al. Novel prokinetic acotiamide reduces transient lower esophageal sphincter relaxation in healthy subjects. Digestion. 2015;92:90–98. doi: 10.1159/000437301. [DOI] [PubMed] [Google Scholar]
- 13.Kwiatek MA, Pandolfino JE, Hirano I, Kahrilas PJ. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP) Gastrointest Endosc. 2010;72:272–278. doi: 10.1016/j.gie.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh SK, Kahrilas PJ, Brasseur JG. Liquid in the gastroesophageal segment promotes reflux, but compliance does not: a mathematical modeling study. Am J Physiol Gastrointest Liver Physiol. 2008;295:G920–G933. doi: 10.1152/ajpgi.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppo T, McMahon BP, Witteman BP, et al. Functional lumen imaging probe to assess geometric changes in the esophagogastric junction following endolumenal fundoplication. J Gastrointest Surg. 2011;15:1112–1120. doi: 10.1007/s11605-011-1562-2. [DOI] [PubMed] [Google Scholar]
- 16.Fukazawa K, Furuta K, Adachi K, et al. Effects of mosapride on esophageal motor activity and esophagogastric junction compliance in healthy volunteers. J Gastroenterol. 2014;49:1307–1313. doi: 10.1007/s00535-013-0876-0. [DOI] [PubMed] [Google Scholar]
- 17.Mikami H, Ishimura N, Fukazawa K, et al. Effects of metoclopramide on esophageal motor activity and esophagogastric junction compliance in healthy volunteers. J Neurogastroenterol Motil. 2016;22:112–117. doi: 10.5056/jnm15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuribayashi S, Iwakiri K, Kawada A, et al. Variant parameter values-as defined by the Chicago Criteria-produced by ManoScan and a new system with Unisensor catheter. Neurogastroenterol Motil. 2015;27:188–194. doi: 10.1111/nmo.12446. [DOI] [PubMed] [Google Scholar]
- 20.Scheurer U, Halter F. Lower esophageal sphincter in reflux esophagitis. Scand J Gastroenterol. 1976;11:629–634. [PubMed] [Google Scholar]
- 21.Kahrilas PJ, Dodds WJ, Hogan WJ, et al. Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology. 1986;91:897–904. doi: 10.1016/0016-5085(86)90692-X. [DOI] [PubMed] [Google Scholar]
- 22.Dodds WJ, Dent J, Hogan WJ, et al. Mechanism of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 307:1547–1552. doi: 10.1056/NEJM198212163072503. 11982. [DOI] [PubMed] [Google Scholar]
- 23.Dodds WJ. The pathogenesis of gastroesophageal reflux disease. AJR Am J Roentgenol. 1988;151:49–56. doi: 10.2214/ajr.151.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Wong WM, Lai KC, Hui WM, et al. Pathophysiology of gastroesophageal reflux diseases in Chinese- role of transient lower esophageal sphincter relaxation and esophageal motor dysfunction. Am J Gastroenterol. 2004;99:2088–2093. doi: 10.1111/j.1572-0241.2004.30417.x. [DOI] [PubMed] [Google Scholar]
- 25.Chitkara DK, Fortunato C, Nurko S. Esophageal motor activity in children with gastro-esophageal reflex disease and esophagitis. J Pediatr Gastroenterol Nutr. 2005;40:70–75. doi: 10.1097/00005176-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Iwakiri K, Kawami N, Sano H, et al. Mechanism of excessive esophageal acid exposure in patients with reflux esophagitis. Dig Dis Sci. 2009;54:1686–1692. doi: 10.1007/s10620-008-0542-1. [DOI] [PubMed] [Google Scholar]
- 27.Kahrilas PJ, Shi G, Manka M, Joehl RJ. Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients with hiatal hernia. Gastroenterology. 2000;118:688–695. doi: 10.1016/S0016-5085(00)70138-7. [DOI] [PubMed] [Google Scholar]
- 28.Pandolfino JE, Curry J, Shi G, Joehl RJ, Brasseur JG, Kahrilas PJ. Restoration of normal distensive characteristics of the esophagogastric junction after fundoplication. Ann Surg. 2005;242:43–48. doi: 10.1097/01.sla.0000167868.44211.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandolfino JE, Shi G, Curry J, Joehl RJ, Brasseur JG, Kahrilas PJ. Esophagogastric junction distensibility: a factor contributing to sphincter incompetence. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1052–G1058. doi: 10.1152/ajpgi.00279.2001. [DOI] [PubMed] [Google Scholar]
- 30.Pandolfino JE, Shi G, Trueworthy B, Kahrilas PJ. Esophagogastric junction opening during relaxation distinguishes nonhernia reflux patients, hernia patients, and normal subjects. Gastroenterology. 2003;125:1018–1024. doi: 10.1016/S0016-5085(03)01210-1. [DOI] [PubMed] [Google Scholar]
- 31.Rohof WO, Bennink RJ, de Jonge H, Boeckxstaens GE. Increased proximal reflux in a hypersensitive esophagus might explain symptoms resistant to proton pump inhibitors in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2014;12:1647–1655. doi: 10.1016/j.cgh.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Tucker E, Sweis R, Anggiansah A, et al. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endoluminal functional lumen imaging probe for the diagnosis of gastroesophageal reflux disease. Neurogastroenterol Motil. 2013;25:904–910. doi: 10.1111/nmo.12218. [DOI] [PubMed] [Google Scholar]
- 33.Maradey-Romero C, Fass R. New and future drug development for gastroesophageal reflux disease. J Neurogastroenterol Motil. 2014;20:6–16. doi: 10.5056/jnm.2014.20.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koshino K, Adachi K, Furuta K, et al. Effects of mosapride on esophageal functions and gastroesophageal reflux. J Gastroenterol Hepatol. 2010;25:1066–1071. doi: 10.1111/j.1440-1746.2010.06280.x. [DOI] [PubMed] [Google Scholar]
- 35.Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray is effective in symptoms of gastroparesis in diabetics compared to conventional oral tablet. Neurogastroenterol Motil. 2014;26:521–528. doi: 10.1111/nmo.12296. [DOI] [PubMed] [Google Scholar]