Abstract
Attrition rates are high during treatment for major depressive disorder (MDD), and patients who drop out are less likely to reach remission. This report evaluates the incidence, timing, and predictors of attrition during second-step medication treatment. Outpatients in the multisite Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study receiving a medication augmentation (n=563) or medication switch (n=723) for non-psychotic MDD after an unsatisfactory outcome with citalopram were evaluated to determine attrition rates and pretreatment sociodemographic or clinical predictors of attrition. Twenty percent of participants receiving a medication augmentation and 27% receiving a medication switch dropped out before 12 wk in the second treatment step. Remission rates were lower for dropouts [7% vs. 43% (medication augmentation); 12% vs. 31% (medication switch)]. For medication augmentation, Black and other non-Caucasian races, Hispanic ethnicity, younger age, family history of drug abuse, concurrent drug abuse, sociodemographic disadvantage, less symptom improvement with initial citalopram treatment, and greater symptom severity when beginning augmentation were associated with attrition. For medication switch, Black and other non-Caucasian races, younger age, more melancholic features, and lower exit doses but more severe side-effects with citalopram treatment were associated with attrition. Minority status, younger age, and greater difficulty with the first treatment step are risk factors for attrition in the second treatment step. Focus on patients with attrition risk factors for medication augmentation or switch strategies may enhance retention and improve outcomes.
Keywords: Adherence, attrition, antidepressants, depression, predictors
Introduction
Leaving treatment prematurely, or treatment attrition, is common in the treatment of major depressive disorder (MDD). Several reports based on clinical trials and naturalistic care indicate that attrition rates range from 20% to 60% (Demyttenaere et al., 2001). This is a very important clinical problem as patients who drop out of treatment are less likely to reach remission (Mel. et al., 1998) (i.e. the virtual absence of symptoms) and remission is associated with improved functioning and prognosis (Hirschfeld et al., 2002; Rush et al., 2006b). The World Health Organization has emphasized that interventions that improve adherence to treatment could have a greater impact on reducing the burden of chronic illnesses, including depression, than improvements in medical treatments (WHO, 2003). The identification of sociodemographic and/or clinical characteristics that predict attrition could aid in treatment retention by enabling clinicians to direct special retention efforts toward patients at risk for attrition.
A few small (n=66–224) studies have identified pretreatment predictors of attrition in clinical trials of initial treatment for MDD. These predictors included minority status (Arnow et al., 2007); younger age (Arnow et al., 2007; Demyttenaere et al., 1998); male gender (Demyttenaere et al., 1998); fewer years of education, poorer social adjustment, unemployment (Last et al., 1985); lower income (Arnow et al., 2007; Last et al., 1985); increased anxiety, and increased severity of depressive symptoms (Arnow et al., 2007; Tedlow et al., 1996). Recently, we reported on attrition during first step (initial) treatment with citalopram in a large ‘real world’ generalizable sample (Warden et al., 2007) (n=4041) as part of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. The overall attrition rate was 26%. Greater attrition was associated with being Black, Hispanic, younger, less educated, having public insurance, having more concurrent Axis I psychiatric comorbidities, and having higher perceived mental health functioning. Recurrent depression was associated with less attrition.
The above studies all report on attrition in first-step treatment of MDD. However, two-thirds of MDD patients do not achieve remission with a first-step treatment (Trivedi et al., 2006b). Therefore, it is useful to identify predictors of attrition for those who need a second treatment step. To our knowledge, however, no studies have attempted to identify predictors of attrition with second-step medication treatments in depressed patients.
This report used STAR*D data to evaluate whether any demographic or clinical characteristics might predict attrition in second-step treatments following initial treatment with citalopram, which either did not result in remission or could not be tolerated. In STAR*D, most participants received either a medication switch or a medication augmentation at the second step. For each of these two medication strategies, the following questions were addressed:
How common was attrition?
When did attrition occur?
What were the remission rates in the attrition and non-attrition groups?
What sociodemographic, clinical, or first-step treatment characteristics individually and independently distinguished the attrition group from the non-attrition group?
Methods
Study overview
The rationale and design of STAR*D are detailed elsewhere (Fava et al., 2003; Rush et al., 2004). In brief, STAR*D aimed to prospectively identify the next best treatment steps for adult outpatients with non-psychotic MDD after an unsatisfactory benefit to the initial, and if necessary, subsequent treatment steps.
Participants who did not reach remission with or who were intolerant to initial treatment with citalopram could elect to be randomized to a second step switch and/or augmentation treatment (level 2). Second-step treatments included four switch options (venlafaxine XR, sertraline, bupropion-SR, and cognitive therapy) and three augmentation options (augmenting citalopram with bupropion-SR, buspirone, or cognitive therapy). Participants could choose to exclude randomization to either the switch or augmentation strategy, to cognitive therapy in either strategy, or choose randomization to only the two cognitive therapy options; they could not, however, exclude medication choices within the switch or randomization arms. This report focuses on 723 participants who entered a medication switch and 563 participants who entered a medication augmentation arm as their second-step treatment.
The Institutional Review Boards at the STAR*D National Coordinating Center (University of Texas Southwestern Medical Center), Data Coordinating Center (University of Pittsburgh), Regional Centers, Clinical Sites, and the Data Safety and Monitoring Board at the National Institute of Mental Health approved and monitored the protocol. All participants gave written informed consent at enrolment into the first treatment step and each of any subsequent treatment steps, including the second treatment step reported here.
Participants
STAR*D enrolled outpatients aged 18–75 yr who met DSM-IV criteria for non-psychotic MDD and had a score of ≥14 (moderate severity) on the 17-item Hamilton Rating Scale for Depression (HAMD17; Hamilton, 1960, 1967). Participants were enrolled from 18 primary and 23 psychiatric care ‘real world’ settings across the USA that serve the public and private sectors. Advertising for volunteers was proscribed.
To maximize the generalizability of findings, broad inclusion and minimal exclusion criteria were used. Patients with most general medical and psychiatric comorbidities were included unless protocol medications were contraindicated. Suicidal participants or those with substance abuse could be included if their clinicians determined outpatient treatment to be appropriate. The study excluded patients with a lifetime history of psychosis or bipolar disorder, current anorexia nervosa or bulimia, a primary diagnosis of obsessive–compulsive disorder, or clear non-response or intolerance (in the current major depressive episode) to medications used in the first two treatment steps. Patients who were breastfeeding, pregnant, or in tending to conceive in the 9 months subsequent to study entry were excluded, as were those taking antipsychotics, anticonvulsants, mood stabilizers, central nervous system stimulants, or antidepressant medications.
Assessments
At entry into the first step of the STAR*D trial, clinical research coordinators (CRCs) obtained sociodemographic information and self-reported psychiatric history. The presence of current general medical conditions was determined using the Cumulative Illness Rating Scale (CIRS) (Linn et al., 1968; Miller et al., 1992), which provides a count of the number of organ systems affected and the severity of each condition. Current Axis I psychiatric comorbidities were identified using 90% specificity (Rush et al., 2005) based on the self-report Psychiatric Diagnostic Screening Questionnaire (PDSQ; Zimmerman and Mattia, 2001a,b). Depressive symptom severity was evaluated at entry into level 1 and each subsequent level using the HAMD17, the 16-item Quick Inventory of Depressive Symptomatology – Clinician-rated (QIDS-C16), and the QIDS Self-Report (QIDS-SR16; Rush et al., 2000, 2003, 2006a; Trivedi et al., 2004).
Within 72 h of the baseline visit and upon exit from each step, a research outcomes assessor, masked to treatment, obtained the HAMD17 (primary outcome measure) and the 30-item Inventory of Depressive Symptomatology – Clinician-rated (IDS-C30; Rush et al., 1996, 2000; Trivedi et al., 2004) via telephone interviews (in English or Spanish) to measure depressive symptom severity. Anxious (Fava et al., 2004), atypical (Novick et al., 2005), and melancholic (Khan et al., 2006) features were defined based on HAMD17 and IDS-C30 items. During each step, the QIDS-SR16 and the Frequency, Intensity and Burden of Side Effects Rating (FIBSER; Wisniewski et al., 2006) were obtained at each clinic visit.
Depressive symptom severity and quality-of-life measures were collected using a telephone-based interactive voice response (IVR) system (Kobak et al., 1999; Mundt, 1997; Rush et al., 2006a) within 72 h of entry into each step. These measures included the QIDS-SR16, the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q; Endicott et al., 1993) to measure satisfaction and enjoyment in daily functioning, the 12-item Short Form Health Survey (SF-12; Ware et al., 1996) to measure perceived mental and physical functioning, and the Work and Social Adjustment Scale (WSAS; Mundt et al., 2002) to measure functional impairment.
Treatment
The goal of STAR*D treatment was remission, defined as an HAMD17 score ≤7 or a QIDS-SR16 score ≤5. Response was defined as a reduction of ≥50% from the baseline QIDS-SR16 score.
In the second step of treatment, as in all STAR*D steps, the protocol recommended treatment visits at treatment initiation (baseline) and at weeks 2, 4, 6, 9, and 12. A week 14 visit could be held based on clinical judgement if a participant experienced response, but not remission, as of week 12.
Manual-based guidance with dosing flexibility based on clinical judgement, in conjunction with measurement-based care (Trivedi et al., 2006b, 2007), helped to ensure a fully adequate but tolerable dose and an adequate treatment duration to maximize the likelihood of improvement and safety, while minimizing side-effects. This guidance included critical decision points based on measurement of symptoms (QIDS-C16) and side-effects (FIBSER) at each clinic visit. A web-based monitoring system (Wisniewski et al., 2004) flagged decisions that deviated from the protocol, and CRCs assisted both participants and clinicians in implementing the protocol.
In the first treatment step, citalopram was started at 20 mg/d and gradually raised to a maximum dose of 60 mg/d. In the second step, dose recommendations for the switch to bupropion-SR began at 150 mg/d and increased to 200 mg/d after 1 wk, 300 mg/d at week 4, and 400 mg/d at week 6. Switch to sertraline began at 50 mg/d for 1 wk and increased to 100 mg/d at week 2, 150 mg/d at week 4, and 200 mg/d at week 9. Switch to venlafaxine XR began at 37.5 mg/d and increased to 150 mg/d after 1 wk with intermediate titration, 225 mg/d at week 4, 300 mg/d at week 6, and 375 mg/d at week 9. Bupropion-SR augmentation to citalopram began at 200 mg/d and was increased to 300 mg/d at week 4 and 400 mg/d at week 6. Buspirone augmentation to citalopram began at 15 mg/d and was increased to 30 mg/d after 1 wk, 45 mg/d at week 4, and 60 mg/d at week 6.
At all levels, participants who reached remission could proceed to a 12-month naturalistic follow-up phase. Those who reached response without remission could proceed to follow-up, but were encouraged to proceed to the next treatment level. Participants without remission or response were encouraged to enter the next treatment level. Those intolerant to treatment could proceed to the next level as early as week 4 or as of week 9 for lack of efficacy at a maximally tolerated dose.
Attrition in STAR*D was to be minimized by both the opportunity for participants to refuse randomization to treatment strategies that were unacceptable or to move to the next treatment level when intolerability was an issue.
Attrition
Attrition was defined as leaving treatment before the week 12 visit without transition to follow-up or to the next treatment step, excluding those who left the study for medical reasons. Examples of leaving for medical reasons would be the participant leaving due to a new pregnancy or development of a medical condition that required medication that disallowed study medications.
Data analysis
Summary statistics are presented as means and standard deviations for continuous variables, and percentages for discrete variables. Within each treatment strategy, bivariate logistic regression models were used to identify factors associated with each type of attrition. There were many factors that had a bivariate association with attrition. However, a number of these factors were highly correlated with each other. In an effort to control for this correlation and identify the factors independently associated with remission, an exploratory stepwise regression model was generated, controlling for regional centre and treatment. Statistical significance was defined as a two-sided p value<0.05. No adjustments were made for multiple comparisons, so results must be interpreted accordingly.
Since a number of comparisons were made for this exploratory report, the primary focus in this report is on the independent predictors of attrition from the stepwise logistic regressions.
Results
Of the group electing to be randomized to a level 2 treatment, 57% were willing to accept a medication switch, 50% were willing to accept an augmentation of citalopram, but only 7% were willing to accept either a switch or an augmentation (Wisniewski et al., 2007). In addition, clinical characteristics in the treatment groups were somewhat different at level 2 baseline. Those who received a medication switch had more side-effects with citalopram than those who received an augmentation (56% vs. 9%), had a little less improvement in symptom severity with citalopram (4.3% vs. 8.7%), and had greater symptom severity on the QIDS-SR16 at entry into the second-step treatment (13.2 vs. 11.3) (Rush et al., 2006c; Trivedi et al., 2006a). The outcomes from the groups, therefore, cannot be compared directly.
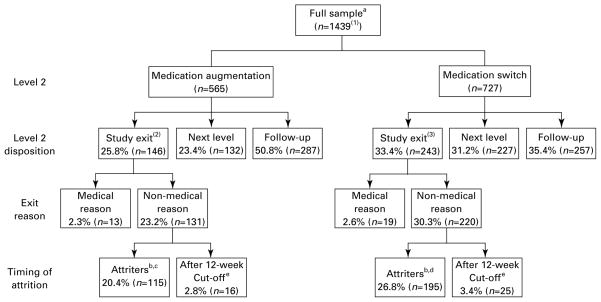
Figure 1 shows the disposition of participants enrolled in the second-step treatments. About 27% (195/727) of those in the medication-switch group dropped out, as did 20% (115/565) of those in the augmentation group. Attrition was often immediate (i.e. right after baseline). For example, 66/195 (34%) of those who dropped out of medication switch did so after only a baseline visit, and 47/115 (41%) of those who dropped out of medication augmentation left immediately after baseline.
Figure 1.
Flow of patients in step 2 medication treatment. aFull sample for which there is no missing data [(1) cognitive therapy switch/augment=147; (2) two participants do not have data that would clarify medical reason for attrition; (3) four participants do not have data that would clarify medical reason for attrition.] b Exited before the week 12 visit. c In the augmentation attrition group, 47 (41%) exited immediately after the baseline visit, 37 (32%) exited before the week 6 visit, and 31 (27%) exited after the week 6 visit. d In the switch attrition group, 66 (34%) exited immediately after the baseline visit, 77 (39%) exited before the week 6 visit and 52 (27%) exited after the week 6 visit. e Attended week 12 visit but did not attend scheduled visits after 12 wk; categorized with the non-attrition group.
Medication augmentation
Attrition rates for the augmenting medications were 20% for bupropion-SR and 21% for buspirone. These were not significantly different. Only 7% of those who dropped out remitted, as compared to 43% of those who remained in treatment (p<0.0001) (Table 1). For medication augmentation, those who dropped out were more likely to be Black or of another non-Caucasian race, Hispanic, have a family history of drug abuse, and have an Axis I psychiatric comorbidity (especially panic disorder or current drug abuse). Those who dropped out were also more likely to be younger, have slightly fewer years of education, and lower household income (Table 2). Importantly, experience in the first-step treatment with citalopram was also related to attrition with augmentation. Those who dropped out began augmentation with somewhat greater depressive symptom severity and less symptom improvement with citalopram (QIDS-SR16). There were no differences in citalopram dose or side-effects between those who did and did not drop out (Table 3).
Table 1.
Remission rates for attrition and non-attrition participants
| In remission |
p value | ||||
|---|---|---|---|---|---|
| Attrition participants |
Non-attrition participants |
||||
| n/N | % | n/N | % | ||
| Medication augmentation | |||||
| Citalopram+bupropion-SR | 5/54 | 9.3 | 102/223 | 45.9 | <0.0001 |
| Citalopram+buspirone | 3/60 | 5.0 | 91/225 | 40.4 | <0.0001 |
| Total | 8/114 | 7.0 | 193/447 | 43.2 | <0.0001 |
| Medication switch | |||||
| Bupropion-SR | 12/70 | 17.1 | 49/168 | 29.2 | 0.0529 |
| Sertraline | 6/57 | 10.5 | 57/179 | 31.8 | 0.0015 |
| Venlafaxine-XR | 6/67 | 9.0 | 56/179 | 31.3 | 0.0003 |
| Total | 24/194 | 12.4 | 162/526 | 30.8 | <0.0001 |
n, Number of participants reaching remission; N, number in the attrition or non-attrition groups.
Bold values denote significant findings.
Attrition participants – Dropped out before their week 12 visit.
Non-attrition participants – Proceeded to follow-up or the next treatment, left the study for medical reasons, or dropped out after the 77-d cut-o. (given a visit window of 6 d around the week 12 visit).
Table 2.
Sociodemographic and clinical characteristics associated with attrition
| Characteristics | Medication augmentation: N=563 (43.8%) |
Medication switch: N=723 (56.2%) |
||||
|---|---|---|---|---|---|---|
| Attrition participantsa (N=115) (20.4%) % |
Non-attrition participantsb (N=448) (79.6%) % |
p value | Attrition participantsa (N=195) (27%) % |
Non-attrition participantsb (N=528) (73%) % |
p value | |
| Sociodemographic characteristics | ||||||
| Setting | 0.1436 | 0.5694 | ||||
| Primary | 23.9 | 76.1 | 28.1 | 71.9 | ||
| Speciality | 18.7 | 81.3 | 26.2 | 73.8 | ||
| Race | 0.0004 | 0.0081 | ||||
| White | 16.9 | 83.1 | 24.1 | 75.9 | ||
| Black | 33.7 | 66.3 | 35.4 | 64.6 | ||
| Othersc | 31.0 | 69.0 | 37.5 | 62.5 | ||
| Ethnicity – Hispanic | 0.0269 | 0.0220 | ||||
| No | 18.9 | 81.1 | 28.3 | 71.7 | ||
| Yes | 29.9 | 70.1 | 16.3 | 83.7 | ||
| Gender | 0.7680 | 0.2397 | ||||
| Male | 19.8 | 80.2 | 24.7 | 75.3 | ||
| Female | 20.8 | 79.2 | 28.6 | 71.4 | ||
| Marital status | 0.4703 | 0.0100 | ||||
| Never married | 22.8 | 77.2 | 32.5 | 67.5 | ||
| Married | 17.5 | 82.5 | 20.6 | 79.4 | ||
| Divorced | 21.7 | 78.3 | 31.4 | 68.6 | ||
| Widowed | 28.6 | 71.4 | 22.9 | 77.1 | ||
| Employment status | 0.6816 | 0.3402 | ||||
| Unemployed | 21.7 | 78.3 | 29.4 | 70.6 | ||
| Employed | 19.9 | 80.1 | 25.5 | 74.5 | ||
| Retired | 14.3 | 85.7 | 20.5 | 79.5 | ||
| Insurance status | 0.6181 | 0.4758 | ||||
| Private insurance | 19.2 | 80.8 | 25.0 | 75.0 | ||
| Public insurance | 21.1 | 78.9 | 30.8 | 69.2 | ||
| No insurance | 22.9 | 77.1 | 27.6 | 72.4 | ||
| Clinical characteristics | ||||||
| Family history of depression | 0.3144 | 0.6325 | ||||
| No | 18.6 | 81.4 | 25.8 | 74.2 | ||
| Yes | 22.0 | 78.0 | 27.3 | 72.7 | ||
| Family history of alcohol abuse | 0.2256 | 0.5251 | ||||
| No | 18.7 | 81.3 | 25.7 | 74.3 | ||
| Yes | 22.9 | 77.1 | 27.8 | 72.2 | ||
| Family history of drug abuse | 0.0001 | 0.0969 | ||||
| No | 16.8 | 83.2 | 25.1 | 74.9 | ||
| Yes | 32.3 | 67.7 | 31.4 | 68.6 | ||
| Attempted suicide | 0.1895 | 0.2339 | ||||
| No | 19.4 | 80.6 | 26.0 | 74.0 | ||
| Yes | 25.2 | 74.8 | 31.2 | 68.8 | ||
| Anxious features | 0.3107 | 0.2432 | ||||
| No | 17.6 | 82.4 | 23.5 | 76.5 | ||
| Yes | 21.5 | 78.5 | 27.6 | 72.4 | ||
| Melancholic features | 0.4087 | 0.1823 | ||||
| No | 18.4 | 81.6 | 24.1 | 75.9 | ||
| Yes | 23.4 | 76.6 | 30.0 | 70.0 | ||
| Atypical features | 0.8512 | 0.2000 | ||||
| No | 19.1 | 80.9 | 26.2 | 73.8 | ||
| Yes | 18.2 | 81.8 | 20.8 | 79.2 | ||
| Chronic depression | 0.6182 | 0.0563 | ||||
| No | 19.8 | 80.2 | 25.2 | 74.8 | ||
| Yes | 21.7 | 78.3 | 32.3 | 67.7 | ||
| Recurrent depression | 0.9469 | 0.3873 | ||||
| 1 episode | 20.0 | 80.0 | 25.0 | 75.0 | ||
| >1 episode | 20.3 | 79.7 | 28.5 | 71.5 | ||
| Onset | 0.6578 | 0.0265 | ||||
| <18 yr | 21.3 | 78.7 | 31.8 | 68.2 | ||
| ≥18 yr | 19.8 | 80.2 | 24.2 | 75.8 | ||
| Concurrent Axis I psychiatric comorbidities (PDSQ)d | ||||||
| Panic | 0.0030 | 0.3812 | ||||
| No | 18.2 | 81.8 | 26.2 | 73.8 | ||
| Yes | 32.2 | 67.8 | 30.3 | 69.7 | ||
| PTSD | 0.0670 | 0.0324 | ||||
| No | 18.9 | 81.1 | 24.9 | 75.1 | ||
| Yes | 26.9 | 73.1 | 33.3 | 66.7 | ||
| Drug Abuse | 0.0030 | 0.9167 | ||||
| No | 18.8 | 81.2 | 26.7 | 73.2 | ||
| Yes | 36.7 | 63.3 | 27.5 | 72.5 | ||
| Number of Axis I comorbidities | 0.0253 | 0.3574 | ||||
| 0 | 12.9 | 87.1 | 22.9 | 77.1 | ||
| 1 | 25.5 | 74.5 | 30.9 | 69.1 | ||
| 2 | 22.0 | 78.0 | 24.6 | 75.4 | ||
| 3 | 27.7 | 72.3 | 26.6 | 73.4 | ||
| ≥4 | 24.0 | 76.0 | 30.2 | 69.8 | ||
| Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | |||
| Age (yr) | 38.4 (13.3) | 41.9 (12.5) | 0.0099 | 40 (13.2) | 42.5 (12.6) | 0.0169 |
| Education (yr) | 12.4 (3.2) | 13.4 (3.3) | 0.0040 | 13 (3) | 13.4 (2.9) | 0.0973 |
| Monthly household income (US$) | 1551 (1635) | 2301 (3025) | 0.0184 | 1931 (2419) | 2047 (2211) | 0.5865 |
| Age at onset (yr) | 24.5 (13.9) | 25.4 (14.1) | 0.5782 | 23.7 (14.3) | 25.4 (13.9) | 0.1445 |
| Number of episodes | 5.2 (7.6) | 5.4 (8.7) | 0.8353 | 5.9 (9.3) | 6.9 (11.7) | 0.3254 |
| Length of MDE episode (months) | 26.3 (51.2) | 27.5 (56.8) | 0.8436 | 30.4 (61.7) | 29.4 (67.6) | 0.8560 |
| Length of illness (yr) | 14 (11.5) | 16.6 (13.5) | 0.0678 | 16.4 (12.8) | 17.1 (13.9) | 0.5213 |
| Symptom severity (step 2 entry) | ||||||
| HAMD17 (ROA) | 17.5 (7.7) | 15.4 (6.9) | 0.0126 | 19.8 (7.3) | 18.6 (7.2) | 0.0690 |
| IDS-C30 (ROA) | 31.8 (13.3) | 27.7 (12.3) | 0.0049 | 35.5 (13.3) | 33.7 (12.8) | 0.1189 |
| QIDS-SR16 | 12.6 (4.8) | 11.0 (4.8) | 0.0032 | 13.5 (5.2) | 13.2 (4.8) | 0.3619 |
| Function and quality of life (step 2 entry) | ||||||
| SF-12 Physical | 45.6 (12.4) | 47.4 (12) | 0.2497 | 43.4 (12.4) | 45.8 (12.7) | 0.0673 |
| SF-12 Mental | 32.2 (9.5) | 32.7 (9.3) | 0.6413 | 30.9 (10.7) | 29 (9.4) | 0.0486 |
| General medical comorbidities (CIRS) | ||||||
| Categories endorsed | 2.9 (2.2) | 3 (2.3) | 0.5586 | 3.1 (2.2) | 3.4 (2.5) | 0.1757 |
| Total score | 4.2 (3.8) | 4.4 (3.9) | 0.6956 | 4.5 (3.8) | 4.9 (3.9) | 0.2785 |
| Severity index | 1.2 (0.7) | 1.2 (0.6) | 0.9229 | 1.3 (0.6) | 1.2 (0.6) | 0.6246 |
CIRS, Cumulative Illness Rating Scale; HAMD17, 17-item Hamilton Rating Scale for Depression; IDS-C30, 30-item Inventory of Depressive Symptomatology – Clinician-rated; MDE, major depressive episode; PTSD, post-traumatic stress disorder; QIDS-SR16, 16-item Quick Inventory of Depressive Symptomatology – Self-Report; ROA, research outcomes assessor; SF-12, 12-item Short Form Health Survey.
Bold values denote significant findings.
Attrition participants – Dropped out before their week 12 visit.
Non-attrition participants – Proceeded to follow-up or the next treatment, left the study for medical reasons, or dropped out after the 77-d cut-o. (given a visit window of 6 d around the week 12 visit).
Asian, American Indian or Alaskan Native, Native Hawaiian/Other Pacific Islander, or multiracial.
Significant findings only shown.
Table 3.
Level 1 exit treatment characteristics associated with level 2 attrition
| Level 1 variables | Medication augmentation: N=563 (43.8%) |
Medication switch: N=723 (56.2%) |
||||
|---|---|---|---|---|---|---|
| Attrition participantsa N=115) (20.4%) % |
Non-attrition participantsb (N=448) (79.6%) % |
p value | Attrition participantsa (N=195) (27%) % |
Non-attrition participantsb (N=528) (73%) % |
p value | |
| Exit dose of citalopram (mg/d) | 0.4845 | 0.0020 | ||||
| <20 mg | 50.0 | 50.0 | 37.3 | 62.7 | ||
| 20–39 mg | 15.2 | 84.8 | 29.6 | 70.4 | ||
| 40–49 mg | 24.0 | 76.0 | 29.6 | 70.4 | ||
| ≥50 mg | 19.9 | 80.1 | 17.7 | 82.3 | ||
| Exit FIBSER frequency | 0.2877 | 0.2863 | ||||
| No side-effects | 24.0 | 76.0 | 25.0 | 75.0 | ||
| 10–25% of the time | 17.0 | 83.0 | 22.7 | 77.3 | ||
| 50–75% of the time | 20.8 | 79.2 | 26.6 | 73.4 | ||
| 90–100% of the time | 16.1 | 83.9 | 31.4 | 68.6 | ||
| Exit FIBSER intensity | 0.1962 | 0.0283 | ||||
| No side effects | 24.4 | 75.6 | 23.7 | 76.3 | ||
| Trivial-mild | 17.1 | 82.9 | 24.2 | 75.8 | ||
| Moderate-marked | 19.3 | 80.7 | 23.6 | 76.4 | ||
| Severe-intolerable | 11.1 | 88.9 | 34.8 | 65.2 | ||
| Exit FIBSER burden | 0.3185 | 0.3229 | ||||
| No impairment | 22.8 | 77.2 | 25.3 | 74.7 | ||
| Minimal-mild impairment | 16.7 | 83.3 | 23.2 | 76.8 | ||
| Moderate-marked impairment | 23.6 | 76.4 | 28.7 | 71.3 | ||
| Severe/unable to function | 12.5 | 87.5 | 31.9 | 68.1 | ||
| Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | Mean (S.D.) | |
| Exit QIDS-SR16 | 12.6(4.8) | 11(4.8) | 0.0023 | 13.5(5.3) | 13.2(4.8) | 0.4597 |
| Exit dose (citalopram) | 54(12.1) | 54.5(11.6) | 0.6646 | 31.5(17.3) | 37(18.4) | 0.0004 |
| Percent change in QIDS-SR16 | −23(32.8) | −30.4(28.3) | 0.0169 | −13.9(31.6) | −15.8(30.2) | 0.4618 |
| Duration in level 1 (wk) | 12.1(3.6) | 11.8(2.8) | 0.3387 | 7.5(4.2) | 8.2(4.2) | 0.0445 |
FIBSER, Frequency, Intensity and Burden of Side Effects Rating; QIDS-SR16, 16-item Quick Inventory of Depressive Symptomatology – Self-Report.
Bold values denote significant findings.
Attrition participants – Dropped out before their week 12 visit.
Non-attrition participants – Proceeded to follow-up or the next treatment, left the study for medical reasons, or dropped out after the 77-d cut-o. (given a visit window of 6 d around the week 12 visit).
Factors independently associated with attrition were Black and other non-Caucasian races, Hispanic ethnicity, and family history of drug abuse. Additional independent factors included younger age and somewhat greater severity of depression (IDS-C30) at the beginning of the medication augmentation trial (Table 4).
Table 4.
Sociodemographic and clinical characteristics independently associated with attrition
| Significant predictors | Medication augmentation |
Medication switch |
||
|---|---|---|---|---|
| OR | p value | OR | p value | |
| Switch treatment (ref. group=SER) | 0.5134 | |||
| BUP-SR | 0.96 | |||
| VEN-XR | 1.27 | |||
| Augment treatment (ref. group=CIT+BUP) | 0.3500 | |||
| CIT+BUS | 1.30 | |||
| Race (ref. group=Caucasian) | 0.0390 | 0.0217 | ||
| Black | 1.87 | 2.30 | ||
| Othersa | 3.70 | 1.28 | ||
| Hispanic (ref. group=No) | 3.32 | 0.0088 | ||
| Family history of drug abuse (ref. group=No) | 3.65 | 0.0001 | ||
| Melancholic features (ref. group=No) | 1.72 | 0.0416 | ||
| Age (units=10) | 0.67 | 0.0012 | ||
| Step 2 baseline IDS-C30 (units=5) | 1.14 | 0.0264 | ||
| Citalopram exit dose (ref. group=20–39 mg) | 0.0046 | |||
| <20 mg | 1.99 | |||
| 40–49 mg | 0.85 | |||
| ≥50 mg | 0.48 | |||
(1) This is based on a stepwise regression.
(2) For each characteristic for which the measurement is categorical, the comparison is with the noted reference group. For example, Hispanics are compared with non-Hispanics.
(3) For characteristics for which the measurement is continuous (age, level 2 baseline IDS-30), the odds ratio is relative to an increase in the measurement by the number of units indicated. For example, the odds ratio for age describes the change in odds relative to a 10-yr increase in age. The estimated odds ratio of 0.67 means that for any 10-yr increase in age (e.g. 25–35 or 47–57), the odds of dropping out are lower by a factor of 0.67. If the odds of a 25-yr-old dropping out are some amount ‘X’, then the odds of a 35-yr-old dropping out are 0.67 times ‘X’.
BUP-SR, bupropion sustained release; CIT+BUP, citalopram plus bupropion sustained release; CIT+BUS, citalopram plus buspirone; IDS-C30, 30-item Inventory of Depressive Symptomatology – Clinician-rated; OR, odds ratio; SER, sertraline; VEN-XR, venlafaxine extended release.
Bold values denote significant findings.
Asian, American Indian or Alaskan Native, Native Hawaiian/Other Pacific Islander, or multiracial.
Medication switch
Attrition rates within the three medication-switch groups were 29% for bupropion-SR, 24% for sertraline, and 27% for venlafaxine-XR. These were not significantly different. Overall, 12% of those in the attrition group remitted, while 31% of those in the non-attrition group remitted (p<0.0001) (Table 1). In the medication-switch group, those who dropped out were more likely to be Black or of other non-Caucasian races, non-Hispanic, divorced or never married, younger, and have an age of onset <18 yr (Table 2). Those who dropped out at the second step had a lower mean exit dose of citalopram (31.0 vs. 37.0 mg/d), but more intense side-effects and somewhat fewer weeks of treatment with citalopram (Table 3).
Factors independently associated with attrition with a medication switch included Black and other non-Caucasian races, melancholic features, and lower mean exit dose of citalopram (Table 4).
Discussion
About a quarter of the participants in the medications-witch group dropped out before the week 12 visit, but only a fifth of those in the medication-augmentation group did so. The overall attrition rate in second-step treatment was similar to the 26% rate of attrition in the STAR*D first-step treatment with citalopram (Warden et al., 2007). Clearly, the challenge of adequately addressing treatment-resistant depression is compounded by attrition in both first- and second-step treatments. Leaving treatment prematurely is associated with significantly poorer outcomes.
The difference in remission rates between those who did or did not drop out was greater in the augmentation group (7% vs. 43%) than in the switch group (12% vs. 31%). A substantial opportunity for remission may have been lost by some of those who prematurely dropped out of both groups.
Despite many dissimilar characteristics of those entering the medication augmentation and switch groups, several predictors of attrition were similar. First, minority status was independently associated with greater attrition in both groups. With medication augmentation, non-Caucasian/non-Blacks were nearly four times as likely, and Blacks were nearly twice as likely to drop out as Caucasians. Hispanics were more than three times as likely as non-Hispanics to drop out. With a medication switch, Blacks were more than twice as likely to drop out as Caucasians, and those of other non-Caucasian races were more likely to drop out as well. Both Black and Hispanic status also predicted attrition in our earlier study of first-step treatment with citalopram (Warden et al., 2007), as well as in another study of first-step MDD treatment (Arnow et al., 2007).
Blacks and Hispanics have been shown to find antidepressants less acceptable than Caucasians (Cooper et al., 2003), and psychotherapy has been reported as preferred by Hispanics (Cooper et al., 2003) and Blacks (Dwight-Johnson et al., 2000). However, in our study Blacks did not accept the possibility of randomization to cognitive therapy more than Caucasians, and Hispanics did not elect this possibility more than non-Hispanics (Wisniewski et al., 2007).
A host of other issues may be related to drop out by minority patients. These may include the nature of the patient/provider alliance, preference for a clinician of the same race or ethnicity, the acceptability of the treatment setting, perception of treatment efficacy, cultural attitudes about health services, income level, and social support.
Second, attrition was independently predicted by younger age in the augmentation group and, although not an independent predictor, age was also a significant finding in the switch group. This was again similar to our findings with citalopram treatment. Third, negative experience with the first medication step also played a key role in attrition for both the augmentation and switch groups. In medication augmentation, those who dropped out had less symptom improvement with citalopram and greater symptom severity when beginning augmentation than those who did not. In medication switch, those who dropped out had more intolerable side-effects with citalopram than those who did not, despite having lower citalopram dosing, suggesting possible metabolic differences leading to intolerance to a range of medications or a negative predisposition towards medication.
Those who dropped out of both the augmentation and switch groups were, therefore, unwilling to remain in second-step treatment long enough to reach remission despite the availability of several other treatment strategies, the option to adjust doses in step 2, and access to concomitant treatments for treatment-emergent symptoms such as anxiety, agitation, or sexual dysfunction.
In the medication-augmentation group, a striking finding is that participants with a family history of drug abuse dropped out almost four times as often as those without this history. Given the significant finding that medication-augmentation participants with concurrent drug abuse also dropped out more often, it may be that individuals with one or both of these experiences have a negative perception of or unwillingness to sustain treatment with polypharmacy. Interestingly, however, those with concurrent drug abuse were initially more likely to accept a randomization to medication augmentation than a switch (Wisniewski et al., 2007).
In the augmentation group significant, although not independent predictors, included sociodemographic disadvantages in addition to younger age, including less education and lower household income, as well as the clinical disadvantage of more frequent Axis I comorbidity (especially concurrent drug abuse or panic disorder). These predictors again were similar to our previously reported predictors of attrition with STAR*D first-step citalopram treatment. We found that those with sociodemographic disadvantages such as public insurance and more psychiatric comorbidities were more likely to drop out of first-step treatment (Warden et al., 2007). Prior studies identified youth and sociodemographic disadvantage as attrition risk factors during initial treatment as well (Arnow et al., 2007; Demyttenaere et al., 1998; Last et al., 1985). Therefore, the theme of sociodemographic or clinical disadvantage continues to predict risk of drop out with a second-step augmentation treatment, although other than younger age, these factors did not appear to be relevant for those with a medication switch.
For the medication-switch group only, there was a significant finding that participants who were never married or divorced were more likely to drop out than those who were married. Since the medication-switch group had more side-effects with citalopram (56% vs. 9%) and greater symptom severity when beginning step 2 than the augmentation group, perhaps social support was helpful in allaying frustration and drop out in this group. Similarly, perhaps melancholic features, an independent predictor of attrition in the switch group only, interfered with these participants’ ability to sustain participation until remission was reached.
Higher doses of citalopram in level 1 were also independently associated with remaining in a second-step medication switch, even though the medication was changed. Perhaps higher dosing with citalopram is a signal that a participant is willing to persist in treatment as options are tried.
Of interest, in first-step treatment with citalopram, recurrent depression and more years since first onset of depression were related to remaining in treatment, perhaps due to experience with depression, awareness of the risk of recurrence, and/or awareness that treatment can work (Warden et al., 2007). This type of experience with depression was not a useful predictor of retention with a second-step medication strategy. In fact, those with an age of onset <18 yr were somewhat more likely to drop out with a medication switch.
In clinical trials and clinical practice, the finding that minority status and younger age are predictors of drop out in both first- and second-step medication treatment highlights the vulnerability of these populations to discontinuation of treatment and the need for implementing strategies to increase retention. Patients with sociodemographic disadvantages or Axis I comorbid conditions are also in need of focused retention efforts at both the first step and second augmentation step. Interventions targeted to these populations, therefore, need not be tailored to different steps in a treatment algorithm, making them easier to implement. Patients with a family history of drug abuse or concurrent drug abuse require careful implementation if medication augmentation is used.
For patients with these and other population- or illness-related risk factors for attrition, it would be advisable for clinicians to elicit and address any biases or barriers to remaining in treatment before treatment begins, especially biases that involve the use of multiple medications if contemplating an augmentation strategy. High risk patients can be directly asked about their likelihood of returning at each visit to signal the need for further discussion of concerns, and retention interventions can then be targeted to the identified issues.
For patients who require a second-step medication strategy following intolerance or lack of efficacy with the first step, it may be useful to provide specific information about the expected probability of and range of times to remission with each subsequent step after the first one. It may also be helpful to specifically encourage these patients to remain in treatment and provide their clinician the opportunity to respond to side-effects, lack of efficacy, the perception of efficacy, or other factors that may drive an inclination to discontinue. These issues are not typically discussed with patients in clinical practices.
Several features of this trial may limit the generalizability of findings. Participants were able to move on to a new treatment option before completing a treatment step if they experienced side-effects or a lack of efficacy. CRCs helped implement the protocol with both clinicians and participants, clinicians were closely monitored to enhance protocol fidelity, and medications and uninsured clinic visits were free to participants. Comorbid Axis I conditions, including substance abuse, were assessed at step 1 entry and not again at step 2 entry and substance abuse was not confirmed or monitored with urine toxicology screens. A number of other patient, clinician, setting, medication, and treatment-related factors that we did not measure may also have impacted attrition. These include the quality of the participant/clinician relationship, clinician experience or commitment, patient perception of regimen complexity, ease of access to the clinic, ability to attend appointments, and adherence to prescribed medications, which was not adequately assessed in STAR*D, among others.
Given the prevalence of attrition despite the high quality of care provided in the STAR*D trial and given the less than acceptable remission rates in those in the attrition groups, there is a clear need to improve efforts to retain patients in treatment and/or deliver more rapidly effective, yet well-tolerated treatments.
Acknowledgments
This project has been funded with Federal funds from the National Institute of Mental Health, National Institutes of Health (NIMH), under Contract N01MH90003 to UT Southwestern Medical Center at Dallas (P.I.: A. J. Rush). NIMH had no involvement in study design; in the collection, analysis, and interpretation of data; nor in the writing of the report or the decision to submit the paper for publication. This analysis was also supported by funds from the National Alliance for Research in Schizophrenia and Depression. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We appreciate the support of Bristol–Myers Squibb, Forest Laboratories, GlaxoSmithKline, King Pharmaceuticals, Organon, Pfizer, and Wyeth in providing medications at no cost for this trial. We also acknowledge the editorial support of Jon Kilner, M.S., M.A.
Footnotes
Portions of this paper were presented in poster form at the International Society for Affective Disorders (ISAD), 4th Biennial Conference, Capetown, South Africa, March 2008.
Statement of Interest
Diane Warden, Ph.D., M.B.A. currently owns stock in Pfizer, Inc. has owned stock in Bristol–Myers Squibb Company within the last 5 years. A. John Rush, M.D. has received research support from the National Institute of Mental Health, the Robert Wood Johnson Foundation, and the Stanley Medical Research Institute; has been on the advisory boards and/or consultant for Advanced Neuromodulation Systems, Inc., AstraZeneca, Best Practice Project Management, Inc., Bristol–Myers Squibb Company, Cyberonics, Inc., Eli Lilly and Company, Gerson Lehman Group, GlaxoSmithKline, Jazz Pharmaceuticals, Magellan Health Services, Merck & Co., Inc., Neuronetics, Ono Pharmaceutical, Organon USA Inc., Pam Lab, Personality Disorder Research Corp., Pfizer Inc., The Urban Institute, and Wyeth–Ayerst Laboratories Inc.; has been on the speakers’ bureaux for Cyberonics, Inc., Forest Pharmaceuticals, Inc., and GlaxoSmithKline; has equity holdings (excluding mutual funds/blinded trusts) in Pfizer Inc.; and has royalty income affiliations with Guilford Publications and Healthcare Technology Systems, Inc. Stephen R. Wisniewski, Ph.D. has been a consultant for Cyberonics, Inc. (2005–2006), ImaRx Therapeutics, Inc. (2006), Bristol– Myers Squibb Company (2007), Organon (2007), and Case-Western University (2007). Ira M. Lesser, M.D. has received grant support from the National Institute of Mental Health and Aspect Medical Systems, and has served on the speakers’ bureau of Medical Education Speakers Network. Susan G. Kornstein, M.D. has received grant/research support from the Department of Health and Human Services, the National Institute of Mental Health, Pfizer, Inc., Bristol–Myers Squibb Company, Eli Lilly and Company, Forest Laboratories, Inc., GlaxoSmithKline, Inc., Mitsubishi-Tokyo, Merck, Inc., Biovail Laboratories, Inc., Wyeth, Inc., Berlex Laboratories, Novartis Pharmaceuticals, Inc., Sepracor, Inc., Boehringer-Ingelheim, Sanofi-Synthelabo, and AstraZeneca. She has served on advisory boards for and/or received honoraria from Pfizer, Inc., Wyeth, Inc., Eli Lilly and Company, Bristol–Myers Squibb Company, Warner-Chilcott, Inc., Biovail Laboratories, Berlex Laboratories, Forest Laboratories, Neurocrine, and Sepracor, Inc., and has received book royalties from Guilford Press. Michael E. Thase, M.D. has served in an advisory or consulting capacity to, or received speaker’s honoraria from, AstraZeneca, Bristol–Myers Squibb, Cephalon, Cyberonics, Inc., Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceutica, MedAvante, Inc., Neuronetics, Inc., Novartis, Organon, Inc., Sanofi-Aventis, Sepracor, Inc., Shire US, Inc., and Wyeth Pharmaceuticals; has equity holdings in MedAvante, Inc.; and has received royalty/patent or other income from American Psychiatric Publishing, Inc., Guilford Publications, and Herald House. Sheldon H. Preskorn, M.D. has served or is serving in one or more of the following capacities: as a principal investigator, on the speakers’ bureau, and/or as a consultant for the following companies: Abbott Laboratories, AstraZeneca, Aventis, Biovail, Boehringer-Ingleheim, Bristol–Myers Squibb, E. Merck, Eisai, Eli Lilly, GlaxoSmithKline, Hoffman LaRoche, Janssen, Johnson & Johnson, Lundbeck, Merck, Neurosearch, Novartis, Organon, Otusak, Pfizer, Inc., Solvay, Somerset, Sumitomo, Wyeth, and Yamanouchi. Andrew A. Nierenberg, M.D. has received research support from Bristol–Myers Squibb, Cederroth, Cyberonics, Forest Pharmaceuticals, Eli Lilly & Co, GlaxoSmithKline, Janssen Pharmaceutica, Lichtwer Pharma, NARSAD, NIMH, Pfizer Pharmaceuticals, Stanley Foundation, and Wyeth–Ayerst. He has served on speakers’ bureaux with Bristol–Myers Squibb, Cyberonics, Forest Pharmaceuticals, Eli Lilly & Co., GlaxoSmithKline, and Wyeth–Ayerst and has provided advisory/consulting services to Abbott Laboratories, Brain Cells Inc., Bristol–Myers Squibb, Cederroth, Eli Lilly & Co, GlaxoSmithKline, Genaissance, Innapharma, Janssen Pharmaceutica, Novartis Pharmaceuticals, Pfizer Pharmaceuticals, Sepracor, Shire, and Somerset. Madhukar H. Trivedi, M.D. has been a consultant for Abbott Laboratories, Inc.; Akzo (Organon Pharmaceuticals Inc.); AstraZeneca; Bayer; Bristol–Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals; Glaxo-SmithKline; Janssen Pharmaceutica Products, LP; Johnson & Johnson PRD; Eli Lilly & Company; Meade Johnson; Neuronetics; Parke-Davis Pharmaceuticals, Inc.; Pfizer, Inc.; Pharmacia & Upjohn; Sepracor; Solvay Pharmaceuticals, Inc.; VantagePoint; and Wyeth–Ayerst Laboratories. He has served on speakers’ bureaux for Abdi Brahim; Akzo (Organon Pharmaceuticals Inc.); Bristol–Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica Products, LP; Eli Lilly & Company; Pharmacia & Upjohn; Solvay Pharmaceuticals, Inc.; and Wyeth–Ayerst Laboratories. He has also received grant support from Bristol–Myers Squibb Company; Cephalon, Inc.; Corcept Therapeutics, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica; Merck; National Institute of Mental Health; National Alliance for Research in Schizophrenia and Depression; Novartis; Pfizer Inc.; Pharmacia & Upjohn; Predix Pharmaceuticals; Solvay Pharmaceuticals, Inc.; and Wyeth–Ayerst Laboratories.
References
- Arnow BA, Blasey C, Manber R, Constantino MJ, Markowitz JC, Klein DN, Thase ME, Kocsis JH, Rush AJ. Dropouts versus completers among chronically depressed outpatients. Journal of Affective Disorders. 2007;97:197–202. doi: 10.1016/j.jad.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Cooper LA, Gonzales JJ, Gallo JJ, Rost KM, Meredith LS, Rubenstein LV, Wang NY, Ford DE. The acceptability of treatment for depression among African-American, Hispanic, and white primary care patients. Medical Care. 2003;41:479–489. doi: 10.1097/01.MLR.0000053228.58042.E4. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Enzlin P, Dewe W, Boulanger B, De Bie J, De Troyer W, Mesters P. Compliance with antidepressants in a primary care setting, 1: Beyond lack of efficacy and adverse events. Journal of Clinical Psychiatry. 2001;62(Suppl 22):30–33. [PubMed] [Google Scholar]
- Demyttenaere K, Van Ganse E, Gregoire J, Gaens E, Mesters P. Compliance in depressed patients treated with fluoxetine or amitriptyline. Belgian Compliance Study Group. International Clinical Psychopharmacology. 1998;13:11–17. doi: 10.1097/00004850-199801000-00002. [DOI] [PubMed] [Google Scholar]
- Dwight-Johnson M, Sherbourne CD, Liao D, Wells KB. Treatment preferences among depressed primary care patients. Journal of General Internal Medicine. 2000;15:527–534. doi: 10.1046/j.1525-1497.2000.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology Bulletin. 1993;29:321–326. [PubMed] [Google Scholar]
- Fava M, Alpert JE, Carmin CN, Wisniewski SR, Trivedi MH, Biggs MM, Shores-Wilson K, Morgan D, Schwartz T, Balasubramani GK, Rush AJ. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychological Medicine. 2004;34:1299–1308. doi: 10.1017/s0033291704002612. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatric Clinics of North America. 2003;26:457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Dunner DL, Keitner G, Klein DN, Koran LM, Kornstein SG, Markowitz JC, Miller I, Nemero CB, Ninan PT, et al. Does psychosocial functioning improve independent of depressive symptoms? A comparison of nefazodone, psychotherapy, and their combination. Biological Psychiatry. 2002;51:123–133. doi: 10.1016/s0006-3223(01)01291-4. [DOI] [PubMed] [Google Scholar]
- Khan AY, Carrithers J, Preskorn SH, Lear R, Wisniewski SR, Rush AJ, Stegman D, Kelley C, Kreiner K, Nierenberg AA, Fava M. Clinical and demographic factors associated with DSM-IV melancholic depression. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists. 2006;18:91–98. doi: 10.1080/10401230600614496. [DOI] [PubMed] [Google Scholar]
- Kobak KA, Greist JH, Jefferson JW, Mundt JC, Katzelnick DJ. Computerized assessment of depression and anxiety over the telephone using interactive voice response. MD Computing. 1999;16:63–68. [PubMed] [Google Scholar]
- Last CG, Thase ME, Hersen M, Bellack AS, Himmelhoch JM. Patterns of attrition for psychosocial and pharmacologic treatments of depression. Journal of Clinical Psychiatry. 1985;46:361–366. [PubMed] [Google Scholar]
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. Journal of the American Geriatrics Society. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Mel CA, Chawla AJ, Croghan TW, Hanna MP, Kennedy S, Sredl K. The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Archives of General Psychiatry. 1998;55:1128–1132. doi: 10.1001/archpsyc.55.12.1128. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., III Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Research. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Mundt JC. Interactive voice response systems in clinical research and treatment. Psychiatric Services. 1997;48:611–612. doi: 10.1176/ps.48.5.611. [DOI] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. British Journal of Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- Novick JS, Stewart JW, Wisniewski SR, Cook IA, Manev R, Nierenberg AA, Rosenbaum JF, Shores-Wilson K, Balasubramani GK, Biggs MM, Zisook S, Rush AJ. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: preliminary findings from STAR*D. Journal of Clinical Psychiatry. 2005;66:1002–1011. doi: 10.4088/jcp.v66n0807. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, Shores-Wilson K, Biggs MM, Woo A, Nierenberg AA, Fava M. An evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: a Sequenced Treatment Alternatives to Relieve Depression trial report. Biological Psychiatry. 2006a;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Carmody TJ, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. International Journal of Methods in Psychiatric Research. 2000;9:45–59. [Google Scholar]
- Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, et al. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): rationale and design. Controlled Clinical Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, Ninan PT, Thase ME, Gelenberg AJ, Kupfer DJ, et al. Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology. 2006b;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [Erratum, p. 585] [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New England Journal of Medicine. 2006c;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Zimmerman M, Wisniewski SR, Fava M, Hollon SD, Warden D, Biggs MM, Shores-Wilson K, Shelton RC, Luther JF, et al. Comorbid psychiatric disorders in depressed outpatients: Demographic and clinical features. Journal of Affective Disorders. 2005;87:43–55. doi: 10.1016/j.jad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Tedlow JR, Fava M, Uebelacker LA, Alpert JE, Nierenberg AA, Rosenbaum JF. Are study dropouts different from completers? Biological Psychiatry. 1996;40:668–670. doi: 10.1016/0006-3223(96)00204-1. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, et al. Medication augmentation after the failure of SSRIs for depression. New England Journal of Medicine. 2006a;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Gaynes BN, Stewart JW, Wisniewski SR, Warden D, Ritz L, Luther JF, Stegman D, DeVeaugh-Geiss J, Howland R. Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR*D measurement-based care. Neuropsychopharmacology. 2007;32:2479–2489. doi: 10.1038/sj.npp.1301390. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychological Medicine. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry. 2006b;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Warden D, Trivedi MH, Wisniewski SR, Davis L, Nierenberg AA, Gaynes BN, Zisook S, Hollon SD, Balasubramani GK, Howland R, et al. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. American Journal of Psychiatry. 2007;164:1189–1197. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-item Short-Form Health Survey. Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- WHO. Adherence to Long-term Therapies: Evidence for Action. Noncommunicable Diseases and Mental Health Adherence to Long-term Therapies Project. Geneva: World Health Organization; 2003. [Google Scholar]
- Wisniewski SR, Eng H, Meloro L, Gatt R, Ritz L, Stegman D, Trivedi M, Biggs MM, Friedman E, Shores-Wilson K, et al. Web-based communications and management of a multi-center clinical trial: the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) project. Clinical Trials (London, England) 2004;1:387–398. doi: 10.1191/1740774504cn035oa. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Fava M, Trivedi MH, Thase ME, Warden D, Niederehe G, Friedman ES, Biggs MM, Sackeim HA, Shores-Wilson K, et al. Acceptability of second-step treatments to depressed outpatients: a STAR*D report. American Journal of Psychiatry. 2007;164:753–760. doi: 10.1176/ajp.2007.164.5.753. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. Journal of Psychiatric Practice. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI. A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Archives of General Psychiatry. 2001a;58:787–794. doi: 10.1001/archpsyc.58.8.787. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Mattia JI. The Psychiatric Diagnostic Screening Questionnaire: development, reliability and validity. Comprehensive Psychiatry. 2001b;42:175–189. doi: 10.1053/comp.2001.23126. [DOI] [PubMed] [Google Scholar]