Abstract
Rationale
Blood gas analysis is often used to assess acid–base, ventilation, and oxygenation status in critically ill patients. Although arterial blood gas (ABG) analysis remains the gold standard, venous blood gas (VBG) analysis has been shown to correlate with ABG analysis and has been proposed as a safer less invasive alternative to ABG analysis.
Objective
The purpose of this study was to evaluate the correlation of VBG analysis plus pulse oximetry (SpO2) with ABG analysis.
Methods
We performed a prospective cohort study of patients in the emergency department (ED) and intensive care unit (ICU) at a single academic tertiary referral center. Patients were eligible for enrollment if the treating physician ordered an ABG. Statistical analysis of VBG, SpO2, and ABG data was done using paired t test, Pearson χ2, and Pearson correlation.
Main Results
There were 156 patients enrolled, and 129 patients completed the study. Of the patients completing the study, 53 (41.1%) were in the ED, 41 (31.8%) were in the medical ICU, and 35 (27.1%) were in the surgical ICU. The mean difference for pH between VBG and ABG was 0.03 (95% confidence interval: 0.03–0.04) with a Pearson correlation of 0.94. The mean difference for pCO2 between VBG and ABG was 4.8 mm Hg (95% confidence interval: 3.7–6.0 mm Hg) with a Pearson correlation of 0.93. The SpO2 correlated well with PaO2 (the partial pressure of oxygen in arterial blood) as predicted by the standard oxygen–hemoglobin dissociation curve.
Conclusion
In this population of undifferentiated critically ill patients, pH and pCO2 on VBG analysis correlated with pH and pCO2 on ABG analysis. The SpO2 correlated well with pO2 on ABG analysis. The combination of VBG analysis plus SpO2 provided accurate information on acid–base, ventilation, and oxygenation status for undifferentiated critically ill patients in the ED and ICU.
Keywords: blood gas analysis, critical care, oximetry
Introduction
Identification of a patient’s arterial pH, pCO2, and pO2 is often critical for diagnostic and treatment purposes. Arterial blood gases (ABGs) are more technically difficult to obtain, more painful, more expensive, and often an extra needle stick that would otherwise have been unnecessary. Venous blood gases (VBGs) are obtained from the venous system and therefore can be obtained along with other blood work. There have been studies published on the correlation between ABGs and VBGs. However, many of these studies have concentrated only on certain patient populations such as diabetic ketoacidosis (DKA),1–3 trauma,4,5 or chronic obstructive pulmonary disease (COPD).6–8 In clinical practice, the exact diagnosis is not always known early on and patients may have multiple underlying pathophysiologic states. Other studies have enrolled patients only in certain areas of the hospital such as the emergency department (ED) or intensive care unit (ICU).9–12 Only one study has compared ABG, VBG, and pulse oximetry (SpO2) in adult populations.13 Despite existing studies, questions remain regarding the use of VBG analysis instead of ABG analysis more broadly in populations of undifferentiated critically ill patients. The goal of this study was to compare venous pH and pCO2 to arterial pH and pCO2 in a diverse group of critically ill patients in the ED and ICU. Additionally, we compared pulse oximetry to arterial pO2. Our hypothesis was that the combination of venous pH, pCO2, and pulse oximetry would correlate with arterial pH, pCO2, and PaO2 independent of disease state.
Methods
This prospective study was conducted in the ED, medical ICU, and surgical ICU at Christiana Hospital, which is an 1100-bed teaching hospital located in Newark, Delaware. The ED has an annual census of over 100,000. This study was approved by the Christiana Care Health System Institutional Review Board. Patients were eligible for the study if they were aged 18 years or older and the treating physician ordered an ABG. Patients were excluded if they had a contraindication to arterial or venous blood draw, if they had already been enrolled in the study during the same hospitalization, if they were pregnant, or if they were a prisoner. Patients were identified and enrolled by trained research nurses and could be enrolled at any time. After informed consent, ABG and VBG samples were obtained as temporally close to each other as possible. The ABG was obtained from either an existing arterial line or from the radial, brachial, or femoral artery. The VBG was obtained from either an existing venous catheter or from a new peripheral venipuncture.
Additional data gathered included patient location and presence of ongoing shock state. For the purposes of this study, we defined shock as the use of vasopressors and/or inotropes or systolic blood pressure <90 mm Hg or mean arterial blood pressure <65 mm Hg. The ABG and VBG analyses were performed either at the bedside or in the laboratory using the i-STAT System (Abbott Point of Care, Princeton, New Jersey). The strength of association between arterial pH, venous pH, arterial pCO2, venous pCO2, and arterial pO2 and SpO2 was assessed using paired t test, Pearson χ2, and Pearson correlation coefficient.
Results
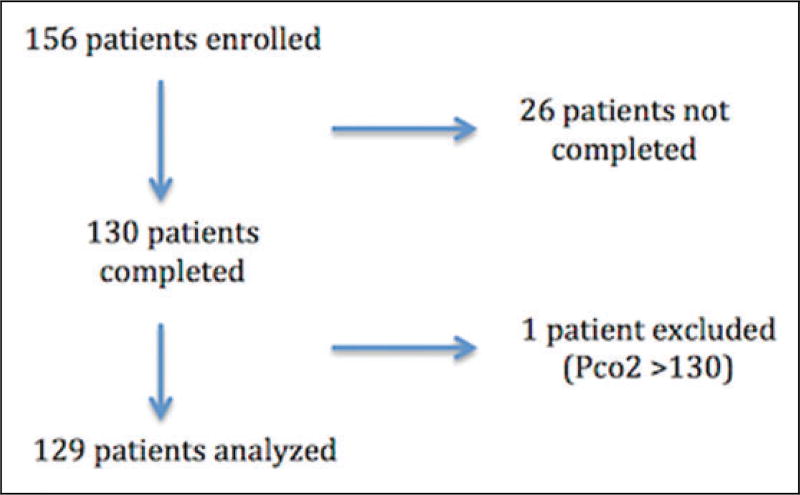
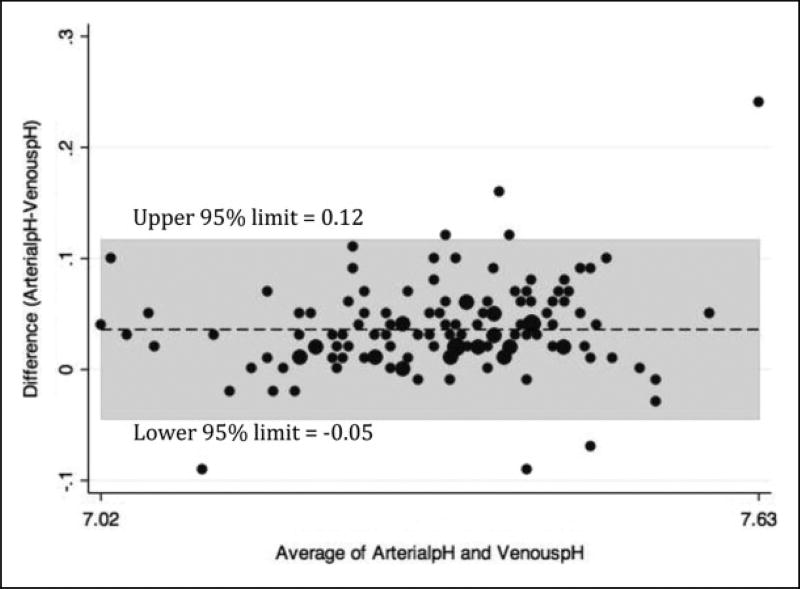
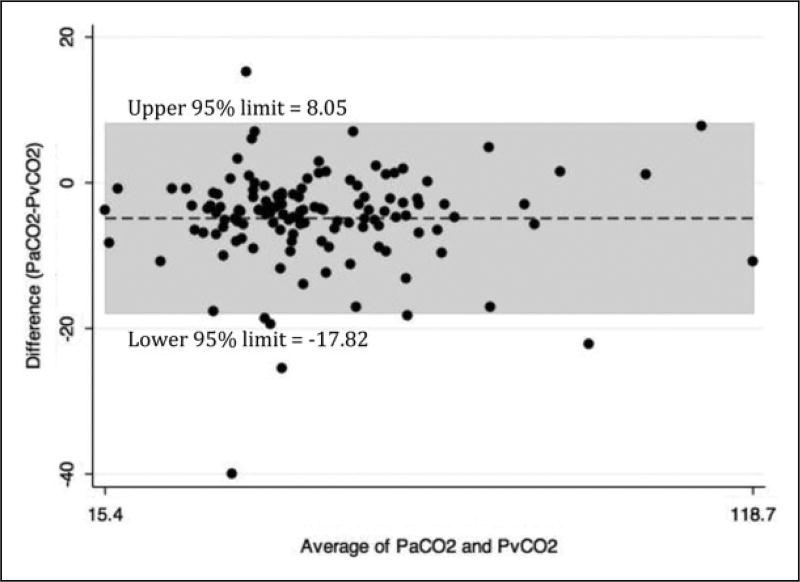
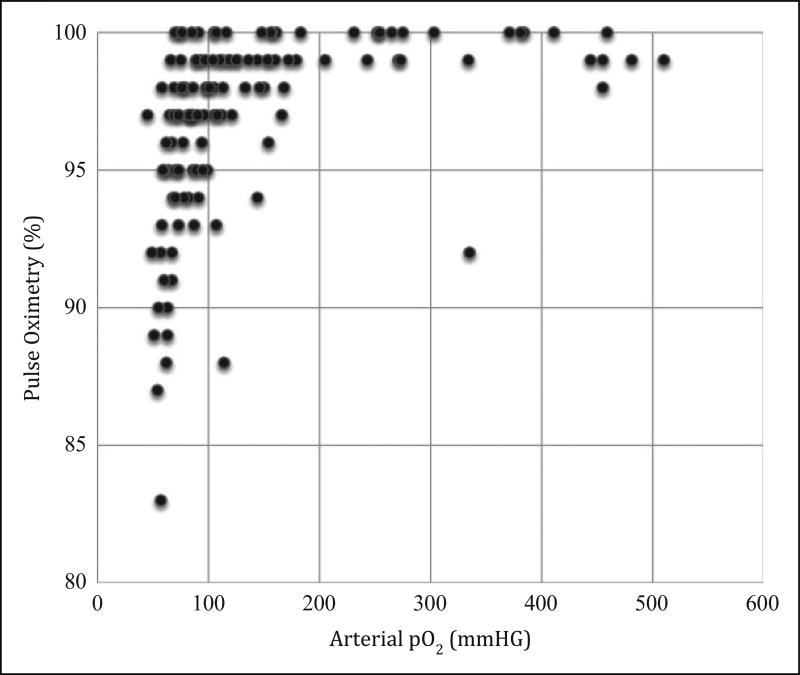
There were 156 patients enrolled and 129 patients completed the study (see Figure 1). The study patients were diverse as evidenced by their demographics, vital signs, and location in the hospital at the time of enrollment (see Table 1). There was excellent correlation between venous pH and arterial pH (see Figure 2) and venous pCO2 and arterial pCO2 (see Figure 3). SpO2 correlated well with arterial pO2 as predicted by the standard oxygen–hemoglobin dissociation curve (see Figure 4). In our cohort, an SpO2 of ≥90% correlated with a PaO2 of ≥60 mm Hg 94.6% of the time. Of the patients who did not correlate, 7 patients had SpO2 ≥90 with a PaO2 <60 (see Table 2). The mean difference for pH between VBG and ABG was 0.03 (95% confidence interval: 0.03–0.04) with a Pearson correlation of 0.94. The mean difference for pCO2 between VBG and ABG was 4.8 mm Hg (95% confidence interval: 3.7–6.0 mm Hg), with a Pearson correlation of 0.93. Subgroup analysis was performed on patients based on location, central versus peripheral VBG, primary working diagnosis, and patients in shock. There were no significant differences noted in any of the subgroups (see Table 3).
Figure 1.
Patient flow.
Table 1.
Patient Characteristics for All 129 Patients.
| Demographics | |
| Age, mean (range), years | 63.0 (21–91) |
| Male, n (%) | 70 (54) |
| Female, n (%) | 59 (46) |
| Location | |
| Emergency department, n (%) | 51 (41) |
| Medical ICU, n (%) | 43 (32) |
| Surgical ICU, n (%) | 35 (27) |
| Clinical Variables | |
| Mechanical ventilation, n (%) | 95 (74) |
| Vasopressors, n (%) | 21 (16) |
| VBG location | |
| VBG central, n (%) | 72 (56) |
| VBG peripheral, n (%) | 57 (44) |
| Vital signs | |
| HR, mean (range), beats per minute | 91.9 (46–143) |
| RR, mean (range), breaths per minute | 23.0 (8–51) |
| Temperature, mean (range), °C | 36.9 (32.6–40.0) |
| SpO2, mean (range), % | 96.9 (83–100) |
| MAP, mean (range), mm Hg | 78.1 (23–154) |
Abbreviations: HR, heart rate; ICU, intensive care unit; MAP, mean arterial pressure; RR respiratory rate; SpO2, pulse oximetry; VBG, venous blood gas.
Figure 2.
Venous blood gas (VBG)–arterial blood gas (ABG) pH (Bland-Altman plot).
Figure 3.
Venous blood gas (VBG)–arterial blood gas (ABG) CO2 (Bland-Altman plot).
Figure 4.
Pulse Oximetry-paO2 (scatterplot).
Table 2.
Relevant Characteristic of the 7 Patients With SpO2 ≥90% and paO2 <60 mm Hg.
| Arterial pH | Temperature, °C | paCO2, mm Hg | SpO2, % | paO2, mm Hg | Hemoglobin, g/dL |
|---|---|---|---|---|---|
| 7.52 | 37.0 | 49.0 | 98 | 58 | 6.7 |
| 7.46 | 36.7 | 55.9 | 92 | 49 | 10.7 |
| 7.46 | 37.0 | 49.8 | 93 | 58 | 8.5 |
| 7.44 | 37.2 | 39.0 | 92 | 57 | 8.6 |
| 7.44 | 37.7 | 46.7 | 95 | 59 | 11.6 |
| 7.40 | 36.4 | 35.1 | 90 | 55 | 15.2 |
| 7.25 | 36.5 | 46.3 | 97 | 45 | 13.5 |
Abbreviations: paCO2, the partial pressure of carbon dioxide in arterial blood; paO2, the partial pressure of oxygen in arterial blood; SpO2, pulse oximetry.
Table 3.
Means, Standard Deviations (SDs), Mean Difference (95% Confidence Interval [CI]), and Correlation of pH and pCO2 Comparing Venous and Arterial Blood Samples by Selected Subgroup Categories for 129 Study Participants.
| Group (N) | Variable | Arterial Mean (SD) |
Venous Mean (SD) |
Mean Difference (Arterial – Venous)a |
95% CI | Correlation Coefficientb |
|---|---|---|---|---|---|---|
| All patients (129) | pH | 7.35 (0.12) | 7.32 (0.11) | 0.03 | 0.03 to 0.04 | 0.94 |
| pCO2 | 46.8 (17.3) | 51.6 (17.1) | −4.8 | −3.7 to −6.0 | 0.93 | |
| Central VBG (72) | pH | 7.35 (0.11) | 7.32 (0.10) | 0.03 | 0.02 to 0.04 | 0.96 |
| pCO2 | 42.3 (11.6) | 47.5 (11.7) | −5.2 | −3.7 to −6.7 | 0.85 | |
| Peripheral VBG (57) | pH | 7.36 (0.13) | 7.31 (0.12) | 0.05 | 0.03 to 0.06 | 0.92 |
| pCO2 | 52.4 (21.3) | 56.9 (21.2) | −4.5 | −2.7 to −6.3 | 0.95 | |
| Shock (37) | pH | 7.30 (0.12) | 7.27 (0.11) | 0.03 | 0.02 to 0.05 | 0.92 |
| pCO2 | 43.7 (16.7) | 49.7 (16.5) | −6.0 | − 3.6 to −8.3 | 0.91 | |
| Not shock (91) | pH | 7.4 (0.11) | 7.36 (0.10) | 0.04 | 0.03 to 0.05 | 0.95 |
| pCO2 | 48.1 (17.5) | 52.5 (17.5) | −4.4 | −3.0 to −5.7 | 0.93 | |
| Emergency department (53) | pH | 7.33 (0.14) | 7.29 (0.13) | 0.04 | 0.03 to 0.06 | 0.93 |
| pCO2 | 51.3 (22.3) | 57.4 (22.1) | −6.1 | −3.8 to −8.3 | 0.93 | |
| Medical intensive care unit (41) | pH | 7.38 (0.11) | 7.35 (0.11) | 0.03 | 0.02 to 0.05 | 0.95 |
| pCO2 | 44.6 (11.4) | 49.2 (10.8) | −4.6 | −2.9 to −6.2 | 0.89 | |
| Surgical intensive care unit (35) | pH | 7.36 (0.08) | 7.33 (0.08) | 0.03 | 0.02 to 0.04 | 0.96 |
| pCO2 | 42.3 (12.2) | 45.8 (11.5) | −3.5 | −1.9 to −5.1 | 0.93 | |
| COPD/asthma (29) | pH | 7.34 (0.10) | 7.32 (0.10) | 0.02 | 0.04 to 0.04 | 0.90 |
| pCO2 | 60.1 (19.9) | 64.4 (21.7) | −4.3 | −1.1 to −7.4 | 0.92 | |
| CHF (14) | pH | 7.40 (0.14) | 7.35 (0.15) | 0.05 | 0.02 to 0.08 | 0.95 |
| pCO2 | 44.7 (12.3) | 52.9 (13.3) | −7.9 | −3.8 to −12.7 | 0.82 | |
| Pneumonia (39) | pH | 7.40 (1.0) | 7.37 (0.09) | 0.03 | 0.02 to 0.04 | 0.96 |
| pCO2 | 46.3 (10.8) | 49.9 (11.5) | −3.6 | −1.8 to −5.3 | 0.89 | |
| Sepsis nonpulmonary (29) | pH | 7.33 (0.13) | 7.29 (0.12) | 0.04 | 0.02 to 0.05 | 0.96 |
| pCO2 | 41.5 (14.1) | 47.7 (12.9) | −6.2 | −3.3 to −9.1 | 0.85 | |
| Neurologic (15) | pH | 7.40 (0.06) | 7.36 (0.06) | 0.04 | 0.02 to 0.06 | 0.83 |
| pCO2 | 43.5 (8.4) | 46.5 (8.0) | −3.0 | −0.4 to −5.7 | 0.83 | |
| ARDS (17) | pH | 7.37 (0.07) | 7.34 (0.07) | 0.03 | 0.01 to 0.04 | 0.96 |
| pCO2 | 42.7 (9.6) | 45.5 (9.4) | −2.8 | −1.3 to −4.3 | 0.95 | |
| Trauma (14) | pH | 7.36 (0.09) | 7.33 (0.08) | 0.03 | 0.02 to 0.04 | 0.99 |
| pCO2 | 40.5 (11.1) | 44.4 (11.8) | −3.9 | −0.9 to −6.8 | 0.90 |
Abbreviations: ARDS acute respiratory distress syndrome; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; VBG, venous blood gas.
All P values for the mean differences <.05.
All P values for the correlation coefficients <.05.
Discussion
Blood gas analysis remains an important tool for evaluation of acid–base and ventilation status of critically ill patients. The results of this study add to a growing body of evidence that supports the use of VBG instead of ABG for determination of pH and pCO2 in these patients. Three studies and 1 meta-analysis have examined VBG values specifically in COPD and have showed good correlation with ABG values.6–8,14 Many studies have evaluated arterial and venous blood gas values in the setting of DKA.1–3,15 Several other studies have also examined the correlation between arterial and venous pH and pO2 in a variety of disease states but in isolated locations such as the ED or medical or surgical ICUs.9–12 This has led to possible limitations where the focus is on one disease state or a specific location, reflecting a more acute process (the ED patient) or subacute or chronic process (the ICU patient). Our study is unique in that, to our knowledge, it is the first to enroll a diverse group of undifferentiated critically ill patients in both medical and surgical ICUs as well as the ED. Often, the underlying pathophysiologic state is not known for certain at the time of blood gas analysis, and multiple disease processes may occur simultaneously. By simplifying the enrollment criteria to a critically ill patient in whom the treating physician ordered an ABG, we captured a wide variety of patients and could not identify a subgroup in which the VBG failed to correlate with the ABG and pulse oximetry. We believe that this study design most realistically replicates real-world clinical practice.
Many other studies that have evaluated the correlation between pulse oximetry and arterial blood gas values have compared SpO2 and SaO2 (saturation of oxygen in hemoglobin as measured from an arterial puncture sample).16–18 We chose to compare SpO2 and PaO2, evaluating the common clinical dictum that when SpO2 is ≥90, PaO2 is ≥60. We also chose to use this comparison because we believe the PaO2 is more clinically relevant.
In this study, SpO2 correlated with PaO2 as predicted by the standard oxygen–hemoglobin dissociation curve. We would still caution that this assumption is only accurate when the typical oxygen–hemoglobin dissociation is present. Clinical factors that may alter this include arterial pH, paCO2, and temperature. A left shift in the oxygen–hemoglobin dissociation curve causes hemoglobin to have a higher affinity for oxygen and more reluctance to unload oxygen in the capillary beds. This could lead to situations where the actual PaO2 is lower than predicted from the pulse oximetry value. Of the 7 patients with SpO2 ≥90% and a PaO2 <60 mm Hg, there were no patients with significant hypothermia or profound hypercarbia. However, 5 of the 7 patients were alkalemic (see Table 3). This shift in the standard oxygen–hemoglobin dissociation curve may be clinically significant and should be considered when interpreting SpO2 in the setting of alkalemia.
Of course pH, pCO2, and temperature can also shift the oxygen dissociation curve to the right, which would lead to a situation where the PaO2 is higher than that predicted by the pulse oximetry value. Another factor that has been described that can cause falsely lower SpO2 values is high venous pressure states. In this scenario, venous pulsations can theoretically be interpreted by the pulse oximeter as arterial flow, which would lead to a markedly low pulse oximeter reading.19 It does not appear that we had any patients in our cohort where this occurred as there were no instances where the SpO2 was markedly low with normal or near normal PaO2 levels.
Anemia has been evaluated and shown to affect pulse oximetry readings. However, the significance of this is not fully known.17–20 It is likely that this is not relevant except at extremes.19,21 The hemoglobin values for our 7 patients with SpO2 ≥90% and PaO2 <60 mm Hg ranged from 6.7 to 13.5 (see Table 3). There were no other patients who had falsely reassuring SpO2 values, so we do not feel anemia played a role in our clinical findings.
Dyshemoglobinemia can cause discrepancies between pulse oximetry and PaO2. With significant carbon monoxide poisoning or methemoglobinemia, pulse oximetry readings would be higher than expected or in a normal range, despite a true low PaO2 value.19 We did not have any patients in our cohort with a known working diagnosis of carbon monoxide poisoning or methemoglobinemia.
It is important to note that the method of using venous blood gas values and pulse oximetry as a surrogate for arterial blood gas values will not identify potentially clinically relevant hyperoxia. Hemoglobin is fully saturated, in other words, SpO2 equals 100% when the PaO2 is approximately 120 mm Hg.19 This means, any higher PaO2 level will go unnoticed by pulse oximetry. More recently, hyperoxia has been associated with increased mortality, particularly in postcardiac arrest patients, although this has been challenged in other studies.22–24 If using the combination of VBG and pulse oximetry without a known PaO2, the possibility of hyperoxia would still need to be considered.
We had hypothesized that the poor perfusion associated with shock states could lead to a weaker correlation between VBGs and ABGs. However, the correlation was just as strong in the rest of the patients as in this cohort. Other studies comparing arterial and venous blood gas analysis in shock states have been mixed.25–28 There is a large body of research evaluating arterial to venous CO2 difference in shock as a marker for inadequate tissue perfusion. An arbitrary number of greater than 6 mm Hg difference between arterial and venous CO2 measurements has often been designated as elevated and potentially a marker of ongoing tissue ischemia.27–30 In cases where there is significant discrepancy, it is likely that the more clinically relevant CO2 and pH comes from the venous system rather than the arterial. This becomes even more apparent when evaluating arterial and venous blood gas analysis during cardiopulmonary resuscitation where much wider discrepancies are seen, sometimes with normal pH or only mild arterial acidemia.27 In the shock subgroup, the mean CO2 difference was −6 mm Hg with a pH difference of 0.03. In terms of pulmonary gas exchange and acid–base status, we do not feel these identified differences in CO2 and pH are clinically relevant.
Another important finding in the subgroups addresses the accuracy of central versus peripheral VBGs compared to ABGs. The best way to answer this question would be to obtain a peripheral VBG, a central VBG, and an ABG in the same patient at the same time as was performed in a prior study.31 Their findings showed very good correlation between all 3 groups. In our study, only 1 VBG was analyzed per patient, either peripheral or central. However, when the peripheral VBGs were compared to ABGs and the central VBGs were compared to ABGs, there was no statistical difference in the correlation. This result seems to be consistent with others’ findings.31
Limitations
This study has limitations that should be considered when interpreting the results. The patients were enrolled as a convenience sample based on when resources were available. The vast majority of patients were enrolled during daytime hours on weekdays. However, the patients still comprised a diverse group with a wide range of disease processes. Therefore, this is unlikely to have resulted in any bias. In regard to subgroup analysis based on diagnosis, we did not find any specific diagnosis where venous blood gas results failed to correlate with arterial analysis. However, this analysis was quite limited given the significant overlap between diagnoses. We had many cases where one patient had multiple diagnoses making it very difficult to characterize various subgroups. Additionally, these subgroups were defined as the “working diagnosis” at the time of enrolling the patient and not the final diagnosis. Further limitations include our analysis of the peripheral versus central blood gases and the shock versus nonshock patients. Both these subgroups were not specified in advance and therefore may be more susceptible to confounding bias.
Conclusion
In this population of undifferentiated critically ill patients, pH and pCO2 on VBG analysis correlated with pH and pCO2 on ABG analysis. The SpO2 correlated well with pO2 on ABG analysis. When compared to ABG, the combination of VBG analysis plus SpO2 provided accurate information on which to make bedside clinical decisions regarding acid–base, ventilation, and oxygenation status for undifferentiated critically ill patients in the ED and ICU.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by an internal grant from Christiana Care Health System.
Footnotes
Author Contributions
Eli Zeserson, Ben Goodgame, Michael Breyer, Vinay Maheshwari, and J. Daniel Hess contributed to conception and design. Mia Papas and James Reed contributed to analysis and interpretation. Cynthia Hoon, Keith Lamb, Ben Goodgame, and Eli Zeserson contributed to data gathering. Eli Zeserson, Ben Goodgame, J. Daniel Hess, Kristine Schultz, Vinay Maheshwari, Steven Johnson, and Michael Breyer drafted the manuscript for important intellectual content.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Brandenburg MA, Dire DJ. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med. 1998;31(4):459–652. doi: 10.1016/s0196-0644(98)70254-9. [DOI] [PubMed] [Google Scholar]
- 2.Kelly AM. The case for venous rather than arterial blood gases in diabetic ketoacidosis. Emerg Med Australas. 2006;18(1):64–67. doi: 10.1111/j.1742-6723.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 3.Gokel Y, Paydas S, Koseoglu Z, Alparslan N, Seydaoglu G. Comparison of blood gas and acid-base measurements in arterial and venous blood samples in patients with uremic acidosis and diabetic ketoacidosis in the emergency room. Am J Nephrol. 2000;20(4):319–323. doi: 10.1159/000013607. [DOI] [PubMed] [Google Scholar]
- 4.Schmelzer TM, Perron AD, Thomason MH, Sing RF. A comparison of central venous and arterial base deficit as a predictor of survival in acute trauma. Am J Emerg Med. 2008;26(2):119–123. doi: 10.1016/j.ajem.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Malinoski DJ, Todd SR, Slone S, Mullins RJ, Schreiber MA. Correlation of central venous and arterial blood gas measurements in mechanically ventilated trauma patients. Arch Surg. 2005;140(11):1122–1125. doi: 10.1001/archsurg.140.11.1122. [DOI] [PubMed] [Google Scholar]
- 6.Ak A, Ogun CO, Bayir A, Kayis SA, Koylu R. Prediction of arterial blood gas values from venous blood gas values in patients with acute exacerbation of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2006;210(4):285–290. doi: 10.1620/tjem.210.285. [DOI] [PubMed] [Google Scholar]
- 7.Razi E, Moosavi GA. Comparison of arterial and venous blood gases analysis in patients with exacerbation of chronic obstructive pulmonary disease. Saudi Med J. 2007;28(6):862–865. [PubMed] [Google Scholar]
- 8.Kelly AM, Kerr D, Middleton P. Validation of venous pCO2 to screen for arterial hypercarbia in patients with chronic obstructive airways disease. J Emerg Med. 2005;28(4):377–379. doi: 10.1016/j.jemermed.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Kelly AM, Kyle E, McAlpine R. Venous pCO2 and pH can be used to screen for significant hypercarbia in emergency patients with acute respiratory disease. J Emerg Med. 2002;22(1):15–19. doi: 10.1016/s0736-4679(01)00431-0. [DOI] [PubMed] [Google Scholar]
- 10.Rang LC, Murray HE, Wells GA, Macgougan CK. Can peripheral venous blood gases replace arterial blood gases in emergency department patients? CJEM. 2002;4(1):7–15. doi: 10.1017/s1481803500006011. [DOI] [PubMed] [Google Scholar]
- 11.Malatesha G, Singh NK, Bharija A, Rehani B, Goel A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24(8):569–571. doi: 10.1136/emj.2007.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly AM, McAlpine R, Kyle E. Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J. 2001;18(5):340–342. doi: 10.1136/emj.18.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toftegaard M, Rees SE, Andreassen S. Evaluation of a method for converting venous values of acid-base and oxygenation status to arterial values. Emerg Med J. 2009;26(4):268–272. doi: 10.1136/emj.2007.052571. [DOI] [PubMed] [Google Scholar]
- 14.Lim BL, Kelly AM. A meta-analysis on the utility of peripheral venous blood gas analyses in exacerbations of chronic obstructive pulmonary disease in the emergency department. Eur J Emerg Med. 2010;17(5):246–248. doi: 10.1097/MEJ.0b013e328335622a. [DOI] [PubMed] [Google Scholar]
- 15.Hale PJ, Nattrass M. A comparison of arterial and non-arterialized capillary blood gases in diabetic ketoacidosis. Diabet Med. 1988;5(1):76–78. doi: 10.1111/j.1464-5491.1988.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 16.Hinkelbein J, Genzwuerker HV, Fiedler F. Detection of a systolic pressure threshold for reliable readings in pulse oximetry. Resuscitation. 2005;64(3):315–319. doi: 10.1016/j.resuscitation.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Van de Louw A, Cracco C, Cerf C, Harf A, Duvaldestin P, Lemaire F, Brochard L. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med. 2001;27(10):1606–1613. doi: 10.1007/s001340101064. [DOI] [PubMed] [Google Scholar]
- 18.Perkins GD, McAuley DF, Giles S, Routledge H, Gao F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care. 2003;7(4):r67. doi: 10.1186/cc2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnapp LM, Cohen NH. Pulse oximetry uses and abuses. Chest. 1990;98(5):1244–1250. doi: 10.1378/chest.98.5.1244. [DOI] [PubMed] [Google Scholar]
- 20.Severinghaus JW, Kelleher JF. Recent developments in pulse oximetry. Anesthesiology. 1992;76(6):1018–1038. doi: 10.1097/00000542-199206000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Tremper KK, Barker SJ. Effects of anemia on pulse oximetry and continuous mixed venous hemoglobin saturation monitoring in dogs. Anesthesiology. 1991;75(1):118–122. doi: 10.1097/00000542-199107000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Kilgannon JH, Jones AE, Shapiro NI, et al. Emergency Medicine Shock Research Network (EMShockNet) Investigators. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 23.Bellomo R, Bailey M, Eastwood GM, et al. Study of Oxygen in Critical Care (SOCC) Group. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):r90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ihle JF, Bernard S, Bailey MJ, Pilcher DV, Smith K, Scheinkestel CD. Hyperoxia in the intensive care unit and outcome after out-of-hospital ventricular fibrillation cardiac arrest. Crit Care Resusc. 2013;15(3):186–190. [PubMed] [Google Scholar]
- 25.Williams KB, Christmas AB, Heniford BT, Sing RF, Messick J. Arterial vs venous blood gas differences during hemorrhagic shock. World J Crit Care Med. 2014;3(2):55–60. doi: 10.5492/wjccm.v3.i2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirani F, Salehi R, Naini AE, Azizkhani R, Gholamrezaei A. The effects of hypotension on differences between the results of simultaneous venous and arterial blood gas analysis. J Res Med Sci. 2011;16(2):188–194. [PMC free article] [PubMed] [Google Scholar]
- 27.Adrogué HJ, Rashad MN, Gorin AB, Yacoub J, Madias NE. Assessing acid-base status in circulatory failure. Differences between arterial and central venous blood. N Engl J Med. 1989;320(20):1312–1316. doi: 10.1056/NEJM198905183202004. [DOI] [PubMed] [Google Scholar]
- 28.Vallée F, Vallet B, Mathe O, et al. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Med. 2008;34(12):2218–2225. doi: 10.1007/s00134-008-1199-0. [DOI] [PubMed] [Google Scholar]
- 29.Mecher CE, Rackow EC, Astiz ME, Weil MH. Venous hypercarbia associated with severe sepsis and systemic hypoperfusion. Crit Care Med. 1990;18(6):585–589. doi: 10.1097/00003246-199006000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Bakker J, Vincent JL, Gris P, Leon M, Coffernils M, Kahn RJ. Veno-arterial carbon dioxide gradient in human septic shock. Chest. 1992;101(2):509–515. doi: 10.1378/chest.101.2.509. [DOI] [PubMed] [Google Scholar]
- 31.Treger R, Pirouz S, Kamangar N, Corry D. Agreement between central venous and arterial blood gas measurements in the intensive care unit. Clin J Am Soc Nephrol. 2010;5(3):390–394. doi: 10.2215/CJN.00330109. [DOI] [PMC free article] [PubMed] [Google Scholar]