Abstract
The human spindle and kinetochore associated (Ska) complex is required for proper mitotic progression. Extensive studies have demonstrated its important functions in both stable kinetochore-microtubule interactions and spindle checkpoint silencing. We suggest a model to explain how various Ska functions might be fulfilled by distinct pools of Ska at kinetochores. The Ndc80-loop pool of Ska is recruited by the Ndc80 loop, or together with some of its flanking sequences, and the recruitment is also dependent on Cdk1-mediated Ska3 phosphorylation. This pool seems to play a more important role in silencing the spindle checkpoint than stabilizing kinetochore-microtubule interactions. In contrast, the Ndc80-N-terminus pool of Ska is recruited by the N-terminal domains of Ndc80 and appears to be more important for stabilizing kinetochore-microtubule interactions. Here, we review and discuss the evidence that supports this model and suggest further experiments to test the functioning mechanisms of the Ska complex.
Keywords: kinetochore, mitosis, microtubule, Ndc80, Ska, spindle checkpoint
1. Introduction
Aneuploidy, usually arising from chromosome missegregation during mitosis, is known to drive the development of many diseases, including cancer under some contexts.[1] One of the major causes of chromosome missegregation is improper kinetochore-microtubule (KT-MT) interactions.[2] Therefore, a better understanding of the molecular mechanisms controlling proper kinetochore-microtubule interactions will help decipher the causes of aneuploidy and tumorigenesis and also benefit for developing the more efficacious anti-cancer therapy.
The kinetochore, a large protein complex that is established on the centromere region of each chromatid, is responsible for microtubule attachments. At early mitosis, the kinetochore is initially captured by the lattice of spindle microtubules (side-on attachments).[3] These initial kinetochore-microtubule attachments are stochastic, unstable, and error-prone. They must be converted to more stable end-on attachments—kinetochores captured by the ends of spindle microtubules—to ensure proper chromosome segregation. In this process, a surveillance system, the spindle checkpoint, is activated to prevent premature mitotic exit, thus allowing cells to establish proper kinetochore-microtubule attachments before sister chromatid segregation.[4] Once all sister chromatids achieve end-on attachments by microtubules emanating from the opposite spindle poles, the spindle checkpoint will be silenced and sister chromatids will segregate from each other to generate two daughter cells with the equal number of chromosomes.[4] The KMN (Knl1, the Mis12, and Ndc80 complexes) network at outer kinetochores constitutes the microtubule-binding sites and is essential for kinetochore-microtubule attachments, but it on its own is not sufficient to establish and/or maintain stable end-on kinetochore-microtubule attachments that support the final chromosome segregation.[5–7] As well required are other factors, among which is the Ska complex, which comprises three subunits, Ska1, 2 and 3, and localizes at both kinetochores and microtubules (Figure 1).[8] Its functional ortholog in yeast is the Dam1 complex although they share no similarity in amino-acid sequences.[8] Extensive studies have revealed at least two major functions of this complete Ska complex in chromosome segregation during mitosis—establishment and/or maintenance of stable kinetochore-microtubule interactions (end-on) and spindle checkpoint silencing.[9–15] Here, we review the recent evidence that sheds light on the molecular mechanisms underlying these Ska functions. Based on the available evidence, we also propose a model to explain how the Ska complex functions to promote stable end-on kinetochore-microtubule interactions and spindle checkpoint silencing.
Figure 1.
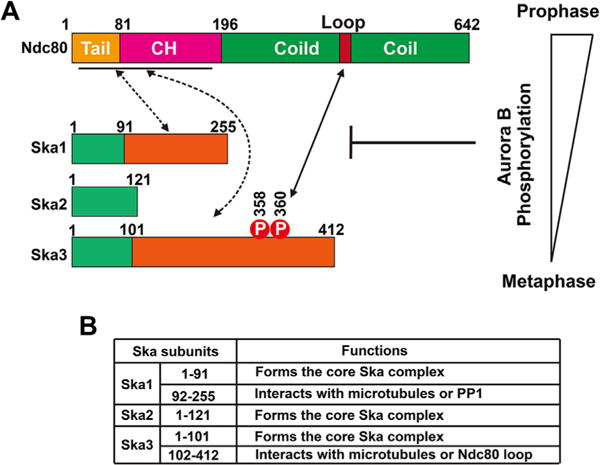
Mapping and regulation of the Ndc80-Ska interactions (A). Schematic drawing of the domains of Ska subunits and Ndc80. The solid line with arrows shows the verified interactions. The dashed lines with arrows show the speculative interactions. The Ska-Ndc80 interactions, including both the verified and speculative ones, are subject to Aurora B regulation in a cell cycle-dependent manner. At prophase/prometaphase, the Ska complex and the Ndc80 complex are spatially close to Aurora B and thereby readily phosphorylated. Aurora B phosphorylations of Ska and Ndc80 might inhibit the Ndc80-Ska interactions, thus preventing Ska kinetochore accumulation. At metaphase, the Ska and Ndc80 complexes are dephosphorylated, leading to a full Ska accumulation at kinetochores. B) Summary of the functions of Ska domains.
2. What Structurally Determines Ska Localization at Microtubules?
The crystal structure work showed that N-terminal domains of Ska1 and Ska3, and Ska2 form trimers of coiled coils, two copies of which assemble into a W-shaped structure with the C-terminal domains of Ska1 and Ska3 protruding from each end.[16] These two C-terminal domains might mediate Ska association with kinetochores and microtubules (Figure 1). In vitro microtubule pelleting assays demonstrated that the C-terminal domains of Ska1 and Ska3 directly bind microtubules.[16,17] Truncation of either or both of these two C-terminal domains significantly compromised chromosome alignment in cells.[16,17] Thus, the Ska-microtubule interactions are essential for proper functions of the Ska complex. The crystal structure of the Ska1 C-terminal domain demonstrated that it forms into a winged-helix-like structure, which usually mediates interactions with DNA or proteins.[18] Further Mutagenesis analysis identified that Ska1 interacts with microtubules in a multipartite mode. The crystal structure of the Ska3 C-terminal domain has not been determined. How this domain helps Ska bind to microtubules is not quite understood. Recent biochemical studies suggested that the C-terminal domain of Ska3 may regulate the microtubule-binding capability of the Ska complex by directly interacting with tubulin monomers and/or indirectly by interacting with tubulin contacting regions of Ska1.[17] Taken all together, the two C-terminal domains of Ska1 and Ska3 collaboratively bind microtubules to mediate the Ska-microtubule interactions, which is essential for Ska functions.
The microtubule localization of the Ska complex is also regulated by other microtubule-binding proteins. siRNA-mediated knockdown of EB1, an important microtubule plus-end tracking proteins (+TIPs), significantly reduced the localizations of Ska3 at both microtubules and kinetochores.[19] It was proposed that EB1 stimulates Ska1 recruitment onto microtubules by forming a complex with Ska1 and by imparting stabilization onto MT, but it is also possible that the spindle structure defects caused by EB1 knockdown might contribute to the decreased Ska localization at spindle microtubules. Regardless, as a microtubule-binding factor, the Ska complex may collaborate with other microtubule-binding proteins to regulate spindle microtubule functions and chromosome segregation.
3. How is Ska Targeted to Kinetochores?
The Ska complex localizes at kinetochores during mitosis, and the kinetochore localization depends on the Ndc80 complex.[12,14,15,20,21] However, how it recruits the Ska complex to the kinetochore is not quite understood. In yeast, it has been shown that the Dam1 and Ndc80 complexes directly bind to each other.[22] Recently, we found that the C-terminal domain of Ska3 mediates the direct Ska-Ndc80 binding and Ska recruitment to the kinetochore.[23] Interestingly, the Ska-Ndc80 binding is dependent on the Cdk1 phosphorylation of Thr358 and Thr360 in the C-terminal domain of Ska3. The non-phosphorylatable Ska3 mutants remained their association with spindle microtubules although they were poorly targeted to kinetochores, suggesting that Cdk1-mediated Ska3 phosphorylation at Thr358 and Thr350 is dispensable for Ska microtubule localization. Thus, these separation-of-function mutants are important and useful for dissecting the distinct functions of the Ska complex in cells.
Our results further demonstrated that the Ndc80 loop, together with some of its flanking sequences, is required for its direct binding to the C-terminal domain of Ska3.[23] These biochemical data are congruent with the finding that the Ndc80 loop is required for Ska kinetochore localization in cells.[21,23] Thus, in addition to conferring Ndc80 structural flexibility, the Ndc80 loop may also provide a platform to directly recruit regulatory factors important for proper chromosome segregation.[24–27] Although the crystal structures of modified versions of Ndc80 complexes (Bonsai and dwarf) and the core Ska complexes have been solved,[16,28,29] a large portion of these two complexes, including the interacting domains, are unfortunately not included in the solved structures. Therefore, it is still unclear how the Ska and Ndc80 complexes interact with one another at the atomic level and how these two complexes are organized in three dimensions. In spite of these findings, Ska kinetochore recruitment seems more complicated because of two recent studies suggesting that the Ndc80 N-terminus also plays a role in recruiting Ska to kinetochores.[30,31] Janczyk et al. isolated an Ndc80 tail mutant that retained a robust microtubule-binding capacity, but was deficient in clustering along microtubule protofilaments.[30] As a result, this mutant recruited less Ska to the kinetochore. They proposed that clusters of Ndc80 proteins recruit Ska complexes to kinetochores. In the other study, Work from the Desai group found the SKA-1 localization at kinetochores in C. Elegan was dependent on the NDC-80 CH domain Toe docking to the microtubule lattice.[31] These observations suggest that the Ndc80 tail and/or CH domain are also important for recruiting the Ska complex to kinetochores. How do the Ndc80 tail and/or CH domain recruit Ska to kinetochores? We suggest that the Ndc80 tail and/or CH domain might directly bind to either or both of the C-termini of Ska1 and Ska3. However, such interaction has not been reported yet, suggesting that this interaction might be too dynamic in cells to be detected by in vitro assays. Alternatively, such interaction might require a microtubule binding-dependent structural change of Ndc80 as suggested by Desai.[31] Taken together, both the Ndc80 loop and N-terminus can recruit the Ska complex to kinetochores. More recently, it has been proposed that kinetochore-associated phosphatases also function to promote Ska kinetochore accumulation in a positive feedback cycle.[32]
How are these Ndc80-Ska interactions coordinated with cell cycle progression? Evidence suggests that Aurora B plays an important role (Figure 1). At prophase/prometaphase when kinetochores are poorly attached and tensionless, the Ska complex and the Ndc80 complex are spatially close to Aurora B and thereby readily phosphorylated.[33,34] The Aurora B phosphorylation of the Ska complex inhibits its binding to the Ndc80 loop and reduces its accumulation at kinetochores.[20] At the same time, the Aurora B phosphorylation on Ndc80 N-terminus might also inhibit its interaction with Ska,[35] thereby further preventing Ska kinetochore recruitment as well. Thus, both types of Ska kinetochore recruitments are negatively regulated by Aurora B at early mitosis. Once kinetochores are bioriented and tension is established at metaphase, the Ska and Ndc80 complexes are dephosphorylated, leading to a full Ska accumulation at kinetochores. In addition, it is worth mentioning that the Ska complex retains at kinetochores through early anaphase until late anaphase, during which, Cdk1/Cyclin B1 activity has significantly decreased.[12,13] In such scenario, the Ndc80 N-terminus might be the major factor that retains Ska at anaphase kinetochores.
4. Ska Promotes End-on Kinetochore Attachments
Kinetochores are initially captured by the lattice of spindle microtubules (side-on).[3] The side-on attachments are unstable and must be converted to more stable end-on attachments, during which kinetochores are captured by the dynamic ends of microtubules, to sustain sister chromatid segregation. Conversion of side-on to end-on attachments will help promote proper chromosome alignment at cell equators, a process called chromosome congression. Extensive studies have revealed the important roles of the Ska complex in promoting end-on kinetochore attachments and chromosome alignment, thereby facilitating chromosome congression. Knockdown of Ska subunits seemed to have little effects on the initial kinetochore attachments by microtubules, but significantly decreased the number of the kinetochores with stable end-on attachments.[15] As a result, the stability of kinetochore-fibers is significantly decreased and chromosome alignment is delayed.[15] These findings suggest that the Ska complex is important for establishing and/or maintaining end-on kinetochore-microtubule attachments. Recently, detailed analysis of kinetochore attachments using live imaging demonstrated that the Ska complex functions to prevent force-dependent detachment of kinetochores from microtubules in the process of chromosome congression, which helps to stabilize the end-on kinetochore attachments and promote proper chromosome alignment.[36]
How does the Ska complex promote end-on kinetochore attachments at the molecular level? Spindle microtubule ends undergo dynamic assembly and disassembly. Disassembly usually results in the peeling away of individual microtubule protofilaments, which assume curved structures. The peeling protofilaments can also generate force.[37] The Ska complex has been demonstrated to bind to both straight and curved microtubule structures in vitro, whereas the Ndc80 complex binds preferentially to straight ones.[14,18,38] Such biochemical property of the Ska complex might allow it to harness the force generated by the peeling protofilaments to track on the depolymerizing microtubules. This microtubule end-tracking capacity has recently been demonstrated to require diverse tubulin-interacting surfaces of Ska1.[39] Interestingly, it has also been shown that the Ska complex confers this microtubule end-tracking capacity to the Ndc80 complex in vitro.[38] This might help establish and stabilize end-on kinetochore-microtubule interactions in cells. In addition, the Ska and Ndc80 complexes can synergistically bind to microtubules. This synergy might further strengthen kinetochore-microtubule interactions. In yeast, the Dam1 complex has also been shown to be able to track on depolymerizing microtubule ends.[40–42] Thus, the Dam1 and Ska complexes have similar functions in kinetochore-microtubule attachments although they share no similarity in amino-acid sequences.[43]
5. Ska Promotes Spindle Checkpoint Silencing
Some studies demonstrated that inhibition of the Ska complex using RNA interference technology did not prevent proper chromosome alignment although it still slightly delayed this process;[9,10,13] instead, these cells were arrested at metaphase, followed by sister chromatid cohesion fatigue.[10,13] The spindle checkpoint kinase Bub1 has been detected at kinetochores in these metaphase-arrested cells, suggesting that the Ska complex might function to promote the silencing of the spindle checkpoint.[10] In contrast to these observations, other studies demonstrated that knockdown of the Ska complex resulted in severe chromosome alignment defects, which arrested cells at prometaphase by activating the spindle checkpoint.[12,14,15] The discrepancy may be explained by distinct siRNA-mediated Ska knock-down efficiency as co-depletion of two Ska subunits exhibited more severe chromosome alignment defects.[15] Therefore, to better dissect these distinct Ska functions, isolation of separation-of-function Ska mutants is needed. We recently identified such mutants (Cdk1-phosphorylation-defient) that largely support chromosome alignment, but fail to promote anaphase onset, strongly suggesting that the Ska complex might function to promote spindle checkpoint silencing.[23] Interestingly, these mutants failed to localize to kinetochores, but still retained their association with spindle microtubules. Thus, the major pool of kinetochore Ska may be more important for promoting anaphase onset, probably through silencing the spindle checkpoint, than for promoting chromosome alignment. This notion is further supported by functional analysis on the Ska3 mutant that mimics the constitutive Mps1 phosphorylation.[44] This mutant localized properly to kinetochores, but failed to promote chromosome alignment. This defect has been attributed to the altered behavior of its association with microtubules. Taken all these findings together, it is likely that distinct pools of the Ska complex fulfill its various functions in cells (Figure 2).
Figure 2.
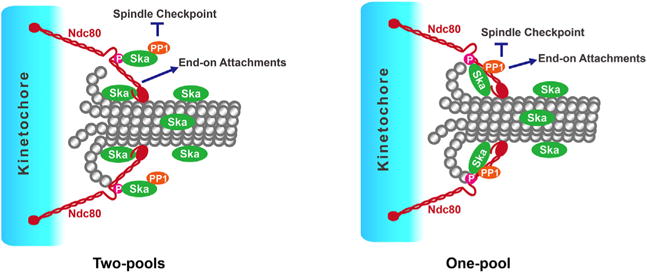
Speculative models of various Ska functions mediated by “One-pool” or “two-pools” of Ska. In “Two-pools” model, the Ndc80-loop pool of Ska is recruited by the Ndc80 loop, or together with some of its flanking sequences, and the recruitment is also dependent on Cdk1-mediated Ska3 phosphorylation. This pool seems to play a more important role in silencing the spindle checkpoint than stabilizing kinetochore-microtubule interactions. In contrast, the Ndc80-N-terminus pool of Ska is recruited by the N-terminal domains of Ndc80 and appears to be more important for stabilizing kinetochore-microtubule interactions. In “One-pool” model, the Ndc80 loop and N-terminus collaborate to recruit and maintain one major Ska pool at kinetochores. This pool might be responsible for both stabilizing kinetochore-microtubule interactions and silencing the spindle checkpoint.
How does the Ska complex promote spindle checkpoint silencing? A recent study shed light by showing that the Ska complex targets PP1 to the kinetochore.[45] It found that the very C-terminal domain of Ska1 that binds microtubules also directly interacts with PP1. Truncation of the Ska1 C-terminus resulted in significant delay in both chromosome alignment and anaphase onset. Fusion of the PP1-binding motif of Knl1 rescued both the defects. Thus, it is likely that the Ska complex targets PP1 to kinetochores to promote spindle checkpoint silencing. In addition to recruiting PP1, the Ska complex might also target APC/C to chromosomes to help checkpoint silencing.[46]
In budding yeast, the Dam1 complex, the functional ortholog of human Ska, has been demonstrated by Wang et al. to prevent premature silencing of the spindle checkpoint.[47] This function of the Dam1 complex is fulfilled through Aurora B-phosphorylation of Dam1, but how this phosphorylation regulates the spindle checkpoint function is unknown. The human Ska complex has also been shown to be an Aurora B substrate.[20] The phosphorylation appears to negatively regulate the Ska-Ndc80 interactions, thus preventing Ska accumulation at kinetochores, which might also prevent premature silencing of the spindle checkpoint. In addition to be regulated by Aurora B, the Ska complex has also been shown to regulate Aurora B activity.[48] This type of mutual regulation might fine-tune kinetochore-microtubule interactions and the spindle checkpoint function to promote proper chromosome segregation.
6. “Distinct Pools” of Ska Might Specify Its Various Functions
Based on the accumulated evidence, we propose a model to explain how the Ska complex functions to promote stable end-on kinetochore attachments and spindle checkpoint silencing. We suggest that distinct pools of Ska might exist at kinetochores to fulfill these two functions—the Ndc80 loop pool promoting spindle checkpoint silencing and the Ndc80 N-terminus pool promoting stable end-on kinetochore-microtubule interactions (Figure 2). In prophase/prometaphase when kinetochores are poorly attached by microtubules, the kinetochore-localized spindle checkpoint kinase Mps1 phosphorylates the MELT domains in Knl1 to recruit downstream effectors, thus activating the spindle checkpoint (Figure 3).[49–51] At this stage, High Aurora B phosphorylation prevents Ska accumulation at kinetochores (Figure 2). At metaphase, kinetochores are properly attached by microtubules and the kinetochore-microtubule interactions are stabilized by the Ska complex recruited by the Ndc80 N-terminus. Microtubule attachments to kinetochores also displace Mps1 from kinetochores to initiate spindle checkpoint silencing[52,53] (Figure 3). At the same time, phospho (p)-MELT domains in Knl1 are dephosphorylated by both the Ndc80-loop-based Ska-PP1 and the Knl1-based PP1.
Figure 3.
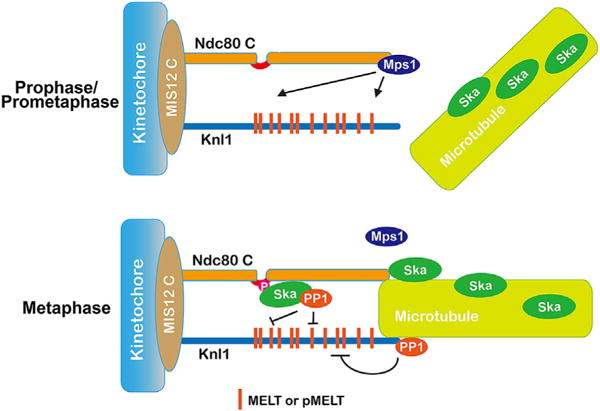
Stabilizing kinetochore-microtubule interactions and silencing the spindle checkpoint by the Ska complex. In prophase/prometaphase when kinetochores are poorly attached by microtubules, the kinetochore-localized Mps1 phosphorylates the MELT domains in Knl1 to recruit downstream effectors, thus activating the spindle checkpoint. At metaphase, microtubule attachments to kinetochores displace Mps1 from kinetochores and the kinetochore-microtubule attachments are stabilized by the Ska complex recruited by the Ndc80 N-terminus. At the same time, multiple copies of phospho (p)-MELT domains distributed along a wide range of amino-acid sequences in Knl1 are dephosphorylated by both the Ndc80-loop pool of Ska-PP1 and the Knl1-based PP1. The Ndc80-loop pool might provide extra dephosphorylating strength to more efficiently remove the phosphorylation from the phospho-MELT domains.
Considering the fact that kinetochore-microtubule interactions and the spindle checkpoint are closely intertwined in functions, the question is raised regarding why distinct Ska pools at kinetochores are needed to fulfill these intertwined functions. At early mitosis, Mps1 activates the spindle checkpoint by phosphorylating the MELT domains of Knl1.[49–51] At metaphase-anaphase transition, these kinase phosphorylations must be removed to silence the spindle checkpoint. It has been suggested that the very N-terminus of Knl1 can serve as a platform to recruit PP1 to reverse these phosphorylations.[54] Because there are many copies of MELT domains distributed along Knl1 in a wide range of amino-acid sequences (150–1200 in human),[55] it is unclear whether the pool of Knl1-based PP1—which localizes at outer kinetochores—could be sufficient for dephosphorylating all the phosphorylated MELT motifs, especially the ones that locate to the more inner side of kinetochores. Therefore, we speculate that the Ska-based PP1 pool recruited by the Ndc80 loop might provide the extra dephosphorylating strength, together with the Knl1-based pool, to more efficiently silence the spindle checkpoint. In addition, it is also worth mentioning that these two pools of Ska are not isolated from one another and they might dynamically communicate to coordinate kinetochore-microtubule attachments with the spindle checkpoint.
Although the evidence strongly suggests that distinct pools of Ska complexes might exist at kinetochores to fulfill distinct functions, it is still possible that the Ndc80 loop and N-terminus collaborate to recruit and maintain one major Ska pool at kinetochores (Figure 2). If this was the case, the C-terminal domains of Ska1 and Ska3 protruding from one end of the coiled coils might bind the Ndc80 loop and the ones protruding from the other end might bind microtubules or Ndc80 N-terminus. In future, to isolate more separation-of-function Ska mutants and characterize their cellular and biochemical functions would be a way to distinguish these distinct models.
7. Conclusion and Outlook
How are kinetochores attached to the dynamic ends of microtubules? In the last decade, great progress has been made to address this fascinating question using a combination of diverse interdisciplinary techniques. The Ska complex, as a central regulator to this process, has been extensively studied. It is evident that Ska has at least two major functions—promoting end-on kinetochore attachments and spindle checkpoint silencing. Based on the recent evidence, we suggest that distinct Ska pools might be responsible for these functions. In future, further experiments are needed to confirm the existence of distinct pools. Structural determination of how Ska interacts with the Ndc80 complex will be an effective way, but it might be technically challenging. Alternatively, a combination of biochemical analysis of the Ska-Ndc80 interactions using recombinant complexes and functional analysis of these interactions in cells will be a more feasible way. In vitro biochemical evidence suggests that the unique property of the Ska complex—tracking with the depolymerizing microtubule ends—might be responsible for its function in end-on kinetochore-microtubule interactions, but whether this is the case in cells is unclear because these in vitro systems used in the studies lack regulators or in vivo post-translational modifications in cells. Therefore, better systems are needed. In the light of work from the Biggins’s lab,[56] it will be tempting to isolate kinetochores from human cells and use them to study how Ska helps stabilizing kinetochore-microtubule interactions. How does Ska promote spindle checkpoint silencing? The current model is that Ska targets PP1 to kinetochores to help inactivate the spindle checkpoint by reversing checkpoint kinase phosphorylations.[45] Mps1-phosphoryalted Knl1 within the MELT domains may be one of such Ska3-bound PP1 substrates.[49–51] In future, it will be important to identify more of these substrates in order to fully understand how the spindle checkpoint is silenced.
Acknowledgments
Research in the Liu laboratory is supported by grants from Tulane Startup and NIH (P20GM103629).
Footnotes
Conflict of Interest
The authors have declared no conflict of interest.
References
- 1.Santaguida S, Amon A. Nat Rev Mol Cell Biol. 2015;16:473. doi: 10.1038/nrm4025. [DOI] [PubMed] [Google Scholar]
- 2.Funk LC, Zasadil LM, Weaver BA. Dev Cell. 2016;39:638. doi: 10.1016/j.devcel.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheerambathur DK, Desai A. Curr Opin Cell Biol. 2014;26:113. doi: 10.1016/j.ceb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S, Yu H. Semin Cell Dev Biol. 2011;22:551. doi: 10.1016/j.semcdb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varma D, Salmon ED. J Cell Sci. 2012;125:5927. doi: 10.1242/jcs.093724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley EA, Kapoor TM. Nat Rev Mol Cell Biol. 2013;14:25. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, Prescott AR, Antony C, Tanaka TU. Nature. 2005;434:987. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 8.Guimaraes GJ, Deluca JG. EMBO J. 2009;28:1375. doi: 10.1038/emboj.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanisch A, Sillje HH, Nigg EA. EMBO J. 2006;25:5504. doi: 10.1038/sj.emboj.7601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daum JR, Wren JD, Daniel JJ, Sivakumar S, McAvoy JN, Potapova TA, Gorbsky GJ. Curr Biol. 2009;19:1467. doi: 10.1016/j.cub.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohta S, Bukowski-Wills JC, Wood L, de Lima Alves F, Chen Z, Rappsilber J, Earnshaw WC. Cold Spring Harb Symp Quant Biol. 2010;75:433. doi: 10.1101/sqb.2010.75.022. [DOI] [PubMed] [Google Scholar]
- 12.Raaijmakers JA, Tanenbaum ME, Maia AF, Medema RH. J Cell Sci. 2009;122:2436. doi: 10.1242/jcs.051912. [DOI] [PubMed] [Google Scholar]
- 13.Theis M, Slabicki M, Junqueira M, Paszkowski-Rogacz M, Sontheimer J, Kittler R, Heninger AK, Glatter T, Kruusmaa K, Poser I, Hyman AA, Pisabarro MT, Gstaiger M, Aebersold R, Shevchenko A, Buchholz F. EMBO J. 2009;28:1453. doi: 10.1038/emboj.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR, 3rd, Cheeseman IM. Dev Cell. 2009;16:374. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaitanos TN, Santamaria A, Jeyaprakash AA, Wang B, Conti E, Nigg EA. EMBO J. 2009;28:1442. doi: 10.1038/emboj.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeyaprakash AA, Santamaria A, Jayachandran U, Chan YW, Benda C, Nigg EA, Conti E. Mol Cell. 2012;46:274. doi: 10.1016/j.molcel.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Abad MA, Zou J, Medina-Pritchard B, Nigg EA, Rappsilber J, Santamaria A, Jeyaprakash AA. Sci Rep. 2016;6:34042. doi: 10.1038/srep34042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abad MA, Medina B, Santamaria A, Zou J, Plasberg-Hill C, Madhumalar A, Jayachandran U, Redli PM, Rappsilber J, Nigg EA, Jeyaprakash AA. Nat Commun. 2014;5:2964. doi: 10.1038/ncomms3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas GE, Bandopadhyay K, Sutradhar S, Renjith MR, Singh P, Gireesh KK, Simon S, Badarudeen B, Gupta H, Banerjee M, Paul R, Mitra J, Manna TK. Nat Commun. 2016;7:11665. doi: 10.1038/ncomms11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan YW, Jeyaprakash AA, Nigg EA, Santamaria A. J Cell Biol. 2012;196:563. doi: 10.1083/jcb.201109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang G, Kelstrup CD, Hu XW, Kaas Hansen MJ, Singleton MR, Olsen JV, Nilsson J. J Cell Sci. 2012;125:3243. doi: 10.1242/jcs.104208. [DOI] [PubMed] [Google Scholar]
- 22.Kim JO, Zelter A, Umbreit NT, Bollozos A, Riffle M, Johnson R, MacCoss MJ, Asbury CL, Davis TN. Elife. 2017;6:e21069. doi: 10.7554/eLife.21069. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Sivakumar S, Chen Y, Gao H, Yang L, Yuan Z, Yu H, Liu H. Curr Biol. 2017;27:1477. doi: 10.1016/j.cub.2017.03.060. [DOI] [PubMed] [Google Scholar]
- 24.Wang HW, Long S, Ciferri C, Westermann S, Drubin D, Barnes G, Nogales E. J Mol Biol. 2008;383:894. doi: 10.1016/j.jmb.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson J. Bioessays. 2012;34:1070. doi: 10.1002/bies.201200096. [DOI] [PubMed] [Google Scholar]
- 26.Tang NH, Toda T. Cell Div. 2013;8:2. doi: 10.1186/1747-1028-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varma D, Chandrasekaran S, Sundin LJ, Reidy KT, Wan X, Chasse DA, Nevis KR, DeLuca JG, Salmon ED, Cook JG. Nat Cell Biol. 2012;14:593. doi: 10.1038/ncb2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valverde R, Ingram J, Harrison SC. Cell Rep. 2016;17:1915. doi: 10.1016/j.celrep.2016.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, Salek M, Rappsilber J, Moores CA, Salmon ED, Musacchio A. Cell. 2008;133:427. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janczyk PL, Skorupka KA, Tooley JG, Matson DR, Kestner CA, West T, Pornillos O, Stukenberg PT. Dev Cell. 2017;41:438. doi: 10.1016/j.devcel.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheerambathur DK, Prevo B, Hattersley N, Lewellyn L, Corbett KD, Oegema K, Desai A. Dev Cell. 2017;41:424. doi: 10.1016/j.devcel.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sivakumar S, Gorbsky GJ. Biol Open. 2017;6:1672. doi: 10.1242/bio.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang E, Ballister ER, Lampson MA. J Cell Biol. 2011;194:539. doi: 10.1083/jcb.201103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Science. 2009;323:1350. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Mol Cell. 2010;38:383. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auckland P, Clarke NI, Royle SJ, McAinsh AD. J Cell Biol. 2017;216:1623. doi: 10.1083/jcb.201607096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grishchuk EL, Molodtsov MI, Ataullakhanov FI, McIntosh JR. Nature. 2005;438:384. doi: 10.1038/nature04132. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt JC, Arthanari H, Boeszoermenyi A, Dashkevich NM, Wilson-Kubalek EM, Monnier N, Markus M, Oberer M, Milligan RA, Bathe M, Wagner G, Grishchuk EL, Cheeseman IM. Dev Cell. 2012;23:968. doi: 10.1016/j.devcel.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monda JK, Whitney IP, Tarasovetc EV, Wilson-Kubalek E, Milligan RA, Grishchuk EL, Cheeseman IM. Curr Biol. 2017;27:3666. doi: 10.1016/j.cub.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westermann S, Wang HW, Avila-Sakar A, Drubin DG, Nogales E, Barnes G. Nature. 2006;440:565. doi: 10.1038/nature04409. [DOI] [PubMed] [Google Scholar]
- 41.Lampert F, Hornung P, Westermann S. J Cell Biol. 2010;189:641. doi: 10.1083/jcb.200912021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tien JF, Umbreit NT, Gestaut DR, Franck AD, Cooper J, Wordeman L, Gonen T, Asbury CL, Davis TN. J Cell Biol. 2010;189:713. doi: 10.1083/jcb.200910142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Hooff JJE, Snel B, Kops G. Genome Biol Evol. 2017;9:1295. doi: 10.1093/gbe/evx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maciejowski J, Drechsler H, Grundner-Culemann K, Ballister ER, Rodriguez-Rodriguez JA, Rodriguez-Bravo V, Jones MJK, Foley E, Lampson MA, Daub H, McAinsh AD, Jallepalli PV. Dev Cell. 2017;41:143. doi: 10.1016/j.devcel.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivakumar S, Janczyk PL, Qu Q, Brautigam CA, Stukenberg PT, Yu H, Gorbsky GJ. Elife. 2016;5:e12902. doi: 10.7554/eLife.12902. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivakumar S, Daum JR, Tipton AR, Rankin S, Gorbsky GJ. Mol Biol Cell. 2014;25:594. doi: 10.1091/mbc.E13-07-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin F, Wang Y. Proc Natl Acad Sci U S A. 2013;110:21036. doi: 10.1073/pnas.1307595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redli PM, Gasic I, Meraldi P, Nigg EA, Santamaria A. J Cell Biol. 2016;215:77. doi: 10.1083/jcb.201603019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. Nat Cell Biol. 2012;14:746. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- 50.Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB. Curr Biol. 2012;22:891. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.London N, Ceto S, Ranish JA, Biggins S. Curr Biol. 2012;22:900. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji Z, Gao H, Yu H. Science. 2015;348:1260. doi: 10.1126/science.aaa4029. [DOI] [PubMed] [Google Scholar]
- 53.Hiruma Y, Sacristan C, Pachis ST, Adamopoulos A, Kuijt T, Ubbink M, von Castelmur E, Perrakis A, Kops GJ. Science. 2015;348:1264. doi: 10.1126/science.aaa4055. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberg JS, Cross FR, Funabiki H. Curr Biol. 2011;21:942. doi: 10.1016/j.cub.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang G, Lischetti T, Nilsson J. J Cell Sci. 2014;127:871. doi: 10.1242/jcs.139725. [DOI] [PubMed] [Google Scholar]
- 56.Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, Biggins S. Nature. 2010;468:576. doi: 10.1038/nature09594. [DOI] [PMC free article] [PubMed] [Google Scholar]


