Abstract
Production of trimethylamine-N-oxide (TMAO), a biomarker of CVD risk, is dependent on intestinal microbiota, but little is known of dietary conditions promoting changes in gut microbial communities. Resistant starches (RS) alter the human microbiota. We sought to determine whether diets varying in RS and carbohydrate (CHO) content affect plasma TMAO levels. We also assessed postprandial glucose and insulin responses and plasma lipid changes to diets high and low in RS. In a cross-over trial, fifty-two men and women consumed a 2-week baseline diet (41 percentage of energy (%E) CHO, 40% fat, 19% protein), followed by 2-week high- and low-RS diets separated by 2-week washouts. RS diets were assigned at random within the context of higher (51–53 %E) v. lower CHO (39–40 %E) intake. Measurements were obtained in the fasting state and, for glucose and insulin, during a meal test matching the composition of the assigned diet. With lower CHO intake, plasma TMAO, carnitine, betaine and γ-butyrobetaine concentrations were higher after the high- v. low-RS diet (P< 0·01 each). These metabolites were not differentially affected by high v. low RS when CHO intake was high. Although the high-RS meal reduced postprandial insulin and glucose responses when CHO intake was low (P<0·01 each), RS did not affect fasting lipids, lipoproteins, glucose or insulin irrespective of dietary CHO content. In conclusion, a lower-CHO diet high in RS was associated with higher plasma TMAO levels. These findings, together with the absence of change in fasting lipids, suggest that short-term high-RS diets do not improve markers of cardiometabolic health.
Keywords: Trimethylamine-N-oxide, Resistant starch, Carbohydrate, Lipids, Insulin, Glucose, CVD
There is growing awareness that gut microbiotas have a substantial influence on human health and disease. Both animal and human studies have shown that gut microbial metabolism of dietary trimethylamines produces trimethylamine-N-oxide (TMAO)(1–3), a metabolite associated with risk of CVD, independent of traditional CVD risk factors(1,2,4). Studies from this group have also established that elevated plasma levels of carnitine, choline and betaine are associated with CVD risk because of their role in formation of TMAO(1,2,5). More recently, another precursor of TMAO, γ-butyrobetaine, was shown to be associated with the development of atherosclerosis in a susceptible mouse model(3). Together, these studies have fuelled interest in the potential for dietary modification to alter TMAO production(6–8). Given the obligatory role of gut microbes in the conversion of trimethylamine-containing nutrients to TMAO(1,2), it is of interest to determine whether dietary components associated with changes in gut microbial communities affect plasma concentrations of TMAO.
Dietary starches differ in their rates of digestion and absorption. Compared with most starches, resistant starches (RS) undergo limited digestion by α-amylases in the small intestine, but may be converted by amylolytic bacterial species in the colon to a range of metabolites including SCFA(9). Differing forms of RS have been shown to rapidly alter the composition of the human gut microbiota (10–12). In view of this, and because production of TMAO is dependent on gut microbes(13), we undertook a study to determine whether diets that differed in RS content affected plasma concentrations of TMAO, and to test whether any such effect was modified by total dietary CHO. In addition, we sought to confirm the attenuation in postprandial glucose and insulin responses by high RS intake (14–18), and to examine changes in plasma lipids and lipoproteins whose associations with RS intake are less well established(15,16).
Methods
Study participants
In all, fifty-two individuals (thirty-two women, twenty men) were recruited from participants of our previous dietary intervention studies and respondents to advertisements on the Internet. The sample included men (>20 years) and post-menopausal women (defined as ≥43 years of age and amenorrhoea for ≥3 years or amenorrhoea for ≥1 but <3 years and plasma follicle-stimulating hormone concentrations elevated to the postmenopausal range) with BMI ≥20 and ≤35 kg/m2. All of them were non-smokers, had no history of CVD or other chronic diseases, and were not taking lipid- or glucose-lowering medications, blood thinning agents or hormones. Moreover, to permit testing of the insulin-lowering effects of RS, we also excluded individuals with relatively high insulin sensitivity as assessed by the homoeostatic model assessment of insulin resistance (HOMA-IR) <50th percentile(19) (based on HOMA-IR distributions of a comparable group of men and women screened for a previous study(20); median: 2·1). Additional selection criteria included fasting glucose <7mmol/l, total and LDL-cholesterol ≤90th percentile for age and sex, fasting TAG <5·65mmol/l, blood pressure (BP) <150/90 mmHg, stable weight (<3% change) for at least 3 months before study onset and willingness to refrain from alcohol and dietary supplements during the study period.
The study protocol was approved by the Institutional Review Board of Children’s Hospital and Research Center of Oakland. All participants gave their written informed consent to take part in the study. Participants were provided with a list of clinical staff to contact should they need to discuss study procedures or report adverse events. This trial was registered at clinicaltrials.gov as NCT01027325.
Study design and diets
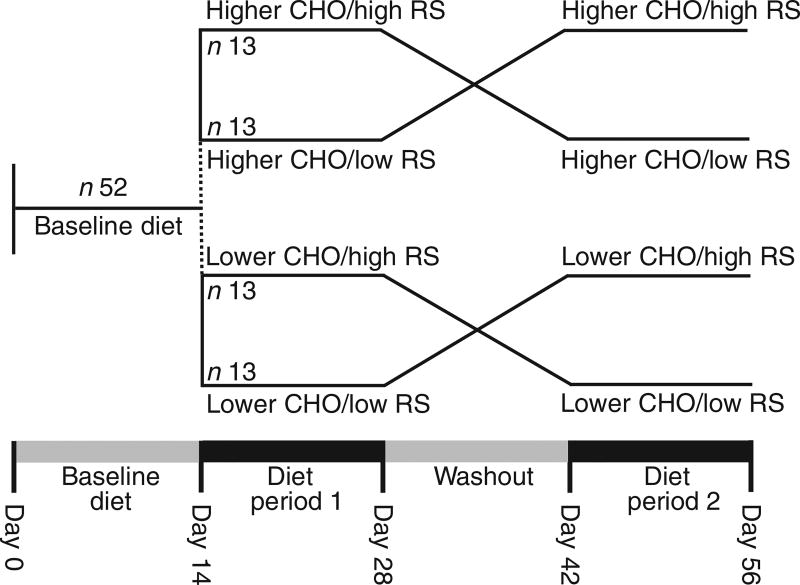
The present study was a controlled, randomised, cross-over dietary intervention conducted in an outpatient setting with weekly visits to our clinic located in Berkeley, CA. The logistical constraints of creating twenty-five different diets (five experimental diets, at five energy levels) required that the first twenty-six subjects enrolled into the study be assigned to the higher-CHO study arm and the second twenty-six to the lower-CHO study arm. Within each diet, a uniform random number generator was used to determine block sizes (two, four, six or eight subjects) and the sequence of the high- v. low-RS diets within each block, which were supplied to the project coordinator in sealed, numbered envelopes. The project statistician was the only person aware of the treatment assignment before subject enrolment.
The study design consisted of two arms: higher and lower total CHO intake with comparison of high RS v. low RS intake in random order in each arm (Fig. 1). All study participants (n 52) first consumed the lower-CHO baseline diet for 2 weeks, after which they followed the higher-CHO diet (the first twenty-six subjects recruited) or the lower-CHO diet (the second twenty-six subjects recruited). The high- and low-RS diets were each consumed for 2 weeks, separated by a 2-week washout, during which they were instructed to consume their habitual diet for 7 d, followed by repeating the baseline diet for an additional 7 d (Fig. 1). Clinic staff met with participants weekly to review and reinforce dietary patterns and ensure that body weight remained within ±3% of initial weight. Investigators, laboratory staff and study participants were blinded to the dietary assignment, whereas staff responsible for provision of food and monitoring of dietary compliance (nutritionist, study coordinator and nurse) was not.
Fig. 1.
Study design. CHO, carbohydrate; RS, resistant starch.
The lower-CHO baseline diet was designed to match the macronutrient distribution of the lower-CHO study arm, but to be low in foods containing naturally occurring RS in order to facilitate limitation of RS intake. High- and low-RS contents of the diets (Table 1) were achieved by incorporating, respectively, a high-amylose maize starch (41·5 g RS/100 g starch, Hi-Maize 260; Ingredion Inc.) or a conventional, high-amylopectin maize starch (2·3 g RS/100 g starch, Melojel; Ingredion Inc.) into recipes. The resulting high-RS diets provided 19g RS/4184 kJ (1000 kcal) for the lower-CHO study arm and 26 g RS/4184kJ (1000 kcal) for the higher-CHO study arm, for an average daily intake of 48–66 g RS. These amounts are within ranges previously shown to affect human faecal microbiota composition(10–12) as well as glycaemic control(22).
Table 1.
Composition of baseline and experimental diets)†
| Higher-CHO arm |
Lower-CHO arm |
||||
|---|---|---|---|---|---|
| Baseline/ washout diet |
High RS |
Low RS |
High RS |
Low RS |
|
| CHO (%E) | 41 | 51 | 53 | 40 | 39 |
| RS (%E) | 10 | 0·6 | 8 | 0·5 | |
| RS (g) | 66 | 4 | 48 | 3 | |
| Protein (%E) | 19 | 22 | 22 | 19 | 21 |
| Fat (%E) | 40 | 27 | 26 | 41 | 40 |
| SFA | 9 | 6 | 7 | 9 | 10 |
| MUFA | 20 | 12 | 12 | 21 | 19 |
| PUFA | 9 | 7 | 6 | 9 | 10 |
| Carnitine (mg))‡ | 118 | 97 | 99 | 115 | 97 |
| Choline (mg))§ | 440 | 439 | 427 | 392 | 433 |
| Choline + carnitine (mg) | 558 | 536 | 526 | 507* | 530 |
| Glycaemic load)║ | 41 | 39 | 27 | 35 | |
| Cholesterol (mg)§ | 302 | 321 | 321 | 327 | 324 |
| Food fibre (g)§ | 28 | 24 | 27 | 28 | 28 |
CHO, carbohydrate; RS, resistant starch; %E, percentage of energy.
P< 0·05 compared with all other diets.
Values shown are for 10460 kJ (2500 kcal) menus.
Estimated values, based on published carnitine content of commonly consumed foods(21).
Calculated values, Nutrition Data System for Research (University of Minnesota).
Calculated values, ProNutra software (Viocare Technologies Inc.). Data were analysed by ANOVA for a cross-over design. %E for macronutrients is based on compositional analysis (Covance Laboratories) and represents an average for 3-d cycle menus (days 1, 3 were consumed twice per week; day 2 was consumed three times per week).
Although rapidly digested maize starch was consumed mostly cooked, in baked goods and entrees, approximately 50% of the high-RS maize starch was consumed raw, mixed into beverages, fruit purees and soups (online Supplementary Table S1). Hi-Maize 260 maize starch was chosen on the basis of its high RS content and because such starches resist losing their granular structure under the range of processing conditions typically used to prepare conventional food products. Maintenance of granular structure and starch polymer association during cooking make high-amylose starch resistant to enzymatic degradation(23). Melojel maize starch was chosen on the basis of its low RS content and because it is widely used in conventional food products.
Dietary control was achieved by provision of standard entrées for home consumption and by having weekly meetings with nutritionists and clinic staff to ensure compliance with the study protocol. Specifically, the clinic provided two standardised entrées (lunch and dinner) during the baseline diet and three standardised meals (entrée, side dish, beverage and occasional dessert) and one to three snacks per day, contributing to approximately 80% of daily energy content. Detailed menus and checklists were provided for the remaining food items that participants were required to purchase (dairy products, fresh produce, fruit juice), mostly during the baseline and low-RS diets. Participants were instructed on procedures to store and, where applicable, thaw and reheat study foods to minimise changes in starch digestibility due to processing. Diets and menus were developed and prepared by the Bionutrition Core of the University of California, San Francisco Clinical and Translational Science Institute. The nutrient composition of the diets was assessed using Nutrition Data System for Research Software (NDSR 2010; Nutrition Coordinating Center, University of Minnesota) and ProNutra software (version 3.3; Viocare Technologies Inc.). Compositional analysis of the menus was validated by Covance Laboratories. Body weight was measured weekly, and energy intake was adjusted when weight fluctuated by more than ±3% of baseline. Participants were required to abstain from alcohol and dietary supplements during the study. The staff nutritionist used menu checklists, grocery receipts and information gathered from weekly interactions to assign a compliance score (1–5-point scale, where 5 is indicative of high compliance) for each study participant.
Fasting blood samples were collected on two consecutive days following completion of the baseline diet (days 13, 14) and at the end of each diet period (days 27, 28 and days 55, 56). A 3-h meal tolerance test was administered on days 28 and 56 (the concluding day of each experimental diet) with blood samples collected in the fasting state and 0·5, 1, 2 and 3h after consumption of a meal representative in macronutrient composition to the assigned experimental diet. The different high-and low-RS meals were designed to correspond to the CHO content of the lower- or higher-CHO study arms (40% v. 52 percentage of energy (%E) as CHO), and to provide one-third of the daily allotted energy and starch (e.g. 16 and 22 g of RS/meal for the high-RS diet for a subject consuming 10 460 kJ/d (2500 kcal/d); and 0·8 and 1·1 g RS/meal for the low-RS diet for a subject consuming 10 460k J/d (2500 kcal/d)).
Laboratory measures
Quantification of resistant starch
The RS content of the test starches was analysed by a modified AOAC method 2009.01, using a Megazyme K-INTDF assay kit (Megazyme International Ireland Ltd).
Plasma measurements
Plasma was prepared from blood samples obtained by venepuncture after an overnight fast, and collected in tubes containing Na2EDTA (1·4 g/l) and a preservative cocktail containing sodium azide, chloramphenicol succinate, gentamicin sulphate, PPACK dihydrochloride and aprotinin. Blood and plasma samples were maintained at 4°C until further processing.
Trimethylamine-N-oxide, choline, betaine, γ-butyrobetaine and carnitine
Analyses were performed in plasma samples stored at −80°C using a stable-isotope dilution HPLC with online electrospray ionisation tandem MS (LC/ESI/MS/MS)(1,24). In brief, four volumes of methanol containing 10 µm-TMAO-trimethyl-d9 (d9-TMAO), betaine-trimethyl-d9 (d9-betaine), choline-trimethyl-d9 (d9-choline), γ-butyrobetaine-trimethyl-d9 (d9-γ-butyrobetaine) and carnitine-trimethyl-d9 (d9-carnitine) were added to plasma as internal standard to precipitate protein. Following centrifugation, the supernatant was collected for LC/MS/MS assay. Supernatants (10 µl) were analysed by injection into a silica column (4·6 × 250 mm, 5 µm Luna silica; cat. no. 00G-4274-E0; Phenomenex) at a flow rate of 0·8 ml/min interfaced with an API 5000 MS (AB SCIEX). Precursor–product ion transitions at m/z 76→58, m/z 104→60, m/z 118→59, m/z 146→60, m/z 162→60, m/z 85→66, m/z 113→69, m/z 127→68, m/z 155→69 and m/z 171→69 were used for TMAO, choline, betaine, γ-butyrobetaine, carnitine, d9-TMAO, d9-choline, d9-betaine, d9-γ-butyrobetaine and d9-carnitine, respectively. Increasing concentrations of TMAO, choline, betaine, γ-butyrobetaine and carnitine standards were spiked to control plasma to generate calibration curves with the y-axis as the peak area ratio to their respective internal standards for determining plasma concentrations of TMAO, betaine, choline, γ-butyrobetaine and carnitine, respectively.
Glucose, insulin and lipids
Plasma insulin concentrations were measured by an ELISA (EZHI-14K Human Insulin ELISA kit; Millipore). HOMA-IR was calculated from plasma insulin and glucose concentrations (insulin (mU/l) × glucose (nmol/l)/22·5)(19).
Total plasma cholesterol, TAG, HDL-cholesterol and glucose concentrations were measured enzymatically on a Liasys 330 Clinical Chemistry System (AMS Diagnostics), and LDL-cholesterol was calculated using the Friedewald formula(25). Quality control of lipid measurements was maintained through the standardisation programme of the Centers for Disease Control-National Heart, Lung and Blood Institute. Plasma apo B and apo AI were analysed on the same machine by immuno-turbidimetric assays using the ITA reagent kit (Bacton Assay Systems)(26,27).
Particle concentrations of VLDL, intermediate-density lipoprotein and LDL subfractions in plasma were determined by ion mobility (IM) as described previously(28). This method uniquely allows for direct particle quantification following brief ultra-centrifugation in D2O to remove albumin. The IM instrument uses an electrospray to create an aerosol of particles that pass through a dynamic mobility analyzer coupled to a particle counter. Particle numbers are measured in pre-specified particle diameter intervals and converted to plasma particle concentrations (nmol). LDL diameter is also measured at the peak of LDL particle distribution(28).
Faecal DNA extraction and sequencing
Faecal samples were collected at the end of each dietary intervention (high RS and low RS) from sixteen participants assigned to the higher-CHO study arm (2 × 16 = 32 faecal samples) and from twenty-three participants assigned to the lower-CHO study arm (2 × 23 = 46 faecal samples). From these samples, total genomic DNA was extracted in duplicate using the MoBio PowerSoil DNA extraction kit with additional heat lysis for 5 min at 60°C (MoBio Laboratories). PCR were used to amplify DNA, using the F515/ R806 primer to target the V3/V4 region of the 16 S rRNA gene, and the reverse primer construct also contained a twelve-base error-correcting Golay code(29). 16 S rRNA was sequenced as described in the online Supplementary Methods. Sequence data were analysed using the Quantitative Insights into Microbial Ecology pipeline version 1.7(30), as described in the online Supplementary Material.
Statistical analysis
On the basis of published data comparing high- v. low-RS diets(15,16), a sample size of fifty-two participants was estimated to provide 80% power (5% significance) to detect a significant metabolic effect of RS, as manifest by 15 and 50% changes in postprandial insulin and glucose responses (AUC), respectively, a 19% change in plasma TAG, and a 13% change in small dense LDL between the high- v. low-RS diets.
Statistical analyses were performed using ANOVA and crossover experiments procedure of Stata 11.1 (StataCorp LP). The effects of high v. low RS were estimated by ANOVA for a crossover design that involved the random assignment of subjects to high and low RS, and included effects due to RS, CHO and their interaction. These analyses also tested effects of dietary sequence (i.e. high- following low-RS diets v. low- following high-RS diets), and no significant diet order effects were observed for any of the measures of response (data not shown). The analyses were repeated within each CHO condition for a simple cross-over design that included only RS effects. Log-transformation of data that were not normally distributed (carnitine, choline, insulin, HOMA-IR, TAG and HDL-cholesterol) did not affect the results.
Results
Participant retention and baseline characteristics
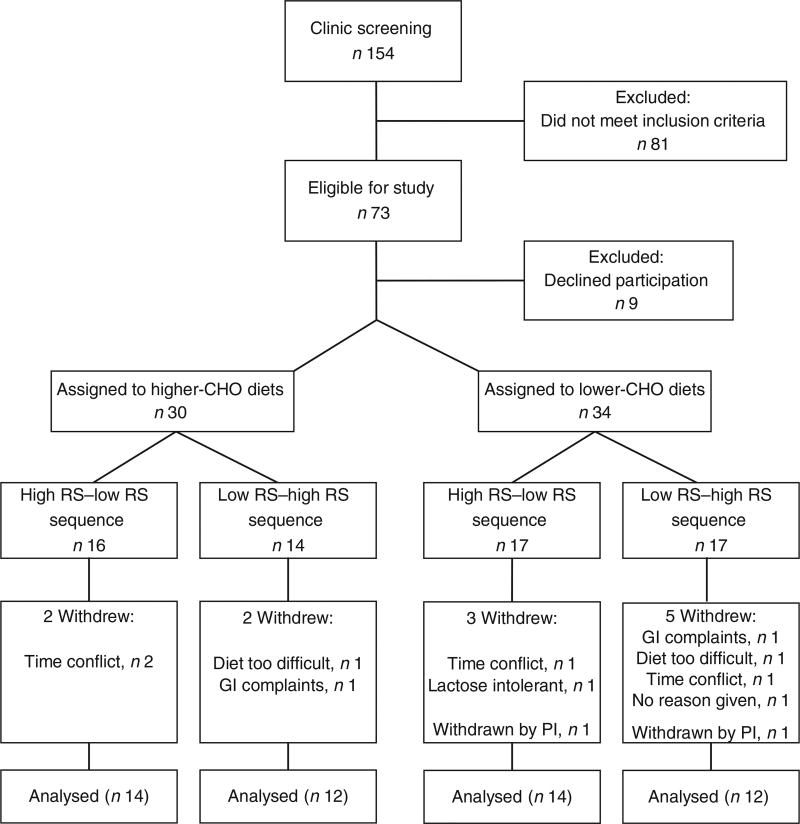
A total of fifty-two participants (twenty men and thirty-two women) completed the study. The flow diagram of participant recruitment and withdrawal is illustrated in Fig. 2. On average, these individuals were middle aged (mean 44 (sd 14) years), normotensive (systolic BP: 119 (sd 14) mmHg, diastolic BP: 70 (sd 8) mmHg), and overweight or obese as characterised by their BMI (31 (sd 2) kg/m2), body fat (38 (sd 7) %) and /or waist circumference (107 (sd 8) cm, men; 101 (sd 7) cm, women). HOMA-IR ranged from 2·1 to 20·4 at screening (mean 3·87 (sd 2·90); median: 3·0).
Fig. 2.
Participant enrolment and withdrawal. CHO, carbohydrate; RS, resistant starch; GI, gastrointestinal; PI, principal investigator.
With the exception of baseline plasma carnitine levels, which were lower in those randomised to the lower-carbohydrate diet, baseline characteristics of participants did not differ significantly between those in the lower- and higher-CHO study arms (Table 2). Adjustment for differences in baseline plasma carnitine levels between high- and low-CHO groups did not affect microbiome metabolite responses to diets high v. low in RS.
Table 2. Baseline characteristics*.
(Mean values and standard deviations; n 10 males and 16 females in each diet group)
| Higher-CHO study arm |
Lower-CHO study arm |
P (high v. low CHO) |
|||
|---|---|---|---|---|---|
| Mean | sd | Mean | sd | ||
| Age (years) | 45·7 | 14·1 | 42·4 | 13·0 | 0·39 |
| BMI (kg/m2) | 30·6 | 2·4 | 30·8 | 2·3 | 0·77 |
| Glucose (mmol/l) | 5·52 | 0·48 | 5·54 | 0·43 | 0·84 |
| Insulin (mU/l) | 11·2 | 5·2 | 10·8 | 8·7 | 0·85 |
| HOMA-IR | 2·74 | 1·26 | 2·69 | 2·35 | 0·93 |
| TMAO (µmol/l) | 5·19 | 3·19 | 4·16 | 2·27 | 0·18 |
| Carnitine (µmol/l) | 33·7 | 6·1 | 30·2 | 5·2 | 0·03 |
| Choline (µmol/l) | 6·57 | 1·37 | 6·60 | 1·62 | 0·93 |
| Betaine (µmol/l) | 36·1 | 8·1 | 32·9 | 7·7 | 0·16 |
| γ-Butyrobetaine (µmol/l) | 0·91 | 0·26 | 0·90 | 0·21 | 0·77 |
| Total cholesterol (mmol/l) | 4·47 | 0·77 | 4·39 | 0·64 | 0·70 |
| LDL-cholesterol (mmol/l) | 2·83 | 0·57 | 2·75 | 0·53 | 0·59 |
| TAG (mmol/l) | 1·11 | 0·29 | 1·15 | 0·50 | 0·76 |
| HDL-cholesterol (mmol/l) | 1·13 | 0·20 | 1·12 | 0·23 | 0·87 |
| Apo B (mg/l) | 790 | 135 | 740 | 154 | 0·22 |
| Apo A1 (mg/l) | 1138 | 129 | 1115 | 114 | 0·51 |
CHO, carbohydrate; HOMA-IR, homoeostatic model assessment of insulin resistance (insulin (mU/l) ×glucose (nmol/l)/22·5).
Data were analysed by ANOVA for a cross-over design.
Compliance with dietary protocol
Participants were highly compliant with the dietary protocol, with nutritionist-reported mean compliance scores of 4·7 (sd 0·7) (on a scale of 1–5). Self-reported gastrointestinal symptoms and perceived satiety during high and low RS intake were consistent with high dietary adherence, with significantly increased frequency of flatulence, fullness, loss of appetite and burping and significantly increased intensity of abdominal cramps, flatulence and fullness after the high-RS diets (online Supplementary Fig. S1). High RS intake also increased the reported number of weekly bowel movements in participants assigned to the higher-CHO diets (12 (sd 7) bowel movements/ week with high RS and 9 (sd 5) bowel movements/week with low RS, P=0·005).
Documentation of adverse events
No serious adverse events were reported (online Supplementary Table S2).
Gut microbiome derived metabolites
Fasting plasma carnitine, betaine, γ-butyrobetaine and TMAO concentrations were significantly higher after the high- v. low-RS diet in the lower-CHO treatment arm (Table 3), but not the higher-CHO treatment arm (P > 0·38 for all metabolites), resulting in a significant CHO by RS interaction for these metabolites. Plasma choline concentration was not significantly affected by starch digestibility. Additional analyses showed that plasma TMAO levels were not correlated with the sum of plasma choline and carnitine, both dietary precursors of TMAO (P=0·53).
Table 3.
Plasma concentrations of carnitine, choline, betaine, γ-butyrobetaine and trimethylamine-N-oxide (TMAO) after 2 weeks of diet with differing amounts of resistant starch (RS) and carbohydrate (CHO)*
(Mean values and standard deviations)
| Higher-CHO study arm
|
Lower-CHO study arm
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High RS
|
Low RS
|
P (high v. low RS) |
High RS
|
Low RS
|
P (high v. low RS) |
P (RS effect) | P (CHO effect) | P (interaction) | |||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | ||||||
| Carnitine (µmol/l) | 32·9 | 7·2 | 32·3 | 4·9 | 0·38 | 30·0 | 5·2 | 27·1 | 5·2 | <00001 | <00001 | 0·47 | 0·007 |
| Choline (µmol/l) | 5·92 | 1·43 | 6·07 | 1·28 | 0·58 | 6·40 | 1·69 | 6·15 | 2·55 | 0·59 | 0·85 | 0·46 | 0·46 |
| Betaine (µmol/l) | 34·1 | 8·3 | 34·5 | 7·6 | 0·68 | 34·9 | 10·3 | 31·2 | 7·9 | 0·002 | 0·05 | 0·13 | 0·008 |
| γ-Butyrobetaine (µmol/l) | 0·92 | 0·25 | 0·91 | 0·23 | 0·66 | 0·93 | 0·21 | 0·86 | 0·19 | <00001 | 0·005 | 0·96 | 0·03 |
| TMAO (µmol/l) | 4·04 | 1·90 | 3·66 | 1·58 | 0·41 | 4·85 | 2·60 | 2·60 | 1·57 | <00001 | 0·0001 | 0·24 | 0·005 |
Data were analysed by ANOVA for a cross-over design.
Gut microbial taxa associated with plasma trimethylamine-N-oxide concentrations
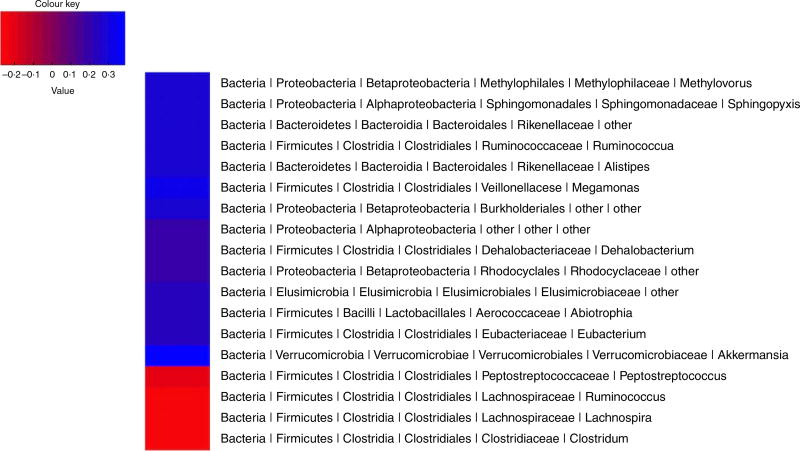
Faecal samples were collected in a subgroup of thirty-nine participants for microbial community analysis. Consistent with findings in the group as a whole (n 52), plasma TMAO concentrations were significantly higher after the high- v. low-RS diets (4·35 (sd 2·35) and 3·14 (sd 1·74) µm, respectively; P=0·0008) in these thirty-nine participants. Microbial community analysis of these samples identified significant positive and negative correlations between multiple taxa and plasma TMAO levels (Fig. 3, P< 0·05 for all taxa).
Fig. 3.
Pearson’s correlations between relative abundance of taxa and plasma trimethylamine-N-oxide (TMAO) concentrations. An OTU table was filtered at a minimum depth of 5000 sequences per sample, summarised at the genus level, and filtered to exclude genera less than 0·05 % abundant. Relative abundances of taxa were correlated to TMAO values for each sample.
Fasting and postprandial insulin and glucose
Although high- and low-RS diets did not affect fasting concentrations of insulin and glucose (Table 4), the high-RS test meals produced significantly lower postprandial insulin and glucose responses, expressed as incremental AUC (IAUC), compared with low-RS test meals (Table 4 and online Supplementary Fig. S2). These differences were largely due to the differential effect of RS on the 0·5-h postprandial glucose response (P=0·0001) and the 1-h postprandial insulin response (P=0·007).
Table 4.
Body weight and plasma insulin, glucose and lipid concentrations after 2 weeks of diet with differing amounts of resistant starch (RS) and carbohydrate (CHO)*
(Mean values and standard deviations)
| Higher-CHO study arm
|
Lower-CHO study arm
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High RS
|
Low RS
|
P (high v. low RS) |
High RS
|
Low RS
|
P (high v. low RS) |
P (RS effect) |
P (CHO effect) |
P (interaction) |
|||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | ||||||
| Weight (kg) | 88·0 | 12·0 | 87·9 | 11·6 | 0·65 | 88·3 | 12·8 | 87·7 | 12·8 | 0·002 | 0·02 | 0·14 | 0·10 |
| Fasting insulin (mU/l) | 8·6 | 4·5 | 9·7 | 5·5 | 0·54 | 8·8 | 4·1 | 8·0 | 3·9 | 0·29 | 0·92 | 0·30 | 0·29 |
| Fasting glucose (mmol/l) | 5·26 | 0·55 | 5·30 | 0·55 | 0·82 | 5·38 | 0·51 | 5·27 | 0·49 | 0·14 | 0·40 | 0·77 | 0·22 |
| IAUC insulin (mU/l per h) | 132·8 | 73·6 | 157·8 | 111·6 | 0·20 | 76·6 | 34·7 | 103·8 | 45·7 | 0·01 | 0·01 | 0·001 | 0·66 |
| IAUC glucose (mmol/l per h) | 1·85 | 1·62 | 2·51 | 1·68 | 0·11 | 1·45 | 1·32 | 2·44 | 1·85 | 0·006 | 0·003 | 0·71 | 0·49 |
| Total cholesterol (mmol/l) | 4·30 | 0·71 | 4·35 | 0·78 | 0·41 | 4·25 | 0·63 | 4·26 | 0·65 | 0·83 | 0·51 | 0·86 | 0·69 |
| LDL-cholesterol (mmol/l) | 2·66 | 0·48 | 2·67 | 0·54 | 0·73 | 2·68 | 0·50 | 2·69 | 0·54 | 0·84 | 0·71 | 0·12 | 0·94 |
| TAG (mmol/l) | 1·23 | 0·42 | 1·24 | 0·44 | 0·78 | 1·08 | 0·51 | 1·13 | 0·74 | 0·52 | 0·44 | 0·02 | 0·65 |
| HDL-cholesterol (mmol/l) | 1·08 | 0·17 | 1·11 | 0·21 | 0·23 | 1·07 | 0·21 | 108 | 0·21 | 0·75 | 0·28 | 0·80 | 0·37 |
| Apo B (mg/l) | 762 | 141 | 768 | 151 | 0·83 | 725 | 174 | 713 | 159 | 0·61 | 0·69 | 0·73 | 0·57 |
| Apo AI (mg/l) | 1121 | 143 | 1154 | 151 | 0·25 | 1083 | 140 | 1075 | 131 | 0·67 | 0·61 | 0·11 | 0·16 |
IAUC, incremental AUC.
Data were analysed by ANOVA for a cross-over design.
Consuming higher-CHO compared with lower-CHO test meals acutely did not affect the IAUC for glucose, but resulted in significantly higher postprandial insulin responses at all time points after the meal (online Supplementary Fig. S2), expressed as IAUC (P=0·001, Table 4). There were no significant CHO by RS interactions for postprandial glucose or insulin responses (P=0·49 and P=0·66, respectively).
Fasting lipids and lipoproteins
With the exception of plasma TAG and large VLDL particles, which were increased by high- v. low-CHO diets (P=0·02 and P=0·002, respectively), fasting plasma lipids, lipoproteins and apoproteins were not affected by starch digestibility or the amount of CHO in the diet (Tables 4 and 5).
Table 5.
Total mass concentrations of plasma lipoprotein subfractions after 2 weeks of diet with differing amounts of resistant starch (RS) and carbohydrate (CHO)*
(Mean values and standard deviations)
| Higher-CHO study arm
|
Lower-CHO study arm
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High RS
|
Low RS
|
P (high v. low RS) |
High RS
|
Low RS
|
P (high v. low RS) |
P (RS effect) | P (CHO effect) | P (interaction) | |||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | ||||||
| Large VLDL (nmol/l) | 12·5 | 5·7 | 12·3 | 4·8 | 0·85 | 10·1 | 5·3 | 10·2 | 4·6 | 0·92 | 0·75 | 0·002 | 0·82 |
| Medium VLDL (nmol/l) | 39·0 | 15·8 | 38·9 | 12·8 | 0·81 | 38·3 | 12·8 | 39·5 | 10·8 | 0·49 | 0·66 | 0·16 | 0·76 |
| Small VLDL (nmol/l) | 53·3 | 21·1 | 55·2 | 19·4 | 0·47 | 58·5 | 18·3 | 60·9 | 21 | 0·39 | 0·33 | 0·59 | 0·89 |
| IDL (nmol/l) | 128 | 40 | 134 | 41 | 0·32 | 144 | 44 | 147 | 45 | 0·70 | 0·41 | 0·67 | 0·70 |
| Large LDL (nmol/l) | 714 | 182 | 718 | 201 | 0·86 | 744 | 225 | 731 | 239 | 0·51 | 0·68 | 0·68 | 0·58 |
| Medium LDL (nmol/l) | 195 | 80 | 198 | 65 | 0·78 | 228 | 95 | 215 | 77 | 0·26 | 0·45 | 0·29 | 0·30 |
| Small LDL (nmol/l) | 117 | 61 | 126 | 70 | 0·29 | 168 | 104 | 155 | 82 | 0·44 | 0·73 | 0·54 | 0·25 |
| Very small LDL (nmol/l) | 106 | 28 | 115 | 38 | 0·05 | 141 | 36 | 135 | 35 | 0·38 | 0·75 | 0·43 | 0·06 |
| LDL ppd (Å) | 223 | 3·5 | 223 | 3·8 | 0·25 | 222 | 4·5 | 222 | 4·6 | 0·89 | 0·41 | 0·26 | 0·52 |
IDL, intermediate-density lipoprotein; LDL ppd, LDL peak particle diameter.
Data were analysed by ANOVA for a cross-over design.
Body weight
Changes in body weight were minimal (Table 4), although a reduction with low v. high RS in the low-CHO arm (87·7 (sd 12·8) v. 88·3 (sd 12·8) kg) was significant at P< 0·05. Adjustment for change in body weight did not significantly affect the gut microbiome-derived metabolite or glycaemic and lipoprotein responses to the high- and low-RS diets (data not shown).
Discussion
We report that intake of dietary RS can modulate circulating levels of TMAO, a metabolite that is associated with increased future risk of major cardiovascular events(1,2,4). The production of TMAO is dependent on gut microbes and arises from dietary precursors such as choline, carnitine, phosphatidylcholine and γ-butyrobetaine, which are first converted by colonic bacteria to trimethylamine(1,2). Trimethylamine is then absorbed and rapidly oxidised to TMAO by hepatic flavin mono-oxygenases(31).
The association of TMAO with CVD has been ascribed in part to inhibition of reverse cholesterol transport, changes in cholesterol and bile acid metabolism, and increased macrophage foam cell formation(1,2). In more recent studies, TMAO has also been linked to development of vulnerable plaque, both through activation of arterial endothelial cells(32), and through a direct effect on intracellular Ca signalling in platelets, promoting a prothrombotic phenotype(33). Notably, in a susceptible mouse model, inhibition of microbial trimethylamine production from choline was recently shown to reduce plasma levels of TMAO and to inhibit the development of atherosclerotic lesions(13). Moreover, in two different mouse models of atherosclerosis, anti-sense oligonucleotide targeting of hepatic flavin mono-oxygenase 3 has been shown to similarly inhibit TMAO formation and development of atherosclerosis(34,35).
Resident gut micro-organisms are rapidly modulated by variation in intake of starches, and these changes may vary in conjunction with differences in starch digestibility(10–12). It is known that starches that are relatively resistant to intestinal digestion are subject to fermentation by amylolytic bacterial species that reside in the colon(36,37). Consistent with this property of RS and the fact that the bacterial production of trimethylamine occurs primarily in the colon(1), we found that plasma levels of TMAO were significantly increased by high v. low RS intake, although this effect was dependent on total dietary CHO. Specifically, the TMAO-raising effect of RS was observed with a CHO intake of 39–40 %E, whereas with CHO intake >50 %E levels of TMAO were yet higher and independent of RS content. This finding suggests that both higher CHO intake alone and high RS intake alone are sufficient to promote the production of trimethylamine by the colonic microbiota, and that both must be reduced in order to attenuate this process.
It is unlikely that the increase in plasma TMAO with the high-RS diet was due to higher dietary intake of carnitine and choline. In fact, the sum of dietary carnitine + choline, both dietary precursors of TMAO, was slightly lower for the high-RS diet v. low-RS diet in the lower-CHO study arm. Moreover, the sum of plasma choline+carnitine levels was not correlated with plasma TMAO levels. Rather, our findings suggest that differential effects of high v. low RS on gut microbial composition led to increased TMAO concentrations with high RS intake.
In keeping with the obligatory role of gut microbiota in producing TMAO(1), analysis of the microbial composition of faecal samples showed that the proportions of certain taxa were correlated with plasma TMAO levels. Although recent studies have delineated biochemical processes involved in the microbial conversion of choline and carnitine to TMAO(38,39), little is known of the diversity of microbial taxa that can contribute to this process. One recent study examined seventy-nine human microbial isolates spanning several common phyla observed in the human gut and identified several human commensals with TMA-producing activity(40). Although it is not possible from the present results to determine the contribution of specific microbial communities to the diet-induced changes in TMAO levels observed here, it is intriguing that some that were inversely correlated with TMAO change – namely, Lachno-spiraceae and Clostridiales (Fig. 3) – were also recently found to be associated with lower plasma TMAO levels in mice(33). Finally, the extent to which products of RS fermentation (e.g. SCFA) may have influenced the associations of these microbial communities with TMAO requires further study – for example, by examination of these associations in conjunction with measurements of faecal fatty acids and other metabolites at multiple time points after RS feeding.
In agreement with earlier observations(14–18,22), we report significantly attenuated insulin and glucose responses to meals providing 16–22 g RS. A strength of our study is that this was observed in the context of physiological meals that were balanced and matched for fat, protein and food fibre, which can markedly affect the digestion and absorption of RS(41). These results suggest a potential utility for RS in improving meal-to-meal regulation of blood glucose. SCFA, particularly acetate and propionate generated from colonic fermentation by resident bacteria, have also been implicated in the insulin sensitising effects of RS(42,43).
Although earlier studies have suggested that the lipid-lowering effects of RS may be dependent upon high levels of intake(15,16), this is not supported by our findings. In the present study, test starches were provided in amounts 1·9–2·6-fold higher than in earlier interventions(44–46), but we found no effect of RS on fasting plasma lipids or lipoproteins. The short-term nature of the current intervention may also be a factor, but earlier studies showing reductions in cholesterol and TAG with high- v. low-RS diets at 4 weeks, but not at 8 and 13 weeks(15,16), suggest that the effects of RS on plasma lipids are transitory. Notably, we observed that, independent of starch digestibility, higher-CHO diets increased plasma TAG and large VLDL particle concentrations, and promoted a shift in LDL particle distribution towards more medium and small LDL (Supplementary Fig. S4), in keeping with the recognised effect of carbohydrates on features of atherogenic dyslipidemia(47–51), and in overall agreement with the recent OmniCarb study(52), which found that plasma TAG were increased by higher CHO intake, but were not influenced by starch quality as assessed by the glycaemic index.
Strengths of our study include a design that, for the first time, allowed testing of high v. low RS in the context of both higher-and lower-CHO diets, lack of confounding effects from other nutrients that were matched across diets, and strict dietary control achieved by the preparation and provision of most study foods. This differs from previous studies in which test starches were provided in the form of supplements that individuals consumed with their self-selected diet(15,46,53,54), with only one intervention conducted in a controlled setting(16).
A limitation of our study is that the protocol and randomisation scheme required that all foods be prepared in advance, flash-frozen and stored until consumed, typically within 1–2 months. Starch processing conditions have been shown to affect their functionality and susceptibility to enzymatic degradation. When heated in excess water, high-amylopectin starches become highly digestible as a result of gelatinisation, a process that results in disruption of starch crystalline structure and swelling of starch granules. Upon cooling and storage, gelatinised starch may undergo retrogradation during which amylose and, to a lesser extent, outer branches of amylopectin re-align into more ordered crystalline structures that are less susceptible to degradation by α-amylases(55). Therefore, we cannot rule out the possibility that in our study freezing and storage may have promoted retrogradation of gelatinised starch products in a manner that rendered them more resistant to digestion, thus attenuating differential metabolic effects of high- and low-RS diets. However, in an earlier study, storage time, freezing, thawing and re-heating did not affect the RS content of high- and low-amylose muffins(45,46). Also of note, re-heating of starch-based foods may promote the re-dispersion of crystallised starch chains and restoration of starch digestibility(56,57). In the present study, regular maize starch in the low-RS diet was incorporated mostly into entrees and baked goods, which, after freezing, were thawed and re-heated before consumption. The diets were otherwise consumed in a manner consistent with how individuals eat on a day-to-day basis. Under these conditions, and despite a 16-fold difference in the RS content of the high- and low-RS diets at time of preparation, we found no differences in their effects on fasting plasma glucose, insulin, lipids and lipoproteins.
Another limitation of our study is the short-term duration of the dietary intervention. As was also shown in an acute feeding study with egg yolks(7), our findings demonstrate that changes in TMAO levels in response to dietary modification can occur rapidly. Our findings are also consistent with earlier demonstrations of rapid alterations in microbial community structure with RS intake(10–12), and suggest that such changes in gut microbiota promote generation of TMAO in a setting of lower carbohydrate, higher fat intake. However, it remains to be determined whether these effects are sustained with longer-term dietary interventions.
In light of the health benefits generally ascribed to RS, and of epidemiological evidence linking high fibre intake to reduced CVD risk(58), the increase in TMAO with RS is contrary to what might be expected. Earlier dietary intervention studies have also shown increased abundance of TMAO after diets high in soya(59) or low glycaemic load carbohydrates(60), typically deemed beneficial to cardiometabolic health. Hence, although there is strong evidence for the relation of TMAO to atherosclerotic CVD, we cannot conclude that the dietary effects on TMAO observed here would translate into changes in risk for CVD. Furthermore, whether increases in TMAO are clinically relevant in the context of a concomitant improvement in glycaemic control, as is commonly observed with RS, remains to be established.
In conclusion, our study showed that, in the context of a lower-CHO diet, high RS intake resulted in significantly higher plasma concentrations of TMAO, a novel CVD risk biomarker. In keeping with earlier findings, RS blunted the postprandial glucose and insulin responses to meals consumed acutely, but average daily intake of 49–68 g RS did not affect fasting plasma lipids, lipoprotein particle concentrations, glucose or insulin. Together, these observations support the conclusion that at least in the short term high RS intake does not improve biomarkers of cardiometabolic health.
Supplementary Material
Acknowledgments
The authors thank the staff of the Cholesterol Research Center for their help with conducting the study. The authors also thank the staff of the Bionutrition Core of the University of California, San Francisco, Clinical and Translational Science Institute for their help in designing the diets and preparing study foods.
The authors received the following financial supports: National Institutes of Health (NIH) (DK086472); NIH National Center for Advancing Translational Sciences, University of California, San Francisco (UCSF) Clinical and Translational Science Unit (UL1 TR000004); Ingredion Inc.; NIH and Office of Dietary Supplements (HL103866 and DK106000); S. L. H. is also partially supported by funds from the Lenard Krieger Endowment.
Abbreviations
- HOMA-IR
homoeostatic model assessment of insulin resistance
- RS
resistant starches
- TMAO
trimethylamine-N-oxide
Footnotes
The authors’ contributions were as follows: N. B., P. T. W. and R. M. K. designed the study, analysed the data, performed statistical analysis and wrote the manuscript; N. B., N. F. and R. M. K. conducted the human study; R. L., A. G., R. K. and J. K. J conducted the microbiome analyses; X. L. and S. L. H. conducted the choline, carnitine and TMAO analyses. N. B. and R. M. K. had primary responsibility for the final content. All the authors reviewed and accepted the final content of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114516004165
References
- 1.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koeth RA, Levison BS, Culley MK, et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of l-carnitine to TMAO. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutagy NE, Neilson AP, Osterberg KL, et al. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring) 2015;23:2357–2363. doi: 10.1002/oby.21212. [DOI] [PubMed] [Google Scholar]
- 7.Miller CA, Corbin KD, da Costa KA, et al. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100:778–786. doi: 10.3945/ajcn.114.087692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohrmann S, Linseisen J, Allenspach M, et al. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr. 2016;146:283–289. doi: 10.3945/jn.115.220103. [DOI] [PubMed] [Google Scholar]
- 9.Birt DF, Boylston T, Hendrich S, et al. Resistant starch: promise for improving human health. Adv Nutr. 2013;4:587–601. doi: 10.3945/an.113.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abell GC, Cooke CM, Bennett CN, et al. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol Ecol. 2008;66:505–515. doi: 10.1111/j.1574-6941.2008.00527.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez I, Kim J, Duffy PR, et al. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behall KM, Scholfield DJ, Canary J. Effect of starch structure on glucose and insulin responses in adults. Am J Clin Nutr. 1988;47:428–432. doi: 10.1093/ajcn/47.3.428. [DOI] [PubMed] [Google Scholar]
- 15.Behall KM, Howe JC. Effect of long-term consumption of amylose vs amylopectin starch on metabolic variables in human subjects. Am J Clin Nutr. 1995;61:334–340. doi: 10.1093/ajcn/61.2.334. [DOI] [PubMed] [Google Scholar]
- 16.Behall KM, Scholfield DJ, Yuhaniak I, et al. Diets containing high amylose vs amylopectin starch: effects on metabolic variables in human subjects. Am J Clin Nutr. 1989;49:337–344. doi: 10.1093/ajcn/49.2.337. [DOI] [PubMed] [Google Scholar]
- 17.Raben A, Tagliabue A, Christensen NJ, et al. Resistant starch: the effect on postprandial glycemia, hormonal response, and satiety. Am J Clin Nutr. 1994;60:544–551. doi: 10.1093/ajcn/60.4.544. [DOI] [PubMed] [Google Scholar]
- 18.Sands AL, Leidy HJ, Hamaker BR, et al. Consumption of the slow-digesting waxy maize starch leads to blunted plasma glucose and insulin response but does not influence energy expenditure or appetite in humans. Nutr Res. 2009;29:383–390. doi: 10.1016/j.nutres.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravata DM, Wells CK, Concato J, et al. Two measures of insulin sensitivity provided similar information in a U.S. population. J Clin Epidemiol. 2004;57:1214–1217. doi: 10.1016/j.jclinepi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Chiu S, Williams PT, Dawson T, et al. Diets high in protein or saturated fat do not affect insulin sensitivity or plasma concentrations of lipids and lipoproteins in overweight and obese adults. J Nutr. 2014;144:1753–1759. doi: 10.3945/jn.114.197624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demarquoy J, Georges B, Rigault C, et al. Radioisotopic determination of l-carnitine content in foods commonly eaten in Western countries. Food Chem. 2004;86:137–142. [Google Scholar]
- 22.Behall KM, Hallfrisch J. Plasma glucose and insulin reduction after consumption of breads varying in amylose content. Eur J Clin Nutr. 2002;56:913–920. doi: 10.1038/sj.ejcn.1601411. [DOI] [PubMed] [Google Scholar]
- 23.Ratnayake WS, Jackson DS. Starch gelatinization. Adv Food Nutr Res. 2009;55:221–268. doi: 10.1016/S1043-4526(08)00405-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Levison BS, Hazen JE, et al. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Rifai N, King ME. Immunoturbidimetric assays of apolipoproteins A, AI, AII, and B in serum. Clin Chem. 1986;32:957–961. [PubMed] [Google Scholar]
- 27.Smith SJ, Cooper GR, Henderson LO, et al. An international collaborative study on standardization of apolipo-proteins A-I and B. Part I. Evaluation of a lyophilized candidate reference and calibration material. Clin Chem. 1987;33:2240–2249. [PubMed] [Google Scholar]
- 28.Caulfield MP, Li S, Lee G, et al. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54:1307–1316. doi: 10.1373/clinchem.2007.100586. [DOI] [PubMed] [Google Scholar]
- 29.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J Am Heart Assoc. 2016;5:e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao J, Ling AV, Manthena PV, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ze X, Duncan SH, Louis P, et al. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macfarlane GT, Englyst HN. Starch utilization by the human large intestinal microflora. J Appl Bacteriol. 1986;60:195–201. doi: 10.1111/j.1365-2672.1986.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 38.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111:4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romano KA, Vivas EI, Amador-Noguez D, et al. Intestinal microbiota composition modulates choline bio-availability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6:e02481. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh J, Dartois A, Kaur L. Starch digestibility in food matrix: a review. Trends Food Sci Technol. 2010;21:168–180. [Google Scholar]
- 42.Robertson MD, Bickerton AS, Dennis AL, et al. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- 43.Maki KC, Pelkman CL, Finocchiaro ET, et al. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr. 2012;142:717–723. doi: 10.3945/jn.111.152975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heijnen ML, van Amelsvoort JM, Deurenberg P, et al. Neither raw nor retrograded resistant starch lowers fasting serum cholesterol concentrations in healthy normolipidemic subjects. Am J Clin Nutr. 1996;64:312–318. doi: 10.1093/ajcn/64.3.312. [DOI] [PubMed] [Google Scholar]
- 45.Jenkins DJ, Vuksan V, Kendall CW, et al. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr. 1998;17:609–616. doi: 10.1080/07315724.1998.10718810. [DOI] [PubMed] [Google Scholar]
- 46.Noakes M, Clifton PM, Nestel PJ, et al. Effect of high-amylose starch and oat bran on metabolic variables and bowel function in subjects with hypertriglyceridemia. Am J Clin Nutr. 1996;64:944–951. doi: 10.1093/ajcn/64.6.944. [DOI] [PubMed] [Google Scholar]
- 47.Krauss RM, Blanche PJ, Rawlings RS, et al. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83:1025–1031. doi: 10.1093/ajcn/83.5.1025. quiz 1205. [DOI] [PubMed] [Google Scholar]
- 48.Dreon DM, Fernstrom HA, Miller B, et al. Low-density lipoprotein subclass patterns and lipoprotein response to a reduced-fat diet in men. FASEB J. 1994;8:121–126. [PubMed] [Google Scholar]
- 49.Dreon DM, Fernstrom HA, Williams PT, et al. LDL subclass patterns and lipoprotein response to a low-fat, high-carbohydrate diet in women. Arterioscler Thromb Vasc Biol. 1997;17:707–714. doi: 10.1161/01.atv.17.4.707. [DOI] [PubMed] [Google Scholar]
- 50.Dreon DM, Fernstrom HA, Williams PT, et al. A very low-fat diet is not associated with improved lipoprotein profiles in men with a predominance of large, low-density lipoproteins. Am J Clin Nutr. 1999;69:411–418. doi: 10.1093/ajcn/69.3.411. [DOI] [PubMed] [Google Scholar]
- 51.LeCheminant JD, Smith BK, Westman EC, et al. Comparison of a reduced carbohydrate and reduced fat diet for LDL, HDL, and VLDL subclasses during 9-months of weight maintenance subsequent to weight loss. Lipids Health Dis. 2010;9:54. doi: 10.1186/1476-511X-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacks FM, Carey VJ, Anderson CA, et al. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial. JAMA. 2014;312:2531–2541. doi: 10.1001/jama.2014.16658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heijnen ML, van den Berg GJ, Beynen AC. Dietary raw versus retrograded resistant starch enhances apparent but not true magnesium absorption in rats. J Nutr. 1996;126:2253–2259. doi: 10.1093/jn/126.9.2253. [DOI] [PubMed] [Google Scholar]
- 54.Park OJ, Kang NE, Chang MJ, et al. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J Nutr Sci Vitaminol (Tokyo) 2004;50:93–99. [PubMed] [Google Scholar]
- 55.Wang S, Copeland L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: a review. Food Funct. 2013;4:1564–1580. doi: 10.1039/c3fo60258c. [DOI] [PubMed] [Google Scholar]
- 56.Yadav BS, Sharma A, Yadav RB. Studies on effect of multiple heating/cooling cycles on the resistant starch formation in cereals, legumes and tubers. Int J Food Sci Nutr. 2009;60(Suppl. 4):258–272. doi: 10.1080/09637480902970975. [DOI] [PubMed] [Google Scholar]
- 57.Englyst H, Wiggins HS, Cummings JH. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1982;107:307–318. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y, Qian Y, Pan Y, et al. Association between dietary fiber intake and risk of coronary heart disease: a meta-analysis. Clin Nutr. 2015;34:603–611. doi: 10.1016/j.clnu.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Solanky KS, Bailey NJ, Beckwith-Hall BM, et al. Biofluid 1H NMR-based metabonomic techniques in nutrition research - metabolic effects of dietary isoflavones in humans. J Nutr Biochem. 2005;16:236–244. doi: 10.1016/j.jnutbio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Barton S, Navarro SL, Buas MF, et al. Targeted plasma metabolome response to variations in dietary glycemic load in a randomized, controlled, crossover feeding trial in healthy adults. Food Funct. 2015;6:2949–2956. doi: 10.1039/c5fo00287g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.