Abstract
Hyperlipidemia leads to the formation of oxidized LDL (oxLDL), vessel dysfunction, atherosclerotic disease, and ultimately to plaque rupture and thrombosis. OxLDL induces tissue factor (TF) expression in various cell types, including monocytes and macrophages. High levels of TF are present in atherosclerotic plaques and this represents that major source of TF that triggers thrombosis after plaque rupture. In addition, increased levels of “circulating TF” are observed in hyperlipidemic animals and patients. This is due to induced TF expression in monocytes and monocyte-derived, TF+ microparticles, which represents a minor source of TF that likely contributes to thrombosis after plaques rupture. This review will summarize the connections between hyperlipidemia and TF expression within atherosclerotic plaques and circulating monocytes, as well as its inhibition by statins.
Introduction
Tissue factor (TF) is a transmembrane glycoprotein that serves as the primary initiator of the coagulation cascade.[1-2] TF forms a complex with factor VII/VIIa (FVII/VIIa) that activates both FIX and FX and this leads to thrombin and fibrin generation.[3] Cross-linked fibrin then acts to stabilize thrombi in the vasculature. TF is constitutively expressed by cells surrounding the vessel wall, including vascular smooth muscle cells (VSMCs) and adventitial fibroblasts,[4-6] but is not expressed by endothelial cells and circulating cells under normal physiologic conditions. However, during hyperlipidemia, TF expression is induced in macrophages within atherosclerotic plaques and circulating monocytes.[7] Microparticles (MPs), also called microvesicles, are sub-micron sized membrane vesicles that are released from activated and apoptotic cells.[8-9] Importantly, levels of TF+ MPs are increased in a variety of pathologic states, including hyperlipidemia.[9-11]
This review will discuss (1) TF and atherosclerosis, (2) the link between hyperlipidemia and coagulation, (3) the contribution of monocyte TF and monocyte derived TF+ MPs to hyperlipidemic activation of coagulation, and (4) the inhibitory effect of HMG-CoA reductase inhibitors (statins) on TF expression and thrombosis (Figure 1).
Figure 1. Pathways that lead to TF expression during hyperlipidemia.
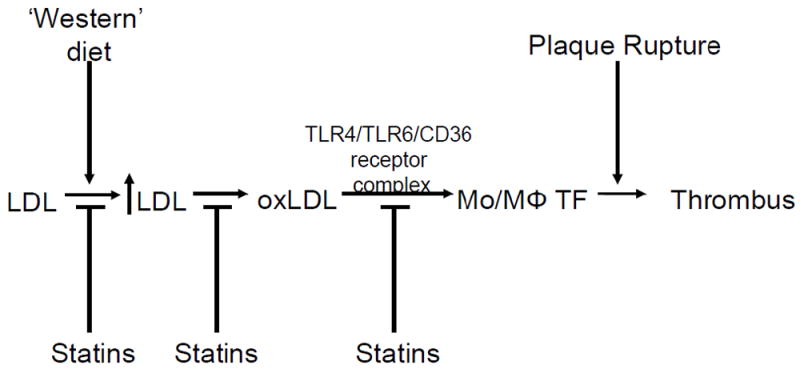
Levels of circulating low density lipoproteins (LDL) are increased with the consumption of a high fat/high cholesterol ‘western’ diet. This results in oxidation of LDL (oxLDL), which can increase the expression of tissue factor (TF) in macrophages (MΦ) and circulating monocytes (Mo), via a TLR4/TLR6/CD36 receptor complex, which release TF+ MPs. Plaque rupture would exposure of high levels of TF to the blood and trigger thrombosis. TF expression in monocytes and monocyte-derived TF+ MPs may also contribute to thrombosis. Statins inhibit increases in LDL, increases in oxLDL, induction of monocyte TF expression, and reduce thrombosis.
TF and Atherosclerosis
Plaque disruption and subsequent arterial thrombosis is a critical event in atherosclerosis resulting in acute vascular syndromes, such as myocardial infarction.[12] TF expression has been found to increase with the progression of human atherosclerotic lesions [13]. For instance, coronary atheroma from patients with unstable angina contained more functional TF than atheroma from patients with stable angina [14]. Furthermore, higher levels of TF activity are observed in plaques with thrombi [15]. Importantly, much of this TF appears to be in the form of TF+ MPs. High levels of TF are also present in atherosclerotic lesions in rabbit and mouse models [16-17]. Macrophages and VSMCs appear to be the major source of TF [18]. Hyperlipidemia is associated with a shorter occlusion time in mouse carotid artery thrombosis model.[9, 19-22] We confirmed these results and further found that inhibition of TF ablates hyperlipidemic-induced thrombosis indicating a key role for TF in this enhanced thrombosis.[23] Taken together, these results suggest that atherosclerotic plaques are the major source of TF that triggers thrombosis after plaque rupture (Figure 1).[4, 18, 24]
Hyperlipidemia and Coagulation
Hyperlipidemia describes a pathological condition in which there are increased lipid concentrations in the blood. These lipids mainly consist of low density lipoprotein (LDL). Previous studies showed that the risk of arterial thrombosis is elevated in patients with increased circulating cholesterol levels in part due to the activation of platelets and the coagulation system.[25-26] Furthermore, patients with type II familial hypercholesterolemia have elevated levels of monocyte TF.[27-28] However, the mechanism of hyperlipidemic induction of systemic coagulation has not been defined.
In the early stages of atherosclerosis, it is thought that circulating LDL infiltrates into the arterial wall due to endothelial dysfunction.[29] The trapped LDL particles become progressively oxidized resulting in the modification of LDL into biologically active lipids termed oxidized LDL (oxLDL). OxLDL is rapidly internalized by infiltrating monocytes/macrophages and this results in the expression of inflammatory mediators that increase endothelial dysfunction, which further increases monocyte infiltration, macrophage foam cell formation, and production of oxLDL.[29] This vicious cycle is the foundation of atherosclerotic disease progression. While the majority of oxLDL is found in the atheroma, small amounts are also detected in the circulation of patients with acute coronary syndromes (ACS).[30-33] Moreover, elevated levels of oxLDL are observed in the plasma of hyperlipidemic mice before atherosclerotic disease is evident.[23, 34] Importantly, oxLDL and other bioactive lipids induce TF expression in monocyte/macrophages, endothelial, and VSMCs.[23, 35-39] These data suggest that oxLDL induction of TF expression may be responsible for the hypercoagulably state observed during hyperlipidemia.
Circulating TF+ MPs in Hyperlipidemic Coagulation
Several studies have reported elevated levels of TF expression in monocytes, monocyte-derived TF+ MPs, and TF in the plasma of patients with hyperlipidemia.[9, 23, 27-28, 40] In addition, several studies have found elevated levels of monocyte/macrophage-derived TF+ MPs and MP TF activity in patients with ACS after a myocardial infarction. These studies have been recently reviewed.[9] These data support the notion that in addition to TF within the atherosclerotic plaque TF+ monocytes and monocyte-derived, TF+ MPs may contribute to the formation of an occlusive thrombus after plaque rupture (Figure 1).
We recently demonstrated that hyperlipidemia resulted in a step-wise increase in plasma MP TF activity, which was associated with elevated levels of thrombin-antithrombin in both mice and monkeys fed a high-fat ‘Western’ diet.[23] In addition, TF expression is induced in peripheral blood mononuclear cells after prolonged hyperlipidemia. Finally, we demonstrated these events coincide with step-wise increases in oxLDL and not total cholesterol, are ablated by in vivo administration of an anti-TF antibody, and are dependent upon hematopoietic cell-derived TF. Together, these studies support the notion that monocyte-derived TF is responsible for hyperlipidemic systemic activation of coagulation and that MP TF activity may serve as a biomarker for this hypercoagulable state.
The carotid artery model of thrombosis is not ideal for studying the role of TF+ MPs in thrombosis because it exposes TF within the vessel wall and hyperlipidemia increases TF expression in the vessel wall and also activates platelets.[9, 41-43] Therefore, we utilized the laser-injury cremaster arteriole model of thrombosis, which is a better model to assess the impact of hematopoietic cell-derived TF+ MPs in thrombus formation.[9] We found that hyperlipidemia significantly augmented the accumulation of fibrin and platelets at the site of arteriole injury.[23] To assess whether hematopoietic cells contributed to this injury, we irradiated LDL receptor deficient mice and repopulated them with bone marrow from mice expressing human TF in place of mouse TF.[44] Importantly, we found that an anti-human TF antibody reduced the amount of fibrin deposition and platelet accumulation in the cremaster thrombosis model versus an irrelevant control antibody in hyperlipidemic mice (Owens, Passam, Furie, Furie and Mackman, unpublished data). These data suggest that the increased levels of monocyte-derived TF+ MPs observed during hyperlipidemia can enhance thrombosis (Figure 1).
Statins and Thrombosis
Statins were originally developed to decrease the amount of plasma cholesterol based on the ‘lipid hypothesis’ of atherosclerosis.[45] However, in addition to reducing the incidence of arterial thrombosis by decreasing plasma LDL or regression of atherosclerosis,[46] statins also have pleiotrophic effects, including anti-inflammatory activity and inhibition of prenylation of intracellular signaling proteins.[47-48] Importantly, statins inhibit TF expression by monocytes and macrophages.[27-28, 36, 38, 49-51] In particular, Ferro and colleagues demonstrate simvastatin treatment of type II familial hypercholesterolemic patients reduces monocyte TF and prothrombin fragment F1+2.[28] Further, treatment of hyperlipidemic mice with either simvastatin or rosuvastatin reduced aortic and atherosclerotic TF expression without reducing lipid levels.[47, 52] In addition, simvastatin and pravastatin treated hyperlipidemic pigs and monkeys had reduced inflammation and thrombogenicity without affecting lipids levels.[53-54] We recently demonstrated that simvastatin administration could completely attenuate hyperlipidemic-induced increases in oxLDL, peripheral blood mononuclear cell TF, MP TF activity and TAT (all independent of changes in plasma cholesterol and LDL) in both mice and monkeys.[23] These studies extend a previous study by Undas and colleagues that demonstrated lipid-independent anti-inflammatory and antithrombotic effects in human hyperlipidemic patients with short-term simvastatin therapy.[55] These data suggest that the anticoagulant activity of simvastatin during hyperlipidemia is in part due to its ability to inhibit monocyte TF expression (Figure 1).
Statin therapy can also reduce the risk of venous thromboembolism (VTE) in patients with established cardiovascular disease [56] and in healthy patients with idiopathic VTE.[57-58] Furthermore, a recent clinical trial called JUPITER evaluated preemptive administration of rosuvastatin to individuals with normal LDL but elevated levels of inflammation.[59] Statin therapy significantly reduced the incidence of major cardiovascular events and symptomatic VTE.[60] It was speculated that the reduction in thrombosis was due to statin inhibition of TF expression, but this was not analyzed.[60] Several publications concur with the findings of the JUPITER study [61-63] and when grouped together in large meta-analyses [64-66] further support the notion that statin treatment reduces the risk of VTE. Statins may reduce TF expression indirectly by inhibiting the expression of inflammatory mediators. However, this appears to be unlikely because cytokines are weak inducers of monocyte TF expression and inflammation is not a risk factor for VTE. Thus it seems more likely that statins are reducing VTE by directly inhibiting monocyte TF expression (Figure 1).
Conclusion
In summary, TF expression within the atherosclerotic plaque is the major source of TF that triggers thrombosis after plaque rupture. In addition, TF expression in circulating monocytes and monocyte-derived TF+ MPs is also likely to contribute to formation of thrombus. TF+ MPs may be a useful biomarker for monitoring the activation of coagulation in hyperlipidemic and atherosclerotic patients. Statin therapy can reduce hyperlipidemia, monocyte TF, TF+ MPs, and the incidence of both arterial and venous thrombosis. This anti-coagulant activity of statins appears to be due to their ability to inhibit monocyte TF expression. However, it is possible that inflammatory mediators also contribute to pathological TF expression. In light of all the aforementioned studies, statins label as ‘the miracle drug’ and ‘new aspirin of the 21st century’ seems to be a profound and prophetic statement.
Acknowledgments
This work was supported by a National Institutes of Health grants 1F32-HL099175-02 (APO III) and PO1-HL006350-34 (NM).
Footnotes
COMPETING INTERESTS STATEMENT: Nigel Mackman is a consultant for Merck.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bach RR. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem. 1988;23:339–68. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 2.Edgington TS, Mackman N, Brand K, Ruf W. The structural biology of expression and function of tissue factor. Thromb Haemost. 1991;66:67–79. [PubMed] [Google Scholar]
- 3.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–22. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 4.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 5.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;59:421–37. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 6.Flossel C, Luther T, Muller M, Albrecht S, Kasper M. Immunohistochemical detection of tissue factor (TF) on paraffin sections of routinely fixed human tissue. Histochemistry. 1994;101:449–53. doi: 10.1007/BF00269495. [DOI] [PubMed] [Google Scholar]
- 7.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 8.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101:439–51. [PubMed] [Google Scholar]
- 9.Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–97. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llorente-Cortes V, Otero-Vinas M, Camino-Lopez S, Llampayas O, Badimon L. Aggregated low-density lipoprotein uptake induces membrane tissue factor procoagulant activity and microparticle release in human vascular smooth muscle cells. Circulation. 2004;110:452–9. doi: 10.1161/01.CIR.0000136032.40666.3D. [DOI] [PubMed] [Google Scholar]
- 11.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–3. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 12.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–8. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatakeyama K, Asada Y, Marutsuka K, Sato Y, Kamikubo Y, Sumiyoshi A. Localization and activity of tissue factor in human aortic atherosclerotic lesions. Atherosclerosis. 1997;133:213–9. doi: 10.1016/s0021-9150(97)00132-9. [DOI] [PubMed] [Google Scholar]
- 14.Annex BH, Denning SM, Channon KM, Sketch MH, Jr, Stack RS, Morrissey JH, et al. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation. 1995;91:619–22. doi: 10.1161/01.cir.91.3.619. [DOI] [PubMed] [Google Scholar]
- 15.Marmur JD, Thiruvikraman SV, Fyfe BS, Guha A, Sharma SK, Ambrose JA, et al. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94:1226–32. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- 16.Kato K, Elsayed YA, Namoto M, Nakagawa K, Sueishi K. Enhanced expression of tissue factor activity in the atherosclerotic aortas of cholesterol-fed rabbits. Thromb Res. 1996;82:335–47. doi: 10.1016/0049-3848(96)00083-7. [DOI] [PubMed] [Google Scholar]
- 17.Aikawa M, Voglic SJ, Sugiyama S, Rabkin E, Taubman MB, Fallon JT, et al. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation. 1999;100:1215–22. doi: 10.1161/01.cir.100.11.1215. [DOI] [PubMed] [Google Scholar]
- 18.Thiruvikraman SV, Guha A, Roboz J, Taubman MB, Nemerson Y, Fallon JT. In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X. Lab Invest. 1996;75:451–61. [PubMed] [Google Scholar]
- 19.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1831–4. doi: 10.1161/01.atv.20.7.1831. [DOI] [PubMed] [Google Scholar]
- 20.Schafer K, Muller K, Hecke A, Mounier E, Goebel J, Loskutoff DJ, et al. Enhanced thrombosis in atherosclerosis-prone mice is associated with increased arterial expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2003;23:2097–103. doi: 10.1161/01.ATV.0000097766.36623.DF. [DOI] [PubMed] [Google Scholar]
- 21.Wilson KM, McCaw RB, Leo L, Arning E, Lhotak S, Bottiglieri T, et al. Prothrombotic effects of hyperhomocysteinemia and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:233–40. doi: 10.1161/01.ATV.0000251607.96118.af. [DOI] [PubMed] [Google Scholar]
- 22.Owens AP, 3rd, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost. 2010;104:432–9. doi: 10.1160/TH09-11-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens AP, 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2011 doi: 10.1172/JCI58969. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86:2839–43. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–7. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 26.Kaul S, Waack BJ, Padgett RC, Brooks RM, Heistad DD. Altered vascular responses to platelets from hypercholesterolemic humans. Circ Res. 1993;72:737–43. doi: 10.1161/01.res.72.4.737. [DOI] [PubMed] [Google Scholar]
- 27.Ferro D, Basili S, Alessandri C, Cara D, Violi F. Inhibition of tissue-factor-mediated thrombin generation by simvastatin. Atherosclerosis. 2000;149:111–6. doi: 10.1016/s0021-9150(99)00291-9. [DOI] [PubMed] [Google Scholar]
- 28.Ferro D, Basili S, Alessandri C, Mantovani B, Cordova C, Violi F. Simvastatin reduces monocyte-tissue-factor expression type IIa hypercholesterolaemia. Lancet. 1997;350:1222. doi: 10.1016/S0140-6736(05)63452-6. [DOI] [PubMed] [Google Scholar]
- 29.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 30.Holvoet P, Theilmeier G, Shivalkar B, Flameng W, Collen D. LDL hypercholesterolemia is associated with accumulation of oxidized LDL, atherosclerotic plaque growth, and compensatory vessel enlargement in coronary arteries of miniature pigs. Arterioscler Thromb Vasc Biol. 1998;18:415–22. doi: 10.1161/01.atv.18.3.415. [DOI] [PubMed] [Google Scholar]
- 31.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–94. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 32.Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, Kronenberg F, et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. 2006;47:2219–28. doi: 10.1016/j.jacc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. doi: 10.1161/01.atv.21.1.95. [DOI] [PubMed] [Google Scholar]
- 34.Palinski W, Tangirala RK, Miller E, Young SG, Witztum JL. Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1569–76. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 35.Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, et al. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99:199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- 36.Schuff-Werner P, Claus G, Armstrong VW, Kostering H, Seidel D. Enhanced procoagulatory activity (PCA) of human monocytes/macrophages after in vitro stimulation with chemically modified LDL. Atherosclerosis. 1989;78:109–12. doi: 10.1016/0021-9150(89)90214-1. [DOI] [PubMed] [Google Scholar]
- 37.Drake TA, Hannani K, Fei HH, Lavi S, Berliner JA. Minimally oxidized low-density lipoprotein induces tissue factor expression in cultured human endothelial cells. Am J Pathol. 1991;138:601–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Meisel SR, Xu XP, Edgington TS, Cercek B, Ong J, Kaul S, et al. Dose-Dependent Modulation of Tissue Factor Protein and Procoagulant Activity in Human Monocyte-Derived Macrophages by Oxidized Low Density Lipoprotein. J Atheroscler Thromb. 2011 doi: 10.5551/jat.7179. [DOI] [PubMed] [Google Scholar]
- 39.Cui MZ, Penn MS, Chisolm GM. Native and oxidized low density lipoprotein induction of tissue factor gene expression in smooth muscle cells is mediated by both Egr-1 and Sp1. J Biol Chem. 1999;274:32795–802. doi: 10.1074/jbc.274.46.32795. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto N, Nomura S, Kamihata H, Kimura Y, Iwasaka T. Increased level of oxidized LDL-dependent monocyte-derived microparticles in acute coronary syndrome. Thromb Haemost. 2004;91:146–54. doi: 10.1160/TH03-04-0247. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh A, Li W, Febbraio M, Espinola RG, McCrae KR, Cockrell E, et al. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J Clin Invest. 2008;118:1934–43. doi: 10.1172/JCI34904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–95. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–13. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, et al. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5:1693–700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res. 2006;47:1339–51. doi: 10.1194/jlr.R600009-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 47.Monetti M, Canavesi M, Camera M, Parente R, Paoletti R, Tremoli E, et al. Rosuvastatin displays anti-atherothrombotic and anti-inflammatory properties in apoE-deficient mice. Pharmacol Res. 2007;55:441–9. doi: 10.1016/j.phrs.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Tuomisto TT, Lumivuori H, Kansanen E, Hakkinen SK, Turunen MP, van Thienen JV, et al. Simvastatin has an anti-inflammatory effect on macrophages via upregulation of an atheroprotective transcription factor, Kruppel-like factor 2. Cardiovasc Res. 2008;78:175–84. doi: 10.1093/cvr/cvn007. [DOI] [PubMed] [Google Scholar]
- 49.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–83. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 50.Baetta R, Camera M, Comparato C, Altana C, Ezekowitz MD, Tremoli E. Fluvastatin reduces tissue factor expression and macrophage accumulation in carotid lesions of cholesterol-fed rabbits in the absence of lipid lowering. Arterioscler Thromb Vasc Biol. 2002;22:692–8. doi: 10.1161/01.atv.0000012802.69414.a8. [DOI] [PubMed] [Google Scholar]
- 51.Colli S, Eligini S, Lalli M, Camera M, Paoletti R, Tremoli E. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol. 1997;17:265–72. doi: 10.1161/01.atv.17.2.265. [DOI] [PubMed] [Google Scholar]
- 52.Bea F, Blessing E, Shelley MI, Shultz JM, Rosenfeld ME. Simvastatin inhibits expression of tissue factor in advanced atherosclerotic lesions of apolipoprotein E deficient mice independently of lipid lowering: potential role of simvastatin-mediated inhibition of Egr-1 expression and activation. Atherosclerosis. 2003;167:187–94. doi: 10.1016/s0021-9150(02)00387-8. [DOI] [PubMed] [Google Scholar]
- 53.Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol. 2002;22:1452–8. doi: 10.1161/01.atv.0000030360.72503.56. [DOI] [PubMed] [Google Scholar]
- 54.Casani L, Sanchez-Gomez S, Vilahur G, Badimon L. Pravastatin reduces thrombogenicity by mechanisms beyond plasma cholesterol lowering. Thromb Haemost. 2005;94:1035–41. doi: 10.1160/TH05-04-0245. [DOI] [PubMed] [Google Scholar]
- 55.Undas A, Celinska-Lowenhoff M, Domagala TB, Iwaniec T, Dropinski J, Lowenhoff T, et al. Early antithrombotic and anti-inflammatory effects of simvastatin versus fenofibrate in patients with hypercholesterolemia. Thromb Haemost. 2005;94:193–9. doi: 10.1160/TH05-01-0067. [DOI] [PubMed] [Google Scholar]
- 56.Herrington DM, Vittinghoff E, Lin F, Fong J, Harris F, Hunninghake D, et al. Statin therapy, cardiovascular events, and total mortality in the Heart and Estrogen/Progestin Replacement Study (HERS) Circulation. 2002;105:2962–7. doi: 10.1161/01.cir.0000019406.74017.b2. [DOI] [PubMed] [Google Scholar]
- 57.Ray JG, Mamdani M, Tsuyuki RT, Anderson DR, Yeo EL, Laupacis A. Use of statins and the subsequent development of deep vein thrombosis. Arch Intern Med. 2001;161:1405–10. doi: 10.1001/archinte.161.11.1405. [DOI] [PubMed] [Google Scholar]
- 58.Yang CC, Jick SS, Jick H. Statins and the risk of idiopathic venous thromboembolism. Br J Clin Pharmacol. 2002;53:101–5. doi: 10.1046/j.0306-5251.2001.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ridker PM, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Khurmi NS, et al. Baseline characteristics of participants in the JUPITER trial, a randomized placebo-controlled primary prevention trial of statin therapy among individuals with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein. Am J Cardiol. 2007;100:1659–64. doi: 10.1016/j.amjcard.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 60.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–61. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Resh M, Mahmoodi BK, Navis GJ, Veeger NJ, Lijfering WM. Statin use in patients with nephrotic syndrome is associated with a lower risk of venous thromboembolism. Thromb Res. 2011;127:395–9. doi: 10.1016/j.thromres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorensen HT, Horvath-Puho E, Sogaard KK, Christensen S, Johnsen SP, Thomsen RW, et al. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2009;7:521–8. doi: 10.1111/j.1538-7836.2009.03279.x. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal V, Phung OJ, Tongbram V, Bhardwaj A, Coleman CI. Statin use and the prevention of venous thromboembolism: a meta-analysis. Int J Clin Pract. 2010;64:1375–83. doi: 10.1111/j.1742-1241.2010.02439.x. [DOI] [PubMed] [Google Scholar]
- 65.Squizzato A, Galli M, Romualdi E, Dentali F, Kamphuisen PW, Guasti L, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248–56. doi: 10.1093/eurheartj/ehp556. [DOI] [PubMed] [Google Scholar]
- 66.Pai M, Evans NS, Shah SJ, Green D, Cook D, Crowther MA. Statins in the prevention of venous thromboembolism: A meta-analysis of observational studies. Thromb Res. 2011;128:422–30. doi: 10.1016/j.thromres.2011.05.012. [DOI] [PubMed] [Google Scholar]


