 In the presence of 5 mol% Pd(OAc)2, 1 equiv. of norbornene, and K2CO3, the reaction of 4-iodo-2-quinolones with tertiary o-bromobenzylic alcohols produced the desired benzopyran-fused 2-quinolones in moderate to high yields.
In the presence of 5 mol% Pd(OAc)2, 1 equiv. of norbornene, and K2CO3, the reaction of 4-iodo-2-quinolones with tertiary o-bromobenzylic alcohols produced the desired benzopyran-fused 2-quinolones in moderate to high yields.
Abstract
In the presence of 5 mol% Pd(OAc)2, 1 equiv. of norbornene, and K2CO3, the reaction of 4-iodo-2-quinolones with tertiary o-bromobenzylic alcohols produced the desired benzopyran-fused 2-quinolones in moderate to high yields. A Catellani-type mechanism involving vinylic C–H cleavage is proposed based on the results of control experiments and density functional theory calculations.
Introduction
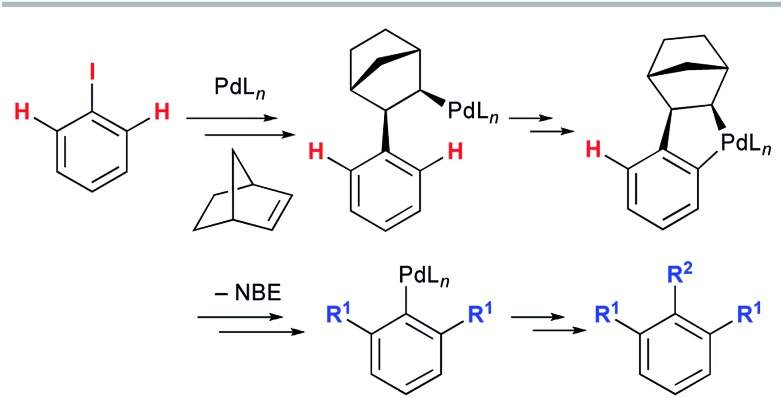
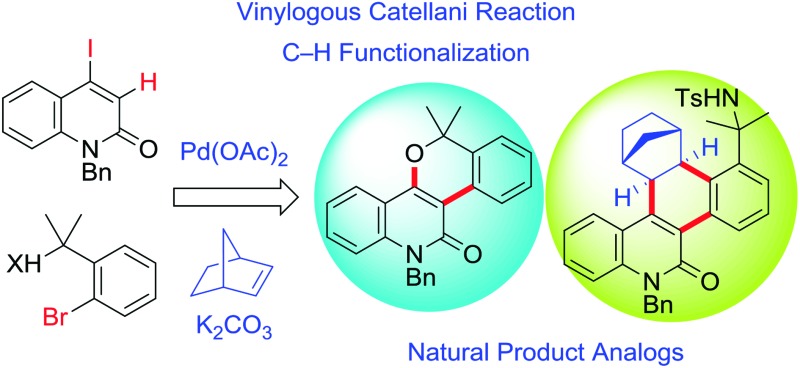
The late-stage functionalization of heterocycles by C–H bond activation has an enormous potential for straightforward access to diverse bioactive compounds.1 However, this strategy has been applied much less frequently to non-aromatic heterocyclic scaffolds,2 because vinylic C–H bonds are generally more challenging to functionalize than (hetero)aromatic C–H bonds.3 Therefore, a method to assemble fused heterocyclic structures by vinylic C–H functionalization remains elusive. We focused on the palladium-catalyzed, norbornene (NBE)-mediated C–H functionalization reaction (the Catellani reaction). This is because this reaction allows multiple functionalizations of contiguous sp2 carbon centers starting from an iodinated carbon, enabling divergent transformations of relatively simple iodo(hetero)arenes (Scheme 1).4 However, the current scope of the Catellani reaction is limited to (hetero)aromatic substrates; no example of a Catellani-type vinylic C–H functionalization has been reported, to the best of our knowledge.4
Scheme 1. The palladium-catalyzed, norbornene-mediated C–H functionalization of an iodoarene (the Catellani reaction).
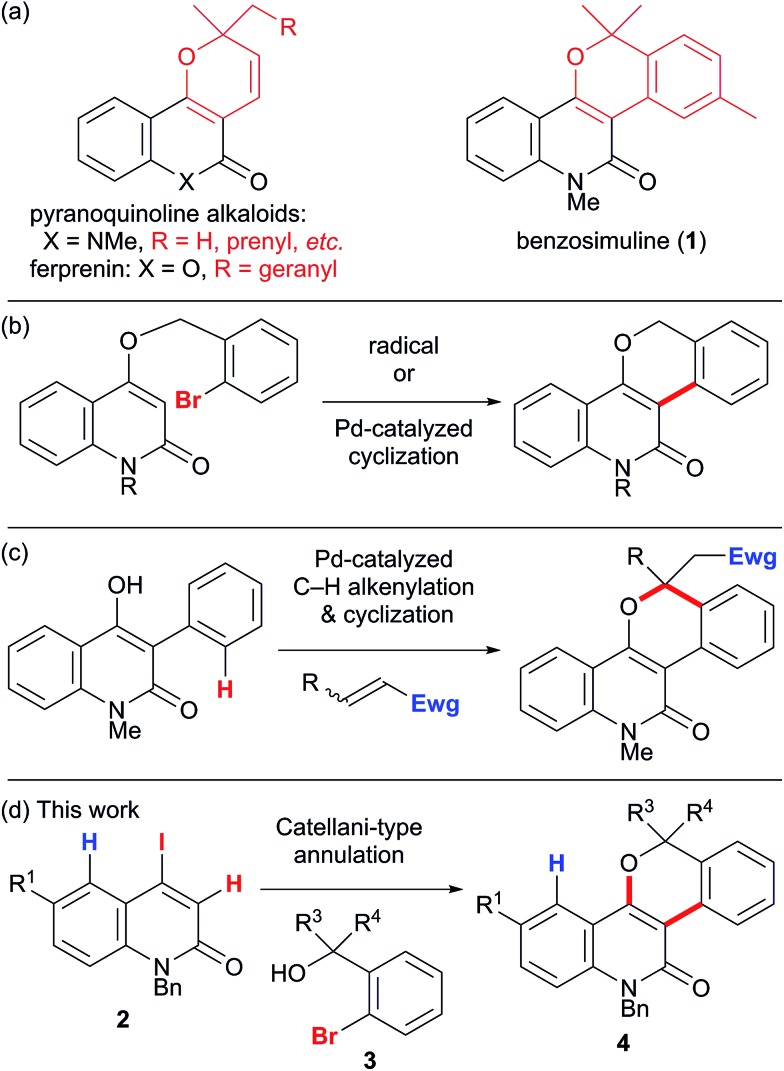
Pyran-fused 2-quinolone derivatives are widespread in nature (pyranoquinoline alkaloids), and the construction of this privileged structure has attracted continual attention, owing to the interesting biological activities of these compounds (Scheme 2a).5 Ferprenin, a related natural product with the pyranocoumarin framework, was also reported to have interesting biological activity.6 In contrast, the synthesis of benzopyran-fused derivatives (e.g. the natural product benzosimuline (1) which shows antiplatelet aggregation activity)7 has been less investigated. Therefore, an efficient synthetic method that enables the divergent synthesis of benzopyran-fused derivatives would be highly beneficial for drug discovery. However, construction of the benzopyran-fused 2-quinolone framework is limited to a few methods. One practical example is the radical or palladium-catalyzed cyclization of 4-(o-bromophenyl)methoxy-2-quinolones (Scheme 2b).8 This route reliably produces the desired products in high yields. Nevertheless, preparation of the cyclization precursors is necessary and no example with a tertiary benzylic center has been reported. Alternatively, a palladium-catalyzed oxidative annulation of 4-hydroxy-3-phenyl-2-quinolones has recently been developed (Scheme 2c).9 This method is remarkable because an aromatic C–H alkenylation is utilized to obviate the use of aryl halides. However, the introduction of the desired aryl group to 4-hydroxyquinolone at the 3-position is required; thus, only a phenyl group has been investigated. Michael acceptors are also required for the final ring-closing step. Therefore, the synthesis of benzosimuline analogs has been severely limited with the existing methods. To address this problem, we envisaged that the Catellani-type annulation of 4-iodo-2-quinolones 2 with o-bromobenzyl alcohols 3 would afford the desired benzosimuline derivatives 4 in a modular manner (Scheme 2d). This method has an advantage over previous examples as the combination of 4-iodo-2-quinolines with o-bromobenzylic alcohols enables the divergent synthesis of benzosimuline analogs. Herein, we report the results of our study on the vinylogous Catellani reaction of 4-iodo-2-quinolones. To elucidate the reaction mechanism, we also performed density functional theory (DFT) calculations.
Scheme 2. (a) Pyran-fused 2-quinolone scaffolds in nature, (b and c) previous syntheses of benzopyran-fused 2-quinolones, and (d) the synthesis of benzopyran-fused 2-quinolones using the vinylogous Catellani reaction.
Results and discussion
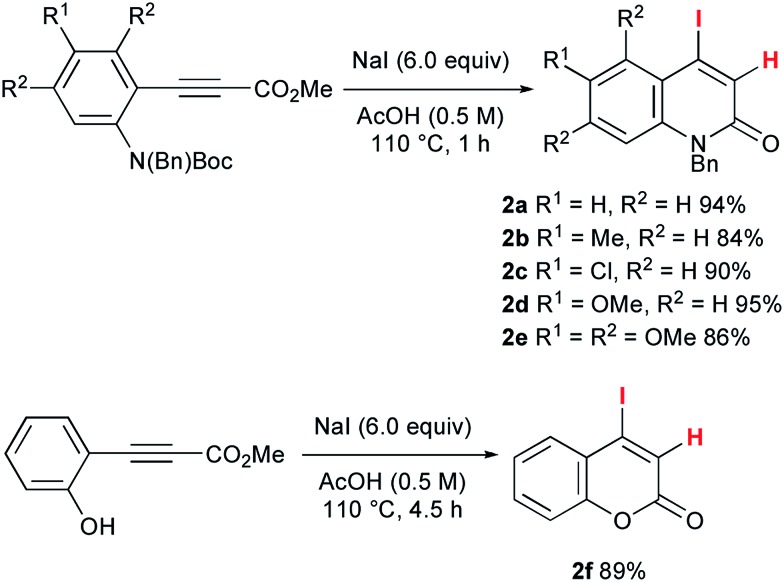
To accomplish the elusive Catellani-type annulation, the use of 4-iodo-2-quinolones, which are expected to be more susceptible to oxidative addition than bromoarenes, is necessary, because the oxidative addition of the 2-quinolone precursors should precede the oxidative addition of o-bromobenzyl alcohols. However, hardly any 4-iodo-2-quinolones have been accessible previously, even though diverse 4-bromo-2-quinolones are available. Therefore, this study commenced with the preparation of unknown 4-iodo-2-quinolones 2 (Scheme 3). In a similar manner to the procedure reported by Piers and co-workers,10 propiolates with orthogonally protected o-aniline terminals, which were previously prepared by our group,11 were treated with NaI in AcOH at 110 °C for 1 h. Under the reaction conditions, hydroiodination with concomitant removal of the Boc group was followed by lactam formation to afford the desired 4-iodo-2-quinolones 2a–e in good yields. Notably, a similar reaction with ethyl 3-phenylpropiolate was reported to give the anti-hydroiodination product ((Z)-ethyl 3-iodo-3-phenylacrylate).10 In this case, the initial syn-hydroiodination product presumably underwent isomerization to the observed anti-hydroiodination product. In striking contrast, lactam formation proceeded faster than the E/Z isomerization to afford 4-iodo-2-quinolones 2. Similarly, 4-iodocoumarin 2f was prepared from (o-hydroxyphenyl)propiolate in 89% yield.
Scheme 3. The synthesis of 4-iodo-2-quinolones 2a–e and 4-iodocoumarin 2f.
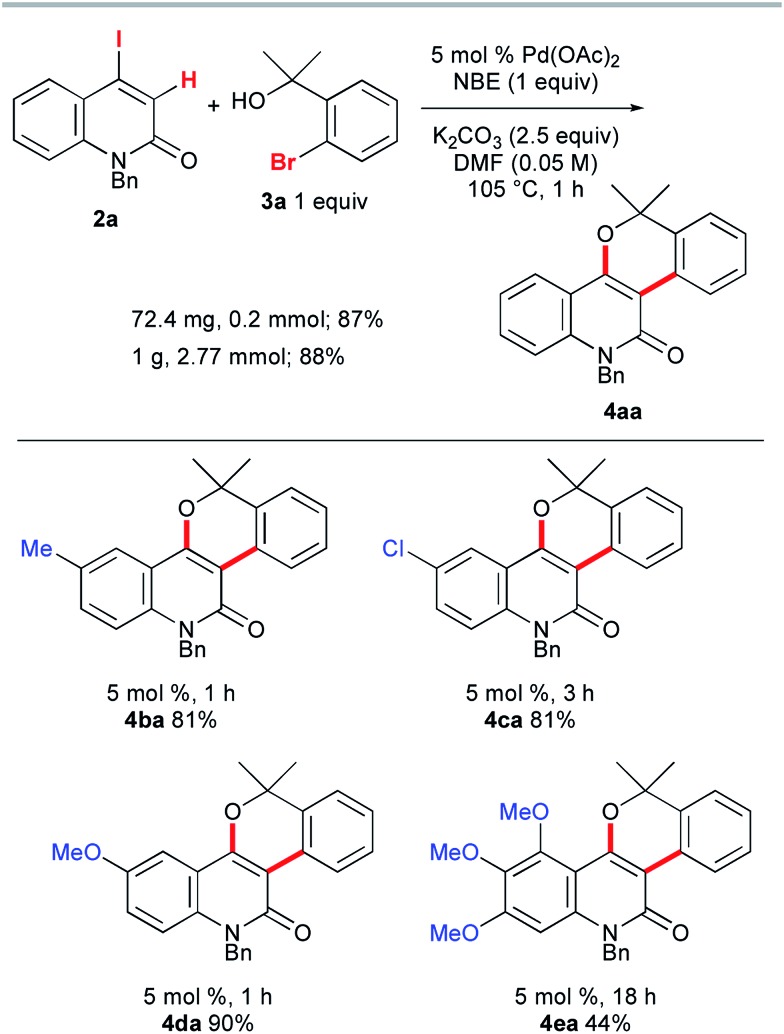
Once we had obtained precursor 2a, its Catellani-type annulation with o-bromobenzyl alcohol 3a was investigated (Scheme 4). Catellani and co-workers reported that the reaction of iodobenzenes with o-bromobenzylic alcohols bearing no benzylic hydrogen proceeded in the presence of a palladium catalyst and NBE to produce dibenzopyrans.12 According to their procedure, 2a and 3a were treated with 5 mol% Pd(OAc)2, 1 equiv. of NBE, and 2.5 equiv. of K2CO3 in DMF at 105 °C for 1 h. Gratifyingly, the desired annulation product 4aa was obtained in 87% yield after purification by silica gel chromatography. The same reaction was conducted using KOAc (2.5 equiv.) instead of K2CO3, affording 4aa with a similar efficiency (93% NMR). Therefore, KOAc functions as the net base. In contrast, the use of Cs2CO3 or Na2CO3 as the base significantly reduced the efficiency (4% and 29% NMR, respectively), indicating that the potassium ion plays an important role. To examine the scalability, the reaction of 2a with 3a was performed at the 1 g scale to obtain 4aa in 88% yield.
Scheme 4. The Catellani-type annulation of 4-iodo-2-quinolones 2a–e with 3a.
Next, the influence of substituents on the quinolone benzene ring was investigated. The reactions of benzyl alcohol 3a with 4-iodo-2-quinolones 2b–d, bearing methyl, chloro, or methoxy substituents at the 6-position, afforded the corresponding annulation products 4ba, 4ca, and 4da in 81–90% yields (Scheme 4). However, the formation of the trimethoxy analog 4ea was sluggish (18 h) and the yield was moderate (44%). In striking contrast, the reaction of 4-iodocoumarin 2f under the same conditions led to a complex product mixture, although a modified procedure enabled the desired annulation of 2f in a high yield (see below).
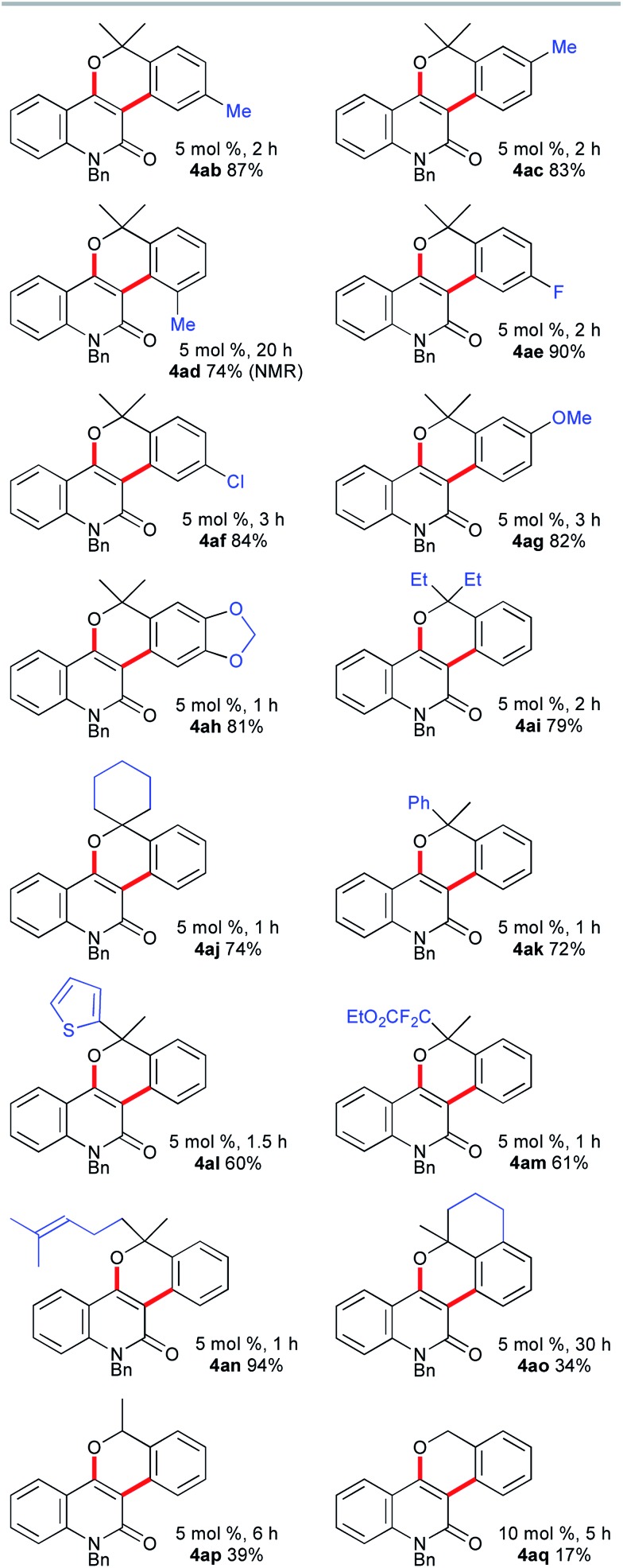
The scope of the synthesis of benzopyran-fused 2-quinolones with the vinylogous Catellani reaction was investigated, and the results are summarized in Fig. 1. The use of 4-methyl- and 5-methyl-2-bromobenzyl alcohols 3b and 3c under similar conditions afforded 4ab, which is an N-benzyl analog of benzosimuline (1), and 4ac in 87% and 83% yields, respectively. In contrast, the reaction with 3d was not complete within 20 h, suggesting that the methyl group next to the bromine substituent slowed the oxidative addition and/or subsequent reductive elimination (see below). Other benzyl alcohols, bearing fluoro, chloro, or methoxy substituents, also underwent smooth annulation to afford the corresponding products 4ae–4ag in high yields. The 1,3-benzodioxole-fused analog 4ah was obtained in 81% yield. The reaction of 2a with benzyl alcohols 3i and 3j bearing a diethylmethylene and cyclohexyl moiety, respectively, successfully afforded 4ai and spirocycle 4aj in 79% and 74% yields. Alcohols 3k–n substituted unsymmetrically at the benzylic position were used, producing 4ak, 4al, 4am and 4an in 60–94% yields. Thus, 2-thienyl, α,α-difluoro ester, and trisubstituted alkene moieties were well tolerated. Although the reaction of the fused benzyl alcohol 3o was very sluggish and required a prolonged reaction time, an interesting polyfused derivative 4ao was obtained in 34% yield. In this case, a small amount of the three-component annulation product was also formed along with unidentified byproducts (see below). Moreover, the reactions of 2a with secondary and primary benzyl alcohols 3p and 3q afforded 4ap and 4aq in much lower yields than that of 4aa. These results suggest that tertiary benzyl alcohols are essential for high-yield formation of the chromeno-2-quinolones because primary and secondary benzyl alcohols undergo oxidation under the reaction conditions, as reported by Catellani and coworkers.12
Fig. 1. The scope of benzylic alcohols 3.
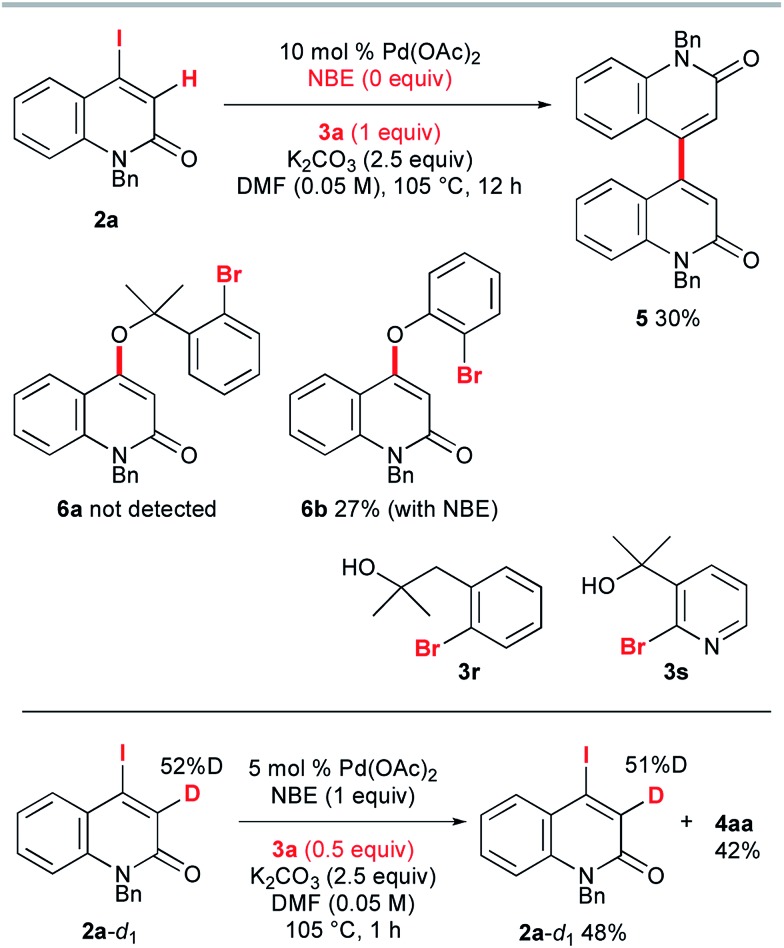
To gain insight into the reaction mechanism, several control experiments were performed (Scheme 5). First, NBE proved to be indispensable for the reaction: in its absence, 2a and 3a were treated with 10 mol% Pd(OAc)2 and 2.5 equiv. of K2CO3 in DMF at 105 °C for 12 h, but no trace of the annulation product was observed. Instead, the homocoupling product 5 was obtained in 30% yield. The C–O coupling product 6a could not be detected. When o-bromophenol was used instead of 3a, 4-aryloxy-2-quinolone 6b was obtained in 27% yield, even when 1 equiv. of NBE was added under the same conditions. Presumably, the acidic proton of o-bromophenol was abstracted by K2CO3 and the conjugate addition of the resultant aryloxide was followed by removal of iodide to produce 6b. These results suggest that the intramolecular Heck pathway via the initial formation of 6a and 6b can be disregarded. No reaction occurred when the homobenzylic alcohol 3r and pyridyl derivative 3s were used. The reaction of 3-deutero-2-quinolone 2a-d1 with 52% deuterium content was performed with 0.5 equiv. of 3a to investigate the kinetic isotope effect. The 1H NMR spectroscopic analysis of recovered 2a-d1 showed that the deuterium content remained almost the same as that of the starting material 2a-d1. Therefore, the reaction rates for 2a and 2a-d1 are almost the same (kH/kD ≈ 1) and it can be concluded that the vinylic C–H cleavage is not the rate-determining step.13
Scheme 5. Control experiments.
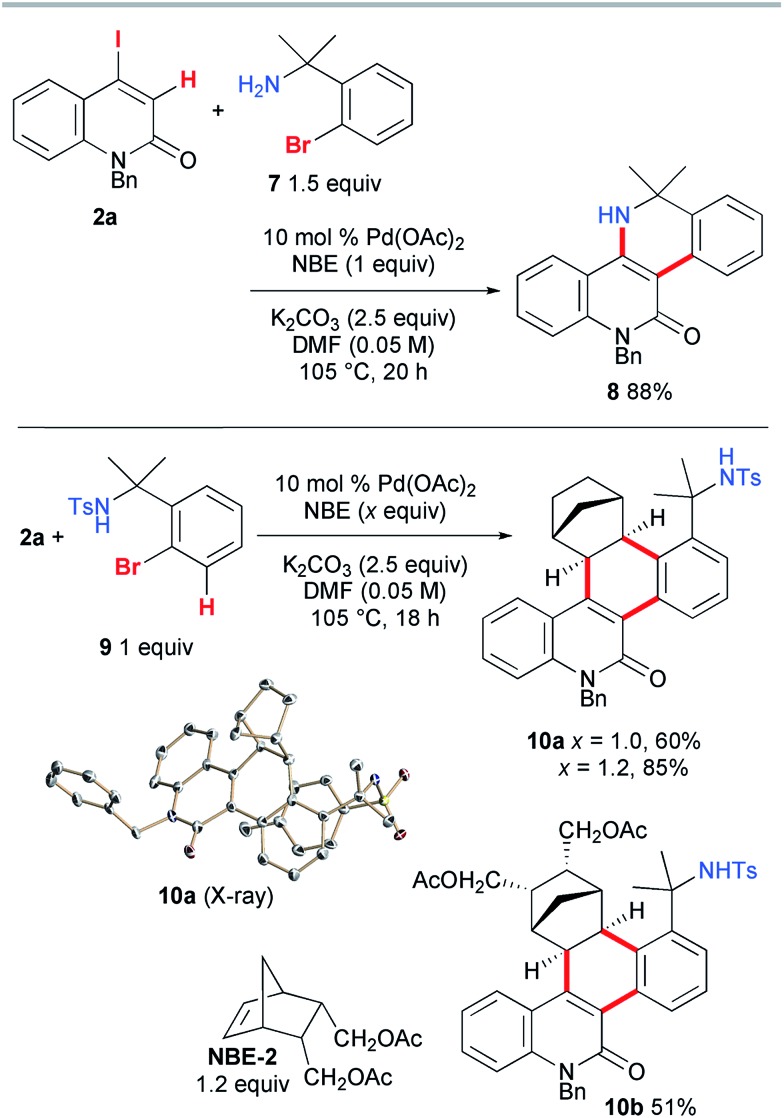
Next, the vinylogous Catellani reaction was performed with benzylamines (Scheme 6). Under similar conditions, except for an increased loading of Pd(OAc)2 (10 mol%), 2a and 1.5 equiv. of 7 were heated for 20 h, affording the desired 5,6-dihydrodibenzo[c,h][1,6]naphthyridin-11(12H)-one 8 in 88% yield. In contrast, the use of tosylamide 9 instead of 7 led to the interrupted Catellani product 10a in 60% yield. A good quality single crystal was obtained, so the structure of 10a was unambiguously confirmed by X-ray diffraction analysis. As expected, NBE reacted from its exo face, and the C–H bond at the 3-position of 9 was involved in the reaction instead of the amide moiety. This indicates that, as proposed for other Catellani reactions, carbopalladation of NBE indeed occurred, and in addition to the vinylic C–H bond of 2a, the aromatic C–H bond of 9 was cleaved, leading to dehydrogenative coupling.14 Because one equivalent of NBE was required for the formation of 10a, the increased loading of NBE (1.2 equiv.) improved the yield to 85%. When a substituted NBE derivative (NBE-2) was used, the corresponding three-component annulation product 10b was obtained in a moderate yield (51%), indicating that the substitution of NBE has a negative effect on the reaction efficiency.
Scheme 6. Vinylic Catellani-type reactions involving benzylamines.
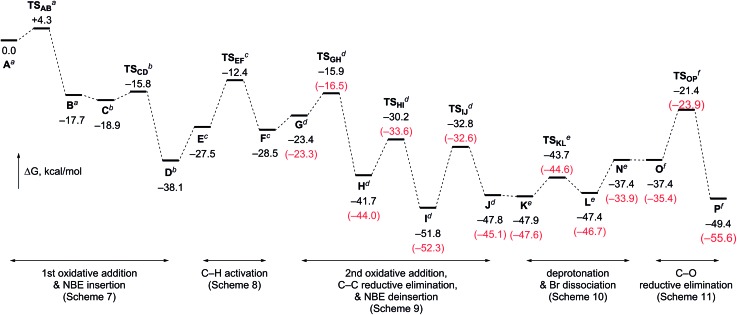
To gain insight into the fundamental steps of the vinylogous Catellani reaction, DFT calculations were performed at the PCM (DMF) rM06L/6-311G++(d,p)-SDD//rB3LYP/6-31G(d)-LanL2DZ level of theory (for details, see ESI†). This method has been reliably used for the computational analysis of carboxylate-assisted C–H activations.15 For computational efficiency, the N-benzyl groups of the real molecules were replaced by smaller methyl groups in the model molecules. In addition, the primary benzyl alcohol 3q was used instead of the tertiary benzyl alcohol 3a for the model reactions, because the inefficiency of 3q can be attributed to its susceptibility to oxidation under the experimental conditions.12 Calculations on simple ligand exchanges were omitted because they are facile and reversible.
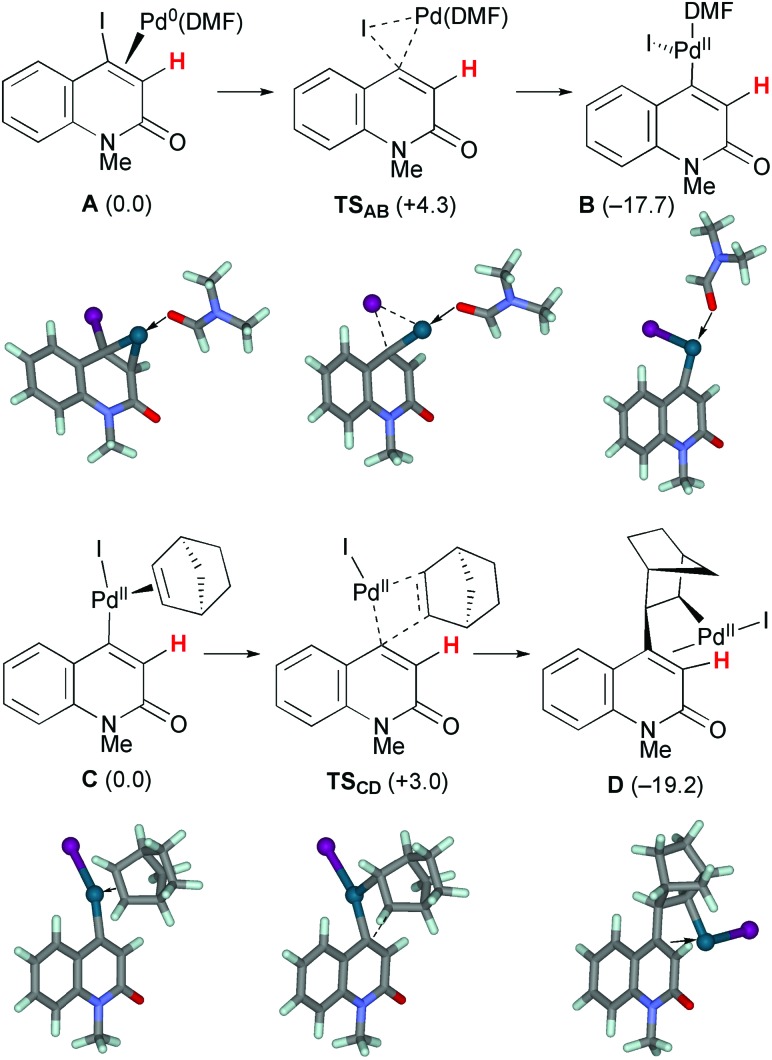
The first oxidative addition step starts with the 4-iodo-2-quinolone Pd(0) complex A with a DMF ligand (Scheme 7). Under ligandless conditions, the abundant solvent molecule, DMF, should act as the supporting ligand for the Pd(0) species. o-Bromobenzyl alcohol 3q can be a bidentate ligand; however, the smaller and more electron-donating DMF ligand more efficiently promotes oxidative addition.16 The Pd(0)(DMF) fragment was coordinated by the 2-quinolone at the electron-deficient alkene moiety. Oxidative addition proceeded viaTSAB with a small activation energy of ΔG‡ = +4.3 kcal mol–1, affording the Pd(ii) complex B which has a T-shape geometry with the 2-quinolone and iodide ligands being mutually trans. The formation of B is exergonic by 17.7 kcal mol–1. The DMF ligand was replaced by NBE to generate complex C, in which the 2-quinoline and iodide ligands occupy mutually trans positions. The subsequent insertion of NBE proceeded viaTSCD with a small activation energy of ΔG‡ = +3.0 kcal mol–1, affording the Pd(ii) alkyl complex D coordinated by the electron-deficient alkene moiety of the 2-quinolone. The iodide ligand occupies the trans position of the alkene ligand. The formation of D is exergonic by 19.2 kcal mol–1. Therefore, these two steps are both kinetically and thermodynamically feasible.
Scheme 7. The oxidative addition and NBE insertion steps of model complexes A and C leading to complexes B and D, respectively. The relative Gibbs free energies in DMF at 298 K and 1 atm are indicated in parentheses.
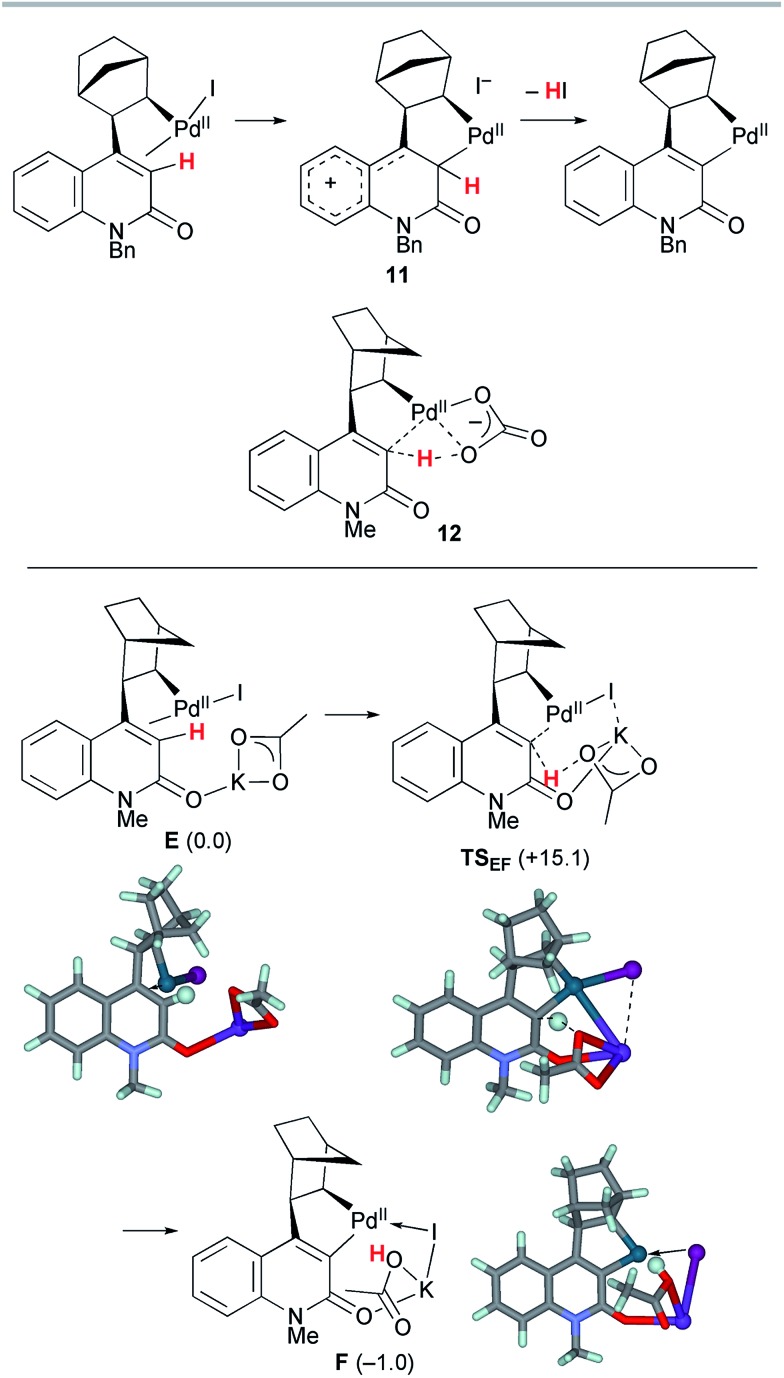
Next, the palladacycle formation was investigated (Scheme 8). As previously proposed,4a electrophilic palladation via the cationic intermediate 11 is operative for the 2-quinolone system; however, such a transition state (TS) could not be located for a model complex. Therefore, concerted metallation–deprotonation (CMD) mechanisms were considered.17,18 Because the activation energy for intramolecular proton abstraction by a κ2-carbonate ligand on the Pd(ii) center (12) was estimated to be as large as 50 kcal mol–1 (Scheme S1, ESI†), an alternative TS for intermolecular proton abstraction by an external base was examined. Such a CMD of complex E with KOAc as a base proceeded viaTSEF with a reasonable activation energy of ΔG‡ = +15.1 kcal mol–1. In this TS, the cleaved C–H and formed Oacetate–H bond distances are 1.346 and 1.318 Å, respectively, while the Pd–Cquinolone distance is 2.103 Å. The potassium ion has interactions with the Br center and carbonyl group, as the K–I and K–Ocarbonyl distances are 3.772 and 2.789 Å, respectively. These values are markedly smaller than the sums of the corresponding van der Waals radii (K/I 4.73 Å, K/O 4.27 Å). Therefore, the amide group plays the important role of a directing group that places KOAc in close proximity to the vinylic proton. The formation of palladacycle complex F with the concomitant extrusion of the iodide ligand is slightly endergonic by 1 kcal mol–1.
Scheme 8. The CMD step of model complex E leading to palladacycle complex F. The relative Gibbs free energies in DMF at 298 K and 1 atm are indicated in parentheses.
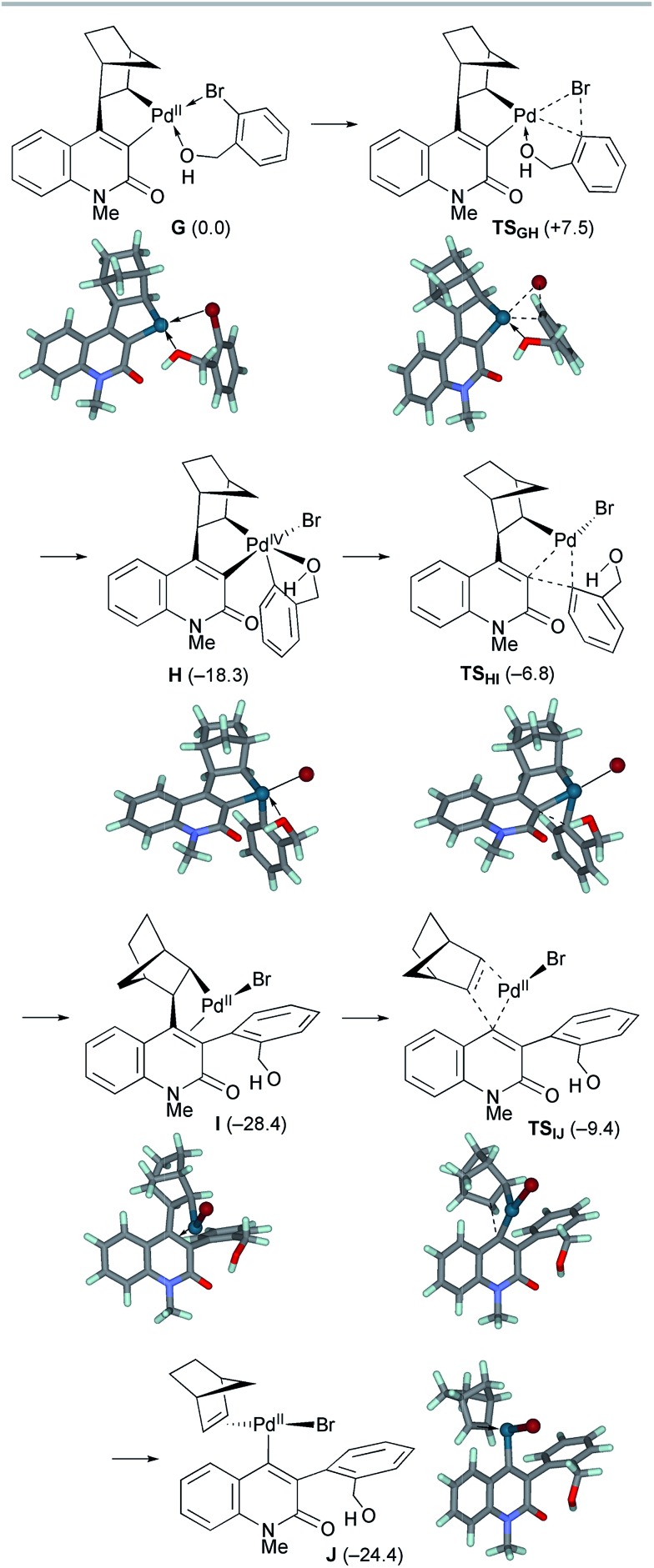
The second oxidative addition of o-bromobenzyl alcohol started from palladacycle complex G (Scheme 9). This step proceeded viaTSGH with a small activation energy of ΔG‡ = +7.5 kcal mol–1, generating the Pd(iv) complex H with an exergonicity of 18.3 kcal mol–1. Because the five-coordinated complex H has a square pyramidal geometry with the o-(hydroxymethyl)phenyl ligand at the apical position, the subsequent reductive elimination facilely proceeded viaTSHI with an activation energy of ΔG‡ = +11.5 kcal mol–1. As a result, the Pd(ii) complex I with a 3-arylquinolone ligand was formed with an exergonicity of 10.1 kcal mol–1. Subsequently, deinsertion of NBE from I proceeded viaTSIJ with an activation energy of ΔG‡ = +19.0 kcal mol–1, generating the vinyl Pd(ii) complex J with a slight endergonicity of 4.0 kcal mol–1. Thus, the overall process from G to J is efficient and highly exergonic by 24.4 kcal mol–1.
Scheme 9. The oxidative addition/reductive elimination steps of model complex G and subsequent deinsertion of NBE from intermediate complex I. The relative Gibbs free energies in DMF at 298 K and 1 atm are indicated in parentheses.
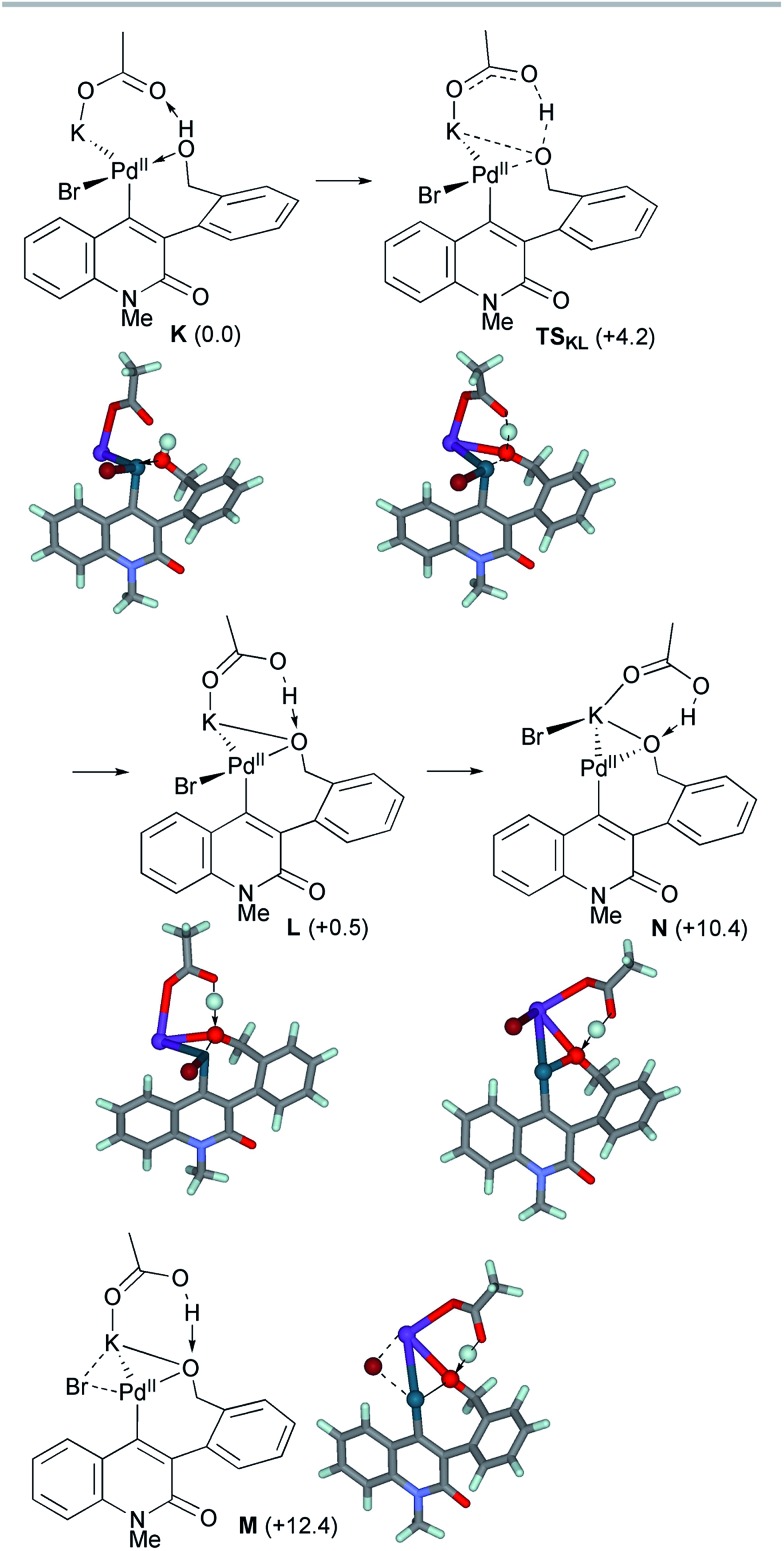
The ligand exchange between the labile NBE ligand and the benzylic hydroxyl group was followed by the association of KOAc to generate intermediate K (Scheme 10). Facile deprotonation of the benzylic hydroxyl group proceeded viaTSKL with a small activation energy of ΔG‡ = +4.2 kcal mol–1, generating the alkoxo complex L with a slight endergonicity of 0.5 kcal mol–1. The Pd–Obenzyl distance was shortened from 2.245 Å in K to 2.081 Å in L, while the Pd–Br bond length remained almost constant (2.492–2.503 Å). Subsequent dissociation of the bromide from the palladium center ultimately produced alkoxo complex N. We could not find the TS for this step, but instead, the TS-like stationary point M with one small imaginary frequency (18.5i cm–1) was located 11.9 kcal mol–1 above L. At this point, the bromide ion is placed at the bridging position between the Pd and K centers (Pd–Br 2.637 Å and K–Br 3.382 Å). In the resulting alkoxo complex N, the Pd–Obenzyl distance was shortened to 2.054 Å. The formation of N from K was endergonic by 10.4 kcal mol–1.
Scheme 10. The deprotonation of the benzylic alcohol of model complex K and subsequent bromide dissociation from the intermediate complex L. The relative Gibbs free energies in DMF at 298 K and 1 atm are indicated in parentheses.
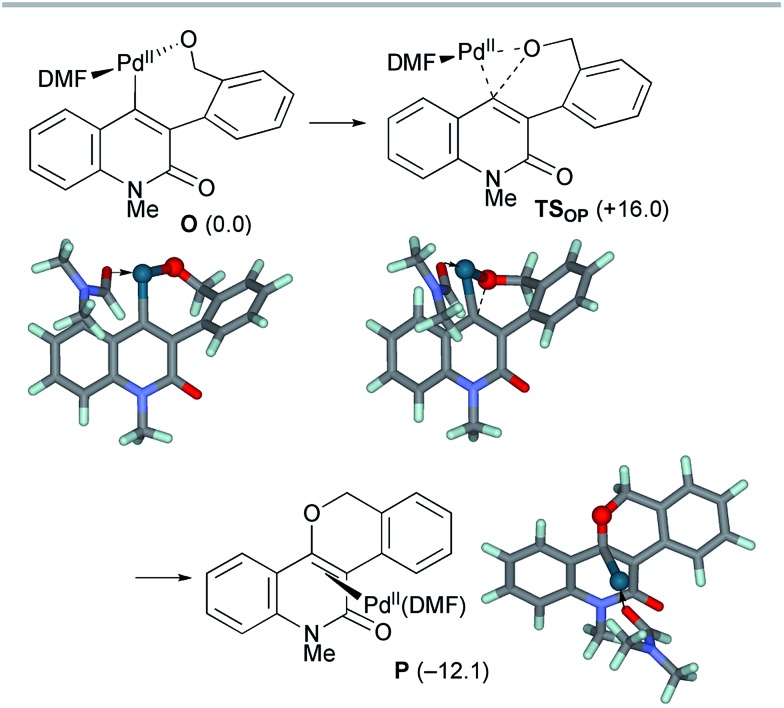
After the dissociation of AcOH·KBr, the C–O bond-forming reductive elimination proceeded from the alkoxo complex OviaTSOP with an activation energy of ΔG‡ = +16.0 kcal mol–1 (Scheme 11). The Calkene–Obenzyl distance decreased from 2.878 Å in O to 1.947 Å in TSOP. The formation of the benzopyranyl-2-quinolone complex P from O is exergonic by 12.1 kcal mol–1. Therefore, the final step is both kinetically and thermodynamically feasible.
Scheme 11. The reductive elimination from model complex O affording the final product complex P. The relative Gibbs free energies in DMF at 298 K and 1 atm are indicated in parentheses.
Fig. 2 outlines the energy surface of the overall process. A reasonable amount of the largest energy span19 (30.4 kcal mol–1) is estimated for steps between I and TSOP, and thus, the later, C–O bond-forming cyclization stage is the rate limiting process. This analysis is in good accordance with the fact that no kinetic isotope effect was observed in the deuterium-labeling experiment shown in Scheme 5. The overall transformation of the starting complex A into the final product complex P is exergonic by 49.4 kcal mol–1, and thus, the vinylogous Cattelani reaction of 4-iodo-2-quinolone is thermodynamically favorable.
Fig. 2. The calculated energy surface for the overall process, with relative Gibbs free energies in DMF at 298 K and 1 atm. The relative Gibbs free energies include those of aNBE/2KOAc/o-bromobenzyl alcohol, bDMF/2KOAc/o-bromobenzyl alcohol, cDMF/KOAc/o-bromobenzyl alcohol, dDMF/KOAc/AcOH·KI, eDMF/NBE/AcOH·KI, and fNBE/KOAc·KI/AcOH·KBr. The relative Gibbs free energies for the reactions associated with 1a are shown in parentheses (red).
The DFT calculations were also performed for the tertiary benzylic alcohol 3a, which experimentally proved to be more efficient compared to primary or secondary benzylic alcohols. Therefore, very similar results were obtained for the steps involving benzylic alcohols (Fig. 1 and Schemes S2–S4, ESI†). Notably, the largest energy span ΔΔG(I–TSOP) was found to be 2 kcal mol–1 smaller for the reaction with 3a than for that with 3q. This result is in good agreement with the experimental results.
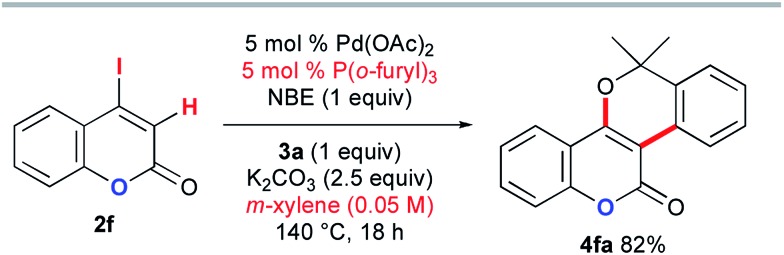
The above DFT study implies that the lactam carbonyl oxygen directs KOAc in close proximity to the abstracted hydrogen atom in the CMD step. Therefore, the inefficiency of the coumarin substrate 2f can be attributed to the weaker coordination ability of its lactone carbonyl group than that of the DMF solvent. To enable the desired annulation with o-bromobenzyl alcohol 3a, modified reaction conditions were investigated using P(o-furyl)3 as the supporting ligand in xylene as a non-coordinating solvent (Scheme 12). Although a higher reaction temperature and a prolonged reaction time were required, the desired benzopyran-fused coumarin 4fa was obtained in 82% yield.
Scheme 12. The Catellani-type annulation of 4-iodocoumarin 2f with 3a.
Conclusions
In conclusion, we successfully realized the first vinylogous Catellani reaction of 4-iodo-2-quinolones with benzyl alcohols bearing a tertiary benzyl moiety. By using this method, a variety of chromeno-2-quinolones, which are analogs of the natural alkaloid benzosimuline, were synthesized in moderate to high yields. Moreover, the reactions with the corresponding benzylamine and its tosylamide produced a dibenzonaphthyridin-11(12H)-one derivative and a three-component annulation product involving norbornene, respectively. The results of the control experiments corroborate the Catellani-type mechanism via norbornene-assisted vinylic C–H functionalization. Moreover, DFT calculations of model complexes suggest that the C–H bond fission proceeds via a CMD mechanism and the later C–O bond-forming cyclization stage is the rate-limiting process.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research is partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research (AMED) and JSPS KAKENHI (Grand Number JP 16KT0051).
Footnotes
†Electronic supplementary information (ESI) available. CCDC 1557549. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc04265e
References
- For recent reviews, see: ; (a) Sandtorv A. H. Adv. Synth. Catal. 2015;357:2403. [Google Scholar]; (b) Iwai T., Sawamura M. ACS Catal. 2015;5:5031. [Google Scholar]; (c) Maes J., Maes B. U. W. Adv. Heterocycl. Chem. 2016;120:137. [Google Scholar]
- For selected examples, see: ; (a) Tamura R., Yamada Y., Nakao Y., Hiyama T. Angew. Chem., Int. Ed. 2012;51:5679. doi: 10.1002/anie.201200922. [DOI] [PubMed] [Google Scholar]; (b) Chen Y., Wanag F., Jia A., Li X. Chem. Sci. 2012;3:3231. [Google Scholar]; (c) Gigant N., Chausset-Boissarie L., Gillaizeau I. Chem.–Eur. J. 2014;20:7548. doi: 10.1002/chem.201402070. [DOI] [PubMed] [Google Scholar]
- Wang K., Hu F., Zhang Y., Wang J. Sci. China: Chem. 2015;58:1252. [Google Scholar]
- For recent reviews, see: ; (a) Martins A., Mariampillai B., Lautens M. Top. Curr. Chem. 2010;292:1. doi: 10.1007/128_2009_13. [DOI] [PubMed] [Google Scholar]; (b) Ferraccioli R. Synthesis. 2013;45:581. [Google Scholar]; (c) Ye J., Lautens M. Nat. Chem. 2015;7:863. doi: 10.1038/nchem.2372. [DOI] [PubMed] [Google Scholar]; (d) Della Ca’ N., Fontana M., Motti E., Catellani M. Acc. Chem. Res. 2016;49:1389. doi: 10.1021/acs.accounts.6b00165. [DOI] [PubMed] [Google Scholar]
- Michael J. P. Nat. Prod. Rep. 1999;16:697. doi: 10.1039/a809408j. [DOI] [PubMed] [Google Scholar]
- (a) Appendino G., Tagliapietra S., Nano G. M., Picci V. Phytochemistry. 1988;27:944. [Google Scholar]; (b) Louvet M.-S., Gault G., Lefebvre S., Popowycz F., Boulven M., Besse S., Benoit E., Lattard V., Grancher D. Phytochemistry. 2015;118:124. doi: 10.1016/j.phytochem.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Chen I.-S., Tsai I.-W., Teng C.-M., Chen J.-J., Chang Y.-L., Ko F.-N., Lu M. C., Pezzuto J. M. Phytochemistry. 1997;46:525. [Google Scholar]
- . Also, see: ; (a) Majumdar K. C., Mukhopadhyay P. P. Synthesis. 2003:97. [Google Scholar]; (b) Majumdar K. C., Pal A. K., Taher A., Debnath P. Synthesis. 2007:1707. [Google Scholar]; (c) Pardo L. M., Prendergast A. M., Nolan M.-T., Muimhneacháin E. Ó., McGlacken G. P. Eur. J. Org. Chem. 2015:3540. [Google Scholar]
- . Also see: ; (a) Dooley J. D., Chidipudi S. R., Lam H. W. J. Am. Chem. Soc. 2013;135:10829. doi: 10.1021/ja404867k. [DOI] [PubMed] [Google Scholar]; (b) Choppakatla S., Dachepally A. K., Bollikolla H. B. Tetrahedron Lett. 2016;57:2488. [Google Scholar]
- Piers E., Wong T., Coish P. D., Rogers C. Can. J. Chem. 1994;72:1816. [Google Scholar]
- Murayama T., Shibuya M., Yamamoto Y. Adv. Synth. Catal. 2016;358:166. [Google Scholar]
- (a) Motti E., Della Ca’ N., Xu D., Piersimoni A., Bedogni E., Zhou Z.-M., Catellani M. Org. Lett. 2012;14:5792. doi: 10.1021/ol302889t. [DOI] [PubMed] [Google Scholar]; (b) Fontana M., Xu D., Cremaschi M., Lucentini R., Zhou Z.-M., Catellani M., Motti E. Tetrahedron. 2015;71:6389. [Google Scholar]
- Similar KIE values were previously observed for relevant Catellani-type reactions, see: ; (a) Jiao L., Herdtweck E., Bach T. J. Am. Chem. Soc. 2012;134:14563. doi: 10.1021/ja3058138. [DOI] [PubMed] [Google Scholar]; (b) Shi H., Wang P., Suzuki S., Farmer M. E., Yu J.-Q. J. Am. Chem. Soc. 2016;138:14876. doi: 10.1021/jacs.6b11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A related three-component coupling of iodobenzenes, norbornene, and 2-bromophenylacetamides was reported, see: Larraufie M.-H., Maestri G., Beaume A., Derat É., Ollivier C., Fensterbank L., Courillon C., Lacôte E., Catellani M., Malacria M., Angew. Chem., Int. Ed., 2011, 50 , 12253 . [DOI] [PubMed] [Google Scholar]
- Davies D. L., Macgregor S. A., McMullin C. L. Chem. Rev. 2017;117:8649. doi: 10.1021/acs.chemrev.6b00839. [DOI] [PubMed] [Google Scholar]
- Muzart J. Tetrahedron. 2009;65:8313. [Google Scholar]
- (a) Lapointe D., Fagnou K. Chem. Lett. 2010;39:1118. [Google Scholar]; (b) Ackermann L. Chem. Rev. 2011;111:1315. doi: 10.1021/cr100412j. [DOI] [PubMed] [Google Scholar]; (c) Roudesly F., Oble J., Poli G. J. Mol. Catal. A: Chem. 2017;426:275. [Google Scholar]
- Recently, a CMD mechanism was suggested for the Catellani reaction of iodobenzenes with aryl carbamic chlorides: Li X., Pan J., Jiao N., Chem. Sci., 2016, 7 , 5384 . [Google Scholar]
- Kozuch S., Shaik S. Acc. Chem. Res. 2011;44:101. doi: 10.1021/ar1000956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.