Abstract
This population-based cohort study examines the association of radiotherapy for Hodgkin lymphoma with risk of ER-positive and ER-negative breast cancers using data from the US National Cancer Institute Surveillance, Epidemiology, and End Results database.
Survivors of Hodgkin lymphoma (HL) have a high risk of developing a subsequent breast cancer, particularly after chest irradiation. Although breast cancer is a heterogeneous disease, the association between HL treatment and the estrogen receptor (ER) status of subsequent breast cancers has not been examined in large cohorts. We explored the association between radiotherapy for HL and risk of ER-positive and ER-negative breast cancer in a large, population-based cohort, accounting for chemotherapy.
Methods
We included 7355 women diagnosed with first primary HL during 1973 to 2009 at ages 10 to 39 years that were reported to 12 US National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registries. Follow-up began at either 5 years following diagnosis (the estimated minimum period for radiation-related solid cancers to develop) or, if diagnosed prior to 1985, January 1, 1990 (ER status was not collected in SEER before 1990). Follow-up ended at the diagnosis of a second invasive breast cancer, last known vital status, death, or December 31, 2014. Patients were classified by radiotherapy and/or chemotherapy status for their first course of HL treatment (yes vs no/unknown). Standardized incidence ratios (SIRs, a measure of relative risk) for ER-positive and ER-negative breast cancers were estimated using expected rates of each subtype in the SEER general population according to attained age, race, and calendar period. Tests of differences between SIRs were conducted using likelihood ratio tests in a Poisson regression model. Data were publically available and institutional review board approval was not required.
Results
The mean age at HL diagnosis was 26 years. Survivors were followed for a mean of 12 years (range, 5-38 years). In total, 377 invasive breast cancers were diagnosed (mean age, 45 years). Fifty-seven percent were ER positive, 34% were ER negative, and 9% had unknown/borderline ER status (Table).
Table. Characteristics of Subsequent Breast Cancer Cases by Estrogen Receptor (ER) Subtype Within a Cohort of 7355 Hodgkin Lymphoma Patients Diagnosed From 1973 to 2009 and Surviving to 1990,a US SEER13 Registries (Excluding Alaska).
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Total | Estrogen Receptor Positive | Estrogen Receptor Negative | Estrogen Receptor Borderline/Unknown | |
| Total | 377 (100) | 214 (100) | 129 (100) | 34 (100) |
| Decade of Hodgkin lymphoma diagnosis | ||||
| 1973-1979 | 103 (27) | 57 (27) | 30 (23) | 16 (47) |
| 1980-1989 | 184 (49) | 104 (49) | 64 (50) | 16 (47) |
| 1990-1999 | 80 (21) | 47 (22) | 31 (24) | 2 (6) |
| 2000-2009 | 10 (3) | 6 (3) | 4 (3) | 0 |
| Age at Hodgkin lymphoma diagnosis, y | ||||
| 10-19 | 96 (25) | 57 (27) | 29 (22) | 10 (29) |
| 20-29 | 176 (47) | 99 (46) | 60 (47) | 17 (50) |
| 30-39 | 105 (28) | 58 (27) | 40 (31) | 7 (21) |
| Radiotherapy receipt | ||||
| Yes | 304 (81) | 183 (86) | 95 (74) | 26 (76) |
| Chemotherapy receipt | ||||
| Yes | 130 (34) | 69 (32) | 50 (39) | 11 (32) |
| Decade of breast cancer diagnosis | ||||
| 1990-1999 | 94 (25) | 42 (20) | 31 (24) | 21 (62) |
| 2000-2009 | 173 (46) | 103 (48) | 60 (47) | 10 (29) |
| 2010-2014 | 110 (29) | 69 (32) | 38 (29) | 3 (9) |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
The first year in which estrogen receptor status was routinely collected.
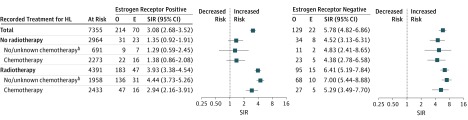
Compared with the general population, survivors of HL had a greater relative risk of ER-negative (SIR, 5.8; 95% CI, 4.8-6.9) than ER-positive breast cancer (SIR, 3.1; 95% CI,2.7-3.5; P < .001 for difference) (Figure). For ER-positive disease, the increased SIR was only observed among women who received radiotherapy (SIR, 3.9; 95% CI, 3.4-4.5). In this group, the SIR for ER-positive disease was lower in the chemotherapy than the no/unknown chemotherapy group (P = .04). In contrast, the SIRs for ER-negative breast cancer did not vary significantly by treatment.
Figure. Number of Observed (O) and Expected (E) Cases and Standardized Incidence Ratiosa (SIR) for Subsequent Breast Cancer by Estrogen Receptor Subtype and Hodgkin Lymphoma (HL) Treatment.
Error bars indicate 95% confidence intervals.
aSIR estimated using SEER Stat software (version 8.3.4).
bInterpret with caution because chemotherapy is known to be underreported in SEER.
Discussion
Breast cancer is a major source of morbidity in young HL survivors; cumulative risk estimates exceed 40% following high-dose mantle radiotherapy. In the current study, radiotherapy was associated with an increased relative risk of ER-positive breast cancer, whereas the risk of ER-negative breast cancer was elevated irrespective of radiotherapy status. This suggests other mechanisms of increased risk for ER-negative cancers, such as genetic predisposition, and warrants further investigation especially given that ER-negative disease has poorer prognosis than ER-positive disease.
A major strength of SEER-based studies is their large sample size and long-term, systematic follow-up. Nonetheless, lack of information on risk factors such as family history, reproductive factors, and hormone replacement therapy is a limitation as is the lack of detailed treatment information such as radiotherapy dose, fields, specific chemotherapeutic agents, and subsequent therapy. Ovarian exposure to radiotherapy and certain chemotherapeutic agents have been associated with reduced risks of subsequent breast cancer.
Despite changes in treatment recommendations, 40% of young women with HL currently receive radiotherapy, which was associated with an increased risk of ER-positive breast cancer in our study. Further investigation of the association between HL treatment and breast cancer subtype in studies with comprehensive treatment records will lead to a better understanding of the risk and etiology of subsequent breast cancers and can ultimately inform treatment and screening decisions to reduce morbidity and mortality among HL survivors.
References
- 1.Swerdlow AJ, Higgins CD, Smith P, et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: a collaborative British cohort study. J Clin Oncol. 2011;29(31):4096-4104. [DOI] [PubMed] [Google Scholar]
- 2.Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97(19):1428-1437. [DOI] [PubMed] [Google Scholar]
- 3.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168(1):1-64. [DOI] [PubMed] [Google Scholar]
- 4.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27(24):3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson TO, Moskowitz CS, Chou JF, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(9):910-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290(4):465-475. [DOI] [PubMed] [Google Scholar]