Key Points
Question
What are the relative and absolute risks of breast anaplastic large-cell lymphoma (ALCL) in women with breast implants?
Findings
In this population-based, case-control study, we identified 32 patients with primary breast-ALCL with ipsilateral breast implants. Relative risk for breast-ALCL in women with breast implants was 421.8 and absolute cumulative risk was 29 per million at age 50 years and 82 per million at age 70 years.
Meaning
Breast implants were associated with an increased relative risk of breast-ALCL, although the absolute risk remains small; the results suggest a need for increased clinical awareness, comprehensive registration of implants and complications, and stimulation of alternative cosmetic/reconstructive procedures.
This population-based, case-control study examines the relative and absolute risks of breast anaplastic large-cell lymphoma in women with breast implants.
Abstract
Importance
Breast implants are among the most commonly used medical devices. Since 2008, the number of women with breast implants diagnosed with anaplastic large-cell lymphoma in the breast (breast-ALCL) has increased, and several reports have suggested an association between breast implants and risk of breast-ALCL. However, relative and absolute risks of breast-ALCL in women with implants are still unknown, precluding evidence-based counseling about implants.
Objective
To determine relative and absolute risks of breast-ALCL in women with breast implants.
Design, Setting, and Participants
Through the population-based nationwide Dutch pathology registry we identified all patients diagnosed with primary non-Hodgkin lymphoma in the breast between 1990 and 2016 and retrieved clinical data, including breast implant status, from the treating physicians. We estimated the odds ratio (OR) of ALCL associated with breast implants in a case-control design, comparing implant prevalence between women with breast-ALCL and women with other types of breast lymphoma. Cumulative risk of breast-ALCL was derived from the age-specific prevalence of breast implants in Dutch women, estimated from an examination of 3000 chest x-rays and time trends from implant sales.
Main Outcomes and Measures
Relative and absolute risks of breast-ALCL in women with breast implants.
Results
Among 43 patients with breast-ALCL (median age, 59 years), 32 had ipsilateral breast implants, compared with 1 among 146 women with other primary breast lymphomas (OR, 421.8; 95% CI, 52.6-3385.2). Implants among breast-ALCL cases were more often macrotextured (23 macrotextured of 28 total implants of known type, 82%) than expected (49 193 sold macrotextured implants of total sold 109 449 between 2010 and 2015, 45%) based on sales data (P < .001). The estimated prevalence of breast implants in women aged 20 to 70 years was 3.3%. Cumulative risks of breast-ALCL in women with implants were 29 per million at 50 years and 82 per million at 70 years. The number of women with implants needed to cause 1 breast-ALCL case before age 75 years was 6920.
Conclusions and Relevance
Breast implants are associated with increased risk of breast-ALCL, but the absolute risk remains small. Our results emphasize the need for increased awareness among the public, medical professionals, and regulatory bodies, promotion of alternative cosmetic procedures, and alertness to signs and symptoms of breast-ALCL in women with implants.
Introduction
Since the introduction of breast implants in the 1960s, their safety has been debated extensively, even resulting in a temporary ban (1992-2006) on silicone-gel implants for cosmetic indications by the US Food and Drug Administration (FDA). However, consistent associations of silicone breast implants with adverse events, such as breast cancer, autoimmune diseases, and connective tissue diseases have not been substantiated, as recently underlined by 2 meta-analyses. The risk for anaplastic large-cell lymphoma (ALCL) in the breast in relation to breast implants was not discussed in these studies.
In 2008, we reported the first epidemiological study showing an increased risk of breast-ALCL in association with breast implants (odds ratio [OR], 18.2; 95% CI, 2.1-156.8), based on 5 exposed cases. Since then, the number of reported cases has increased to 173 unique cases reported in the literature by 2015, and 359 international Medical Device Reports (MDRS) received by the FDA by February 2017. Breast-ALCL has been included as a provisional new disease entity in the most recent update of the World Health Organization’s lymphoma classification.
Epidemiological studies with appropriate comparison groups have not been published after 2008, likely owing to the rarity of breast-ALCL. Most reports discussing risk estimations for breast-ALCL rely on clinical reporting of breast-ALCL cases with implants and lack valid data on the prevalence of women with implants in the population. Estimating the prevalence of (type of) breast implants has proven to be a true international challenge because sales data are generally not released by companies and information on unilateral vs bilateral usage as well as use for prosthesis revisions are not known. Consequently, the precise relative and absolute risks of breast-ALCL in women with implants are unknown.
The Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands (PALGA) of the Netherlands provides the unique opportunity for complete nationwide ascertainment of all cases of breast-ALCL and other classes of primary breast lymphomas as a comparison population. To estimate absolute risks of breast-ALCL in women with breast implants, we determined age- and calendar year-specific implant prevalence rates using a large, random sample of chest x-rays from 2015.
Methods
Design and Study Population
We performed a case-control study comparing the prevalence of breast implants between women with primary breast-ALCL and women with primary breast lymphomas other than ALCL. This fully deidentified study was centrally approved by the ethics board of PALGA and locally by all institutions submitting at least 1 patient with breast-ALCL. It was determined that the Medical Research Involving Human Subjects Act (WMO) does not apply to the study. We identified 782 female patients diagnosed with a histologically or cytologically proven non-Hodgkin lymphoma (NHL) of the breast in the Netherlands during 1990 to 2016. Identification was based on data from PALGA, with nationwide coverage of all academic and nonacademic centers since 1990 (eFigure 1 in the Supplement).
Breast-ALCL Cases
For the 47 primary breast-ALCL cases among the identified patients, all available pathology samples (cytological and histological slides and/or blocks) and reports were retrieved from the original pathology laboratories for review, including immunohistochemical analyses and T-cell receptor gene rearrangements. All patients with a previously reported lymphoma diagnosis prior to lymphoma diagnosis in the breast, were excluded. In addition, anonymized clinical information was collected from treating physicians via PALGA. Breast as the primary site of involvement was confirmed in 43 patients.
Controls With Other Types of Breast Lymphoma
Control selection procedures were performed using methods as previously described. From 735 non-ALCL breast NHL cases, full pathology reports from the laboratories were reviewed to confirm the diagnosis. All patients with a previously reported lymphoma diagnosis, prior to lymphoma diagnosis in the breast (n = 325) and with chronic and acute leukemia as disseminated diseases per definition were excluded (n = 220). Only patients classified as diffuse large B-cell lymphoma, Burkitt lymphoma, follicular lymphoma, nodal and mucosa-associated lymphoid tissue-type marginal zone lymphoma, and peripheral T-cell lymphoma not otherwise specified were included (n = 190).
Questionnaire on Lymphoma and Breast Implant Characteristics
Through PALGA, a standardized questionnaire was sent to the treating physicians (oncologists, surgeons, or plastic surgeons) of all potential breast-ALCL cases (n = 47) and potential controls (n = 190). The questionnaire assessed whether the breast was the primary site of involvement, features at lymphoma presentation including clinicopathological variants (ie, tumor-forming or seroma-associated breast-ALCL), lymphoma treatment and outcome, and breast implant presence and history. Physicians were asked to review the full medical history, interdisciplinary correspondence, and chest imaging for any breast implant surgery. In addition, information was collected on breast implant indications, type of breast implant, and implant revisions. Physician response was 100% for breast-ALCL cases and 92% for controls. Breast as primary site of involvement was confirmed in 43 breast-ALCL cases and 146 controls (eFigure 1 in the Supplement).
Prevalence of Breast Implants in the General Dutch Population 1965 to 2016
We determined regional age-specific breast implant prevalence in 3000 women aged 20 to 70 years by review of chest x-rays performed in 2015 in 2 large hospitals in different regions of the Netherlands. X-rays were sampled randomly from radiology records, stratified by age (eFigure 2 in the Supplement). The validity of assessing breast implant presence from chest x-rays was first examined using a chest x-ray series of patients with simultaneously performed computed tomographic (CT) scans that had demonstrated the presence of breast implants (eMethods in the Supplement). Chest x-rays were assessed by 3 reviewers who had demonstrated high sensitivity and specificity in the validation study (eMethods in the Supplement).
To account for regional variation in breast implant prevalence rates in the chest x-ray study, we used differences between region-specific breast implant prevalence rates from the National Breast Cancer Screening Program (BCSP) to derive nationwide breast implant prevalence rates (eFigure 2 in the Supplement). Implant prevalence rates from the BCSP were not used as such because these are underestimates, owing to the fact that women with breast implants participate less in population screening as a result of discomfort, risk of implant rupture, and suboptimal mammography.
Breast implant prevalence prior to 2015 was estimated by applying changes in implant sales to the 2015 age- and region-specific prevalence rates. On request to all currently active breast implant vendors, we obtained nearly complete sales data for the period 2010 to 2015, covering more than 95% of the Dutch market share for this period. The change in implant prevalence by calendar year was determined from the 2010 to 2015 nationwide sales data by calculating the average annual percentage change (AAPC) in a regression of the log-transformed number of sold implants per year on calendar year. The AAPCs for the period 1965 to 2010 were calculated assuming a linear decrease of the log-transformed number of sold implants to zero in 1965, the start of breast implant use in the Netherlands (eFigure 3 in the Supplement). The age-specific size of the female Dutch population was obtained from Statistics Netherlands (CBS).
Statistical Analysis
For assessment of the association between breast implants and breast-ALCL, we calculated the OR between case-control status and breast implant status (in the ipsilateral breast) as an approximation of the relative risk, using unconditional logistic regression with adjustment for age and calendar year (continuous). The distribution of microtextured and macrotextured silicone-filled implants was compared between breast-ALCL cases and Dutch sales data between 2010 and 2015 using the Fisher exact test. P < .05 were considered statistically significant.
We calculated the cumulative risk of breast-ALCL by age in women with breast implants and in the general Dutch female population without breast implants, using the number of breast-ALCL cases with or without breast implants and the age-specific denominator of women with breast implants or the complete female Dutch population, respectively (eMethods in the Supplement). Cumulative risk to develop breast-ALCL up to age z was calculated as Pcri = 1-exp(−Σxlx*cx/nx) where cx and nx are the numbers of cases and person-years in age-category x, respectively, lx is the width of the x-th age interval and z is the upper limit of the last age category. As a sensitivity analysis, cumulative risk of breast-ALCL in women with implants was also calculated by multiplying the background incidence of breast-ALCL without implants in the general Dutch female population with the OR from our case-control study.
The number needed to harm was calculated as the inverse of the difference between the cumulative risk with breast implants and the cumulative risk in the general population at age 75 years. Statistical analyses were performed with SAS statistical software (version 9.4, SAS Institute).
Results
Case-Control Study: Relative Risk for ALCL Associated With Breast Implants
Of 43 breast-ALCL patients (median age, 59 years; range, 24-87 years), 32 had an ipsilateral breast implant (median age, 56 years; range, 29-73 years), whereas in 11 patients no implant or implant history was noted. Of 146 controls (median age, 61 years; range, 24-89 years), 1 patient had a breast implant in the lymphoma-affected breast for a cosmetic indication and 1 other patient had a breast implant for reconstructive purposes in the contralateral (not lymphoma-affected) breast (Table 1). This resulted in an OR of 421.8 (95% CI, 52.6-3385.2; P < .001) for breast-ALCL associated with a breast implant. The implant-related log OR increased by about 10% when adjusted for age and calendar year. A sensitivity analysis restricted to cases and controls not included in our previous report, showed 27 exposed cases and no exposed controls, resulting in an infinite OR (P < .001). Seven of 43 ALCL cases had previous breast cancer (all 7 with breast implants), whereas 3 of 147 control patients had previous breast cancer, of whom none had implants.
Table 1. Diagnostic Characteristics of 43 Patients With Primary ALCL in the Breast and 146 Patients With Primary Breast Lymphomas Other Than ALCL Included in the Case-Control Study.
| Characteristic | No. (%) | |
|---|---|---|
| Primary Breast ALCL | Primary Breast Lymphomas Other Than ALCLa |
|
| Year of diagnosis | ||
| 1990-1995 | 1 (2.3) | 5 (3.4) |
| 1996-2000 | 6 (14.0) | 20 (13.7) |
| 2001-2005 | 3 (7.0) | 12 (8.2) |
| 2006-2010 | 9 (20.9) | 56 (38.4) |
| 2011-2016 | 24 (55.8) | 53 (36.3) |
| Age at diagnosis, y | ||
| 18-35 | 4 (9.3) | 13 (8.9) |
| 36-50 | 14 (32.6) | 36 (24.7) |
| 51-75 | 23 (53.5) | 74 (50.7) |
| >75 | 2 (4.7) | 23 (15.8) |
| Breast implant | ||
| Yes | 32 (74.4) | 1 (0.7) |
| No | 11 (25.6) | 145 (99.3) |
Abbreviation: ALCL, anaplastic large-cell lymphoma.
Including diffuse large B-cell lymphoma (n = 95), Burkitt lymphoma (n = 7), marginal zone lymphoma, mucosa-associated lymphoid tissue-type (n = 22), follicular lymphoma (n = 10), nodal marginal zone lymphoma (n = 1), indolent B-cell lymphoma, unclassifiable (n = 9), peripheral T-cell lymphoma, not otherwise classified (CD30 negative, n = 3).
Implant Characteristics in Patients With Primary Breast-ALCL
Patients received their first breast implants at a median interval of 13 years before lymphoma diagnosis (range, 1-39 years). In 21 of 32 (65%) patients, bilateral breast implants were placed for cosmetic reasons. Ten of 32 (31%) had implants for reconstruction after mastectomy for breast cancer, including 3 patients with contralateral prophylactic procedures with implants, of whom 2 patients received breast implants after bilateral prophylactic mastectomy owing to BRCA mutation carriership. One patient received breast implants as part of a gender transition program (Table 2) (eTable 1 and eFigure 4 in the Supplement). Twenty-one patients received implants only once, whereas single (n = 3) or multiple implant revisions (n = 8) for leakage, rupture, or pain were necessary in 11 patients (Table 2) (eTable 1 in the Supplement).
Table 2. Implant Characteristics of 32 Patients With Breast-ALCL With Breast Implants.
| Characteristic | Breast-ALCL Cases, No. |
|---|---|
| Age at breast implant, y | |
| 21-30 | 10 |
| 31-40 | 7 |
| 41-50 | 8 |
| 51-60 | 6 |
| >60 | 1 |
| Indications for implants | |
| Cosmetic | 22 |
| Reconstruction after breast cancer surgery | 7 |
| Reconstruction after prophylactic mastectomy | 3 |
| Type of implant | |
| Macrotexture | |
| Allergan/Inamed/McGhan | 22 |
| Nagor | 1 |
| Microtexture | |
| Eurosilicone | 2 |
| Mentor | 1 |
| PIP | 1 |
| Sebbin | 1 |
| Unknown | 4 |
| Interval between first implant and ALCL diagnosis, ya | |
| 1-5 | 6 |
| 6-10 | 5 |
| 11-20 | 14 |
| 21-30 | 5 |
| 31-40 | 2 |
Abbreviation: ALCL, anaplastic large-cell lymphoma.
Median interval, 13 years (range, 1-39 years).
We examined whether a specific type of implant was more strongly associated with breast-ALCL. Of the 28 patients with breast-ALCL with known implant type, 23 (82%) had macrotextured implants on diagnosis, whereas only 45% of all implants sold in the Netherlands in 2010 to 2015 were macrotextured (49 193 of 109 449, P < .001) (eFigures 5A and 5B in the Supplement). Based on sales data, macrotextured breast implants were introduced on the Dutch market around 1995. Eighteen percent of implants in patients with breast-ALCL were microtextured with a market share of 54%. No smooth or polyurethane covered implants were observed in patients with breast-ALCL. All implants were permanent and silicone-filled; none were saline or hydrocellulose-filled. However, it should be noted that the use of such implants was very limited (0.1%-1%) (eFigures 5A and 5B in the Supplement).
Clinicopathological Characteristics of Patients With Breast-ALCL
Clinical and pathological characteristics did not differ significantly between implant-exposed patients and breast-ALCL patients without implant exposure, except for seroma-associated features uniquely in patients with implants (Fisher-exact test, P < .001) (eTable 2, eResults in the Supplement). With a median follow-up of 33 months in the implant-exposed group (range, 2-240 months), 29 women were in complete remission after first-line (n = 23) or second-line treatment (n = 6). Two patients died of disseminated disease after second-line treatment (eTable 3 in the Supplement). In the nonimplant-exposed breast-ALCL group, 8 were in complete remission after first-line treatment (n = 8), and 3 patients died of disseminated disease (eTable 2 in the Supplement).
Absolute Risk Assessment for ALCL Associated With Breast Implants
The estimated prevalence of 20- to 70-year-old women with a breast implant in 2015 was 3.3%, ranging from 2.3% between 20 to 30 years, 4.0% between 31 to 40 years, 4.2% between 41 to 50 years, 3.6% between 51 to 60 years, and 2.1% between 61 to 70 years (eFigure 2 in the Supplement).
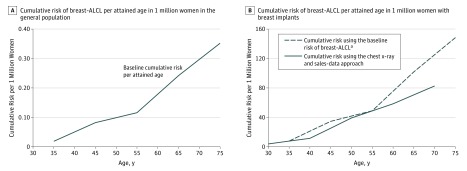
Cumulative risk of breast-ALCL in the general population increased with age and reached about 0.35 per million at an attained age of 75 years (Figure, A). Among women with an implant, cumulative risk increased from about 29 per million at 50 years and 82 per million at 70 years (Figure, B). The cumulative risks estimated using the alternative approach (based on the breast-ALCL background incidence and the breast implant-associated OR) did not essentially differ from this estimate (Figure, B).
Figure. Cumulative Risk of Breast-ALCL per Attained Age in 1 Million Women in the General Population and With Breast Implants.
ALCL Indicates anaplastic large-cell lymphoma. Cumulative risk of breast-ALCL in the general female population was calculated using the number of breast-ALCL cases without breast implants from the Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands, and the age-specific size of the female Dutch population from 1990 to 2016 per age category. Cumulative risk of breast-ALCL associated with breast implants was calculated using the number of breast-ALCL cases with breast implants and the cumulative number of women with breast implants in the Netherlands from 1965 to 2016 per age category.
aThe sensitivity analysis for cumulative risk of breast-ALCL in women with breast implants was calculated by multiplying the background incidence of breast-ALCL without implants in the general female population with the OR for breast-ALCL in women with breast implants from the case-control study.
The number needed to harm, ie, the number of women with implants needed to cause 1 breast-ALCL case before the age of 75 years, was 6920. The lack of reliable denominator data on textures precluded calculation of separate risks per implant type or vendor.
Discussion
In 2008, we reported the first relative risk estimate for breast-ALCL associated with breast implants, based on only 5 exposed cases. Based on what is the largest population-based study conducted thus far, with nationwide coverage of breast-ALCL cases in the period from 1990 to 2016, we now confirm that implants strongly increase the risk of this rare type of lymphoma. Our relative risk estimate of over 400, implying an attributable risk approaching 100%, is highly suggestive of a direct or indirect causal role of the breast implant in breast implant-associated ALCL (BIA-ALCL). So far, various, not mutually exclusive causal factors have been suggested. Specifically, a local inflammatory response, elicited by silicone-derived products or specific bacterial species adherent to the prosthesis surface (biofilm) may play a role, possibly via an auto-immune response. Toxic products related to the production of breast implants have been implicated as direct mutagens. Whether certain groups of women have a genetically determined increased risk to develop lymphoma when exposed to breast implants, eg, via a genetically determined altered or exaggerated local immunological response, remains hypothetical. A major increase in breast-ALCL incidence over time was noted, especially over the last 3 to 4 years (eFigure 4 in the Supplement). Apart from a truly increased incidence, this rise may also have been influenced by increased awareness and earlier diagnosis of breast-ALCL in the context of breast implants among plastic surgeons and pathologists alike, since the subject has drawn major attention in the medical and lay literature in the past few years.
Rather than the relative risk of developing a rare disease, albeit of impressive magnitude, for women with breast implants, it is the absolute risk estimates by physicians and governmental organizations, as well as associations with specific types of implants, that are of most interest to possibly avoid or reduce risks. Thus far absolute risk estimates have not been based on large population-based studies and reliable information on the prevalence of women with breast implants over time. Therefore, results of previous absolute risk calculations should be considered rough and potentially biased estimates. Using 3 complementary data sources (1-year point prevalence data for 2 Dutch regions, data on regional variation in breast implant prevalence based on the BCSP and national sales data), we could, for the first time, make an unbiased estimate of the age- and period-specific prevalence of breast implants in Dutch women. This key ingredient provided absolute risks of 29 BIA-ALCL cases per million women with implants at 50 years and about 82 per million at 70 years. This risk exceeds previous estimates by us and others 10 to 20 fold, but is unlikely to be an overestimation. Remarkably, our sensitivity analysis using another statistical approach resulted in very similar estimates.
Most BIA-ALCL cases were associated with macrotextured implants (24 of 32 with known implant type), provided by various distributors over time. The current market share was 45%, indicating a possible increased risk of developing BIA-ALCL with macrotextured implants, as suggested by others based on case-reports and series. It should be noted, however, that BIA-ALCL has also been observed in patients with microtextured implants both in our study and by others, as well as possibly in smooth implants. Furthermore, our sales data lack historical information on market shares before 2010, as a result of bankruptcy or changing distributors with loss of product data files. These considerations preclude reliable conclusions on associations between implant types or vendors and the risk of developing BIA-ALCL.
Clinical information on the 32 BIA-ALCL patients with implants shows that the disease is not restricted to specific indications for receiving implants because affected patients had implants for cosmetic reasons alone, for reconstruction in transgender surgery, after breast cancer surgery, and after prophylactic mastectomy for high breast cancer risk. Because 3 of 32 patients with BIA-ALCL were from families with high breast cancer risk, of whom 2 had proven BRCA mutations, future studies should investigate the possibility that BRCA1 and BRCA2 mutations might increase the risk of BIA-ALCL.
Limitations
Retrospective data on the prevalence of breast implants were not available owing to the absence of a breast implant registration, which started only in 2016 in the Netherlands, and lack of reliable and complete historical implant sales data. Therefore our absolute risks of breast-ALCL in implant carriers were based on extrapolated data. Even in this nationwide study, numbers were too small to allow definite conclusions on modifying factors, such as duration of implant exposure and implant types.
Conclusions
Considering absolute risks of breast-ALCL of 1 per 35 000 at age 50 years, 1 per 12 000 at 70 years, and 1 per 7000 at 75 years, with 3.3% of all women in the Netherlands having implants, our results affect a relatively large group of women, and therefore have multiple implications. First, comprehensive counseling of women considering breast implants for cosmetic or reconstructive reasons should be mandatory, including communication of risks and symptoms (late seroma or mass) of BIA-ALCL, especially because outcomes in early-stage disease are usually excellent. Second, in our opinion, alternative (autologous) breast surgery procedures should be stimulated, and importantly, be reimbursed in specific groups of women, ie, healthy women at high genetic breast cancer risk considering prophylactic mastectomy, women who had mastectomy for breast cancer, and women who underwent explantation after silicone breast implant-related problems. Third, the fact that the use of silicone breast implants—more than fifty years after their introduction—is again under debate owing to increased risk of BIA-ALCL, implies a call for support of registry programs for breast implants and other medical devices, supported and funded independently as postmarket monitoring systems. Risk-benefit evaluations on breast implants will vary by indication and fall under the responsibility of national governmental and regulatory bodies. Collaboration between international research groups, registries, and governmental organizations to pool multidisciplinary data on BIA-ALCL cases and breast implant prevalence are essential to support these efforts.
eMethods
eResults
eReferences
Supplementary Legends
eTable 1. Implant characteristics of 32 patients with breast-ALCL with breast implants
eTable 2. Clinical characteristics and treatment of 43 patients with breast-ALCL with and without breast implants
eTable 3. Clinicopathological characteristics of 32 patients with breast-ALCL with breast implants, diagnosed between 1990 and 2016
eFigure 1. Selection strategy
eFigure 2. Assessment of breast implant prevalence in the Netherlands in 2015, by A: Regional breast implant prevalence in 2015 in women between 20-70 as estimated from 3000 chest X-rays in two large regional medical centers in the East and South of the Netherlands, B: Region-specific breast implant prevalence rates from the National Breast Cancer Screening Program (BCSP) and C: Derived estimation of breast implant prevalence in the Netherlands in 2015 in women between 20-70
eFigure 3. Estimated number of breast implants sold in the Netherlands by calendar year
eFigure 4. Incidence of breast-ALCL in patients with breast implants and reasons for breast implantation
eFigure 5. Sales data of breast implants in the Netherlands between 2010 and 2015 by A: surface properties and B: filling properties
References
- 1.Angell M. Shattuck Lecture—evaluating the health risks of breast implants: the interplay of medical science, the law, and public opinion. N Engl J Med. 1996;334(23):1513-1518. [DOI] [PubMed] [Google Scholar]
- 2.Balk EM, Earley A, Avendano EA, Raman G. Long-term health outcomes in women with silicone gel breast implants: a systematic review. Ann Intern Med. 2016;164(3):164-175. [DOI] [PubMed] [Google Scholar]
- 3.Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med. 2000;342(11):781-790. [DOI] [PubMed] [Google Scholar]
- 4.de Jong D, Vasmel WL, de Boer JP, et al. . Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300(17):2030-2035. [DOI] [PubMed] [Google Scholar]
- 5.Brody GS, Deapen D, Taylor CR, et al. . Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135(3):695-705. [DOI] [PubMed] [Google Scholar]
- 6.Medical Device Reports of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Silver Spring, Maryland, U.S. Food & Drug Administration (FDA), 2017. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm481899.htm. Accessed March 23, 2017.
- 7.Swerdlow SH, Campo E, Pileri SA, et al. . The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doren EL, Miranda RN, Selber JC, et al. . U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139(5):1042-1050. [DOI] [PubMed] [Google Scholar]
- 9.Loch-Wilkinson A, Beath KJ, Knight RJW, et al. . Breast implant associated anaplastic large cell lymphoma in Australia and New Zealand—high surface area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140(4):645-654. [DOI] [PubMed] [Google Scholar]
- 10.Casparie M, Tiebosch AT, Burger G, et al. . Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda RN, Aladily TN, Prince HM, et al. . Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32(2):114-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paap E, Witjes M, van Landsveld-Verhoeven C, Pijnappel RM, Maas AH, Broeders MJ. Mammography in females with an implanted medical device: impact on image quality, pain and anxiety. Br J Radiol. 2016;89(1066):20160142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fracheboud J, de Koning HJ, Boer R, et al. ; National Evaluation Team for Breast cancer screening in The Netherlands . Nationwide breast cancer screening programme fully implemented in The Netherlands. Breast. 2001;10(1):6-11. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335-351. [DOI] [PubMed] [Google Scholar]
- 15.Population in the Netherlands; by sex and age and marital status, 1 January, 1950-2016. Heerlen, the Netherlands: Statistics Netherlands, 2017. http://statline.cbs.nl/Statweb/. Accessed February 13, 2017.
- 16.Schouten LJ, Straatman H, Kiemeney LA, Verbeek AL. Cancer incidence: life table risk versus cumulative risk. J Epidemiol Community Health. 1994;48(6):596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SAS Institute Inc 2011. Base SAS 9.3 Procedures Guide. Cary, NC. [Google Scholar]
- 18.De Boer M, van der Sluis WB, de Boer JP, et al. . BIA-ALCL in a transgender woman. Aesthet Surg J. 2017;37(8):NP83-NP87. [DOI] [PubMed] [Google Scholar]
- 19.Blombery P, Thompson ER, Jones K, et al. . Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica. 2016;101(9):e387-e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadin ME, Deva A, Xu H, et al. . Biomarkers provide clues to early events in the pathogenesis of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2016;36(7):773-781. [DOI] [PubMed] [Google Scholar]
- 21.Hu H, Johani K, Almatroudi A, et al. . Bacterial biofilm infection detected in breast implant associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2016;137(6):1659-1669. [DOI] [PubMed] [Google Scholar]
- 22.Ye X, Shokrollahi K, Rozen WM, et al. . Anaplastic large cell lymphoma (ALCL) and breast implants: breaking down the evidence. Mutat Res Rev Mutat Res. 2014;762:123-132. [DOI] [PubMed] [Google Scholar]
- 23.Thomas A, Link BK, Altekruse S, Romitti PA, Schroeder MC. Primary breast lymphoma in the United States: 1975-2013. J Natl Cancer Inst. 2017;109(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deva AK. Discussion: U.S. Epidemiology of Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Plast Reconstr Surg. 2017;139(5):1051-1052. [DOI] [PubMed] [Google Scholar]
- 25.Khouri RK, Rigotti G, Khouri RK Jr, et al. . Tissue-engineered breast reconstruction with Brava-assisted fat grafting: a 7-year, 488-patient, multicenter experience. Plast Reconstr Surg. 2015;135(3):643-658. [DOI] [PubMed] [Google Scholar]
- 26.Tuinder S, Baetens T, De Haan MW, et al. . Septocutaneous tensor fasciae latae perforator flap for breast reconstruction: radiological considerations and clinical cases. J Plast Reconstr Aesthet Surg. 2014;67(9):1248-1256. [DOI] [PubMed] [Google Scholar]
- 27.Faris O, Shuren J. An FDA Viewpoint on Unique Considerations for Medical-Device Clinical Trials. N Engl J Med. 2017;376(14):1350-1357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eResults
eReferences
Supplementary Legends
eTable 1. Implant characteristics of 32 patients with breast-ALCL with breast implants
eTable 2. Clinical characteristics and treatment of 43 patients with breast-ALCL with and without breast implants
eTable 3. Clinicopathological characteristics of 32 patients with breast-ALCL with breast implants, diagnosed between 1990 and 2016
eFigure 1. Selection strategy
eFigure 2. Assessment of breast implant prevalence in the Netherlands in 2015, by A: Regional breast implant prevalence in 2015 in women between 20-70 as estimated from 3000 chest X-rays in two large regional medical centers in the East and South of the Netherlands, B: Region-specific breast implant prevalence rates from the National Breast Cancer Screening Program (BCSP) and C: Derived estimation of breast implant prevalence in the Netherlands in 2015 in women between 20-70
eFigure 3. Estimated number of breast implants sold in the Netherlands by calendar year
eFigure 4. Incidence of breast-ALCL in patients with breast implants and reasons for breast implantation
eFigure 5. Sales data of breast implants in the Netherlands between 2010 and 2015 by A: surface properties and B: filling properties