Key Points
Question
Are pretreatment derived neutrophils/(leukocytes minus neutrophils) ratio (dNLR) and lactate dehydrogenase (LDH) level associated with resistance to immunotherapy in patients with advanced non–small cell lung cancer (NSCLC)?
Findings
In this cohort study evaluating 466 patients with advanced NSCLC, the Lung Immune Prognostic Index (LIPI), combining baseline dNLR and LDH, was associated with the outcomes of immunotherapy but not chemotherapy.
Meaning
Poor baseline LIPI, combining dNLR greater than 3 and LDH greater than upper limit of normal, was correlated with worse outcomes for immune checkpoint inhibitor treatment in patients with NSCLC, but not with chemotherapy.
This cohort study investigates whether the pretreatment derived neutrophils/(leukocytes minus neutrophils) ratio and lactate dehydrogenase level are associated with resistance to immunotherapy in patients with advanced non–small cell lung cancer.
Abstract
Importance
Derived neutrophils/(leukocytes minus neutrophils) ratio (dNLR) and lactate dehydrogenase (LDH) level have been correlated with immune checkpoint inhibitor (ICI) outcomes in patients with melanoma.
Objective
To determine whether pretreatment dNLR and LDH are associated with resistance to ICIs in patients with advanced non–small cell lung cancer (NSCLC).
Design, Setting, and Participants
Multicenter retrospective study with a test (n = 161) and a validation set (n = 305) treated with programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors in 8 European centers, and a control cohort (n = 162) treated with chemotherapy only. Complete blood cell counts, LDH, and albumin levels were measured before ICI treatment. A lung immune prognostic index (LIPI) based on dNLR greater than 3 and LDH greater than upper limit of normal (ULN) was developed, characterizing 3 groups (good, 0 factors; intermediate, 1 factor; poor, 2 factors).
Main Outcomes and Measures
The primary end point was overall survival (OS). Secondary end points were progression-free survival (PFS) and disease control rate (DCR).
Results
In the pooled ICI cohort (N = 466), 301 patients (65%) were male, 422 (90%) were current or former smokers, and 401 (87%) had performance status of 1 or less; median age at diagnosis was 62 (range, 29-86) years; 270 (58%) had adenocarcinoma and 159 (34%) had squamous histologic subtype. Among 129 patients with PD-L1 data, 96 (74%) had PD-L1 of at least 1% by immunohistochemical analysis, and 33 (26%) had negative results. In the test cohort, median PFS and OS were 3 (95% CI, 2-4) and 10 (95% CI, 8-13) months, respectively. A dNLR greater than 3 and LDH greater than ULN were independently associated with OS (hazard ratio [HR] 2.22; 95% CI, 1.23-4.01 and HR, 2.51; 95% CI, 1.32-4.76, respectively). Median OS for poor, intermediate, and good LIPI was 3 months (95% CI, 1 month to not reached [NR]), 10 months (95% CI, 8 months to NR), and 34 months (95% CI, 17 months to NR), respectively, and median PFS was 2.0 (95% CI, 1.7-4.0), 3.7 (95% CI, 3.0-4.8), and 6.3 (95% CI, 5.0-8.0) months (both P < .001). Disease control rate was also correlated with dNLR greater than 3 and LDH greater than ULN. Results were reproducible in the ICI validation cohort for OS, PFS, and DCR, but were nonsignificant in the chemotherapy cohort.
Conclusions and Relevance
Pretreatment LIPI, combining dNLR greater than 3 and LDH greater than ULN, was correlated with worse outcomes for ICI, but not for chemotherapy, suggesting that LIPI can serve as a potentially useful tool when selecting ICI treatment, raising the hypothesis that the LIPI might be useful for identifying patients unlikely to benefit from treatment with an ICI.
Introduction
Immunotherapy, principally represented by programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors, has been approved worldwide as treatment for advanced non–small cell lung cancer (NSCLC) and has been hailed as an important addition to the treatment armamentarium for this population. In an unselected previously treated NSCLC population, response rates with single-agent immune checkpoint inhibitors (ICIs) range between 14% and 20%, with median overall survival (OS) of 10 to 12 months. However, when this population is stratified by specific biomarkers, notably positive PD-L1 expression by immunohistochemical analysis, these outcomes improved, reaching up to 30% response with a median OS of 20 months, in contrast to patients with negative or weak PD-L1 expression (1%-49% positive tumor cells, accounting for approximately 67% of the population) for whom response rates between 8% and 19% have been reported with median OS slightly below 10 months. However, even within the PD-L1–positive stratum, the benefit with immunotherapy is not seen for the entire population, making the identification of biomarkers for patients likely to respond to ICI therapy a critical step in selecting the candidate population.
The inflammation process has been proposed as a mechanism of immunoresistance in patients with cancer, promoting cancer growth and dissemination, and activating oncogenic signaling pathways. Furthermore, a peripheral pro-inflammatory status has been associated with worse outcomes in patients with cancer. Numerous routine blood parameters have been investigated as potential inflammatory biomarkers in patients with cancer, such as elevated concentration of circulating white blood cells, absolute neutrophil count, absolute platelet count, and lactate dehydrogenase (LDH) level, which are associated with poor outcomes in several cancer types. Novel potential biomarkers such as the neutrophil to lymphocyte ratio (NLR) and derived neutrophil to lymphocyte ratio (dNLR; absolute neutrophil count/[white blood cell concentration − absolute neutrophil count]) have been investigated to measure inflammatory status in various cancers, including NSCLC. These ratios are simple, easy-to-calculate instruments, combining at least 2 blood cell subpopulations, and are accessible from routine complete blood cell counts.
However, with the exception of melanoma, the prognostic and predictive value of circulating inflammatory biomarkers for ICIs is unknown in most tumor types (eTable 1 in the Supplement). The 2 largest published retrospective analyses in advanced melanoma cohorts, one in 720 patients treated with ipilimumab and the other in 512 patients treated with pembrolizumab, reported the independent prognostic value of the dNLR (dNLR ≥3, hazard ratio [HR], 4.10; 95% CI, 3.08-5.46; P < .001) and LDH of at least 2.5 times upper limit of normal (ULN; HR, 2.8; 95% CI, 2.0-3.9; P = .001). Both studies reflected that a pro-inflammatory status was correlated with poor outcomes in patients with melanoma treated with ICIs. We hypothesized that the combination of baseline dNLR and LDH could be correlated with resistance to ICI therapy in patients with advanced NSCLC and could be used to develop a lung immune prognostic index (LIPI).
Methods
Patients
We conducted a multicentric retrospective study of a cohort of 466 patients with advanced NSCLC receiving treatment with PD-1/PD-L1 inhibitors in a variety of settings covering routine clinical care, expanded access, and compassionate-use programs, as well as clinical trials (nivolumab, pembrolizumab, atezolizumab, durvalumab, and durvalumab-ipilimumab). Initially, patients from Gustave Roussy treated with PD-1/PD-L1 inhibitors between November 2012 and July 2016 were included in a monocentric test set (n = 161) to establish the potential of the score. The hypothesis was then validated in a larger multicentric validation set (n = 305) that included patients treated with PD-1/PD-L1 inhibitors between November 2012 and January 2017 from 8 European academic centers (including patients from Gustave Roussy from July 2016 to January 2017) (eFigure 1 in the Supplement). To determine whether the LIPI is ICI specific, a control cohort of 162 patients with advanced NSCLC exclusively treated with chemotherapy, composed of 128 patients from Gustave Roussy treated between December 2011 and September 2015, and 34 from the Hospital 12 de Octubre treated between November 2012 and July 2016 were also evaluated for LIPI.
Complete blood cell counts, LDH, and albumin levels at baseline before ICI treatment (within 30 days before the first treatment) were extracted from electronic medical records. Demographic, clinical, pathological, and molecular data were also collected.
Data for PD-L1 expression were analyzed on tumor cells by immunohistochemistry, according to standard practice for each center. Expression of at least 1% was considered positive.
Radiological assessments were performed every 8 weeks per RECIST (Response Evaluation Criteria in Solid Tumors) v1.1 in the test set and per the investigator’s discretion in the validation set and the chemotherapy cohort. Radiological atypical response patterns per the investigator were collected including mixed or dissociated response (progressive disease in one location with objective response at another site).
This study was approved by the Institutional Review Board of Gustave Roussy (Commission Scientifique des Essais Thérapeutiques) and informed consent was not required because of the retrospective character of the study.
The LIPI was developed on the basis of dNLR greater than 3 and LDH greater than ULN, characterizing 3 groups (good, 0 factors; intermediate, 1 factor; poor, 2 factors). The cutoff for dNLR was greater than 3 (according to the cutoff from the largest published study with ICIs in patients with cancer), and the ULN for LDH was defined according the limit of each center.
Statistical Analysis
Disease control rate (DCR) was defined as complete plus partial response plus stable disease, and overall response rate as complete plus partial response. Overall survival was calculated from the date of first immunotherapy administration until death due to any cause. Progression-free survival (PFS) was calculated from the date of first immunotherapy administration until disease progression or death due to any cause. Comparisons between patient characteristics were performed using χ2 or Fisher exact test for discrete variables and the unpaired t test, Wilcoxon sign-rank test, or analysis of variance for continuous variables. Survival analyses were performed using the Kaplan-Meier method and the log-rank test. All P values inferior to .05 were considered statistically significant.
A Cox proportional hazards regression model was used to evaluate factors independently associated with OS and PFS. Variables included in the final multivariate model were selected according to their clinical relevance and statistical significance in a univariate analysis (cutoff, P = .10). The proportional hazard hypothesis was verified with the Schoenfeld residual method. Factors associated with disease control were tested with logistic regression in univariate and multivariate analyses. The α level was 5%. Internal validation of the final multivariate model for OS was performed on the overall LIPI population with a bootstrap sample procedure (n = 1000 samples). Statistical analyses were performed with R (free software environment for statistical computing and graphics).
Results
Test Set
dNLR and LDH
Baseline characteristics of the 161 test set patients are summarized in eTable 2 in the Supplement. Median follow-up was 12 months (95% CI, 11-14 months). Median OS was 10 months (95% CI, 8-13 months) and median PFS was 3 months (95% CI, 2-4 months). A dNLR greater than 3 and LDH greater than ULN were associated with shorter OS (HR, 1.98; 95% CI, 1.27-3.10; P = .002 and HR, 2.44; 95% CI, 1.47-4.04; P < .001, respectively, log-rank test) and shorter PFS (HR, 1.61; 95% CI, 1.1-2.34 and HR, 1.77; 95% CI, 1.16-2.69; both P = .01, log-rank test). A dNLR greater than 3 and LDH greater than ULN were also associated with progressive disease (non-DCR) (OR, 2.12; 95% CI, 1.07-4.21; P = .03 and OR, 3.14; 95% CI, 1.47-6.71; P = .003, respectively, Cox regression).
In a multivariate analysis, LDH greater than ULN (HR, 2.51; 95% CI, 1.32-4.76) and dNLR greater than 3 (HR, 2.22; 95% CI, 1.23-4.01) were independently associated with OS, while performance status of 2 or greater (HR; 2.21, 95% CI, 1.04-4.67) and dNLR greater than 3 (HR, 1.83; 95% CI, 1.12-2.98) were independently associated with PFS (eTable 3 in the Supplement).
Lung Immune Prognostic Index (LIPI)
Baseline dNLR greater than 3 and LDH greater than ULN, both independently associated with OS in the Cox proportional hazard model, were combined for the LIPI calculation. Thirty-seven patients without baseline LDH or dNLR were excluded from the LIPI analysis. Among the 126 evaluable patients, 45 (36%) had good LIPI, 62 (49%) had intermediate LIPI, and 19 (15%) had poor LIPI.
Median OS was 3 months (95% CI, 1 month to not reached [NR]) vs 10 months (95% CI, 8 months to NR) vs 34 months (95% CI, 17 months to NR) for the poor, intermediate, and good LIPI groups, respectively (P < .001) (eFigure 2A in the Supplement). Median PFS was 1 month (95% CI, 2 to 4 months) vs 3 months (95% CI, 2 to 5 months) vs 6 months (95% CI, 4 months to NR) for the poor, intermediate, and good LIPI groups, respectively (P = .001) (eFigure 2B in the Supplement). Disease control rate was also significantly correlated with the LIPI groups (P = .004) (eTable 4 in the Supplement). Intermediate and poor LIPI groups were associated with progressive disease as the best response to ICI (non-DCR), with an odds radio (OR) of 3.21 (95% CI, 1.12-9.18; P = .03) and 8.03 (95% CI, 1.82-35.36; P = .006), respectively. Performance status of at least 2 at baseline was also associated with non-DCR, with an OR of 7.89 (95% CI, 1.46-42.50).
Validation Set
Baseline characteristics in the 305 patients in the validation set were comparable to those of the test set (eTable 1 in the Supplement). Median follow-up was 12.8 months (95% CI, 11.9-14.4 months), median OS was 10.5 months (95% CI, 8.3-12.6 months), and median PFS was 4.6 months (95% CI, 4.0-6.3 months).
Lung Immune Prognostic Index was inversely correlated with OS and PFS. For the poor (high LIPI), intermediate, and good (low LIPI) LIPI groups, median OS was 6.2 months (95% CI, 4.3 to 13.9 months) vs 10.0 months (95% CI, 7.2 to 12.9 months) vs 14.2 months (95% CI, 10.8 months to NR), respectively (P = .004) (eFigure 2C in the Supplement). For the poor, intermediate, and good LIPI groups, median PFS was 3.6 months (95% CI, 2.1-6.7 months) vs 4.2 months (95% CI, 3.1-6.3 months) vs 6.7 months (95% CI, 4.5-8.0 months) (P = .005) (eFigure 2D in the Supplement). The response rate (according to the investigator) and the DCR were also correlated with the LIPI groups (P < .001 and P = .01, respectively) (eFigure 3 and eTable 4 in the Supplement).
Pooled LIPI Population
Baseline characteristics are summarized in eTable 2 in the Supplement, and in eTable 5 in the Supplement according to LIPI group. Median follow-up was 12.8 months (95% CI, 11.9-13.5 months). Median dNLR was 2.42 (interquartile range, 1.73-3.61) and was greater than 3 in 163 patients (35%). Median LDH was 248.5 U/L (interquartile range, 189-350 U/L; to convert to microkatals per liter, multiply by 0.0167), and greater than ULN in 179 patients (41%). For the overall population, LDH and dNLR data were missing for 7% and 3% of the population, respectively. Overall, PD-L1 status was positive in 96 patients (21%), negative in 33 (7%) and not available in 337 patients (72%). The high rate of missing PD-L1 status was due to the fact that it was not mandatory for ICI prescription. The median number of prior lines of therapy administered before ICI therapy was 1 (range, 0-11). The overall response rate with ICI was 27% (n = 126 patients), and 32 patients (7%) had additional atypical or dissociated response patterns.
The LIPI was evaluable for 431 patients, giving 63 patients (15%) in the poor group, 206 (48%) in the intermediate group, and 162 (38%) in the good LIPI group. Median OS was 10.1 months (95% CI, 9.0-11.7 months) and median PFS was 4.0 months (95% CI, 3.4-5.0 months).
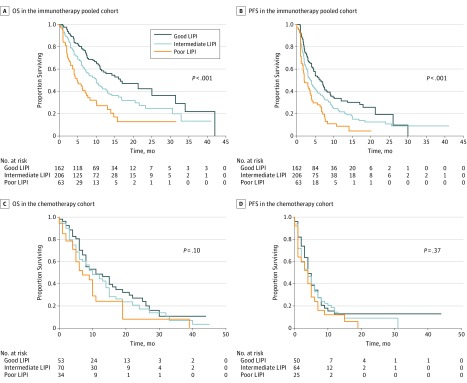
Median OS was 4.8 (95% CI, 3.6-7.7) vs 10.0 (95% CI, 7.3-12.6) vs 16.5 (95% CI, 11.4-34.0) months for the poor, intermediate, and good LIPI groups, respectively (Figure, A), and median PFS was 2.0 (95% CI, 1.7-4.0) vs 3.7 (95% CI, 3.0-4.8) vs 6.3 (95% CI, 5.0-8.0) months (both P < .001) (Figure, B).
Figure. Overall Survival (OS) and Progression-Free Survival (PFS) According to Lung Immune Prognostic Index (LIPI) Groups, in the Immunotherapy Pooled Cohort and in the Chemotherapy Cohort.
The role of dNLR greater than 3 and LDH greater than ULN were further validated with a resampling bootstrap procedure (1000 replications) in which all statistical analyses were replicated on each bootstrapped sample (Table 1), with this internal validation procedure reflecting the robustness of the final model. A dNLR greater than 3 and LDH greater than ULN were associated with significantly shorter OS (HR, 1.70; 95% CI, 1.27-2.28; P < .001 and HR, 1.36; 95% CI, 1.02-1.83; P = .04, respectively). No differences were observed in our cohort according to PD-L1 expression (positive/negative/unknown) in terms of PFS and OS.
Table 1. Multivariate Analysis for Overall Survival in the Immunotherapy Pooled Cohort (Bootstrap Internal Validation).
| Variable | HR (95% CI) | P Value | Internal Validation BCA HR (95% CI) |
|---|---|---|---|
| Age, y | |||
| <70 | 1 [Reference] | .10 | |
| ≥70 | 1.35 (0.94-1.93) | 0.93-1.96 | |
| Smoking history | |||
| Nonsmoker | 1 [Reference] | .89 | |
| Smoker | 0.96 (0.58-1.60) | 0.60-1.66 | |
| Histologic subtype | |||
| Nonsquamous | 1 [Reference] | .10 | |
| Squamous | 1.30 (0.95-1.79) | 0.94-1.87 | |
| No. of metastatic sites | |||
| <2 | 1 [Reference] | .004 | |
| ≥2 | 1.56 (1.15-2.12) | 1.11-2.22 | |
| Lines of immunotherapy | |||
| <2 | 1 [Reference] | .56 | |
| ≥2 | 0.91 (0.67-1.24) | 0.65-1.25 | |
| Performance status before immunotherapy | |||
| 0 or 1 | 1 [Reference] | <.001 | |
| ≥2 | 2.08 (1.38-3.13) | 1.27-3.70 | |
| Lactate dehydrogenase (ULN) | |||
| Low or normal | 1 [Reference] | .04 | |
| High | 1.36 (1.02-1.83) | 0.98-1.92 | |
| Derived neutrophil/lymphocyte ratio | |||
| ≤3 | 1 [Reference] | <.001 | |
| >3 | 1.70 (1.27-2.28) | 1.29-2.41 | |
| Albumin level, g/dL | |||
| ≥3.5 | 1 [Reference] | .001 | |
| <3.5 | 1.69 (1.23-2.34) | 1.17-2.48 |
Abbreviations: BCA, bias-corrected and accelerated bootstrap interval; ULN, upper limit of normal.
SI conversion factors: To convert lactate dehydrogenase to microkatals per liter, multiply by 0.0167.
The LIPI was also correlated with response rate (P < .001). In a multivariate analysis, the intermediate and poor groups were associated with progressive disease, with an OR of 2.20 (95% CI, 1.26-3.84; P = .005) and 3.04 (95% CI, 1.46-6.36; P = .003), respectively (Table 2).
Table 2. Logistic Regression of Clinical Benefit (Disease Control Rate) in the Immunotherapy Pooled Cohort.
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Age, y | ||
| <70 | .84 | |
| ≥70 | 1.06 (0.58-1.96) | |
| Smoking history | ||
| Nonsmoker | 1 [Reference] | .21 |
| Smoker | 0.57 (0.24-1.37) | |
| Histologic subtype | ||
| Nonsquamous | 1 [Reference] | .82 |
| Squamous | 1.06 (0.62-1.81) | |
| No. of metastatic sites | ||
| ≤2 | 1 [Reference] | .12 |
| >2 | 1.49 (0.90-2.46) | |
| Lines of immunotherapy | ||
| ≤2 | 1 [Reference] | .25 |
| >2 | 1.34 (0.81-2.22) | |
| Performance status | ||
| 0 or 1 | 1 [Reference] | .03 |
| ≥2 | 2.10 (1.06-4.16) | |
| LIPI groups | ||
| Good | 1 [Reference] | |
| Intermediate | 2.20 (1.26-3.84) | .005 |
| Poor | 3.04 (1.46-6.36) | .003 |
Abbreviations: LIPI, lung immune prognostic index; OR, odds ratio.
Analysis of OS according to population subgroups revealed significant correlations with the LIPI group, regardless of histologic subtype (eFigure 4 in the Supplement) and age (eFigure 5 in the Supplement). In the smoker population, LIPI was correlated with OS (HR for intermediate-risk group, 1.68; 95% CI, 1.21-2.32; HR for poor-risk group, 2.82; 95% CI, 1.88-4.24; P < .001) (eFigure 6 in the Supplement). In the performance status 0 to 1 population, LIPI was correlated with OS (HR for intermediate-risk group, 1.70; 95% CI, 1.22-2.37; HR for poor-risk group, 3.30; 95% CI, 2.18-4.99; P < .001) (eFigure 7 in the Supplement). In addition, LIPI was correlated with OS for PD-L1 positive patients (HR for intermediate-risk group, 1.87; 95% CI, 0.90-3.85; HR for poor-risk group, 4.49; 95% CI, 1.97-10.24; P = .001) (n = 96 [74%]) (eFigure 8 in the Supplement). In the population without available PD-L1 status, LIPI was also associated with OS and PFS (HR for intermediate-risk group, 1.51; 95% CI, 1.07-2.14; HR for poor-risk group, 2.58; 95% CI, 1.65-4.04; P < .001 and HR for intermediate-risk group, 1.39; 95% CI, 1.04-1.84; HR for poor-risk group, 1.98; 95% CI, 1.34-2.92; P = .001, respectively; n = 337).
Chemotherapy-Only Cohort
In the chemotherapy cohort, the 162 patients with advanced NSCLC had a median follow-up of 25.0 months (95% CI, 21.0 months to NR). Baseline characteristics are summarized in eTable 2 in the Supplement. Patients received a median of 1 chemotherapy line (range, 1-3), which was first-line for 91 (56%) of them. Median PFS and OS were 4.0 (95% CI, 3.0-5.0) and 9.0 (95% CI, 8.0-13.0) months, respectively. Median dNLR and LDH were 3.02 (range, 0.78-43.11) and 218 U/L (range, 109-3408 U/L), respectively. No correlation was observed in the control cohort between dNLR or LDH and OS or PFS.
Among the 157 patients evaluable for LIPI, 53 patients (34%) had a good LIPI score, 70 (45%) intermediate, and 34 (22%) poor (eTable 6 in the Supplement). No differences were observed in terms of survival according to LIPI group. Median OS was 7 (95% CI, 5-19) vs 10 (95% CI, 7-15) vs 11 (95% CI, 8-19) months for the poor, intermediate, and good groups, respectively (P = .10, log-rank test) (Figure, C). Median PFS was 4 months (95% CI, 1-6 months) for poor vs 4 months (95% CI, 2-6 months) for intermediate vs 4 months (95% CI, 4-7 months) for good (P = .37, log-rank test) (Figure, D). No correlation was observed between LIPI and response rate or DCR.
Discussion
The LIPI, based on dNLR and LDH, was used to stratify our NSCLC population into 3 groups, good, intermediate, and poor. In our overall population of 431 patients treated with ICI, median OS and PFS were 10.1 and 4.0 months, respectively, consistent with prior reports in patients with NSCLC treated with PD-1 inhibitors in second or later lines. The 15% of the population with a poor (high) LIPI were more likely to have progressive disease as their best response to immunotherapy (OR, 3.04; P = .003, and had both shorter PFS (median, 2.0 months) and OS (median, 4.8 months) than those with an intermediate or good (low) LIPI (P < .001). On the other hand, LIPI was not associated with outcome in patients treated with chemotherapy only, providing support that it might be a predictor of benefit from ICI, a hypothesis that requires prospective validation.
In lung cancer, systemic inflammatory status has been closely correlated with worse prognosis, but mainly in early disease stages. In advanced disease, a negative impact has been observed in patients treated with platinum-based chemotherapy and targeted therapies; however, the effect of inflammatory status on immunotherapy benefit is not well known. Numerous routine blood parameters have been investigated as potential inflammatory biomarkers, including elevated neutrophils, platelets, LDH, and hypoalbuminemia, all of which are associated with poor outcomes in cancer. More recently, novel parameters, such as dNLR, have been investigated. The NLR is a well-known prognostic factor in patients with NSCLC. In a monocentric retrospective cohort of 175 patients treated with nivolumab, NLR of 5 or greater was associated with poor prognosis. The dNLR may be more relevant than NLR because it includes monocytes and other granulocyte subpopulations. High dNLR has been associated with shorter survival in patients with several tumor types, including melanoma, pancreas, bladder, and renal cancer. In melanoma, Ferruci et al reported that dNLR of 3 or greater had an independent negative effect on survival (HR, 4.10; P < .001) in 720 patients with melanoma treated with ipilimumab. To our knowledge, our study is the first to explore this parameter in NSCLC. Elevated dNLR was found in 35% of the patients with NSCLC, slightly higher than the 22.5% reported by Ferruci et al in melanoma.
Lactate dehydrogenase level is a classic inflammatory marker in patients with cancer, widely studied in lung cancer treated with chemotherapy or targeted therapies, as well as in patients with EGFR-mutant NSCLC; LDH has been found to be associated with shorter survival when increased from 1 to 2.5 times ULN. In melanoma, it has a potentially predictive effect on patients treated with PD-1 and CTLA-4 (cytotoxic T lymphocyte–associated antigen 4) inhibitors. Interestingly, Diem et al reported that in 66 patients with melanoma treated with PD-1 inhibitors, a greater than 10% increase in LDH was significantly associated with shorter OS, reflecting the potential value of the monitoring these markers.
Recently Kargl et al showed that neutrophils dominate the NSCLC immune landscape, being responsible for treatment failure under ICIs. In the tumor microenvironment, neutrophils can be manipulated, including early in their differentiation process, to develop different phenotypic and functional polarization states inducing antitumor or protumor effects. In a pro-inflammatory status, this induces an “emergency granulopoiesis” that rapidly increases neutrophil generation, releasing immature or poorly differentiated neutrophils, which has been associated with tumor progression. Derived NLR may reflect this negative inflammation.
Limitations
The retrospective nature of this study implies various limitations, including missing clinical and pathological data (albeit for a low proportion of patients); a high rate of patients had unknown PD-L1 status, and when data were available, the percentage of positive expression was often not provided. It should be noted that much of the population was treated in a second- or third-line setting, in which PD-L1 status was not mandatory for prescribing the therapy in Europe. Furthermore, for patients with known status, as this was not centrally performed, methodology was heterogeneous (eg, antibodies, measurements). Another limitation is that in the validation set and chemotherapy cohort, radiological assessment was performed locally, lowering the strength of the DCR and PFS results. Finally, some patients from the poor LIPI group achieved clinical benefit—possibly because automated neutrophil counts do not discriminate between the different subpopulations of neutrophils that could have protumor or antitumor functions.
Conclusions
Poor baseline LIPI, combining dNLR greater than 3 and LDH greater than ULN, was correlated with poor outcomes with immunotherapy, but not chemotherapy, raising the hypothesis that LIPI might be useful for identifying patients unlikely to benefit from treatment.
The assessment of LIPI in association with PD-L1 expression should be explored in future prospective clinical trials and will contribute to defining the prognostic and/or predictive value of LIPI. Moreover, LIPI could also be used to stratify patients in randomized studies.
eTable 1. Summary of publications of routine inflammatory markers in cancer patients treated with PD1/PD-L1 inhibitors
eTable 2. Baseline characteristics of the test set, validation set, and pooled immunotherapy cohorts, and the chemotherapy cohort
eTable 3. Multivariate analysis in the test cohort: hazard ratios (HR) for overall survival (OS) and progression-free survival (PFS)
eFigure 1. Study flowchart for A) the GR test set and B) validation set
eFigure 2. Survival according to LIPI groups in the GR test set for A) overall survival (OS), and B) progression-free survival (PFS); and in the ICI validation set for OS (C) and PFS (D)
eFigure 3. Progressive disease rate (%) according to LIPI group in the test and validation sets
eTable 4. Response rate and disease control rate according to LIPI groups in the GR test and validation set
eTable 5. Baseline characteristics according to LIPI group, in the pooled cohort
eTable 6. Baseline characteristics according to LIPI group, in the chemotherapy cohort
eFigure 4. OS according to histology subtypes and LIPI groups in the pooled ICI cohort: A) OS in the non-squamous population and B) OS in the squamous population
eFigure 5. A) OS in age subgroups according to LIPI groups, in the pooled cohort: A) OS in the younger population and B) OS in the older population
eFigure 6. OS according to smoking status and LIPI in the pooled cohort: A) OS in smoker and B) OS in the non-smoker population
eFigure 7. OS according to performance status and LIPI in the pooled cohort A) OS in PS 0-1, and B) OS in PS ≥2 populations
eFigure 8. Survival by PDL1 status, according to LIPI groups, in the pooled cohort: A) OS in the PD-L1 positive and B) OS in the PD-L1 negative population
eReferences
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst RS, Baas P, Kim D-W, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. [DOI] [PubMed] [Google Scholar]
- 5.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L, Li X, Shen Y, et al. . A new prognostic score based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Onco Targets Ther. 2016;9:4879-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laird BJA, Fallon M, Hjermstad MJ, et al. . Quality of life in patients with advanced cancer: differential association with performance status and systemic inflammatory response. J Clin Oncol. 2016;34(23):2769-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534-540. [DOI] [PubMed] [Google Scholar]
- 11.Petrelli F, Cabiddu M, Coinu A, et al. . Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54(7):961-970. [DOI] [PubMed] [Google Scholar]
- 12.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23(1):31-39. [DOI] [PubMed] [Google Scholar]
- 13.Templeton AJ, Ace O, McNamara MG, et al. . Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204-1212. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci PF, Ascierto PA, Pigozzo J, et al. . Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732-738. [DOI] [PubMed] [Google Scholar]
- 15.Weide B, Martens A, Hassel JC, et al. . Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22(22):5487-5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y-J, Wang L-X, Hong Y-Q, et al. . Lymphocyte to monocyte ratio is associated with response to first-line platinum-based chemotherapy and prognosis of early-stage non-small cell lung cancer patients. Tumour Biol. 2016;37(4):5285-5293. [DOI] [PubMed] [Google Scholar]
- 17.Cannon NA, Meyer J, Iyengar P, et al. . Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10(2):280-285. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y-M, Lai C-H, Chang H-C, et al. . Baseline and trend of lymphocyte-to-monocyte ratio as prognostic factors in epidermal growth factor receptor mutant non-small cell lung cancer patients treated with first-line epidermal growth factor receptor tyrosine kinase inhibitors. PLoS One. 2015;10(8):e0136252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Y, Wang J, Hong L, et al. . Platelet-lymphocyte ratio is an independent prognostic factor in patients with ALK-positive non-small-cell lung cancer. Future Oncol. 2017;13(1):51-61. [DOI] [PubMed] [Google Scholar]
- 20.Gu X-B, Tian T, Tian X-J, Zhang X-J. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep. 2015;5:12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagley SJ, Kothari S, Aggarwal C, et al. . Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1-7. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki R, Takagi T, Hikichi T, et al. . Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett. 2016;11(5):3441-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Kessel KEM, de Haan LM, Fransen van de Putte EE, et al. . Elevated derived neutrophil-to-lymphocyte ratio corresponds with poor outcome in patients undergoing pre-operative chemotherapy in muscle-invasive bladder cancer. Bladder Cancer. 2016;2(3):351-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amato RJ, Flaherty A, Zhang Y, Ouyang F, Mohlere V. Clinical prognostic factors associated with outcome in patients with renal cell cancer with prior tyrosine kinase inhibitors or immunotherapy treated with everolimus. Urol Oncol. 2014;32(3):345-354. [DOI] [PubMed] [Google Scholar]
- 25.Fiala O, Pesek M, Finek J, et al. . Change in serum lactate dehydrogenase is associated with outcome of patients with advanced-stage NSCLC treated with erlotinib. Anticancer Res. 2016;36(5):2459-2465. [PubMed] [Google Scholar]
- 26.Inomata M, Hayashi R, Tanaka H, et al. . Elevated levels of plasma lactate dehydrogenase is an unfavorable prognostic factor in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer, receiving treatment with gefitinib or erlotinib. Mol Clin Oncol. 2016;4(5):774-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diem S, Kasenda B, Spain L, et al. . Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114(3):256-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelderman S, Heemskerk B, van Tinteren H, et al. . Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63(5):449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kargl J, Busch SE, Yang GHY, et al. . Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8:14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8(3):125-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431-446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of publications of routine inflammatory markers in cancer patients treated with PD1/PD-L1 inhibitors
eTable 2. Baseline characteristics of the test set, validation set, and pooled immunotherapy cohorts, and the chemotherapy cohort
eTable 3. Multivariate analysis in the test cohort: hazard ratios (HR) for overall survival (OS) and progression-free survival (PFS)
eFigure 1. Study flowchart for A) the GR test set and B) validation set
eFigure 2. Survival according to LIPI groups in the GR test set for A) overall survival (OS), and B) progression-free survival (PFS); and in the ICI validation set for OS (C) and PFS (D)
eFigure 3. Progressive disease rate (%) according to LIPI group in the test and validation sets
eTable 4. Response rate and disease control rate according to LIPI groups in the GR test and validation set
eTable 5. Baseline characteristics according to LIPI group, in the pooled cohort
eTable 6. Baseline characteristics according to LIPI group, in the chemotherapy cohort
eFigure 4. OS according to histology subtypes and LIPI groups in the pooled ICI cohort: A) OS in the non-squamous population and B) OS in the squamous population
eFigure 5. A) OS in age subgroups according to LIPI groups, in the pooled cohort: A) OS in the younger population and B) OS in the older population
eFigure 6. OS according to smoking status and LIPI in the pooled cohort: A) OS in smoker and B) OS in the non-smoker population
eFigure 7. OS according to performance status and LIPI in the pooled cohort A) OS in PS 0-1, and B) OS in PS ≥2 populations
eFigure 8. Survival by PDL1 status, according to LIPI groups, in the pooled cohort: A) OS in the PD-L1 positive and B) OS in the PD-L1 negative population
eReferences