Abstract
Importance
There have been substantial improvements in the early detection, treatment, and survival from cancer in the United States, but it is not clear to what extent patients with different types of health insurance have benefitted from these advancements.
Objective
To examine trends in cancer survival by health insurance status from January 1997 to December 2014.
Design, Setting, and Participants
California Cancer Registry (a statewide cancer surveillance system) data were used to estimate population-based survival by health insurance status in 3 calendar periods: January 1997 to December 2002, January 2003 to December 2008, and January 2009 to December 2014 with follow-up through 2014. Overall, 1 149 891 patients diagnosed with breast, prostate, colorectal, or lung cancer, or melanoma in California were included in the study.
Main Outcomes and Measures
Five-year all-cause and cancer-specific survival probabilities by insurance category and calendar period for each cancer site and sex; hazard ratios (HRs) and 95% CIs for each insurance category (none, Medicare, other public) compared with private insurance in each calendar period.
Results
According to data from 1 149 891 patients diagnosed with breast, prostate, colorectal, or lung cancer, or melanoma gathered from the California Cancer Registry, improvements in survival were almost exclusively limited to patients with private or Medicare insurance. For patients with other public or no insurance, survival was largely unchanged or declined. Relative to privately insured patients, cancer-specific mortality was higher in uninsured patients for all cancers except prostate, and disparities were largest from 2009 to 2014 for breast (HR, 1.72; 95% CI, 1.45-2.03), lung (men: HR, 1.18; 95% CI, 1.06-1.31 and women: HR, 1.32; 95% CI, 1.15-1.50), and colorectal cancer (women: HR, 1.30; 95% CI, 1.05-1.62). Mortality was also higher for patients with other public insurance for all cancers except lung, and disparities were largest from 2009 to 2014 for breast (HR, 1.25; 95% CI, 1.17-1.34), prostate (HR, 1.17; 95% CI, 1.04-1.31), and colorectal cancer (men: HR, 1.16; 95% CI, 1.08-1.23 and women: HR, 1.11; 95% CI, 1.03-1.20).
Conclusions and Relevance
After accounting for patient and clinical characteristics, survival disparities for men with prostate cancer and women with lung or colorectal cancer increased significantly over time, reflecting a lack of improvement in survival for patients with other public or no insurance. To mitigate these growing disparities, all patients with cancer need access to health insurance that covers all the necessary elements of health care, from prevention and early detection to timely treatment according to clinical guidelines.
This population-based study of data from the California Cancer Registry examines trends in cancer survival by health insurance status from January 1997 to December 2014.
Key Points
Question
Have patients with different types of health insurance benefitted equally from recent improvements in cancer survival?
Findings
In this large population-based study, improvements in survival between January 1997 and December 2014 were limited to patients with private or Medicare insurance. Survival disparities for uninsured or other publicly insured patients with prostate, lung, or colorectal cancer increased significantly over time.
Meaning
To mitigate these growing disparities, patients with cancer need access to health insurance that covers all the necessary elements of health care, from prevention and early detection to timely treatment according to clinical guidelines.
Introduction
There have been substantial improvements in the early detection and treatment of cancer in the United States over the last 2 decades, with corresponding improvements in survival and reductions in cancer mortality. However, it is not clear to what extent patients with different types of health insurance have benefitted from these advancements.
Disparities in cancer outcomes by health insurance status have been well documented in the contemporary literature. Patients who are uninsured or insured through the Medicaid program are more likely to present with later stage disease, less likely to receive cancer-directed surgery and/or radiation therapy, and have lower survival than patients who are privately insured. Some of the largest disparities are seen among patients with cancers that have the potential to be diagnosed early through screening (breast and colorectal). The impact of health insurance on survival from cancer has also been shown to differ according to patient race/ethnicity and socioeconomic status (SES). For example, some of the largest differences in cancer survival for black patients vs white patients are seen among patients with private or Medicare insurance, but these disparities are absent among those with no insurance, suggesting that white patients derive greater benefit from health insurance than black patients. In contrast, health insurance has been shown to mediate the effects of poverty and social determinants on cancer survival and reduce disparities in outcome.
These studies are cross-sectional in nature and do not assess how temporal trends in cancer survival for patients with different types of health insurance are affecting the magnitude of these survival disparities. We leveraged population-based data from the California Cancer Registry (CCR) to examine trends in survival by health insurance status from January 1997 to December 2014, for the 5 most common cancers in California: breast, prostate, lung, colorectal, and melanoma. The CCR is 1 of only 5 population-based cancer registries in the United States to have collected payer information from as far back as the 1990s, enabling a comprehensive examination of trends in cancer survival disparities by health insurance status over a period spanning nearly 2 decades.
Methods
This study received institutional review board approval and was included under the protocol for the Greater Bay Area Cancer Registries (ie, Surveillance, Epidemiology, and End Results/CCR Regions 1 and 8).
Data from the CCR were used to estimate trends in all-cause and cancer-specific survival by health insurance status for 5 common cancers. Analyses included all patients in California diagnosed between January 1997 and December 2014 with either female breast, prostate, colorectal, or lung cancer, or melanoma as a first, primary malignancy. Of the 1 240 571 cases eligible for inclusion, we excluded cases diagnosed at autopsy or from death certificate only (n = 11 635) and those with unknown follow-up time (n = 7763). A further 65 443 cases (5%) were excluded because of unknown health insurance status.
Three 6-year calendar periods of diagnosis were defined to allow 5-year survival to be estimated using the most contemporary data available: January 1997 to December 2002 (1997-2002), January 2003 to December 2008 (2003-2008), and January 2009 to December 2014 (2009-2014). Patient vital status was determined by routine linkage to state and national mortality files. Follow-up time for all-cause mortality was computed as the number of days between date of diagnosis and the earliest of: date of death, date of last known contact, or end date of follow-up (5 years after date of diagnosis for patients diagnosed during 1997-2009, or December 31, 2014, for patients diagnosed 2010-2014). In the 2009-2014 calendar period, only patients diagnosed in 2009 were able to be followed for 5 years; median follow-up for this period was 2.1 years. For the analysis of cancer-specific mortality, the underlying cause of death was obtained from death certificates, and follow-up time was censored at date of death for those who died from an underlying cause other than the primary cancer. Cases with an unknown cause of death were excluded from the analysis of cancer-specific mortality (n = 5839). There were 367 543 deaths from any cause (32% of 1 155 730 included cases) and 256 465 cancer-specific deaths (22% of the 1 149 891 included cases) within 5 years.
Patient-level health insurance status was based on primary and secondary payer source and categorized as no insurance; private insurance only; Medicare only or Medicare plus private insurance (Medicare); any public, military, or any Medicaid and/or Medi-Cal insurance (other public). The validity of health insurance status in the CCR has been independently verified, achieving over 80% agreement with 3 other data sources. Patient address at diagnosis was geocoded and assigned to a census block group then linked to an index of neighborhood SES (nSES). The nSES index uses principal components analysis of 2000 Census or 2007 to 2011 American Community Survey data on education, occupation, employment, household income, poverty, and rent and house values. The composite nSES score is categorized according to quintiles of the statewide distribution.
Statistical Analysis
All-cause and cancer-specific survival probabilities at 5 years since diagnosis were estimated at age 65 years for each cancer site, sex, and health insurance category in each calendar period. Temporal trends in survival by insurance status were assessed using linear regression of survival by calendar period. Multivariable Cox proportional hazard regression models were used to examine the relative effect of insurance status on all-cause and cancer-specific mortality in each calendar period. Proportionality of hazards for key covariates was tested by examining the correlation between time and scaled Schoenfeld residuals. The assumption of proportional hazards was violated for age and/or stage at diagnosis for all cancer sites. Cox models were therefore age stratified and stage stratified to allow the baseline hazards to vary by these variables. Models were adjusted for possible prognostic factors including tumor characteristics, treatment (surgery, radiotherapy, chemotherapy), marital status, race/ethnicity, institutional-level factors, neighborhood-level characteristic (SES, racial/ethnic composition, urban/rural status), and diagnosis year.
For each cancer site and sex, factors that were statistically significantly (P < .05) associated with 5-year cancer-specific mortality in multivariable models in any of the 3 calendar periods were included in the models for all 3 periods. All models were adjusted for clustering by hospital, using a sandwich estimator of the covariance structure that accounts for intracluster dependence. Wald P values for individual cross-product interaction terms between insurance status and period (reference: 1997-2002) were computed from an overall model adjusted for all significant interactions between adjustment factors and period. All analyses were performed in SAS version 9.4 (SAS Institute, Inc).
Results
Approximately half of the patients included in the analysis had private health insurance only. From 1997 to 2014, the proportion of patients with Medicare fell by 5 percentage points while the proportion of patients with other public health insurance increased by 8 percentage points. Less than 2% of patients had no health insurance (eTable 1 in the Supplement). Patients with other public or no insurance were more likely to be diagnosis with later stage disease (eTable 2 in the Supplement). The majority of patients in the other public insurance category had Medicaid insurance (74%).
Trends in 5-Year Survival
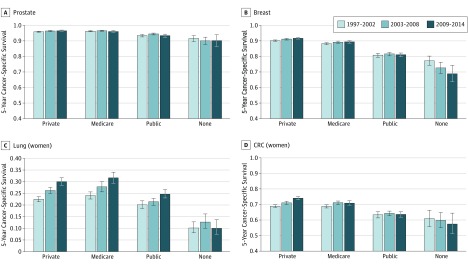
Five-year cancer-specific survival for men with prostate cancer was over 90%, and improvements in survival from 1997 to 2014 were negligible (Figure, A). Survival for women with breast cancer was similarly high, with small improvements for those with private insurance only and evidence of a decline in survival for those with no insurance (Figure, B). For patients with lung cancer, survival improved for those with private, Medicare, and to a lesser extent, other public insurance, but there was no change in survival for the patients who were uninsured (Figure, C). For patients with colorectal cancer, improvements in survival were seen for those with private insurance (men and women) and for uninsured men. In contrast, survival for uninsured women with colorectal cancer declined (Figure, D). There was little change in survival for patients with melanoma from January 1997 to December 2014, with the exception of uninsured men, for whom survival improved slightly, and uninsured women, for whom survival declined (eTable 3 in the Supplement for estimates of 5-year cancer-specific survival by insurance status and calendar period for each cancer site and sex).
Figure. Cancer-Specific Survival Probabilities at 5 Years Since Diagnosis for Patients Age 65 Years by Insurance Status and Calendar Period in California From 1997 to 2014.
Survival probabilities are shown for patients at age 65 years by insurance status and calendar period (A) prostate cancer, (B) breast cancer, (C) lung cancer (women), and (D) colorectal cancer (CRC) (women); for lung and colorectal cancer in men and melanoma in men and women see the eFigure in the Supplement.
Disparities by Insurance Status
Differences in all-cause and cancer-specific mortality between patients with Medicare, other public, or no insurance, relative to those with private insurance, were assessed in each calendar period and adjusted for a number of potential prognostic factors. Among men with prostate cancer, disparities in cancer-specific mortality for those with other public insurance compared with private insurance increased from nonsignificant levels in 1997-2002 to 17% among those diagnosed during 2009-2014 (HR, 1.17; 95% CI, 1.04-1.31) (Table). There were no significant differences in cancer-specific mortality between men with prostate cancer who were uninsured or privately insured.
Table. Five-Year Cause-Specific Mortality by Health Insurance Status in Each Calendar Period of Diagnosis, by Cancer Site and Sex in California From 1997 to 2014a.
| Cancer | HR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| 1997-2002 | 2003-2008 | 2009-2014 | 1997-2002 | 2003-2008 | 2009-2014 | |
| Breast cancer | ||||||
| No insurance | ND | ND | ND | 1.45 (1.26-1.67)b | 1.61 (1.37-1.89)b | 1.72 (1.45-2.03)b |
| Medicare | ND | ND | ND | 1.02 (0.96-1.09) | 1.07 (0.99-1.15) | 1.07 (0.98-1.17) |
| Other public | ND | ND | ND | 1.21 (1.14-1.31)b | 1.32 (1.24-1.40)b | 1.25 (1.17-1.34)b |
| Prostate cancer | ||||||
| No insurance | 0.89 (0.73-1.09) | 1.08 (0.81-1.43) | 1.17 (0.88-1.56) | ND | ND | ND |
| Medicare | 0.88 (0.81-0.96)b | 0.94 (0.87-1.01)c | 1.03 (0.94-1.13)c | ND | ND | ND |
| Other public | 1.02 (0.91-1.13) | 1.06 (0.97-1.16) | 1.17 (1.04-1.31)b,c | ND | ND | ND |
| Lung cancer | ||||||
| No insurance | 1.14 (1.03-1.26)b | 1.14 (0.99-1.31 | 1.18 (1.06-1.31)b | 1.08 (0.97-1.21) | 1.05 (0.88-1.25) | 1.32 (1.15-1.50)c |
| Medicare | 1.00 (0.97-1.03) | 1.02 (0.98-1.05) | 1.02 (0.98-1.07) | 1.00 (0.96-1.03) | 0.97 (0.93-1.00) | 1.01 (0.97-1.05) |
| Other public | 1.00 (0.96-1.05) | 1.03 (0.98-1.08) | 1.00 (0.95-1.05) | 1.00 (0.95-1.04) | 1.00 (0.96-1.05) | 1.02 (0.97-1.07) |
| Colorectal cancer | ||||||
| No insurance | 1.17 (1.03-1.32)b | 1.32 (1.10-1.57)b | 1.32 (1.13-1.54)b | 1.15 (0.95-1.39) | 1.13 (0.91-1.39) | 1.30 (1.05-1.62)b |
| Medicare | 1.02 (0.96-1.07) | 1.05 (0.99-1.11)c | 1.06 (0.99-1.14)c | 1.01 (0.96-1.06) | 0.99 (0.94-1.05) | 1.07 (1.01-1.14)b,c |
| Other public | 1.11 (1.04-1.20)b | 1.11 (1.04-1.20)b | 1.16 (1.08-1.23)b | 1.00 (0.92-1.08) | 1.08 (1.02-1.15)b | 1.11 (1.03-1.20)b,c |
| Melanoma | ||||||
| No insurance | 2.04 (1.56-2.67)b | 1.66 (1.22-2.25)b | 1.92 (1.38-2.67)b | 0.81 (0.45-1.46) | 0.98 (0.56-1.72) | 1.85 (0.98-3.50) |
| Medicare | 1.10 (0.94-1.28) | 1.11 (0.95-1.29) | 1.03 (0.86-1.23) | 0.99 (0.79-1.24) | 1.23 (0.93-1.62) | 1.23 (0.93-1.62) |
| Other public | 1.53 (1.18-1.98)b | 1.30 (1.07-1.59)b | 1.37 (1.14-1.64)b | 1.66 (1.21-2.27)b | 1.25 (0.99-1.58) | 1.76 (1.38-2.24)b |
Abbreviations: HR, hazard ratio; ND, no data.
Patients with private insurance only were used as the reference throughout. This table is expanded and includes specification of stratification and adjustment variables as eTable 4 in the Supplement.
The HR is statistically significantly (P < .05) different to the reference group.
Wald P value for individual cross-product interaction terms between health insurance category and time-period (reference, 1997-2002) was P < .05 in the overall model.
In contrast, uninsured men with lung cancer had 14% higher cancer-specific mortality in 1997-2002 (HR, 1.14; 95% CI, 1.03-1.26) and 18% higher mortality in 2009-2014 (HR, 1.18; 95% CI, 1.06-1.31) relative to men who were privately insured. There were no differences in lung cancer survival between men with Medicare, other public, or private insurance in any calendar period. Uninsured men with colorectal cancer had 32% higher cancer-specific mortality than privately insured men in both 2003-2008 (HR, 1.32; 95% CI, 1.10-1.57) and 2009-2014 (HR, 1.32; 95% CI, 1.13-1.54). Men with other public insurance also had higher mortality from colorectal cancer than those with private insurance; disparities were evident in each calendar period but were widest in 2009-2014 (HR, 1.16; 95% CI, 1.08-1.23).
Uninsured and other publically insured men with melanoma experienced some of the largest survival disparities with little change over time. Among those diagnosed during 2009-2014, cancer-specific mortality was 92% higher in uninsured men (HR, 1.92; 95% CI, 1.38-2.67) and 37% higher in those with other public insurance (HR, 1.37; 95% CI, 1.14-1.64) relative to privately insured men.
Among women with breast cancer, disparities in cancer-specific mortality between those with no insurance and those with private insurance increased from 45% in 1997-2002 (HR, 1.45; 95% CI, 1.26-1.67) to 72% in 2009-2014 (HR, 1.72; 95% CI, 1.45-2.03) (Table). For women with lung cancer, disparities in cancer-specific mortality between the uninsured and privately insured increased from nonsignificant levels in 1997-2002 and 2003-2008 to 32% among those diagnosed during 2009-2014 (HR, 1.32; 95% CI, 1.15-1.50). Similarly for colorectal cancer, significant disparities in mortality for women who were uninsured were evident only among those diagnosed during 2009-2014 (HR, 1.30; 95% CI, 1.05-1.62). Among women with other public insurance, disparities in mortality from colorectal cancer increased from 8% among those diagnosed in 2003-2008 (HR, 1.08; 95% CI, 1.02-1.15) to 11% for those diagnosed in 2009-2014 (HR, 1.11; 95% CI, 1.03-1.20) relative to women with private insurance.
In contrast to men, there were no significant differences in survival between women with melanoma who were uninsured or privately insured, though women with other public insurance had significantly higher cancer-specific mortality than those with private insurance in all 3 calendar periods (2009-2014: HR, 1.76; 95% CI, 1.38-2.24).
Disparities in all-cause mortality for uninsured and other publically insured patients largely mirrored disparities in cancer-specific mortality. For patients with Medicare, survival was equivalent to those with private insurance, with the exception of breast and colorectal cancer, where all-cause mortality for patients with Medicare was consistently higher than for patients who were privately insured (eTable 5 in the Supplement).
Discussion
In a contemporary, population-based sample of 1 149 891 patients with cancer diagnosed over an 18-year period, we found substantial and persistent disparities in survival for patients with either no or other public insurance compared with patients with private insurance for all 5 of the cancer sites examined. With few exceptions, survival disparities were largest among those diagnosed during 2009-2014 relative to the 2 earlier time periods. For men with prostate cancer and women with lung or colorectal cancer, this represents a significant increase in survival disparities over time. To our knowledge, this is the first study to examine survival disparities by type of health insurance over time, for a number of different cancer sites.
Examination of the survival trends revealed that improvements in survival from January 1997 to December 2014 were almost exclusively limited to patients with private or Medicare insurance. For patients with other public or no insurance, survival was often stubbornly unchanged, or in some cancers, declining. While these survival trends were not fully adjusted for the various prognostic factors included in the multivariable analysis, they provide insight into which patient groups are benefitting from recent advances in cancer care, and which are not.
The finding that patients who are uninsured or have Medicaid have higher cancer-specific mortality than patients with private insurance or Medicare has been replicated for cancers of the breast, prostate, lung, and colorectum. Survival disparities among patients with melanoma have been less well studied, but similarly high cancer-specific mortality in patients who are uninsured or have Medicaid has been noted previously. For the first time, we show that the overall lack of improvement in survival for uninsured and other publically insured patients has resulted in increasing disparities for some cancers.
There are numerous mechanisms through which lack of adequate health insurance can lead to poorer survival outcomes, including higher prevalence of comorbidities and behavioral risk factors (poor diet, obesity, smoking), lower uptake of cancer screening, and lack of access to health care and recommended treatment regimens.
Lack of access to preventive health care is likely to have played a key role in the survival disparities reported here, especially for breast and colorectal cancer, for which established screening practices exist. Cancer screening rates among patients who are uninsured and patients with Medicaid are substantially lower than among patients who are privately insured, with estimates suggesting that patients who are uninsured are half as likely to receive breast or colorectal cancer screening.
In our study, patients with other public insurance (74% of whom had Medicaid) had significantly lower survival than patients who were privately insured for all cancers except lung, though disparities were smaller than for patients who were uninsured. Previous studies have reported findings of equivalent, or only marginally improved survival in patients with cancer who have Medicaid compared with patients who are uninsured after accounting for differences in stage and treatment. Medicaid insurance is not uniformly accepted by many health care providers because of low reimbursement levels, potentially making it difficult for these patients to access the same high-quality cancer care as those with private insurance. Rising cancer drug costs and formulary restrictions may also result in patients who are publicly insured being denied access to novel and/or high-cost therapeutics. Our findings suggest that, while survival falls short of that achieved by patients with private insurance, public insurance such as Medicaid does confer a survival benefit over no insurance for breast, prostate, and lung cancer. However, there was little or no benefit of public insurance over no insurance for colorectal cancer or melanoma, and the lack of improvement in survival is a concern. These findings suggest that the health care provided to publically insured patients with cancer in California is not adequately meeting their needs.
The Patient Protection and Affordable Care Act (ACA), signed into law in March 2010, provided insurance coverage for an estimated 30 million previously uninsured Americans through subsidized private health insurance and an expansion of the Medicaid program. The proportion of the US population with no health insurance fell from 16% in 2010 to 10% in 2014 and continues to fall. In California, Medicaid expansion began in late 2013 and enrolled an additional 4.9 million people over the following 3 years (a 57% increase). An extra 1.4 million people gained private insurance through the health care marketplace. It remains to be seen what impact the ACA will have on long-term outcomes for patients with cancer at a population level, but our findings of persistently inferior survival for patients with public insurance in California suggests that a more widespread redesign of cancer care delivery, as opposed to insurance expansion, may need to be considered as an alternative solution to the disparities reported here.
Limitations
Our study may be affected by several limitations inherent to cancer registry data. Patient health insurance status is determined by primary and secondary payer source, which can change over time. Some patients who were uninsured at diagnosis may acquire insurance soon after, such as through enrollment in Medicaid. While this could lead to misclassification, the impact is likely to be minimal as payer information in the CCR is collected at both initial diagnosis and treatment, providing an accurate reflection of patient insurance status over the course of their care. It could, however, explain the small numbers of patients who were uninsured in our study. We did not directly investigate changes in insurance status, but evidence suggests that patients who enroll in Medicaid around the time of a cancer diagnosis present with later-stage disease and have worse survival than patients who were enrolled prior to diagnosis. Our study was also limited in its ability to account for some factors that may influence the relationship between health insurance and survival, such as income, comorbidity, receipt of guideline concordant treatment, or managed and/or fee-for-service health care systems. Differences in treatment, in particular, have been well established as an important contributing factor. The treatment landscape for many cancers evolved dramatically over the period examined, and differences in the dissemination and availability of emerging treatment modalities for patients with different types of health insurance are likely to have influenced the trends reported here.
We found that survival disparities for patients who were uninsured or other publically insured patients with breast, prostate, lung, and colorectal cancer were largest in the most recent calendar period, but our ability to assess whether this represents a statistically significant increase in disparity over time was limited. For the period 2009-2014, only patients diagnosed in 2009 had complete follow-up for 5 years, resulting in greater variance of survival estimates, and potentially a lack of power to detect a significant change from the previous calendar period. Our study may also be affected by limitations inherent to survival analysis. For breast and colorectal cancer, the differential uptake of screening by insurance status may introduce lead-time bias (the artificial lengthening of survival time consequent to earlier detection with no additional life span), though any effect is likely to have been minimized through adjustment for stage at diagnosis.
Conclusions
Despite these limitations, we found significant differences in cancer survival by type of health insurance. From January 1997 to December 2014, survival disparities for uninsured and other publically insured patients were static at best and, at worst, increasing. To address the stark disparities in cancer survival reported here, patients need access to health insurance that covers all the necessary elements of health care, from prevention and early detection, through to timely treatment according to clinical guidelines and long-term follow-up.
eTable 1. Number of cases and distribution (%) of key demographic and clinical characteristics of 1,149, 891 patients diagnosed with breast, prostate, lung, or colorectal cancer or melanoma between 1997 and 2014 in California, by insurance status and sex.
eTable 2. Distribution (%) of stage at diagnosis by insurance status, for each cancer site, sex and calendar period of diagnosis: California, 1997-2014.
eTable 3. Five-year cancer-specific survival at age 65 by insurance status and calendar period, for each cancer site and sex: California, 1997-2014.
eTable 4. Five-year cancer-specific mortality by health insurance status (reference: Private only) in each calendar period of diagnosis, by cancer site and sex: California, 1997-2014 (full table).
eTable 5. Five-year all-cause mortality by health insurance status (reference: Private only) in each calendar period of diagnosis, by cancer site and sex: California, 1997-2014.
eFigure. Five-year cancer-specific survival at age 65 by insurance status and calendar period, for A) lung cancer (men), B) colorectal cancer (men), C) melanoma (men) and D) melanoma (women).
References
- 1.Jemal A, Ward EM, Johnson CJ, et al. . Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109(9):djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker GV, Grant SR, Guadagnolo BA, et al. . Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32(28):3118-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward EM, Fedewa SA, Cokkinides V, Virgo K. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J. 2010;16(6):614-621. [DOI] [PubMed] [Google Scholar]
- 5.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231. [DOI] [PubMed] [Google Scholar]
- 6.Pan HY, Walker GV, Grant SR, et al. . Insurance status and racial disparities in cancer-specific mortality in the United States: a population-based analysis. Cancer Epidemiol Biomarkers Prev. 2017;26(6):869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelsattar ZM, Hendren S, Wong SL. The impact of health insurance on cancer care in disadvantaged communities. Cancer. 2017;123(7):1219-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorey KM, Luginaah IN, Holowaty EJ, Zou G, Hamm C, Balagurusamy MK. Mediation of the effects of living in extremely poor neighborhoods by health insurance: breast cancer care and survival in California, 1996 to 2011. Int J Equity Health. 2013;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorey KM, Luginaah IN, Holowaty EJ, et al. . Effects of being uninsured or underinsured and living in extremely poor neighborhoods on colon cancer care and survival in California: historical cohort analysis, 1996-2011. BMC Public Health. 2012;12:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger N, Chen JT, Ebel G. Can we monitor socioeconomic inequalities in health? A survey of U.S. health departments’ data collection and reporting practices. Public Health Rep. 1997;112(6):481-491. [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Schupp CW, Harrati A, Clarke C, Keegan THM, Gomez SL. Developing an area-based socioeconomic measure from American Community Survey data. Fremont, California: Cancer Prevention Institute of California; 2014. [Google Scholar]
- 12.Lin DY, Wei LJ. The robust inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84:1074-1078. [Google Scholar]
- 13.Roetzheim RG, Gonzalez EC, Ferrante JM, Pal N, Van Durme DJ, Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89(11):2202-2213. [DOI] [PubMed] [Google Scholar]
- 14.Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Van Durme DJ, Krischer JP. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health. 2000;90(11):1746-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins AS, Chen AY, Stewart AK, Staley CA, Virgo KS, Ward EM. Insurance status and survival disparities among nonelderly rectal cancer patients in the National Cancer Data Base. Cancer. 2010;116(17):4178-4186. [DOI] [PubMed] [Google Scholar]
- 16.Robbins AS, Pavluck AL, Fedewa SA, Chen AY, Ward EM. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009;27(22):3627-3633. [DOI] [PubMed] [Google Scholar]
- 17.Mahal BA, Aizer AA, Ziehr DR, et al. . The association between insurance status and prostate cancer outcomes: implications for the Affordable Care Act. Prostate Cancer Prostatic Dis. 2014;17(3):273-279. [DOI] [PubMed] [Google Scholar]
- 18.Slatore CG, Au DH, Gould MK; American Thoracic Society Disparities in Healthcare Group . An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med. 2010;182(9):1195-1205. [DOI] [PubMed] [Google Scholar]
- 19.McDavid K, Tucker TC, Sloggett A, Coleman MP. Cancer survival in Kentucky and health insurance coverage. Arch Intern Med. 2003;163(18):2135-2144. [DOI] [PubMed] [Google Scholar]
- 20.Amini A, Rusthoven CG, Waxweiler TV, et al. . Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74(2):309-316. [DOI] [PubMed] [Google Scholar]
- 21.Bittoni MA, Wexler R, Spees CK, Clinton SK, Taylor CA. Lack of private health insurance is associated with higher mortality from cancer and other chronic diseases, poor diet quality, and inflammatory biomarkers in the United States. Prev Med. 2015;81:420-426. [DOI] [PubMed] [Google Scholar]
- 22.Ward E, Halpern M, Schrag N, et al. . Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9-31. [DOI] [PubMed] [Google Scholar]
- 23.Wu XC, Lund MJ, Kimmick GG, et al. . Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30(2):142-150. [DOI] [PubMed] [Google Scholar]
- 24.Bradley CJ, Given CW, Dahman B, Fitzgerald TL. Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med. 2008;168(5):521-529. [DOI] [PubMed] [Google Scholar]
- 25.Harlan LC, Greene AL, Clegg LX, Mooney M, Stevens JL, Brown ML. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol. 2005;23(36):9079-9088. [DOI] [PubMed] [Google Scholar]
- 26.Coughlin SS, Uhler RJ, Bobo JK, Caplan L. Breast cancer screening practices among women in the United States, 2000. Cancer Causes Control. 2004;15(2):159-170. [DOI] [PubMed] [Google Scholar]
- 27.Sambamoorthi U, McAlpine DD. Racial, ethnic, socioeconomic, and access disparities in the use of preventive services among women. Prev Med. 2003;37(5):475-484. [DOI] [PubMed] [Google Scholar]
- 28.Shi L, Lebrun LA, Zhu J, Tsai J. Cancer screening among racial/ethnic and insurance groups in the United States: a comparison of disparities in 2000 and 2008. J Health Care Poor Underserved. 2011;22(3):945-961. [DOI] [PubMed] [Google Scholar]
- 29.American Cancer Society Cancer Prevention and Early Detection Facts and Figures. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 30.Moy B, Polite BN, Halpern MT, et al. . American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011;29(28):3816-3824. [DOI] [PubMed] [Google Scholar]
- 31.Polite BN, Griggs JJ, Moy B, et al. . American Society of Clinical Oncology policy statement on medicaid reform. J Clin Oncol. 2014;32(36):4162-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patient Protection and Affordable Care Act, 42 U.S.C. § 18001 (2010)
- 33.United States Census Bureau Health Insurance Coverage in the United States: 2015. https://www.census.gov/library/publications/2016/demo/p60-257.html. Published September 13, 2016. Accessed September 26, 2017.
- 34.California Department of Healthcare Services Research and Analytic Studies Division Medi-Cal Monthly Enrollment Fast Facts, December 2016. California Department of Health Care Services. http://www.dhcs.ca.gov/dataandstats/statistics/Documents/Fast_Facts_December_2016.pdf. Published April 2017. Accessed September 26, 2017.
- 35.Bradley CJ, Given CW, Roberts C. Late stage cancers in a Medicaid-insured population. Med Care. 2003;41(6):722-728. [DOI] [PubMed] [Google Scholar]
- 36.Koroukian SM, Bakaki PM, Raghavan D. Survival disparities by Medicaid status: an analysis of 8 cancers. Cancer. 2012;118(17):4271-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Number of cases and distribution (%) of key demographic and clinical characteristics of 1,149, 891 patients diagnosed with breast, prostate, lung, or colorectal cancer or melanoma between 1997 and 2014 in California, by insurance status and sex.
eTable 2. Distribution (%) of stage at diagnosis by insurance status, for each cancer site, sex and calendar period of diagnosis: California, 1997-2014.
eTable 3. Five-year cancer-specific survival at age 65 by insurance status and calendar period, for each cancer site and sex: California, 1997-2014.
eTable 4. Five-year cancer-specific mortality by health insurance status (reference: Private only) in each calendar period of diagnosis, by cancer site and sex: California, 1997-2014 (full table).
eTable 5. Five-year all-cause mortality by health insurance status (reference: Private only) in each calendar period of diagnosis, by cancer site and sex: California, 1997-2014.
eFigure. Five-year cancer-specific survival at age 65 by insurance status and calendar period, for A) lung cancer (men), B) colorectal cancer (men), C) melanoma (men) and D) melanoma (women).