Key Points
Question
Do patients with elevated preoperative carcinoembryonic antigen (CEA) levels that normalize after colon cancer resection have a higher risk of recurrence than patients with normal preoperative CEA?
Findings
In this cohort analysis of 1027 patients, the 3-year rate of recurrence-free survival (RFS) in patients with elevated preoperative CEA that normalized after surgery was similar to the RFS rate in patients with normal preoperative CEA. Patients with elevated postoperative CEA had significantly lower RFS than patients with normal postoperative CEA.
Meaning
Routine measurement of postoperative, rather than preoperative, CEA is warranted.
This cohort analysis examined 3-year recurrence-free survival in patients with varying levels of preoperative and postoperative carcinoembryonic antigen.
Abstract
Importance
Guidelines recommend measuring preoperative carcinoembryonic antigen (CEA) in patients with colon cancer. Although persistently elevated CEA after surgery has been associated with increased risk for metastatic disease, prognostic significance of elevated preoperative CEA that normalized after resection is unknown.
Objective
To investigate whether patients with elevated preoperative CEA that normalizes after colon cancer resection have a higher risk of recurrence than patients with normal preoperative CEA.
Design, Setting, and Participants
This retrospective cohort analysis was conducted at a comprehensive cancer center. Consecutive patients with colon cancer who underwent curative resection for stage I to III colon adenocarcinoma at the center from January 2007 to December 2014 were identified.
Exposures
Patients were grouped into 3 cohorts: normal preoperative CEA, elevated preoperative but normalized postoperative CEA, and elevated preoperative and postoperative CEA.
Main Outcomes and Measures
Three-year recurrence-free survival (RFS) and hazard function curves over time were analyzed.
Results
A total of 1027 patients (461 [50.4%] male; median [IQR] age, 64 [53-75] years) were identified. Patients with normal preoperative CEA had 7.4% higher 3-year RFS (n = 715 [89.7%]) than the combined cohorts with elevated preoperative CEA (n = 312 [82.3%]) (P = .01) but had RFS similar to that of patients with normalized postoperative CEA (n = 142 [87.9%]) (P = .86). Patients with elevated postoperative CEA had 14.9% lower RFS (n = 57 [74.5%]) than the combined cohorts with normal postoperative CEA (n = 857 [89.4%]) (P = .001). The hazard function of recurrence for elevated postoperative CEA peaked earlier than for the other cohorts. Multivariate analyses confirmed that elevated postoperative CEA (hazard ratio [HR], 2.0; 95% CI, 1.1-3.5), but not normalized postoperative CEA (HR, 0.77; 95% CI, 0.45-1.30), was independently associated with shorter RFS.
Conclusions and Relevance
Elevated preoperative CEA that normalizes after resection is not an indicator of poor prognosis. Routine measurement of postoperative, rather than preoperative, CEA is warranted. Patients with elevated postoperative CEA are at increased risk for recurrence, especially within the first 12 months after surgery.
Introduction
First described in 1965, carcinoembryonic antigen (CEA) is a colorectal cancer tumor marker that the National Comprehensive Cancer Network, the American Society of Clinical Oncology, and the European Group on Tumour Markers recommend be measured preoperatively in patients with nonmetastatic colorectal cancer. Elevated CEA levels are associated with metastases and recurrence, and some investigators have suggested that it be included in the American Joint Committee on Cancer staging system.
Before widespread use of modern imaging, an elevated preoperative CEA prompted additional investigation, such as liver scintigraphy, to search for metastases. In the era of high-quality computed tomography (CT), the utility of measuring preoperative CEA is less obvious because an elevated preoperative CEA with a normal CT scan does not preclude surgery with curative intent. An elevated preoperative CEA can normalize after resection of the primary tumor. In this study we sought to determine whether preoperative or postoperative CEA is more prognostic. Specifically, we asked whether patients with elevated preoperative CEA that normalizes after resection of the primary tumor had a risk of recurrence similar to that of patients with normal preoperative CEA.
Methods
Study Design and Patient Cohort
With institutional review board approval and a waiver of the requirement for patient consent, prospectively maintained databases were queried for all consecutive patients who underwent curative resection for stage I to III colon adenocarcinoma from January 2007 through December 2014 at Memorial Sloan Kettering Cancer Center. The exclusion criteria were treatment for malignancy within the last 5 years, preoperative chemotherapy or radiotherapy, noncurative palliative resection, and lack of preoperative CEA data. Data on patient demographics, perioperative clinical outcomes, pathologic outcomes, and disease status at last follow-up were collected from the database, and the electronic medical record was reviewed. Tumor location was categorized as right colon (cecum, ascending, and transverse colon) or left colon (descending, sigmoid, and rectosigmoid colon).
Preoperative CEA was defined as the CEA value closest to the time of surgery, and postoperative CEA was defined as the last CEA value within 12 weeks after surgery and before starting adjuvant chemotherapy. Patients were grouped by CEA status as follows: (1) patients with normal (≤5.0 ng/mL [to convert to μg/L, multiply by 1.0]) preoperative CEA (normal preoperative group); (2) patients with elevated (>5.0 ng/mL) preoperative CEA but normal postoperative CEA (normalized postoperative group); and (3) patients whose preoperative and postoperative CEA levels were both elevated (elevated postoperative group). The CEA assay was performed at Memorial Sloan Kettering using a Tosoh AIA-2000 automated analyzer (Tosoh Bioscience, Inc). The reference range for the assay was 0.0 to 5.0 ng/mL.
Staging and Surveillance Protocol
Preoperative staging included colonoscopy and contrast-enhanced CT of the chest, abdomen, and pelvis. Adjuvant chemotherapy was administered to patients with stage III or high-risk stage II disease after histological evaluation of the surgical specimen as recommended in national guidelines. The general practice for postoperative surveillance of stage I to III colon cancer at Memorial Sloan Kettering (in accordance with national guidelines) included physical examination, interval history, and serum CEA testing at 3- to 6-month intervals for the first 2 to 3 years and at 6-month intervals thereafter for 5 years. Imaging, most frequently CT of the chest, abdomen, and pelvis with oral and intravenous contrast, was performed at a minimum of every 12 months for at least 3 years. Colonoscopy was typically performed at 1 year after surgery and then repeated every 3 to 5 years unless advanced adenomas were identified. Radiographic reports were reviewed, and a definitive diagnosis of recurrence was based on the appearance of new lesions on CT, magnetic resonance imaging (MRI), and/or positron emission tomography (PET) images and/or histological confirmation through biopsy.
Statistical Analysis
Continuous variables were compared using the Mann-Whitney U test. Categorical variables were compared using the χ2 test. Recurrence-free survival (RFS) was estimated using the Kaplan-Meier method. Recurrence-free survival time was calculated from the date of surgery until the date of recurrence, death, or last follow-up. Death and disease recurrence were treated as events in the analysis. Patients who were alive without recurrence at last follow-up were censored. Differences in RFS were assessed by the log-rank test (univariate analysis). Hazard ratios (HRs) and 95% CIs were estimated using Cox regression models and assessed by the Wald test. Association with RFS was evaluated by multivariable Cox regression. Variables with P values of less than .05 on univariate analysis were included in the final multivariable model. The hazard function of recurrence or death was plotted using the kernel-smoothing method. All statistical analyses were performed using JMP version 10.1.2 (SAS Institute Inc) or R version 3.2.4 (R Project). All tests were 2-sided, and P values of less than .05 were considered significant.
Results
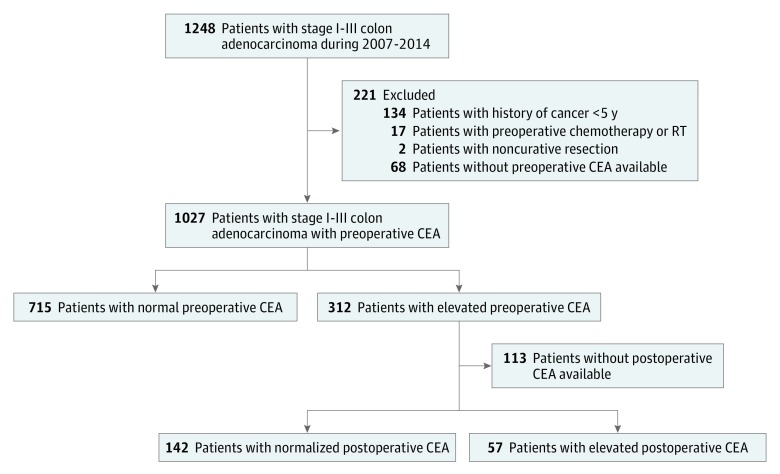
A total of 1248 consecutive patients (461 [50.4%] male; median [IQR] age, 64 [53-75] years) who underwent curative resection for stage I to III colon cancer were identified. Patients were excluded if they had prior cancer treatment within 5 years (n = 134), preoperative chemotherapy or radiotherapy (n = 17), noncurative palliative resection (n = 2), or missing preoperative CEA (n = 68). Among the remaining 1027 patients, preoperative CEA was normal in 715 patients (69.6%) and elevated in 312 patients (30.4%). Of the 312 patients with elevated preoperative CEA, 199 had postoperative CEA data available; 142 of these patients had normalized postoperative CEA levels and 57 had elevated postoperative CEA levels (Figure 1).
Figure 1. Study Design .
Databases were queried for patients who underwent curative resection for stage I to III colon adenocarcinoma from January 2007 through December 2014. Those with history of treatment for malignancy within the last 5 years, preoperative chemotherapy or radiotherapy, noncurative palliative resection, or lack of preoperative CEA (carcinoembryonic antigen) data were excluded. The remaining patients were grouped into 3 cohorts: those with normal preoperative CEA, those with elevated preoperative CEA but normal postoperative CEA, and those whose preoperative and postoperative CEA levels were both elevated.
Descriptive statistics for the 914 patients with either normal preoperative CEA or elevated preoperative CEA with evaluable postoperative CEA are shown in eTable 1 in the Supplement. Tumors were located in the right colon in 468 patients (51.2%) and in the left colon in 446 patients (48.8%). Median (IQR) preoperative and postoperative CEA levels were 2.8 (1.9-4.6) ng/mL and 2.5 (1.7-3.7) ng/mL, respectively. Median (IQR) interval from surgery to postoperative CEA testing was 35 (26-49) days. Median (IQR) follow-up was 38 (21-56) months. A total of 94 patients (10.3%) had recurrences, and 42 patients (4.6%) died before the last follow-up. The 3-year RFS rate for all patients was 88.4% (95% CI, 85.9%-90.5%).
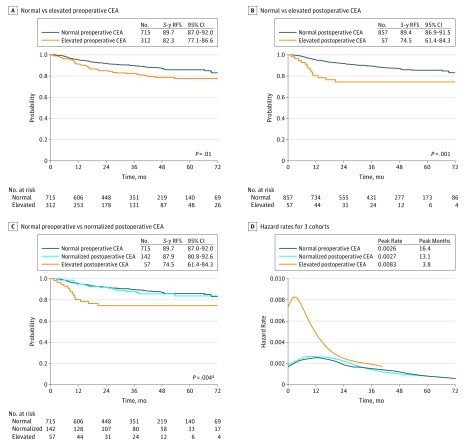
The 3-year RFS rate for the 312 patients with elevated preoperative CEA was 82.3% (95% CI, 77.1%-86.6%) compared with 89.7% (95% CI, 87.0%-92.0%) for the 715 patients with normal preoperative CEA (HR, 1.68; 95% CI, 1.19-2.39; P = .05) (Figure 2A). The 3-year RFS was 74.5% (95% CI, 61.4%-84.3%) for the 57 patients whose CEA levels remained elevated after surgery compared with 89.4% (95% CI, 86.9%-91.5%) for the 857 patients with either normal preoperative CEA (n = 715) or normalized postoperative CEA (n = 142) (HR, 2.53; 95% CI, 1.44-4.44; P = .001) (Figure 2B). The 3-year RFS for the 142 patients with normalized postoperative CEA was 87.9% (95% CI, 80.8%-92.6%), which was statistically indistinguishable from the 89.7% (95% CI, 87.0%-92.0%) 3-year RFS in the 715 patients who had normal preoperative CEA levels (HR, 1.05; 95% CI, 0.63-1.76; P = .85) (Figure 2C). In contrast the 3-year RFS rate of the 57 patients with persistently elevated CEA after surgery was 74.5%, which was significantly lower than that of the other 2 groups (elevated postoperative CEA vs normal preoperative CEA: HR, 2.56; 95% CI, 1.44-4.52; P = .001 and elevated postoperative CEA vs normalized postoperative CEA: HR, 2.43; 95% CI, 1.21-4.89; P = .02) (overall log-rank P = .004) (Figure 2C). Repeat analyses using a CEA cutoff of 10.0 ng/mL (rather than 5.0 ng/mL) produced similar results (eFigure 1 in the Supplement).
Figure 2. Recurrence-Free Survival by Preoperative and Postoperative CEA Levels.
A, Patients with normal vs elevated preoperative CEA. B, Patients with normal vs elevated postoperative CEA. C, Patients with normal preoperative, normalized postoperative, or elevated postoperative CEA. D, Hazard functions for disease recurrence in the 3 patient cohorts. Figure 2A includes all 1027 patients. Figure 2B and 2C include 914 patients and exclude 113 patients with elevated preoperative CEA and no postoperative CEA. CEA indicates carcinoembryonic antigen; RFS, recurrence-free survival.
a Normal preoperative CEA vs elevated postoperative CEA, P = .001; normalized postoperative CEA vs elevated postoperative CEA, P = .018.
The smoothed curve of the hazard function for each CEA group indicated that the risk of recurrence was higher and also peaked earlier in the elevated postoperative CEA group (Figure 2D). The hazard function curves for the normal preoperative and normalized postoperative CEA groups are indistinguishable, confirming that the risk and timing of recurrence in these 2 groups are similar.
Stage-specific RFS based on CEA is shown in eFigure 2 in the Supplement. In patients with stage I (eFigure 2A in the Supplement) or stage II (eFigure 2B in the Supplement) disease, the RFS of the 3 cohorts (normal preoperative, normalized postoperative, and elevated postoperative CEA) did not differ significantly. However, among patients with stage III disease, the RFS was significantly lower in the elevated postoperative CEA group than in the normal preoperative or normalized postoperative CEA groups (elevated postoperative CEA vs normal preoperative CEA: HR, 2.78; 95% CI, 1.44-5.38; P = .002 and elevated postoperative CEA vs normalized postoperative CEA: HR, 4.37; 95% CI, 1.81-10.58; P = .002) (overall log-rank P = .001) (eFigure 2C in the Supplement).
Univariate and multivariate analyses of factors associated with RFS are shown in eTable 2 in the Supplement. In univariate analysis older age, presence of lymphovascular invasion, higher TNM stage, and elevated postoperative CEA were associated with shorter RFS. Multivariate analyses revealed that elevated postoperative CEA (HR, 2.0; 95% CI, 1.1-3.5), but not normalized postoperative CEA (HR, 0.77; 95% CI, 0.45-1.30), was independently associated with shorter RFS as well as with higher TNM stage, older age, and presence of lymphovascular invasion. Repeat analyses using a cutoff of 10 ng/mL produced similar results (eFigure 1 in the Supplement).
No association was noted between normal or elevated perioperative CEA and site of initial recurrence (eTable 3 in the Supplement).
Discussion
Consistent with the literature, our data show that postoperative CEA is more informative than preoperative CEA. Patients with an elevated preoperative CEA had an absolute 7.4% lower 3-year RFS than those with normal preoperative CEA. However, CEA normalized in more than 70% of patients following surgery, and the outcome of patients with normalized postoperative CEA is similar to that of patients with normal preoperative CEA. Conversely, those patients with persistently elevated CEA following surgery had an absolute 14.9% lower 3-year RFS than those with either normal preoperative CEA or normalized postoperative CEA. The impact of elevated postoperative CEA is further demonstrated in a review of the hazard function curves over time (Figure 2D), which show an earlier and higher hazard rate peak in the elevated postoperative CEA cohort compared with normal preoperative and normalized postoperative groups. Multivariate modeling also confirms that elevated postoperative CEA was more prognostic than elevated preoperative CEA. When evaluating the results by stage, it is clear that postoperative CEA is able to stratify patients with stage III disease rather than stage I and II, likely because of limited recurrence in the latter groups, with 3-year disease-free survival greater than 98% and 91% for stage I and II, respectively.
These findings suggest that with regard to prognosis, measuring postoperative rather than preoperative CEA is more instructive. Elevated preoperative CEA is not informative when postoperative CEA is normal. Can preoperative CEA measurements be eliminated? Does CEA assist in identifying metastatic disease? It is unlikely that CEA is of great use in identifying metastatic disease in the setting of modern imaging. Clinically, patients with an elevated preoperative CEA and an otherwise normal contrast-enhanced CT of the chest, abdomen, and pelvis proceed to surgery with the assumption that the primary lesion is the source of the elevated CEA. Some may argue that an elevated preoperative CEA is a useful marker in follow-up. That is, clinicians are more likely to assume a rise in CEA is the result of metastases if the patient had an elevated preoperative CEA. However, recurrence can be accompanied by normal or elevated CEA irrespective of preoperative CEA. For example, in a report of 1542 patients treated from 2000 to 2007, 56% of patients had normal CEA at recurrence despite elevated CEA at time of initial diagnosis. In another study of 1321 patients with colorectal cancer, 54% presented with elevated CEA at recurrence despite normal CEA at time of initial resection. In the present cohort, there were 64 patients with recurrence who had normal preoperative CEA, and 24 of them (38%) had elevated CEA upon recurrence (data not shown). Thus, preoperative CEA does not influence surveillance guidelines that include routine CEA measurement and CT for stage II and III disease.
Our results indicate that postoperative CEA may inform frequency of surveillance. Patients with elevated postoperative CEA have a hazard function that rises quickly and peaks within 12 months before declining to meet that of patients with normal preoperative CEA or normalized postoperative CEA. This may support using postoperative CEA to stratify patients for graded surveillance as outlined by the National Comprehensive Cancer Network. However, we cannot conclude from this data set if additional immediate imaging, such as MRI, ultrasound, or 18F-fluorodeoxyglucose PET and CT, would be beneficial in patients with postoperative elevation in CEA. Often, a short interval follow-up CT scan is performed. Although some studies have found that disease recurrence in patients with colorectal cancer and elevated preoperative or postoperative CEA is more common in the liver than at other sites, we did not find a significant difference among sites in the likelihood of recurrence.
Current guidelines do not support the use of CEA as an indicator for adjuvant chemotherapy. However, studies from Taiwan and Korea suggest that elevated postoperative CEA in patients with stage II colorectal cancer is prognostic, while a study from Korea found no association between postoperative CEA and disease-free survival in stage II disease. We did not find preoperative or postoperative CEA to be predictive in patients with stage II disease. With a 91% 3-year RFS and only 6.3% of patients with stage II disease having elevated postoperative CEA, we did not have the power to determine the significance of postoperative CEA in patients with stage II disease. Proportion of high-risk stage II as defined in the National Comprehensive Cancer Network guideline was also similar between the groups (data not shown).
The major strengths of this study include the large size of the cohort of patients staged with high-quality preoperative CT; the uniform treatment patients received, with complete mesocolic excision technique by specialized colorectal surgeons at a high-volume comprehensive cancer center; and the use of a standard chemotherapy regimen in all patients. Unlike past studies, routine use of high-quality CT scan very likely improved staging by enabling identification of low-volume metastases that may have been undetectable with older imaging technology. Furthermore, improved nodal clearance with complete mesocolic excision technique optimizes tumor resection and nodal staging. Oncological outcomes in this study were similar to those seen in other reports of complete mesocolic excision, confirming that the cohort is a representative sample of stage I to III colon cancer in the era of complete mesocolic excision and modern preoperative imaging. We do not know if the results can be applied to centers that do not perform high-quality preoperative CT scans or complete mesocolic excisions. Confirmatory studies would be necessary.
Limitations
The analysis is subject to the limitations and bias inherent in observational retrospective studies. For example, postoperative CEA data were not available for 113 patients with elevated preoperative CEA. Patients who had postoperative CEA measured were more likely to have stage III disease (43% vs 29%; P = .002), suggesting that the patients with a higher risk had postoperative CEA testing. Timing of postoperative CEA measurement was not controlled, although it was limited to within 12 weeks after surgery and before starting adjuvant chemotherapy. Care was taken to allow sufficient time following surgery before measuring CEA (IQR, 26-49 days) to allow CEA normalization (the half-life of CEA is 3-7 days.) We did not evaluate optimal CEA cutoff but did find similar results when using 5 and 10 ng/mL cutoff values. Postoperative surveillance was in accordance with national guidelines; however, in this retrospective study, intervals and completeness of the follow-up undoubtedly varied. That being said, the hazard function curve (Figure 2D) showed an earlier peak of recurrence in the elevated postoperative CEA cohort (3.8 months) compared with normal preoperative CEA (16.4 months) and normalized postoperative CEA (13.1 months) groups. Such differences are unlikely to be the result of follow-up bias. Confirmation of these results using multicenter trial data with uniform surveillance would be beneficial.
We did not control for tobacco use, a known factor that raises CEA, as this is hard to truthfully ascertain from patients. Similarly, we did not control for other factors that can lead to false-positive CEA elevation, such as gastritis, peptic ulcer disease, diverticulitis, liver diseases, chronic obstructive pulmonary disease, diabetes, and acute or chronic inflammatory conditions. Rather this study is pragmatic and represents what is likely to be seen in real-world colorectal cancer care.
Conclusions
The prognostic value of baseline postoperative CEA exceeds that of preoperative CEA. Carcinoembryonic antigen often normalizes after surgery, and we have shown that patients with elevated preoperative CEA that normalizes after surgery have similar outcome as patients with normal preoperative CEA. Emphasis should be placed on postoperative CEA, and in the setting of modern high-quality imaging we question the utility of measuring preoperative CEA. Patients with elevated postoperative CEA tend to experience recurrence early, which might justify a risk-adjusted and individualized surveillance strategy.
eTable 1. Patient and Tumor Characteristics
eTable 2. Univariate and Multivariate Analyses of Recurrence Free Survival
eTable 3. Analysis of Sites of Metastatic Recurrence in Relation to CEA Status
eFigure 1. Kaplan-Meier Curves for RFS in Patients With Normal Preoperative, Normalized Postoperative, or Elevated Postoperative CEA Using a Cutoff of 10 ng/mL
eFigure 2. Kaplan-Meier Curves for RFS in Patients With Normal Preoperative, Normalized Postoperative, or Elevated Postoperative CEA, Grouped by TNM Disease Stage
References
- 1.Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122(3):467-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CC, Yang SH, Lin JK, et al. Is it reasonable to add preoperative serum level of CEA and CA19-9 to staging for colorectal cancer? J Surg Res. 2005;124(2):169-174. [DOI] [PubMed] [Google Scholar]
- 4.Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. 2010;101(5):396-400. [DOI] [PubMed] [Google Scholar]
- 5.Takagawa R, Fujii S, Ohta M, et al. Preoperative serum carcinoembryonic antigen level as a predictive factor of recurrence after curative resection of colorectal cancer. Ann Surg Oncol. 2008;15(12):3433-3439. [DOI] [PubMed] [Google Scholar]
- 6.Chu DZ, Erickson CA, Russell MP, et al. Prognostic significance of carcinoembryonic antigen in colorectal carcinoma. Arch Surg. 1991;126(3):314-316. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Colon Cancer. Version 2.2016. Fort Washington, PA: NCCN; 2016. [Google Scholar]
- 8.Duffy MJ, van Dalen A, Haglund C, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43(9):1348-1360. [DOI] [PubMed] [Google Scholar]
- 9.Locker GY, Hamilton S, Harris J, et al. ; American Society of Clinical Oncology . ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24(33):5313-5327. [DOI] [PubMed] [Google Scholar]
- 10.Kim CW, Yoon YS, Park IJ, Lim SB, Yu CS, Kim JC. Elevation of preoperative s-CEA concentration in stage IIA colorectal cancer can also be a high risk factor for stage II patients. Ann Surg Oncol. 2013;20(9):2914-2920. [DOI] [PubMed] [Google Scholar]
- 11.Park YJ, Park KJ, Park JG, Lee KU, Choe KJ, Kim JP. Prognostic factors in 2230 Korean colorectal cancer patients. World J Surg. 1999;23(7):721-726. [DOI] [PubMed] [Google Scholar]
- 12.Thirunavukarasu P, Sukumar S, Sathaiah M, et al. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103(8):689-697. [DOI] [PubMed] [Google Scholar]
- 13.Szymendera JJ, Wilczyńska JE, Nowacki MP, Kamińska JA, Szawowski AW. Serial CEA assays and liver scintigraphy for the detection of hepatic metastases from colorectal carcinoma. Dis Colon Rectum. 1982;25(3):191-197. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Kim NK, Sohn SK, et al. Prognostic value of postoperative CEA clearance in rectal cancer patients with high preoperative CEA levels. Ann Surg Oncol. 2009;16(10):2771-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JK, Lin CC, Yang SH, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. 2011;26(9):1135-1141. [DOI] [PubMed] [Google Scholar]
- 16.Wang JY, Lu CY, Chu KS, et al. Prognostic significance of pre- and postoperative serum carcinoembryonic antigen levels in patients with colorectal cancer. Eur Surg Res. 2007;39(4):245-250. [DOI] [PubMed] [Google Scholar]
- 17.Tsai HL, Huang CW, Chen CW, Yeh YS, Ma CJ, Wang JY. Survival in resected stage II colorectal cancer is dependent on tumor depth, vascular invasion, postoperative CEA level, and the number of examined lymph nodes. World J Surg. 2016;40(4):1002-1009. [DOI] [PubMed] [Google Scholar]
- 18.Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. J Clin Oncol. 1996;14(10):2843-2877. [DOI] [PubMed] [Google Scholar]
- 19.Hess KR, Serachitopol DM, Brown BW. Hazard function estimators: a simulation study. Stat Med. 1999;18(22):3075-3088. [DOI] [PubMed] [Google Scholar]
- 20.Müller HG, Wang JL. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50(1):61-76. [PubMed] [Google Scholar]
- 21.Choi JS, Min JS. Significance of postoperative serum level of carcinoembryonic antigen (CEA) and actual half life of CEA in colorectal cancer patients. Yonsei Med J. 1997;38(1):1-7. [DOI] [PubMed] [Google Scholar]
- 22.Oussoultzoglou E, Rosso E, Fuchshuber P, et al. Perioperative carcinoembryonic antigen measurements to predict curability after liver resection for colorectal metastases: a prospective study. Arch Surg. 2008;143(12):1150-1158. [DOI] [PubMed] [Google Scholar]
- 23.Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer. Ann Surg Oncol. 2009;16(11):3087-3093. [DOI] [PubMed] [Google Scholar]
- 24.Wichmann MW, Lau-Werner U, Müller C, Hornung HM, Stieber P, Schildberg FW; Colorectal Cancer Study Group . Carcinoembryonic antigen for the detection of recurrent disease following curative resection of colorectal cancer. Anticancer Res. 2000;20(6D):4953-4955. [PubMed] [Google Scholar]
- 25.Yakabe T, Nakafusa Y, Sumi K, et al. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol. 2010;17(9):2349-2356. [DOI] [PubMed] [Google Scholar]
- 26.Zeng Z, Cohen AM, Urmacher C. Usefulness of carcinoembryonic antigen monitoring despite normal preoperative values in node-positive colon cancer patients. Dis Colon Rectum. 1993;36(11):1063-1068. [DOI] [PubMed] [Google Scholar]
- 27.Primrose JN, Perera R, Gray A, et al. ; FACS Trial Investigators . Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer. JAMA. 2014;311(3):263-270. [DOI] [PubMed] [Google Scholar]
- 28.Bertelsen CA, Neuenschwander AU, Jansen JE, et al. ; Danish Colorectal Cancer Group . Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16(2):161-168. [DOI] [PubMed] [Google Scholar]
- 29.Litvak A, Cercek A, Segal N, et al. False-positive elevations of carcinoembryonic antigen in patients with a history of resected colorectal cancer. J Natl Compr Canc Netw. 2014;12(6):907-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Patient and Tumor Characteristics
eTable 2. Univariate and Multivariate Analyses of Recurrence Free Survival
eTable 3. Analysis of Sites of Metastatic Recurrence in Relation to CEA Status
eFigure 1. Kaplan-Meier Curves for RFS in Patients With Normal Preoperative, Normalized Postoperative, or Elevated Postoperative CEA Using a Cutoff of 10 ng/mL
eFigure 2. Kaplan-Meier Curves for RFS in Patients With Normal Preoperative, Normalized Postoperative, or Elevated Postoperative CEA, Grouped by TNM Disease Stage