Key Points
Question
What postapproval changes occur to Class III dermatologic devices approved by the US Food and Drug Administration via the premarket approval pathway?
Findings
In this cross-sectional database study of 27 dermatologic devices, manufacturers are shown to increasingly modify devices via supplement pathways that require minimal supporting clinical data.
Meaning
Postapproval changes to Class III dermatologic devices may result in inadequately studied modifications that could influence device effectiveness and safety.
Abstract
Importance
The US Food and Drug Administration approves Class III medical devices via the premarket approval pathway, often requiring clinical data on safety and efficacy. Manufacturers can submit incremental device changes via supplemental applications, which are not subjected to such vetting measures and can cause understudied changes that lead to drift from a device’s original design.
Objectives
To characterize the postapproval changes to Class III dermatologic devices and to evaluate inconsistencies in the use of the premarket approval pathway.
Design, Setting, and Participants
This study was a cross-sectional retrospective cohort analysis of a public US Food and Drug Administration database for premarket approval of devices. Included were dermatologic devices approved by the US Food and Drug Administration between January 1, 1980, and November 1, 2016, through the premarket pathway for device approval.
Main Outcomes and Measures
Original devices were identified, and their supplements were characterized chronologically, by review track, and by modification category.
Results
The 27 dermatologic devices studied consisted of 14 injectables, 11 photodynamic therapies, a dermal replacement matrix, and a diagnostic imaging instrument. Supplemental applications are increasingly used: the data-requiring panel-track pathway was the least common approach (2.8% [16 of 562 supplements]), while the 30-day track, which does not require clinical data, was most frequently used (42.5% [239 of 562 supplements]). Four devices (14.8%) underwent low-risk recalls (Class II or Class III), and 10 devices (37.0%) were voluntarily withdrawn.
Conclusions and Relevance
As manufacturers make increasing use of supplemental applications, minor device changes may occur without supporting clinical data, which could pose a safety risk to patients.
This cross-sectional database study characterizes the postapproval changes to Class III dermatologic devices and evaluates inconsistencies in the use of the premarket approval pathway.
Introduction
The US Food and Drug Administration (FDA) evaluates and approves Class III medical devices through its premarket approval (PMA) process. The agency classifies devices that “support or sustain human life, are of substantial importance in preventing impairment of human health, or present a potential unreasonable risk of illness or injury” as Class III devices. Such devices pose a greater risk to patient health than Class I or Class II devices and are therefore subject to the FDA’s most stringent regulatory requirements.
The PMA pathway requires that device manufacturers provide preclinical and clinical data to demonstrate the effectiveness and safety of their products, thus allowing for informed decision making at the level of the FDA. The pathway is categorized into 5 review tracks, ranging from minor design modifications to more substantial changes, such as adding a new use indication. However, the FDA has a lower standard of approval for design changes (or drifting modifications), which do not require clinical supporting data. Such changes away from approved design may be referred to as “device drift.” Recently, an increasing number of Class III device failures and product recalls have occurred because of design and manufacturing changes of FDA-approved devices. In cardiology, for instance, supplemental applications approved for St Jude Riata and Sprint Fidelis implantable cardioverters-defibrillators led to safety issues that underscored the importance of appropriate oversight of PMA supplements. While the PMA pathway was designed to ensure the safety of Class III devices, device modifications that are subject to less regulatory oversight may allow for postmarket introduction of risks to patient health.
The PMA process is important for the regulation of commonly used dermatologic devices, many of which are Class III devices. Advances in material science, coupled with continual change in our understanding of skin biology and physiology, have resulted in an expansion of devices that diagnose and treat skin disorders or address issues of cosmesis. The affordability and noninvasiveness of Class III dermatologic products, which include soft-tissue filler injections, dermal matrix implants, bioimaging modalities, and photodynamic therapy, have increased their use by dermatologists. Adverse effects, some serious, associated with injectable Class III devices are well documented and include inflammatory nodules, nerve damage, and sequelae of vascular occlusion, such as tissue necrosis, stroke, or vision loss. While some of these adverse effects may be operator dependent, a careful understanding of the processes that govern the regulation of these products is especially relevant to the practicing dermatologist.
Our study characterizes the pattern of dermatologic device approvals and subsequent reviews of device modifications to better understand the safety implications of the PMA process. Herein, we sought to review original and supplemental PMAs for dermatologic devices approved between 1980 and 2016, identify the number of supplements emerging from each original PMA, characterize the modifications sought in each supplement, and evaluate whether PMA pathways are used as originally intended.
Methods
A cross-sectional retrospective cohort analysis was conducted using a sample of all Class III dermatologic devices that received marketing clearance within the public FDA database for PMA of devices. The registry was accessed on November 1, 2016. The database compiles approved summaries of original PMA applications and supplements for each device. Novel Class III devices are cleared for marketing with an original PMA application, with supplemental applications corresponding to incremental modifications after initial device approval. To identify dermatologic devices, we searched for approved applications categorized by dermatology-related Class III product codes (LMH, LMI, MDD, MVF, MVG, MYH, OYD, and PKY [please see code explanations in footnote of the Table]) and assigned to the FDA General and Plastic Surgery Devices Panel, approved between January 1, 1980, and November 1, 2016 (Table). This study was exempt from institutional review board approval owing to the public nature of the FDA database.
Table. Summary of Class III Dermatologic Devices by Premarket Approval (PMA) Identifier.
| PMA Identifier | Trade Name | Description | Company | Product Code | Device-years | No. of Supplements | Year Withdrawn | Recalls (Class Types) |
|---|---|---|---|---|---|---|---|---|
| P010061 | Curelight Broadband | Photodynamic therapy light | Photocure ASA | MYH | 4.1 | 0 | 2008 | 0 |
| P020012 | Artefill/Bellafill | Dermal implant | Artes Medical USA, Inc, Biologics Consulting Group, Suneva Medical, Inc | LMH | 10.1 | 7 | NA | 0 |
| P020021 | Wizard X-Cell Photodynamic Therapy | Photodynamic therapy balloon catheter and light delivery system, photodynamic therapy | Cook Endoscopy | MVG | 6.0 | 0 | 2009 | 0 |
| P020023 | Restylane Injectable | Injectable gel | Q-Med Scandinavia, Inc | LMH | 12.9 | 12 | NA | 0 |
| P030032 | Hylaform (Hylan B Gel) | Hyaluronic acid filler | Genzyme Corp | LMH | 11.8 | 14 | 2016 | 0 |
| P030050 | Sculptra | Injectable poly-l-lactic acid | Aventis Pharmaceuticals Inc, Galderma Laboratories LP, Q-Med AB, Sanofi-Aventis US LLC, Valeant International | LMH | 12.3 | 23 | NA | 0 |
| P040024 | Restylane-L/Perlane-L Injectable Gels | Implant, dermal for aesthetic use | Galderma Laboratories LP, Medicis Pharmaceutical Corporation, Q-Med AB, Valeant Pharmaceuticals North America, LLC | LMH | 11.7 | 84 | NA | 0 |
| P050021 | Ceralas I Laser | Diode laser | Biolitec, Inc | MVF | 2.8 | 0 | 2008 | 0 |
| P050026 | Quantel Activis Laser System | Diode laser | Valeant Pharmaceuticals Luxembourg SARL | MVF | 10.6 | 1 | NA | 0 |
| P050033 | Hydrelle Cosmetic Tissue Augmentation Product | Hyaluronic acid/lidocaine filler | Anika Therapeutics, Inc | LMH | 9.9 | 19 | NA | 0 |
| P050037 | Radiesse (Lipoatrophy/HIV) | Injectable calcium hydroxylapatite implant (facial lipoatrophy) | Bioform Medical, Inc, Merz North America, Inc | LMH | 8.9 | 73 | NA | 2 (II, III) |
| P050047 | Juvederm | Juvederm gel implants | Allergan | LMH | 10.4 | 49 | NA | 0 |
| P050052 | Radiesse (Wrinkles) | Injectable calcium hydroxylapatite implant | Bioform Medical, Inc, Merz North America, Inc | LMH, PKY | 8.8 | 83 | NA | 3 (II, II, III) |
| P070013 | Evolence | Dermal collagen filler | Colbar Lifescience Ltd | LMH | 2.5 | 4 | 2010 | 0 |
| P090012 | Melafind | Imaging device for melanoma detection | Mela Sciences, Inc | OYD | 4.3 | 11 | NA | 1 (II) |
| P090016 | Belotero Balance | Hyaluronic acid dermal filler | Merz North America, Inc | LMH | 5.0 | 19 | NA | 0 |
| P110033 | Juvederm XC (Cheek/Midface Augmentation) | Hyaluronic acid/lidocaine gel filler | Allergan | LMH | 3.1 | 21 | NA | 0 |
| P800022 | Cosmoderm/Zyplast/Zyderm | Dermal implants of collagen for aesthetic use | Allergan, Collagen Corp | LMH | 30.3 | 45 | 2011 | 0 |
| P850053 | Fibrel | Gelatin matrix implant | Mentor Corp, Serono Laboratories, Inc | LMH | 20.0 | 4 | 2008 | 0 |
| P900033 | Integra Dermal Regeneration Template | Artificial skin | Integra LifeScience Corp | MGR | 10.6 | 52 | NA | 2 (II, III) |
| P940010 | Optiguide Photodynamic Therapy | Laser for photodynamic therapy | Concordia Laboratories, Inc, Dio Medical Corp, Pinnacle Biologics, QLT Phototherapeutics, Inc | MVG | 20.9 | 11 | NA | 0 |
| P940011 | Coherent PDL1 and PDL2 Lambda Plus Photodynamic Lasers | Laser | Lumenis, Inc | MVF | 18.1 | 0 | 2014 | 0 |
| P940012 | 600/700/800 Series Dye Modules KTP/532 and KTP/YAG Surgical Lasers | Laser for photodynamic therapy | American Medical Systems, Inc | MVF | 18.3 | 0 | 2014 | 0 |
| P990019 | Blu-U Blue Light Photodynamic Therapy Illuminator | Blue light photodynamic therapy illuminator | DUSA Pharmaceuticals, Inc | MVF | 16.9 | 7 | NA | 0 |
| P990021 | Diomed/AngioDynamics Laser System | Diode laser, surgical laser | Concordia Laboratories, Inc, Dio Medical Corp | MVF | 16.4 | 3 | NA | 0 |
| P990048 | Zeiss VISULAS/VISULINK System | Diode laser | Carl Zeiss Meditec AG | MVF | 16.6 | 3 | NA | 0 |
| P990049 | Coherent Opal Photoactivator Laser System | Diode laser | Lumenis | MVF | 10.4 | 2 | 2010 | 0 |
Abbreviations: LMH, Implant, Dermal, for Aesthetic Use; LMI, Implant, Collagen for Non-Aesthetic Use; MDD, Device, Dermal Replacement; MVF, System, Laser, Photodynamic Therapy; MVG, System, Laser, Fiber Optic, Photodynamic Therapy; MYH, System, Non-Coherent Light, Photodynamic Therapy; NA, not applicable; OYD, Optical Diagnostic Device For Melanoma Detection; PKY, Implant, Dermal, For Aesthetic Use In The Hands.
In this study, we classified devices based on FDA coding database descriptions, including implants or fillers, dermal replacement, phototherapy devices, and bioimaging systems. Each PMA supplement entry was classified by its associated review track (180-day track, real-time tract, 135-day track, 30-day track, special track, and panel track). Changes in device design or components are submitted via the 180-day track for major design changes or the real-time track for minor design changes. Postapproval study protocol changes are also linked to the 180-day supplement track. Changes in the production process are intended for review via the 30-day notice track, which may be converted to a 135-day track pending FDA discretion and the complexity of the modification. Labeling changes undergo review by a panel track if expanding indications or removing contraindications. This requires clinical data and includes a formal review committee process. A special (or “immediate”) track is designated for other labeling changes, such as expanding contraindications.
Changes in the annual rate of new device approvals was evaluated using linear regression. Postmarket device changes approved via PMA supplement pathways were reported per active device and device-year. Intervals, such as time between device approval and withdrawal, were rounded to the nearest month and converted to years. Change in the rate of postmarket modifications per device over the study period was also studied using linear regression.
We reported the total entries for each supplement track and further classified supplements into manufacturing, design, or labeling changes. For purposes of simplified data interpretation, the database label changes in design as change design/components/specifications/material; labeling changes are labeled as either labeling change–indications/instructions/shelf life/trade name or labeling change–PAS (postapproval study); and manufacturing changes are divided into procedure (process change–manufacturer/sterilizer/packager/supplier) or location (location change–manufacturer/sterilizer/packager/supplier) changes. Postapproval study protocols, approved via 180-day supplements, are listed and categorized by the administrative entities approving the proposed changes (Office of Device Evaluation, Office of In Vitro Diagnostics and Radiological Health, and Office of Surveillance and Biometrics). The database contains supplement entries before 1990 that are linked to an original PMA device but are not attributable to a specific review track and are thus referred to as “unclassified” in this study.
Recalls were obtained from the FDA Medical Device Recalls Database, which reports all recalls since November 2002. Recall date, classification, and reason for recall were reported. Class I recalls are designed for situations in which there is a “reasonable probability” that a violative device will cause serious adverse health consequences or death. Class II recalls occur when a violative device may cause “temporary or medically reversible adverse health consequences” or where probability of serious adverse consequences is minimal. A Class III recall is used for violative devices that are not likely to cause adverse health consequences. Device withdrawals from the market were also reported, although reasons for withdrawal are not included in the PMA database.
All statistical analyses were performed using JMP Pro (version 11; SAS Institute) and verified with Prism 6 (GraphPad Inc). Figures were rendered using data visualization software (Tableau Software). Statistical tests were 2-tailed, and P < .05 was considered statistically significant.
Results
Dermatologic Devices Approved Through the PMA Process
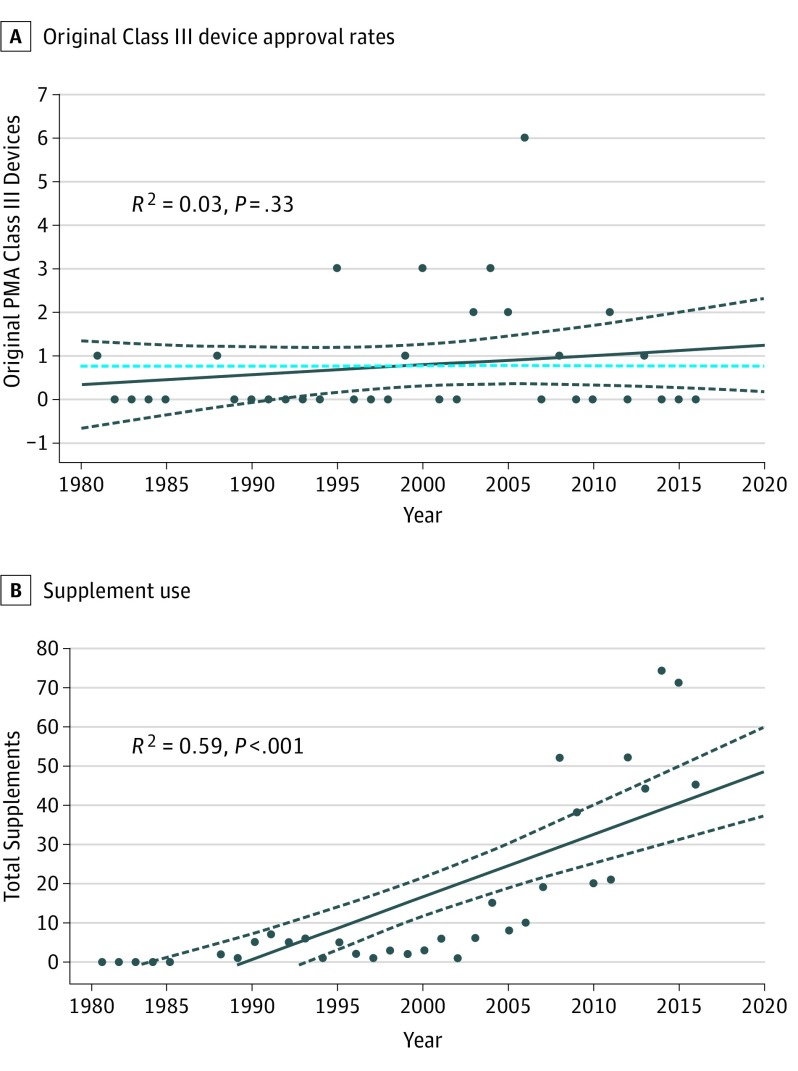
Between January 1, 1980, and November 1, 2016, the FDA approved 27 original PMA applications for dermatologic devices (Table), consisting of 14 implants or fillers (51.9%), 11 photodynamic therapy devices (40.7%), 1 dermal replacement matrix (3.7%), and 1 diagnostic imaging instrument (3.7%). Our linear regression analysis indicates that the rate of original PMA device approvals per year for dermatologic devices has not significantly changed since 1980 (R2 = 0.03, P = .33) (Figure 1A).
Figure 1. Statistical Trends in Device Approvals and Supplement Use.
A, Original Class III device approval rates show no observable trend over the study period (January 1980-November 2016). B, Supplement use during the same period has statistically significantly increased, with a stronger observable trend over the past decade. PMA indicates premarket approval.
Trends in the Use of PMA Supplements
During the study period, a total of 562 PMA supplements were approved for the 27 dermatologic devices. Of these supplements, 467 (83.1%) were for implants or fillers, 37 (6.6%) were for photodynamic therapy devices, 46 (8.2%) were for a dermal replacement device, and 12 (2.1%) were for a diagnostic imaging device (Table). The rate of supplements approved has increased over the lifetime of the PMA program. In the first decade of the PMA program, between 1980 and 1989, supplements were approved at a rate of 3.7 per year. In comparison, the FDA approved 36.7 supplements per year between 2006 and 2015, representing an 891.9% increase in approved supplements (Figure 1B).
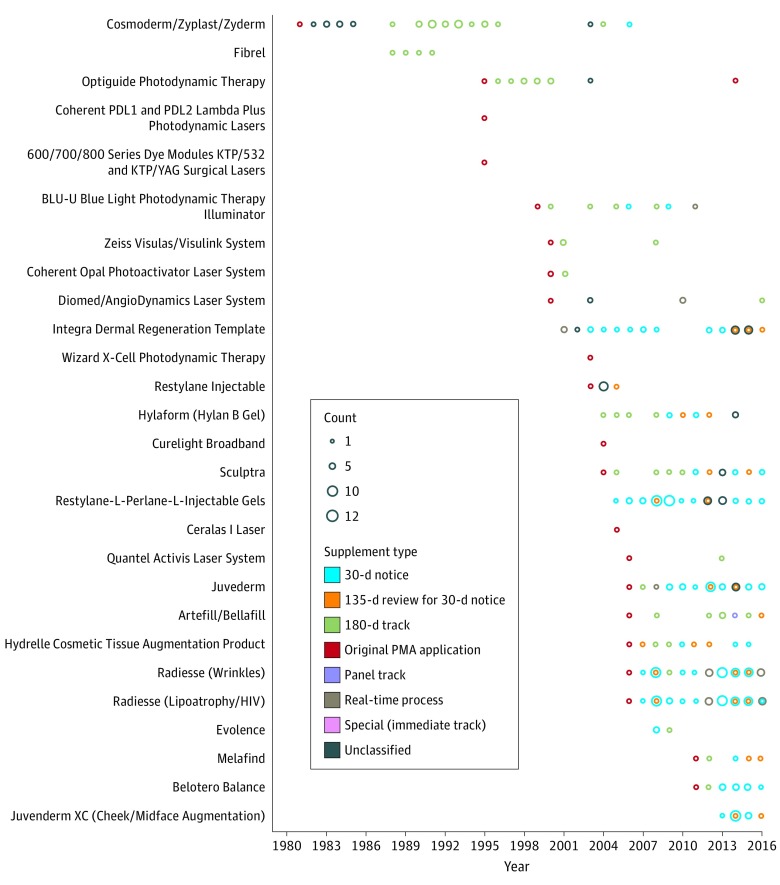
The median number of approved postmarket changes per device was 11 (interquartile range [IQR], 2.5-22) (Figure 2). Of the 27 devices, 5 laser or photodynamic therapy devices (18.5%) had no approved supplements after an original PMA. The only dermal replacement therapy device (P900033) in this study was initiated into its current device class with a panel-track application detailing its indications for use, which were expanded in early 2016 without the necessity of an additional study (Figure 2). The devices with the highest total number of postmarket supplements were P040024 (Restylane/Perlane) and P050052 (Radiesse) injectables, totaling 84 and 83 supplements, respectively. P050037 and P050052 (Radiesse products) each had the highest rate of postmarket supplements per device-year, with a mean of 8.2 and 9.4 changes, respectively, per device-year over 9 device-years, which excludes periods of recall.
Figure 2. Postmarked Modifications per Approved Dermatologic Device.
Class III devices are ordered chronologically by date of original premarket approval (PMA) application. Injectable agents undergo a larger number of supplement routes that require minimal clinical effectiveness data. Panel track and 180-day tracks, which require such studies, are less commonly used compared with earlier devices. Lasers and photodynamic therapies are observed to have lesser postmarket modifications compared with injectables.
PMA Supplement Approvals by Track
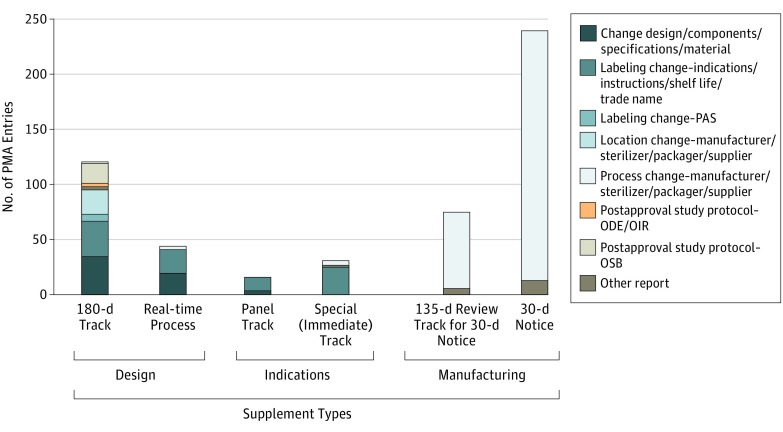
Supplement tracks are not implemented entirely as they were originally intended, with several discrepancies seen on closer analysis (Figure 3). Overall, the 30-day notice has been the most popular track since its introduction in 1997, with 239 approved 30-day notices, comprising 42.5% of 562 PMA entries since 1980. The least used supplement has been the more rigorous panel-track supplement, accounting for only 16 of 562 (2.8%) approved supplements. Of the other types among 562 supplements, there were 120 approved 180-day supplements (21.4%), 75 approved 135-day review supplements (13.3%), 44 approved real-time supplements (7.8%), 31 approved special-track supplements (5.5%), and 11 supplements (2.0%) that were not classified by track in the database. The 180-day process, which was originally indicated for design and component changes, was used for 41.7% (40 of 96 supplements) of all label indications changes for which the panel track was originally intended. In addition, 75 of the 314 (23.8%) submitted 30-day notice applications were converted to the 135-day review track, requiring additional supporting data and FDA discretion before approval.
Figure 3. Types of Changes in Dermatologic Devices by Supplement Track.
Unclassified supplements are not included in this graph. A sizable proportion of the 180-day track supplements are used by manufacturing changes, rather than design and labeling changes. A significant level of the real-time process applications was approved for labeling and manufacturing changes, rather than its intended use for minor design modifications. Panel track and special (immediate track) supplements were used for labeling changes, with few exceptions. Last, 30-day and 135-day tracks were appropriately used for manufacturing changes. ODE indicates Office of Device Evaluation; OIR, Office of In Vitro Diagnostics and Radiological Health; OSB, Office of Surveillance and Biometrics; PAS, postapproval study; and PMA, premarket approval.
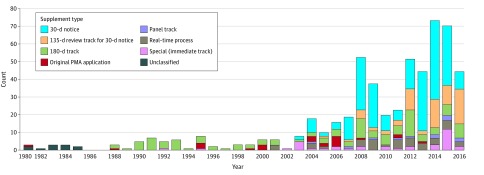
In the decade after the 1990 introduction of the panel-track supplement (1990-1999), there were no approved panel-track supplements. While the number of panel-track supplements has increased to 16, this represents only 2.8% of all 562 supplements. A more dramatic increase was seen for the 30-day notice: frequency of use has more than doubled since its introduction in 1997, comprising 52.7% (217 of 411) of supplements between 2005 and 2015, up from 27.9% (12 of 43) of supplements during the prior 5-year period (2000-2005) (Figure 4).
Figure 4. Postmarket Modification Trends Over Time.
Over time, the overall share of supplements subjected to 180-day review tracks has decreased. The introduction of the 30-day and 135-day and real-time tracks in 1997 resulted in an increased popularity of their use and overall number of postmarket modifications per device. Panel-track supplements remain rarely used. PMA indicates premarket approval.
Recalls
Of the 27 original PMA applications approved by the FDA, a total of 4 devices had product recalls since December 2015. Four devices had more than one recall. In all, the FDA issued 8 separate recalls on dermatologic devices, consisting of 5 Class II and 3 Class III recalls (Table). Reasons for recall of these devices were categorized as software design, employee error, noncomforming material or component, or other.
Manufacturers also voluntarily withdrew 10 of the 27 devices from the market. Notably, both injectable products from Merz (P050037 and P050052) had recalls after undergoing 135-day (n = 18) and 30-day (n = 85) applications. The median time from PMA approval to withdrawal was 11.1 years (IQR, 4.6-18.3; range, 2.5-30.3).
Discussion
We characterized patterns of device approval and supplement use in the PMA process. Our findings show that the use of supplement pathways, which facilitate expedited approval of postmarket device changes, has increased substantially over the lifetime of the PMA program and 891.9% in the past 10 years. This indicates a higher rate of supplements per device-year in dermatology than other reported specialties, including orthopedics, otolaryngology, and cardiology. While the 180-day track represents the most popular track for postmarket changes since the implementation of the PMA program, use patterns since the early 2000s have favored 30-day and 135-day tracks, both of which have lower data requirements for approval.
Therefore, when a manufacturer uses the 30-day PMA track to get approval for manufacturing process modifications, there is a potential for issues to be overlooked. This may result in increased risk to patients, as was the case in the 2011 recall of Radiesse injectable implants. In this case, faulty manufacturing resulted in a reported increased risk of infection among implant recipients. Such device failures may present a significant risk to patients and may reflect inadequate safety assessments of postmarket changes by the FDA. In addition, understudied changes of devices over time may result in end products that vary significantly from the devices initially approved based on clinical data.
This study also demonstrates that discrepancies exist between the intended use of PMA tracks as prescribed by the FDA and the manner in which these pathways are used by manufacturers. Although the real-time process is intended for minor design changes, such modifications amounted to only 45.4% (20 of 44 supplements) of the supplement applications. The 180-day process was used for 41.7% (40 of 96) of all label indications changes, instead of the more comprehensive panel track that would require clinical data and a formal review process. Orthopedic and cardiac devices share similar trends for modifications through the 180-day process, while otolaryngology device manufacturers have a tendency to use the special-track approval for changes in indication. Although applications are reviewed on a case-by-case basis, supplement pathways may allow for inadequately supported changes in product design or indications.
The current regulatory environment does not provide sufficient information necessary for dermatologists to make informed clinical judgment regarding potential device problems. There is a dearth of FDA review memos in dermatology, which are normally published to disclose information about modifications. Increasing disclosure about device modifications across all supplementary pathways may promote more reliable safety in Class III dermatologic devices.
Limitations and Future Directions
Our cross-sectional study contains important limitations. While there are several unclassified supplements before 1990, such entries are a minority of the analyzed entries. In addition, there are several devices in the moderate risk category not included in this study that are approved through the less expensive and less stringent 510(k) pathway, which is known to contain some devices that are marketed with Class III indications. Another limitation is that our analysis was based on the FDA online PMA database, which contains limited information regarding specific changes implemented using supplements. As a result, it is challenging to discern the reasons for device failures, recalls, or withdrawals. Clinicians and researchers are limited to generalized categorization of changes (manufacturing, labeling, and design), without more specific details. Finally, supporting evidence is not linked to each supplement, limiting the evaluation of such studies by physicians and patients.
Although there is a possibility that some minor changes may be proprietary and thus not disclosable, enhanced transparency will better equip health care professionals to understand product risk. Although device drift exists in dermatology, its influence on patient care may not be as substantial as in other specialties.
Improvement of preapproval studies may help reduce the reliance on postapproval surveillance. For example, studies designed with a larger number of participants and longer follow-up period may be beneficial, although more costly in the short term. Ongoing efforts to improve postmarket studies, such as using unique device identifiers and outcome registries, exist in other subspecialties. These steps could help ensure that the Class III device regulatory framework can keep pace with the increase in supplement use, while maintaining appropriate transparency and preventing incremental changes that may result in product recalls, withdrawals, or adverse clinical effects.
Although complications and adverse effect estimates for soft-tissue fillers, for example, remain below 1%, the flexibility of the device approval pathway must be balanced with a need to protect patient safety by minimizing the potential for device drift. This may be accomplished with the creation of an advisory board tasked with reviewing a product for clinically significant device drift after a defined number of years or PMA supplement applications. Such an oversight body would focus on major changes and avoid those that are judged to be minor so as not to impede patient access.
Conclusions
Greater transparency and higher standards of review may improve the inconsistencies in usage of the premarket pathways for device approvals. These enhancements will allow better assessment of the safety of device modifications.
References
- 1.Hauser RG, Maron BJ. Lessons from the failure and recall of an implantable cardioverter-defibrillator. Circulation. 2005;112(13):2040-2042. [DOI] [PubMed] [Google Scholar]
- 2.Rome BN, Kramer DB, Kesselheim AS. FDA approval of cardiac implantable electronic devices via original and supplement premarket approval pathways, 1979-2012. JAMA. 2014;311(4):385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardaugh BM, Graves SE, Redberg RF. The 510(k) ancestry of a metal-on-metal hip implant. N Engl J Med. 2013;368(2):97-100. [DOI] [PubMed] [Google Scholar]
- 4.Garrod AE, Hurtley WH. On the supposed occurrence of uroleucic acid in the urine in some cases of alkaptonuria. J Physiol. 1907;36(2-3):136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser RG, Almquist AK. Learning from our mistakes? testing new ICD technology. N Engl J Med. 2008;359(24):2517-2519. [DOI] [PubMed] [Google Scholar]
- 6.Kay GN, Ellenbogen KA. An ICD lead advisory: a plea for more diligence and more data. Pacing Clin Electrophysiol. 2012;35(6):648-649. [DOI] [PubMed] [Google Scholar]
- 7.Rathi VK, Ross JS, Samuel AM, Mehra S. Postmarket modifications of high-risk therapeutic devices in otolaryngology cleared by the US Food and Drug Administration. Otolaryngol Head Neck Surg. 2015;153(3):400-408. [DOI] [PubMed] [Google Scholar]
- 8.Samuel AM, Rathi VK, Grauer JN, Ross JS. How do orthopaedic devices change after their initial FDA premarket approval? Clin Orthop Relat Res. 2016;474(4):1053-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day CS, Park DJ, Rozenshteyn FS, Owusu-Sarpong N, Gonzalez A. Analysis of FDA-approved orthopaedic devices and their recalls. J Bone Joint Surg Am. 2016;98(6):517-524. [DOI] [PubMed] [Google Scholar]
- 10.Phillips AT, Rathi VK, Ross JS. Publication of clinical studies supporting FDA premarket approval for high-risk cardiovascular devices between 2011 and 2013: a cross-sectional study. JAMA Intern Med. 2016;176(4):551-552. [DOI] [PubMed] [Google Scholar]
- 11.Bliznakov Z, Mitalas G, Pallikarakis N Analysis and classification of medical device recalls. In: World Congress on Medical Physics and Biomedical Engineering 2006 Berlin, Germany: Springer Verlag; 2007:3782-3785. [Google Scholar]
- 12.Wong S, Street D, Delgado SI, Klontz KC. Recalls of foods and cosmetics due to microbial contamination reported to the U.S. Food and Drug Administration. J Food Prot. 2000;63(8):1113-1116. [DOI] [PubMed] [Google Scholar]
- 13.Zuckerman DM, Brown P, Nissen SE. Medical device recalls and the FDA approval process. Arch Intern Med. 2011;171(11):1006-1011. [DOI] [PubMed] [Google Scholar]
- 14.Koneru JN, Gunderson BD, Sachanandani H, et al. Diagnosis of high-voltage conductor fractures in Sprint Fidelis leads. Heart Rhythm. 2013;10(6):813-818. [DOI] [PubMed] [Google Scholar]
- 15.Mitkowski P, Grabowski M, Kowalski O, et al. National Consultant in Cardiology Experts’ Group Guidelines on dealing with patients implanted with some St. Jude Medical Riata and Riata ST leads [in Polish]. Kardiol Pol. 2014;72(6):576-582. [DOI] [PubMed] [Google Scholar]
- 16.Carruthers J, Carruthers A, Humphrey S. Introduction to fillers. Plast Reconstr Surg. 2015;136(5)(suppl):120S-131S. [DOI] [PubMed] [Google Scholar]
- 17.Carruthers JD, Fagien S, Rohrich RJ, Weinkle S, Carruthers A. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134(6):1197-1201. [DOI] [PubMed] [Google Scholar]
- 18.Dayan SH, Arkins JP, Mathison CC. Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol. 2011;10(9):1007-1012. [PubMed] [Google Scholar]
- 19.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. Safety. https://www.fda.gov/safety/recalls/ucm165546.htm. Updated June 24, 2009. Accessed January 31, 2016.
- 21.Klein A. Filler follies. http://www.huffingtonpost.com/arnold-william-klein/filler-follies_b_410987.html. Published 2011. Accessed January 31, 2016.
- 22.Klein AW. The American filler experience: a response to Dr Baumann. J Cosmet Dermatol. 2005;4(2):129. [DOI] [PubMed] [Google Scholar]
- 23.Zheng SY, Redberg RF. Premarket approval supplement pathway: do we know what we are getting? Ann Intern Med. 2014;160(11):798-799. [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration Premarket approval (PMA) summary review memos for 180-day design changes. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHTransparency/ucm206289.htm. Published 2016. Accessed February 12, 2016.
- 25.US Food and Drug Administration 510(k) premarket notification. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm. Published 2015. Accessed January 31, 2016.
- 26.Newburger AE. Cosmetic medical devices and their FDA regulation. Arch Dermatol. 2006;142(2):225-228. [DOI] [PubMed] [Google Scholar]
- 27.Lohman ME, Ghobadi CW, Xu S. Device safety implications of the clinical data leading to US Food and Drug Administration approval of soft-tissue fillers: a systematic review. JAMA Facial Plast Surg. 2017;19(5):421-429. [DOI] [PubMed] [Google Scholar]
- 28.Shuren J, Califf RM. Need for a national evaluation system for health technology. JAMA. 2016;316(11):1153-1154. [DOI] [PubMed] [Google Scholar]
- 29.Alam M, Kakar R, Nodzenski M, et al. Multicenter prospective cohort study of the incidence of adverse events associated with cosmetic dermatologic procedures: lasers, energy devices, and injectable neurotoxins and fillers. JAMA Dermatol. 2015;151(3):271-277. [DOI] [PubMed] [Google Scholar]
- 30.Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33(6):862-877. [DOI] [PubMed] [Google Scholar]