Abstract
This case series presents 3 cases of patients with Hailey-Hailey disease demonstrating varying responses to naltrexone.
Hailey-Hailey disease (HHD) is a genetic intraepidermal blistering disorder characterized by chronic macerated erosions in intertriginous areas. The recent report of 2 case series demonstrating the efficacy of low-dose naltrexone in the treatment of HHD represents exciting progress in the management of a disease with limited therapeutic options. Herein we present 3 additional cases of HHD demonstrating varying responses to naltrexone.
Methods
Three patients with biopsy-proven HHD were started on naltrexone based on patient request and followed every 4 to 6 weeks or as needed for physician assessment of clinical response, subjective quality of life, and incidence of adverse effects. This case-report study was granted exemption by the Partners Healthcare institutional review board.
Results
Case 1
A woman in her 30s presented with a 10-year history of HHD. Past treatments included topical steroids and oral antibiotics. The patient was started on naltrexone 4.5 mg nightly, topical clobetasol for flares, and topical tacrolimus for maintenance therapy. After 18 months she experienced 95% clearance of her erosions and ulcerations with relief in pruritus and pain, requiring only minimal amounts of clobetasol.
Case 2
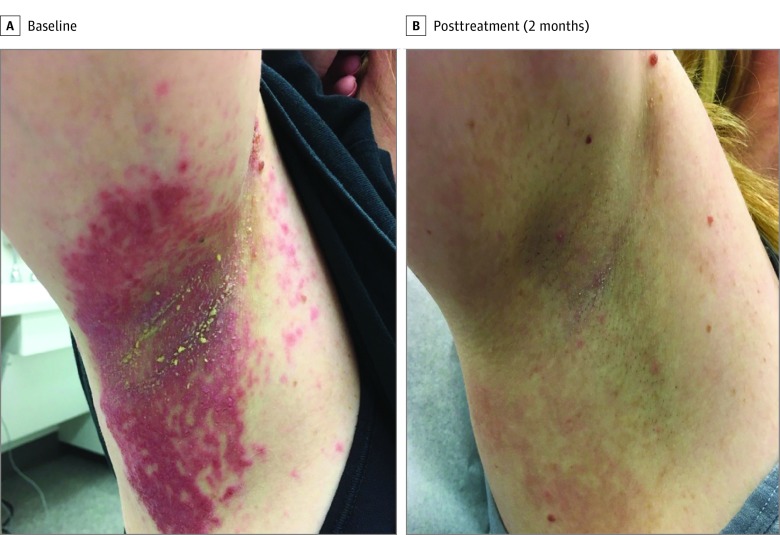
A woman in her 30s with a 15-year history of HHD poorly responsive to topical antibiotics, antifungals, and steroids, was started on naltrexone 12.5 mg nightly, given the ability to quarter the commercially available 50-mg tablets covered by her insurance. The patient demonstrated complete resolution of erosions and ulcerations within 2 months (Figure). However, she stopped treatment owing to concerns that 12.5 mg was higher than dosages discussed on HHD online forums. Three weeks later she experienced a flare in the inframammary and inguinal areas and restarted naltrexone at 4.5 mg. After 1 month, she reported that her erosions were improving but at a slower rate than on the 12.5-mg dose.
Figure. Clinical Response to Naltrexone 12.5 mg Nightly.
Clinical photographs show (A) the right axilla of a patient at baseline with large macerated plaques with superficial erosions, and (B) the patient’s right axilla after 2 months of treatment with naltrexone 12.5 mg nightly demonstrating complete resolution.
Case 3
A woman in her 60s with a 17-year history of HHD resistant to topical, intralesional, intramuscular, and systemic steroids, topical antifungals, and oral antibiotics, was started on naltrexone 50 mg nightly, as she also had brachioradialis pruritus. After 1 month, her HHD had neither improved nor worsened, and she was started on acitretin 10 mg daily to improve disease control. The patient experienced 30% clearance in the extent of disease within 1 month. However, she discontinued naltrexone owing to cost, resulting in a flare 2 weeks later. Naltrexone 4.5 mg was started and acitretin increased to 25 mg daily, but her flare progressed over the next 10 days, requiring methylprednisolone, an oral prednisone taper, and intralesional triamcinolone to achieve control. Since then, she has initiated glycopyrrolate 1 mg 3 times per day and naltrexone 4.5 mg and achieved 30% clearance of HHD over the last 3 months. The patient is pleased because no lesions are exudative, eroded, or symptomatic.
Discussion
Online testimonials and recent publications have documented dramatic responses of HHD to naltrexone. However, we demonstrate response variability and the possible use of adjunctive agents to achieve a desired effect. Clinicians should carefully manage patient expectations and explain the potential use of naltrexone in a multipronged approach to treatment. When used with topical steroids or acitretin, positive results can be observed with low frequency and dosages of these adjunctive agents, allowing for simplified regimens.
Our cases suggest that the previously published 4.5-mg dose may not be sufficient, and that 12.5-mg to 50-mg doses can be safely attempted for an adequate response. Caution should be taken with immediate cessation of 12.5-mg to 50-mg doses, given evidence of flares following discontinuation. While no adverse effects were noted, we suggest monitoring patients on 12.5-mg to 50-mg doses for adverse effects such as depression, increased pain, and elevated liver function tests. Additional studies are needed to fully characterize optimal dosing regimens of naltrexone for HHD given the variability of response.
References
- 1.Arora H, Bray FN, Cervantes J, Falto Aizpurua LA. Management of familial benign chronic pemphigus. Clin Cosmet Investig Dermatol. 2016;9:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim O, Hogan SR, Vij A, Fernandez AP. Low-dose naltrexone treatment of familial benign pemphigus (Hailey-Hailey disease). JAMA Dermatol. 2017;153(10):1015-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albers LN, Arbiser JL, Feldman RJ. Treatment of Hailey-Hailey disease with low-dose naltrexone. JAMA Dermatol. 2017;153(10):1018-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]