Abstract
Importance
The ongoing opioid epidemic in the United States has been fueled by prescription opioids. Increases in opioid-related deaths and complications mandate clinicians in all fields to scrutinize their prescribing patterns.
Objective
To characterize the current status and potential complications of opioid prescribing practices among dermatologists for Medicare beneficiaries.
Design, Setting, and Participants
A cross-sectional study used Medicare Part D prescriber data to evaluate opioid prescriptions by dermatologists from January 1 to December 31, 2014. The number of prescribers, opioid claims, beneficiaries, and days supplied as well as the type of opioid and geographic location of prescribers were extracted and analyzed. The top 1% of dermatologists prescribing opioids were identified and compared with a random sample of the same size among the remaining dermatologists based on sex, geographic location, type of practice, and time in practice. A systematic literature review was conducted to estimate the outcome of opioid prescribing practices on the exposed population.
Main Outcome and Measures
Practice characteristics, epidemiologic factors, and consequences of opioids prescribed by dermatologists.
Results
Of the 12 537 dermatologists in the study, 5305 (42.3%) prescribed no opioid claims, 5408 (43.1%) prescribed 1 to 10 opioid claims, and 1824 (14.5%) prescribed more than 10 opioid claims. Among dermatologists prescribing at least 10 opioid claims, a mean of 1.0 opioid claims was given to each beneficiary, with a supply lasting a mean of 4.4 days. A total of 108 dermatologists (93.9%) in the top 1% of opioid prescribers (n = 115) work in a surgical practice. Estimates suggest that opioids prescribed by dermatologists could annually lead to 3877 to 7602 beneficiaries continuing to use opioids at 1 year and 1825 to 4209 continuing to use opioids at 3 years. A total of 9882 to 22 806 beneficiaries could experience gastrointestinal tract or central nervous system adverse effects and 588 to 999 could experience fractures.
Conclusions and Relevance
Opioid prescribing among dermatologists is limited and concentrated in the surgical setting, but it may be associated with a substantial number of adverse events that serve as a reminder to emphasize nonopioid pain medications in the postoperative setting.
This cross-sectional study characterizes the current status and potential complications of opioid prescribing practices among dermatologists for Medicare beneficiaries.
Key Points
Question
What is the nature and outcome of opioid prescriptions by dermatologists among Medicare patients?
Findings
In this cross-sectional study using 2014 Medicare Part D prescriber data, opioid prescriptions by dermatologists were few and concentrated among dermatologists in surgical practices. Estimates project that this use could place more than 7000 Medicare beneficiaries at risk for addiction, more than 22 000 at risk for gastrointestinal tract or central nervous system adverse effects, and close to 1000 at risk for fractures.
Meaning
Opioid prescribing among dermatologists is limited but associated with potential adverse effects for elderly patients; therefore, nonopioid alternatives should be emphasized.
Introduction
The opioid epidemic in the United States is a national emergency. From 2000 to 2014, nearly half a million persons died from drug overdoses, with 61% of overdose-related deaths in 2014 attributable to opioids. In 2015, an estimated 1.9 million people in the United States met the criteria for opioid abuse or dependence. The rise in opioid misuse, overdoses, deaths, and hospital admissions has increased in parallel with opioid sales, which quadrupled between 1999 and 2010.
Little is known about opioid prescribing patterns in dermatology. Evaluations of opioid prescriptions in other fields have encouraged changes in practice, as even a short course of opioids can place patients at risk for addiction. Current dermatology guidelines recommend oral opioids as second-line agents after a trial of nonsteroidal anti-inflammatory drugs and/or acetaminophen after surgical excisions and Mohs microsurgery (MMS). Prior studies on opioid prescribing practices in dermatology suggest limited use of opioids after MMS but are derived from observational studies with limited cohorts of dermatologists and patients.
In this study, we sought to broaden this evaluation by examining the use and potential complications associated with opioid prescriptions within the US Medicare population.
Methods
Data Collection
The Part D Prescriber Public Use File (PUF) is a public Centers for Medicare & Medicaid Services data set that contains information on prescription drug events incurred by Medicare beneficiaries with a Part D prescription drug plan. The Part D prescription drug plan covers approximately 72% of the roughly 57 million people on Medicare, a federal insurance plan for Americans who are 65 years or older or have certain disabilities. Part D Prescriber PUFs are currently available for 2013-2015 and contain information organized by specific drug events on physician information (National Provider Identifier, name, geographic location, and specialty), drug name, drug use (beneficiary count, claim count, and days’ supply), and total drug costs. To protect the privacy of Medicare beneficiaries, drug events derived from 10 or fewer claims are excluded from the Part D Prescriber PUFs. In addition, any beneficiary counts, claim counts, 30-day fill counts, drug costs, and days’ supply with values between 0 and 10 are suppressed. This study was granted institutional review board exemption by the Partners Healthcare Institutional Review Board because all patient information is deidentified.
The Centers for Medicare & Medicaid Services offer additional opioid-specific data via the Medicare Part D Opioid Prescribing Mapping Tool. Among these data are the Medicare Part D Opioid Prescriber Summary Files available for 2013-2014, which combine all individual opioid drug events to calculate the opioid prescribing rates of physicians in the Medicare Part D program. These files contain physician information on those prescribing 0 opioid claims, 1 to 10 opioid claims, and greater than 10 opioid claims. Compared with the Part D Prescriber PUFs, opioid drug events are approached as a class rather than as individual drugs in this data set.
The top 1% of opioid prescribers and a random sample of the same size from the remaining dermatologists prescribing more than 10 opioid claims were identified using the Part D Opioid Prescriber Summary File for 2014, covering January 1 to December 31, 2014. Information on sex and geographic location were collected using the Part D Prescriber Look-Up Tool offered by the Centers for Medicare & Medicaid Services. Graduation year from medical school and subspecialization were obtained from the prescriber’s state medical board website. The type of each prescriber’s practice was assessed by searching for descriptions of the practice on Google. Practices were designated as surgical if the prescriber practiced MMS or cosmetic surgery. Telephone calls were made to individual offices when clarification about the type of practice was needed.
Statistical Analysis
The number of dermatologists prescribing 0 opioid claims, 1 to 10 opioid claims, and greater than 10 opioid claims was calculated using the Part D Opioid Prescriber Summary File from 2014. All subsequent calculations for prescribing characteristics of dermatologists prescribing greater than 10 opioid claims and dermatologists in the top 1% of opioid prescribers were performed using the Part D Prescriber PUF for 2014. The mean number of days’ supply per opioid claim was obtained by dividing the total days’ supply for all opioid claims by the total opioid claim count. The mean number of opioid claims per beneficiary was determined by dividing the total opioid claim count by the total number of beneficiaries receiving opioids. The sex breakdown, geographic distribution, and type of practice among the top 1% of opioid prescribers and a random sample of remaining dermatologists prescribing more than 10 opioid claims were compared using a χ2 test. The mean number of years since medical school graduation between these 2 groups was compared using a 2-sided t test. P < .05 was considered significant.
Mapping Geographic Variation
The opioid claim count was summed for each state and divided by the number of Medicare beneficiaries for that state to yield the number of opioid claims per 1000 Medicare beneficiaries per state. The same calculation was repeated for states grouped by geographic regions. State-based data were translated into a map with different colors corresponding to different amounts of opioid claims per 1000 Medicare beneficiaries.
Modeling of Risks
We performed a comprehensive literature search for observational studies and systematic reviews reporting the risks associated with opioid use in the setting of acute pain control, particularly in elderly patients (eTable 1 in the Supplement). Search terms included opioids, risks, elderly, acute, postoperative, and outpatient. References of articles were also manually searched for additional relevant studies. The rate of each adverse event was summarized as a range and then multiplied by the total number of beneficiaries receiving opioids in the 2014 Part D Prescriber PUF to obtain a projected number of affected Medicare beneficiaries. The predicted incidence of fracture for our population was calculated by taking the total days’ supply derived from opioid claims in 2014 and dividing by 365 to obtain the number of person-years, which was then multiplied by the incidence of fracture reported per 1000 person-years in the literature. Harris et al report a mean of 5.4 leftover pills among patients who filled a prescription for opioids after dermatologic surgery. The number of pills per claim is not available in the Part D data sets. We therefore multiplied the number of leftover pills found by Harris et al by the total number of opioid claims for 2014 to obtain an estimate of the number of leftover pills.
Results
A total of 12 537 dermatologists were identified using the Part D Opioid Prescriber Summary File: 5305 (42.3%) prescribed 0 opioid claims, 5408 (43.1%) prescribed 1 to 10 opioid claims, and 1824 (14.5%) prescribed more than 10 opioid claims.
Among dermatologists prescribing at least 10 opioid claims in the Part D Prescriber PUF, a total of 91 334 opioid claims were prescribed to 76 019 beneficiaries (Table 1). Each dermatologist prescribed a mean (SD) of 63 (109) opioid claims to 61 (89) beneficiaries. Each beneficiary received a mean of 1.0 opioid claims, with a supply lasting a mean of 4.4 days. Combination hydrocodone bitartrate and acetaminophen accounted for 55 166 opioid claims (60.4%), followed by combination codeine phosphate and acetaminophen (17 445 [19.1%]), combination oxycodone hydrochloride and acetaminophen (9042 [9.9%]), and tramadol hydrochloride (7581 [8.3%]).
Table 1. Current Opioid Prescribing Practices Among Dermatologists Prescribing More Than 10 Opioid Claims and the Top 1% of Opioid Prescribers Using the 2014 Part D Prescriber Public Use File.
| Characteristic | Dermatologists Prescribing >10 Opioid Claims | Top 1% of Opioid Prescribers |
|---|---|---|
| No. (%) of dermatologistsa | 1449 (12.6) | 115 (1.0) |
| No. of opioid claims | 91 334 | 38 520 |
| No. of beneficiariesb | 76 019 | 32 205 |
| Opioid claims per dermatologist, mean (SD) [median] | 63 (109) [27] | 335 (234) [285] |
| Beneficiaries per dermatologist, mean (SD) [median] | 61 (89) [28] | 280 (148) [246] |
| Mean days’ supply per opioid claim | 4.4 | 4.2 |
| Mean opioid claims per beneficiaryb | 1.0 | 1.2 |
| Type of opioid, No. (%) | ||
| Hydrocodone bitartrate and acetaminophen | 55 166/91 334 (60.4) | 22 881/38 520 (59.4) |
| Codeine phosphate and acetaminophen | 17 445/91 334 (19.1) | 6933/38 520 (18.0) |
| Oxycodone hydrochloride and acetaminophen | 9042/91 334 (9.9) | 4006/38 520 (10.4) |
| Tramadol hydrochloride | 7581/91 334 (8.3) | 3313/38 520 (8.6) |
| Otherc | 2101/91 334 (2.3) | 655/38 520 (1.7) |
The total number of dermatologists was 11 526.
Value may be underestimated owing to suppression of beneficiary counts with values below 10.
Other opioids include: oxycodone hydrochloride; tramadol hydrochloride and acetaminophen; fentanyl; morphine sulfate; butorphanol tartrate; hydrocodone bitartrate and ibuprofen; methadone hydrochloride; hydromorphone hydrochloride; and codeine phosphate, butalbital, aspirin, and caffeine.
We next identified 115 dermatologists in the top 1% of dermatologists prescribing opioids. These dermatologists accounted for 42.2% of opioid claims and prescribed a total of 38 520 opioid claims to 32 205 beneficiaries. Each dermatologist prescribed a mean (SD) of 335 (234) opioid claims to 280 (148) beneficiaries, and each beneficiary received a mean of 1.2 opioid claims, with a supply of 4.2 days.
Among the top 1% of dermatologists prescribing opioids, 99 (86.1%) were male physicians, compared with 89 of 114 (78.1%) in a random sample of the same size from the remaining dermatologists prescribing more than 10 opioid claims (P = .11) (Table 2). A total of 108 dermatologists (93.9%) in the top 1% of prescribers worked in a surgical practice, vs 85 of 114 (74.6%) in the comparison group (P < .001). A total of 83 dermatologists (72.2%) in the top 1% had practices located in Southern states, with less of a geographic predominance among the random sample of remaining dermatologists (P = .003). The mean number of years since medical school graduation was 24 for both groups (P > .99).
Table 2. Characteristics of Dermatologists Prescribing More Than 10 Opioid Claims and the Top 1% of Prescribers Using the 2014 Part D Prescriber Public Use File.
| Characteristic | Dermatologists Prescribing >10 Opioid Claims (n = 114) | Top 1% of Opioid Prescribers (n = 115) | P Value |
|---|---|---|---|
| Sex, No. (%) | |||
| Female | 25 (21.9) | 16 (13.9) | .11 |
| Male | 89 (78.1) | 99 (86.1) | |
| Geographic distribution, No. (%)a | |||
| Northeast | 9 (7.9) | 7 (6.1) | .003 |
| Midwest | 13 (11.4) | 9 (7.8) | |
| South | 55 (48.2) | 83 (72.2) | |
| West | 37 (32.5) | 17 (14.8) | |
| Type of practice, No. (%) | |||
| Surgical | 85 (74.6) | 108 (93.9) | <.001 |
| General dermatology | 29 (25.4) | 7 (6.1) | |
| Years since medical school graduation, mean (SD) [median] | 24 (11.5) [23] | 24 (8.6) [24] | >.99 |
Northeast: Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont; South: Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia; Midwest: Indiana, Illinois, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin; and West: Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, New Mexico, Nevada, Oregon, Utah, Washington, and Wyoming.
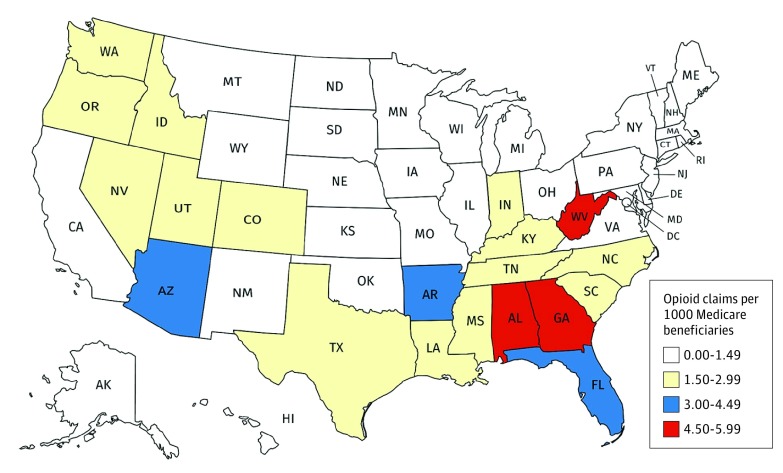
Dermatologists in Southern states prescribed higher numbers of opioid claims per 1000 Medicare beneficiaries, compared with dermatologists in the rest of the United States (Figure). Aggregated by region, dermatologists in the South prescribed 2.77 opioid claims per 1000 Medicare beneficiaries, compared with 1.60 in the West, 0.89 in the Midwest, and 0.83 in the Northeast (eTable 2 in the Supplement).
Figure. Opioid Claims by State.
Shaded by number of opioid claims per 1000 Medicare beneficiaries for 2014 (accompanying values aggregated by region listed in eTable 2 in the Supplement).
The frequencies of several risks associated with opioid use in the elderly, including addiction, gastrointestinal tract adverse effects (including constipation, nausea, and vomiting), central nervous system adverse effects (including dizziness, somnolence, and unsteadiness), and fracture, were identified from the literature (Table 3). We estimate that 3877 to 7602 beneficiaries could continue to use opioids 1 year after their prescription and 1825 to 4029 may continue to use them 3 years after their prescription. A total of 9882 to 22 806 beneficiaries could experience gastrointestinal tract or central nervous system adverse effects and 588 to 999 could experience fractures. We also estimate a remainder of 493 204 unused opioid pills in the community.
Table 3. Projections of Risks Associated With Opioid Prescriptions From Dermatologists.
| Risk | Frequency of Risk | No. Affected |
|---|---|---|
| Opioid use at 1 y, % | 5.1-10 | 3877-7602 |
| Opioid use at 3 y, % | 2.4-5.3 | 1825-4029 |
| Gastrointestinal tract adverse effects: constipation, nausea, and vomiting, % | 13-30 | 9882-22 806 |
| CNS adverse effects: dizziness, unsteadiness, tiredness, and somnolence, % | 13-30 | 9882-22 806 |
| Fracture, No. per 1000 person-years | 531-902 | 588-999 |
| Leftover pills, No. of leftover pills per claim | 5.4 | 493 204 |
Abbreviation: CNS, central nervous system.
Discussion
In this study, we investigate current opioid prescribing practices among dermatologists within the Medicare system and explore their implications for the Medicare population. These data suggest that opioid prescribing within dermatology is limited. Approximately 1 in 8 dermatologists listed in the 2014 Part D Prescriber PUF prescribe more than 10 opioid claims, typically providing one 4-day course per beneficiary. A closer evaluation of the top 1% of opioid prescribers reveals their concentration in surgical practices, suggesting that opioid use in dermatology stems primarily from the need for acute pain control after surgical procedures. Our findings confirm the results of prior observational studies demonstrating limited use of opioids after MMS, but extend these conclusions on a larger scale by examining prescribing practices among all dermatologists practicing within Medicare Part D.
Dermatologists in southern states exhibit heavier use of opioids than those in other regions of the United States. Most dermatologists among the top 1% of opioid prescribers have practices located in southern states, exceeding the proportion practicing in the South among a random sample of dermatologists prescribing more than 10 opioid claims. Although we were not able to control for the volume of patients seen by these dermatologists, we concurrently found that dermatologists in southern states exhibit a higher opioid prescribing rate per 1000 Medicare beneficiaries, more than tripling the number prescribed in the Northeast. These observations are in line with previously reported increased prescribing rates among physicians in southern states, pointing to regional influences on attitudes toward opioids that may benefit from region-specific interventions.
Although overall use of opioids may be low, short courses of opioids are associated with adverse effects, including addiction. The risk is likely potentiated in older adults, given age-related changes in drug pharmacokinetics and the tendency toward polypharmacy among older adults. Our calculations estimate that more than 7000 Medicare patients may be at risk for long-term opioid use from prescriptions they receive from dermatologists. Many more may be harmed from other adverse effects, including gastrointestinal tract adverse effects, central nervous system side effects, and fractures.
The substantial number of potentially affected Medicare beneficiaries serves as a reminder to carefully weigh the risks of opioids when selecting agents for acute pain management. As suggested by current guidelines, nonsteroidal anti-inflammatory drugs and acetaminophen should be used as first-line pain control agents, followed by opioids for additional control in certain high-risk patients. After MMS, acetaminophen plus ibuprofen achieves lower pain scores with fewer adverse effects when compared with acetaminophen plus codeine. Furthermore, 25% of patients undergoing MMS do not use any of their prescribed opioids and those who use their prescription take only 41% of their course, suggesting that patients may not require these drugs. Based on these consumption habits, we calculated that a total of 493 204 pills could be left unused by Medicare patients receiving prescriptions from their dermatologist, creating a large opioid reservoir that poses a substantial risk for future misuse.
Overall, the combination of patient and societal risks combined with the superior efficacy of nonopioid pain medications strongly suggests that dermatologists revisit habitual practices of prescribing opioids and consider the use of nonopioid pain medications alone in the management of acute pain.
Limitations
Our data must be interpreted in the context of the study design. The use of Medicare Part D data files limits our results to opioid prescribing practices among dermatologists participating in Medicare Part D and thus does not reflect prescribing practices applying to the general population of patients. Given the higher rates of skin cancer and the greater need for MMS among older adults, our findings may overestimate opioid prescribing by dermatologists to the general population. In addition, in our creation of the Figure, we used the number of Medicare beneficiaries enrolled in Parts A and B to calculate the number of opioid claims per 1000 Medicare beneficiaries per state. The use of data from different Medicare plans for this calculation may incorrectly estimate the rate of opioids per 1000 Medicare beneficiaries.
There are specific limitations associated with the use of Medicare Part D data files. The exclusion of data for claim counts or beneficiary counts with values below 10 in the 2014 Part D Prescriber PUFs prevents more accurate descriptions of opioid prescribing practices within Medicare. The data files also lack information on pill count per claim. We assume a mean of 5.4 leftover pills per claim based on the findings by Harris et al, which limits the accuracy of our calculations for the number of leftover pills. The studies we used to model the risks of opioid use focused only on opioid-naive patients. However, we lack patient-level information to know the opioid status of our patients. Our extrapolations using data in opioid-naive patients may therefore misrepresent the number of patients affected by adverse events. Finally, although our analysis of the top 1% of opioid prescribers in dermatology suggests the use of prescription opioids in the setting of acute pain control, Medicare Part D data files do not provide information on the specific indications for opioid prescriptions to confirm our findings. Additional studies are needed to evaluate the full spectrum of indications for prescription opioids and to understand the decision making behind opioid prescribing practices within dermatology. Research in these areas will help to identify more specific recommendations to limit opioid prescribing by dermatologists.
Conclusions
Our study, in conjunction with the current literature, suggests that the prescription of opioids by dermatologists is limited and concentrated among dermatologists in surgical practices primarily in Southern states. Despite modest opioid prescribing practices, efforts must be made to reduce opioid prescriptions and minimize the risks associated with opioid use in the elderly Medicare population. When clinically appropriate, dermatologists should follow current guidelines recommending an initial approach using nonopioid agents alone for pain control in the postsurgical setting.
eTable 1. Literature Review to Model Risks of Opioid Use
eTable 2. Number of Opioid Claims per 1000 Medicare Beneficiaries for 2014 Calculated by Geographic Region
References
- 1.Gostin LO, Hodge JG Jr, Noe SA. Reframing the opioid epidemic as a national emergency. JAMA. 2017;318(16):1539-1540. [DOI] [PubMed] [Google Scholar]
- 2.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. [DOI] [PubMed] [Google Scholar]
- 3.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293-301. [DOI] [PubMed] [Google Scholar]
- 4.Paulozzi LJ, Jones CM, Mack KA, Rudd RA; Centers for Disease Control and Prevention (CDC) . Vital signs: overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492. [PubMed] [Google Scholar]
- 5.Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177(9):1265-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields LB, Nasraty S, Bell AD, et al. Primary care providers’ prescribing practices of opioid controlled substances. J Opioid Manag. 2016;12(6):397-403. [DOI] [PubMed] [Google Scholar]
- 7.Broida RI, Gronowski T, Kalnow AF, Little AG, Lloyd CM. State emergency department opioid guidelines: current status. West J Emerg Med. 2017;18(3):340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson SR, Harding AM, Taylor SE, Vally H, Greene SL. Evaluation of a targeted prescriber education intervention on emergency department discharge oxycodone prescribing. Emerg Med Australas. 2017;29(4):400-406. [DOI] [PubMed] [Google Scholar]
- 9.Ruder J, Wally MK, Oliverio M, Seymour RB, Hsu JR; PRIMUM Group . Patterns of opioid prescribing for an orthopaedic trauma population. J Orthop Trauma. 2017;31(6):e179-e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamvu G, Feranec J, Blanton E. Perioperative pain management: an update for obstetrician-gynecologists. [published online June 27, 2017]. Am J Obstet Gynecol. 2017;S0002-9378(17)30790-1. doi: 10.1016/j.ajog.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 11.Dionne R, Moore PA. Opioid prescribing in dentistry: keys for safe and proper usage. Compend Contin Educ Dent. 2016;37(1):29-32. [PubMed] [Google Scholar]
- 12.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glass JS, Hardy CL, Meeks NM, Carroll BT. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73(4):543-560. [DOI] [PubMed] [Google Scholar]
- 14.Harris K, Calder S, Larsen B, et al. Opioid prescribing patterns after Mohs micrographic surgery and standard excision: a survey of American Society for Dermatologic Surgery members and a chart review at a single institution. Dermatol Surg. 2014;40(8):906-911. [DOI] [PubMed] [Google Scholar]
- 15.Firoz BF, Goldberg LH, Arnon O, Mamelak AJ. An analysis of pain and analgesia after Mohs micrographic surgery. J Am Acad Dermatol. 2010;63(1):79-86. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services Medicare provider utilization and payment data: Part D prescriber. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Part-D-Prescriber.html. Updated May 25, 2017. Accessed Aug 23, 2017.
- 17.The Henry J Kaiser Family Foundation The Medicare Part D prescription drug benefit. https://www.kff.org/medicare/fact-sheet/the-medicare-prescription-drug-benefit-fact-sheet/. Updated September 16, 2016. Accessed August 11, 2017.
- 18.Centers for Medicare & Medicaid Services, Office of Enterprise Data and Analytics. Medicare fee-for service provider utilization & payment data: Part D prescriber public use file : a methodological overview. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Downloads/Prescriber_Methods.pdf. Updated May 25, 2017. Accessed August 2, 2017.
- 19.Centers for Medicare & Medicaid Services. Medicare Part D opioid prescribing mapping tool . https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/OpioidMap.html. Updated March 28, 2017. Accessed Aug 23, 2017.
- 20.Centers for Medicare & Medicaid Services. Medicare provider utilization and payment data : 2015 Part D prescriber look-up tool. https://data.cms.gov/part-d-prescriber. Accessed Aug 23, 2017.
- 21.Centers for Medicare & Medicaid Services MDCR ENROLL AB 8: total Medicare enrollment : Part A and/or Part B enrollees by type of entitlement and area of residence, calendar year 2014. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2014/Downloads/MDCR_ENROLL_AB/2014_CPS_MDCR_ENROLL_AB_8.pdf. Accessed August 23, 2017.
- 22.Harris K, Curtis J, Larsen B, et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149(3):317-321. [DOI] [PubMed] [Google Scholar]
- 23.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. [DOI] [PubMed] [Google Scholar]
- 24.Hunold KM, Esserman DA, Isaacs CG, et al. Side effects from oral opioids in older adults during the first week of treatment for acute musculoskeletal pain. Acad Emerg Med. 2013;20(9):872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papaleontiou M, Henderson CR Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller M, Stürmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59(3):430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170(22):1979-1986. [DOI] [PubMed] [Google Scholar]
- 28.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain. 2012;13(10):988-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther. 2013;35(11):1659-1668. [DOI] [PubMed] [Google Scholar]
- 30.Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37(7):1007-1013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Literature Review to Model Risks of Opioid Use
eTable 2. Number of Opioid Claims per 1000 Medicare Beneficiaries for 2014 Calculated by Geographic Region