This observational cohort study assesses the effectiveness and drug discontinuation rates of rituximab among patients with newly diagnosed relapsing-remitting multiple sclerosis compared with injectable disease-modifying treatments, dimethyl fumarate, fingolimod, or natalizumab.
Key Points
Question
How does traditional initial disease-modifying treatment choices compare with rituximab in relapsing-remitting multiple sclerosis regarding drug discontinuation and clinical efficacy?
Findings
In this cohort study that included a population-based sample of 494 patients from 2 Swedish counties, both drug survival and rate of sufficient treatment effect were significantly higher for rituximab compared with injectable disease-modifying treatments (interferons, glatiramer acetate), dimethyl fumarate, and, in most comparisons, fingolimod and natalizumab.
Meaning
Rituximab can be considered an option for treatment-naive patients with relapsing-remitting multiple sclerosis.
Abstract
Importance
Comparative real-world effectiveness studies of initial disease-modifying treatment (DMT) choices for relapsing-remitting multiple sclerosis (RRMS) that include rituximab are lacking.
Objective
To assess the effectiveness and drug discontinuation rates of rituximab among patients with newly diagnosed RRMS compared with injectable DMTs, dimethyl fumarate, fingolimod, or natalizumab.
Design, Setting, and Patients
This retrospective cohort study used prospectively collected data to examine specialized care of 2 Swedish county–based community samples of patients with RRMS. Patients with RRMS who received diagnoses from January 1, 2012, to October 31, 2015, who resided in Stockholm or Västerbotten Counties were identified from a Swedish multiple sclerosis registry.
Main Outcomes and Measures
All reasons for drug discontinuation of initial treatment choice (main outcome) and specific reasons for switching (secondary outcomes) were analyzed with multivariable Cox regression, including propensity scores.
Results
Among 494 patients (median [interquartile range] age, 34.4 [27.4-43.4] years; 158 men [32.0%]), 215 received an injectable DMT (43.5%); 86 (17.4%), dimethyl fumarate; 17 (3.4%), fingolimod; 50 (10.1%), natalizumab; 120 (24.3%), rituximab; and 6 (1.2%), other DMT. Regional preferences were pronounced, with 42 of 52 (81%) and 78 of 442 (18%) receiving rituximab in Västerbotten and Stockholm, respectively. The annual discontinuation rate for rituximab, injectable DMTs, dimethyl fumarate, fingolimod, and natalizumab were 0.03, 0.53, 0.32, 0.38, and 0.29, respectively. Continued disease activity was the main reason for discontinuation of injectable DMTs, dimethyl fumarate, and fingolimod; positive John Cunningham virus serology results were the main reason for discontinuation of natalizumab. Rate of clinical relapses and/or neuroradiologic disease activity were significantly lower for rituximab compared with injectable DMTs and dimethyl fumarate, with a tendency for lower relapse rates also compared with natalizumab and fingolimod. The annual discontinuation rate of initial treatment choice was significantly lower in Västerbotten compared with Stockholm (0.09 and 0.37, respectively).
Conclusions and Relevance
Rituximab was superior to all other DMT in terms of drug discontinuation and displayed better clinical efficacy compared with injectable DMTs and dimethyl fumarate with borderline significance compared with natalizumab and fingolimod. The county where rituximab constituted the main initial treatment choice displayed better outcomes in most measured variables. Collectively, our findings suggest that rituximab performs better than other commonly used DMTs in patients with newly diagnosed RRMS.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system that often results in significant neurological disability over time. The MS treatment landscape has changed considerably during the last few years with the addition of several new disease-modifying treatments (DMTs). This provides better opportunities for personalized treatment, but detailed knowledge about how to tailor therapy in practice is still largely lacking.
It is difficult to accurately extrapolate drug survival and reasons to switch therapy based on data from randomized clinical trials, with selected patient populations running for limited periods of time. However, data from real-world populations indicate poor drug survival (ie, proportion of patients remaining on drug) for traditional first-line options (eg, interferon beta and glatiramer acetate—referred to as injectable DMTs combined), with less than half of patients remaining on therapy after 2 years. Similar studies have been performed for highly effective therapies, arguably comprising fingolimod, natalizumab, and alemtuzumab. In contrast, studies on newer oral DMTs (ie, dimethyl fumarate [DMF] and teriflunomide) are still rare.
Anti-CD20 B-cell depleting agents are likely to become an additional treatment option for relapsing-remitting MS (RRMS) and also primary progressive MS. The anti-CD20 class comprises rituximab (RTX) (immunoglobulin G1, mouse chimeric), ocrelizumab (immunoglobulin G1, humanized), and ofatumumab (immunoglobulin G1, fully human). Off-label use of RTX in patients with MS has increased considerably in Sweden but with large regional differences. We used these differences to compare outcomes for patients with RRMS receiving their first DMT in a region using a traditional escalating strategy (ie, Stockholm) with a region using a sustained induction strategy, initiating and maintaining treatment with highly efficient therapies (ie, Västerbotten, where RTX was predominately used). Furthermore, we compared outcomes for RTX with all other frequent DMTs in in the combined cohort.
Methods
Study Population
The source population comprised all individuals in Stockholm and Västerbotten Counties, respectively, who received a diagnosis of RRMS from January 1, 2012, to October 31, 2015, starting their first DMT (ie, first-line treatment). Patients were identified through a national web-based MS registry (http://www.neuroreg.se) from which data were collected along with social security numbers used to access corresponding local medical records. Patient data were collected from Karolinska University Hospital on April 11, 2016, and from Danderyds Hospital on May 3, 2016, in Stockholm County. In Västerbotten County, patient data were collected from the University Hospital of Umeå on October 18, 2015, with no additional incident cases occurring until October 31, 2015. Patients were observed until April 30, 2016, after which data were censored. All clinics adhere to the guidelines of the Swedish MS Association (http://www.mssallskapet.se/Checklistor.html) for the clinical follow-up of patients. Relapsing-remitting multiple sclerosis was diagnosed according to the 2010 revision of the McDonald criteria.
Exclusion criteria were (1) patients who received a diagnosis and/or treatment initiation outside of Stockholm or Västerbotten Counties, (2) participation in randomized clinical trials with unknown treatment allocation, (3) lack of follow-up data, and (4) migration to another county or country. Patients who had a diagnosis of radiologically isolated syndrome or clinically isolated syndrome were included if their symptoms instead fulfilled the diagnostic criteria of RRMS within the follow-up period and otherwise fulfilled inclusion and exclusion criteria. Patients were censored at treatment discontinuation regarding drug survival. For secondary outcomes, for natalizumab, fingolimod, and RTX, the follow-up was extended 1, 3, and 6 months, respectively, or until the end of the observation period, corresponding with the estimated duration of treatment effect. Cases of conversion to secondary progressive MS during the observation period were censored at the date of conversion to secondary progressive MS. Patients with treatment interruption due to planned pregnancy were censored according to DMT, as described above.
The study was a part of the Stockholm Prospective Assessment of MS project, approved by the regional ethical committee of Stockholm (2009/2107-31/2) and Umeå (2013/445-31). Patients provided oral consent.
Data Collection and Outcomes
Data collected from the MS registry with manual cross-referring and additional data retrieval from medical records comprised age, sex, hospital, DMT, relapses, presence of gadolinium-enhancing (Gd+) lesions based on original magnetic resonance imaging (MRI) reports, discontinuation date and stated cause, Expanded Disability Status Scale (EDSS) score, and adverse events. Magnetic resonance imaging scans were performed according to standard follow-up guidelines and MRI protocols, using 1.5-T or 3-T MRI scanners (GE Healthcare, Siemens Healthcare, and Philips Medical Systems). Relapses and Gd+ lesions were included if occurring at least 3 months after the first DMT dose. Suspected/registered relapses were adjudicated based on clinical record data by an evaluator (F.P.) who was blinded to treatment allocation and patient identity. Information on adverse events were collected through medical record review of the respective hospital electronic medical records system, which does not contain data from family practitioners. Furthermore, uncomplicated upper respiratory tract and lower urinary tract infections were not included. Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events.
The primary outcome was discontinuation of therapy due to any reason, including conversion to secondary progressive MS and pregnancy. Secondary outcomes were relapses and Gd+ lesions on MRI scans, adverse events, and stated cause of therapy discontinuation. All outcomes were specified before data analysis.
Statistical Analyses
Statistical analyses and data processing were performed in R, version 3.4.0 (R Foundation) with the survival (version 2.41-3), cmprsk (version 2.2-7), and ggplot2 (version 2.2.1) packages. Significance was set at a P value of less than .05. Baseline characteristics were compared using Wilcoxon rank sum test for the continuous variables age, MS duration since debut and diagnosis, and follow-up time. Fisher exact test was used for the categorical variables sex, EDSS score, relapse during the year before treatment, and region. Kaplan-Meier curves and Cox proportional hazards models were used to visualize and compare drug survival and relapse rates using time from first day of drug administration to outcome of interest used as timescale. The relative odds of experiencing Gd+ lesions were assessed in logistic regression models. The potential confounding variables age, sex, baseline EDSS score (as a second-degree polynomial), MS duration after debut and diagnosis, relapse in the year before treatment initiation, region, and follow-up time (odds ratio [OR] only) were examined through sequential regression models. Cumulative incidences were estimated to compare reasons for therapy discontinuation over time between drug categories and counties. Propensity scores were estimated for each treatment group in comparison with RTX and were separately adjusted for as stratified quintiles in the regression models. The same covariates as stated above were used, omitting follow-up time. Cases with incomplete sets of covariates were discarded from analysis.
Results
Study Population
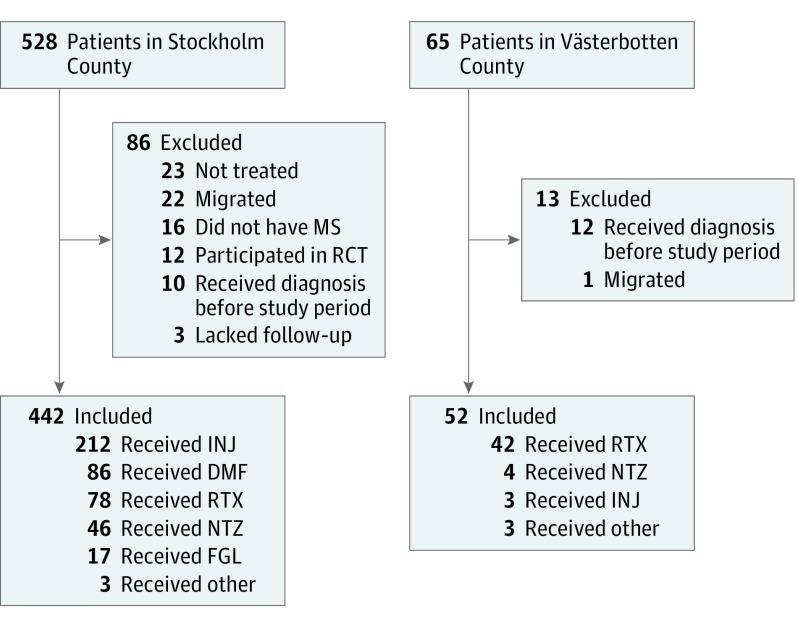
The final study cohort comprised 494 patients after exclusion of 99 patients not fulfilling inclusion criteria (Figure 1). The median (interquartile) age of included patients were 34.4 (27.4-43.4) years, and 158 (32.0%) were men. The annual incidence of RRMS was slightly higher in Västerbotten (eTable 1 in the Supplement).
Figure 1. Cohort Selection and Treatment Groups.
Study individuals were patients who received a diagnosis of relapsing-remitting MS between January 1, 2012, and October 30, 2015, who began disease-modifying treatment in 2 counties. Other treatments included 3 patients treated with teriflunomide in Stockholm County and 3 with alemtuzumab in Västerbotten County. DMF indicates dimethyl fumarate; FGL, fingolimod; INJ, interferon beta and glatiramer acetate; MS, multiple sclerosis; NTZ, natalizumab; RCT, randomized clinical trial; RTX, rituximab.
Baseline characteristics for all DMT groups are given in eTable 2 in the Supplement. Compared with injectable DMTs, RTX-treated patients had slightly higher baseline EDSS scores. In comparison with DMF and fingolimod, patients receiving RTXs were older but did not differ in EDSS scores. Compared with RTX-treated patients, natalizumab-treated patients were younger and had a shorter MS duration since debut, and a higher frequency had a relapse the year before treatment.
Patients in Stockholm compared with Västerbotten County were slightly older and had a longer delay in treatment initiation after MS onset (eTable 3 in the Supplement).
The median number of annual valid MRI scans per patient was lower in the RTX group compared with DMF and natalizumab but not injectable DMTs and fingolimod. The frequency of scanning did not differ between regions.
Disease-modifying treatment had in all but a few cases been administered according to clinical routine. Rituximab off-label had in all but a few cases (4 of 120 [3.3%]) been administered as a single intravenous infusion of 500 mg or 1000 mg every 6 months; however, in some cases, the first infusion had been repeated after 2 weeks.
Drug Discontinuation
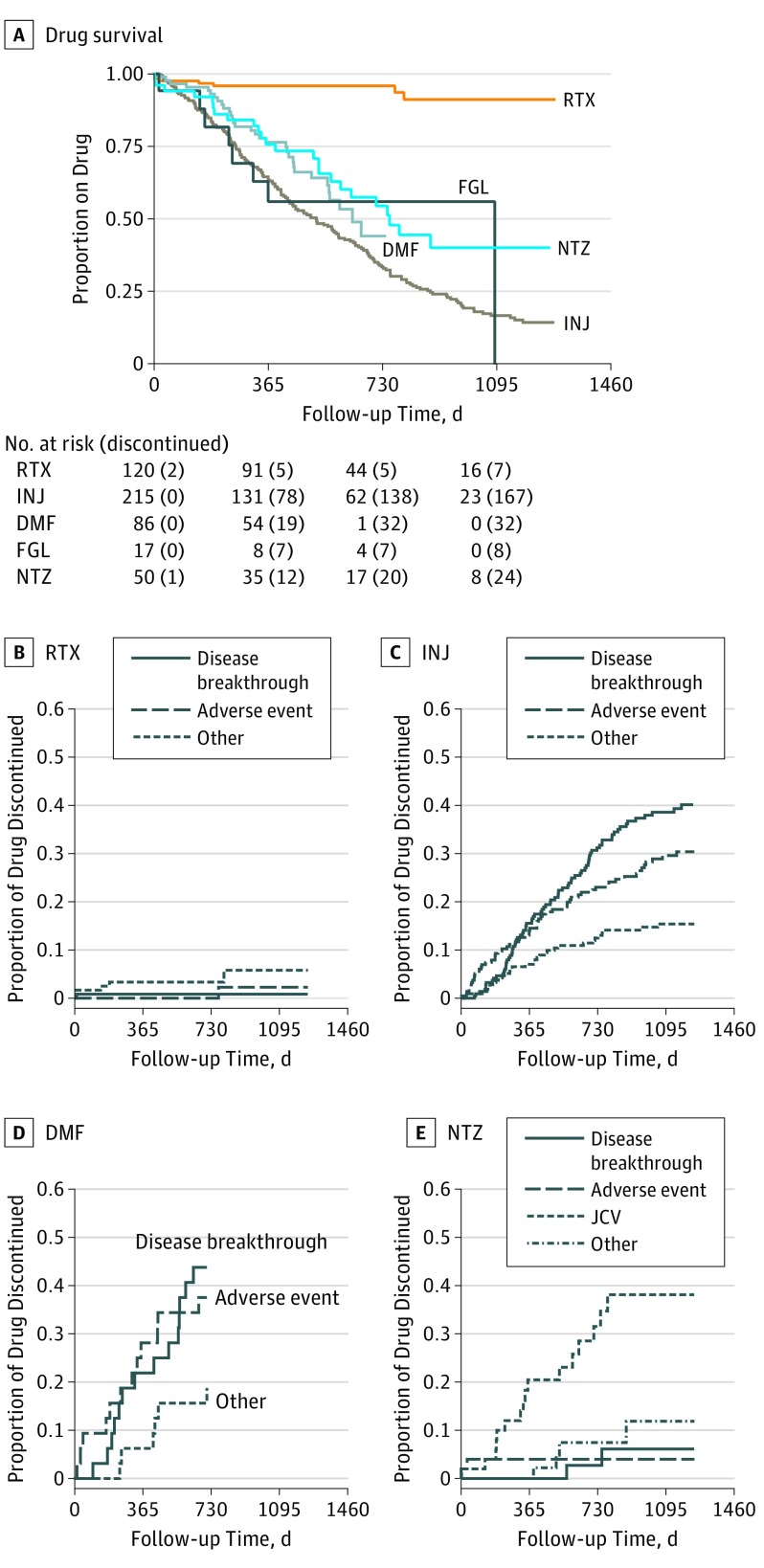
The proportion of patients remaining on therapy was significantly higher for RTX compared with injectable DMTs, DMF, fingolimod, and natalizumab (Figure 2 and Table 1). Cox proportional hazards model for drug discontinuation during the entire follow-up period yielded a higher hazard rate for drug discontinuation for all drug categories in comparison with RTX (Table 1), and it was significant for all groups after adjusting for covariates and propensity scores separately. The causes of therapy discontinuation differed between treatment groups. Notably, the most common cause of therapy discontinuation among RTX-treated patients was pregnancy (4 of 120 [3.3%]), and 1 of 120 (0.8%) discontinued treatment because of disease breakthrough (eTable 4 in the Supplement). Among patients treated with injectable DMTs, DMF, and fingolimod, disease breakthrough and adverse events were the most common causes of discontinuation (82 of 215 [38.1%] and 60 of 215 [27.9%], respectively, for injectable DMTs; 14 of 86 [16.3%] and 12 of 86 [14.0%], respectively, for DMF; and 4 of 17 [23.5%] and 3 of 17 [17.6%] for fingolimod, respectively). Natalizumab-treated patients discontinued treatment because of positive John Cunningham virus serology results in 16 of 50 cases (32%), followed by disease breakthrough, adverse events, and pregnancy at 4% each (2 of 50).
Figure 2. Drug Survival and Reasons for Therapy Discontinuation for Treatment Groups.
Kaplan-Meier curve for drug survival (A) and cumulative incidence reasons for therapy discontinuation for rituximab (RTX) (B), interferon beta and glatiramer acetate (INJ) (C), dimethyl fumarate (DMF) (D), and natalizumab (NTZ) (E). The most frequent reason to stop INJ and DMF was disease breakthrough; the most common reason to stop NTZ was John Cunningham virus (JCV) titers. Owing to the smaller size of the fingolimod (FGL) group (n = 17) reasons for therapy discontinuation are not presented.
Table 1. Distribution of Outcomes for Treatment Groups.
| Outcomes | Treatment Group | ||||
|---|---|---|---|---|---|
| RTX (n = 120) |
INJ (n = 215) |
DMF (n = 86) |
FGL (n = 17) |
NTZ (n = 50) |
|
| Drug discontinuation | |||||
| Patients who discontinued therapy, No. (%) | 7 (5.8) | 173 (80.5) | 32 (37.2) | 8 (47.1) | 24 (48.0) |
| Person-years | 206.9 | 326.9 | 99.6 | 21.0 | 83.6 |
| Annual drug discontinuation rate | 0.03 | 0.53 | 0.32 | 0.38 | 0.29 |
| HR, crude (95% CI) | NA | 16.0 (7.5-34.1) | 14.5 (5.1-41.4) | 10.3 (3.7-28.6) | 8.7 (3.7-20.2) |
| HR, adjusted (95% CI) | NA | 14.4 (4.9-42.3) | 12.4 (3.1-49.4) | 8.7 (2.0-38.0) | 13.9 (3.8-50.6) |
| HR, propensity score (95% CI) | NA | 11.4 (4.7-27.4) | 15.1 (3.9-58.0) | 5.9 (1.5-23.4) | 11.3 (3.2-39.4) |
| Clinical relapse | |||||
| Patients with clinical relapse, No. (%) | 6 (5.0) | 58 (27.0) | 10 (11.6) | 3 (17.6) | 10 (20.0) |
| Person-years | 183.9 | 275.6 | 78.8 | 19.1 | 73.7 |
| Annual rate of clinical relapses | 0.03 | 0.21 | 0.12 | 0.16 | 0.14 |
| HR, crude (95% CI) | NA | 7.1 (3.1-16.6) | 3.8 (1.3-11.2) | 5.3 (1.3-21.4) | 4.9 (1.8-13.4) |
| HR, adjusted (95% CI) | NA | 4.3 (1.6-11.2) | 3.2 (0.9-10.6) | 6.0 (0.8-44.1) | 5.1 (1.2-22.2) |
| HR, propensity score (95% CI) | NA | 7.0 (2.5-19.6) | 3.4 (1.0-11.8) | 3.8 (0.6-24.2) | 4.1 (1.0-17.2) |
| Gd+ lesions | |||||
| Patients with positive scan, No. (%) | 2 (1.7) | 27 (12.6) | 11 (12.8) | 1 (5.9) | 3 (6.0) |
| Patients with valid scan, No. (%)a | 104 (86.7) | 159 (74.0) | 73 (84.9) | 15 (88.2) | 43 (86.0) |
| Patients with positive scan/patients with valid scan | 0.02 | 0.17 | 0.15 | 0.07 | 0.07 |
| OR, crude (95% CI) | NA | 10.5 (3.0-65.8) | 9.1 (2.3-59.7) | 3.7 (0.2-40.9) | 3.8 (0.6-30.2) |
| OR, adjusted (95% CI) | NA | 9.3 (2.0-87.0) | 8.8 (1.5-168.2) | 2.7 (0.1-116.3) | 6.6 (0.1-94.7) |
| OR, propensity score (95% CI) | NA | 10.1 (2.3-73.0) | 8.4 (1.7-72.1) | 3.0 (0.1-85.0) | 8.5 (0.9-109.1) |
| AE | |||||
| Patients with AE, No. (%) | 28 (23.3) | 93 (43.3) | 43 (50.0) | 5 (29.4) | 19 (38.0) |
| Person-years | 173.0 | 218.0 | 55.6 | 19.3 | 57.3 |
| Incidence of AE/y | 0.16 | 0.43 | 0.77 | 0.26 | 0.33 |
| First-dosing AEs | |||||
| Patients with first-dosing AE, No. (%) | 25 (20.8) | 91 (42.3) | 52 (60.5) | 3 (17.6) | 2 (4.0) |
Abbreviations: AE, adverse event; DMF, dimethyl fumarate; FGL, fingolimod; Gd+, gadolinium enhancing magnetic resonance imaging scan; HR, Hazard Ratio; INJ, interferon beta and glatiramer acetate; NA, not applicable; NTZ, natalizumab; OR, odds ratio; RTX, rituximab.
A magnetic resonance imaging scan done at least 3 months after treatment started and before treatment ended.
Relapses
Relapse rates were significantly lower in RTX-treated patients compared with injectable DMTs and natalizumab in the Cox proportional hazards model (95% CI, 1.6-11.2; P < .01 and 95% CI, 1.2-22.2; P < .05, respectively) (Table 1). However, when adjusting for propensity score, the difference between RTX and natalizumab was no longer significant (95% CI, 1.0-17.2; P = .05) (Table 1). Comparing RTX with DMF and fingolimod did not result in significant differences after adjusting for confounders (95% CI, 1.0-11.8; P = .06 and 95% CI, 0.6-24.2; P = .15, respectively) (eTable 5 in the Supplement).
Contrast Enhancing MRI Lesions
Compared with RTX, the odd ratio for Gd+ lesions was higher with injectable DMTs and DMF (95% CI, 2.0-87.0; P = .02 and 95% CI, 1.5-168.2; P = .05, respectively) but not with fingolimod and natalizumab (95% CI, 0.1-116.3; P = .57 and 95% CI, 0.1-94.7; P = .12, respectively) (Table 1). The statistical significance for all comparisons remained unchanged after adjusting only for propensity scores (injectable DMTs: 95% CI, 2.3-73.0; P < .01; DMF: 95% CI, 1.7-72.1; P = .05; fingolimod: 95% CI, 0.1-85.0; P = .46; natalizumab: 95% CI, 0.9-109.1; P = .07, respectively).
Adverse Events
Milder adverse events (Common Terminology Criteria for Adverse Events grade 1 and 2) were more frequent with injectable DMTs compared with RTX, whereas moderate to severe adverse events were similarly low (eTable 6 in the Supplement). Likewise, grade 1 treatment–associated adverse events were more common with DMF, with no observed difference for severe grades (eTable 6 in the Supplement). Adverse events did not differ significantly between RTX, fingolimod, and natalizumab (eTable 2 in the Supplement). However, 1 patient treated with natalizumab developed a grade 4 sepsis (gram-negative Fusobacteriae in blood cultures) but made a full recovery after treatment with intravenous antibiotics.
Comparison Between Regions
The conspicuous difference in treatment strategy between the 2 regions provided a possibility to study outcomes irrespective of DMT choice. Patients in Västerbotten were younger and had a higher EDSS score than those in Stockholm, suggesting a more active disease course, and received treatment earlier from suspected onset of MS (eTable 3 in the Supplement). These differences were controlled for in subsequent analyses.
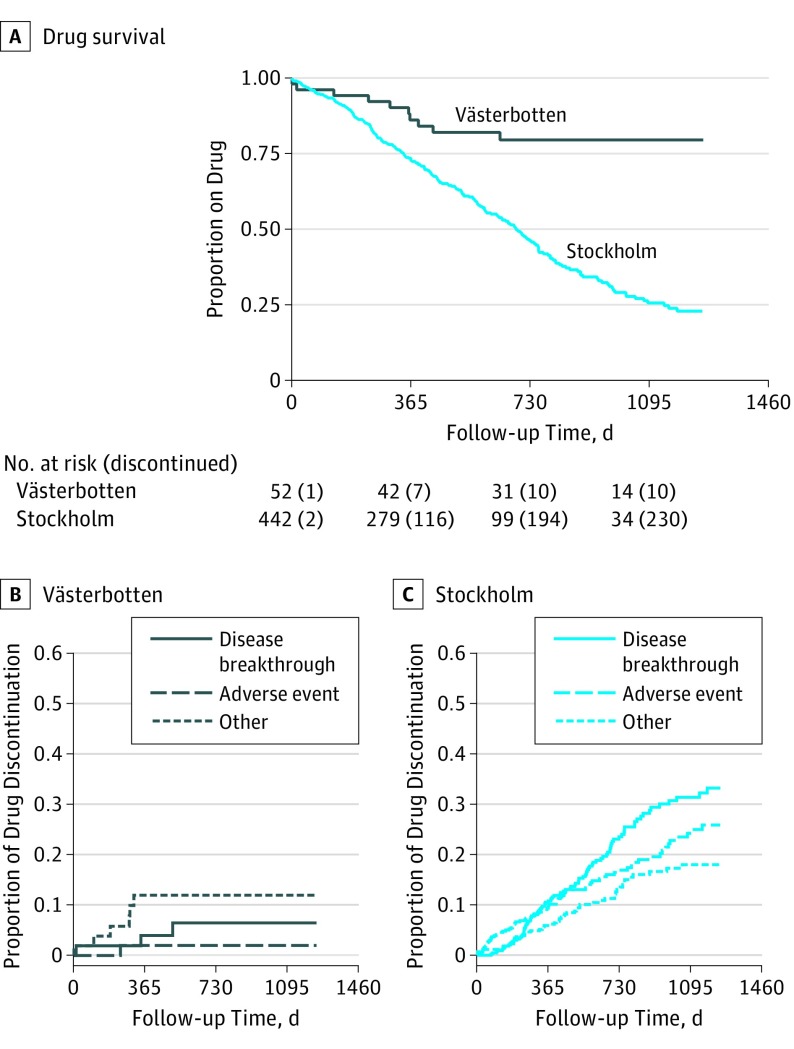
Overall drug survival was higher in Västerbotten compared with Stockholm (95% CI, 2.6-10.9; P < .001) (Figure 3 and Table 2). Relapses were less frequent in Västerbotten, with no recorded relapses over the first year and 3 during the second year (7.7%; 1 patient treated with RTX) compared with 51 (13.9%) and 55 (25.5%), respectively, in Stockholm (95% CI, 2.3-123.1; P < .01) (Table 2). In contrast, there was no significant difference between counties regarding Gd+ lesions (95% CI, 0.8-10.5; P = .19) (Table 2). There were fewer adverse events of all grades in Västerbotten compared with Stockholm (eTable 6 in the Supplement).
Figure 3. Drug Survival and Reasons for Therapy Discontinuation for Stockholm and Västerbotten Counties.
Plots depicting the different outcomes for the cohorts in Stockholm and Västerbotten Counties: (A) Kaplan-Meier curve for drug survival, cumulative incidence curves of reasons for therapy discontinuation in Västerbotten (B) and Stockholm Counties (C).
Table 2. Outcomes for Patients Treated in Västerbotten and Stockholm Counties.
| Results | Region | |
|---|---|---|
| Västerbotten (n = 52) |
Stockholm (n = 442) |
|
| Drug discontinuation | ||
| Patients who discontinued therapy, No. (%) | 10 (19.2) | 236 (53.4) |
| Person-years | 115.0 | 636.2 |
| Annual drug discontinuation rate | 0.087 | 0.37 |
| HR, crude (95% CI) | NA | 4.6 (2.4-8.7) |
| HR, adjusted (95% CI) | NA | 5.3 (2.6-10.9) |
| Clinical relapse | ||
| Patients with clinical relapse, No. (%) | 3 (5.8) | 84 (19.0) |
| Person-years | 103.2 | 534.7 |
| Annual rate of clinical relapses | 0.03 | 0.16 |
| HR, crude (95% CI) | NA | 5.5 (1.7-17.4) |
| HR, adjusted (95% CI) | NA | 16.9 (2.3-123.1) |
| Gd+ lesions | ||
| Patients with positive scan, No. (%) | 3 (5.8) | 44 (10.0) |
| Patients with valid scan, No. (%)a | 50 (96.2) | 350 (79.2) |
| Patients with positive scan/patients with valid scan | 0.06 | 0.13 |
| OR, crude (95% CI) | NA | 2.2 (0.8-9.5) |
| OR, adjusted (95% CI) | NA | 2.4 (0.8-10.5) |
| AE | ||
| Patients with AE, No. (%) | 2 (3.8) | 188 (42.5) |
| Person-years | 111.2 | 420.7 |
| Incidence of AE per year | 0.02 | 0.45 |
| First-dosing AEs | ||
| Patients with first-dosing AE | 6 | 169 |
| First-dosing AEs/patient | 0.12 | 0.39 |
Abbreviations: AE, adverse event; Gd+, gadolinium enhancing magnetic resonance imaging scan; NA, not applicable; HR, hazard ratio; NA, not applicable; OR, odds ratio.
A magnetic resonance imaging scan done at least 3 months after treatment start and before treatment ended.
Outcomes for all treatment groups with patients only from Stockholm County were similar to those of Västerbotten and Stockholm Counties combined (eTable 7 in the Supplement).
Discussion
We compared outcomes for patients with newly diagnosed RRMS receiving their first DMT in 2 population-based samples and found that RTX was associated with superior drug survival compared with all more frequently used MS-approved DMT included in the analysis. Patients treated with RTX also had lower rate of clinical relapses and/or neuroradiologic disease activity and occurrence of adverse events compared with patients treated with injectable DMTs and DMF. Compared with fingolimod and natalizumab, relapse rates and Gd+ lesions were numerically lower but did not reach statistical significance in all analyses.
The retrospective observational design of the study is inherently sensitive to confounding by indication (ie, all factors affecting choice of therapy cannot be accounted for). On the other hand, observational studies can provide important information on comparative drug performance in a real-world context in which randomized clinical trials are lacking. Although our main analysis is likely to at least partly have residual confounding by indication, we also took advantage of the major differences in DMT strategies between the 2 studied regions. In Stockholm, a traditional treatment algorithm was used, while in Västerbotten, almost 95% of patients were initiated on highly effective DMTs. Notably, there is no difference in reimbursement policy across Sweden because all DMTs are covered by the national health insurance, including off-label medications. Our results demonstrate that a traditional treatment approach, including novel oral treatment options, is associated with a relatively poor medium term performance. In most cases, the reason to switch was breakthrough disease activity manifested as clinical relapses and/or active inflammation on MRI, with the second most common reason being side effects. John Cunningham virus serology results was the most frequent cause to interrupt natalizumab treatment.
Our findings regarding drug survival of traditional initial treatment choices are in line with previous data obtained in other real-world populations demonstrating that up to two thirds of patients terminate their first-line DMT within 2 years. The introduction of novel oral DMT has fueled hopes of better outcomes in this regard. In Stockholm, a drastic shift from injectable DMTs to DMF occurred in May 2014 when DMF became reimbursed in Sweden. Baseline characteristics for patients treated with injectable DMTs and DMF were also similar, except for a younger age in the DMF group. Drug survival of DMF was statistically better than injectable therapies, but differences in real terms were moderate (a 1-year drug survival of 63.9% and 79.4% for injectable DMTs and DMF, respectively). In both cases, lack of effect was the main reason for switching therapy, with nonserious adverse events being the second most common, reflecting their inferior effectiveness and tolerability compared with highly effective DMT.
Patients starting RTX were older and had a longer delay between diagnosis and start of therapy than those starting natalizumab. However, after correcting for baseline differences, RTX still displayed a more favorable outcome regarding drug survival rates compared with natalizumab. Rituximab also displayed a significantly lower frequency of relapses compared with natalizumab in the regression model (95% CI, 1.2-22.2; P = .03) but lost significance when adjusted for propensity score (95% CI, 1.0-17.2; P = .05). Rituximab displayed a higher rate of drug survival compared with fingolimod, but the power to detect differences in other outcome measures was low because of the small number of patients in the fingolimod group. However, we have previously shown a lower risk of rebound disease activity in patients switching from natalizumab to RTX owing to John Cunningham virus test results, compared with those switching to fingolimod.
The argument for starting with less effective DMT concerns mainly 2 aspects: safety and price. Indeed, newer and more effective DMTs have been associated with risks of serious adverse events, such as progressive multifocal leukoencephalopathy, and usually command a substantially higher price than platform therapies. Documentation of the safety profile of natalizumab and fingolimod in patients with MS is more extensive than for RTX, including long-term extensions of large clinical trials and observational postmarketing studies. Although there is extensive experience of long-term use of RTX in patients with rheumatoid arthritis with a good tolerability profile and a low risk of malignancies and progressive multifocal leukoencephalopathy, extrapolation to MS should be done with caution owing to differences in patient populations and dose and administration regimens. Hence, it is undisputed that the safety record of first-line injectable DMTs in use since more than 2 decades is superior to that of newer DMT, which is of high relevance in particular for the risk-benefit assessment of long-term treatment in patients with mild to moderate disease. Regarding price, RTX used at a single dose of 500 mg or 1000 mg twice yearly results in a lower cost than even platform therapies. The status of being an off-label drug, and therefore subject to variable insurance regulations in countries other than Sweden, remains as a barrier for the use of RTX in patients with MS.
Limitations
Limitations of the present study include its nonrandomized design and smaller group sizes for some of the treatment groups. The initial therapy choice, as well as the threshold for therapy switching, also depends on physicians’ opinions. We cannot exclude the existence of additional factors influencing therapy choice between the 2 studied regions. Regarding switching for efficacy reasons, our data support that such switches have been prompted by objective evidence of relapses and/or signs of breakthrough inflammation on MRI. However, switching owing to safety or tolerability issues cannot be demonstrated in objective terms, and there may also be geographical imbalances in the recording of adverse events given the lack of formal study visits and prospective adverse event logging, likely mostly having an effect on milder adverse events. Furthermore, it would have been preferable with more detailed data on the history of other diseases and other patient characteristics that may influence the choice of using RTX rather than a conventional therapy, including patients’ treatment satisfaction. Guidelines for follow-up differ to some degree between different DMTs. Thus, until 2014, 2 yearly visits were recommended for RTX, natalizumab, and fingolimod, while 1 visit per year was recommended for injectable DMTs, which may have affected the sensitivity to detect adverse outcomes in the latter group. We note that this would rather have led to a bias against highly effective therapies, including RTX. Since 2015, guidelines regarding follow-up have been identical across all therapies. Expanding Disability Status Scale ratings were included in the analysis, but ratings are likely to be less reliable when performed in clinical routine, and not all raters had formal EDSS rating qualifications. The relatively limited follow-up time diminished the possibility to analyze long-term disability outcomes, and the lack of volumetric MRI data prevented the detection of differences in atrophy rate, an objective parameter associated with long-term prognosis.
Conclusions
This observational study of patients with newly diagnosed RRMS who are new to treatment demonstrates that patients receiving RTX displayed a significantly better drug survival compared with all DMT included in the analysis and a lower risk of switching because of disease breakthrough compared with platform DMT. Furthermore, DMF was found to be only moderately better than injectable therapies. Further studies are needed to shed light on long-term comparative safety and effectiveness outcomes of the studied DMT, especially given the excellent safety records of first-generation injectable DMTs.
eTable 1. Estimated RRMS Incidence in Västerbotten and Stockholm Counties
eTable 2. Patient Baseline Characteristics
eTable 3. Baseline Characteristics for Västerbotten and Stockholm Counties
eTable 4. Reasons for Drug Discontinuation
eTable 5. Outcomes Comparisons Adjusted Through Sequential Addition of Baseline Factors
eTable 6. Adverse Events Separated After Treatment Group and County
References
- 1.Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. . Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(suppl 1):S5-S48. [DOI] [PubMed] [Google Scholar]
- 2.Piehl F. A changing treatment landscape for multiple sclerosis: challenges and opportunities. J Intern Med. 2014;275(4):364-381. [DOI] [PubMed] [Google Scholar]
- 3.Wong J, Gomes T, Mamdani M, Manno M, O’Connor PW. Adherence to multiple sclerosis disease-modifying therapies in Ontario is low. Can J Neurol Sci. 2011;38(3):429-433. [DOI] [PubMed] [Google Scholar]
- 4.Hansen K, Schüssel K, Kieble M, et al. . Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort study. PLoS One. 2015;10(7):e0133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisell T, Forsberg L, Nordin N, et al. . Comparative analysis of first-year fingolimod and natalizumab drug discontinuation among Swedish patients with multiple sclerosis. Mult Scler. 2016;22(1):85-93. [DOI] [PubMed] [Google Scholar]
- 6.Hersh CM, Love TE, Cohn S, et al. . Comparative efficacy and discontinuation of dimethyl fumarate and fingolimod in clinical practice at 12-month follow-up. Mult Scler Relat Disord. 2016;10:44-52. [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL. The Charcot Lecture—beating MS: a story of B cells, with twists and turns. Mult Scler. 2015;21(1):8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauser SL, Bar-Or A, Comi G, et al. ; OPERA I and OPERA II Clinical Investigators . Ocrelizumab vs interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. [DOI] [PubMed] [Google Scholar]
- 9.Montalban X, Hauser SL, Kappos L, et al. ; ORATORIO Clinical Investigators . Ocrelizumab vs placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220. [DOI] [PubMed] [Google Scholar]
- 10.Salzer J, Svenningsson R, Alping P, et al. . Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology. 2016;87(20):2074-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 data file. 2015. https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed November 6, 2017.
- 13.Alping P, Frisell T, Novakova L, et al. . Rituximab vs fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950-958. [DOI] [PubMed] [Google Scholar]
- 14.Trojano M, Tintore M, Montalban X, et al. . Treatment decisions in multiple sclerosis: insights from real-world observational studies. Nat Rev Neurol. 2017;13(2):105-118. [DOI] [PubMed] [Google Scholar]
- 15.Kalincik T, Butzkueven H. Observational data: understanding the real MS world. Mult Scler. 2016;22(13):1642-1648. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor P, Goodman A, Kappos L, et al. . Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology. 2014;83(1):78-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butzkueven H, Kappos L, Pellegrini F, et al. ; TYSABRI Observational Program (TOP) Investigators . Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry. 2014;85(11):1190-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappos L, O’Connor P, Radue EW, et al. . Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015;84(15):1582-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies. Arthritis Rheum. 2012;64(9):3043-3051. [DOI] [PubMed] [Google Scholar]
- 20.Clifford DB, Ances B, Costello C, et al. . Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68(9):1156-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Vollenhoven RF, Fleischmann RM, Furst DE, Lacey S, Lehane PB. Longterm safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. J Rheumatol. 2015;42(10):1761-1766. [DOI] [PubMed] [Google Scholar]
- 22.Wadström H, Frisell T, Askling J; Anti-Rheumatic Therapy in Sweden (ARTIS) Study Group . Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med. 2017;177(11):1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Flon P, Laurell K, Söderström L, Gunnarsson M, Svenningsson A. Improved treatment satisfaction after switching therapy to rituximab in relapsing-remitting MS. Mult Scler. 2017;23(9):1249-1257. [DOI] [PubMed] [Google Scholar]
- 24.Radue EW, Barkhof F, Kappos L, et al. . Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology. 2015;84(8):784-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. 2014;75(1):43-49. [DOI] [PubMed] [Google Scholar]
- 26.Schlaeger R, Papinutto N, Panara V, et al. . Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Ann Neurol. 2014;76(4):568-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jong HJI, Kingwell E, Shirani A, et al. ; British Columbia Multiple Sclerosis Clinic Neurologists . Evaluating the safety of β-interferons in MS: a series of nested case-control studies. Neurology. 2017;88(24):2310-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford C, Goodman AD, Johnson K, et al. . Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler. 2010;16(3):342-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Estimated RRMS Incidence in Västerbotten and Stockholm Counties
eTable 2. Patient Baseline Characteristics
eTable 3. Baseline Characteristics for Västerbotten and Stockholm Counties
eTable 4. Reasons for Drug Discontinuation
eTable 5. Outcomes Comparisons Adjusted Through Sequential Addition of Baseline Factors
eTable 6. Adverse Events Separated After Treatment Group and County