Key Points
Question
Has the overall prognosis of epilepsy improved after the introduction of more than a dozen new antiepileptic drugs in the past 2 decades?
Findings
This longitudinal cohort study followed up a succession of newly diagnosed patients with epilepsy presenting at a single health care center over 30 years. The seizure-free rate observed was virtually unchanged over the study period, and the probability of achieving seizure freedom declined for each unsuccessful antiepileptic drug regimen prescribed.
Meaning
Despite availability of many new antiepileptic drugs with differing mechanisms of action, outcomes in newly diagnosed epilepsy have not improved; developing new disease-modifying compounds to current add to seizure-suppressing drug treatments may be necessary.
This longitudinal cohort study assessed treatment outcomes in patients with newly diagnosed epilepsy and calculated the probability of achieving seizure freedom with first, second, and successive drug regimens prescribed.
Abstract
Importance
A study published in 2000 showed that more than one-third of adults with epilepsy have inadequate control of seizures with antiepileptic drugs (AEDs). This study evaluates overall treatment outcomes in light of the introduction of more than 1 dozen new AEDs in the past 2 decades.
Objective
To assess long-term treatment outcome in patients with newly diagnosed and treated epilepsy.
Design, Setting, and Participants
This longitudinal observational cohort study was conducted at the Epilepsy Unit of the Western Infirmary in Glasgow, Scotland. A total of 1795 individuals who were newly treated for epilepsy with AEDs between July 1, 1982, and October 31, 2012, were included in this analysis. All patients were followed up for a minimum of 2 years (until October 31, 2014) or until death, whichever came sooner. Data analysis was completed between March 2015 and May 2016.
Exposures
Treatment with antiepileptic drugs for patients newly diagnosed with epilepsy.
Main Outcomes and Measures
Seizure control was assessed at the end of the study period. Probability of achieving 1-year seizure freedom was estimated for each AED regimen prescribed. Multivariable models assessed the associations between risk factors and AED treatment outcome after adjustments were made for demographic and clinical characteristics.
Results
Of the 1795 included patients, 964 (53.7%) were male; the median age was 33 years (range, 9-93 years). At the end of the study period, 1144 patients (63.7%) had been seizure free for the previous year or longer. Among those achieving 1-year seizure freedom, 993 (86.8%) were taking monotherapy and 1028 (89.9%) had achieved seizure control with the first or second AED regimens. Of the total patient pool, 906 (50.5%) remained seizure free for 1 year or longer with the initial AED. If this AED failed, the second and third regimens provided an additional 11.6% and 4.4% likelihoods of seizure freedom, respectively. Only 2.12% of patients attained optimal seizure control with subsequent AEDs. Epilepsy that was not successfully controlled with the first AED had 1.73 times greater odds of not responding to treatment for each subsequent medication regimen (odds ratio, 1.73; 95% CI, 1.56-1.91; P < .001).
Conclusions and Relevance
Despite the availability of many new AEDs with differing mechanisms of action, overall outcomes in newly diagnosed epilepsy have not improved. Most patients who attain control do so with the first or second AED. The probability of achieving seizure freedom diminishes substantially with each subsequent AED regimen tried. More than one-third of patients experience epilepsy that remains uncontrolled.
Introduction
Epilepsy is 1 of the most common serious chronic neurological disorders, estimated to affect approximately 68 million people worldwide.1 Antiepileptic drugs (AEDs) are the mainstay of treatment and suppress seizure occurrence without rectifying the underlying neuropathological process. As a result, people with epilepsy often require lifelong AED treatment. (Additional therapeutic options include resective surgery, brain stimulation, and dietary therapy.2) To formulate a rational management plan for these patients, it is crucial to understand the long-term clinical courses and patterns of responses to AEDs.
In an initial study,3 we followed up 470 patients with newly diagnosed epilepsy treated in the Epilepsy Unit at the Western Infirmary in Glasgow, Scotland, from 1982 to 1998. We found that more than one-third of patients continued to have seizures despite AED treatment, and those who had an inadequate response to the first or second AED regimen were likely to develop refractory epilepsy.3 Since then, the introduction of many AEDs with different mechanisms of action has greatly expanded the range of treatment options.4 Newer AEDs are generally effective, and many have favorable safety profiles,5,6 but all have been reported to have efficacy similar to the established AEDs when used as monotherapy or adjunctive treatment.7,8 Whether their availability has improved the overall prognosis of epilepsy therefore remains controversial.9
To examine whether the overall treatment outcomes have changed with the advent of these additional drugs, we analyzed an expanded cohort of 1795 newly diagnosed patients followed up from 1982 to 2014 at the Epilepsy Unit at the Western Infirmary.
Methods
Patients
Our study included consecutive individuals in whom epilepsy was diagnosed and a first AED prescribed at the Epilepsy Unit of the Western Infirmary in Glasgow, Scotland, between July 1, 1982, and October 31, 2012. All individuals were prospectively followed up until October 31, 2014, or until their deaths. The study population included the patients described in previous studies (which had cohort sizes of 470,3 890,10 and 1098 persons,11 respectively). Most of the patients (90%) were referred by their primary care physicians, with the remainder coming from the accident and emergency department of the hospital (which is the equivalent in Scotland of an emergency department in the United States).
This study protocol was ruled exempt by the institutional review board of Western Infirmary, Glasgow. The informed consent requirement was waived because all data were deidentified prior to analysis for clinical audit and quality assurance purposes.
Data were collected in the course of standard clinic care. As described in our initial report,3 general physical and neurologic examinations were performed at the first clinical visit; in addition, clinicians used a predesigned questionnaire developed from standard clinical practice to collect demographic and clinical information from patients and accounts from those who witnessed the seizures (if any).3 Scalp electroencephalography was performed to investigate interictal changes that might support the diagnosis and classification of the epilepsy. Brain computed tomographic scans and/or magnetic resonance imaging were undertaken to screen for underlying structural abnormalities that might have contributed to the generation of seizures.3 We excluded from the analysis all patients who had exhibited persistent poor treatment adherence unrelated to drug efficacy or tolerability, those who were thought to have seizures only because of alcohol intake or recreational drug use, and those presenting with nonepileptic seizures.
Treatment
After diagnosing a patient with epilepsy, clinicians considered seizure type, adverse drug effects, and interaction profiles in selecting an appropriate AED.12 In general, an AED was prescribed after 2 or more seizures.11 Infrequently, patients who experienced a single seizure were offered treatment if there was clear evidence of an epileptogenic abnormality in the brain that portended recurrent seizures.13 A staged approach for epilepsy management was subsequently adopted. Generally, if the initial AED induced intolerable adverse effects at a low daily dose, or if it failed to improve seizure control, an alternative was substituted. If the first well-tolerated AED significantly improved seizure numbers, but failed to provide complete control, combination therapy was considered.14 A combination of AEDs could be used if the epilepsy remained uncontrolled despite treatment with 1 or more monotherapy trials.15 Patients were treated with a single AED whenever possible, and drug doses were adjusted as clinical indications dictated.
Follow-up and Ascertainment of Outcomes
For the first 6 months after commencing treatment, patients were seen at the epilepsy clinic every 2 to 6 weeks. Patients attended follow-up visits at least every 4 months thereafter. At each follow-up visit, clinical information and response to AED therapy were recorded. Patients were provided with customized charts and asked to record seizure descriptions and the number of seizures that occurred between clinic visits. The patients, their relatives, and their primary care physicians could contact the Epilepsy Unit directly via a dedicated telephone line if problems arose.
Definitions
Epilepsy was diagnosed and seizures and syndromes classified according to the guidelines of the International League Against Epilepsy (ILAE).13,16 Epilepsy was broadly classified as generalized or focal according to the putative cause and depending on factors such as age, seizure type, family history, interictal electroencephalographic changes, and the presence or absence of a potential epileptogenic lesion or injury visible on brain imaging. Generalized epilepsies, such as juvenile absence epilepsy and juvenile myoclonic epilepsy, were presumed to be of genetic origin. On the basis of clinical information and results of investigations, focal epilepsy was regarded as resulting from either demonstrated epileptogenic lesions or underlying but unidentified focal abnormalities. Because of the duration of the study, some analyses were made using the previous classification of idiopathic, symptomatic, and cryptogenic epilepsies.17
Seizure freedom was defined as a patient experiencing no seizures for the previous 12 months or longer. Time to seizure freedom was expressed as the time from treatment initiation to the day that the seizure freedom definition was met (ie, the first day after 1 full year without seizures had elapsed). Patients who had experienced seizure-free periods of longer than 1 year were not counted as seizure free if a relapse had occurred before the end of the study. Treatment outcomes for patients who died or were lost to follow-up were assessed as of the last clinic visit.
An AED regimen was defined as either a single drug (monotherapy) or a combination of 2 or more drugs. The first AED regimen was always monotherapy, while all subsequent regimens could be an alternative monotherapy or a dual therapy combining the first AED with a second medication. For comparability reasons, potential risk factors associated with AED therapy outcomes were assessed using the traditional 1-year seizure-free period.3,10,11
In 2010, an ILAE task force proposed a new “rule of three” definition for seizure freedom. This defines seizure freedom as the absence of seizures for at least the previous year or for 3 times the longest pretreatment interval between seizures, whichever was greater18 (eAppendix and eReferences in the Supplement).
Statistical Analysis
The Pearson χ2 test was performed to compare categorical data, and the Mann-Whitney test was used for comparisons of nonparametric continuous data. Logistic regression was completed to assess the association between the number of AED regimens prescribed and the outcomes of treatment. Survival analysis was performed to assess differences in the time required for the use of each AED regimen to result in seizure freedom. Cox regression was completed to estimate the time for the patients to become seizure free while taking each AED regimen. The Kaplan-Meier survivor function was used to estimate the seizure control benefit provided by each successive AED regimen.
To explore potential changes in patient characteristics, choice of AEDs, and treatment outcomes across the 30-year study period, the study cohort was divided into 3 subgroups based on the year that they started initial AED treatment (ie, patients who started the first AED between 1982 and 1991, those who started between 1992 and 2001, and those who started between 2002 and 2012). Terminal seizure control for these subgroups was defined as seizure freedom at a specified end point of follow-up of that subgroup, which was defined to be a minimum of 2-years after the end of the decade (ie, at the end of 1993, 2003, and 2014, respectively). Logistic regression was undertaken to assess the association between terminal seizure outcome and potential risk factors. Covariates with P < .20 were selected for a full multivariable model. Covariates with P < .05 in the full multivariable model were then included in the final fitted model.
All statistical tests were performed by using Stata, version 14 (StataCorp). Data analysis was completed between March 2015 and May 2016.
Results
A total of 2282 individuals were newly treated with AEDs during the study period. Of these, 487 were excluded from analysis (of whom 287 had exhibited poor drug adherence; 122 had seizures as a result of alcohol or recreational drug use; and 78 had been diagnosed with nonepileptic seizures), and 1795 were included. Of the included patients, 969 (53.7%) were male (Table 1). The median age at referral (defined at the first time the patient was seen at the clinic where data was collected) was 33 years (range, 9-93 years; interquartile range [IQR], 21-50 years).
Table 1. Clinical Characteristics of Patients With Epilepsy Stratified by 1-Year Seizure Freedom.
| Characteristics | No. (%) | |
|---|---|---|
| Seizure Free (n = 1144) |
Uncontrolled (n = 651) |
|
| Sex | ||
| Male | 634 (55) | 335 (51) |
| Female | 510 (45) | 316 (49) |
| Age at onset, y | ||
| Median (IQR) | 32 (19-53) | 29 (19-43) |
| Range | 1-92 | 1-88 |
| Age at referral, y | ||
| Median (IQR) | 32 (20-54) | 34 (22-47) |
| Range | 9-93 | 11-88 |
| First-degree family history of epilepsy | ||
| Yes | 132 (12) | 117 (18) |
| No | 1012 (88) | 534 (82) |
| History of febrile convulsions | ||
| Yes | 58 (5.1) | 31 (4.7) |
| No | 1086 (95) | 620 (95) |
| Type of epilepsy | ||
| Idiopathic | 255 (22) | 131 (20) |
| Symptomatic | 307 (27) | 160 (25) |
| Cryptogenic | 582 (51) | 360 (55) |
| Seizures at baseline | ||
| ≤20 | 946 (83) | 473 (73) |
| >20 | 198 (17) | 178 (27) |
Abbreviation: IQR, interquartile range.
All but 22 of the patients (98.8%) had experienced 2 or more seizures prior to commencing AED treatment. The 22 patients treated after a single seizure (1.2%) had epileptogenic brain abnormalities or evidence of brain injury. Epilepsy was classified as generalized in 386 patients (21.5%) and focal in 1409 patients (78.5%).
The median duration of follow-up was 11 years (IQR, 7-16 years). There were no clinically significant changes in patient characteristics over the study period, apart from slightly increased median age in the overall cohort (eTable 1 in the Supplement).
Treatment Outcomes
At the last follow-up, 1440 patients (80.2%) took AED monotherapy and 355 (19.8%) were receiving combinations of 2 or more AEDs. A total of 1144 patients (63.7%) had been seizure free for the last 12 months or longer, and 993 patients (55.3%) had achieved this by taking a single AED; the remainder had controlled their seizures by taking 2 or more drugs. A total of 820 patients (45.7%) achieved at least 1 year of seizure freedom by taking their first AED, and 208 patients (28.0%) had achieved it while taking the second regimen (Table 2). When combined, the first and second regimens accounted for 1028 of the 1144 patients (89.9%) who achieved at least 1 year of seizure freedom. A higher proportion of patients with generalized epilepsy (n = 263/386; 68.1%) were seizure free at the last follow-up than those with focal epilepsy (n = 881/1409; 62.5%) (P = .04). Analysis of treatment outcomes in accordance with the recent ILAE definition yielded similar findings (eTable 2 in the Supplement).18
Table 2. Rates of 1-Year Seizure Freedom With Successive Antiepileptic Drug Regimens.
| Successive Antiepilepsy Drug Regimens | Total Patients Trying These Regimens, No. | Seizure Freedom | |||
|---|---|---|---|---|---|
| Total, No. | % of Patients Achieving Seizure Freedom With AED Regimen | % of the Total Achieving Seizure Freedom (n = 1144) | % of the Total Study Cohort (n = 1795) | ||
| First | 1795 | 820 | 45.7 | 71.7 | 45.7 |
| Second | 742 | 208 | 28.0 | 18.2 | 11.6 |
| Third | 330 | 78 | 23.6 | 6.82 | 4.35 |
| Fourth | 140 | 21 | 15.0 | 1.84 | 1.17 |
| Fifth | 71 | 10 | 14.1 | 0.87 | 0.56 |
| Sixth | 43 | 6 | 14.0 | 0.52 | 0.33 |
| Seventh | 15 | 1 | 6.67 | 0.09 | 0.06 |
| Eighth | 9 | 0 | 0 | 0 | 0 |
| Ninth | 5 | 0 | 0 | 0 | 0 |
| Tenth | 2 | 0 | 0 | 0 | 0 |
| Eleventh | 1 | 0 | 0 | 0 | 0 |
| Total | 1795 | 1144 | NA | 100.04a | 63.7 |
Abbreviations: AED, antiepileptic drug; NA, not applicable.
Total percentage totals to slightly more than 100% owing to rounding.
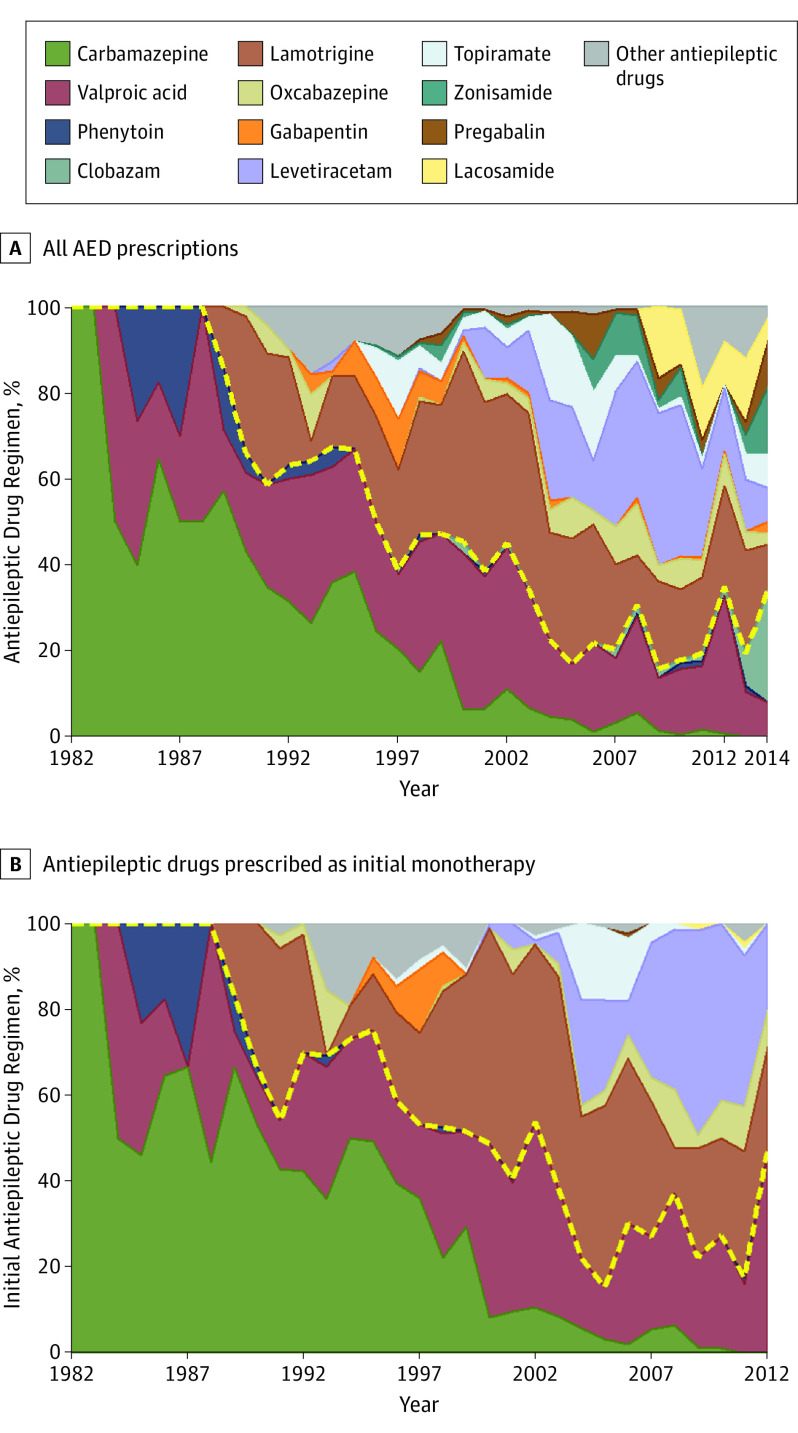
There was marked increase in the use of the newer AEDs over the study period (Figure 1A), both as initial monotherapy (Figure 1B) and in the subsequent treatment regimens (eFigure 1 in the Supplement). The proportion of patients who were seizure free at the last follow-up was similar in the 3 time period subgroups (61%–64%), with or without adjusting for patient characteristics (eTable 1 in the Supplement), as was the cumulative probability of 1 year of seizure freedom (eFigure 2 in the Supplement).
Figure 1. Antiepileptic Drug Regimens Over the Study Period.
A, All antiepileptic drug (AED) regimens. B, All AEDs prescribed as a first monotherapy. The colored areas represent each antiepileptic drug as a proportion of all the antiepileptic drugs given to the full study cohort (n = 1795) in the corresponding years. The category “Other antiepileptic drugs” includes vigabatrin, felbamate, tiagabine, rufinamide, eslicarbazepine, retigabine, perampanel, and unnamed trial drugs. The yellow dashed lines divide the established AEDs from the new AEDs.
Response to Successive AEDs
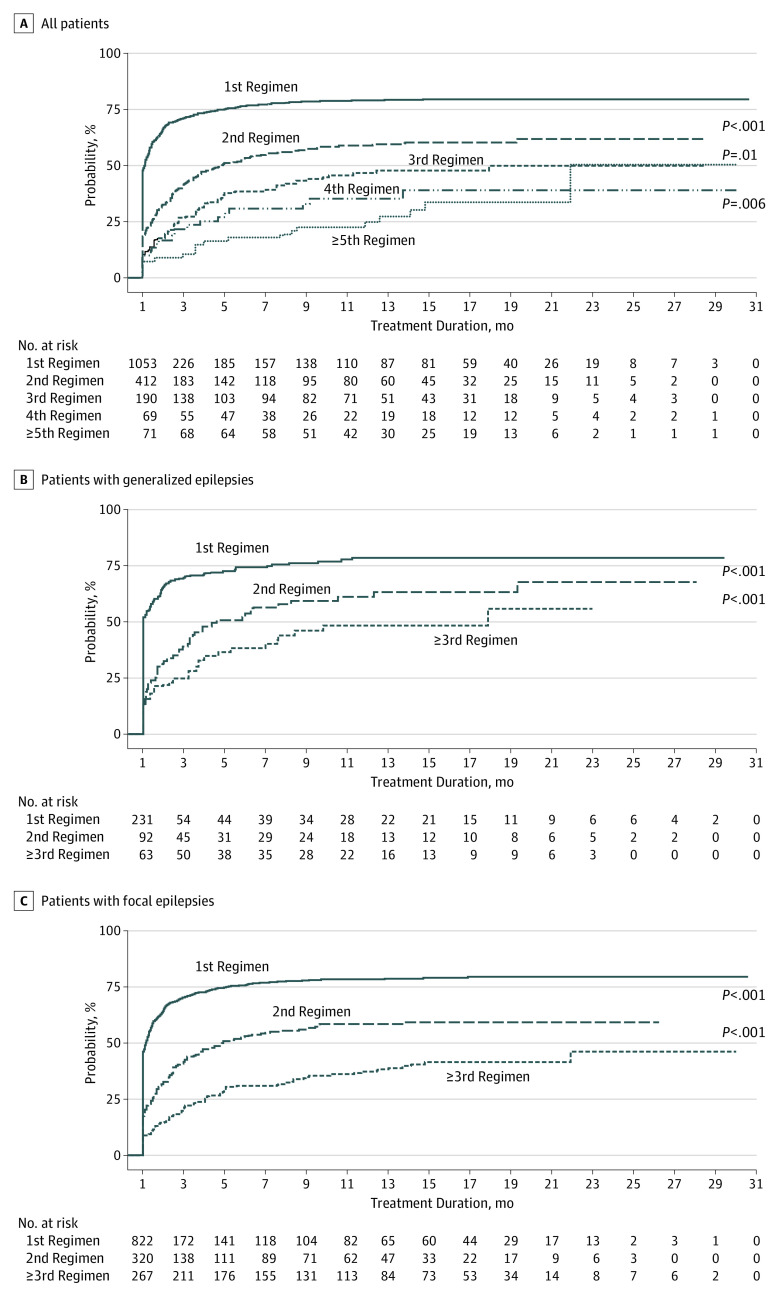
Patients who did not achieve 1 year of seizure freedom by taking the first AED had a greater likelihood of uncontrolled epilepsy for each additional AED tried (odds ratio [OR], 1.73; 95% CI, 1.56-1.91), after adjusting for disease classification, age at onset, and sex. Survival analysis over time confirmed that the probability of seizure freedom diminished with the number of AED regimens received. There was a significant difference in overall probability of seizure freedom between patients treated with the first and second AED regimens (hazard ratio [HR], 0.52; 95% CI, 0.45-0.60; P < .001; Figure 2A). The difference was also significant when comparing those treated with their second and third AED regimen (HR, 0.71; 95% CI, 0.55-0.92; P = .01). There were no significant differences between the third, fourth, and fifth and greater numbers of AED regimens. Similar effects were also observed in both generalized and focal epilepsy syndromes (Figure 2B and C).
Figure 2. Cumulative Probability of 1-Year Seizure Freedom by Treatment Duration and Number of Antiepileptic Drugs Regimens Tried.
A, Data for all patients; B, Patients with generalized epilepsy; C, Patients with focal epilepsy. In A, probabilities of seizure freedom differ significantly between first and second regimens, between second and third regimens, and between third and fourth regimens. In both B and C, patients who tried more than 3 antiepileptic drugs were grouped together in the subanalyses owing to small sample sizes; probabilities varied significantly between both first and second regimens and second and third regimens.
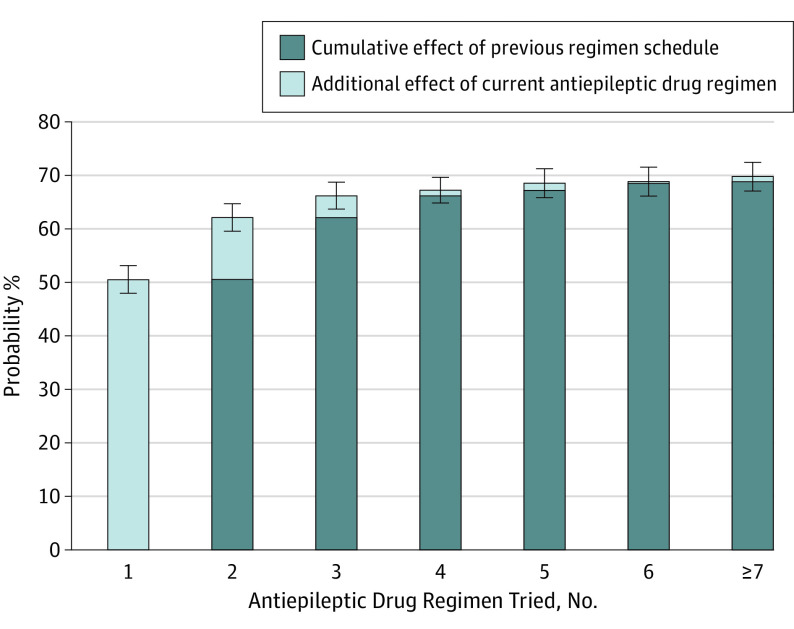
The effect of additional seizure control diminished with each successive AED regimen tried (Figure 3). There were 820 patients who were seizure free 1 year or longer with the initial AED, so the predicted probability was 50.5% (95% CI, 47.9%-53.1%). If the first treatment failed, the second AED regimen only provided an additional 11.6% probability of seizure freedom (n = 208; 95% CI, 10.1%-13.1%; P < .001). If the first 2 drugs failed to control all seizures, the third AED regimen offered only a 4.1% additional probability of seizure freedom. From the fourth AED regimen onwards, each additional AED only added an approximate 1% or less probability of seizure freedom. After 2 AEDs were tried, the cumulative probabilities of seizure freedom were not significantly different with each successive AED regimen.
Figure 3. Increases in Probability of 1-Year Seizure Freedom for Each Additional Antiepileptic Drug Regimen Tried.
The percentage of patients achieving seizure freedom via the first, second, third, fourth, fifth, sixth, and seventh AED regimens were 50.5%, 11.6%, 0.99%, 1.34%, 0.28%, and 0.94%, respectively. Please see Table 2 for numbers of patients achieving seizure freedom and total patients in each subgroup.
Analysis of treatment outcomes was repeated in accordance with the 2010 ILAE rule of three. This yielded similar findings (eFigures 3 and 4 in the Supplement).
Risk Factors for Treatment Response
After adjustment for sex and epilepsy classification, logistic regression analysis demonstrated that a high number of seizures in the year prior to the initiation of treatment, a history of smoking, previous recreational drug use, a family history of epilepsy in first-degree relatives, previous brain injury, and the presence of psychiatric comorbidity were significantly associated with an adverse prognosis (eTable 3 in the Supplement). Multivariate analysis involving all of these variables confirmed that the number of seizures occurring in the year before treatment began, previous recreational drug use, and a family history of epilepsy in first-degree relatives were associated with poorer terminal seizure outcome (eTable 4 in the Supplement). For each increase in the numbers of seizures in the year prior to treatment, there was a 6% decrease in likelihood of being seizure free at the last clinic visit. Patients with a history of recreational drug use had a 64% reduced chance of achieving terminal seizure freedom, and patients with a family history of seizure in first-degree relatives had a 55% reduced likelihood of attaining this end point.
Discussion
In this expanded and time-extended cohort of patients with newly diagnosed epilepsy, the overall 1-year seizure freedom rate was 63.7%. The majority of patients (86.8%) who achieved seizure freedom did so by taking a single AED. Patients with generalized epilepsy demonstrated a better response to AED therapy than those with focal epilepsy.
Despite the introduction of more than a dozen new AEDs in the past 2 decades, there remain no robust data to suggest improvement in treatment outcomes.9 In our cohort, there had been a continual increase in the use of the new AEDs, both as initial and subsequent treatments, since the early 1990s. Yet compared with our initial analysis in the first 470 patients, the seizure-free rate observed in the present study (63.7%) was virtually unchanged from that of 16 years ago (64.0%).3 However, the overall seizure-free rate in this study was slightly lower than the rate (68.2%) reported in our last analysis of a smaller cohort, which was performed in 2008.11 Similarly, there was a decrease in the proportion of patients whose seizures were controlled with monotherapy among those who were seizure free, from 90.5% in 2008 (n = 678/749) to 86.8% in the present study (n = 993/1144). Given the lack of significant changes in patient characteristics over the study period, these differences are likely owing to the fluctuation in seizure control identified in multiple studies.11,19,20
The present study confirmed our previous observations that the likelihood of becoming seizure-free decreases with each unsuccessful AED regimen. The changes were statistically significant for the first 3 AED regimens, but owing to the small number of patients who took more than 3 AEDs (n = 286), no significant difference was observed thereafter. Overall, patients who did not become seizure free while taking the first AED had 1.73 times the likelihood of having uncontrolled epilepsy with each successive AED. While a second AED regimen could render approximately 11% additional patients seizure-free, the benefit was reduced by more than half for the third regimen. Trying a fourth AED or more provided less than 5% additional probability of seizure freedom.
As previously reported, the prognosis of AED treatment was associated with the number of seizures that occurred prior to treatment, a family history of epilepsy in first-degree relatives, and a history of recreational drug use.21 A high number of pretreatment seizure numbers might correlate with the severity of epilepsy, and this might be more likely to have poor response to pharmacotherapy.22 Although the result was adjusted for classification, patients with a family history of epilepsy in first-degree relatives demonstrated greater odds of developing refractory seizures.
Similarly, recreational drug use is a well-recognized risk factor for seizures. The association between this problem and poor AED response may be partly owing to misreporting of provoked seizures and to suboptimal treatment adherence.
Limitations
To our knowledge, this is the largest long-term outcome study of newly diagnosed and treated epilepsy patients. Several limitations warrant mention. First, because this study was based on patients attending a single health care center in Glasgow, there may be selection bias in our population. Second, despite the size of the overall cohort, there were small numbers in some subgroups (eg, patients taking their third or greater AED regimen), which limited the statistical power for these analyses. Finally, to provide a more accurate estimation of the effects of AEDs when adequately tried, the analysis excluded patients who had persistently poor drug adherence, which is a common cause of treatment failure.23 Hence, the overall seizure outcomes might have been even poorer if the study design had involved an intention-to-treat analysis.
Conclusions
Despite the increased use of many new AEDs with differing mechanisms of action over the past 2 decades, long-term outcomes in adolescent and adult patients who are diagnosed with common, newly diagnosed epilepsies have not improved. The probability of achieving seizure freedom diminishes sharply with each unsuccessful AED regimen. As a result, our findings lend support to the definition of drug-resistant epilepsy as failure of 2 appropriately selected and tolerated AED regimens.18
A paradigm shift in treatment and research strategies is needed to improve the long-term outcomes of newly diagnosed epilepsy. Patients with drug-resistant epilepsy should be considered early for nonpharmacological therapies, such as resective surgery and brain stimulation techniques.2
While some modern AEDs have novel antiseizure mechanisms, their increasing use did not seem to have improved overall long-term seizure control. This may be attributed to deficiencies in the preclinical and clinical strategies of AED development.6 This finding also suggests that the modern AEDs still lack the capacity to rectify the underlying neuropathological processes and reverse epileptogenesis. Our results provide further evidence that current AEDs are seizure suppressing and not disease modifying. Future research should focus on novel treatments that can modify the development or progression of epilepsy, ideally guided by biomarkers.24
eAppendix. “Rule-Of-Three” Seizure-Free Rate
eReferences. Online only references.
eTable 1. Demographics and treatment outcome of patients recruited in different time periods.
eTable 2. "Rule-of-Three" seizure-free rates with successive antiepileptic drug schedules
eFigure 1. Antiepileptic drug prescriptions over the study period for antiepileptic drugs prescribed in subsequent treatment schedules.
eFigure 2. Cumulative probability of one-year seizure freedom by time from start of treatment in the three subgroups.
eFigure 3. Cumulative probability of “Rule-of-Three” seizure freedom by time from start of treatment and number of antiepileptic drug tried for (A) all patients; (B) patients with generalised epilepsies; and (C) patients with focal epilepsies.
eFigure 4. Additional effects on probability of “Rule-of-Three” seizure freedom for each antiepileptic drug schedule.
eTable 3. Associations between terminal seizure outcome and individual potential risk factors after adjustment for gender and epilepsy classification, univariate analysis
eTable 4. Associations between terminal seizure outcome and potential risk factors after adjustment for sex and epilepsy classification
References
- 1.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365(10):919-926. [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. [DOI] [PubMed] [Google Scholar]
- 4.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9(1):68-82. [DOI] [PubMed] [Google Scholar]
- 5.Brodie MJ, Kwan P. Newer drugs for focal epilepsy in adults. BMJ. 2012;344:e345. [DOI] [PubMed] [Google Scholar]
- 6.Golyala A, Kwan P. Drug development for refractory epilepsy: the past 25 years and beyond. Seizure. 2017;44:147-156. [DOI] [PubMed] [Google Scholar]
- 7.Marson AG, Al-Kharusi AM, Alwaidh M, et al. ; SANAD Study group . The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1000-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glauser T, Ben-Menachem E, Bourgeois B, et al. ; ILAE Subcommission on AED Guidelines . Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551-563. [DOI] [PubMed] [Google Scholar]
- 9.Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 2011;52(4):657-678. [DOI] [PubMed] [Google Scholar]
- 10.Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. 2006;13(3):277-282. [DOI] [PubMed] [Google Scholar]
- 11.Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephen LJ, Brodie MJ. Selection of antiepileptic drugs in adults. Neurol Clin. 2009;27(4):967-992. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-482. [DOI] [PubMed] [Google Scholar]
- 14.Brodie MJ, Kwan P. Staged approach to epilepsy management. Neurology. 2002;58(8)(suppl 5):S2-S8. [DOI] [PubMed] [Google Scholar]
- 15.Kwan P, Brodie MJ. Combination therapy in epilepsy: when and what to use. Drugs. 2006;66(14):1817-1829. [DOI] [PubMed] [Google Scholar]
- 16.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676-685. [DOI] [PubMed] [Google Scholar]
- 17.Commission on Classification and Terminology of the International League Against Epilepsy . Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30(4):389-399. [DOI] [PubMed] [Google Scholar]
- 18.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069-1077. [DOI] [PubMed] [Google Scholar]
- 19.Mohanraj R, Brodie MJ. Early predictors of outcome in newly diagnosed epilepsy. Seizure. 2013;22(5):333-344. [DOI] [PubMed] [Google Scholar]
- 20.Callaghan B, Schlesinger M, Rodemer W, et al. Remission and relapse in a drug-resistant epilepsy population followed prospectively. Epilepsia. 2011;52(3):619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75(2-3):192-196. [DOI] [PubMed] [Google Scholar]
- 22.Rogawski MA, Johnson MR. Intrinsic severity as a determinant of antiepileptic drug refractoriness. Epilepsy Curr. 2008;8(5):127-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perucca E. Pharmacoresistance in epilepsy: how should it be defined? CNS Drugs. 1998;10:171-179. [Google Scholar]
- 24.Pitkänen A, Engel J Jr. Past and present definitions of epileptogenesis and its biomarkers. Neurotherapeutics. 2014;11(2):231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. “Rule-Of-Three” Seizure-Free Rate
eReferences. Online only references.
eTable 1. Demographics and treatment outcome of patients recruited in different time periods.
eTable 2. "Rule-of-Three" seizure-free rates with successive antiepileptic drug schedules
eFigure 1. Antiepileptic drug prescriptions over the study period for antiepileptic drugs prescribed in subsequent treatment schedules.
eFigure 2. Cumulative probability of one-year seizure freedom by time from start of treatment in the three subgroups.
eFigure 3. Cumulative probability of “Rule-of-Three” seizure freedom by time from start of treatment and number of antiepileptic drug tried for (A) all patients; (B) patients with generalised epilepsies; and (C) patients with focal epilepsies.
eFigure 4. Additional effects on probability of “Rule-of-Three” seizure freedom for each antiepileptic drug schedule.
eTable 3. Associations between terminal seizure outcome and individual potential risk factors after adjustment for gender and epilepsy classification, univariate analysis
eTable 4. Associations between terminal seizure outcome and potential risk factors after adjustment for sex and epilepsy classification