Key Points
Question
What is the rate of cardiovascular adverse events (CVAE) among patients receiving carfilzomib for multiple myeloma?
Findings
In this systematic review and meta-analysis of 24 prospective clinical trials including 2594 patients, all-grade and high-grade CVAE were seen in 18.1% and 8.2% of patients, respectively; cardiovascular adverse event rates were higher in trials using carfilzomib doses of 45 mg/m2 or more. In randomized clinical trials, the rate of high-grade CVAEs was more than double in carfilzomib treated patients than in controls.
Meaning
Carfilzomib is associated with a high rate of clinically significant CVAEs, which underscores a need for increased awareness of these adverse events and further study into optimal patient selection and risk mitigation strategies.
This systematic review with meta-analysis examines the rates of cardiovascular adverse events among patients receiving carfilzomib for the treatment of multiple myeloma.
Abstract
Importance
Cardiovascular adverse events (CVAE) with carfilzomib in patients with multiple myeloma can be potentially life-threatening and remain incompletely characterized. We performed the first systematic review and meta-analysis of carfilzomib-associated CVAE.
Objective
To determine the incidence of carfilzomib-associated CVAE and to compare the rates of carfilzomib CVAE among different doses and companion therapies.
Data Sources
PubMed, EMBASE, Web of Science, and clinicaltrials.gov were queried for the keywords “carfilzomib,” “Kyprolis,” and “PX-171” through January 1, 2017.
Study Selection
Phase 1 to 3 prospective clinical trials of carfilzomib in patients with multiple myeloma with evaluable toxic effects data were eligible for meta-analysis.
Data Extraction and Synthesis
Data were independently extracted by 2 reviewers following Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Pooled incidence rates and relative risks (for randomized trials) and 95% confidence intervals were calculated using a random effects model. Subgroup analyses were performed to assess study-level characteristics associated with CVAE.
Main Outcomes and Measures
Cardiovascular adverse events were defined as heart failure, hypertension, ischemia, and arrhythmia. All-grade and grades 3 or higher AEs and study characteristics were recorded.
Results
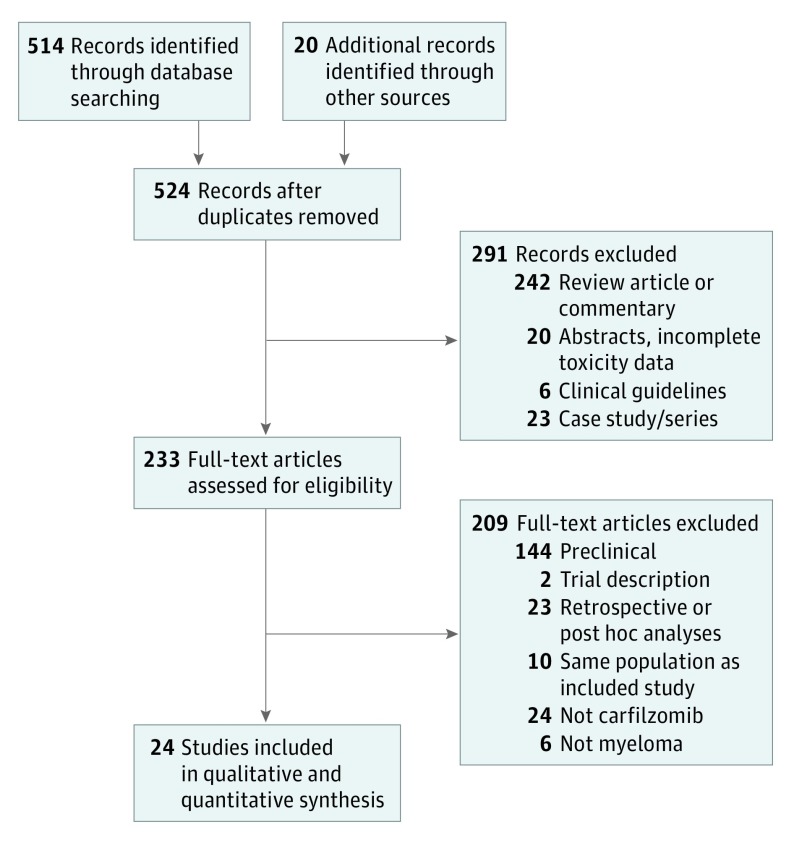
A total of 514 studies were assessed for eligibility. Of those, 24 studies were eligible, including a total of 2594 patients with multiple myeloma. All-grade and grades 3 and higher CVAE were seen in 617 (18.1%) and 274 (8.2%), respectively. Phase 2 or 3 studies and carfilzomib doses of 45 mg/m2 or higher were associated with high-grade CVAE. Median age older than 65 years, prior myeloma therapies, and concurrent myeloma therapies were not associated with CVAE. For the 3 randomized clinical trials, the summary relative risk of all-grade and grade 3 or higher CVAE for patients receiving carfilzomib compared with noncarfilzomib-receiving control patients were 1.8 and 2.2, respectively.
Conclusions and Relevance
Carfilzomib was associated with a significant incidence of CVAE, with higher rates seen with higher doses of carfilzomib. Phase 1 studies may be underdetecting CVAE. Future studies are needed to identify patients at high risk for CVAE, develop optimal monitoring strategies, and explore strategies to mitigate these risks.
Introduction
Survival rates for patients with multiple myeloma (MM) have dramatically improved with the clinical development of proteasome inhibitors, thalidomide analogues, and monoclonal antibodies. Cardiovascular adverse events (CVAE) are particularly important in MM given the overlapping risk factors for both MM and cardiovascular disease, such as older age and obesity, as well as a significant prevalence of coexisting cardiovascular disease in patients with MM. In a study of over 30 000 patients with MM, nearly two-thirds had cardiovascular disease at baseline and 70% developed cardiovascular events in a 6-year time period. Thus, it is imperative to understand CVAE in this population, who are living longer with MM, have high rates of baseline cardiovascular disease, and are being exposed to an expanding portfolio of therapeutic agents with potential for cardiovascular toxic effects.
With patients with MM living longer, and with treatment options expanding, adverse event profiles are increasingly important in providing personalized care. A systematic review and meta-analysis of CVAE in patients receiving bortezomib, a first-in-class reversible proteasome inhibitor, showed a 3.8% rate of all-grade CVAE and a 2.3% rate of high-grade CVAE. An analysis of randomized studies did not find a significantly increased risk of CVAE in bortezomib arms compared with control arms. Carfilzomib, the first irreversible proteasome inhibitor, is approved in the United States in combination with dexamethasone with or without lenalidomide for the treatment of patients with MM who have received at least 1 prior therapy based on the results of multiple clinical trials. Since its approval in 2012, there have been increasing reports of carfilzomib-associated CVAE, including heart failure, severe hypertension, cardiac arrhythmias, ischemic events, and cardiac arrest. Proposed mechanisms for these proteasome inhibitor-associated CVAE include oxidative stress on cardiac myocytes, endothelial effects as a result of proteasome inhibition leading to hypertension and vascular dysfunction, and an increase in coronary vascular tone and reactivity. Further underlying the importance of endothelial dysfunction in cardiorenal dysfunction, direct endothelial toxic effects have been suggested to underlie the mechanism of carfilzomib-associated acute kidney injury and thrombotic microangiopathy as well.
The nature and incidence of carfilzomib-associated CVAE is not currently known. Clinical trials performed in different patient populations and with varying treatment parameters (eg, dose, infusion duration, combination regimens) have shown varying rates of CVAE. We have to our knowledge performed the first systematic review and meta-analysis to specifically address carfilzomib-associated CVAE to determine the rate of CVAE and to explore potential reasons for varying CVAE rates reported among trials.
Methods
Search Methods and Study Selection
This systematic review and meta-analysis was performed in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. We performed a systematic review of studies cited in Medline (via PubMed) from January 1, 2007, to January 1, 2017, using the search terms “carfilzomib,” “Kyprolis”, and “PX-171.” Because the purpose of this study was to summarize the incidence of overall and high-grade CVAE in patients with MM receiving carfilzomib, we restricted our study to prospective clinical trials in which adult, human participants received carfilzomib for MM. To allow for comparability between trials, only studies in which adverse events were reported using the Common Terminology Criteria for Adverse Events (CTCAE) version 3 or 4 in the English language were included. To avoid additional selection or ascertainment bias, studies in which toxic effects data were collected retrospectively and substudies of separately reported prospective trials were excluded from analysis. Preclinical studies and studies with nonhuman subjects were excluded. The EMBASE and Web of Science databases were also queried with the same search terms to identify additional studies. All studies were selected and reviewed by 2 independent reviewers (A.J.W. and S.C.).
Data Extraction
Data were extracted for all studies reporting nonhematologic toxic effects associated with carfilzomib. The primary outcome was an aggregate outcome of overall CVAE rates including heart failure, hypertension, cardiac ischemia, arrhythmia, and cardiac arrest. Outcomes of heart failure, hypertension, cardiac ischemia, arrhythmia, and cardiac arrest were treated as secondary outcomes. All-grade and high-grade (grade ≥3) treatment-emergent CVAE were abstracted on the basis of sets of CTCAE definitions (eTable in the Supplement). These outcomes were abstracted for each eligible study, counting participants documented as having multiple CVAE once when that information was available. In addition, CTCAE outcomes of dyspnea and edema were abstracted but not included in the aggregate endpoint owing to the possibility that these could represent a mixture of pulmonary and cardiovascular adverse events. Study-level characteristics were abstracted (Table 1). For randomized clinical trials, data on CVAE in control groups that did not receive carfilzomib were also abstracted. Data were collected using a standardized data collection instrument by both reviewers. Results were reconciled during a consensus meeting. When consensus could not be reached, a third reviewer (B.M.W.) was consulted to resolve discrepancies. Preconsensus inter-rater reliability was assessed using both absolute inter-rater agreement and the κ statistic.
Table 1. Study and Patient Characteristics.
| Study Characteristic | Studies, No. | Patients, No. |
|---|---|---|
| Total | 24 | 2594 |
| Phase | ||
| 1 | 5 | 144 |
| 1/2 | 8 | 488 |
| 2 | 8 | 945 |
| 3 | 3 | 1017a |
| Median age, y | ||
| ≤60 | 4 | 251 |
| 60-65 | 15 | 2019 |
| >65 | 5 | 324 |
| Myeloma status | ||
| Newly diagnosed | 6 | 391 |
| Relapsed/Refractory | 18 | 2203 |
| Prior therapies | ||
| <3 | 10 | 1407 |
| ≥3 | 13 | 1139 |
| Infusion length, minutes | ||
| ≤10 | 13 | 1642 |
| 30 | 11 | 952 |
| Carfilzomib dose, mg/m2 | ||
| <45 | 14 | 1603 |
| ≥45 | 10 | 991 |
| Carfilzomib duration, mo | ||
| <6 | 11 | 1068 |
| ≥6 | 11 | 1385 |
| Not available | 2 | 141 |
| Regimen | ||
| Carfilzomib alone | 11 | 1552 |
| Combination regimen | 13 | 1042 |
| Randomization | ||
| Not randomized | 21 | 1577 |
| Randomized | 3 | 1017 |
For randomized clinical trials, these characteristics reflect only patients from carfilzomib receiving arms.
Meta-analysis
The rates of CVAE in each study were calculated by dividing the number of each CVAE by the total sample size. Binomial exact 95% confidence intervals (CIs) were calculated. To avoid inducing bias toward a higher event rate from employing a fixed continuity correction for studies with a 0 event rate, data were transformed using the Freeman-Tukey Double Arcsine transformation without continuity correction. The transformed CVAE rates were pooled using a random effects model as described by DerSimonian and Laird. A random effects model was selected a priori owing to expected treatment-effect heterogeneity from differences in study designs. Heterogeneity was quantified using the τ2 and I2 statistics. Prespecified subgroup analyses were performed using study characteristic stratification and differences were assessed using the Q-test, which tests the null hypothesis that there is no between-group heterogeneity. For the randomized clinical trials, relative risk (RR) of CVAE were calculated by dividing the CVAE rate in the experimental carfilzomib group by the CVAE rate in the control group. These individual study RRs were pooled using the Peto method to compute a summary RR.
Publication bias was assessed by querying a national clinical trials registry (clinicaltrials.gov) for unreported completed or discontinued trials and by review of abstracts presented at national meetings. Publication bias and small study effects were also assessed graphically via funnel plot and mathematically using Begg’s rank correlation test. All statistical analyses were performed using STATA statistical software (version 14.2, StataCorp).
Results
Eligible Studies and Characteristics
Our initial literature search revealed 524 potential studies for review (Figure 1). Following exclusion of review articles, preclinical and animal studies, case reports and case series, clinical guidelines and editorials, studies without toxic effects data, retrospective analyses and studies for alternate indications, we identified 24 prospective studies of patients receiving carfilzomib for MM.A total of 2594 patients with MM were included. Interrater agreement for study inclusion was 99.2% (κ = .92, P < .001). The 24 included studies included a range of trial sizes (7-513 patients receiving carfilzomib), phases, number of prior myeloma therapies, maximum doses of carfilzomib administered (15 mg/m2 to 88 mg/m2), and companion therapy. High-grade CVAE data was available for all 24 included studies whereas all-grade CVAE data was available in 22 studies. All studies excluded patients with amyloidosis and had at least some exclusion criteria on the basis of cardiac function or comorbidities. Summary study and patient level characteristics are listed in Table 1.
Figure 1. PRISMA Flow Diagram of Study Selection.
Rates of CVAE
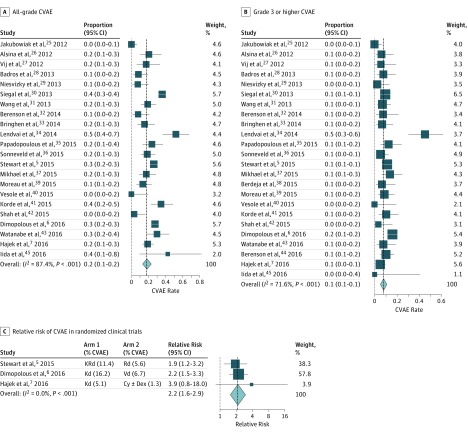
The rate of all-grade CVAE ranged from 0% to 52% and the rate of high-grade CVAE ranged from 0% to 45%. Interrater agreement for CVAE was 95.6%. On pooling the data using the random effects model (Figure 2A and B), summary all-grade and high-grade CVAE rates were 18.1% and 8.2%, respectively. Both estimates showed substantial heterogeneity as quantified by I2 statistics of 87.4% and 71.6% for all-grade and high-grade CVAE, respectively, which represent the fraction of variability in studies not attributable solely to sample size. Heart failure (4.1%) and hypertension (12.2%) were the most common types of CVAE, whereas arrhythmias (2.4%) and ischemic events (1.8%) were comparatively less common (Table 2). Of 2594 patients, there were 6 reported carfilzomib-associated cardiac arrests. This was not significantly different from 0 (P > .99). Reports of all-grade dyspnea (23.9%) and edema (24.7%) were relatively common, but information on the underlying causes of these adverse events were limited.
Figure 2. Forest Plots for CVAE.
A, Forest plots of all-grade and B, high-grade cardiovascular adverse events for all studies. C, Forest plot of relative risk for cardiovascular adverse events in randomized trials. The gray dotted lines represent the null hypothesis (either 0 events or a relative risk of 1) and the black dashed lines represent the summary event rate obtained from the random effects meta-analysis. CVAE indicates cardiovascular adverse events.
Table 2. Summary Estimates of Percentages of Participants With All-Grade or High-Grade Cardiovascular Adverse Events by Type of Event.
| Outcome | All-Grade Adverse Events | Grade ≥3 Adverse Events | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | % (95% CI) | P Value | I2 |
I2 P Value |
No. of Studies | % (95% CI) | P Value | I2 |
I2 P Value |
|
| All events | 22 | 18.1 (13.5-23.3) |
<.001 | 87.4 | <.001 | 24 | 8.2 (5.9-10.7) |
<.001 | 71.6 | <.001 |
| Congestive heart failure | 17 | 4.1 (2.3-6.2) |
<.001 | 65.2 | <.001 | 23 | 2.5 (1.5-3.8) |
<.001 | 49.2 | .004 |
| Hypertension | 16 | 12.2 (9.8-14.9) |
<.001 | 54.1 | .004 | 17 | 4.3 (2.6-6.4) |
<.001 | 60.3 | .001 |
| Arrhythmia | 13 | 2.4 (0.4-5.6) |
.004 | 84.4 | <.001 | 17 | 0.8 (0.3-1.4) |
<.001 | 0 | .86 |
| Ischemia | 13 | 1.8 (0.8-3.0) |
<.001 | 38.0 | .08 | 18 | 0.8 (0.4-1.4) |
<.001 | 0 | .78 |
| Cardiac arrest | NA | NA | NA | NA | 24 | 0.0 (0.0-0.1) |
>.99 | 0 | .98 | |
| Dyspnea | 17 | 23.9 (18.4-29.9) |
<.001 | 88.4 | <.001 | 18 | 3.2 (2.2-4.3) |
<.001 | 29.5 | .11 |
| Edema | 12 | 24.7 (21.0-28.6) |
<.001 | 64.2 | .001 | 12 | 0.4 (0.1-0.9) |
<.001 | 0 | .61 |
Abbreviation: NA, not applicable.
Subgroup Analysis
Heterogeneity was explored using subgroup analysis on the basis of study-level patient- and therapy-associated characteristics (Table 3). We found that the phase of trial and dose of carfilzomib were both significantly associated with higher rates of high-grade CVAE, with phase 1 trials having a rate of 2.3% compared with 9.5% for phase 2 and 3 trials (P = .02) and doses of carfilzomib smaller than 45 mg/m2 having a rate of 6.4% compared with 11.9% for doses of 45 mg/m2 or more (P = .02). Median age of study participants, whether MM was newly diagnosed or relapsed/refractory, number of prior therapies, and duration of carfilzomib exposure were not associated with differing rates of CVAE or reduced heterogeneity. Longer infusion duration (30 minutes vs ≤10 minutes) demonstrated a trend toward higher rates of high-grade CVAE (11.0% vs 6.7%, P = .06). However, studies with a 30-minute infusion length were far more likely to have a higher dose of carfilzomib (OR, 8.6; 95% CI, 1.4-51.7; P = .02).
Table 3. Subgroup Analysis of High-Grade Cardiovascular Adverse Events by Study Characteristics .
| Study Characteristic | Estimate, % (95% CI) | P Value | |
|---|---|---|---|
| No | Yes | ||
| Median age >65 years | 8.1 (5.4-11.2) | 8.5 (5.6-11.9) | .95 |
| Phase 1 trial | 9.5 (6.9-12.3) | 2.3 (0.1-6.2) | .02a |
| Randomized trial | 7.7 (5.2-10.5) | 10.8 (5.8-17.0) | .48 |
| Newly diagnosed MM | 8.7 (6.1-11.8) | 6.7 (2.9-11.8) | .38 |
| ≥3 Prior therapies | 8.4 (5.4-12.0) | 8.2 (4.6-12.5) | .87 |
| ≥6 Months carfilzomibb | 9.9 (5.7-15.0) | 7.1 (4.2-10.7) | .26 |
| Dose ≥45 mg/m2 | 6.4 (3.3-8.6) | 11.9 (7.25-17.49) | .02a |
| 30-Minute infusion | 6.7 (4.9-8.8) | 11.0 (6.4-16.5) | .06 |
| Combination regimen | 10.6 (6.6-15.2) | 6.5 (4.1-9.2) | .08 |
Statistically significant.
The median duration of carfilzomib treatment was available for 22 of the 24 studies. All other study characteristics were available for all 24 studies.
Relative Rates of CVAE in Randomized Clinical Trials
To assess the relative rate of carfilzomib CVAE compared with CVAE in otherwise identical control arms, we calculated the relative risk of developing CVAE in the 3 randomized clinical trials in which carfilzomib was administered in the experimental arm and computed a summary relative risk estimate (Figure 2C). The following trials in patients with relapsed MM were included: ASPIRE (carfilzomib, lenalidomide, and dexamethasone vs lenalidomide and dexamethasone), ENDEAVOR (carfilzomib and dexamethasone vs bortezomib and dexamethasone), and FOCUS (carfilzomib and dexamethasone vs dexamethasone with or without cyclophosphamide). All 3 trials were of high quality and followed CONSORT 2010 guidelines. For high-grade CVAE, receipt of carfilzomib was associated with a greater than 2-fold increase in risk (summary relative risk, 2.2; 95% CI, 1.6-2.9; P < .001; I2 = 0%). Results for all-grade CVAE were similar, with a summary relative risk of 1.8 (95% CI, 1.4-2.2; P < .001, I2 = 14.8%). A sensitivity analysis was performed excluding hypertension to assess whether these findings were driven by hypertension. Excluding hypertension yielded similar results; the summary relative risk was 2.6 (95% CI, 1.4-4.6; P = .002) for high-grade events and 1.9 (95% CI, 1.1-3.3; P = .02) for all-grade events.
Publication Bias Assessment
Studies were assessed for publication bias and small-study effects. Review of clinicaltrials.gov did not reveal additional completed studies with results that were not found in the published literature. Visual inspection of funnel plots (eFigure in the Supplement) and Begg’s test revealed asymmetry with a moderate correlation between greater sample size and more high-grade CVAE (P = .03). Significant asymmetry was not found for all-grade CVAE (P = .07).
Discussion
To our knowledge, this is the first systematic review and meta-analysis specifically assessing carfilzomib-associated CVAE. We note 4 key findings. First, from 24 studies including 2594 patients, we determined robust estimates of the rates of all-grade and high-grade CVAE with carfilzomib of 18.1% and 8.2%, respectively. Second, higher doses of carfilzomib were associated with higher rates of CVAE whereas infusion rate was not associated with different rates of CVAE. Third, rates of CVAE differ according to trial phase, suggesting a possible systematic underdetection or underreporting in early phase studies. Finally, we found that carfilzomib was associated with an elevated risk of high-grade and all-grade CVAE compared with noncarfilzomib control groups. Taken together, these findings indicate that carfilzomib confers an elevated risk of CVAE.
These findings are largely consistent with those recently discussed in an extensive review of safety issues of new treatments for multiple myeloma by Brioli et al. These include higher rates of CVAE noted in carfilzomib arms and greater CVAE rates in studies in which patients received higher doses of carfilzomib. Our study provides a rigorous, systematic, and quantitative analysis that meaningfully adds to the literature by confirming, quantifying, and statistically assessing these effects as well as exploring other important differences between trials on the rates of CVAE in patients receiving carfilzomib.
The pathogenesis of CVAE in the population of patients receiving proteasome inhibitors is not currently well understood. A prenatal mouse model has shown that exposure to proteasome inhibitors can induce oxidative stress leading to myocardial dysfunction, particularly in conjunction with anthracyclines. Further support for proteasome inhibitor-induced oxidative stress has been shown by Imam et al, who demonstrated that apremilast, an inhibitor of phosphdiesterase 4, reduces oxidative stress and carfilzomib cardiotoxic effects in a rat model. Other studies have shown that echocardiographic findings are not necessarily indicative or predictive of CVAE, raising the concern for endothelial effects as a potential mechanism. Carfilzomib is also known to cause renal toxic effects and microangiopathy that is also believed to be mediated by endothelial dysfunction. An in-vitro study showed increased coronary vascular tone and decreased coronary relaxation in response to nitroglycerin in hearts exposed to carfilzomib. Taken together, these studies reveal a complex mechanism of proteasome inhibitor-associated CVAE that involves changes to both the myocardium and vasculature that may be more severe with carfilzomib than bortezomib owing to the irreversible nature of proteasome inhibition with carfilzomib.
Our study found a lower rate of CVAE in early phase compared with later phase studies. In addition, we found that smaller study size correlated with fewer CVAE. One possible explanation for this would be nonpublication of small studies showing high rates of CVAE. However, our review of trial registries did not demonstrate nonpublication of small studies, which would be unlikely given that CVAE are not the primary endpoint of any of these studies. Rather, this relationship may be owing to systematic underdetection or underreporting of CVAE in smaller and earlier phase studies or exclusion of patients at elevated risk for CVAE from phase 1 studies. Furthermore, limitations in reporting of low-grade and longitudinal toxic effects has been described in the setting of limited follow-up at the time of publication. In either case, this may indicate that our summary effect size may underestimate the true mean rate of carfilzomib-associated CVAE in the general population.
We included hypertension in our aggregate endpoint of CVAE. Treatment-emergent hypertension is clinically significant because hypertension often precedes left ventricular diastolic and systolic dysfunction. Mechanistically, the association between carfilzomib use and hypertension is important given previously reported endothelial effects of proteasome inhibitors. The relationship between hypertension leading to diastolic dysfunction and clinical heart failure may explain the finding of low rates of reduced ejection fraction by echocardiography in a substudy of ENDEAVOR despite elevated rates of clinical heart failure in the carfilzomib arm because heart failure with preserved ejection fraction may account for many observed events. Although a considerable proportion of all-grade CVAE in our meta-analysis could be attributed to hypertension, our finding of greater rates of CVAE in participants receiving carfilzomib compared with controls is consistent whether hypertension is included in the aggregate outcome or not. This suggests a need for further study to explore methods of monitoring and treating hypertension among patients receiving carfilzomib.
Limitations
There are several limitations to the present study. Because this is a study-level analysis, it is not possible to make inferences regarding which individual patients are at higher risk of CVAE. Because subgroup analysis is powered on the basis of number of studies rather than total number of patients, we were only able to assess 1 characteristic at a time, precluding a multivariate analysis to assess multiple potential factors simultaneously. Ascertainment of CVAE may be limited by variable reporting between studies because it is possible to have different grades for the same event dependent on the adjudicator. For example, an asymptomatic reduction in ejection fraction may be ascertained as either a grade 1 heart failure event or a grade 3 decline in ejection fraction. Moreover, some events adjudicated as dyspnea or edema may represent underlying CVAE that were not diagnosed owing to lack of ejection fraction monitoring, leading to underestimation. Other events that may be clinically important but are uncommon, such as treatment-emergent pulmonary hypertension, are not well represented in the data. In addition, all studies included explicit and often broad exclusion criteria on the basis of cardiovascular history and function, limiting the generalizability of our results to patients with cardiac comorbidities, which accounts for a significant proportion of real-world patients with MM receiving carfilzomib.
Prior studies have suggested that patients with MM have elevated rates of CVAE compared with the general population. This finding may be related to the overlap between cardiovascular disease risk factors and the population of patients with MM, including older age and obesity, as well as disease-related factors, such as anemia and renal dysfunction. Our study demonstrates a rate of high-grade CVAE approximately twice as high among patients receiving carfilzomib compared with noncarfilzomib-receiving controls. This is of particular importance given that the MM patient population is at an elevated baseline risk of CVAE, underscoring the importance of delineating the cardiovascular risk of therapies for MM.
Conclusions
Our systematic review and meta-analysis demonstrates an 8.2% rate of high-grade carfilzomib CVAE and 18.1% risk of carfilzomib CVAE with higher doses of carfilzomib associated with more CVAE. It is critical that clinicians caring for patients with MM be aware of early signs of CVAE and promptly refer such patients for additional evaluation and to hold carfilzomib. Further study is needed to assess patient-level risk factors to predict which patients are at highest risk of developing carfilzomib CVAE as well as to establish optimal monitoring strategies for patients receiving carfilzomib and to develop strategies to mitigate this risk, including determining which patients may be able to safely restart the drug at a reduced dose.
eMethods
eFigure. Funnel plot to assess for publication bias or small study effects
eTable. Common terminology criteria for adverse events (CTCAE) definitions used to define classes of cardiovascular adverse events
References
- 1.Kristinsson SY, Anderson WF, Landgren O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia. 2014;28(6):1346-1348. [DOI] [PubMed] [Google Scholar]
- 2.Kistler KD, Kalman J, Sahni G, et al. Incidence and risk of cardiac events in patients with previously treated multiple myeloma versus matched patients without multiple myeloma: an observational, retrospective, cohort study. Clin Lymphoma Myeloma Leuk. 2017;17(2):89-96.e3. [DOI] [PubMed] [Google Scholar]
- 3.Kistler KD, Rajangam K, Faich G, Lanes S. Cardiac event rates in patients with newly diagnosed and relapsed multiple myeloma in US clinical practice. Blood. 2012;120(21)(suppl). Abstract 2916. [Google Scholar]
- 4.Xiao Y, Yin J, Wei J, Shang Z. Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: a systematic review and meta-analysis. PLoS One. 2014;9(1):e87671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. ; ASPIRE Investigators . Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142-152. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Moreau P, Palumbo A, et al. ; ENDEAVOR Investigators . Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. [DOI] [PubMed] [Google Scholar]
- 7.Hájek R, Masszi T, Petrucci MT, et al. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia. 2017;31(1):107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food & Drug Administration. Approved Drugs > Carfilzomib. http://www.fda.gov/Drugs/-InformationOnDrugs/ApprovedDrugs/ucm312945.htm. Accessed November 3, 2016.
- 9.Grandin EW, Ky B, Cornell RF, Carver J, Lenihan DJ. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail. 2015;21(2):138-144. [DOI] [PubMed] [Google Scholar]
- 10.Tjionas H, Gupta AK. Heart failure secondary to carfilzomib-induced heart block in multiple myeloma patients. J Oncol Pharm Pract. 2017;23(2):152-156. [DOI] [PubMed] [Google Scholar]
- 11.Jain T, Narayanasamy H, Mikhael J, et al. Reversible cardiotoxicity associated with carfilzomib use in patients with multiple myeloma. Blood. 2016;128(22)(suppl). Abstract 2126. [Google Scholar]
- 12.Chari A, Aggarwal SK, Mezzi K, et al. Cardiac events in real-world multiple myeloma patients treated with carfilzomib: a retrospective claims database analysis. Blood. 2016;128(22)(suppl). Abstract 3319. [Google Scholar]
- 13.Imam F, Al-Harbi NO, Al-Harbia MM, et al. Rutin attenuates carfilzomib-induced cardiotoxicity through inhibition of NF-κB, hypertrophic gene expression and oxidative stress. Cardiovasc Toxicol. 2017;17(1):58-66. [DOI] [PubMed] [Google Scholar]
- 14.Hasinoff BB, Patel D, Wu X. Molecular mechanisms of the cardiotoxicity of the proteasomal-targeted drugs bortezomib and carfilzomib. Cardiovasc Toxicol. 2017;17(3):237-250. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal A, Luthi J, Belohlavek M, et al. Carfilzomib and the cardiorenal system in myeloma: an endothelial effect? Blood Cancer J. 2016;6:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen-Scarabelli C, Corsetti G, Pasini E, et al. Spasmogenic effects of the proteasome inhibitor carfilzomib on coronary resistance, vascular tone and reactivity. EBioMedicine. 2017;21:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yui JC, Van Keer J, Weiss BM, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91(9):E348-E352. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamseer L, Moher D, Clarke M, et al. ; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 20.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335-371. [DOI] [PubMed] [Google Scholar]
- 23.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897-903. [DOI] [PubMed] [Google Scholar]
- 24.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsina M, Trudel S, Furman RR, et al. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;18(17):4830-4840. [DOI] [PubMed] [Google Scholar]
- 27.Vij R, Siegel DS, Jagannath S, et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol. 2012;158(6):739-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badros AZ, Vij R, Martin T, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia. 2013;27(8):1707-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niesvizky R, Martin TG III, Bensinger WI, et al. Phase Ib dose-escalation study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res. 2013;19(8):2248-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel D, Martin T, Nooka A, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98(11):1753-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M, Martin T, Bensinger W, et al. Phase 2 dose-expansion study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122(18):3122-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berenson JR, Hilger JD, Yellin O, et al. Replacement of bortezomib with carfilzomib for multiple myeloma patients progressing from bortezomib combination therapy. Leukemia. 2014;28(7):1529-1536. [DOI] [PubMed] [Google Scholar]
- 33.Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124(1):63-69. [DOI] [PubMed] [Google Scholar]
- 34.Lendvai N, Hilden P, Devlin S, et al. A phase 2 single-center study of carfilzomib 56 mg/m2 with or without low-dose dexamethasone in relapsed multiple myeloma. Blood. 2014;124(6):899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadopoulos KP, Siegel DS, Vesole DH, et al. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2015;33(7):732-739. [DOI] [PubMed] [Google Scholar]
- 36.Sonneveld P, Asselbergs E, Zweegman S, et al. Phase 2 study of carfilzomib, thalidomide, and dexamethasone as induction/consolidation therapy for newly diagnosed multiple myeloma. Blood. 2015;125(3):449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikhael JR, Reeder CB, Libby EN, et al. Phase Ib/II trial of CYKLONE (cyclophosphamide, carfilzomib, thalidomide and dexamethasone) for newly diagnosed myeloma. Br J Haematol. 2015;169(2):219-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berdeja JG, Hart LL, Mace JR, et al. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica. 2015;100(5):670-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau P, Kolb B, Attal M, et al. Phase 1/2 study of carfilzomib plus melphalan and prednisone in patients aged over 65 years with newly diagnosed multiple myeloma. Blood. 2015;125(20):3100-3104. [DOI] [PubMed] [Google Scholar]
- 40.Vesole DH, Bilotti E, Richter JR, et al. Phase I study of carfilzomib, lenalidomide, vorinostat, and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Br J Haematol. 2015;171(1):52-59. [DOI] [PubMed] [Google Scholar]
- 41.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1(6):746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah JJ, Stadtmauer EA, Abonour R, et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015;126(20):2284-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe T, Tobinai K, Matsumoto M, et al. A phase 1/2 study of carfilzomib in Japanese patients with relapsed and/or refractory multiple myeloma. Br J Haematol. 2016;172(5):745-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berenson JR, Cartmell A, Bessudo A, et al. CHAMPION-1: a phase 1/2 study of once-weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood. 2016;127(26):3360-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iida S, Tobinai K, Taniwaki M, Shumiya Y, Nakamura T, Chou T. Phase I dose escalation study of high dose carfilzomib monotherapy for Japanese patients with relapsed or refractory multiple myeloma. Int J Hematol. 2016;104(5):596-604. [DOI] [PubMed] [Google Scholar]
- 46.Brioli A, Mügge LO, Hochhaus A, Von Lilienfeld-Toal M. Safety issues and management of toxicities associated with new treatments for multiple myeloma. Expert Rev Hematol. 2017;10(3):193-205. [DOI] [PubMed] [Google Scholar]
- 47.Imam F, Al-Harbi NO, Al-Harbi MM, et al. Apremilast reversed carfilzomib-induced cardiotoxicity through inhibition of oxidative stress, NF-κB and MAPK signaling in rats. Toxicol Mech Methods. 2016;26(9):700-708. [DOI] [PubMed] [Google Scholar]
- 48.Russell SD, Lyon A, Lenihan DJ, et al. Serial Echocardiographic Assessment of Patients (Pts) with Relapsed Multiple Myeloma (RMM) Receiving Carfilzomib and Dexamethasone (Kd) Vs Bortezomib and Dexamethasone (Vd): A Substudy of the Phase 3 Endeavor Trial (NCT01568866). Blood. 2015;126(23)(suppl). Abstract 4250. [Google Scholar]
- 49.Hobeika L, Self SE, Velez JC. Renal thrombotic microangiopathy and podocytopathy associated with the use of carfilzomib in a patient with multiple myeloma. BMC Nephrol. 2014;15:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thanarajasingam G, Hubbard JM, Sloan JA, Grothey A. The imperative for a new approach to toxicity analysis in oncology clinical trials. J Natl Cancer Inst. 2015;107(10):djv216. [DOI] [PubMed] [Google Scholar]
- 51.Narayan V, Keefe S, Haas N, et al. Prospective evaluation of sunitinib-induced cardiotoxicity in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2017;23(14):3601-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witteles RM, Telli M. Underestimating cardiac toxicity in cancer trials: lessons learned? J Clin Oncol. 2012;30(16):1916-1918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure. Funnel plot to assess for publication bias or small study effects
eTable. Common terminology criteria for adverse events (CTCAE) definitions used to define classes of cardiovascular adverse events