Key Points
Question
What is the prevalence of high-risk human papillomavirus (HPV) in otherwise healthy adult tonsil tissue, and does HPV colocalize in biofilm of tonsillar crypts?
Findings
In this retrospective, cross-sectional study, prevalence of HPV in tonsils of otherwise healthy adults is consistent with previously published studies. In our sample population, in situ hybridization colocalized HPV virus to the biofilm of the tonsillar crypts and demonstrated extranuclear presence of viral capsid.
Meaning
Colocalization of HPV in bacterial biofilm of human tonsil tissue has considerable implications with respect to the determination of HPV prevalence rates in the oropharynx and may also play a role in the pathogenesis of HPV-related oropharyngeal carcinoma.
This retrospective, cross-sectional study examines the prevalence of oropharyngeal human papillomavirus and the spatial relationship between the virus and crypt biofilm in tonsil tissue of otherwise healthy adults.
Abstract
Importance
The pathogenesis of human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma is currently an important topic of elucidation. The presence of latent HPV infection in tonsil tissue of healthy adults may provide an explanation for a component of this process and contribute to the understanding of HPV-associated squamous cell carcinoma oncogenesis of the oropharynx.
Objective
To determine the prevalence of oropharyngeal HPV and to determine the spatial relationship between the virus and crypt biofilm in tonsil tissue.
Design, Setting, and Participants
A retrospective, cross-sectional study was carried out using samples obtained from tonsils that were archived at a university hospital following elective nononcologic tonsillectomy from 2012 to 2015. Samples consisted of formalin-fixed paraffin embedded samples of tumor-free tonsil tissue from 102 adults between the ages of 20 and 39 years.
Exposures
Human papillomavirus status was assessed by polymerase chain reaction, and high-risk subtypes 16 and 18 were assessed with quantitative polymerase chain reaction assay. Samples that demonstrated presence of HPV were then analyzed by in situ hybridization to localize the viral capsid protein. These samples were then stained with concanavalin A to establish biofilm presence and morphology. These samples were also stained with diamidino-phenylindole (DAPI) to visualize location of the virus in relation to cell nuclei. These data were then assembled for aggregate analysis to colocalize HPV in the biofilm of the tonsillar crypts.
Main Outcomes and Measures
Outcome measurements were determined prior to data collection and include prevalence of high-risk HPV types 16 and 18 in tonsil tissue of otherwise healthy adults, as well as demonstration with immunohistochemistry of HPV in tonsillar crypt biofilm.
Results
In 102 otherwise healthy adults (55 [53.9%] female; age range, 20-39 years), the overall prevalence of HPV in tonsils was 4.9% (n = 5); and high-risk type 16 or 18, 3.9% (n = 4). In this sample population, in situ hybridization colocalized HPV virus to the biofilm of the tonsillar crypts.
Conclusions and Relevance
Biofilm is present in the tonsillar crypts in a considerable proportion of tonsil tissues and may be reproducibly identified. Human papillomavirus is demonstrated to colocalize to the crypt biofilm. This has important implications with respect to the determination of HPV prevalence rates in the oropharynx. It may also play a role in the pathogenesis of HPV-related oropharyngeal carcinoma.
Introduction
In the evolving landscape of head and neck squamous cell carcinomas (HNSCC), human papillomavirus (HPV) is emerging as a uniquely significant etiologic component. Although traditionally alcohol consumption and tobacco use have been seen as primary risk factors in developing HNSCC, HPV is now recognized as the causative agent behind most HNSCC, and specifically oropharyngeal squamous cell carcinomas (OPSCC).
Human papillomavirus is a nonenveloped virus of double-stranded DNA with approximately 8000 base pairs that generally infects squamous epithelia. The genome can be divided into 3 major regions: the early (E) region encodes nonstructural proteins, the late (L) region that encodes 2 capsid proteins, and a noncoding long controlling region, which regulates viral replication and gene expression. Overall, the replication of the viral genome depends largely on the host’s DNA synthesis machinery.
More than 200 unique types of HPV have been identified, 13 of which are considered high-risk owing to their oncogenic potential. It is these high-risk types that are associated with the increased incidence of HPV-positive oropharyngeal squamous cell carcinoma, especially among young, white males. Incidence of this cancer more than tripled between 1988 and 2010 in the United States, United Kingdom, and Sweden. In 2012 the Centers for Disease Control reported incidence of HPV-associated OPSCC to be up to 4.5 per 100 000. At least 70% of these cancers have now been associated with HPV, especially the subtype HPV 16, which is found in more than 90% of such cases. In addition, it is expected that the prevalence of HPV-related HNSCC will surpass the incidence of cervical cancer by 2020. Patients with HPV-positive cancers have improved survival compared with their HPV-negative counterparts. This is owing in part to multiple patient factors, including age at presentation, lack of exposure to carcinogens, and absence of comorbidities. However, even after correcting for these issues, HPV status remains an independent prognostic factor in the improved survival of this cohort.
The risk of developing OPSCC from an HPV infection is thought to be similar to the risk of developing cervical cancer from genital HPV. Recent estimates of cervicovaginal prevalence of HPV 6, 11, 16, and/or 18 is estimated to be 4.3% among females aged 14 to 19 years, and 12.1% among women aged 20 to 24 years. However, although there are reliable and validated clinical methods to detect HPV in asymptomatic cervical carriers, an equivalent test does not exist for the oral cavity and pharynx. The screening methods employed to date have been unreliable and not reproducible. Moreover, there have been limited studies exploring this question. Consequently, the true prevalence of oral/oropharyngeal HPV infection/carriage remains largely unknown. According to a study by Gillison et al, the prevalence of oral HPV among both men and women aged 14 to 69 years was 6.9% (as determined by a mouth rinse technique). In their cohort, HPV infection was more prevalent in men than women and more common among those who were sexually active. A pilot study performed in the UK by Knight et al explored the utility of buccal swabs as a screening tool for oral HPV. The prevalence of detectable oral HPV in 124 participants, most aged between 18 and 25 years, was tested. The participants provided a swab of buccal cells from which the DNA was extracted for polymerase chain reaction (PCR) to detect HPV L1 protein, a highly conservative protein among different HPV strains. The study supported previous epidemiological studies that reported the prevalence of HPV oral infections to be between 2.4% and 5.4% among young adults aged 18 to 24 years.
Seroprevalence of high-risk HPV types ranges from 20% to 45% among nonvaccinated individuals. If the prevalence data for cervical and oral HPV infection is accurate, it follows that in most exposed individuals, HPV is being detected and readily cleared by host defenses. A component of the human oral and oropharyngeal environment that may contribute to the ability of HPV to escape immune detection and/or clearance—thereby facilitating oncogenesis—is biofilm located in the tonsillar crypts. Biofilm is defined as a community of immotile bacteria encased in a self-produced glycocalyx matrix. Free-living or planktonic bacteria become adherent to the surface, followed by development of bacterial microcolonies in this glycocalyx matrix. Important clinical features of biofilm include resistance to both antibiotic treatment, as well as host defense. Therefore, it is believed to play a role in the process of chronic and/or recurrent infections. The presence of biofilm in various sites of the head and neck has been well documented. Biofilm of the paranasal sinuses has been identified in patients with chronic rhinosinusitis. Biofilm was also demonstrated on the surface and in the crypts of human adenoid tissue using confocal laser scanning microscopy in conjunction with scanning electron microscopy. Similarly, bacterial cells and the glycocalyx in biofilm have been demonstrated to be located preferentially in crypts of tonsil tissue. Another critical component to consider is the viability of the virus in biofilm. Nonenveloped viruses, such as coxsackievirus type B5, have been demonstrated to persist in fungal biofilm in quantities that suggest virus particles are protected from degradation and retain their infectivity. In addition, virus-exposed biofilm can release virus that remains viable and presumably infectious.
Another important contribution to latent infection with HPV is chronic inflammation of oral and oropharyngeal tissue. Chronic inflammation of oral tissue has been proposed to be a risk factor for HPV infection and subsequent association with tumor growth. Tezal et al, investigated the role of periodontitis in predicting the HPV status of tongue base SCC tumors. As a chronic oral infection caused by inflammatory reactions to bacteria in the dental plaque, periodontitis results in the destruction of tissues around teeth. This allows HPV to gain access to basal epithelial cells through abrasions and alveolar bone destruction in these periodontal pockets. This was associated with a nearly 4-fold increased risk of HPV-positive tumor status with every millimeter of alveolar bone loss. Dental biofilm itself has also been shown to harbor the HPV virus, which may contribute to the development of periodontitis and perpetuate infection with HPV.
If biofilm is indeed a reservoir of HPV, these factors may also facilitate the ability of HPV to reinfect tonsil epithelia, which may increase risk of malignant transformation. We therefore evaluated the prevalence of HPV in tumor-free tonsil tissue of healthy adults, and we hypothesize that biofilm in the crypts of tonsil tissue act as a reservoir for HPV.
Methods
This was a retrospective cross-sectional study. Tonsil tissue was obtained from archived tissue in the Department of Pathology at the University of Rochester Medical Center. Samples for this study were derived from formalin-fixed paraffin-embedded (FFPE) tonsil tissue from adults aged 18 to 39 years following elective tonsillectomies performed at a single institution between 2011 and 2013. Patient characteristics are presented in the Table.
Table. Sample Population Characteristics.
| Characteristic | Sex (M/F) | Total, No. (%) | With HPV Infection, No. | HPV Prevalence, % | HPV16 E6/E7 | HPV18 E6/E7 | High-Risk HPV Prevalence, % |
|---|---|---|---|---|---|---|---|
| Age, y | |||||||
| 18-19 | M | 2 (1.9) | 1 | 50 | 0 | 1 | 50 |
| F | 1 (1.0) | 0 | 0 | 0 | 0 | 0 | |
| 20-29 | M | 34 (33.3) | 2 | 5.9 | 2 | 0 | 5.9 |
| F | 45 (44.1) | 2 | 4.4 | 0 | 1 | 2.2 | |
| 30-39 | M | 11 (10.8) | 0 | 0 | 0 | 0 | 0 |
| F | 9 (8.8) | 0 | 0 | 0 | 0 | 0 | |
| Categorical total | M | 47 (46.1) | 3 | 2.9 | 2 | 1 | 3.0 |
| F | 55 (53.9) | 2 | 2.0 | 0 | 1 | 1.0 | |
| Total, % (95% CI) | 102 | 5 | 4.9 (0.7-9.1) | 2.0 | 2.0 | 4.0 (0.2-7.8) |
Abbreviation: HPV, human papillomavirus.
For our study, 102 cases stratified for sex and age were randomly sampled from the archive. The study had a greater than 80% power to detect a difference between a prevalence rate of 1% and 5% between individuals in the entire cohort. Research subjects’ review board approval was obtained from the University of Rochester Medical Center prior to commencing with the study.
Laboratory Analyses
Human papillomavirus infection was assessed from sections cut from FFPE blocks of tonsil tissue. This method was chosen to allow for retrospective analyses, as well as permit further histopathological evaluations for positive samples. Sections from FFPE p16-positive tonsil cancer were included as positive controls. DNA was extracted from these sections using the QIAmp Mini Kit protocol as provided (Qiagen). DNA purity and yield were determined by Nanodrop spectrophotometry. Human papillomavirus detection was performed by nested PCR using MY09/11 and G5+/6+ primers (able to detect over 30 subtypes, including 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). Amplicon size was reported to be 450 bp. Positive (HPV16-positive) and negative (water) controls were included for each PCR run. This was an end-point assay and was assessed by visual examination of agarose gels including appropriate standards. The HPV 16, 18, 6, and 11 viral loads were determined with the use of sensitive real-time qPCR targeted to the E6 coding region. The viral load was assessed in each sample after completion of 40 cycles of SYBR green-based real-time qPCR assays. Positive samples were defined as detectable HPV DNA at concentrations of 1.0 × 108 copies per microliter or greater within 30 cycles derived from a reference standard curve. A PCR mixture without template DNA was used as a negative control in all reactions. The DNA derived from HPV16-positive tissue samples were used as positive controls.
Concanavalin A
Sections were slide-mounted and underwent multiple treatments in xylene followed by 96% and 70% ethanol. Slides were rinsed with sterile phosphate-buffered saline (PBS) and incubated in 50 mM TE buffer containing lysozyme for 20 minutes at 37° C to permeabilize the cells. Following another rinse with PBS, sections were again incubated in 50 mM TE buffer with proteinase K at 37°C for 20 minutes. They were then rinsed in distilled water, 70% and 96% ethanol and air dried. They were then preincubated at 48° C for 20 minutes in hybridization buffer containing 0.9 M sodium chloride, 20 mM tromethamine (Tris) hydrochloride, and 0.5% (w/v) sodium dodecyl sulfate. This was incubated in the dark for 3.5 hours at 46°C, rinsed with distilled water, and air dried in the dark. Sections were then counterstained with 0.025% (w/v) concanavalin A-Alexa Fluor 594 conjugate for 20 minutes. These were again rinsed and dried, then mounted with FluoroSave. Hybridized sections were viewed using an Olympus BX43 microscope with built-in light fluorescence illuminator. Images were obtained using an attached DP80 digital camera and the associated software (Cellsens).
HPV Immunohistochemical Analysis
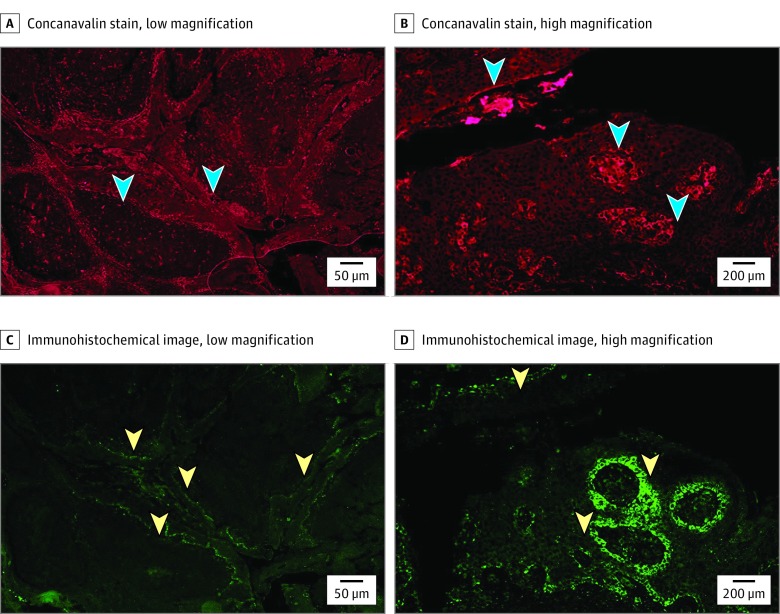
Sections from tissue identified as HPV positive by nested PCR, as previously described, were slide-mounted and incubated at 60° C for 30 minutes. The sections were deparaffinized in 3 successive baths of xylene for 5 minutes each. The tissue was rehydrated by incubation in decreasing grades of ethanol, and finally in distilled water. Epitope retrieval entailed rinsing sections in 3 successive baths of PBS for 2 minutes each. The slides were then placed in a canister containing a high-pH sodium citrate buffer (pH 9). This was placed in a decloaking chamber and cycled through at 110° C for 15 minutes. Slides were rinsed in distilled water after cooling. Sections were rinsed in PBS in 3 successive washes for 2 minutes each. Excess PBS was tapped off and drops of DAKO serum-free protein block was added to the tissue sections until completely covered. These were then incubated at room temperature for 30 minutes in a humidified chamber. Excess block was then removed and primary antibody (Dako monoclonal mouse anti-HPV clone K1H8) diluted 1:50 in DAKO antibody diluent was applied and incubated overnight at 4 C in a humidified chamber. Antibody was then rinsed in 3 successive baths of PBS for 2 minutes each. Biotinylated goat anti-mouse antibody diluted in 1:200 DAKO antibody diluent was added to the sections and incubated at room temperature in a humidified chamber for 30 minutes. The slides were then rinsed in 3 successive baths of PBS for 2 minutes each. The tissue was fixed with antifade mounting media with DAPI (Vectashield) followed by placement of a coverslip. The sections were then viewed under light microscopy, as described previously (Figure 1).
Figure 1. Histopathologic Images.
A, Tonsil tissue stained with concanavalin A (arrowheads indicate glycocalyx matrix, original magnification ×4). B, Tonsil tissue stained with concanavalin A (arrowheads indicate glycocalyx matrix, original magnification ×20). C, Tonsil tissue immunohistochemistry with human papillomavirus (HPV) L1 Ab (arrowheads indicate HPV L1 Ab, original magnification ×4). D, Tonsil tissue immunohistochemistry with HPV L1 Ab (arrowheads indicate HPV L1 Ab, original magnification ×20).
Results
Prevalence
Most of our samples were from the 20 to 29 years age group. The 102 samples were roughly split into male and female, with 47 (46.1%) men and 55 (53.9%) women. Of the total 102 patients in this sample, 5 were found to have evidence of HPV infection by PCR. And of these, 4 were positive for high-risk types 16 or 18 by qPCR. Thus, the overall prevalence of HPV in our sample population is 4.9%, and prevalence of high-risk HPV in this same population is 3.9%.
Immunofluorescence
Cross sections of tonsil tissue were stained with concanavalin A as described. These were then visualized under low-, medium-, and high-powered magnification. The areas of high saturation represent areas of bacterial biofilm. The architecture of the tonsil is such that invaginations into the tissue seen in these images correspond to crypts in the tonsil seen grossly. At low magnification, there is high saturation of concanavalin, representing glycocalyx matrix of biofilm, along the tonsillar crypts. The biofilm is most concentrated within the squamous epithelial layer, but is also noted as a distinct entity along the superficial border of this layer, as well.
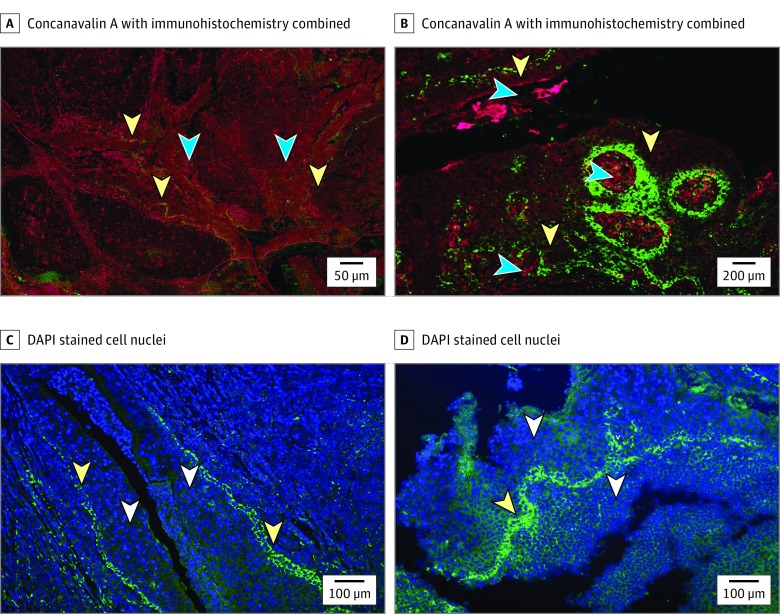
Human papillomavirus capsid protein is visualized with HPV-1 antibody. The HPV-1 antibody binds the L1 capsid protein of the viral particle. The areas of fluorescence that denote HPV correspond to the basal layer of squamous epithelium that lines the crypts (Figure 2). When biofilm matrix and HPV capsid protein are viewed simultaneously, HPV is clearly associated with the bacterial biofilm that is concentrated along the tonsillar crypt region. In addition, HPV can be seen outside of the basal layer freely associated in crypt biofilm (Figure 2).
Figure 2. Histopathologic Images .
A, Tonsil crypts with human papillomavirus (HPV) in epithelial and biofilm layers. Glycocalyx stained with concanavalin A, HPV L1 stained with HPV L1 Ab (anti-HPV clone K1H8), cell nuclei stained with diamidino-phenylindole (DAPI) (yellow arrowheads indicate glycocalyx matrix; blue arrowheads, HPV L1 Ab; original magnification ×4) and B, (yellow arrowheads indicate glycocalyx matrix; blue arrowheads, HPV L1 Ab; original magnification ×20). C, Tonsil tissue with DAPI and HPV L1 immunohistochemistry (white arrowheads indicate cell nuclei; yellow arrowheads, HPV L1 immunohistochemistry; original magnification ×10). D, Tonsil tissue with DAPI and HPV L1 immunohistochemistry (white arrowheads indicate cell nuclei; yellow arrowheads, HPV L1 IHC; original magnification ×10).
At high magnification, DAPI has been used to stain cell nuclei. The HPV continued to follow the linear pattern seen in previous images. Interestingly, the capsid protein is located outside of the cell nuclei. Without concurrent hematoxylin-eosin staining of the tissue, it is difficult to determine whether the HPV is in the cell cytoplasm vs an extracellular location. It may also be residing in the biofilm found dispersed across the squamous epithelial layer.
Discussion
The prevalence of oropharyngeal HPV in this small retrospective sample is similar to previously reported figures for oral HPV infection. The oral mucosa and tonsillar crypts are lined with squamous epithelium, which HPV is known to infect. It is therefore reasonable to expect that prevalence of oral cavity and oropharyngeal HPV would be similar.
Evidence of glycocalyx matrix did not appear to be a factor influencing the presence or absence of HPV. Tonsil tissue that was negative for HPV by PCR still demonstrated evidence of biofilm glycocalyx matrix lining the tonsillar crypts on immunofluorescent imaging. Qualitatively, these matrices across various tonsil tissue samples appeared to be similar.
Tonsil tissue that was HPV positive by PCR demonstrated concentration of HPV virions, as detected by capsid protein, in the crypt epithelial layers. Importantly, virus was also demonstrated to be freely associated in the crypt biofilm and outside of the epithelial cell layer. This suggests that as the virus is shed from the superficial epithelial layer during active infection, it may become trapped in the surrounding biofilm—thereby creating a reservoir of latent virions. This may allow the virus to remain quiescent, escaping immune surveillance in close proximity to squamous epithelium of tonsillar crypts and thereby facilitating a mechanism for reinfection at a later time. Human papillomavirus infection requires basal epithelial cells capable of proliferation. The virus gains access to the basal layer of stratified squamous epithelium through structural breaks in the stratified epithelial superstructure. Tonsillar crypt reticulated epithelium itself has been shown to contain numerous small blood vessels and has a discontinuous basement membrane, which may facilitate this infection and reinfection process. Notably, HPV-associated tonsillar squamous cell carcinomas have been reported to arise largely from the tonsillar crypts. These histologic features of the crypt epithelium may be implicated in both the infectious precursor and invasion potential of these malignant abnormalities.
An unexpected finding in the immunofluorescent evaluation of HPV was the presence of HPV virus outside of the cell nuclei. It is well known that HPV DNA is typically an intranuclear finding. Whether the finding of HPV capsid protein outside of the nuclei represents complete virions is unclear at this time. Further studies using hematoxylin-eosin staining, as well as electron microscopy, are warranted to further evaluate this relationship. Currently, it is difficult to determine if these HPV capsids are in the cell cytoplasm, or are extracellular in location.
Limitations
Prevalence of HPV may be greater than appreciated in this study because this study protocol may underestimate the prevalence of HPV in tonsil tissue. Formalin fixation may cause extensive DNA damage, including cross-linking and fragmentation. Successful amplification of HPV sequences from FFPE specimens is inversely correlated with the length of amplicon of the PCR method. Specimen age may contribute to degradation. MY09/11 has been shown to have lower sensitivity compared with other L1 consensus primer sets. Owing to limitations of retrospective analyses, the significance of reported prevalence in this study is unclear. Risk factors, such as sexual history, were unable to be ascertained. Further investigation into the correlation of these factors is pending.
This study establishes the ability to use FFPE specimens to detect biofilm and colocalize HPV successfully using formalin-fixed tissue. The reproducibility of this process is therefore feasible in a much larger population sample. This may in turn allow for a better understanding of the role of biofilm as a reservoir for HPV, the relationship between HPV and biofilm and a broader array of demographic variables, and the overall pathogenesis of OPSCC.
The role of the HPV vaccine in generating significant effect on prevalence of oral HPV in this population of interest is unknown. As of 2011 only 1.3% of males and 34.8% of females in the United States between the ages of 13 and 17 had received 3 or more doses. After the introduction of the vaccine into the routine immunization schedule of adolescent girls in 2006, there was a 56% reduction in the prevalence of vaccine-targeted cervical HPV infections. However, it is projected that the incidence of HPV-associated OPSCC has yet to peak. Establishing a consistent and reproducible method for testing HPV in the oral cavity and/or oropharynx will be a key component in evaluating the impact of HPV vaccination on HPV prevalence in these areas, and this relationship to HPV-associated OPSCC.
This study successfully demonstrates the concept that HPV colocalizes in bacterial biofilm of tonsillar crypts. Further studies are warranted to explore the significance of this finding. Electron microscopy studies would be able to confirm presence of HPV virions in biofilm. In addition, concurrent HPV DNA in situ hybridization and hematoxylin-eosin staining of these tissues could further elucidate the relationship of the virus to the surrounding host cells.
Conclusions
We have found that the prevalence of HPV in the tonsil tissue of asymptomatic individuals is consistent with previous reports. Human papillomavirus DNA may be demonstrated in both intracellular and extracellular locales in tonsil tissue of healthy, asymptomatic adults. Specifically the use of immunohistochemical and immunofluorescent staining has successfully colocalized HPV capsid protein to crypt biofilm in FFPE tonsil tissue. The significance of these findings is yet unclear. However, further studies based on these data may demonstrate implications for oncogenesis of HPV-related OPSCC. Based on our findings, it is plausible that HPV existing in the biofilm and extracellular space represents a reservoir of latent oncovirus that can functionally escape immune surveillance and clearance. Reactivation of these virions with subsequent infection of the basal epithelial layer may result in the cascade of intracellular events leading to OPSCC.
References
- 1.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407-420. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944-1956. [DOI] [PubMed] [Google Scholar]
- 3.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429-1435. [DOI] [PubMed] [Google Scholar]
- 4.Gooi Z, Chan JYK, Fakhry C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope. 2016;126(4):894-900. [DOI] [PubMed] [Google Scholar]
- 5.Münger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451-11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellanger S, Tan CL, Xue YZ, Teissier S, Thierry F. Tumor suppressor or oncogene? A critical role of the human papillomavirus (HPV) E2 protein in cervical cancer progression. Am J Cancer Res. 2011;1(3):373-389. [PMC free article] [PubMed] [Google Scholar]
- 7.Viens LJ, Henley SJ, Watson M, et al. Human Papillomavirus-Associated Cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666. [DOI] [PubMed] [Google Scholar]
- 8.Palmer E, Newcombe RG, Green AC, et al. Human papillomavirus infection is rare in nonmalignant tonsil tissue in the UK: implications for tonsil cancer precursor lesions. Int J Cancer. 2014;135(10):2437-2443. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3):e20151968. doi: 10.1542/peds.2015-1968 [DOI] [PubMed] [Google Scholar]
- 10.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813-819. [DOI] [PubMed] [Google Scholar]
- 11.Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789-1799. [DOI] [PubMed] [Google Scholar]
- 12.Gillison ML, Broutian T, Pickard RKL, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight GL, Needham L, Ward D, Roberts S. Pilot study investigating the prevalence of oral Human Papilloma Viral (HPV) infection in young adults. Public Health. 2016;132:105-107. [DOI] [PubMed] [Google Scholar]
- 14.Liu G, Markowitz LE, Hariri S, Panicker G, Unger ER. Seroprevalence of 9 human papillomavirus types in the United States, 2005-2006. J Infect Dis. 2016;213(2):191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kania RE, Lamers GEM, Vonk MJ, et al. Characterization of mucosal biofilms on human adenoid tissues. Laryngoscope. 2008;118(1):128-134. [DOI] [PubMed] [Google Scholar]
- 16.Kania RE, Lamers GEM, Vonk MJ, et al. Demonstration of bacterial cells and glycocalyx in biofilms on human tonsils. Arch Otolaryngol Head Neck Surg. 2007;133(2):115-121. [DOI] [PubMed] [Google Scholar]
- 17.Chole RA, Faddis BT. Anatomical evidence of microbial biofilms in tonsillar tissues: a possible mechanism to explain chronicity. Arch Otolaryngol Head Neck Surg. 2003;129(6):634-636. [DOI] [PubMed] [Google Scholar]
- 18.Cryer J, Schipor I, Perloff JR, Palmer JN. Evidence of bacterial biofilms in human chronic sinusitis. ORL J Otorhinolaryngol Relat Spec. 2004;66(3):155-158. [DOI] [PubMed] [Google Scholar]
- 19.Mazaheritehrani E, Sala A, Orsi CF, et al. Human pathogenic viruses are retained in and released by Candida albicans biofilm in vitro. Virus Res. 2014;179:153-160. [DOI] [PubMed] [Google Scholar]
- 20.Tezal M, Sullivan Nasca M, Stoler DL, et al. Chronic periodontitis-human papillomavirus synergy in base of tongue cancers. Arch Otolaryngol Head Neck Surg. 2009;135(4):391-396. [DOI] [PubMed] [Google Scholar]
- 21.Cavalcanti EFF, Silva CR, Ferreira CF, et al. Detection of human papillomavirus in dental biofilm and the uterine cervix of a pregnant adolescent. Sao Paulo Medical J. 2016;134(1):88-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11(16):5694-5699. [DOI] [PubMed] [Google Scholar]
- 23.Castro FA, Koshiol J, Quint W, et al. Detection of HPV DNA in paraffin-embedded cervical samples: a comparison of four genotyping methods. BMC Infect Dis. 2015;15:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen CT, Lewis JS Jr, El-Mofty SK, Haughey BH, Nussenbaum B. Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope. 2010;120(9):1756-1772. [DOI] [PubMed] [Google Scholar]
- 25.Chernock RD, Nussenbaum B, Thorstad WL, et al. Extensive HPV-related carcinoma in situ of the upper aerodigestive tract with ‘nonkeratinizing’ histologic features. Head Neck Pathol. 2014;8(3):322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Koo BS, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120(7):1418-1425. [DOI] [PubMed] [Google Scholar]
- 27.Kajitani N, Satsuka A, Kawate A, Sakai H. Productive lifecycle of human papillomaviruses that depends upon squamous epithelial differentiation. Front Microbiol. 2012;3(152):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Depuydt CE, Boulet GAV, Horvath CAJ, Benoy IH, Vereecken AJ, Bogers JJ. Comparison of MY09/11 consensus PCR and type-specific PCRs in the detection of oncogenic HPV types. J Cell Mol Med. 2007;11(4):881-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Şahiner F, Kubar A, Gümral R, et al. Efficiency of MY09/11 consensus PCR in the detection of multiple HPV infections. Diagn Microbiol Infect Dis. 2014;80(1):43-49. [DOI] [PubMed] [Google Scholar]
- 30.Thavaraj S, Stokes A, Guerra E, et al. Evaluation of human papillomavirus testing for squamous cell carcinoma of the tonsil in clinical practice. J Clin Pathol. 2011;64(4):308-312. [DOI] [PubMed] [Google Scholar]