Key Points
Question
Are serial in-office intralesional steroid injections (ISI) effective in reducing the surgical burden in patients with subglottic and proximal tracheal stenosis (SGS/PTS)?
Findings
In this case series of 24 patients with SGS/PTS of multiple etiologies underwent serial in-office ISI either alone or following endoscopic dilation. Mean surgery-free interval (SFI) compared favorably with those reported by others. Patients with previous dilations underwent fewer dilations after ISI than before, and overall had longer SFI after than before ISI. Most patients did not require surgery after ISI.
Meaning
Serial in-office ISI can be effective both as a primary treatment and as an adjunct to dilation in patients with SGS/PTS, and are safe and well tolerated.
This case-series study examines the association of serial in-office intralesional steroid injection after endoscopic dilation with surgery-free interval in adults with subglottic and proximal tracheal stenosis.
Abstract
Importance
Endoscopic dilation is the mainstay treatment strategy for subglottic and proximal tracheal stenosis (SGS/PTS). Its major limitation is restenosis requiring repeated surgery. Intralesional steroid injection (ISI) is a promising adjunctive treatment aimed at prolonging the effects of dilation.
Objective
To evaluate the association of serial in-office ISI after endoscopic dilation with surgery-free interval (SFI) in adults with SGS/PTS.
Design, Setting, and Participants
A retrospective study of adults with SGS/PTS who underwent at least 2 consecutive in-office ISI at the University of Southern California, Keck School of Medicine, over a 3-year period was conducted.
Exposure
Serial ISI with triamcinolone 40 mg/mL using topical anesthesia, spaced 3 to 6 weeks apart.
Main Outcomes and Measures
Surgery-free interval, number of dilations, need for open airway surgery, decannulation rate, and adverse events. Patients with previous dilations and sufficient follow-up time were included in a comparative analysis of SFI before and after ISI. The Mann-Whitney U test was applied for comparisons.
Results
Twenty-four patients met eligibility criteria. Mean (SD) age was 50.1 (15.1) years; 18 (75%) were female. Ten (42%) patients had idiopathic, 8 (33%) had traumatic, and 6 (25%) had rheumatologic-related SGS/PTS. Mean (SD) follow-up time was 32.3 (33.4) months. Patients underwent mean (SD) 4.08 (1.91) injections. Seventeen (71%) patients have not undergone further surgery after ISI. Mean (SD) SFI was 17.8 (12.8) months overall and was 15.7 (10.6) months for idiopathic, 13.8 (9.9) for traumatic, and 26.7 (16.9) for rheumatologic-related SGS/PTS. Twenty-one (88%) patients underwent dilation(s) prior to ISI. Among patients who fulfilled eligibility criteria for comparison of SFI before and after ISI, SFI improved from 10.1 months before, to 22.6 months after ISI (mean difference, 12.5 months; 95% CI, −2.1 to 27.2 months). Three of 6 patients (all with traumatic SGS/PTS) presenting with a tracheotomy were decannulated. No patients required open airway surgery after ISI. There were no adverse events associated with ISI.
Conclusions and Relevance
Serial in-office ISI are safe and well-tolerated in adults with SGS/PTS. This technique can reduce the surgical burden on these patients and may obviate the need for future airway intervention.
Introduction
Subglottic and proximal tracheal stenosis (SGS/PTS) in adults has 3 main etiologies. The most common of these is trauma from a long-term indwelling endotracheal or tracheotomy tube, which causes pressure necrosis of the respiratory mucosa. This results in an inflammatory response, producing granulation tissue and eventually mature scar. Subglottic and proximal tracheal stenosis also occurs in an idiopathic form (iSGS), or as a manifestation of rheumatologic disease (most commonly granulomatosis with polyangiitis [GPA]). Regardless of etiology, untreated SGS/PTS causes dyspnea that in its mildest forms limits activity and negatively impacts patients’ quality of life, and in its most severe form is life-threatening.
Broadly, surgical treatment of SGS/PTS includes endoscopic and open procedures. Endoscopic treatment entails scar resection, scar lysis, dilation, or a combination of these. Endoscopic treatment has become increasingly common in recent years with the incorporation of the carbon dioxide (CO2) laser into the practice of otolaryngology, as well as advances in laryngeal instrumentation. The major shortcoming of endoscopic treatment of SGS/PTS is the frequent need for repeated surgery owing to restenosis.
Previous work on iSGS has found increased levels of proinflammatory cytokines. In addition, animal models of airway stenosis have revealed decreased responsiveness of submucosal fibroblasts to anti-inflammatory mediators in stenotic lesions. Thus, stenosis and restenosis after dilation likely occur in an altered wound-healing environment resulting from a hyperinflammatory response to injury. Given this pathophysiology, adjunctive treatments to modulate the inflammatory response have been investigated as methods of prolonging the effects of dilation and reducing the need for additional operations. These include systemic antibiotics; topical or intralesional antineoplastic compounds, such as mitomycin C (MMC) or fluorouracil; and systemic, inhaled, or intralesional corticosteroids. Each of these adjuncts has shown mixed results in the treatment of SGS/PTS; none have become standard treatment owing to the small numbers of patients and short follow-up periods in most studies, as well as concern for toxic effects associated with some of these agents. In summary, a widely accepted treatment algorithm has not been defined, and as a result treatment varies considerably among clinicians and institutions.
Bonchek first described the use of intralesional steroid injection (ISI) in combination with serial dilations in a tracheotomy-dependent pediatric patient with SGS/PTS, eventually achieving decannulation with no residual respiratory symptoms. Since then, other studies have suggested that ISI at the time of dilation in the operating room may prolong the effects of dilation in patients with GPA-related SGS/PTS. More recently, the concept that serial ISI may be more effective than a single ISI in the operating room was adopted from the treatment of keloids and hypertrophic scars. Serial in-office ISI in airway stenosis were first described by Franco et al and have been shown to be effective as a sole treatment strategy or as an adjunct to surgery in patients with iSGS. To our knowledge, there are no other reports of the safety and efficacy of serial in-office ISI. This technique is particularly appealing because it can be performed in unsedated patients with routinely available equipment, and may reduce the surgical burden on patients with SGS/PTS. In this study, we describe our experience with serial in-office ISI as an adjunctive treatment to endoscopic dilation, and in some cases a sole treatment strategy, for SGS/PTS of multiple etiologies.
Methods
Under a protocol approved by the institutional review board at the University of Southern California we conducted a retrospective review of all adult patients with SGS/PTS who underwent at least 2 consecutive in-office ISI at the Keck Hospital of USC between September 2013 (adoption date of in-office ISI at our institution) and November 2016 (beginning of data collection for this study). Given the retrospective nature of the study, the institutional review board granted a waiver of informed consent. Billing records were queried by International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis codes (ICD-9 478.47 and 519.19, ICD-10 J38.6 and J39.8) to identify patients with SGS/PTS. Records generated via this search were reviewed for eligibility and cross-referenced with individual records of surgeons performing ISI during the study period. Patients with SGS extending into the proximal trachea were included; patients with distal tracheal, glottic, or supraglottic stenosis were excluded.
Subglottic steroid injections were performed as follows. The nose was anesthetized and decongested with a pressurized aerosol nasal sprayer with 4% lidocaine and phenylephrine. Two to 4 mL of 4% lidocaine was delivered via transtracheal injection or laryngeal gargle for topical laryngeal anesthesia. A flexible distal chip nasolaryngoscope (Kay-PENTAX, Olympus) was inserted transnasally to the level of the subglottis. For most patients, a 1.5-inch 25-gauge needle was passed through the cricothyroid space and used to inject 1 to 2 mL of triamcinolone 40 mg/mL into multiple locations in the region of stenosis. This technique is depicted in Figure 1A-C. In a few cases, the injection was performed via the working channel of a laryngoscope using a 200-cm 25-gauge needle. Triamcinolone was chosen for this purpose given its wide use and demonstrated efficacy as an intralesional treatment for keloids and hypertrophic scars, as well as prior use in iSGS.
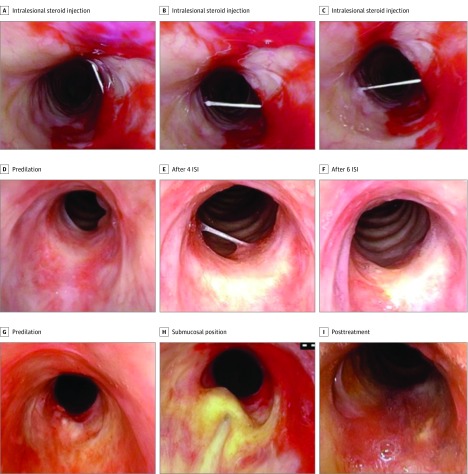
Figure 1. Procedural Images.
A-C, Intralesional steroid injection technique, demonstrating access to multiple places in the stenotic region with a single needle entry site. D, Patient with GPA-related SGS/PTS predilation; E, after 4 ISI; and F, after completing ISI series of 6 injections. G, Patient with idiopathic SGS/PTS predilation; H, undergoing ISI with needle in submucosal position; and I, posttreatment with dilation and ISI. GPA Indicates granulomatous polyangiitis; ISI, intralesional steroid injections; SGS/PTS, subglottic and proximal tracheal stenosis.
Per institutional protocol, patients who underwent endoscopic dilation were treated with inhaled corticosteroids for 1 month and oral trimethoprim-sulfamethoxazole for 2 to 4 weeks postoperatively. The ISI were performed as a planned series of 3 to 6 injections spaced 3 to 6 weeks apart. Patients were then followed with serial examinations, and injections were reinitiated if there was evidence of recurrent stenosis.
Outcome measures included surgery-free interval (SFI), number of endoscopic dilations performed, need for open airway surgery, decannulation rate, and adverse events. The SFI was defined as the time between dilation and the first subsequent dilation or open surgery for treatment of airway stenosis. Follow-up time was defined as the time between the first visit with any otolaryngologist at our institution and the last documented follow-up date. Patients who did not undergo additional airway surgery before their last follow-up date were censored at this date. For patients with known surgical history before the course of dilation with serial ISI, pre-ISI and post-ISI SFI were calculated and compared. Patients with previous dilations with follow-up time longer than prior SFI were included in a comparative analysis of SFI before and after ISI.
Categorical variables were described using frequency and percentage. Continuous variables were described using mean, standard deviation, and range. Statistical analysis was performed using Microsoft Excel 2010 (version 14, Microsoft Inc) and SPSS Statistics software (version 24.0, IBM). Effect sizes were estimated with the use of 95% confidence intervals (CI).
Results
Twenty-four patients met eligibility criteria. Eighteen (75%) were women, 6 (25%) were men. The mean (SD) age was 50.1 (15.2) years (range, 19-76 years). Mean (SD) follow-up time was 32.3 (33.4) months (range, 5-168 months). Ten patients (42%) had idiopathic SGS/PTS, 8 (33%) had traumatic SGS/PTS, and 6 (25%) had SGS/PTS related to rheumatologic disease. Of these 6 patients, 4 (67%) had GPA-related SGS/PTS, 1 (17%) patient had rheumatoid arthritis/systemic lupus erythematosus overlap syndrome, and 1 (17%) patient had rheumatoid factor/cyclic citrullinated peptide-positive, cytoplasmic-antineutrophil cytoplasmic antibody (C-ANCA)–negative inflammatory arthritis.
Ten (41.7%) patients had Cotton-Myer grade 1 stenosis, 3 (12.5%) had grade 2, 5 (20.8%) had grade 3, and 5 (20.8%) had grade 4 stenosis. In 1 (4%) patient, grade of stenosis was unable to be determined from the available records. Mean (SD) length of stenosis was 1.38 (0.54) cm (range, 0.5-2.75 cm). Most patients (18, 75%) had stenosis limited to the subglottis. Six (25%) patients had subglottic stenosis extending into the proximal trachea. Patient characteristics are summarized in the eTable in the Supplement.
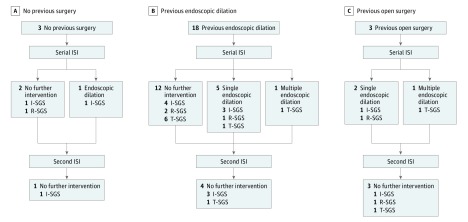
Patients underwent a mean (SD) of 4 (2) injections (range 2-9). Two patients received at least 1 additional ISI several months after their planned initial series of injections. None of these patients have gone on to require subsequent endoscopic dilation or open airway surgery. Figure 1D-I, depicts representative images before and after serial ISI treatment. Figure 2 is a flowchart depicting the clinical course of ISI and surgical interventions for all patients in this series.
Figure 2. Flowchart Depicting All Possible Permutations of ISI and Surgical Interventions for Patients in This Series.
ISI Indicates intralesional steroid injections; I-SGS, idiopathic subglottic/proximal tracheal stenosis; R-SGS, rheumatologic related subglottic/proximal tracheal stenosis; T-SGS, traumatic subglottic/proximal tracheal stenosis.
Mean (SD) SFI was 17.8 (10.5) months overall, and was 15.7 (10.6) months for idiopathic, 13.8 (9.9) months for traumatic, and 26.7 (16.9) months for rheumatologic-related SGS/PTS. Mean SFI was longer for rheumatologic compared with traumatic SGS/PTS (mean difference, 12.9 months; 95% CI, −0.8 to 26.5 months) and compared with idiopathic SGS/PTS (mean difference, 11.0 months; 95% CI for difference, −2.1 to 24.1 months). Mean SFI was marginally longer for idiopathic compared with traumatic SGS/PTS (mean difference, 1.9 months; 95% CI, −10.1 to 13.9 months).
Seventeen (70.8%) of 24 patients overall have not required surgery after the ISI series. By subgroup, 6 (60%) of 10 patients with idiopathic, 6 (75%) of 8 with traumatic, and 5 (83.3%) of 6 with rheumatologic-related stenosis have not required surgery after ISI.
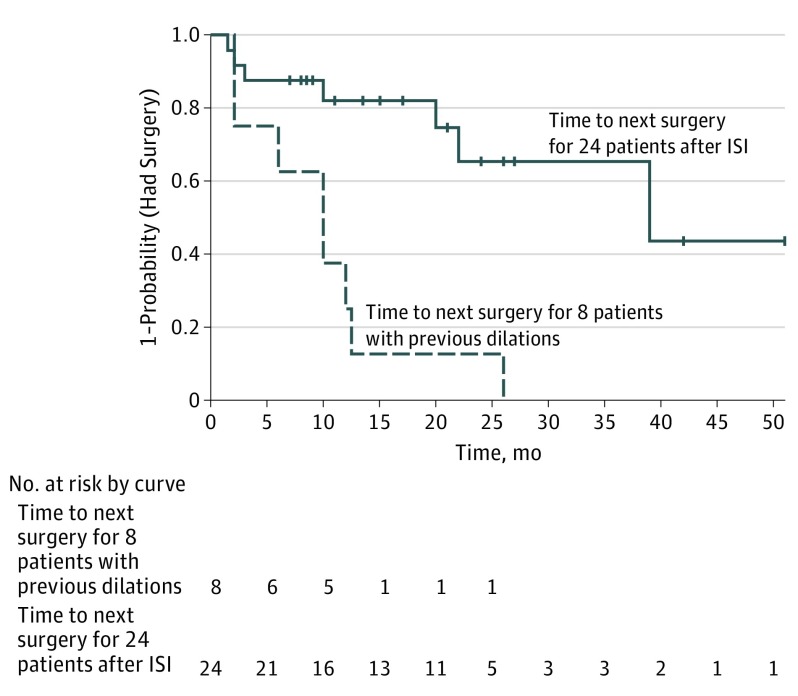
Twenty-one (87.5%) of 24 patients had undergone dilation prior to ISI (Table 1). For 19 (90.5%) of these 21 patients, numbers and dates of prior surgeries were known. Of these 19 patients, 8 had undergone multiple previous dilations and had sufficient follow-up time to compare SFI before and after ISI. Six (75%) of 8 had longer SFI after than before ISI; mean (SD) SFI increased from 10.1 (7.7) months before to 22.6 (15.6) months after ISI (mean difference, 12.5 months; 95% CI, −2.1 to 27.2 months). For six of these 8 patients, history of treatment with adjuvants to dilation is known. Mean SFI after ISI was longer for the 3 patients who had been previously treated with topical MMC than the 3 who had not (mean difference, 23.3 months; 95% CI, −6.5 to 53.1 months). There were insufficient numbers of patients to make comparisons based on prior treatment with intralesional steroid injection in the operating room at the time of dilation. Figure 3 is a Kaplan-Meier curve depicting time to next dilation for the 8 patients with multiple dilations pre-ISI (dashed line), and for the entire cohort (solid line).
Table 1. SFI for Individual Patients Pre-ISI and Post-ISI for Patients With Sufficient Follow-up Time to Be Included in the Comparative Analysis.
| Variable | Pre-ISI SFI | Post-ISI SFI |
|---|---|---|
| Participant | ||
| 1 | 26 | 10 |
| 2 | 10 | 51 |
| 3 | 10 | 22 |
| 4 | 13 | 39 |
| 5 | 12 | 17 |
| 6 | 6 | 15 |
| 7 | 2 | 25 |
| Mean (SD) | 10.1 (7.6) | 22.6 (15.8) |
Abbreviations: ISI, intralesional steroid injections; SFI, surgery-free interval.
Figure 3. Kaplan-Meier Curve Showing Time to Surgery Pre-ISI and Post-ISI Series.
The dashed line indicates time to next surgery for 8 patients with previous dilations and sufficient follow-up time for comparison of SFI pre-ISI and post-ISI series. The solid line indicates time to next surgery for 24 patients after ISI. Data markers represent censoring at date of last follow-up for patients not undergoing repeat surgery in the follow-up period. ISI indicates intralesional steroid injection; SFI, surgery-free interval.
The 19 patients with known surgical history had undergone a mean (SD) 2.32 (2.30) dilations prior to the series of ISI. For all 24 patients in this series, post-ISI surgical history is known. Patients have undergone a mean (SD) of 0.79 (2.26) dilations after ISI (mean difference, 1.53; 95% CI, −0.14 to 3.19). Excluding the 2 nonresponders described below, the mean (SD) number of dilations after ISI was 0.23 (0.53).
Three patients in our series with idiopathic SGS/PTS, including 1 who had undergone 10 prior dilations, underwent serial ISI as sole treatment for their stenosis/recurrence at our institution.
We classified 2 (8.3%) patients in this study as nonresponders based on SFI before and after ISI. One patient was a man with Cotton-Myer grade 3 traumatic stenosis of the subglottis and proximal trachea 2.4 cm in length. This patient underwent ISI and several subsequent dilations, and is currently T-tube dependent. He has cartilaginous tracheal collapse and several severe medical comorbidities, which have precluded open airway surgery.
The second nonresponder was a man with GPA-related Cotton-Myer grade 1 stenosis of the distal subglottis and proximal trachea 1.5 cm in length. He underwent 2 dilations at approximately 2-year intervals prior to presentation at our center. He subsequently underwent 3 ISI, 1 dilation, and 3 additional ISI. He has not required further surgery for the past 21 months of follow-up.
Three (12.5%) patients underwent open airway surgery prior to ISI; none required open airway surgery after ISI. Six patients with traumatic SGS/PTS presented with a tracheotomy. Three (50%) were decannulated in the course of treatment with dilation and ISI. There were no adverse events associated with ISI. Table 2 presents patient characteristics and key outcome measures by etiologic subgroup.
Table 2. Patient Characteristics and Results by Etiologic Subgroup.
| Parameter/Category | Idiopathic (n = 10) |
Traumatic (n = 8) |
Rheumatologic (n = 6) |
|---|---|---|---|
| Grade | 7 | 0 | 3 |
| 1 | 1 | 1 | 1 |
| 2 | 2 | 3 | 0 |
| 3 | 0 | 4 | 1 |
| 4 | 0 | 0 | 1 |
| Unknown | 0 | 0 | 1 |
| Sex | |||
| Male | 0 | 4 | 2 |
| Female | 10 | 4 | 4 |
| Location of stenosis | 10 | 4 | 4 |
| Subglottis | 0 | 4 | 2 |
| Subglottis and proximal trachea | |||
| Length, mean (SD), cm | 1.06 (0.28) | 1.83 (0.57) | 1.16 (0.23) |
| SFI, mean (SD), months | 15.7 (10.6) | 13.8 (9.9) | 26.7 (16.9) |
| No surgery after ISI, No. (%) | 6 (60) | 6 (75) | 5 (83.3) |
| Nonresponders, No. (%) | 0 | 1 (12.5) | 1 (16.7) |
| Decannulated, No. (%) | NA | 3 (50) | NA |
Abbreviations: ISI, intralesional steroid injections; NA, not applicable; SFI, surgery-free interval.
Discussion
Our experience supports the use of in-office ISI as an effective adjunct to endoscopic dilation for SGS/PTS as evidenced by a mean SFI that compares favorably with those reported by others. In addition, this study provides evidence that ISI reduces the surgical burden on patients with SGS/PTS. On average, patients underwent fewer dilations after ISI than before, and most patients did not require surgery after ISI. Among patients with multiple previous dilations and sufficient follow-up time to compare SFI before and after ISI, mean SFI was longer after than before ISI. No patients required open airway surgery after ISI. These findings applied to patients with SGS/PTS of all etiologic subgroups.
Furthermore, although evidence is preliminary, serial ISI may be used in lieu of dilation for patients presenting early in the inflammatory stage or without critically narrow stenosis. This was demonstrated in our cohort by 2 patients from the idiopathic SGS/PTS subgroup who achieved symptom control with serial ISI alone and did not require airway surgery. Three additional patients who had initial endoscopic dilation have undergone serial ISI alone for recurrence of disease and have not required further surgery. This includes 1 patient who had undergone 10 previous dilations at outside institutions at 1-year intervals. Taken together, these data suggest that ISI have clinical benefit independent of dilation. In patients with idiopathic or rheumatologic-related SGS/PTS without cartilaginous framework collapse, serial in-office ISI is a safe and well-tolerated treatment option that may control symptoms and obviate the need for airway surgery under general anesthesia. In patients with noncritical recurrence, we recommend considering a series of 2 to 3 ISI spaced 3 to 4 weeks apart, with progression to surgery if symptoms fail to improve.
Inflammation plays a key role in the pathogenesis of all 3 SGS/PTS subgroups. In traumatic SGS/PTS, indwelling endotracheal or tracheostomy tubes provoke an inflammatory response by submucosal fibroblasts, which eventually results in scarring. There is evidence that the fibroblasts in subglottic scar tissue are phenotypically distinct from those in normal airway mucosa, suggesting that stenosis arises from an altered inflammatory milieu. Similarly, GPA-related SGS/PTS arises from granulomatous inflammation of the subglottis. Disease predilection for the subglottis is thought to be owing to the narrowing of the upper airway at the level of the cricoid ring resulting in turbulent airflow, exposure of the upper airways to gastric acid, and susceptibility to ischemia at the watershed region between laryngeal and tracheal blood supplies. Granulomatosis with polyangiitis-related SGS/PTS is often difficult to differentiate from idiopathic SGS/PTS because airway stenosis may be the presenting or only symptom of GPA, and biopsy and serology results can be negative. Some have suggested that idiopathic SGS is part of a continuum of autoimmune-mediated SGS that also includes GPA-related SGS. Regardless, in all cases, a chronic inflammatory process leads to fibrosis in the subglottis and proximal trachea. The persistence of this abnormal inflammatory environment likely explains restenosis after dilation.
Like SGS/PTS, hypertrophic scars and keloids are thought to result from a hyperactive inflammatory response by skin connective tissue. Research on dermal wound healing has demonstrated important physiologic differences between fibroblasts in keloids vs those in normal skin, including hypersensitivity to transforming growth factor β (TGF-β), and resulting wound contraction and collagen synthesis. Intralesional therapies have been developed to target these aberrant signaling and proliferation pathways. In keloids, ISI have been shown to inhibit TGF-β expression, promote fibroblast apoptosis, flatten and soften scars, and decrease recurrence rates after surgical excision. It follows from the common pathogenesis underlying both keloids and airway stenosis that ISI would be similarly effective in both entities; our results support this conclusion.
As of this writing, serial in-office ISI in airway stenosis have only been described for idiopathic SGS/PTS. To our knowledge, this is the first report on the efficacy of serial in-office ISI for SGS/PTS of multiple etiologies. At our institution, the treatment protocol for SGS/PTS amenable to endoscopic intervention involves either endoscopic dilation followed by ISI, or a trial of ISI alone if the stenosis is not critical. The ISI series consists of 3 to 6 in-office injections spaced 3 to 6 weeks apart. Patients are also treated with inhaled corticosteroids for 1 month and oral antibiotics for 2 to 4 weeks postoperatively. In establishing this protocol, we have attempted to target the inflammatory process responsible for both the development and recurrence of stenosis in all 3 subgroups of SGS/PTS.
Although most SGS/PTS patients respond to serial in-office ISI, select patients may not. Patients with cartilaginous tracheal collapse are known poor responders to endoscopic dilation, which does not address the cartilaginous framework. These patients may not be ideal candidates for either ISI or endoscopic treatment of SGS/PTS and should be considered for open airway surgery if endoscopic treatment is not possible or rapid restenosis occurs. There may be other disease characteristics that influence clinical course and response to ISI; more studies are necessary to elucidate this possibility.
Generally, unsedated ISI are well tolerated by most patients with the use of topical anesthesia. The preparation for and performance of ISI are similar to the techniques used for other in-office laryngologic procedures. There were no adverse events associated with ISI. These findings demonstrate that ISI is a quick and safe procedure to perform. Finally, work by others has shown that shifting procedures from the operating room to the clinic results in considerable cost savings. Although a comprehensive cost-effectiveness analysis is beyond the scope of this work, it is likely that the short procedure time and lower expense of in-office ISI, combined with the reduced need for surgical intervention in the operating room, reduces the expenses associated with treatment of SGS/PTS. Further studies are needed to formally evaluate the cost-effectiveness of this procedure.
Limitations
Our study is limited by its retrospective nature and small size. A shortcoming of our case series is the lack of a well-matched control group not undergoing ISI. Finally, the study is limited by the short duration of follow-up in some patients, which results in an artificially short SFI. Given airway patency and lack of symptoms in these patients at their most recent follow-up visits, we believe that SFI will increase with additional follow-up time.
Conclusions
This technique reduces the surgical burden on patients with SGS/PTS and may obviate the need for future airway intervention. The impact of serial in-office ISI was demonstrated for SGS/PTS of idiopathic, rheumatologic, and traumatic etiology. In our practice, we found that a treatment algorithm conjoining serial ISI with dilation provides immediate symptom relief with durable airway patency in most SGS/PTS patients, regardless of etiology. Furthermore, we believe ISI may be used as the sole treatment strategy in select patients.
eTable 1. Patient Characteristics
References
- 1.Lorenz RR. Adult laryngotracheal stenosis: etiology and surgical management. Curr Opin Otolaryngol Head Neck Surg. 2003;11(6):467-472. [DOI] [PubMed] [Google Scholar]
- 2.Nouraei SA, Singh A, Patel A, Ferguson C, Howard DJ, Sandhu GS. Early endoscopic treatment of acute inflammatory airway lesions improves the outcome of postintubation airway stenosis. Laryngoscope. 2006;116(8):1417-1421. [DOI] [PubMed] [Google Scholar]
- 3.Gnagi SH, Howard BE, Anderson C, Lott DG. Idiopathic subglottic and tracheal stenosis: a survey of the patient experience. Ann Otol Rhinol Laryngol. 2015;124(9):734-739. [DOI] [PubMed] [Google Scholar]
- 4.Patel HH, Goldenberg G, McGinn J Surgical management of upper airway stenosis. In: Cummings CW, Ed. Cummings Otolaryngology Head & Neck Surgery. 5th Ed. Philadelphia, PA: Mosby/Elsevier, 2010. [Google Scholar]
- 5.Gelbard A, Katsantonis NG, Mizuta M, et al. Idiopathic subglottic stenosis is associated with activation of the inflammatory IL-17A/IL-23 axis. Laryngoscope. 2016;126(11):E356-E361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chafin JB, Sandulache VC, Dunklebarger JL, et al. Graded carbon dioxide laser-induced subglottic injury in the rabbit model. Arch Otolaryngol Head Neck Surg. 2007;133(4):358-364. [DOI] [PubMed] [Google Scholar]
- 7.Mankarious LA, Adams AB, Pires VL. Patterns of cartilage structural protein loss in human tracheal stenosis. Laryngoscope. 2002;112(6):1025-1030. [DOI] [PubMed] [Google Scholar]
- 8.Parker NP, Bandyopadhyay D, Misono S, Goding GS Jr. Endoscopic cold incision, balloon dilation, mitomycin C application, and steroid injection for adult laryngotracheal stenosis. Laryngoscope. 2013;123(1):220-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichert LK, Zhao AS, Galati LT, Shapshay SM. The efficacy of mitomycin C in the treatment of laryngotracheal stenosis: results and experiences with a difficult disease entity. ORL J Otorhinolaryngol Relat Spec. 2015;77(6):351-358. [DOI] [PubMed] [Google Scholar]
- 10.Roediger FC, Orloff LA, Courey MS. Adult subglottic stenosis: management with laser incisions and mitomycin-C. Laryngoscope. 2008;118(9):1542-1546. [DOI] [PubMed] [Google Scholar]
- 11.Hirshoren N, Eliashar R. Wound-healing modulation in upper airway stenosis-myths and facts. Head Neck. 2009;31(1):111-126. [DOI] [PubMed] [Google Scholar]
- 12.Bonchek LI. Successful treatment of postintubation subglottic stenosis with intralesional steroid injections. Ann Thorac Surg. 1973;15(1):84-87. [DOI] [PubMed] [Google Scholar]
- 13.Cobb WB, Sudderth JF. Intralesional steroids in laryngeal stenosis: a preliminary report. Arch Otolaryngol. 1972;96(1):52-56. [DOI] [PubMed] [Google Scholar]
- 14.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope. 2016;126(6):1390-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol. 2003;30(5):1017-1021. [PubMed] [Google Scholar]
- 16.Wolter NE, Ooi EH, Witterick IJ. Intralesional corticosteroid injection and dilatation provides effective management of subglottic stenosis in Wegener’s granulomatosis. Laryngoscope. 2010;120(12):2452-2455. [DOI] [PubMed] [Google Scholar]
- 17.Wierzbicka M, Tokarski M, Puszczewicz M, Szyfter W. The efficacy of submucosal corticosteroid injection and dilatation in subglottic stenosis of different aetiology. J Laryngol Otol. 2016;130(7):674-679. [DOI] [PubMed] [Google Scholar]
- 18.Ledon JA, Savas J, Franca K, Chacon A, Nouri K. Intralesional treatment for keloids and hypertrophic scars: a review. Dermatol Surg. 2013;39(12):1745-1757. [DOI] [PubMed] [Google Scholar]
- 19.Franco RA, Hussain I, Reder L, Paddle P. Awake serial intralesional steroid injections without surgery as a novel targeted treatment for idiopathic subglottic stenosis [published online October 8, 2017]. Laryngoscope. doi: 10.1002/lary.26874 [DOI] [PubMed] [Google Scholar]
- 20.Mazhar K, Gunawardana M, Webster P, et al. Bacterial biofilms and increased bacterial counts are associated with airway stenosis. Otolaryngol Head Neck Surg. 2014;150(5):834-840. [DOI] [PubMed] [Google Scholar]
- 21.Sandulache VC, Chafin JB, Li-Korotky HS, Otteson TD, Dohar JE, Hebda PA. Elucidating the role of interleukin 1beta and prostaglandin E2 in upper airway mucosal wound healing. Arch Otolaryngol Head Neck Surg. 2007;133(4):365-374. [DOI] [PubMed] [Google Scholar]
- 22.Supance JS, Reilly JS, Doyle WJ, Bluestone CD, Hubbard J. Acquired subglottic stenosis following prolonged endotracheal intubation: a canine model. Arch Otolaryngol. 1982;108(11):727-731. [DOI] [PubMed] [Google Scholar]
- 23.Marshak G, Doyle WJ, Bluestone CD. Canine model of subglottic stenosis secondary to prolonged endotracheal intubation. Laryngoscope. 1982;92(7 Pt 1):805-809. [DOI] [PubMed] [Google Scholar]
- 24.Singh T, Sandulache VC, Otteson TD, et al. Subglottic stenosis examined as a fibrotic response to airway injury characterized by altered mucosal fibroblast activity. Arch Otolaryngol Head Neck Surg. 2010;136(2):163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gluth MB, Shinners PA, Kasperbauer JL. Subglottic stenosis associated with Wegener’s granulomatosis. Laryngoscope. 2003;113(8):1304-1307. [DOI] [PubMed] [Google Scholar]
- 26.Taylor SC, Clayburgh DR, Rosenbaum JT, Schindler JS. Clinical manifestations and treatment of idiopathic and Wegener granulomatosis-associated subglottic stenosis. JAMA Otolaryngol Head Neck Surg. 2013;139(1):76-81. [DOI] [PubMed] [Google Scholar]
- 27.Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98(5):827-833. [DOI] [PubMed] [Google Scholar]
- 28.Xu SJ, Teng JY, Xie J, Shen MQ, Chen DM. [Comparison of the mechanisms of intralesional steroid, interferon or verapamil injection in the treatment of proliferative scars]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2009;25(1):37-40. [PubMed] [Google Scholar]
- 29.Insalaco L, Saxon S, Spiegel JH. What is the role of intralesional corticosteroid injections for keloids before considering surgery? Laryngoscope. 2016;126(3):549-550. [DOI] [PubMed] [Google Scholar]
- 30.Darzi MA, Chowdri NA, Kaul SK, Khan M. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg. 1992;45(5):374-379. [DOI] [PubMed] [Google Scholar]
- 31.Chowdri NA, Masarat M, Mattoo A, Darzi MA. Keloids and hypertrophic scars: results with intraoperative and serial postoperative corticosteroid injection therapy. Aust N Z J Surg. 1999;69(9):655-659. [DOI] [PubMed] [Google Scholar]
- 32.Simpson GT, Strong MS, Healy GB, Shapshay SM, Vaughan CW. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol. 1982;91(4 Pt 1):384-388. [DOI] [PubMed] [Google Scholar]
- 33.Rees CJ, Postma GN, Koufman JA. Cost savings of unsedated office-based laser surgery for laryngeal papillomas. Ann Otol Rhinol Laryngol. 2007;116(1):45-48. [DOI] [PubMed] [Google Scholar]
- 34.Kuo CY, Halum SL. Office-based laser surgery of the larynx: cost-effective treatment at the office’s expense. Otolaryngol Head Neck Surg. 2012;146(5):769-773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Patient Characteristics