Key Points
Question
In ICU patients receiving invasive ventilation, is a strategy using on-demand nebulization of acetylcysteine or salbutamol inferior to using routine nebulization of acetylcysteine with salbutamol with respect to days ventilator-free and alive at day 28?
Findings
In this randomized clinical trial that included 922 ICU patients receiving invasive ventilation who were expected to not be extubated within 24 hours, on-demand compared with routine nebulization of acetylcysteine with salbutamol resulted in 20 vs 21 days ventilator-free and alive at day 28, a difference that met the noninferiority margin of −0.5 days.
Meaning
Among ICU patients receiving invasive ventilation, on-demand nebulization was not inferior to routine nebulization of acetylcysteine with salbutamol.
Abstract
Importance
It remains uncertain whether nebulization of mucolytics with bronchodilators should be applied for clinical indication or preventively in intensive care unit (ICU) patients receiving invasive ventilation.
Objective
To determine if a strategy that uses nebulization for clinical indication (on-demand) is noninferior to one that uses preventive (routine) nebulization.
Design, Setting, and Participants
Randomized clinical trial enrolling adult patients expected to need invasive ventilation for more than 24 hours at 7 ICUs in the Netherlands.
Interventions
On-demand nebulization of acetylcysteine or salbutamol (based on strict clinical indications, n = 471) or routine nebulization of acetylcysteine with salbutamol (every 6 hours until end of invasive ventilation, n = 473).
Main Outcomes and Measures
The primary outcome was the number of ventilator-free days at day 28, with a noninferiority margin for a difference between groups of −0.5 days. Secondary outcomes included length of stay, mortality rates, occurrence of pulmonary complications, and adverse events.
Results
Nine hundred twenty-two patients (34% women; median age, 66 (interquartile range [IQR], 54-75 years) were enrolled and completed follow-up. At 28 days, patients in the on-demand group had a median 21 (IQR, 0-26) ventilator-free days, and patients in the routine group had a median 20 (IQR, 0-26) ventilator-free days (1-sided 95% CI, −0.00003 to ∞). There was no significant difference in length of stay or mortality, or in the proportion of patients developing pulmonary complications, between the 2 groups. Adverse events (13.8% vs 29.3%; difference, −15.5% [95% CI, −20.7% to −10.3%]; P < .001) were more frequent with routine nebulization and mainly related to tachyarrhythmia (12.5% vs 25.9%; difference, −13.4% [95% CI, −18.4% to −8.4%]; P < .001) and agitation (0.2% vs 4.3%; difference, −4.1% [95% CI, −5.9% to −2.2%]; P < .001).
Conclusions and Relevance
Among ICU patients receiving invasive ventilation who were expected to not be extubated within 24 hours, on-demand compared with routine nebulization of acetylcysteine with salbutamol did not result in an inferior number of ventilator-free days. On-demand nebulization may be a reasonable alternative to routine nebulization.
Trial Registration
clinicaltrials.gov Identifier: NCT02159196
This noninferiority randomized trial compares the effects of on-demand nebulization of acetylcysteine or salbutamol vs routine nebulization of acetylcysteine and salbutamol on duration of invasive ventilation and mortality in adult intensive care unit (ICU) patients.
Introduction
Mucus plugging can increase morbidity and mortality in intensive care unit (ICU) patients receiving invasive ventilation. Mucociliary clearance could be impaired because of the presence of the endotracheal tube. Ventilation with relatively dry gases may lead to dehydration of the mucosa, increasing bronchial mucus production. Furthermore, an inadequate cough reflex resulting from depressed levels of consciousness, sedation, and paralysis puts ICU patients receiving invasive ventilation at increased risk of airway obstruction and atelectasis, eventually leading to airway colonization and respiratory infections.
Use of nebulized mucolytics may provide benefit in ICU patients receiving invasive ventilation, but evidence is limited. Because of its ability to hydrolyze and break the disulfide ponds in mucin, acetylcysteine is one of the most commonly used mucolytics. Two small randomized clinical trials have evaluated this drug in ICU patients using physiologic but not clinical outcomes. To avoid a possible increase of airway resistance, acetylcysteine is frequently combined with a bronchodilator. Bronchodilators can further improve mucus clearance through an increase in small airways diameter.
The literature on practice of nebulization of mucolytics is limited and suggests inconsistent use. Several publications have reported preventive use of inhaled drugs in ICU patients receiving invasive ventilation who do not have evidence of reversible airway disease, although with large variations in frequency and dosages.
The Preventive Nebulization of Mucolytic Agents and Bronchodilating Drugs in Intubated and Ventilated Intensive Care Unit Patients (NEBULAE) randomized clinical trial was conducted to test whether a strategy of on-demand nebulization of acetylcysteine or salbutamol is noninferior to a strategy of routine nebulization of acetylcysteine with salbutamol with respect to duration of invasive ventilation and mortality.
Methods
Study Design and Oversight
The NEBULAE study was a randomized clinical trial conducted at the ICUs of 7 hospitals in the Netherlands. The study design and statistical analysis plan have been published; the approved study protocol is available in Supplement 1. The institutional review boards of all centers approved the trial. Written informed consent was obtained from all patients’ representatives. An independent data and safety monitoring committee monitored safety data.
Patients
The trial enrolled patients receiving invasive ventilation that started shortly before admission to or in the ICU of a participating hospital and who were expected to not be extubated within 24 hours after randomization. Exclusion criteria were age younger than 18 years; pregnancy; ventilation lasting more than 24 hours before randomization; previous invasive ventilation in another ICU; a known allergy to acetylcysteine or salbutamol; a medical history mandating use of mucolytics or bronchodilators; expected need for long-term ventilation because of a known neuromuscular disease or suspected complete spinal cord lesions; patients receiving palliative care only; or previously included in this trial.
Randomization and Masking
Patients were randomized in a 1:1 ratio to a strategy of nebulization of acetylcysteine or salbutamol on strict indications (on-demand nebulization) or a strategy of routine nebulization of acetylcysteine with salbutamol (routine nebulization). The local investigators randomized patients using a central, dedicated, password-protected, secure socket layer–encrypted, web-based, automated randomization system (ALEA; TenALEA Consortium, Amsterdam, the Netherlands). The randomization sequence was generated using a permuted block design with random block sizes of minimum 2 and maximum 8 patients and was stratified per center. Attending nurses and independent physicians were aware of the nebulization strategy in individual patients, but they were not aware of the primary outcome of the trial.
Interventions
Patients assigned to the on-demand nebulization group received nebulization of 5-mL solutions containing acetylcysteine (300 mg) when thick or tenacious secretions were noted or nebulization of 5-mL solutions containing salbutamol (2.5 mg) when wheezing was clinically suspected or observed or when typical abnormalities of ventilator waves or end-tidal CO2 curves suggested obstruction of the lower airways. The continued need for nebulization of acetylcysteine or salbutamol was reassessed daily by independent physicians. Patients assigned to the routine nebulization group received acetylcysteine with salbutamol 4 times daily, from start to end of invasive ventilation and, in the case of ventilation through a tracheostomy tube, until ventilator support was discontinued for longer than 24 hours.
The assigned strategies were continued for a maximum of 28 days. If a patient required reintubation and additional invasive ventilation within this period, the nebulization strategy to which the patient had been randomized was resumed. The nebulization technique was similar in all centers, as described in the eMethods in Supplement 2.
Standard Care
Standard care followed strict local clinical guidelines, as described in the eMethods in Supplement 2.
Weaning From Ventilator
At least every 8 hours the attending nurses tested whether patients triggered the ventilator, to permit a switch to a spontaneous ventilation mode. Next, and again at least every 8 hours, the attending nurses assessed readiness for extubation by lowering the pressure support level stepwise to 5 cm H2O. Attending physicians present around the clock made the decision to extubate a patient based on general extubation criteria, including adequate patient responsiveness and cooperation, appropriate cough reflex, oxygenation saturation greater than 90% with a ratio of Pao2 to fraction of inspired oxygen (Fio2) greater than 200 mm Hg at Fio2 of 0.4 or less, and respiratory rate between 8/min and 30/min with no signs of respiratory distress such as marked accessory muscle use, abdominal paradox, diaphoresis, or dyspnea.
Tracheostomy was preferably not performed in the first 10 days after start of invasive ventilation. Indications included expected duration of ventilation of more than 14 days, a persistent Glasgow Coma Score less than 7 with inadequate swallow or cough reflex or retention of sputum, severe ICU-acquired weakness evaluated by clinical inspection, or repeated respiratory failure after successive tracheal extubations.
Outcomes
The primary outcome was the number of ventilator-free days and alive at day 28, defined as the number of days that a patient was alive and free from invasive ventilation, calculated from the moment of start of invasive ventilation in the ICU, if the period of unassisted breathing lasted longer than 24 consecutive hours. In case of repeated intubation and extubation, all periods free from invasive ventilation and lasting at least 24 consecutive hours were calculated and summed. Timing of intubation and extubation was captured in hours, and numbers of hours a patient received invasive ventilation were used to calculate duration of ventilation. However, the primary end point was expressed in rounded days. Patients who died before day 28, regardless of whether they achieved unassisted breathing, or patients who received invasive ventilation for more than 28 days, were assigned to have zero ventilator-free days. In patients without tracheostomy, successful unassisted breathing was defined as extubated and without the need for reintubation within 24 consecutive hours. In patients with tracheostomy, successful unassisted breathing was defined as breathing without ventilatory assistance lasting for the same period.
Secondary outcomes included ICU and hospital length of stay and mortality; pulmonary complications (development of moderate or severe acute respiratory distress syndrome, ventilator–associated pneumonia, development of severe atelectasis, pneumothorax, and tube occlusion requiring endotracheal tube replacement). Nebulization-related adverse events were hypoxemia, dyspnea, bronchospasm, apnea, self-extubation, agitation, nausea or vomiting, and tachyarrhythmia, as described in the eMethods in Supplement 2. One of the secondary outcomes concerned health care–related costs, for which we collected costs of ventilation, stay in ICU and hospital, cumulative use of sedative drugs and neuromuscular blocking agents, and use of tracheostomies, as well as costs related to treatment of ventilator-associated pneumonia. This outcome, however, is not reported in this article.
Statistical Analysis
A sample size of 890 patients (445 patients per group) would have 80% statistical power to show noninferiority of on-demand compared with routine nebulization, using a 1-sided significance level of .05 and a noninferiority margin of 10% of the median duration of invasive ventilation, assuming no difference in the number of ventilator-free days between the 2 randomization groups. Nine-hundred fifty patients were to be enrolled (475 in each group) to allow for an anticipated dropout or loss to follow-up of 5%. This power calculation was based on the duration of invasive ventilation of 5 days, with an associated coefficient of variation of 0.7 days in 2 large representative patient cohorts in the Netherlands that included patients fulfilling the same inclusion and exclusion criteria as patients in the present trial.
The choice of a noninferiority margin of 10% (ie, 0.5 days) was motivated by what could be considered acceptable from a clinical point of view. Practically, this margin meant that a difference of less than 12 hours with on-demand nebulization was considered noninferior to routine nebulization.
The primary analysis followed a modified intention-to-treat principle and used a 1-sided 95% CI for noninferiority. The per-protocol analysis excluded patients who received nebulization for a reason other than allowed by the study protocol and patients in whom nebulization was started or withheld without a reported reason in the on-demand group and the routine group, respectively. If noninferiority could be confirmed, superiority of on-demand nebulization was tested, using a 90% CI, for the difference in the primary outcome. Because this approach used a hierarchical closed-testing procedure examining a single CI, there was no adjustment of the overall type I error. Analyses of secondary outcomes were performed using common 2-tailed superiority hypothesis tests with α = .05 and with 2-sided 95% CIs. Because the analyses of the secondary outcomes were considered exploratory, we did not correct for type I error.
The amount of missing data was low, and no assumptions were made for missing data. Because the Medical Research Human Subjects Act in the Netherlands does not allow collection of data from patients not enrolled in a study, we were unable to compare clinical characteristics of enrolled patients with those of eligible patients.
For the primary analysis, the number of ventilator-free days was compared using the Mann-Whitney U test. The estimate of the difference between the randomization groups was calculated using a Hodges-Lehman statistic; estimates were reported using a distribution-free 1-sided 95% CI. Noninferiority was established if the lower bound of the 1-sided 95% CI did not cross the margin of −0.5 days.
Duration of ventilation, ICU and hospital length of stay, and 90-day mortality were compared using Kaplan-Meier survival curves. Survival time was calculated from start of invasive ventilation until time of death from any cause or from time to censoring if lost to follow-up with regard to hospital and 90-day mortality.
The number of patients developing at least 1 pulmonary complication was compared using counts and percentages of single events for the randomization groups. Likewise, the number of patients having at least 1 nebulization-related adverse event was compared using counts and percentages of events per type of nebulization in each randomization group. Differences between groups were analyzed using a 2-tailed Fisher exact test and presented as differences in proportions, with 95% CIs.
In a predefined exploratory analysis, treatment effects on the primary outcome were investigated in prespecified subgroups based on patient categories, including patients with pneumonia vs patients without pneumonia, surgical vs medical admissions, type of nebulizer (jet nebulizer or vibrating mesh), and humidification method (active or passive). The analysis deviated from the original analysis plan by also comparing patients after out-of-hospital cardiac arrest with other patients. The homogeneity of treatment effects on the primary outcome across subgroups was examined at a 2-sided significance level of .05 using a test for the treatment × subgroup interaction by adding this term and the subgroup as covariates in a generalized linear model. As a sensitivity analysis, we conducted a generalized linear mixed-effects model with the stratification variable center as random effect.
R version 3.4.3 (R Core Team) was used for all statistical analyses.
Results
Patients
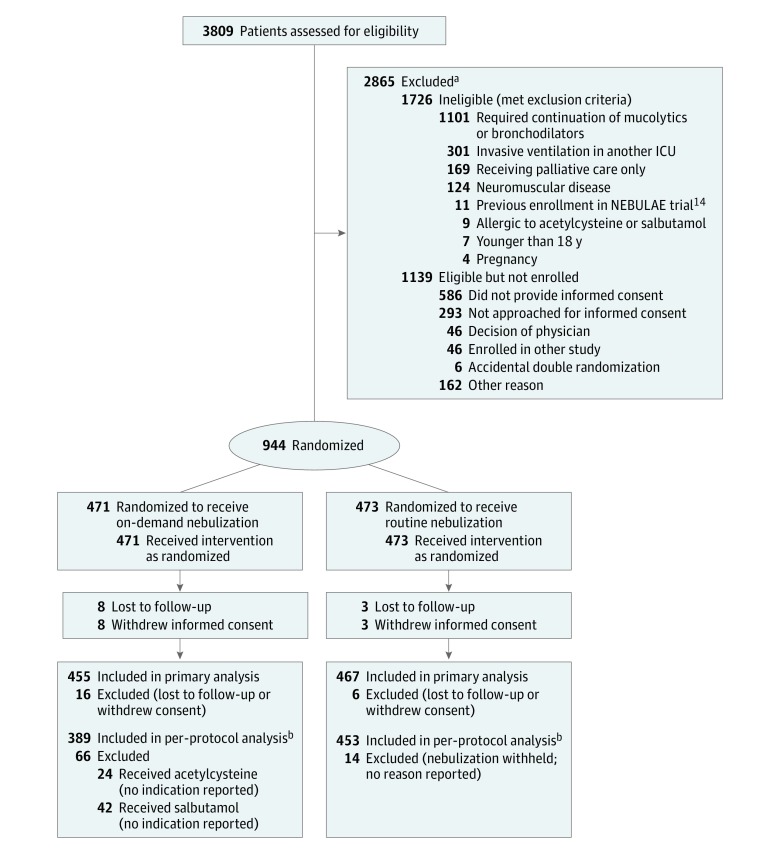
From June 22, 2014, to November 24, 2016, 3809 ICU patients receiving invasive ventilation were screened. A total of 2865 were not enrolled, of whom 1726 (60.2%) met exclusion criteria and 1139 (39.8%) were eligible but not enrolled for other reasons. Nine hundred forty-four patients were randomized, 471 to the on-demand nebulization group and 473 to the routine nebulization group. Representatives of 11 patients withdrew consent to use study data. Follow-up until day 28 was incomplete for 11 patients. Thus, data for 922 patients (455 in the on-demand group and 467 in the routine group) were considered for the primary analysis (Figure 1).
Figure 1. Flow of Patients in the NEBULAE Trial.
ICU indicates intensive care unit; NEBULAE, Preventive Nebulization of Mucolytic Agents and Bronchodilating Drugs in Intubated and Ventilated Intensive Care Unit Patients.
aPatients could have more than 1 reason for exclusion; the most important reason was reported.
bPatients in the on-demand group and routine group who had at least 1 protocol deviation were excluded from the per-protocol analysis.
Of 455 patients supposed to receive on-demand nebulization, 66 were excluded for per-protocol analysis, since these patients received nebulization with acetylcysteine or salbutamol for reasons not reported. Of 467 patients randomized to receive routine nebulization, 14 patients were excluded for per-protocol analysis because nebulization was withheld without any reason reported. The data for 842 patients (389 in the on-demand group and 453 in the routine group) were considered for the per-protocol analysis.
The data and safety monitoring committee evaluated safety data while remaining blinded to the primary end point at 2 predefined time points and recommended the trial be continued.
Baseline characteristics were well balanced between the randomization groups (Table 1). More than half of the patients were admitted for a medical reason. The most frequent reason for invasive ventilation was cardiac arrest. In the on-demand and routine group, baseline tidal volume, positive end-expiratory pressure, and other airway pressure levels were not significantly different.
Table 1. Baseline Patient Characteristics.
| Characteristic | On–Demand Nebulization (n = 455) | Routine Nebulization (n = 467) |
|---|---|---|
| Age, median (IQR), y | 66 (56 to 75) | 65 (54 to 74) |
| Women, No. (%) | 155 (34.1) | 165 (35.3) |
| BMI, median (IQR), kg/m2 | 26 (23 to 29) | 26 (24 to 29) |
| APACHE II, median (IQR)a | 24 (18 to 31) | 23 (17 to 30) |
| Reason of ICU admission, No. (%) | ||
| Medical | 350 (76.9) | 349 (74.7) |
| Surgical | 105 (23.1) | 118 (25.3) |
| Reason of invasive ventilation, No. (%) | ||
| OHCA | 139 (30.5) | 126 (27.0) |
| Postoperative ventilation | 59 (13.0) | 77 (16.5) |
| Head trauma or brain surgery | 53 (11.6) | 52 (11.1) |
| Pneumonia | 46 (10.1) | 41 (8.8) |
| Sepsis | 41 (9.0) | 49 (10.5) |
| Cardiac failure | 28 (6.2) | 23 (4.9) |
| Trauma | 25 (5.5) | 27 (5.8) |
| Respiratory insufficiency | 15 (3.3) | 13 (2.8) |
| Aspiration | 11 (2.4) | 14 (3.0) |
| Airway protection | 6 (1.3) | 13 (2.8) |
| ARDS | 2 (0.4) | 3 (0.6) |
| Other | 25 (5.5) | 29 (6.2) |
| Comorbidity, No, (%) | ||
| Diabetes mellitus | 86 (18.9) | 89 (19.1) |
| Cardiovascular disease | 77 (16.9) | 86 (18.4) |
| Pulmonary disease | 33 (7.3) | 40 (8.6) |
| Immunosuppression | 37 (8.1) | 32 (6.9) |
| Duration of invasive ventilation prior to randomization, median (IQR), hours | 9 (4-15) | 9 (3-15) |
| Nebulization prior to randomization, median (IQR), No. | 0 (0-0) | 0 (0-0) |
| Respiratory measures, median (IQR) | ||
| Pao2 to Fio2 ratio | 204 (133-307) | 199 (129-303) |
| Fio2, % | 50 (40-70) | 50 (40-62) |
| Tidal volume, mL/kg predicted body weight | 6.9 (6.1-7.9) | 6.9 (6.1-7.8) |
| Plateau airway pressure, cm H2Ob | 22 (18-27) | 22 (18-27) |
| Positive end-expiratory pressure, cm H2O | 8 (5-10) | 8 (5-10) |
| Respiratory rate, breaths/min | 19 (15-22) | 18 (15-22) |
Abbreviations: APACHE, acute physiology and chronic health evaluation; ARDS, acute respiratory distress syndrome; BMI, body mass index; Fio2, fraction of inspired oxygen; ICU, intensive care unit; IQR, interquartile range; OHCA, out-of-hospital cardiac arrest.
The range of APACHE II is 0 to 71; an APACHE II score of 23 to 24 indicates a risk of hospital mortality of 46% to 50%, depending on the reason for admission.
Or maximum airway pressure with pressure assist–controlled ventilation.
Nebulization
In the on-demand group, 187 patients (41%) received nebulization, totaling 748 nebulization days. In the routine group, 463 patients (99%) received nebulization, totaling 2944 nebulization days. Details are reported in the eResults in Supplement 2.
Ventilator Settings and Respiratory Variables
Median tidal volumes, positive end-expiratory pressure, and other airway pressures from day 1 through day 3 were not significantly different (eTable 1 in Supplement 2). Partial pressure of carbon dioxide, arterial pH, and Pao2 to Fio2 ratios did not significantly differ between groups. Controlled ventilation lasted a median of 2 days (interquartile range [IQR], 1-3days), irrespective of nebulization group.
Outcomes
At day 28, there were a median of 21 (IQR, 0-26) ventilator-free days in the on-demand group and a median of 20 (IQR, 0-26) ventilator-free days in the routine group (1-sided 95% CI, −0.00003 to ∞), indicating on-demand nebulization to be noninferior to routine nebulization (Table 2). The superiority analysis showed no statistically significant difference between the randomization groups (P = .78). The per-protocol analysis showed a median of 23 (IQR, 0-26) ventilator-free days in the on-demand group and a median of 20 (IQR, 0-26) ventilator-free days in the routine group. Noninferiority was again established, as the 1-sided 95% CI and did not cross the margin of −0.5 days (1-sided 95% CI, −0.00005 to ∞).
Table 2. Primary Analysis and Subgroup Analyses.
| No. of Patients | Ventilator-Free Days | P Value for Interaction | ||||
|---|---|---|---|---|---|---|
| On-Demand Nebulization | Routine Nebulization | Median (IQR) | Estimate of Difference (95% 1-Sided CI)a | |||
| On-Demand Nebulization | Routine Nebulization | |||||
| Primary Analysis | ||||||
| Intention-to-treat | 455 | 467 | 21 (0-26) | 20 (0-26) | –0.00001 (–0.00003 to ∞) | NA |
| Per-protocol | 389 | 453 | 23 (0-26) | 20 (0-26) | –0.00004 (–0.00005 to ∞) | NA |
| Subgroup Analyses | ||||||
| Medical vs surgical | ||||||
| Medical | 350 | 349 | 20 (0-26) | 18 (0-26) | –0.00009 (–0.0001 to ∞) | .95 |
| Surgical | 105 | 118 | 22 (0-26) | 21 (0-26) | –0.00006 (–0.25 to ∞) | |
| Pneumonia vs other | ||||||
| No pneumonia | 409 | 426 | 21 (0-26) | 20 (0-26) | –0.00006 (–0.0001 to ∞) | .42 |
| Pneumonia | 46 | 41 | 17 (0-25) | 0 (0-24) | –0.000008 (–2.5 to ∞) | |
| OHCA vs other | ||||||
| No OHCA | 311 | 336 | 20 (0-26) | 19 (0-25) | –0.00003 (–0.00007 to ∞) | .44 |
| OHCA | 144 | 131 | 21 (0-26) | 21 (0-26) | 0.00007 (0.00003 to ∞) | |
| Type of humidification | ||||||
| Active | 236 | 231 | 19 (0-25) | 15 (0-24) | –0.00007 (–0.23 to ∞) | .12 |
| Passive | 219 | 236 | 23 (0-26) | 23 (0-26) | 0.00001 (–0.00002 to ∞) | |
| Type of nebulizer | ||||||
| Jetb | 175 | 181 | 23 (0-26) | 23 (0-26) | –0.00005 (–0.00009 to ∞) | .55 |
| Meshc | 280 | 286 | 18 (0-25) | 14 (0-25) | –0.000001 (–0.01 to ∞) | |
Abbreviations: IQR, interquartile range; NA, not applicable; OHCA, out-of-hospital cardiac arrest.
The estimated difference between the groups in ventilator-free days was calculated with a 1-sided 95% CI using the Hodges-Lehmann estimator. The margin for noninferiority was set at −0.5 days. A lower bound of the CI that did not exceed this margin indicated noninferiority.
A nebulizer in which compressed air or oxygen creates a flow through a liquid medicine at high velocity to turn it into an aerosol.
A nebulizer in which a vibrating mesh or perforated membrane on top of a liquid medicine reservoir creates an aerosol.
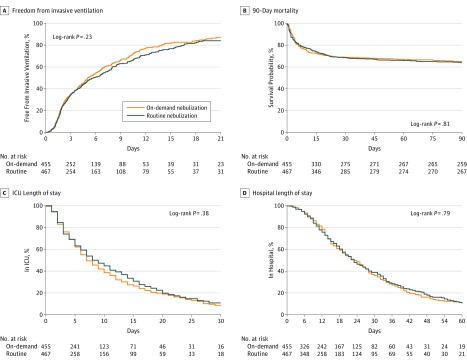
Mortality rates at 28 days, at hospital discharge, and at 90 days, as well as median length of ICU and hospital stay, did not significantly differ between groups (Table 3, Figure 2).
Table 3. Secondary Outcomes of Patients Receiving On-Demand Nebulization vs Routine Nebulization.
| Outcome | On-Demand Nebulization (n = 455) | Routine Nebulization (n = 467) | Absolute Difference (95% CI) | P Valuea |
|---|---|---|---|---|
| Mortality, No. of events/total No. (%) | ||||
| 28-d | 141/455 (31.0) | 149/467 (31.9) | –0.9 (–6.9 to 5.1) | .78 |
| ICU | 135/455 (29.7) | 137/467 (29.3) | 0.3 (–5.6 to 6.2) | .94 |
| 90-db | 160/415 (38.5) | 156/425 (36.7) | 1.8 (–4.7 to 8.4) | .62 |
| Hospitalb | 161/416 (38.7) | 165/438 (37.7) | 1.0 (–5.5 to 7.5) | .78 |
| Duration of invasive ventilation, median (IQR) | 4 (2-8) | 4 (2-10) | –0.5 (–1.3 to 0.2) | .28 |
| Length of stay, median (IQR), d | ||||
| ICU | 5 (2-10) | 5 (2-13) | –0.8 (–1.8 to 0.2) | .49 |
| Hospital | 15 (7-27) | 14 (6-27) | –0.9 (–3.0 to 1.2) | .57 |
| Pulmonary complications, No. (%)c | 204 (44.8) | 228 (48.8) | –4.0 (–10.4 to 2.4) | .24 |
| Moderate or severe ARDSd | 23 (5.1) | 30 (6.4) | –1.4 (–4.4 to 1.6) | .40 |
| VAPe | 14 (3.1) | 10 (2.1) | 0.9 (–1.1 to 3.0) | .41 |
| Severe atelectasisf | 175 (38.5) | 200 (42.8) | –4.4 (–10.7 to 2.0) | .18 |
| Pneumothoraxg | 25 (5.5) | 22 (4.7) | 0.8 (–2.1 to 3.6) | .65 |
| Tube occlusionh | 1 (0.2) | 2 (0.4) | -0.2 (–0.9 to 0.5) | .99 |
| Adverse events, No. (%)i | 63 (13.8) | 137 (29.3) | –15.5 (–20.7 to –10.3) | <.001 |
| Tachyarrhythmia | 57 (12.5) | 121 (25.9) | –13.4 (–18.4 to –8.4) | <.001 |
| Agitation | 1 (0.2) | 20 (4.3) | –4.1 (–5.9 to –2.2) | <.001 |
| Hypoxemia | 9 (2.0) | 20 (4.3) | –2.3 (–4.5 to –0.1) | .06 |
| Dyspnea | 1 (0.2) | 5 (1.1) | –0.9 (–1.9 to 0.2) | .22 |
| Bronchospasm | 1 (0.2) | 4 (0.9) | –0.6 (–1.6 to 0.3) | .37 |
| Apnea | 0 | 3 (0.6) | –0.6 (–1.4 to 0.1) | .25 |
| Self-extubation | 0 | 4 (0.9) | –0.9 (–1.7 to 0.0) | .06 |
| Vomiting | 1 (0.2) | 6 (1.3) | –1.1 (–2.2 to 0.0) | .12 |
Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IQR, interquartile range; VAP, ventilator-associated pneumonia.
Not corrected for multiple comparisons.
Follow-up was not complete for 90-day and hospital mortality.
Severe pulmonary complications that were not present at start of the study.
Moderate or severe ARDS according to the Berlin criteria.
New infiltrate on chest radiograph after more than 48 hours of invasive ventilation, for which antibiotics were started.
Atelectasis in more than 1 lobe.
Pneumothorax with or without the need for a pleural drain.
Tube occlusion requiring tube replacement.
Adverse events during and within the first hour after start of nebulization.
Figure 2. Freedom From Invasive Ventilation, 90-Day Mortality, and ICU and Hospital Length of Stay in the Randomization Groups.
ICU, intensive care unit.
The proportion of patients developing 1 or more pulmonary complications was not significantly different between the nebulization groups (44.8% vs 48.8%; difference, −4.0% [95% CI, −10.4% to 2.4%]; P = .24) (Table 3), whereas the proportion of patients developing 1 or more nebulization-related adverse events was higher in the routine group (13.8 vs 29.3%; difference, −15.5 [95% CI, −20.7% to −10.3%]; P < .001), with events mainly related to tachyarrhythmia (12.5% vs 25.9%; difference, −13.4 [95% CI, −18.4% to −8.4%]; P < .001) and agitation (0.2% vs 4.3%; difference, −4.1% [95% CI, −5.9% to −2.2%]; P < .001). There were no significant differences in adverse events directly related to nebulization with acetylcysteine or salbutamol (eTable 2 in Supplement 2).
Exploratory Analyses
With the exception of patients receiving invasive ventilation for pneumonia, the exploratory analyses demonstrated noninferiority for on-demand nebulization in all subgroups. There was no significant interaction between treatment assignment and subgroups (Table 2). The sensitivity analyses with the addition of the stratification variable center as random effect were consistent with the results of the primary end point.
Discussion
In this trial enrolling adult ICU patients receiving invasive ventilation and expected to not be extubated within 24 hours, on-demand nebulization of acetylcysteine or salbutamol was noninferior to routine nebulization of acetylcysteine with salbutamol with regard to the number of ventilator-free days and alive at day 28. Per-protocol analysis confirmed this finding. In addition, there was no significant difference in length of stay or mortality rates or in the proportion of patients developing pulmonary complications. Adverse events occurred more frequently with routine nebulization.
To our knowledge, no previous studies have addressed whether on-demand nebulization is as effective as routine nebulization in a general population of ICU patients receiving invasive ventilation. A noninferiority design was chosen because routine nebulization could be considered standard of care (at least in the Netherlands) and because it was hypothesized that a strategy in which nebulization needed strict indications would reduce the number of nebulization-related adverse effects, while not or only slightly affecting duration of ventilation. A patient-centered primary end point of ventilator-free days and alive at day 28 was chosen because pulmonary complications may affect duration of invasive ventilation as well as mortality.
One possible explanation for the finding that the 2 strategies of nebulization resulted in a not significantly different proportion of patients with acute respiratory distress syndrome, ventilator-associated pneumonia, and severe atelectasis is that prevention of mucus plugging with on-demand nebulization is noninferior to that achieved with routine nebulization. However, it could also be that mucus plugging plays a minor role in the pathophysiology of pulmonary complications in these patients. This hypothesis is supported by the fact that 59% of the patients in the on-demand group never received nebulization of acetylcysteine or salbutamol. It could still be that mucus plugging played a role, but concentrations of acetylcysteine in the smaller airways with nebulization were not high enough or did not work. Although nebulized salbutamol reduces the resistance of the airways, short-term widening of the smaller airways may not necessarily translate into improved mobilization of sputum in this population.
Another potential explanation for the findings observed in this trial could be the specific care for patients with invasive ventilation in the participating ICUs, possibly resulting in a high number of ventilator-free days. Care was provided by board-certified caregivers, who were present in all centers. Ventilators were switched to assisted ventilation early. Care followed strict protocols for lung-protective ventilation and weaning, sedation and pain management, infection prevention, and fluid management. Nurses in the participating centers performed standard airway care consisting of endotracheal suction if necessary and appropriate conditioning of inhaled air. In addition, all centers used infection prevention strategies against ventilator-associated pneumonia, including selective gut decontamination.
The most frequent nebulization-related adverse event was tachyarrhythmia, followed by hypoxemia and agitation, in both groups. The proportion of patients with nebulization-related agitation was higher in the routine group as compared with the on-demand group. Self-extubation, reported in 4 patients receiving routine nebulization and possibly related to agitation, may be considered potentially life-threatening.
Noninferiority could not be confirmed in patients with pneumonia. Conceivably, sputum plugging is more prominent in these patients than in patients without pulmonary symptoms, contributing to morbidity. Whether routine nebulization improves outcome in patients with pneumonia should be addressed in a future trial, as the present study was not powered to test this hypothesis.
This trial has strengths. The study design attempted to limit bias by using concealed allocation and intention-to-treat analysis using robust protocols; loss to follow-up was minimal; and the trial involved 7 centers, contributing to its generalizability. The on-demand group was designed to receive the minimal number of nebulizations per patient according to clinical needs. It was deliberately decided not to combine the 2 drugs in the on-demand group, allowing conclusions with regard to the specific adverse effects. Patients were enrolled in the trial over a period of 2 years, during which care had not changed. In addition, care for patients in the participating centers was standardized using clinical protocols, and attending nurses were skilled in nebulization therapy.
Limitations
This study also has several limitations. Because of the nature of the intervention tested, attending nurses and physicians could not be blinded to the intervention; this could be a major concern, since this knowledge could have affected adjunctive treatment and study findings. However, no differences in adjunctive respiratory care as provided by nurses were noted between the 2 groups. A large proportion of patients was admitted after out-of-hospital cardiac arrest, with known differences in outcome, but the exploratory analysis showed noninferiority in this subgroup. There was no significant interaction between treatment assignment and subgroups; however, the subgroup analysis may be underpowered and should be considered exploratory. Another limitation is that the results of this trial may only apply to those ICUs in which a strategy of routine nebulization is in place or being considered for implementation. In addition, the results of this trial cannot be extrapolated to other mucolytic and bronchodilating drugs.
Conclusions
Among ICU patients receiving invasive ventilation who were expected to not be extubated within 24 hours, on-demand compared with routine nebulization of acetylcysteine with salbutamol did not result in an inferior number of ventilator-free days. On-demand nebulization may be a reasonable alternative to routine nebulization.
Study Protocol
eMethods
eResults
eReferences
eTable 1. Ventilator Settings and Respiratory Variables
eTable 2. Nebulization-Related Adverse Events
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Icard BL, Rubio E. The role of mucoactive agents in the mechanically ventilated patient: a review of the literature. Expert Rev Respir Med. 2017;11(10):807-814. [DOI] [PubMed] [Google Scholar]
- 2.Konrad F, Schreiber T, Brecht-Kraus D, et al. . Mucociliary transport in ICU patients. Chest. 1994;105:237-241. [DOI] [PubMed] [Google Scholar]
- 3.Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4(1):59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin BK. The pharmacologic approach to airway clearance: mucoactive agents. Respir Care. 2002;47(7):818-822. [PubMed] [Google Scholar]
- 5.Konrad F, Schoenberg MH, Wiedmann H, et al. . The application of N-acetylcysteine as an antioxidant and mucolytic in mechanical ventilation in intensive care patients: a prospective, randomized, placebo-controlled, double-blind study. Anaesthesist. 1995;44(9):651-658. [DOI] [PubMed] [Google Scholar]
- 6.Masoompour SM, Anushiravani A, Tafaroj Norouz A. Evaluation of the effect of nebulized N-acetylcysteine on respiratory secretions in mechanically ventilated patients: randomized clinical trial. Iran J Med Sci. 2015;40(4):309-315. [PMC free article] [PubMed] [Google Scholar]
- 7.Rao S, Wilson DB, Brooks RC, et al. . Acute effects of nebulization of N-acetylcysteine on pulmonary mechanics and gas exchange. Am Rev Respir Dis. 1970;102(1):17-22. [DOI] [PubMed] [Google Scholar]
- 8.Grivans C, Lindgren S, Aneman A. A Scandinavian survey of drug administration through inhalation, suctioning and recruitment maneuvers in mechanically ventilated patients. Acta Anaesthesiol Scand. 2009;53(6):710-716. [DOI] [PubMed] [Google Scholar]
- 9.Dhand R. How should aerosols be delivered during invasive mechanical ventilation? Respir Care. 2017;62(10):1343-1367. [DOI] [PubMed] [Google Scholar]
- 10.Erhmann S, Roche-Campo F, Sferrazza Papa GF, et al. . Aerosol therapy during mechanical ventilation: an international survey. Intensive Care Med. 2013;39(6):1048-1056. [DOI] [PubMed] [Google Scholar]
- 11.Camamo JM, Weibel K, O’Keeffe T, et al. . Cost savings with interventions to reduce aerosolized bronchodilator use in mechanically ventilated patients. J Crit Care. 2014;29(5):814-816. [DOI] [PubMed] [Google Scholar]
- 12.Chang LH, Honiden S, Haithcock JA, et al. . Utilization of bronchodilators in ventilated patients without obstructive airways disease. Respir Care. 2007;52(2):154-158. [PubMed] [Google Scholar]
- 13.Ehrmann S, Roche-Campo F, Bodet-Contentin L, et al. . Aerosol therapy in intensive and intermediate care units: prospective observation of 2808 critically ill patients. Intensive Care Med. 2016;42(2):192-201. [DOI] [PubMed] [Google Scholar]
- 14.van der Hoeven SM, Binnekade JM, de Borgie CAJM, et al. . Preventive nebulization of mucolytic agents and bronchodilating drugs in invasively ventilated intensive care unit patients (NEBULAE): study protocol for a randomized controlled trial. Trials. 2015;16:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526-2533. [DOI] [PubMed] [Google Scholar]
- 16.van Vught LA, Klein Klouwenberg PM, Spitoni C, et al. . Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016;315(14):1469-1479. [DOI] [PubMed] [Google Scholar]
- 17.Graat ME, Choi G, Wolthuis EK, et al. . The clinical value of daily routine chest radiographs in a mixed medical-surgical intensive care unit is low. Crit Care. 2006;10(1):R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutch National Intensive Care Evaluation (NICE) Foundation website. https://www.stichting-nice.nl/. Accessed March 16, 2015.
- 19.Committee for Proprietary Medicinal Products Points to consider on switching between superiority and non-inferiority. Br J Clin Pharmacol. 2001;52(3):223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;(12):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment For Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 22.Schoenfeld DA, Bernard GR; ARDS Network . Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772-1777. [DOI] [PubMed] [Google Scholar]
- 23.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oostdijk EAN, Kesecioglu J, Schultz MJ, et al. . Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial [retracted and replaced in JAMA 2014;312(14):1429-1437]. JAMA. 2017;317(15):1583-1584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eMethods
eResults
eReferences
eTable 1. Ventilator Settings and Respiratory Variables
eTable 2. Nebulization-Related Adverse Events